Chemical Insights into Oxidative and Nitrative Modifications of DNA
Abstract
1. Introduction
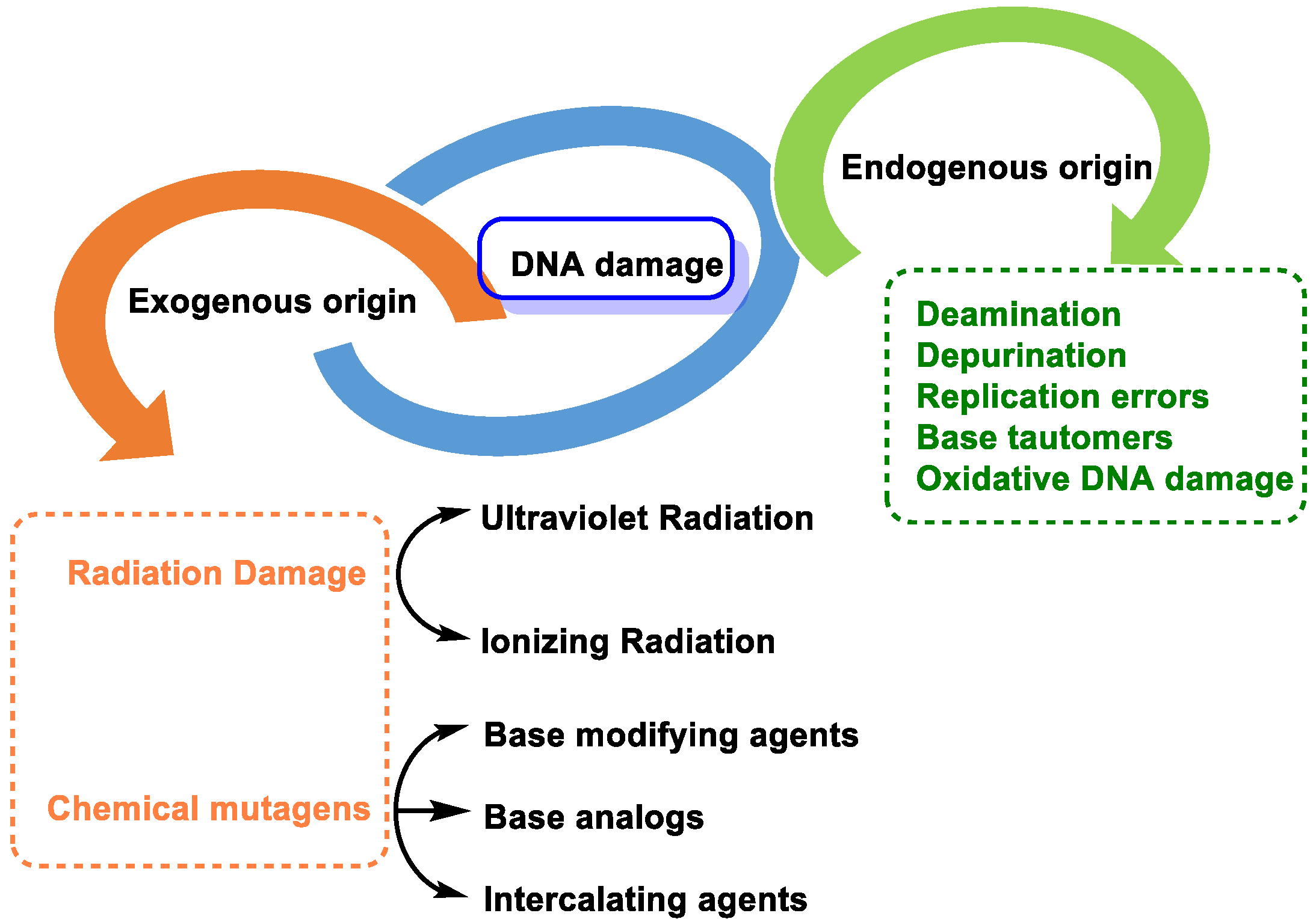
2. Causes of DNA Damage
2.1. DNA Damage by Endogenous Cellular Processes
- -
- Replication errors: DNA polymerase can incorrectly select the nucleotide for insertion into the complementary strand during replication. The substitution of one nucleotide for another is a point mutation, which is the simplest form of DNA damage [8].
- -
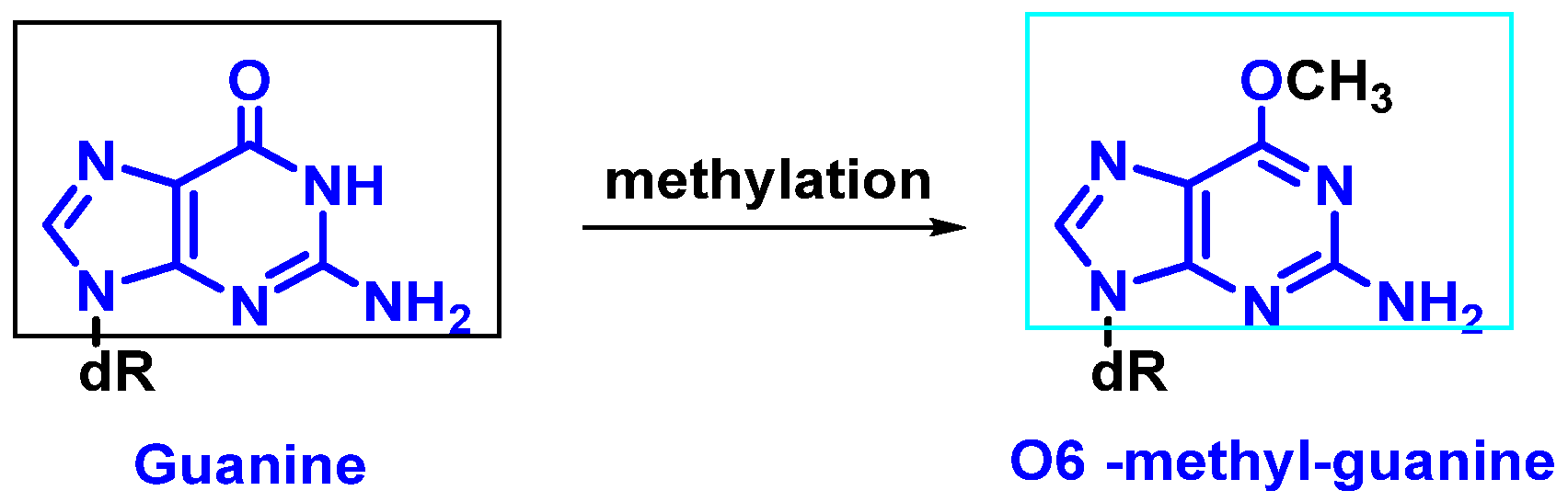
- Modification of nitrogenous bases by methylation: Atypical methylation of nitrogenous bases produces thousands of DNA lesions per cell per day. An example is the formation of a methylated derivative of guanine (O6-methyl-guanine). This modified base can generate a post-replicative mutation because it can pair with the same probability with cytosines or thymines [9] (Figure 4).
- -
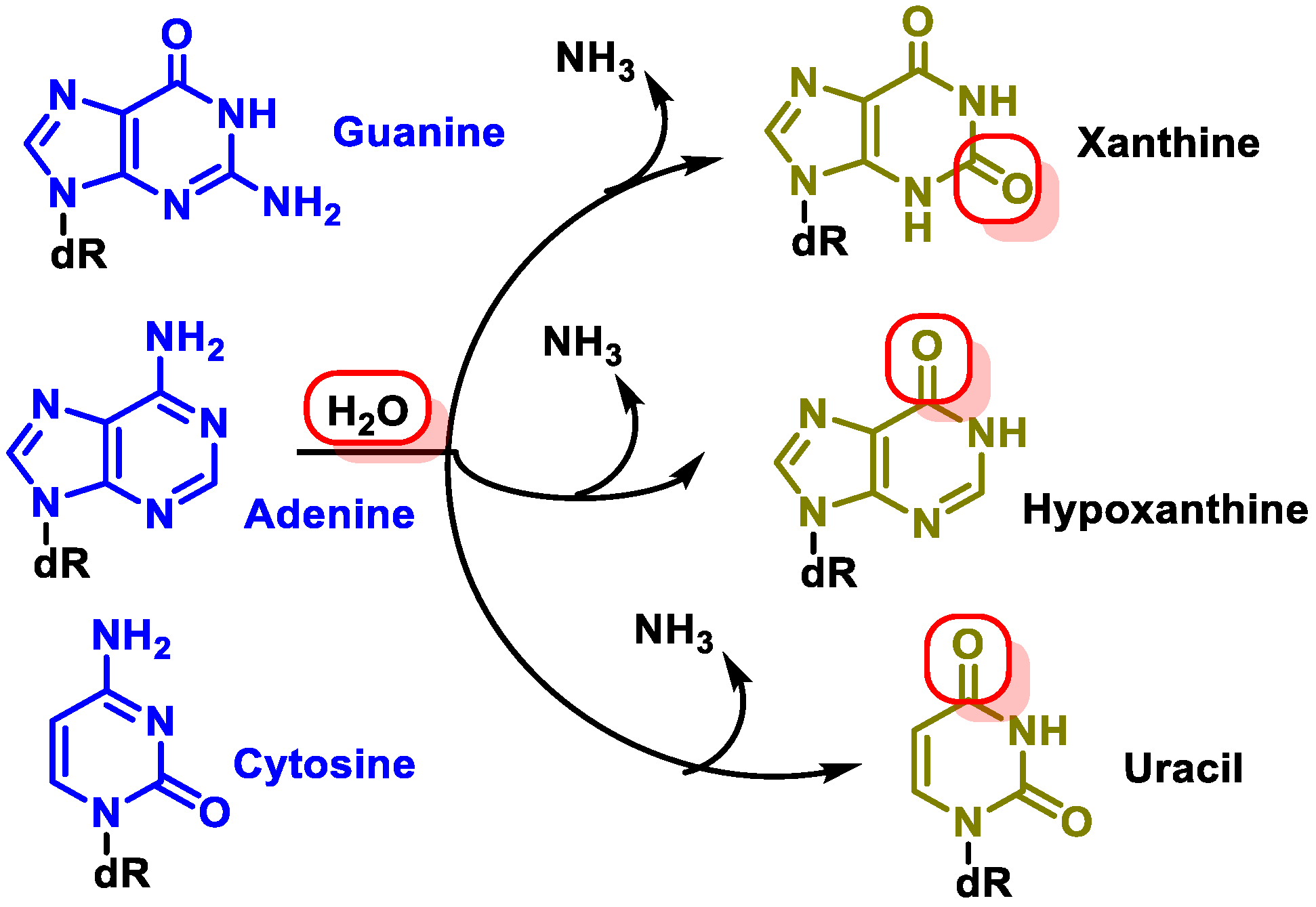
- Changes in DNA’s nitrogenous bases by deamination: Another type of alteration that DNA undergoes is the loss of amino groups from its nitrogenous bases. Three of the four DNA bases, adenine, guanine, and cytosine, contain amino groups that can be lost in a variety of temperature- and pH-dependent reactions, thereby transforming them into hypoxanthine, xanthine, and uracil [10] (Figure 5).
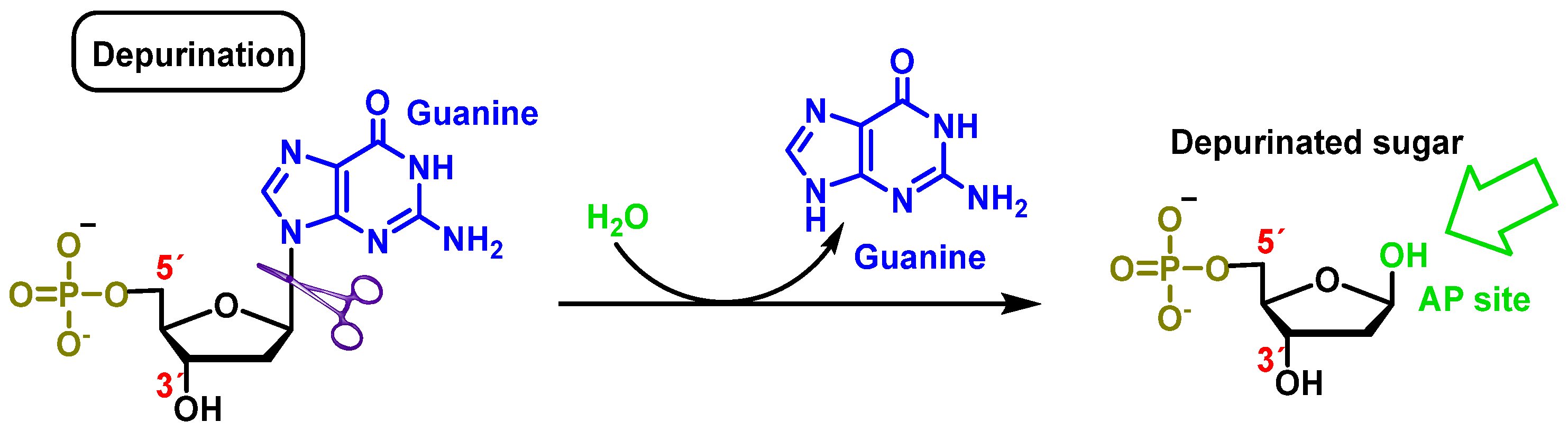
2.2. Loss of Nitrogenous Bases by Depurination or Depyrimidination
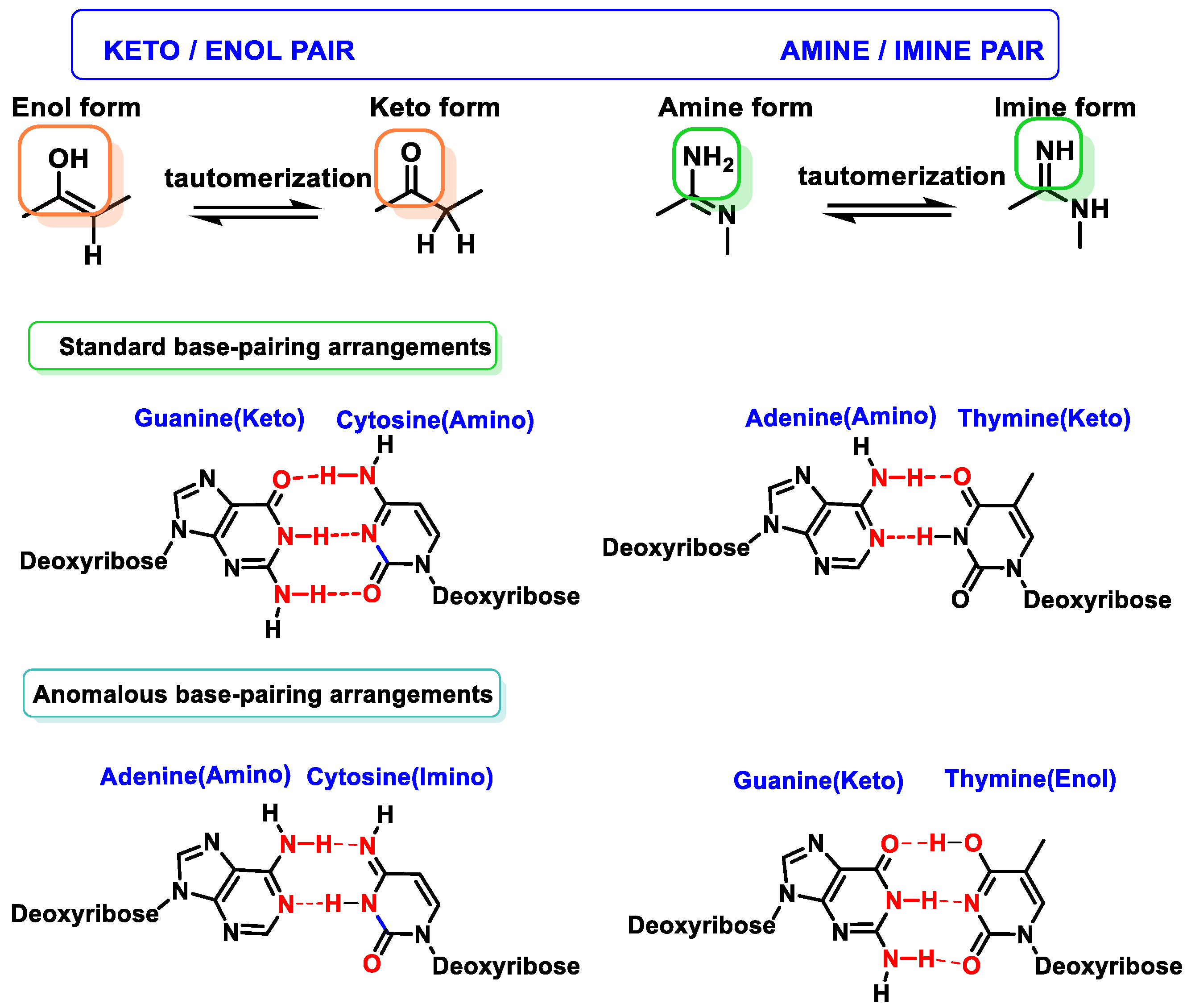
2.3. Base Tautomers
2.4. DNA Damage by Exogenous Agents
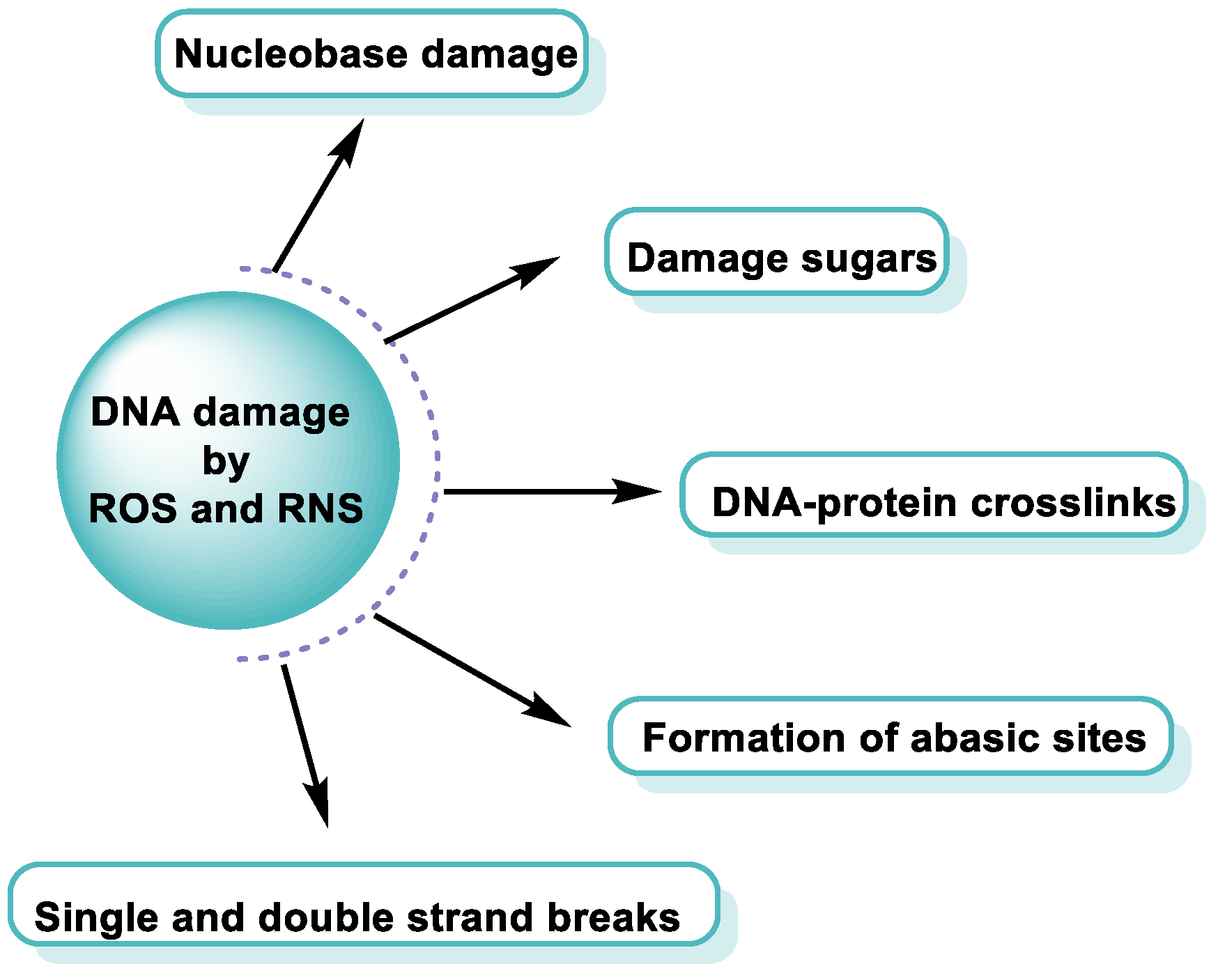
3. Damage to DNA by ROS and RNS
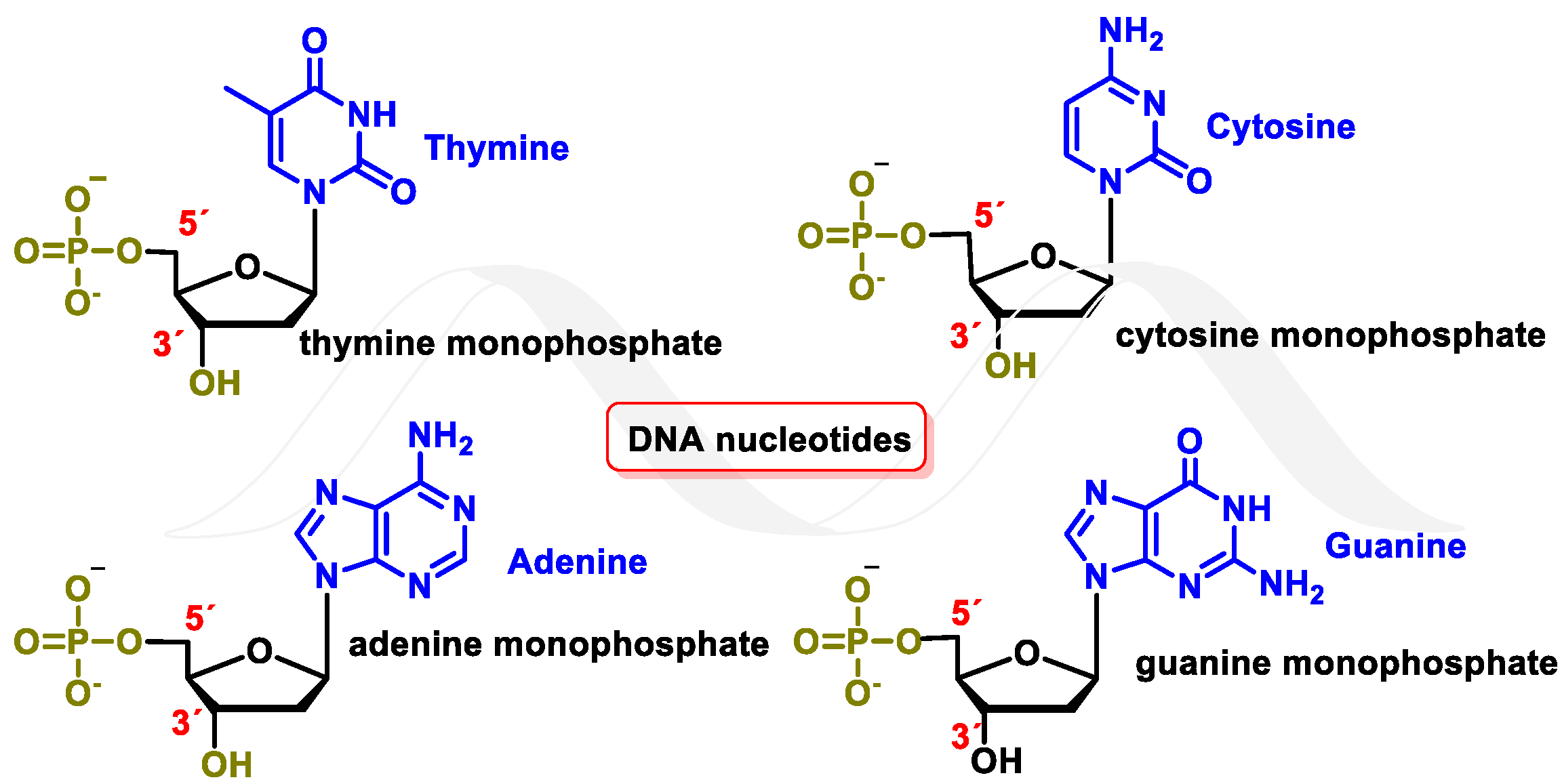
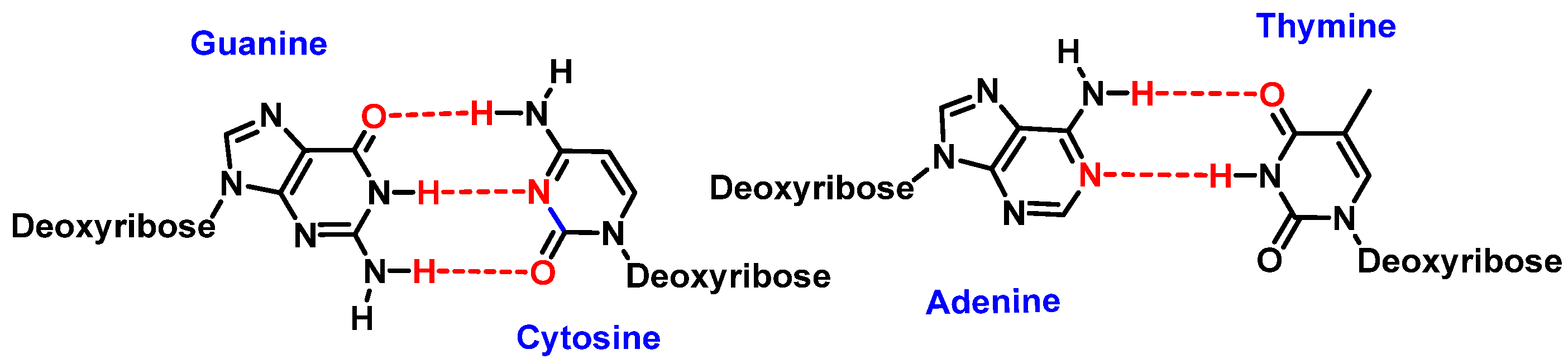
4. Major Damage to the DNA Structure
Modifications of Nitrogenous Bases
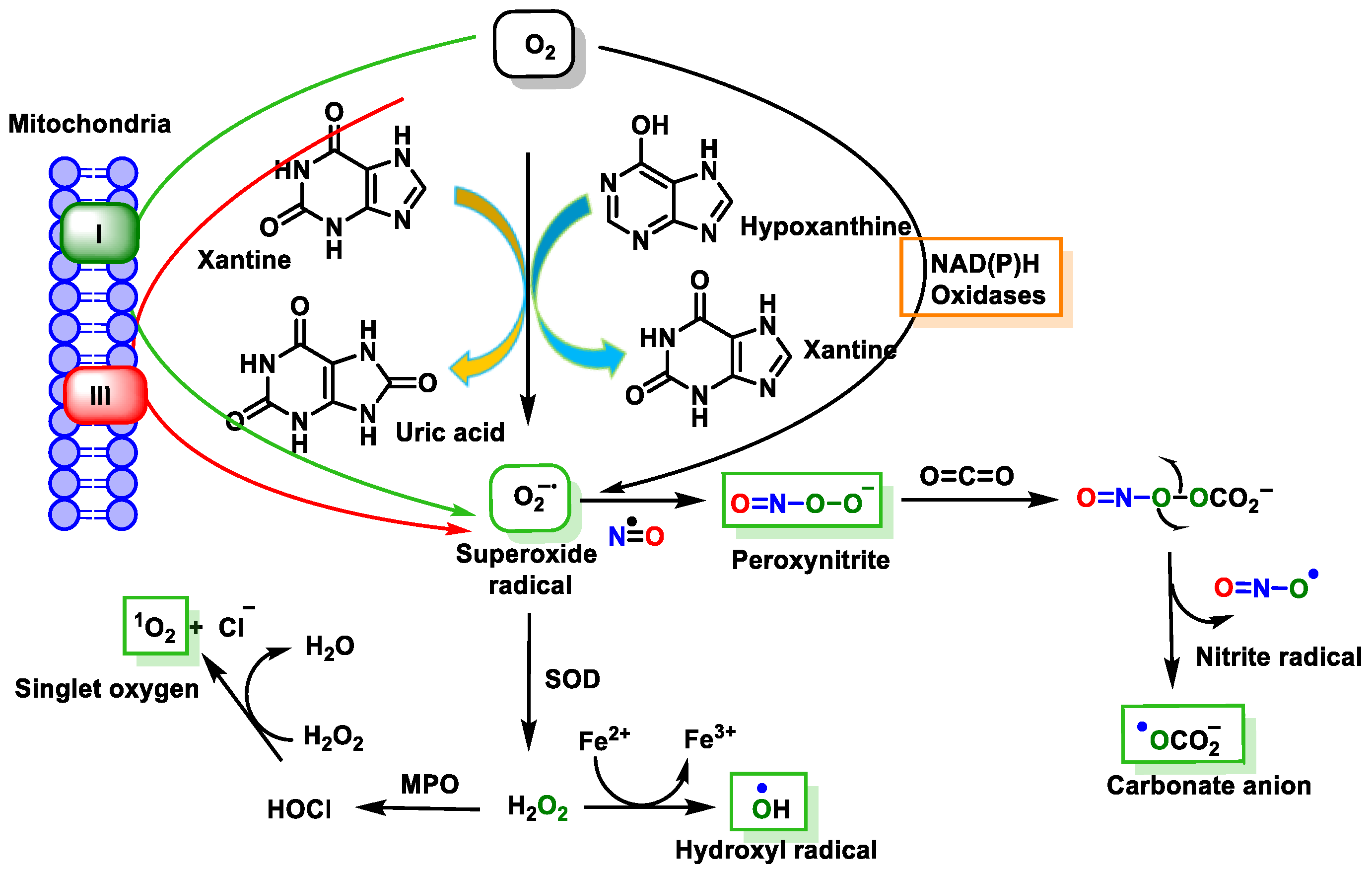
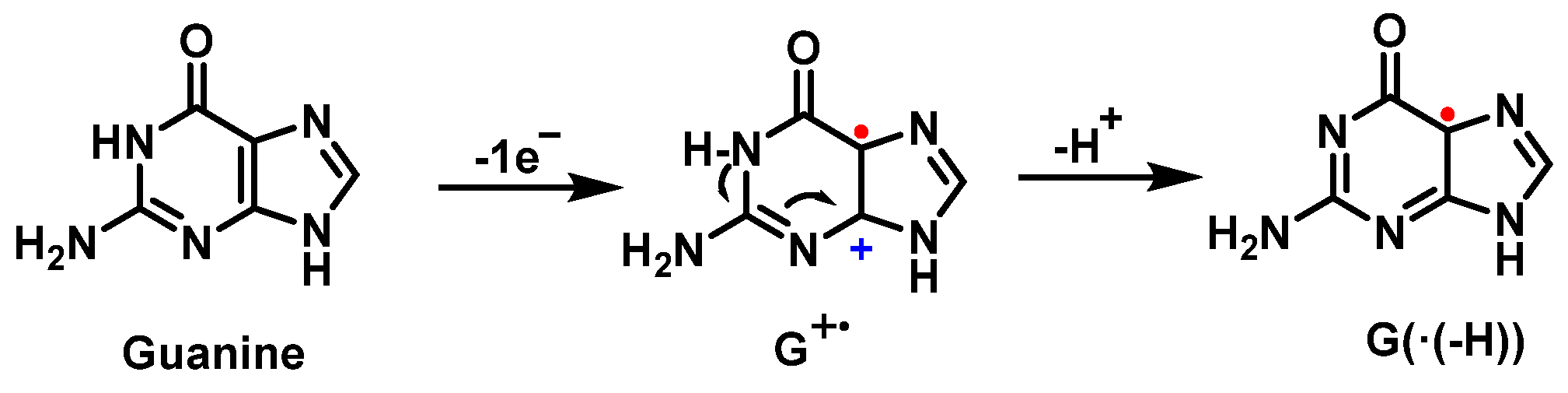
5. Superoxide Anion and DNA Damage
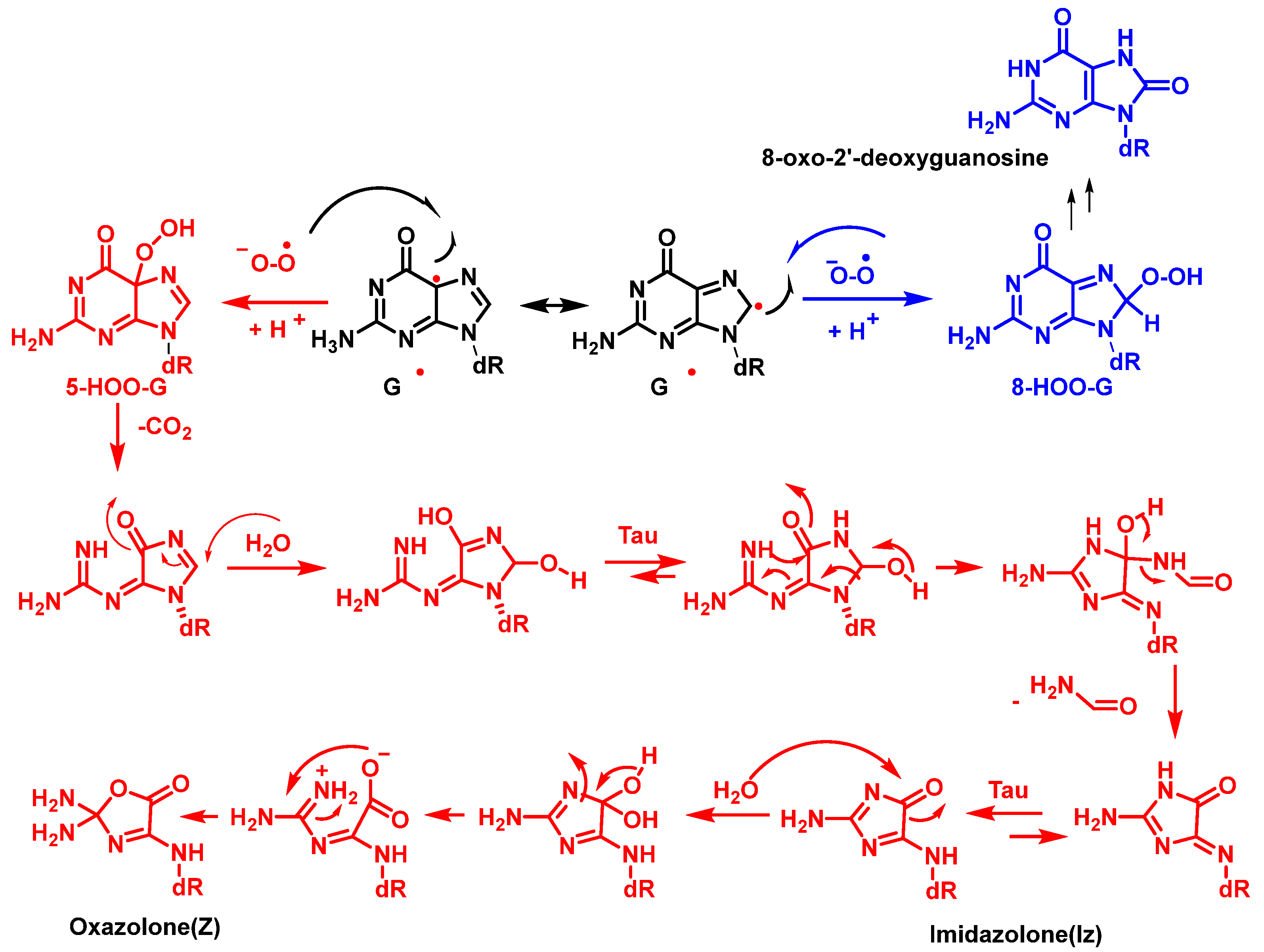
6. Singlet Oxygen and DNA Damage
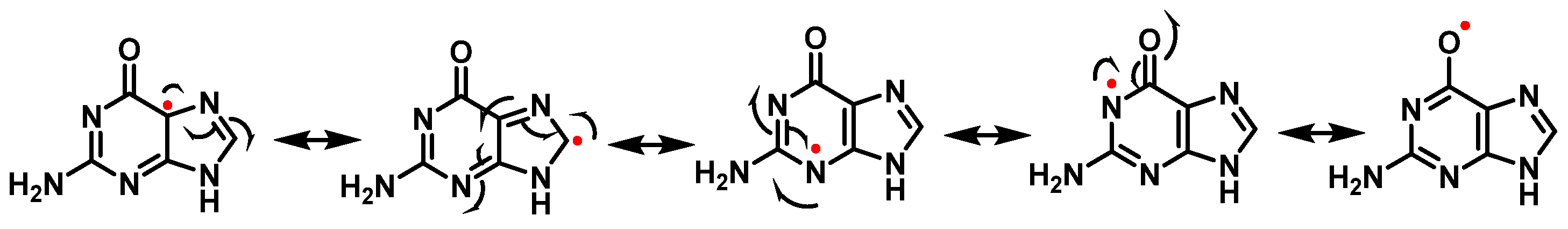
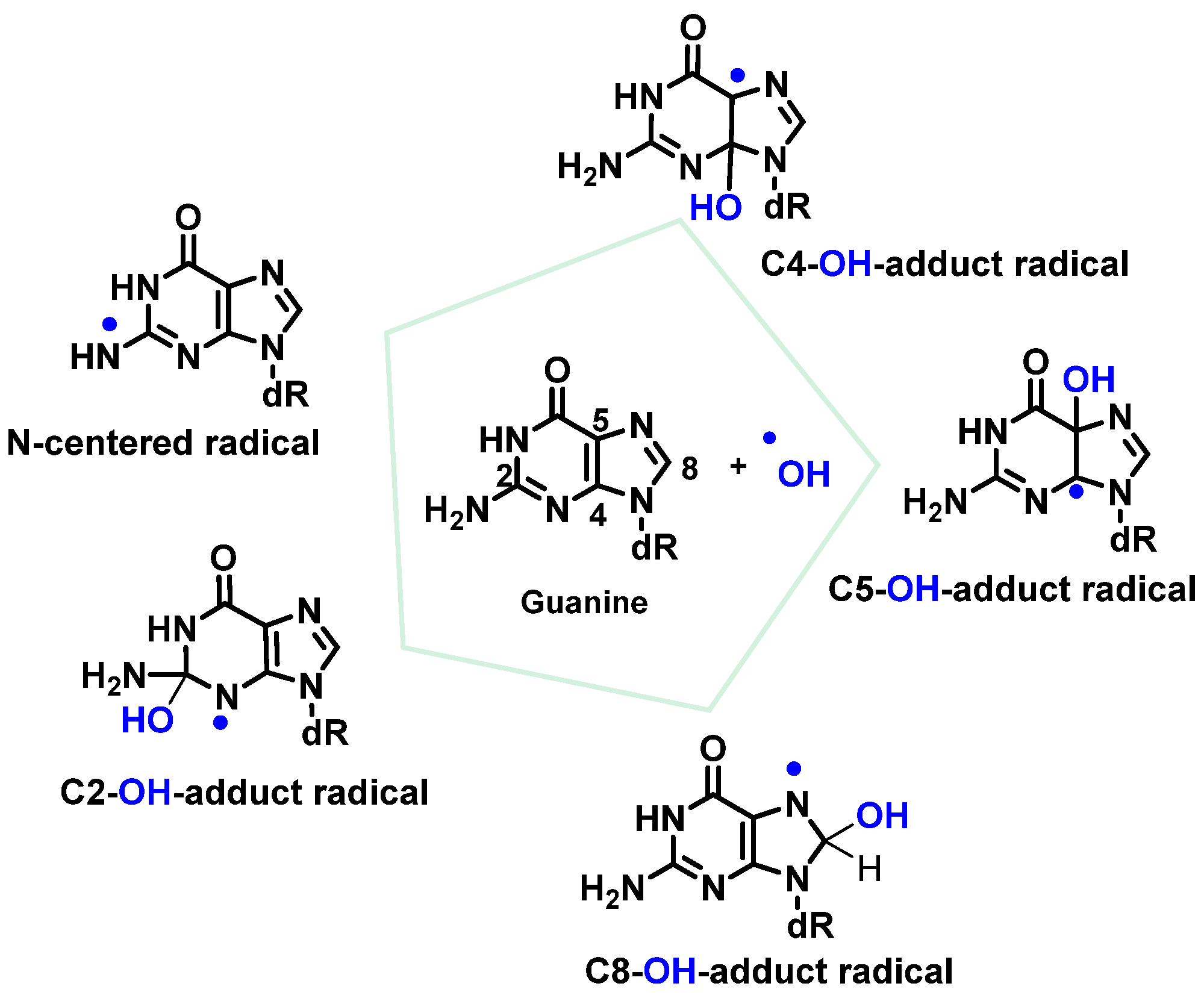
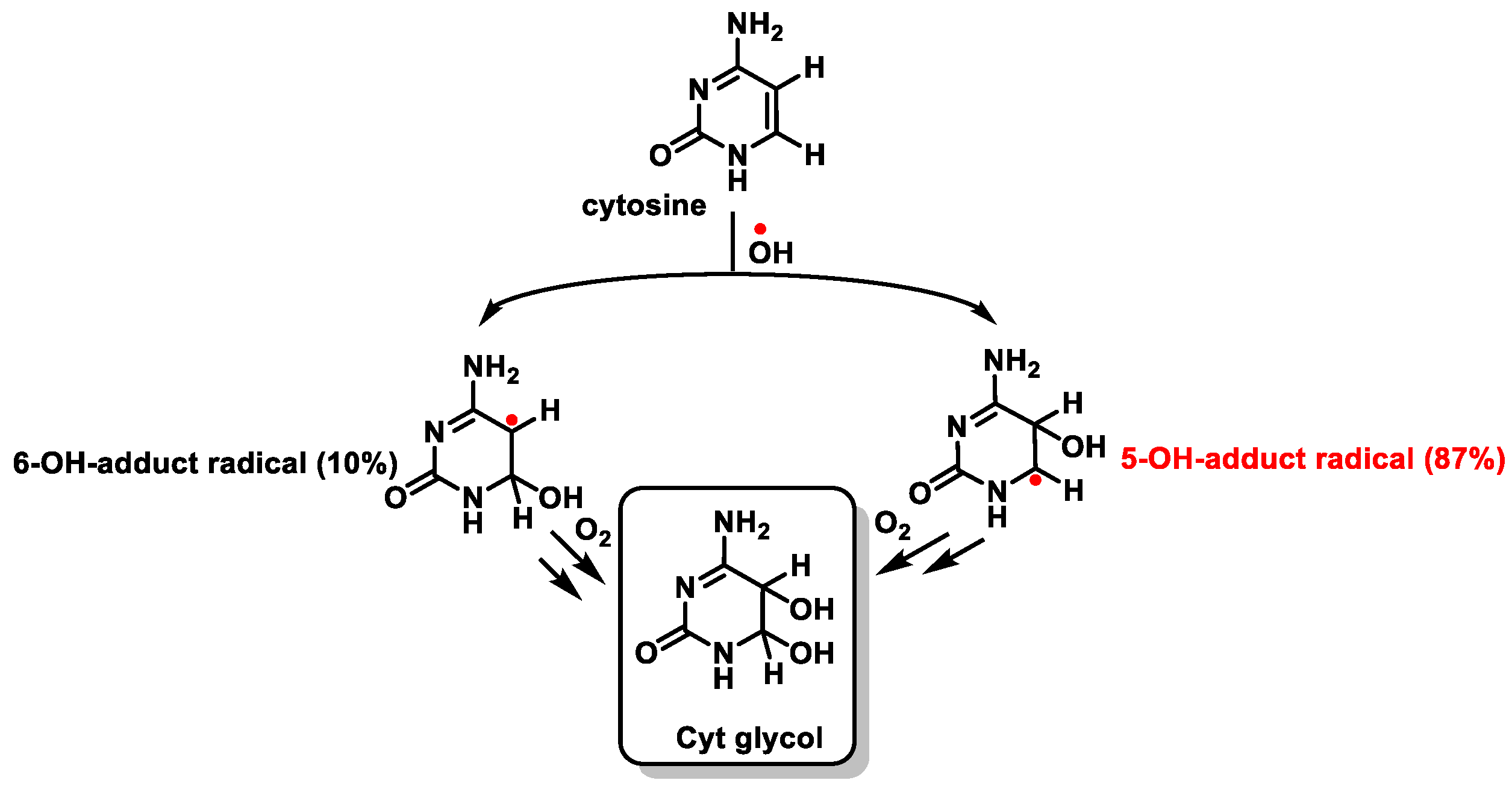
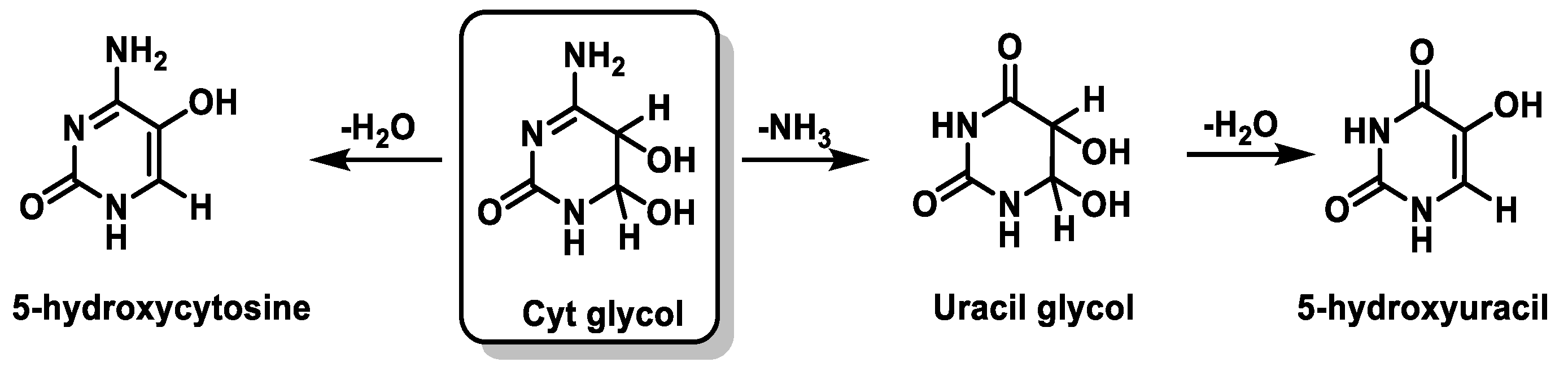
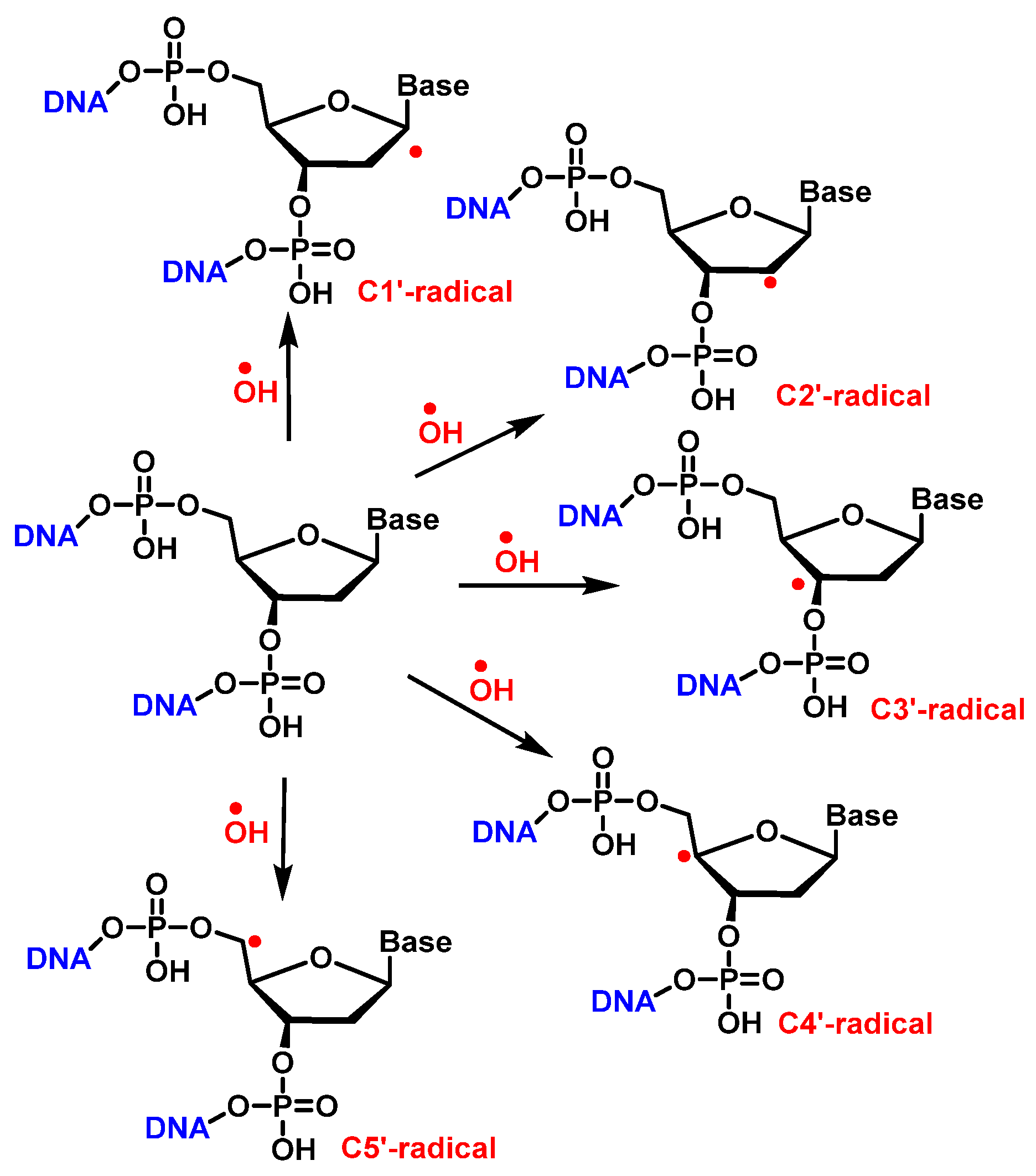
7. Hydroxyl Radical and DNA Damage
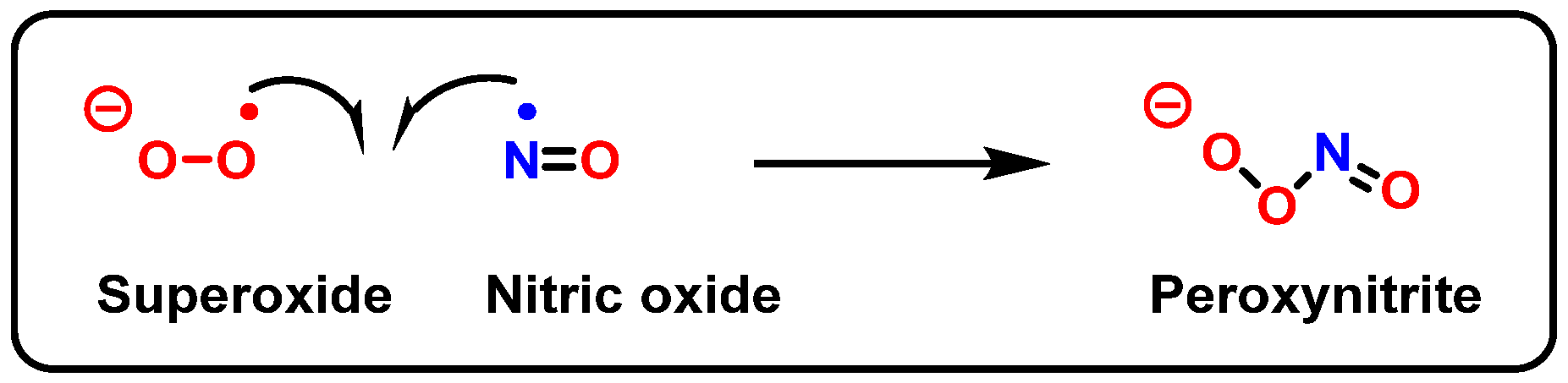
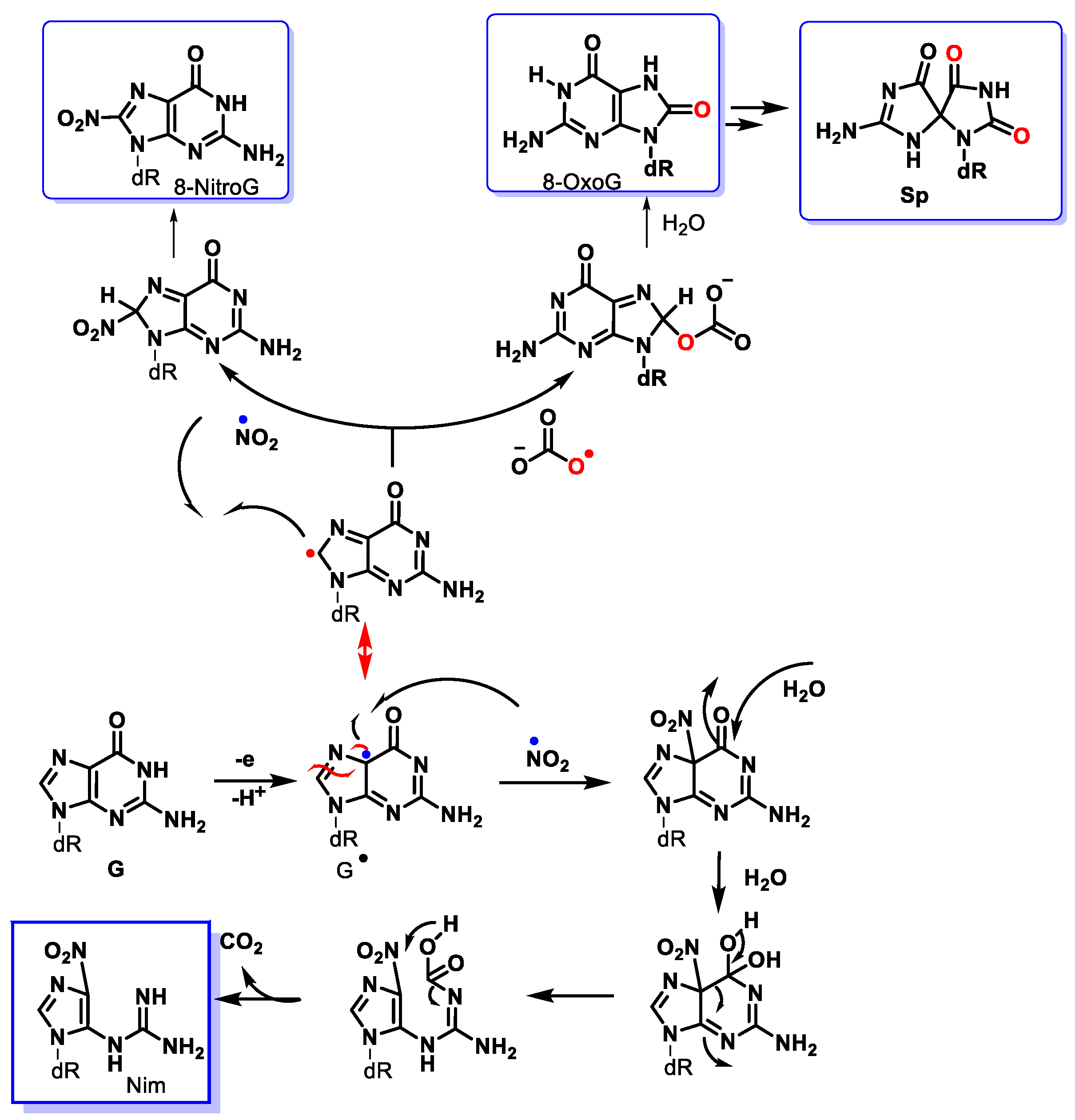
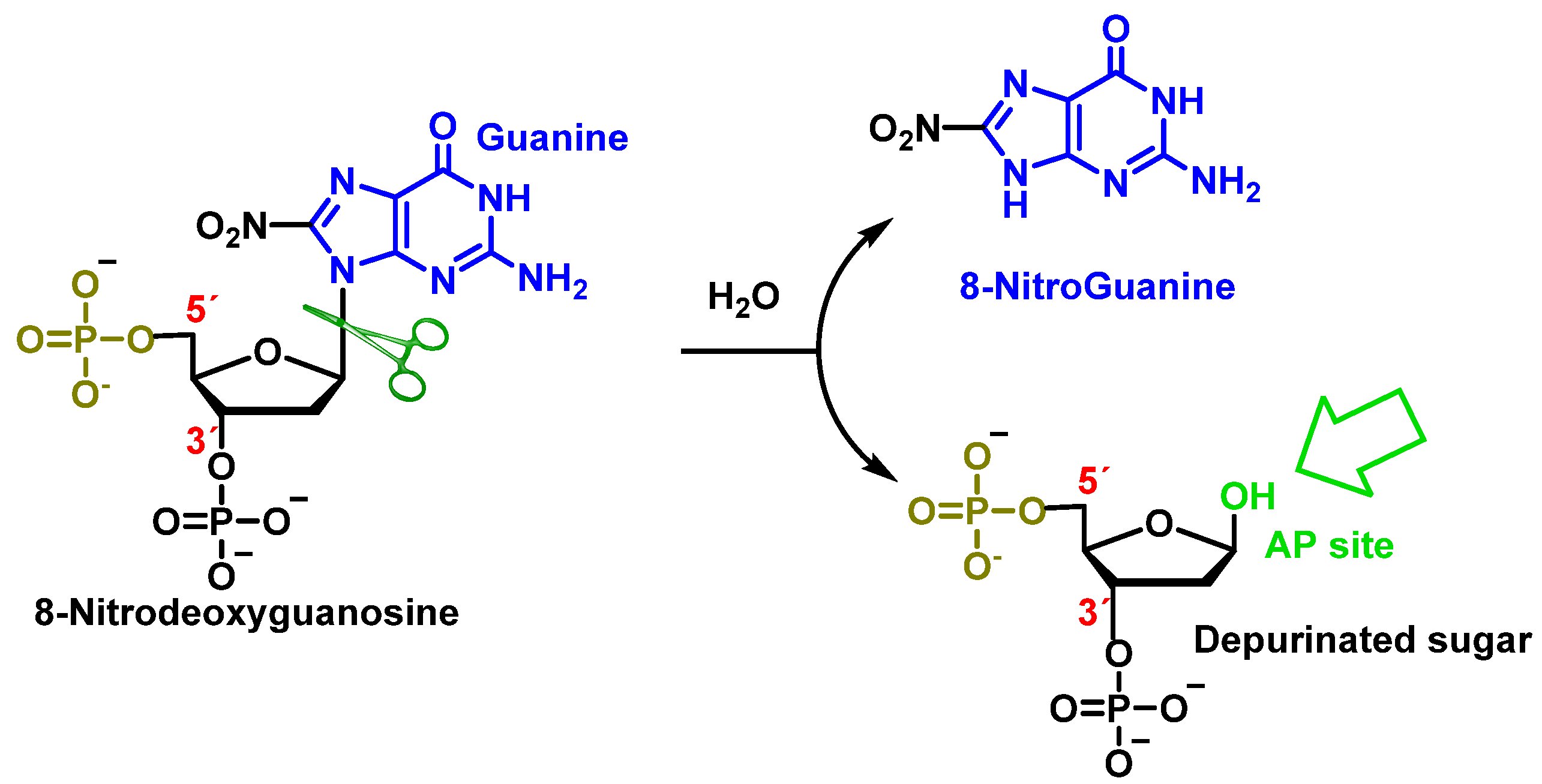
8. Peroxynitrite and DNA Damage
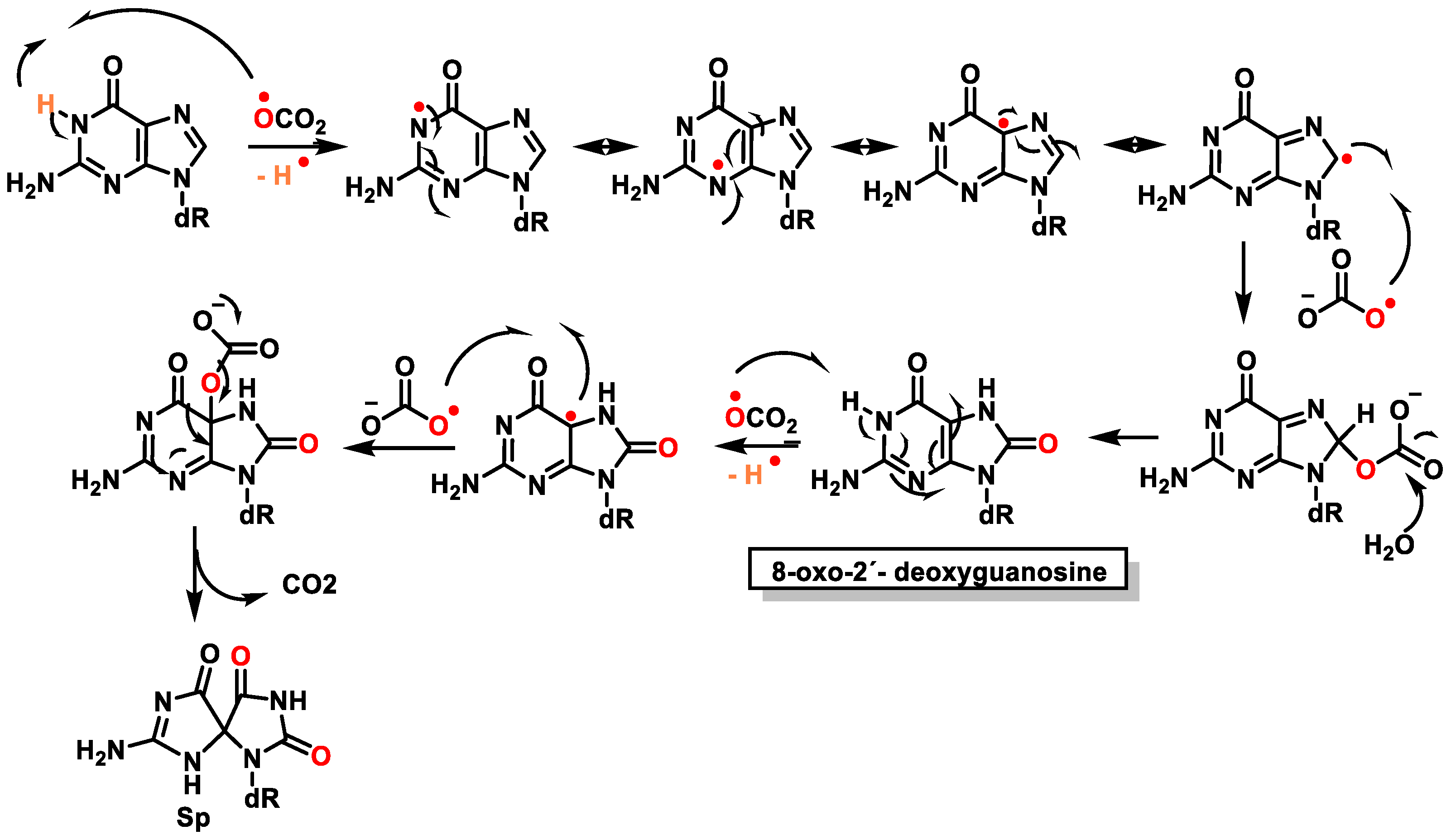
9. Carbonate Radical Anion and DNA Damage
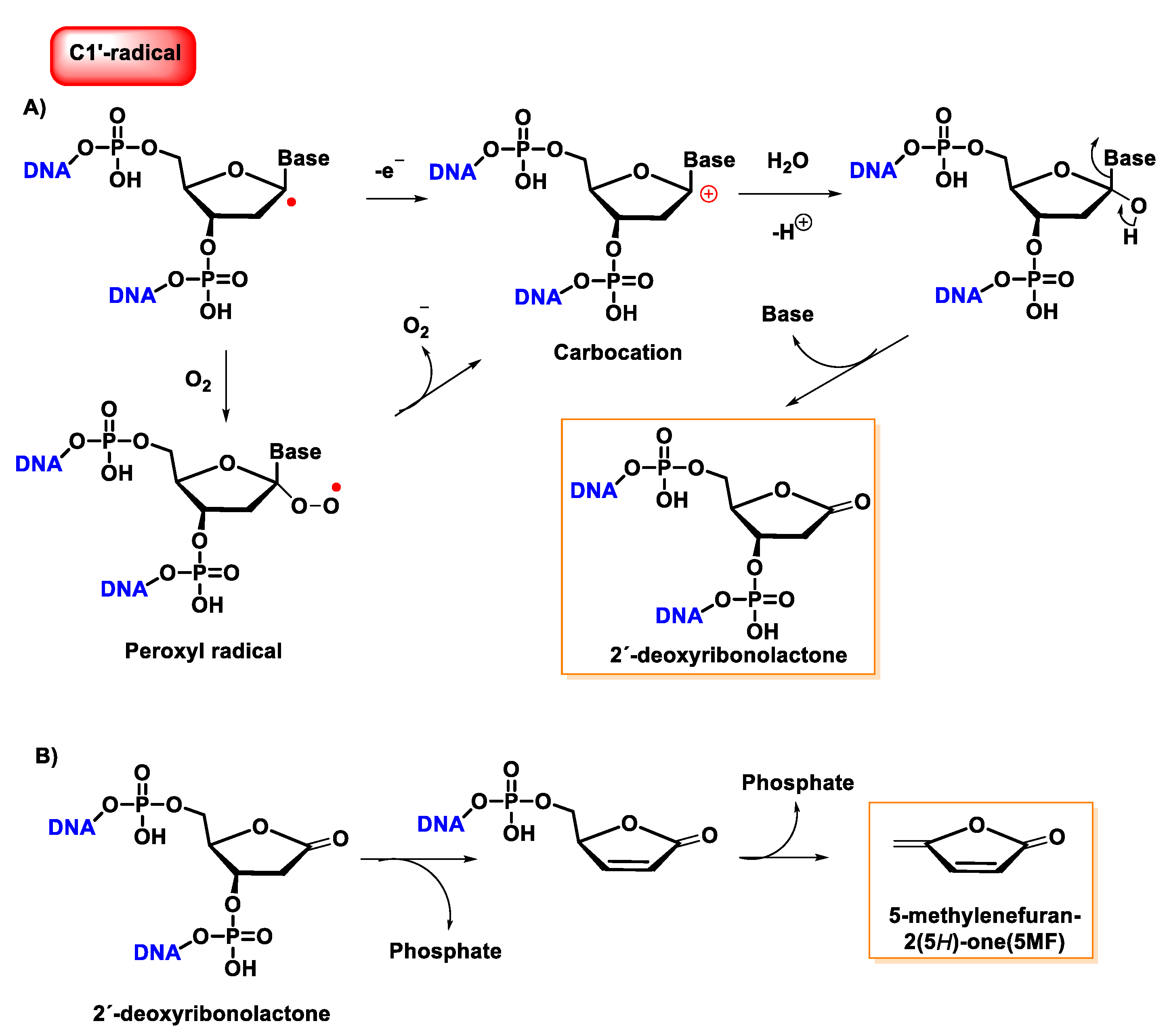
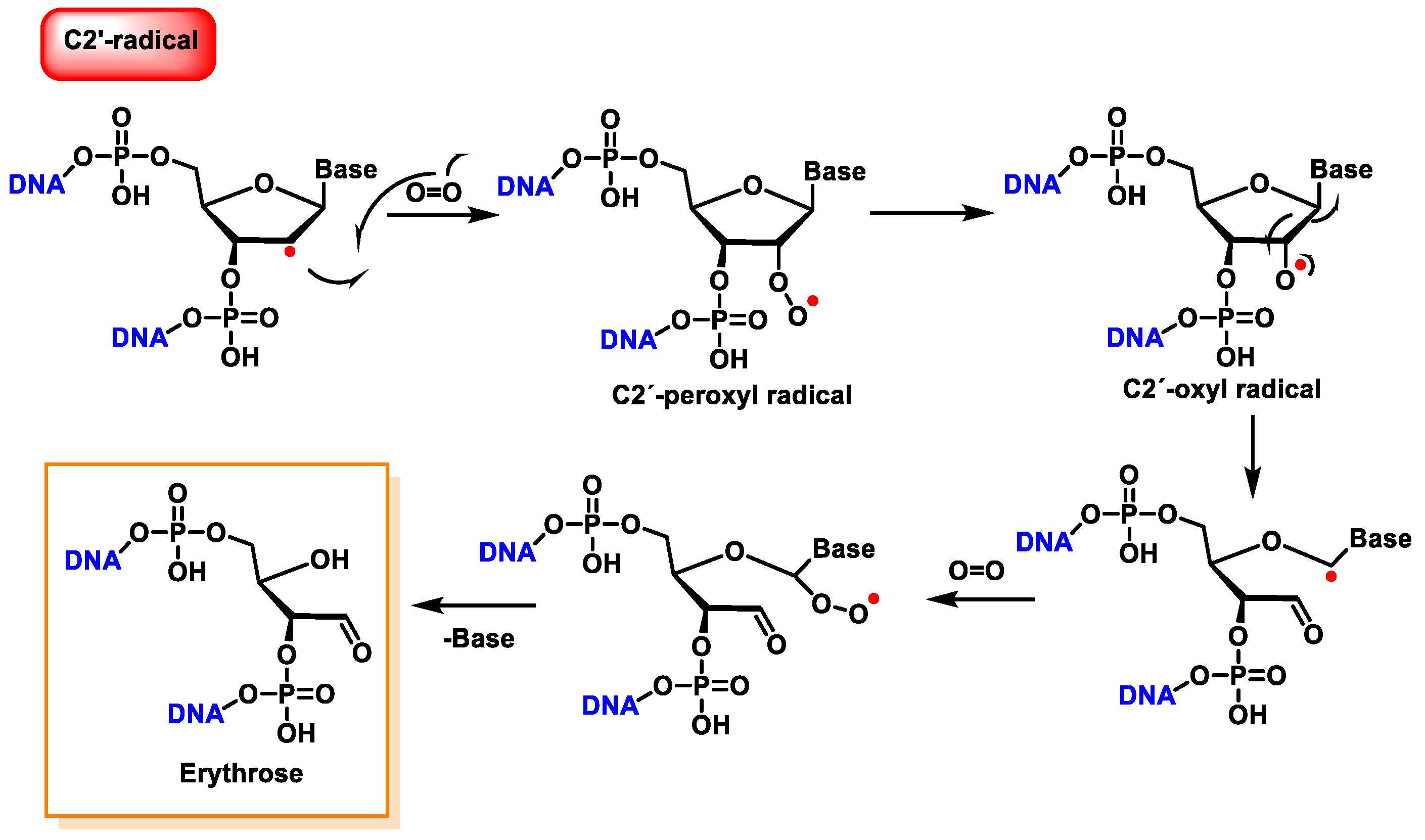
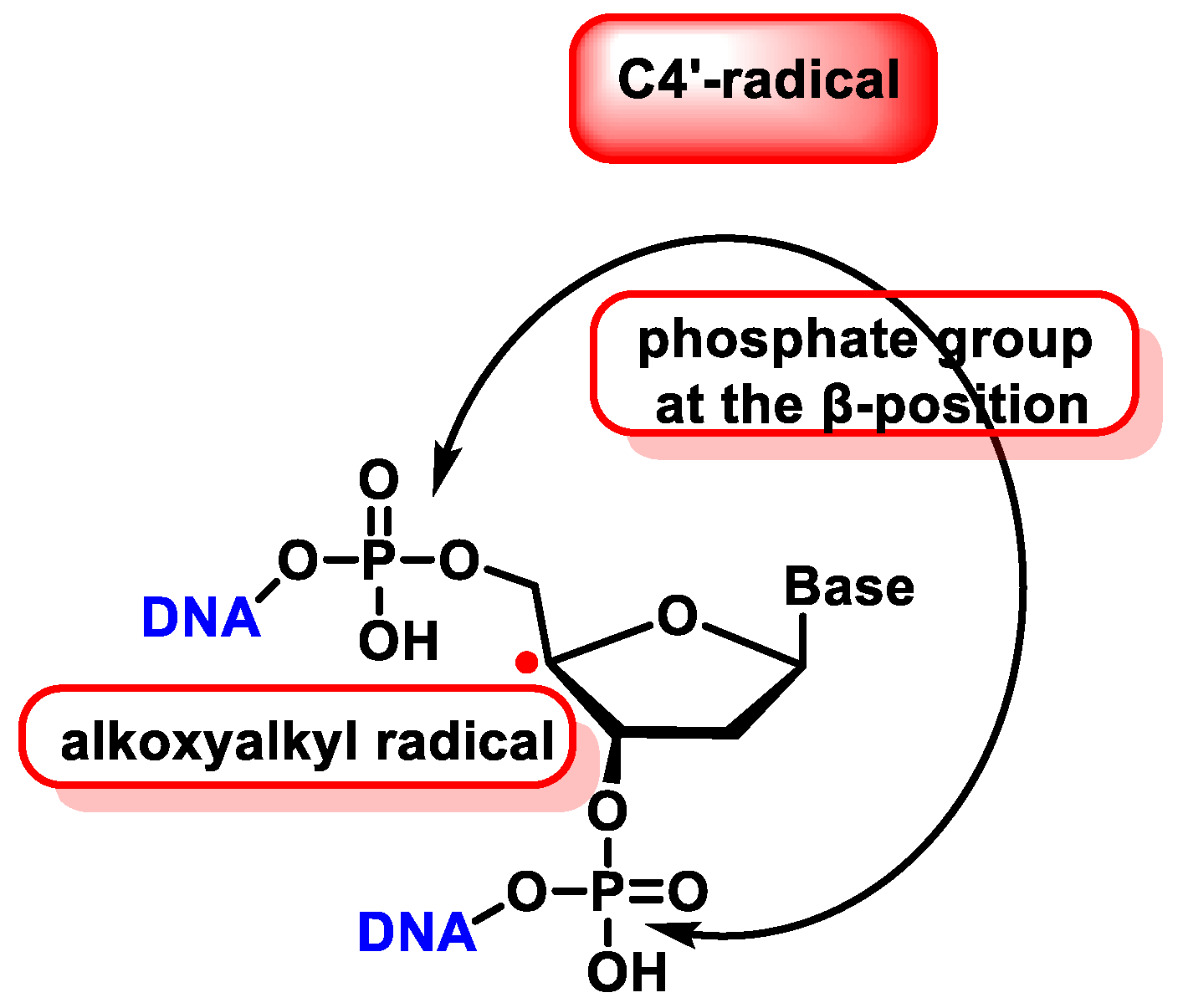
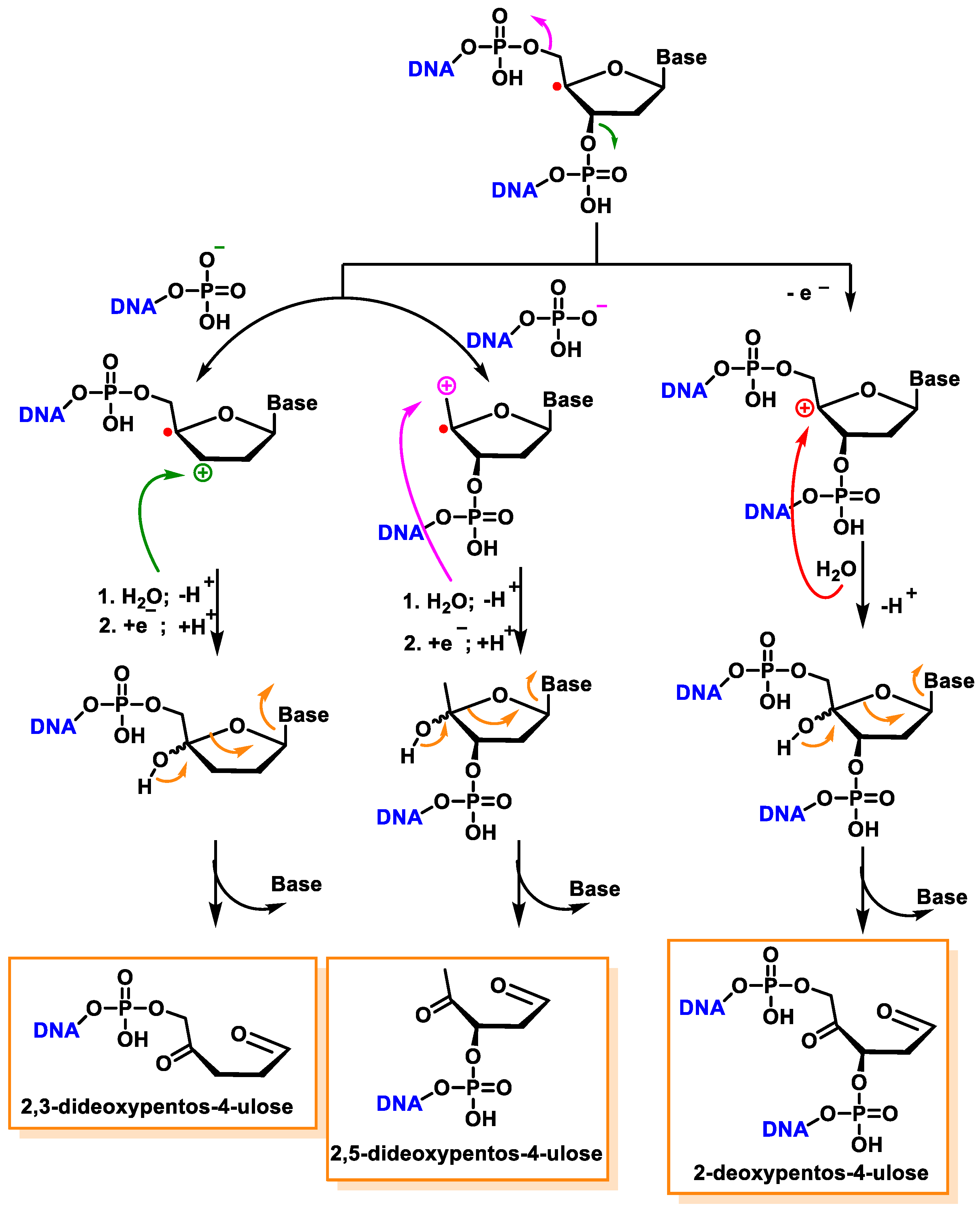
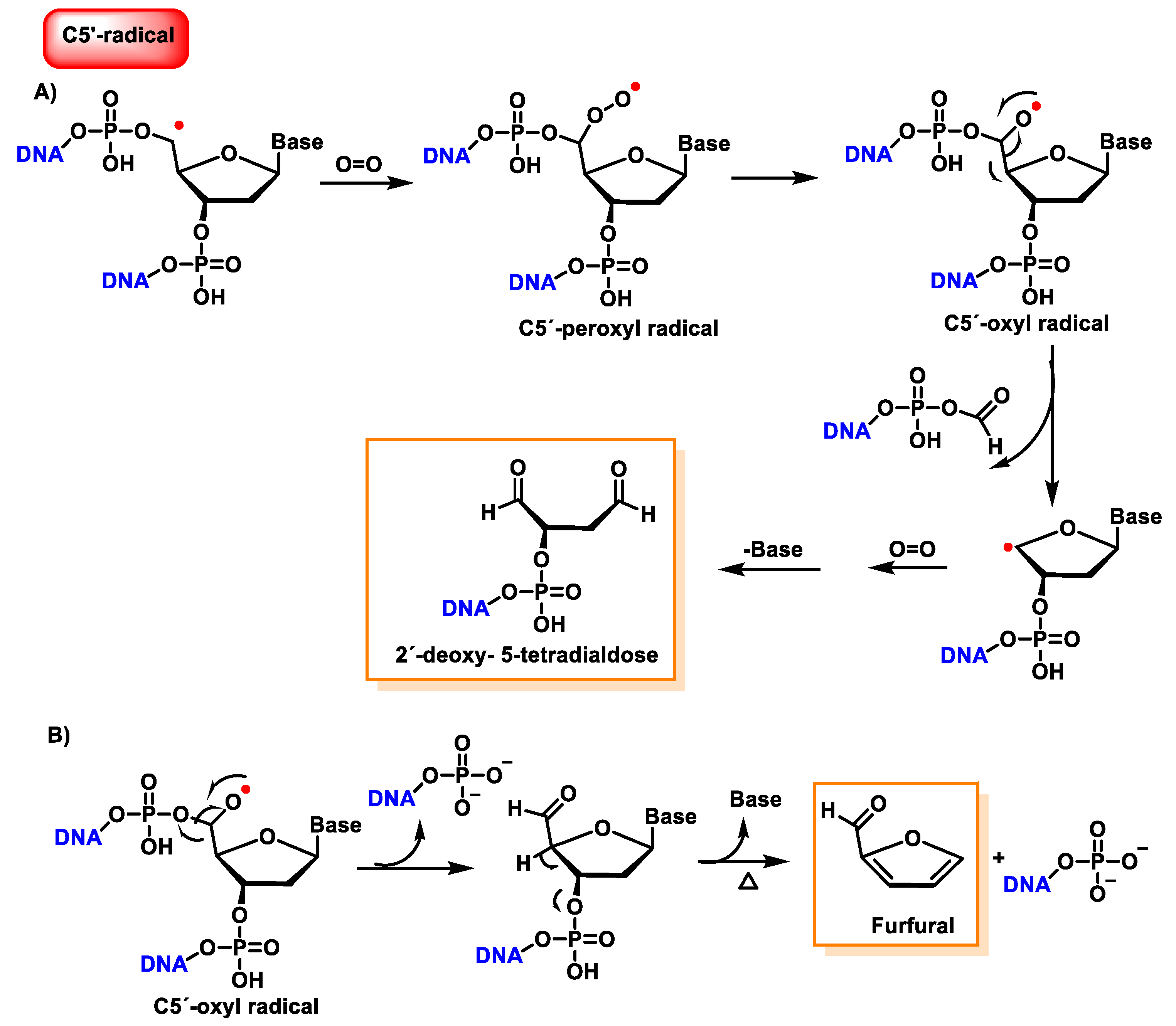
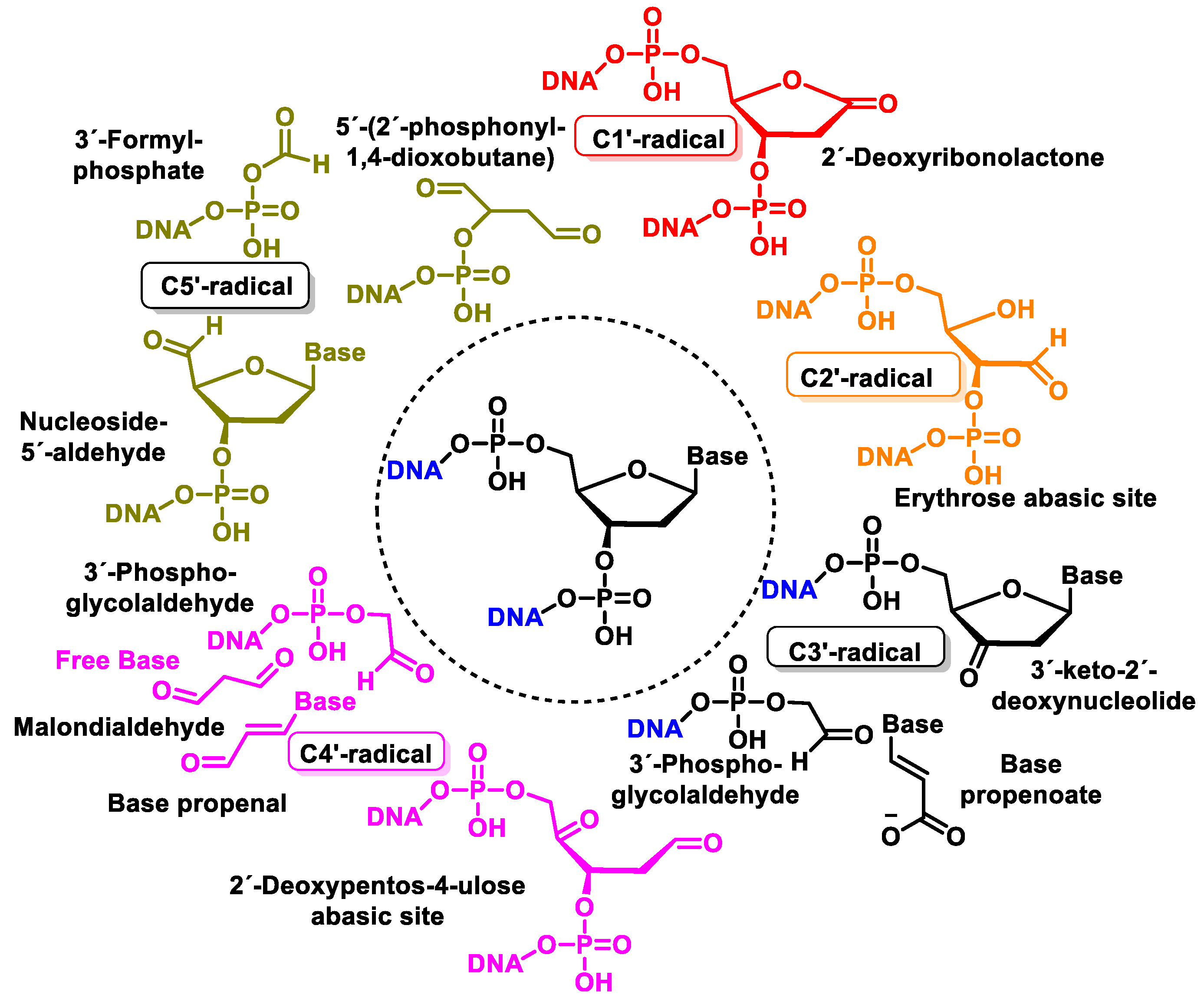
10. Damage to the Sugar of DNA
11. Concomitant Damage to the Base and Sugar Moiety of the Same Nucleoside
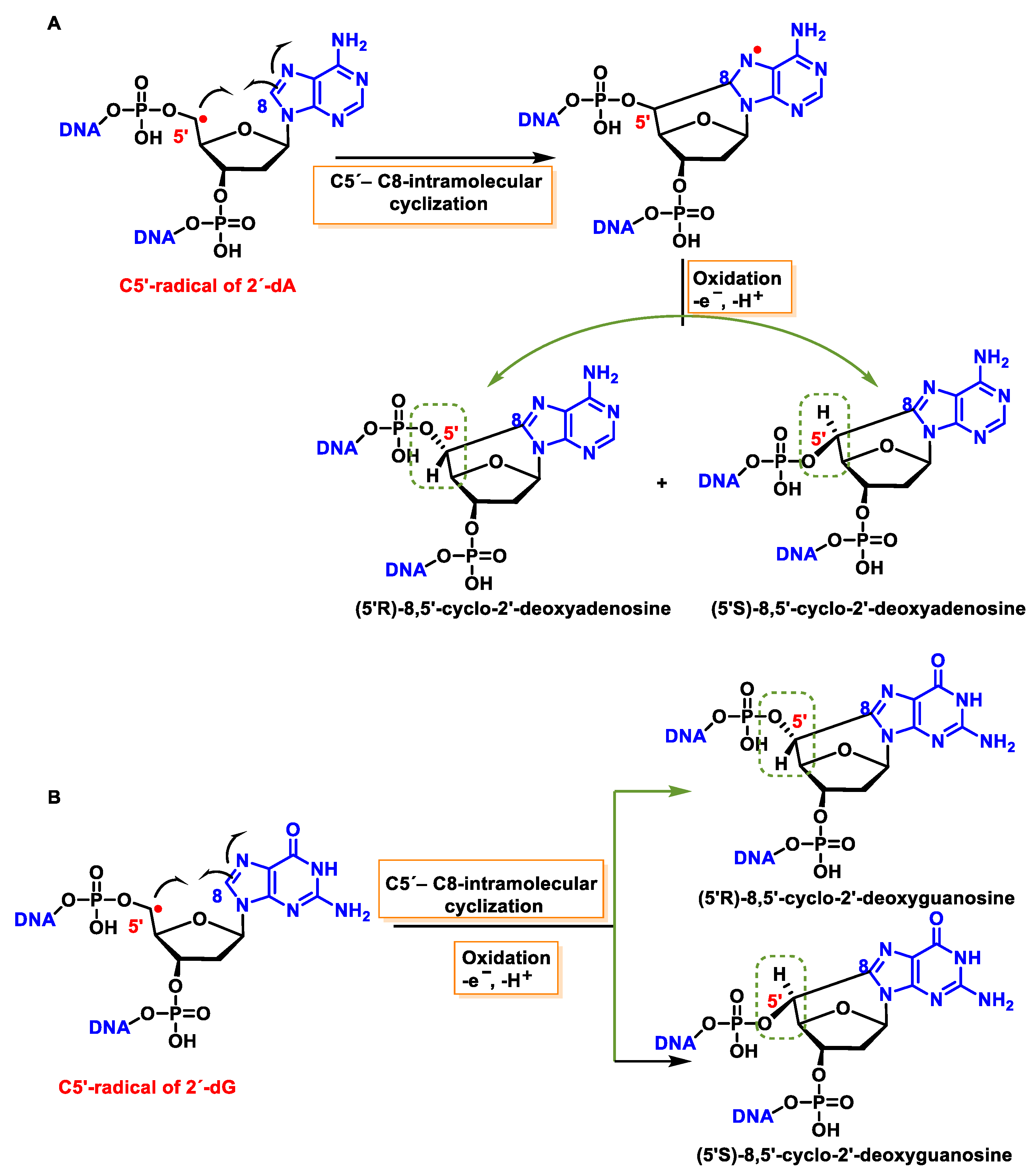
11.1. 8,5′-Cyclopurine-2-deoxynucleosides
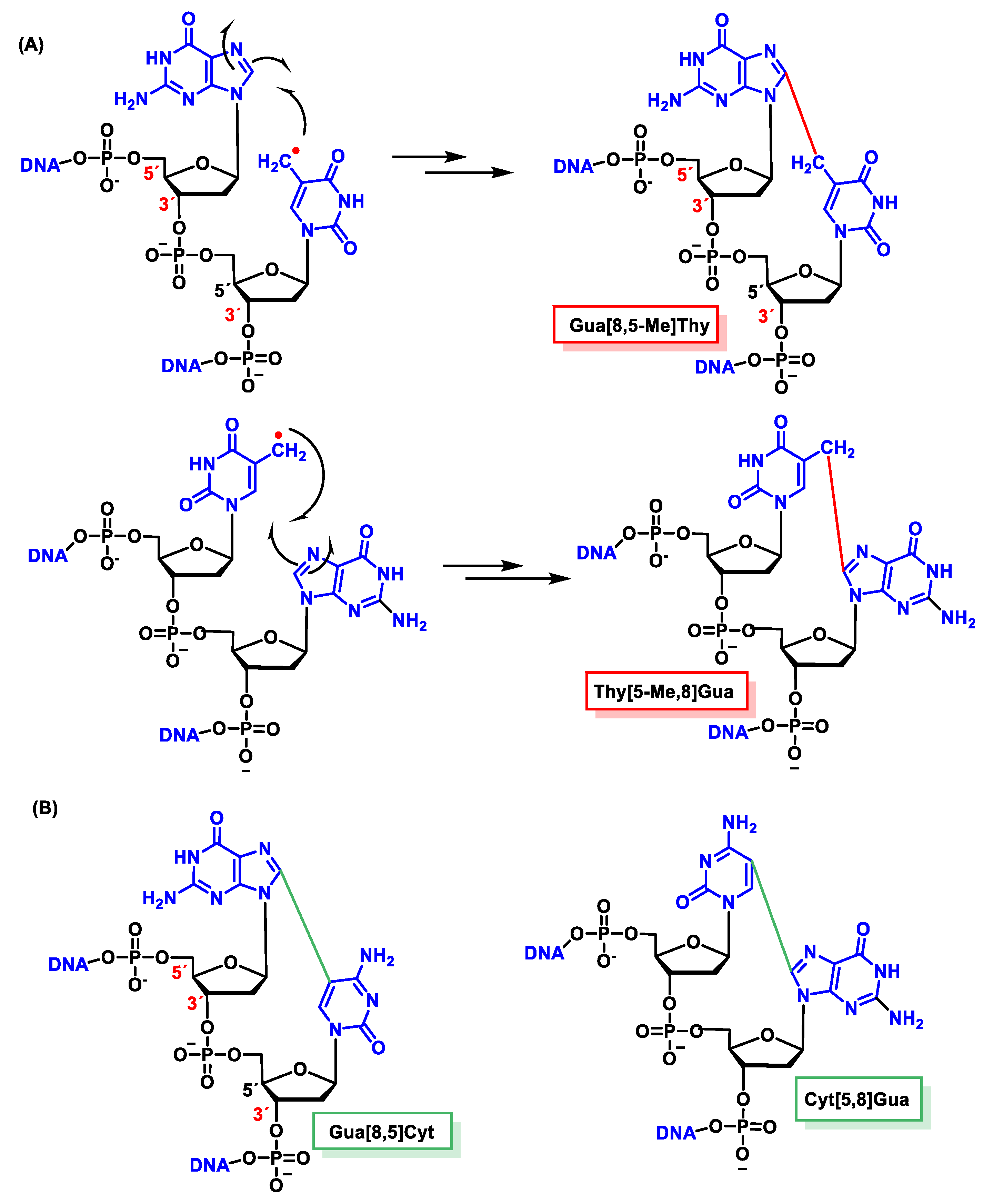
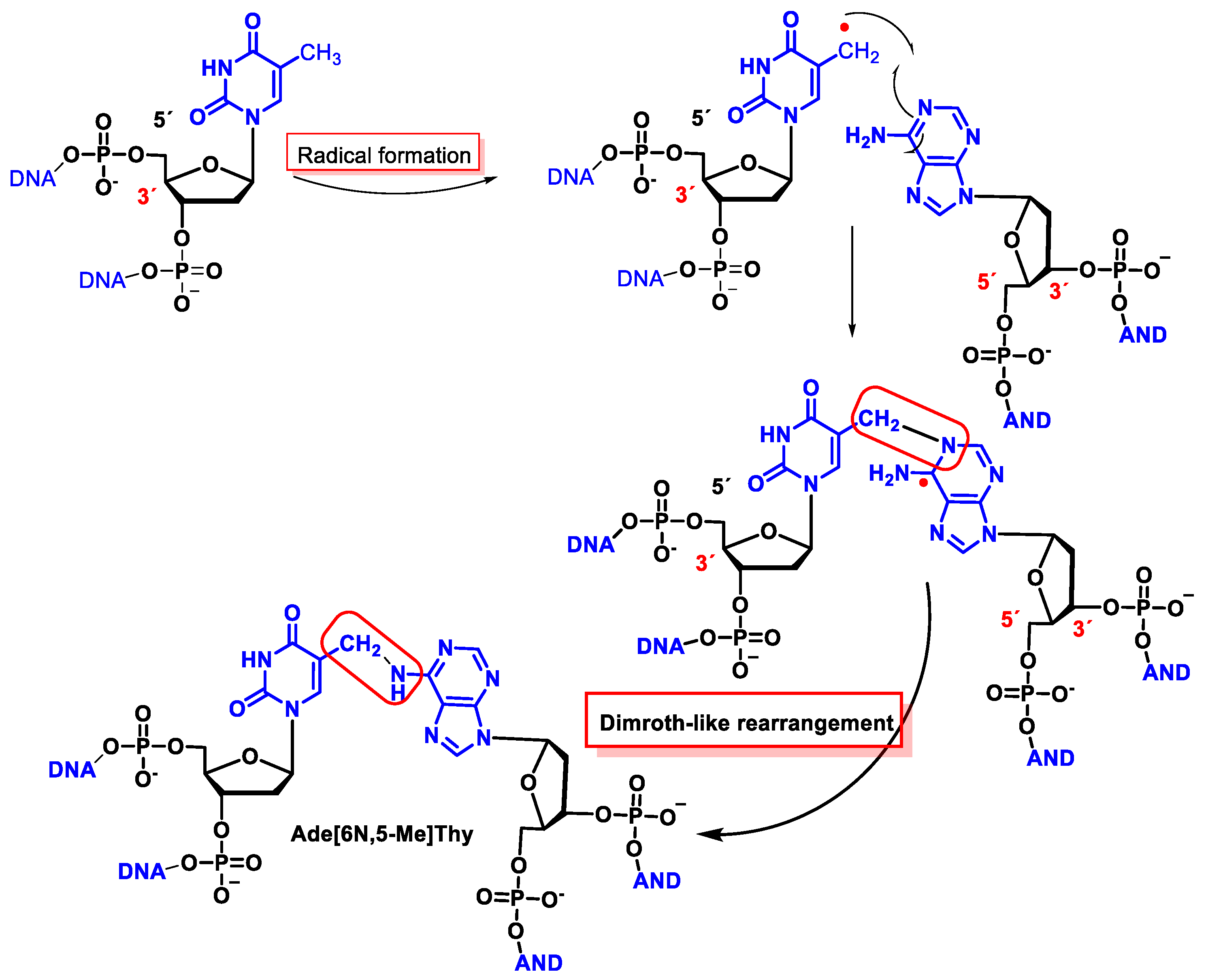
11.2. Intrastrand Base–Base Tandem Lesions
