Exploring the Prognosis-Related Genetic Variation in Gastric Cancer Based on mGWAS
Abstract
1. Introduction
2. Results
2.1. Basic Characteristics of Research Subjects
2.2. Metabolic Profiles of Plasma Samples
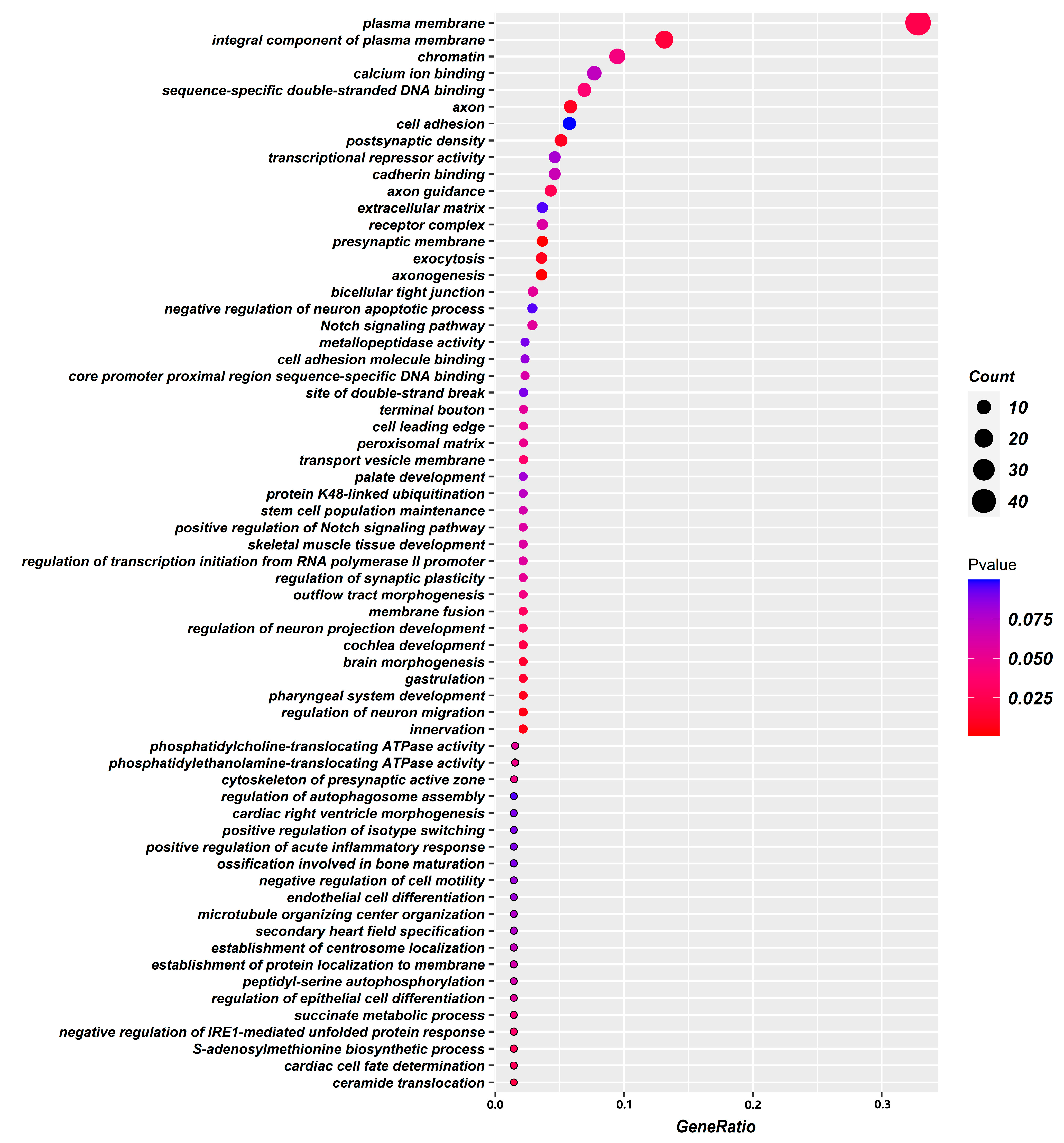
2.3. Functional Gene GO Pathway Enrichment Analysis
2.4. Analysis of the Association between Functional Gene Expression and Survival of Gastric Cancer
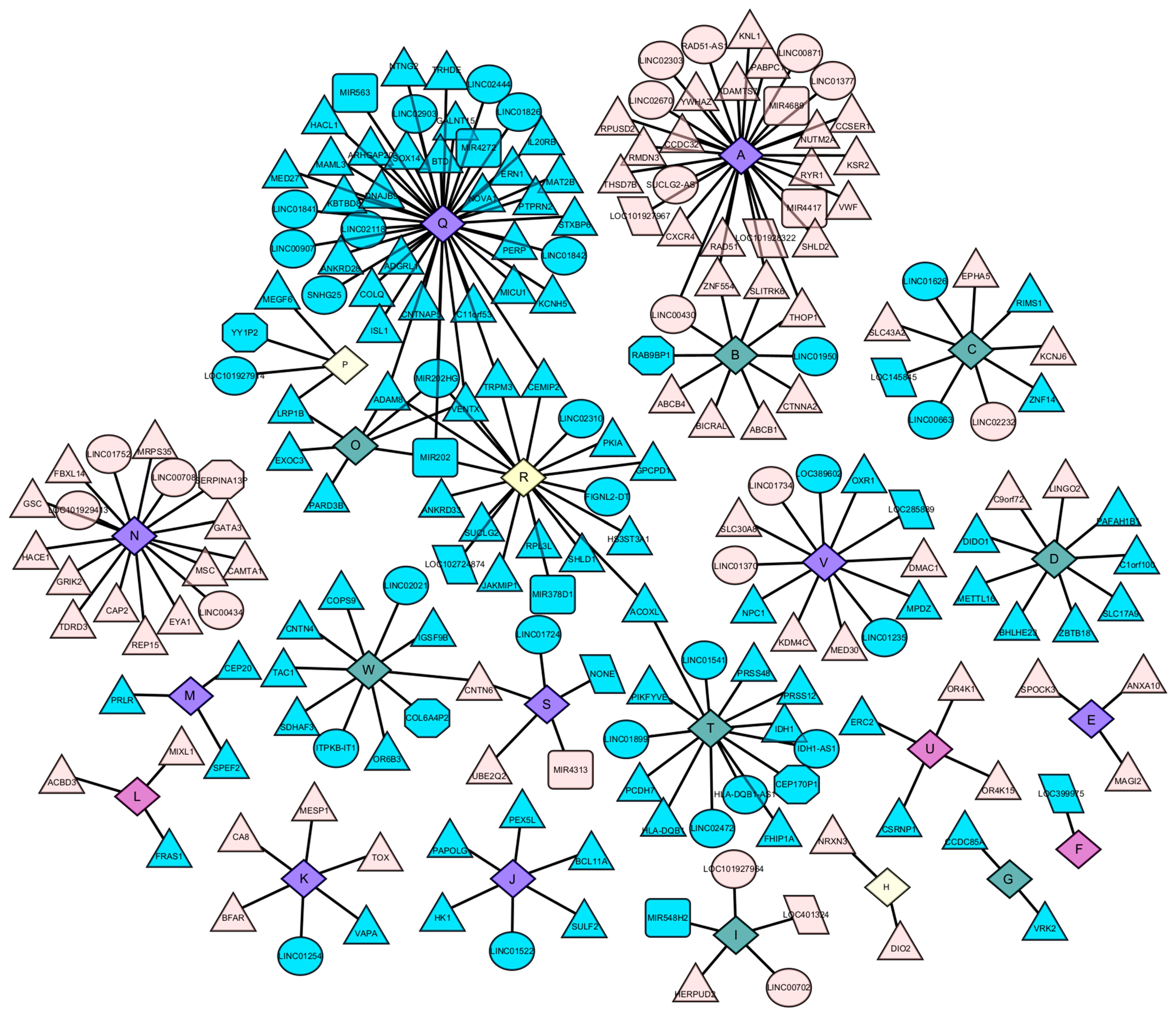
2.5. Construction of Metabolite Gene Interaction Networks
3. Discussion
4. Materials and Methods
4.1. Study Population and Sample
4.2. Peripheral Blood Genotyping
4.3. Non-Targeted Plasma Metabolite Assays
4.4. Quality Control
4.5. Data Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yan, C.; Zhu, M.; Ding, Y.; Yang, M.; Wang, M.; Li, G.; Ren, C.; Huang, T.; Yang, W.; He, B.; et al. Meta-analysis of genome-wide association studies and functional assays decipher susceptibility genes for gastric cancer in Chinese populations. Gut 2020, 69, 641–651. [Google Scholar] [CrossRef]
- Di Virgilio, F.; Adinolfi, E. Extracellular purines, purinergic receptors and tumor growth. Oncogene 2017, 36, 293–303. [Google Scholar] [CrossRef]
- Sathe, A.; Grimes, S.M.; Lau, B.T.; Chen, J.; Suarez, C.; Huang, R.J.; Poultsides, G.; Ji, H.P. Single-Cell Genomic Characterization Reveals the Cellular Reprogramming of the Gastric Tumor Microenvironment. Clin. Cancer Res. 2020, 26, 2640–2653. [Google Scholar] [CrossRef] [PubMed]
- Reimann, E.; Kõks, S.; Ho, X.D.; Maasalu, K.; Märtson, A. Whole exome sequencing of a single osteosarcoma case--integrative analysis with whole transcriptome RNA-seq data. Hum. Genom. 2014, 8, 20. [Google Scholar]
- Cao, N.; Li, M.; Han, J.; Wang, Y.; Wang, X. rs61991156 in miR-379 is associated with low capability of glycolysis of gastric cancer by enhanced regulation of PKM2. Cancer Cell Int. 2018, 18, 92. [Google Scholar] [CrossRef] [PubMed]
- Xiao, S.; Zhou, L. Gastric cancer: Metabolic and metabolomics perspectives (Review). Int. J. Oncol. 2017, 51, 5–17. [Google Scholar] [CrossRef] [PubMed]