11.3. Interstrand Base–Base Tandem Lesions
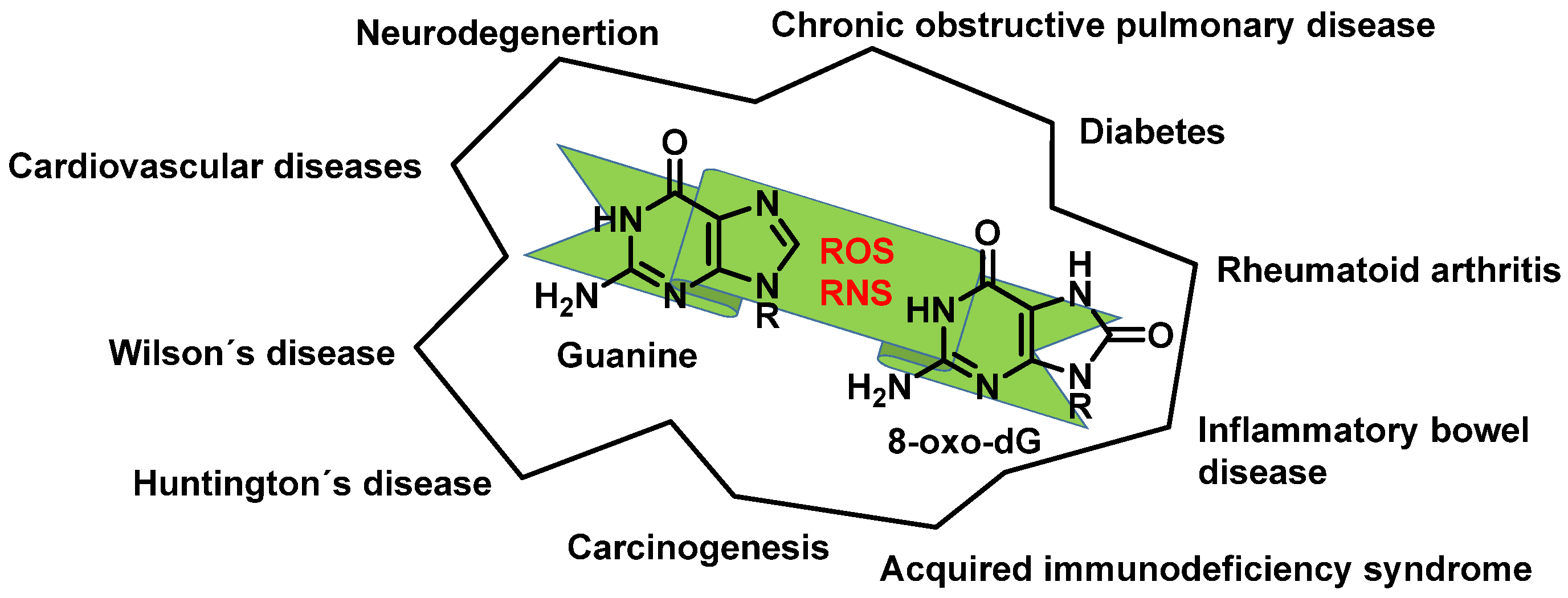
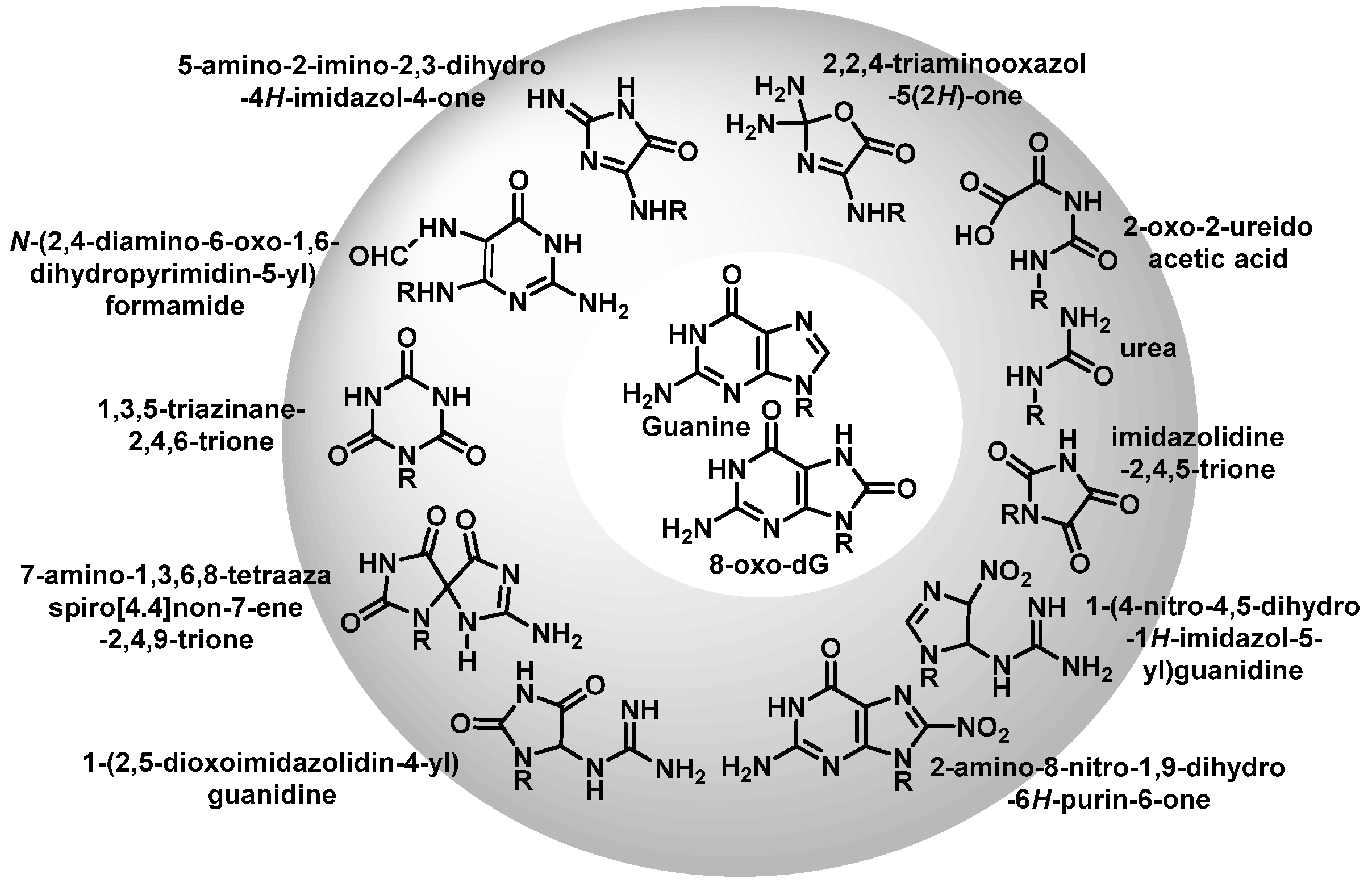
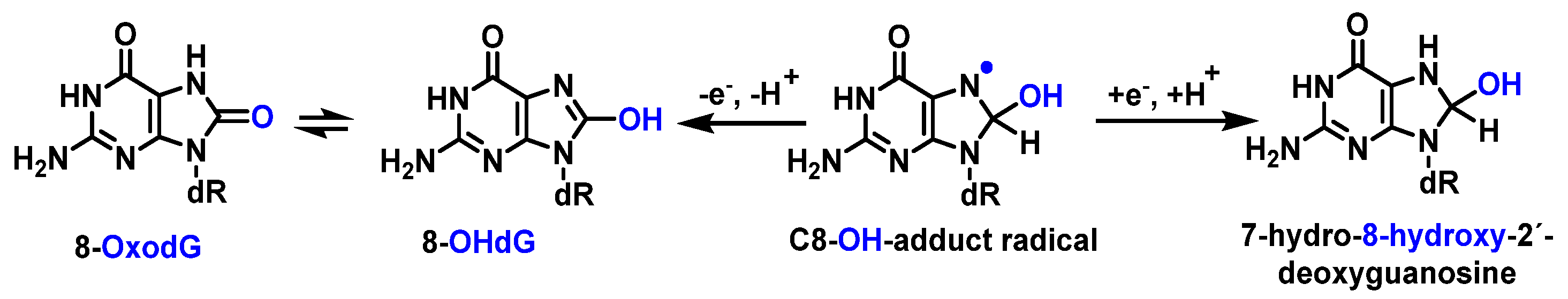
12. 8-Oxo-dG in DNA
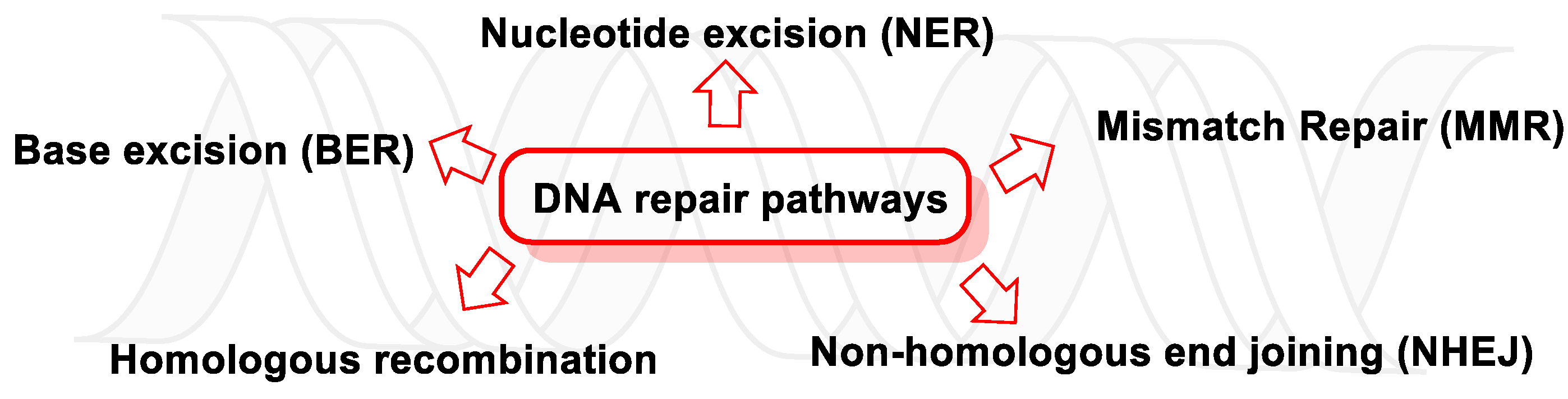
13. Major DNA Repair Pathways
13.1. DNA Damage Response (DDR)
13.1.1. Base Excision Repair (BER)
13.1.2. Nucleotide Excision Repair (NER)
13.1.3. Mismatch Repair (MMR)
13.1.4. Homologous Recombination (HR)
13.1.5. Non-Homologous end Joining (NHEJ)
13.2. DNA Damage Tolerance Pathways (DDT)
14. DNA Oxidation as a Strategic Antimicrobial Mechanism
15. Methods for DNA Damage Assessment
15.1. Gel Electrophoresis-Based Methods
15.2. Radioactive-Based Methods
15.3. Fluorescence-Based Methods
15.4. Next-Generation Sequencing-Based Methods
16. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Alberts, B. Molecular Biology of the Cell; WW Norton & Company: New York, NY, USA, 2017. [Google Scholar]
- Watson, J.D.; Crick, F.H. Molecular structure of nucleic acids: A structure for deoxyribose nucleic acid. Nature 1953, 171, 737–738. [Google Scholar] [CrossRef] [PubMed]
- Tubbs, A.; Nussenzweig, A. Endogenous DNA Damage as a Source of Genomic Instability in Cancer. Cell 2017, 168, 644–656. [Google Scholar] [CrossRef] [PubMed]
- Katerji, M.; Duerksen-Hughes, P.J. DNA damage in cancer development: Special implications in viral oncogenesis. Am. J. Cancer Res. 2021, 11, 3956–3979. [Google Scholar] [PubMed]
- Di Meo, S.; Reed, T.T.; Venditti, P.; Victor, V.M. Role of ROS and RNS Sources in Physiological and Pathological Conditions. Oxidative Med. Cell. Longev. 2016, 2016, 1245049. [Google Scholar] [CrossRef]
- Chatterjee, N.; Walker, G.C. Mechanisms of DNA damage, repair, and mutagenesis. Environ. Mol. Mutagen. 2017, 58, 235–263. [Google Scholar] [CrossRef]
- Lindahl, T.; Barnes, D.E. Repair of endogenous DNA damage. Cold Spring Harb. Symp. Quant. Biol. 2000, 65, 127–133. [Google Scholar] [CrossRef]
- Lujan, S.A.; Williams, J.S.; Kunkel, T.A. DNA Polymerases Divide the Labor of Genome Replication. Trends Cell Biol. 2016, 26, 640–654. [Google Scholar] [CrossRef]
- Sriraman, A.; Debnath, T.K.; Xhemalce, B.; Miller, K.M. Making it or breaking it: DNA methylation and genome integrity. Essays Biochem. 2020, 64, 687–703. [Google Scholar] [CrossRef]
- Villa, S.M.; Altuna, J.C.; Ruff, J.S.; Beach, A.B.; Mulvey, L.I.; Poole, E.J.; Campbell, H.E.; Johnson, K.P.; Shapiro, M.D.; Bush, S.E.; et al. Rapid experimental evolution of reproductive isolation from a single natural population. Proc. Natl. Acad. Sci. USA 2019, 116, 13440–13445. [Google Scholar] [CrossRef]
- Frederico, L.A.; Kunkel, T.A.; Shaw, B.R. A sensitive genetic assay for the detection of cytosine deamination: Determination of rate constants and the activation energy. Biochemistry 1990, 29, 2532–2537. [Google Scholar] [CrossRef]
- Hsu, C.W.; Sowers, M.L.; Baljinnyam, T.; Herring, J.L.; Hackfeld, L.C.; Tang, H.; Zhang, K.; Sowers, L.C. Measurement of deaminated cytosine adducts in DNA using a novel hybrid thymine DNA glycosylase. J. Biol. Chem. 2022, 298, 101638. [Google Scholar] [CrossRef] [PubMed]
- Thompson, P.S.; Cortez, D. New insights into abasic site repair and tolerance. DNA Repair. 2020, 90, 102866. [Google Scholar] [CrossRef]
- Lindahl, T. Instability and decay of the primary structure of DNA. Nature 1993, 362, 709–715. [Google Scholar] [CrossRef]
- Tropp, B.E. Molecular Biology: Genes to Proteins; Jones & Bartlett Publishers: Burlington, MA, USA, 2012. [Google Scholar]
- Díaz-Muñoz, M.; Hernández-Muñoz, R.; Butanda-Ochoa, A. Structure-activity features of purines and their receptors: Implications in cell physiopathology. Mol. Biomed. 2022, 3, 5. [Google Scholar] [CrossRef]
- Fedeles, B.I.; Li, D.; Singh, V. Structural Insights Into Tautomeric Dynamics in Nucleic Acids and in Antiviral Nucleoside Analogs. Front. Mol. Biosci. 2021, 8, 823253. [Google Scholar] [CrossRef] [PubMed]
- Cadet, J.; Davies, K.J.A. Oxidative DNA damage & repair: An introduction. Free Radic. Biol. Med. 2017, 107, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Cooke, M.S.; Evans, M.D.; Dizdaroglu, M.; Lunec, J. Oxidative DNA damage: Mechanisms, mutation, and disease. FASEB J. 2003, 17, 1195–1214. [Google Scholar] [CrossRef]
- Hoeijmakers, J.H. DNA damage, aging, and cancer. N. Engl. J. Med. 2009, 361, 1475–1485. [Google Scholar] [CrossRef]
- Andrés, C.M.C.; Pérez de la Lastra, J.M.; Juan, C.A.; Plou, F.J.; Pérez-Lebeña, E. The Role of Reactive Species on Innate Immunity. Vaccines 2022, 10, 1735. [Google Scholar] [CrossRef]
- Kawanishi, S.; Hiraku, Y.; Pinlaor, S.; Ma, N. Oxidative and nitrative DNA damage in animals and patients with inflammatory diseases in relation to inflammation-related carcinogenesis. Biol. Chem. 2006, 387, 365–372. [Google Scholar] [CrossRef]
- Mu, H.; Sun, J.; Li, L.; Yin, J.; Hu, N.; Zhao, W.; Ding, D.; Yi, L. Ionizing radiation exposure: Hazards, prevention, and biomarker screening. Environ. Sci. Pollut. Res. 2018, 25, 15294–15306. [Google Scholar] [CrossRef] [PubMed]
- Ciccia, A.; Elledge, S.J. The DNA damage response: Making it safe to play with knives. Mol. Cell 2010, 40, 179–204. [Google Scholar] [CrossRef] [PubMed]
- D’Orazio, J.; Jarrett, S.; Amaro-Ortiz, A.; Scott, T. UV radiation and the skin. Int. J. Mol. Sci. 2013, 14, 12222–12248. [Google Scholar] [CrossRef]
- Martincigh, B.S.; Allen, J.M.; Allen, S.K. Sunscreens: The Molecules and Their Photochemistry. In Sunscreen Photobiology: Molecular, Cellular and Physiological Aspects; Gasparro, F.P., Ed.; Springer: Berlin, Heidelberg, 1997; pp. 11–45. [Google Scholar]
- Guan, L.L.; Lim, H.W.; Mohammad, T.F. Sunscreens and photoaging: A review of current literature. Am. J. Clin. Dermatol. 2021, 22, 819–828. [Google Scholar] [CrossRef] [PubMed]
- Ravanat, J.L.; Douki, T.; Cadet, J. Direct and indirect effects of UV radiation on DNA and its components. J. Photochem. Photobiol. B 2001, 63, 88–102. [Google Scholar] [CrossRef] [PubMed]