- Adamski, J.; Suhre, K. Metabolomics platforms for genome wide association studies--linking the genome to the metabolome. Curr. Opin. Biotechnol. 2013, 24, 39–47. [Google Scholar] [CrossRef]
- Zhang, G.; Saito, R.; Sharma, K. A metabolite-GWAS (mGWAS) approach to unveil chronic kidney disease progression. Kidney Int. 2017, 91, 1274–1276. [Google Scholar] [CrossRef]
- Gao, H.; Le, Y.; Wu, X.; Silberstein, L.E.; Giese, R.W.; Zhu, Z. VentX, a novel lymphoid-enhancing factor/T-cell factor-associated transcription repressor, is a putative tumor suppressor. Cancer Res. 2010, 70, 202–211. [Google Scholar] [CrossRef]
- Le, Y.; Gao, H.; Bleday, R.; Zhu, Z. The homeobox protein VentX reverts immune suppression in the tumor microenvironment. Nat. Commun. 2018, 9, 2175. [Google Scholar] [CrossRef]
- Le, Y.; Gao, H.; Richards, W.; Zhao, L.; Bleday, R.; Clancy, T.; Zhu, Z. VentX expression in tumor-associated macrophages promotes phagocytosis and immunity against pancreatic cancers. JCI Insight 2020, 5, e137088. [Google Scholar] [CrossRef]
- Hirayama, T.; Yagi, T. Regulation of clustered protocadherin genes in individual neurons. Semin. Cell Dev. Biol. 2017, 69, 122–130. [Google Scholar] [CrossRef]
- Zhang, S.; Fu, X. The Clinical Significance and Biological Function of PCDH7 in Cervical Cancer. Cancer Manag. Res. 2021, 13, 3841–3847. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Shen, L.; Chen, B.; Han, X.; Yu, Y.; Yuan, X.; Zhong, L. The predictive prognostic values of CBFA2T3, STX3, DENR, EGLN1, FUT4, and PCDH7 in lung cancer. Ann. Transl. Med. 2021, 9, 843. [Google Scholar] [CrossRef]
- Okuno, K.; Akiyama, Y.; Shimada, S.; Nakagawa, M.; Tanioka, T.; Inokuchi, M.; Yamaoka, S.; Kojima, K.; Tanaka, S. Asymmetric dimethylation at histone H3 arginine 2 by PRMT6 in gastric cancer progression. Carcinogenesis 2019, 40, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Sun, X.; Ge, W.; Qian, Y.; Bai, R.; Zheng, S. A seven-gene signature predicts overall survival of patients with colorectal cancer. Oncotarget 2017, 8, 95054–95065. [Google Scholar] [CrossRef] [PubMed]
- Penney, J.; Tsai, L.H. JAKMIP1: Translating the Message for Social Behavior. Neuron 2015, 88, 1070–1072. [Google Scholar] [CrossRef][Green Version]