- Jackson, S.P.; Bartek, J. The DNA-damage response in human biology and disease. Nature 2009, 461, 1071–1078. [Google Scholar] [CrossRef]
- Lee, J.W.; Ratnakumar, K.; Hung, K.F.; Rokunohe, D.; Kawasumi, M. Deciphering UV-induced DNA Damage Responses to Prevent and Treat Skin Cancer. Photochem. Photobiol. 2020, 96, 478–499. [Google Scholar] [CrossRef]
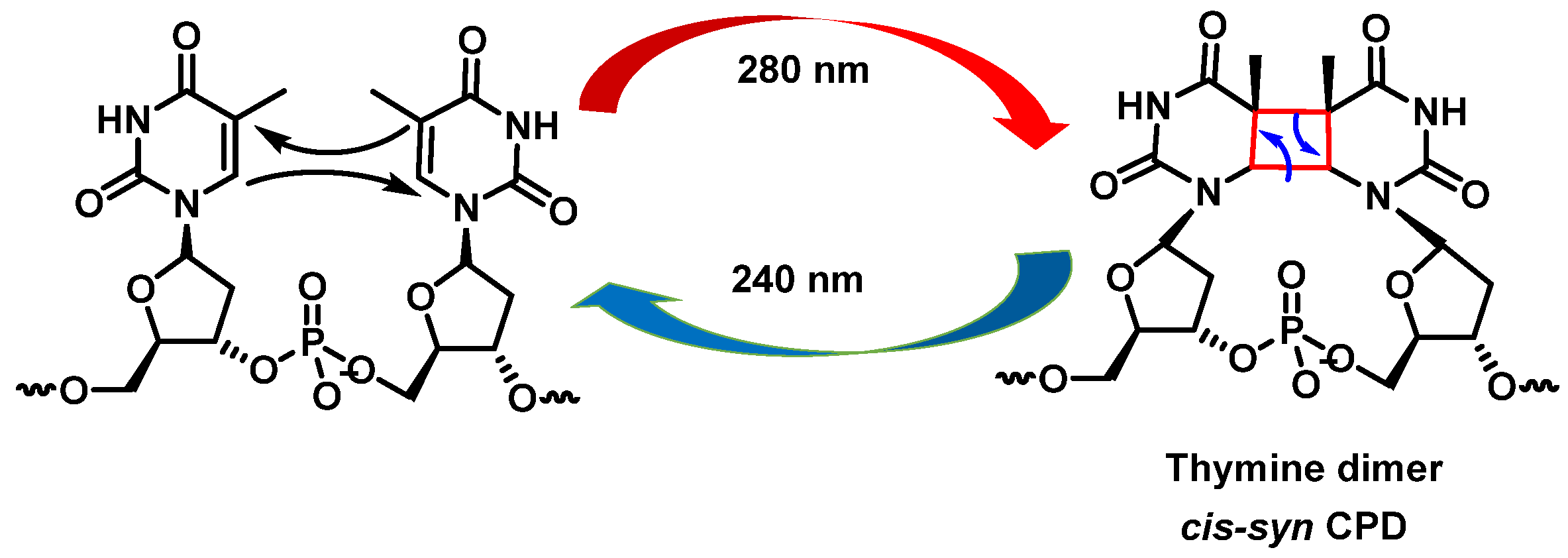
- Goodsell, D.S. The molecular perspective: Ultraviolet light and pyrimidine dimers. Oncologist 2001, 6, 298–299. [Google Scholar] [CrossRef]
- You, Y.H.; Lee, D.H.; Yoon, J.H.; Nakajima, S.; Yasui, A.; Pfeifer, G.P. Cyclobutane pyrimidine dimers are responsible for the vast majority of mutations induced by UVB irradiation in mammalian cells. J. Biol. Chem. 2001, 276, 44688–44694. [Google Scholar] [CrossRef]
- Carpenter, M.A.; Ginugu, M.; Khan, S.; Kemp, M.G. DNA Containing Cyclobutane Pyrimidine Dimers Is Released from UVB-Irradiated Keratinocytes in a Caspase-Dependent Manner. J. Investig. Dermatol. 2022, 142, 3062–3070. [Google Scholar] [CrossRef]
- Mitchell, D.L.; Nairn, R.S. The biology of the (6-4) photoproduct. Photochem. Photobiol. 1989, 49, 805–819. [Google Scholar] [CrossRef]
- Alhmoud, J.F.; Woolley, J.F.; Al Moustafa, A.-E.; Malki, M.I. DNA Damage/Repair Management in Cancers. Cancers 2020, 12, 1050. [Google Scholar] [CrossRef]
- Andrés, C.M.C.; Pérez de la Lastra, J.M.; Andrés Juan, C.; Plou, F.J.; Pérez-Lebeña, E. Impact of Reactive Species on Amino Acids;Biological Relevance in Proteins and Induced Pathologies. Int. J. Mol. Sci. 2022, 23, 14049. [Google Scholar] [CrossRef]
- Pérez de la Lastra, J.M.; Juan, C.A.; Plou, F.J.; Pérez-Lebeña, E. The Nitration of Proteins, Lipids and DNA by Peroxynitrite Derivatives-Chemistry Involved and Biological Relevance. Stresses 2022, 2, 53–64. [Google Scholar] [CrossRef]
- Curieses Andrés, C.M.; Pérezdela Lastra, J.M.; Andrés Juan, C.; Plou, F.J.; Pérez-Lebeña, E. From reactive species to disease development: Effect of oxidants and antioxidants on the cellular biomarkers. J. Biochem. Mol. Toxicol. 2023, e23455. [Google Scholar] [CrossRef] [PubMed]
- Martemucci, G.; Costagliola, C.; Mariano, M.; D’andrea, L.; Napolitano, P.; D’Alessandro, A.G. Free radical properties, source and targets, antioxidant consumption and health. Oxygen 2022, 2, 48–78. [Google Scholar] [CrossRef]
- Phaniendra, A.; Jestadi, D.B.; Periyasamy, L. Free radicals: Properties, sources, targets, and their implication in various diseases. Indian J. Clin. Biochem. 2015, 30, 11–26. [Google Scholar] [CrossRef]
- Chaudhary, P.; Janmeda, P.; Docea, A.O.; Yeskaliyeva, B.; Abdull Razis, A.F.; Modu, B.; Calina, D.; Sharifi-Rad, J. Oxidative stress, free radicals and antioxidants: Potential crosstalk in the pathophysiology of human diseases. Front. Chem. 2023, 11, 1158198. [Google Scholar] [CrossRef] [PubMed]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxidative Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef]
- Zarkovic, N. Roles and Functions of ROS and RNS in Cellular Physiology and Pathology. Cells 2020, 9, 767. [Google Scholar] [CrossRef]
- Juan, C.A.; Pérez de la Lastra, J.M.; Plou, F.J.; Pérez-Lebeña, E. The Chemistry of Reactive Oxygen Species (ROS) Revisited: Outlining Their Role in Biological Macromolecules (DNA, Lipids and Proteins) and Induced Pathologies. Int. J. Mol. Sci. 2021, 22, 4642. [Google Scholar] [CrossRef] [PubMed]
- Arjunan, K.P.; Sharma, V.K.; Ptasinska, S. Effects of Atmospheric Pressure Plasmas on Isolated and Cellular DNA—A Review. Int. J. Mol. Sci. 2015, 16, 2971–3016. [Google Scholar] [CrossRef] [PubMed]
- Steenken, S.; Jovanovic, S.V. How Easily Oxidizable Is DNA? One-Electron Reduction Potentials of Adenosine and Guanosine Radicals in Aqueous Solution. J. Am. Chem. Soc. 1997, 119, 617–618. [Google Scholar] [CrossRef]
- Delaney, S.; Jarem, D.A.; Volle, C.B.; Yennie, C.J. Chemical and biological consequences of oxidatively damaged guanine in DNA. Free Radic. Res. 2012, 46, 420–441. [Google Scholar] [CrossRef] [PubMed]
- Morikawa, M.; Kino, K.; Oyoshi, T.; Suzuki, M.; Kobayashi, T.; Miyazawa, H. Analysis of guanine oxidation products in double-stranded DNA and proposed guanine oxidation pathways in single-stranded, double-stranded or quadruplex DNA. Biomolecules 2014, 4, 140–159. [Google Scholar] [CrossRef] [PubMed]
- Lewis, R.S.; Deen, W.M. Kinetics of the reaction of nitric oxide with oxygen in aqueous solutions. Chem. Res. Toxicol. 1994, 7, 568–574. [Google Scholar] [CrossRef] [PubMed]
- Manoj, P.; Mohan, H.; Mittal, J.P.; Manoj, V.M.; Aravindakumar, C.T. Charge transfer from 2-aminopurine radical cation and radical anion to nucleobases: A pulse radiolysis study. Chem. Phys. 2007, 331, 351–358. [Google Scholar] [CrossRef]
- Giorgio, M.; Dellino, G.I.; Gambino, V.; Roda, N.; Pelicci, P.G. On the epigenetic role of guanosine oxidation. Redox Biol. 2020, 29, 101398. [Google Scholar] [CrossRef]
- Hahm, J.Y.; Park, J.; Jang, E.S.; Chi, S.W. 8-Oxoguanine: From oxidative damage to epigenetic and epitranscriptional modification. Exp. Mol. Med. 2022, 54, 1626–1642. [Google Scholar] [CrossRef]
- David, S.S.; O’Shea, V.L.; Kundu, S. Base-excision repair of oxidative DNA damage. Nature 2007, 447, 941–950. [Google Scholar] [CrossRef] [PubMed]
- van Loon, B.; Markkanen, E.; Hübscher, U. Oxygen as a friend and enemy: How to combat the mutational potential of 8-oxo-guanine. DNA Repair. 2010, 9, 604–616. [Google Scholar] [CrossRef]
- Aerssens, D.; Cadoni, E.; Tack, L.; Madder, A. A Photosensitized Singlet Oxygen (1O2) Toolbox for Bio-Organic Applications: Tailoring 1O2 Generation for DNA and Protein Labelling, Targeting and Biosensing. Molecules 2022, 27, 778. [Google Scholar] [CrossRef]
- Martinez, G.R.; Loureiro, A.P.; Marques, S.A.; Miyamoto, S.; Yamaguchi, L.F.; Onuki, J.; Almeida, E.A.; Garcia, C.C.; Barbosa, L.F.; Medeiros, M.H.; et al. Oxidative and alkylating damage in DNA. Mutat. Res. 2003, 544, 115–127. [Google Scholar] [CrossRef]