- Okai, I.; Wang, L.; Gong, L.; Arko-Boham, B.; Hao, L.; Zhou, X.; Qi, X.; Hu, J.; Shao, S. Overexpression of JAKMIP1 associates with Wnt/β-catenin pathway activation and promotes cancer cell proliferation in vitro. Biomed. Pharmacother. 2013, 67, 228–234. [Google Scholar] [CrossRef]
- Libri, V.; Schulte, D.; van Stijn, A.; Ragimbeau, J.; Rogge, L.; Pellegrini, S. Jakmip1 is expressed upon T cell differentiation and has an inhibitory function in cytotoxic T lymphocytes. J. Immunol. 2008, 181, 5847–5856. [Google Scholar] [CrossRef]
- Ennour-Idrissi, K.; Dragic, D.; Issa, E.; Michaud, A.; Chang, S.L.; Provencher, L.; Durocher, F.; Diorio, C. DNA Methylation and Breast Cancer Risk: An Epigenome-Wide Study of Normal Breast Tissue and Blood. Cancers 2020, 12, 3088. [Google Scholar] [CrossRef]
- Wang, S.; Tong, Y.; Zong, H.; Xu, X.; Crabbe, M.J.C.; Wang, Y.; Zhang, X. Multi-Level Analysis and Identification of Tumor Mutational Burden Genes across Cancer Types. Genes 2022, 13, 365. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Xu, Z.Y.; Ruan, S.M.; Mo, S.; Qin, J.J.; Cheng, X.D. Long non-coding RNAs towards precision medicine in gastric cancer: Early diagnosis, treatment, and drug resistance. Mol. Cancer 2020, 19, 96. [Google Scholar] [CrossRef]
- Ouyang, J.; Xie, Z.; Lei, X.; Tang, G.; Gan, R.; Yang, X. Clinical crosstalk between microRNAs and gastric cancer (Review). Int. J. Oncol. 2021, 58, 7. [Google Scholar] [CrossRef]
- Peng, J.; Shi, S.; Yu, J.; Liu, J.; Wei, H.; Song, H.; Wang, S.; Li, Z.; He, S.; Li, L.; et al. miR-378d suppresses malignant phenotype of ESCC cells through AKT signaling. Cancer Cell Int. 2021, 21, 702. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.C.; Tian, L.L.; Fan, C.X.; Duo, C.H.; Xu, K.M. The Adaptive Responses in Non-Small Cell Lung Cancer A549 Cell Lines Induced by Low-Dose Ionizing Radiation and the Variations of miRNA Expression. Dose Response 2021, 19, 15593258211039931. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Zhao, S.; Shi, Z.; Cao, L.; Liu, J.; Pan, T.; Zhou, D.; Zhang, J. Chemotherapy-elicited exosomal miR-378a-3p and miR-378d promote breast cancer stemness and chemoresistance via the activation of EZH2/STAT3 signaling. J. Exp. Clin. Cancer Res. 2021, 40, 120. [Google Scholar] [CrossRef]