- Jena, N.R.; Mishra, P.C. Formation of ring-opened and rearranged products of guanine: Mechanisms and biological significance. Free Radic. Biol. Med. 2012, 53, 81–94. [Google Scholar] [CrossRef] [PubMed]
- Cadet, J.; Delatour, T.; Douki, T.; Gasparutto, D.; Pouget, J.P.; Ravanat, J.L.; Sauvaigo, S. Hydroxyl radicals and DNA base damage. Mutat. Res. 1999, 424, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Cadet, J.; Berger, M.; Buchko, G.W.; Joshi, P.C.; Raoul, S.; Ravanat, J.-L. 2,2-Diamino-4-[(3,5-di-O-acetyl-2-deoxy-.beta.-D-erythro- pentofuranosyl)amino]-5-(2H)-oxazolone: A Novel and Predominant Radical Oxidation Product of 3′,5′-Di-O-acetyl-2′-deoxyguanosine. J. Am. Chem. Soc. 1994, 116, 7403–7404. [Google Scholar] [CrossRef]
- Candeias, L.P.; Steenken, S. Structure and acid-base properties of one-electron-oxidized deoxyguanosine, guanosine, and 1-methylguanosine. J. Am. Chem. Soc. 1989, 111, 1094–1099. [Google Scholar] [CrossRef]
- Fleming, A.M.; Burrows, C.J. Formation and processing of DNA damage substrates for the hNEIL enzymes. Free Radic. Biol. Med. 2017, 107, 35–52. [Google Scholar] [CrossRef]
- Andrés, C.M.C.; Pérez de la Lastra, J.M.; Andrés Juan, C.; Plou, F.J.; Pérez-Lebeña, E. Superoxide Anion Chemistry;Its Role at the Core of the Innate Immunity. Int. J. Mol. Sci. 2023, 24, 1841. [Google Scholar] [CrossRef]
- Reynafarje, B.; Brand, M.D.; Lehninger, A.L. Evaluation of the H+/site ratio of mitochondrial electron transport from rate measurements. J. Biol. Chem. 1976, 251, 7442–7451. [Google Scholar] [CrossRef] [PubMed]
- Reynafarje, B.; Brand, M.D.; Alexandre, A.; Lehninger, A.L. Determination of the H+/site and Ca2+/site ratios of mitochondrial electron transport. Methods Enzymol. 1979, 55, 640–656. [Google Scholar] [CrossRef] [PubMed]
- Martínez, A.; Prolo, C.; Estrada, D.; Rios, N.; Alvarez, M.N.; Piñeyro, M.D.; Robello, C.; Radi, R.; Piacenza, L. Cytosolic Fe-superoxide dismutase safeguards Trypanosoma cruzi from macrophage-derived superoxide radical. Proc. Natl. Acad. Sci. USA 2019, 116, 8879–8888. [Google Scholar] [CrossRef] [PubMed]
- Cadet, J.; Treoule, R. Comparative study of oxidation of nucleic acid components by hydroxyl radicals, singlet oxygen and superoxide anion radicals. Photochem. Photobiol. 1978, 28, 661–667. [Google Scholar] [CrossRef]
- Misiaszek, R.; Crean, C.; Joffe, A.; Geacintov, N.E.; Shafirovich, V. Oxidative DNA damage associated with combination of guanine and superoxide radicals and repair mechanisms via radical trapping. J. Biol. Chem. 2004, 279, 32106–32115. [Google Scholar] [CrossRef]
- Devasagayam, T.P.; Steenken, S.; Obendorf, M.S.; Schulz, W.A.; Sies, H. Formation of 8-hydroxy(deoxy)guanosine and generation of strand breaks at guanine residues in DNA by singlet oxygen. Biochemistry 1991, 30, 6283–6289. [Google Scholar] [CrossRef]
- Kvam, E.; Berg, K.; Steen, H.B. Characterization of singlet oxygen-induced guanine residue damage after photochemical treatment of free nucleosides and DNA. Biochim. Biophys. Acta 1994, 1217, 9–15. [Google Scholar] [CrossRef]
- Cadet, J.; Berger, M.; Douki, T.; Morin, B.; Raoul, S.; Ravanat, J.L.; Spinelli, S. Effects of UV and visible radiation on DNA-final base damage. Biol. Chem. 1997, 378, 1275–1286. [Google Scholar]
- Ravanat, J.L.; Martinez, G.R.; Medeiros, M.H.; Di Mascio, P.; Cadet, J. Mechanistic aspects of the oxidation of DNA constituents mediated by singlet molecular oxygen. Arch. Biochem. Biophys. 2004, 423, 23–30. [Google Scholar] [CrossRef]
- Niles, J.C.; Wishnok, J.S.; Tannenbaum, S.R. Spiroiminodihydantoin is the major product of the 8-oxo-7,8-dihydroguanosine reaction with peroxynitrite in the presence of thiols and guanosine photooxidation by methylene blue. Org. Lett. 2001, 3, 963–966. [Google Scholar] [CrossRef]
- Sies, H.; Menck, C.F.M. Singlet oxygen induced DNA damage. Mutat. Res./DNAging 1992, 275, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Schneider, J.E.; Price, S.; Maidt, L.; Gutteridge, J.M.; Floyd, R.A. Methylene blue plus light mediates 8-hydroxy 2′-deoxyguanosine formation in DNA preferentially over strand breakage. Nucleic Acids Res. 1990, 18, 631–635. [Google Scholar] [CrossRef] [PubMed]
- Sheu, C.; Foote, C.S. Reactivity toward Singlet Oxygen of a 7,8-Dihydro-8-oxoguanosine (“8-Hydroxyguanosine”) Formed by Photooxidation of a Guanosine Derivative. J. Am. Chem. Soc. 1995, 117, 6439–6442. [Google Scholar] [CrossRef]
- Ravanat, J.-L.; Cadet, J. Reaction of singlet oxygen with 2′-deoxyguanosine and DNA. Isolation and characterization of the main oxidation products. Chem. Res. Toxicol. 1995, 8, 379–388. [Google Scholar] [CrossRef]
- Luo, W.; Muller, J.G.; Rachlin, E.M.; Burrows, C.J. Characterization of spiroiminodihydantoin as a product of one-electron oxidation of 8-Oxo-7,8-dihydroguanosine. Org. Lett. 2000, 2, 613–616. [Google Scholar] [CrossRef]
- Chatgilialoglu, C.; D’Angelantonio, M.; Guerra, M.; Kaloudis, P.; Mulazzani, Q.G. A reevaluation of the ambident reactivity of the guanine moiety towards hydroxyl radicals. Angew. Chem. Int. Ed. Engl. 2009, 48, 2214–2217. [Google Scholar] [CrossRef]
- Candeias, L.P.; Steenken, S. Reaction of HO* with guanine derivatives in aqueous solution: Formation of two different redox-active OH-adduct radicals and their unimolecular transformation reactions. Properties of G(-H)*. Chemistry 2000, 6, 475–484. [Google Scholar] [CrossRef]
- Pryor, W.A. Why is the hydroxyl radical the only radical that commonly adds to DNA? Hypothesis: It has a rare combination of high electrophilicity, high thermochemical reactivity, and a mode of production that can occur near DNA. Free Radic. Biol. Med. 1988, 4, 219–223. [Google Scholar] [CrossRef]
- Haq, K.U.; Rusdipoetra, R.A.; Siswanto, I.; Suwito, H. Elucidation of reactive oxygen species scavenging pathways of norbergenin utilizing DFT approaches. R. Soc. Open Sci. 2022, 9, 221349. [Google Scholar] [CrossRef]