- Li, Y.Y.; Yang, C.; Zhou, P.; Zhang, S.; Yao, Y.; Li, D. Genome-scale analysis to identify prognostic markers and predict the survival of lung adenocarcinoma. J. Cell Biochem. 2018, 119, 8909–8921. [Google Scholar] [CrossRef]
- Zhao, W.; Wang, J.; Luo, Q.; Peng, W.; Li, B.; Wang, L.; Zhang, C.; Duan, C. Identification of LINC02310 as an enhancer in lung adenocarcinoma and investigation of its regulatory network via comprehensive analyses. BMC Med. Genom. 2020, 13, 185. [Google Scholar] [CrossRef]
- Lyu, Y.; Yang, S.; Lyu, X.; Wang, Y.L.; Ji, S.; Kang, S.; Jiang, Y.; Xiang, J.; He, C.; Li, P.; et al. lncRNA polymorphism affects the prognosis of gastric cancer. World J. Surg. Oncol. 2022, 20, 273. [Google Scholar] [CrossRef]
- Cao, K.; Lyu, Y.; Chen, J.; He, C.; Lyu, X.; Zhang, Y.; Chen, L.; Jiang, Y.; Xiang, J.; Liu, B.; et al. Prognostic Implication of Plasma Metabolites in Gastric Cancer. Int. J. Mol. Sci. 2023, 24, 12774. [Google Scholar] [CrossRef]
- Yang, S.; Wang, Y.L.; Lyu, Y.; Jiang, Y.; Xiang, J.; Ji, S.; Kang, S.; Lyu, X.; He, C.; Li, P.; et al. mGWAS identification of six novel single nucleotide polymorphism loci with strong correlation to gastric cancer. Cancer Metab. 2021, 9, 34. [Google Scholar] [CrossRef]
- Dennis, E.A.; Norris, P.C. Eicosanoid storm in infection and inflammation. Nat. Rev. Immunol. 2015, 15, 511–523. [Google Scholar] [CrossRef]
- Serhan, C.N.; Savill, J. Resolution of inflammation: The beginning programs the end. Nat. Immunol. 2005, 6, 1191–1197. [Google Scholar] [CrossRef]
- Liu, F.; Zuo, X.; Liu, Y.; Deguchi, Y.; Moussalli, M.J.; Chen, W.; Yang, P.; Wei, B.; Tan, L.; Lorenzi, P.L.; et al. Suppression of Membranous LRP5 Recycling, Wnt/β-Catenin Signaling, and Colon Carcinogenesis by 15-LOX-1 Peroxidation of Linoleic Acid in PI3P. Cell Rep. 2020, 32, 108049. [Google Scholar] [CrossRef]
- Chen, D.; Zhang, Y.; Wang, W.; Chen, H.; Ling, T.; Yang, R.; Wang, Y.; Duan, C.; Liu, Y.; Guo, X.; et al. Identification and Characterization of Robust Hepatocellular Carcinoma Prognostic Subtypes Based on an Integrative Metabolite-Protein Interaction Network. Adv. Sci. 2021, 8, e2100311. [Google Scholar] [CrossRef]
- Ma, Q.L.; Zhu, C.; Morselli, M.; Su, T.; Pelligrini, M.; Lu, Z.; Jones, M.; Denver, P.; Castro, D.; Gu, X.; et al. The Novel Omega-6 Fatty Acid Docosapentaenoic Acid Positively Modulates Brain Innate Immune Response for Resolving Neuroinflammation at Early and Late Stages of Humanized APOE-Based Alzheimer’s Disease Models. Front. Immunol. 2020, 11, 558036. [Google Scholar] [CrossRef]
- Wu, F.Z.; Xu, W.J.; Deng, B.; Liu, S.D.; Deng, C.; Wu, M.Y.; Gao, Y.; Jia, L.Q. Wen-Luo-Tong Decoction Attenuates Paclitaxel-Induced Peripheral Neuropathy by Regulating Linoleic Acid and Glycerophospholipid Metabolism Pathways. Front. Pharmacol. 2018, 9, 956. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Ren, W.; Huang, X.; Deng, J.; Li, T.; Yin, Y. Potential Mechanisms Connecting Purine Metabolism and Cancer Therapy. Front. Immunol. 2018, 9, 1697. [Google Scholar] [CrossRef] [PubMed]
- Frenguelli, B.G.; Dale, N. Purines: From Diagnostic Biomarkers to Therapeutic Agents in Brain Injury. Neurosci. Bull. 2020, 36, 1315–1326. [Google Scholar] [CrossRef] [PubMed]
- Illes, P.; Rubini, P.; Yin, H.; Tang, Y. Impaired ATP Release from Brain Astrocytes May be a Cause of Major Depression. Neurosci. Bull. 2020, 36, 1281–1284. [Google Scholar] [CrossRef]
- Niesler, B.; Kuerten, S.; Demir, I.E.; Schäfer, K.H. Disorders of the enteric nervous system—A holistic view. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 393–410. [Google Scholar] [CrossRef]
- Human Metabolome Database. Available online: https://hmdb.ca (accessed on 15 September 2021).
- Das, S.; Forer, L.; Schönherr, S.; Sidore, C.; Locke, A.E.; Kwong, A.; Vrieze, S.I.; Chew, E.Y.; Levy, S.; McGue, M.; et al. Next-generation genotype imputation service and methods. Nat. Genet. 2016, 48, 1284–1287. [Google Scholar] [CrossRef] [PubMed]
- R: A Language and Environment for Statistical Computing. Available online: https://www.R-project.org/ (accessed on 1 June 2020).
- Turner, S. qqman: An R package for visualizing GWAS results using Q-Q and manhattan plots. J. Open Source Softw. 2018, 3, 731. [Google Scholar] [CrossRef]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef] [PubMed]


| N | Kaplan–Meier | Cox Regression | ||||
|---|---|---|---|---|---|---|
| 5-Year of Survival Rate (%) | Log-Rank p | HR (95% CI) | p | |||
| Gender | 0.617 | |||||
| male | 162 | 37.49 | 1.00 | |||
| female | 56 | 37.50 | 0.906 (0.617–1.33) | 0.615 | ||
| Age | 0.043 * | |||||
| <65 | 45 | 51.11 | 1.00 | |||
| ≥65 | 173 | 33.97 | 1.588 (1.007–2.506) | 0.047 * | ||
| TNM staging | <0.001 *** | |||||
| early | 41 | 87.80 | 1.00 | |||
| middle | 74 | 53.40 | 4.223 (1.771–10.072) | 0.001 ** | ||
| late | 103 | 5.83 | 24.457 (10.481–57.069) | <0.001 *** | ||
| Surgery | <0.001 *** | |||||
| no | 78 | 16.67 | 1.00 | |||
| yes | 140 | 49.06 | 0.345 (0.245–0.484) | <0.001 *** | ||
| Chemotherapy | 0.007 ** | |||||
| no | 102 | 30.39 | 1.00 | |||
| yes | 116 | 43.76 | 0.634 (0.454–0.885) | 0.007 ** | ||
| Radiotherapy | 0.420 | |||||
| no | 189 | 36.34 | 1.00 | |||
| yes | 29 | 44.83 | 0.809 (0.48–1.364) | 0.427 | ||
| Tumor location | 0.184 | |||||
| Not in Cardiac | 71 | 46.48 | 1.00 | |||
| In Cardiac | 147 | 33.10 | 1.287 (0.89–1.861) | 0.179 | ||
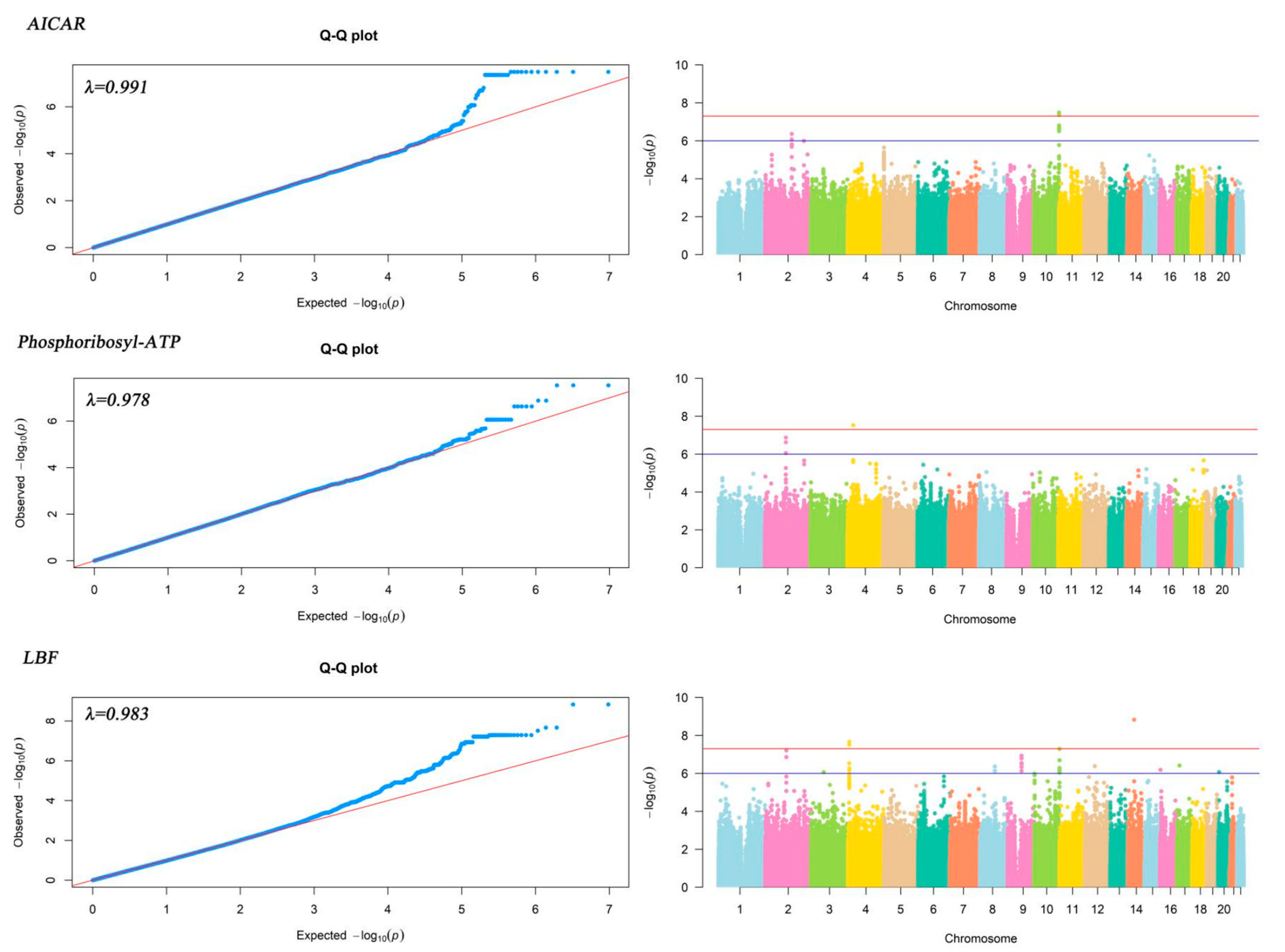
| Metabolites | dbSNP * | Chromosome | Alt | Ref | p-Value | Gene.refGene |
|---|---|---|---|---|---|---|
| AICAR | rs7076167 | 10 | T | C | 3.26 × 10−8 | VENTX |
| rs6537595 | 10 | A | T | 3.26 × 10−8 | VENTX; MIR202HG | |
| rs4838708 | 10 | A | G | 4.40 × 10−8 | VENTX; MIR202HG | |
| Phosphoribosyl-ATP | rs11947372 | 4 | T | A | 2.93 × 10−8 | LINC02472; PCDH7 |
| rs73107710 | 4 | T | G | 2.93 × 10−8 | LINC02472; PCDH7 | |
| rs4346608 | 4 | C | T | 2.93 × 10−8 | LINC02472; PCDH7 | |
| LBF | rs7682973 | 4 | A | G | 2.17 × 10−8 | MIR378D1; JAKMIP1 |
| rs7157739 | 14 | G | A | 1.49 × 10−9 | LINC02310 | |
| rs7158729 | 14 | T | G | 1.49 × 10−9 | LINC02310 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Lyu, Y.; Chen, L.; Cao, K.; Chen, J.; He, C.; Lyu, X.; Jiang, Y.; Xiang, J.; Liu, B.; et al. Exploring the Prognosis-Related Genetic Variation in Gastric Cancer Based on mGWAS. Int. J. Mol. Sci. 2023, 24, 15259. https://doi.org/10.3390/ijms242015259
Zhang Y, Lyu Y, Chen L, Cao K, Chen J, He C, Lyu X, Jiang Y, Xiang J, Liu B, et al. Exploring the Prognosis-Related Genetic Variation in Gastric Cancer Based on mGWAS. International Journal of Molecular Sciences. 2023; 24(20):15259. https://doi.org/10.3390/ijms242015259
Chicago/Turabian StyleZhang, Yuling, Yanping Lyu, Liangping Chen, Kang Cao, Jingwen Chen, Chenzhou He, Xuejie Lyu, Yu Jiang, Jianjun Xiang, Baoying Liu, and et al. 2023. "Exploring the Prognosis-Related Genetic Variation in Gastric Cancer Based on mGWAS" International Journal of Molecular Sciences 24, no. 20: 15259. https://doi.org/10.3390/ijms242015259
APA StyleZhang, Y., Lyu, Y., Chen, L., Cao, K., Chen, J., He, C., Lyu, X., Jiang, Y., Xiang, J., Liu, B., & Wu, C. (2023). Exploring the Prognosis-Related Genetic Variation in Gastric Cancer Based on mGWAS. International Journal of Molecular Sciences, 24(20), 15259. https://doi.org/10.3390/ijms242015259