- Cadet, J.; Wagner, J.R. Oxidatively generated base damage to cellular DNA by hydroxyl radical and one-electron oxidants: Similarities and differences. Arch. Biochem. Biophys. 2014, 557, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Balasubramanian, B.; Pogozelski, W.K.; Tullius, T.D. DNA strand breaking by the hydroxyl radical is governed by the accessible surface areas of the hydrogen atoms of the DNA backbone. Proc. Natl. Acad. Sci. USA 1998, 95, 9738–9743. [Google Scholar] [CrossRef]
- Dizdaroglu, M.; Jaruga, P.; Birincioglu, M.; Rodriguez, H. Free radical-induced damage to DNA: Mechanisms and measurement. Free Radic. Biol. Med. 2002, 32, 1102–1115. [Google Scholar] [CrossRef]
- Steenken, S. Purine bases, nucleosides, and nucleotides: Aqueous solution redox chemistry and transformation reactions of their radical cations and e- and OH adducts. Chem. Rev. 1989, 89, 503–520. [Google Scholar] [CrossRef]
- O’Neill, P. Pulse Radiolytic Study of the Interaction of Thiols and Ascorbate with OH Adducts of dGMP and dG: Implications for DNA Repair Processes. Radiat. Res. 1983, 96, 198–210. [Google Scholar] [CrossRef]
- Mundy, C.J.; Colvin, M.E.; Quong, A.A. Irradiated Guanine: A Car-Parrinello Molecular Dynamics Study of Dehydrogenation in the Presence of an OH Radical. J. Phys. Chem. A 2002, 106, 10063–10071. [Google Scholar] [CrossRef]
- Wu, Y.; Mundy, C.J.; Colvin, M.E.; Car, R. On the Mechanisms of OH Radical Induced DNA-Base Damage: A Comparative Quantum Chemical and Car−Parrinello Molecular Dynamics Study. J. Phys. Chem. A 2004, 108, 2922–2929. [Google Scholar] [CrossRef]
- Chatgilialoglu, C.; Krokidis, M.G.; Masi, A.; Barata-Vallejo, S.; Ferreri, C.; Terzidis, M.A.; Szreder, T.; Bobrowski, K. New Insights into the Reaction Paths of Hydroxyl Radicals with Purine Moieties in DNA and Double-Stranded Oligodeoxynucleotides. Molecules 2019, 24, 3860. [Google Scholar] [CrossRef]
- Valavanidis, A.; Vlachogianni, T.; Fiotakis, C. 8-hydroxy-2′ -deoxyguanosine (8-OHdG): A critical biomarker of oxidative stress and carcinogenesis. J. Environ. Sci. Health C Environ. Carcinog. Ecotoxicol. Rev. 2009, 27, 120–139. [Google Scholar] [CrossRef]
- Crespo-Hernández, C.E.; Close, D.M.; Gorb, L.; Leszczynski, J. Determination of Redox Potentials for the Watson−Crick Base Pairs, DNA Nucleosides, and Relevant Nucleoside Analogues. J. Phys. Chem. B 2007, 111, 5386–5395. [Google Scholar] [CrossRef] [PubMed]
- Ravanat, J.L.; Douki, T.; Duez, P.; Gremaud, E.; Herbert, K.; Hofer, T.; Lasserre, L.; Saint-Pierre, C.; Favier, A.; Cadet, J. Cellular background level of 8-oxo-7,8-dihydro-2′-deoxyguanosine: An isotope based method to evaluate artefactual oxidation of DNA during its extraction and subsequent work-up. Carcinogenesis 2002, 23, 1911–1918. [Google Scholar] [CrossRef]
- Munk, B.H.; Burrows, C.J.; Schlegel, H.B. Exploration of Mechanisms for the Transformation of 8-Hydroxy Guanine Radical to FAPyG by Density Functional Theory. Chem. Res. Toxicol. 2007, 20, 432–444. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, M.M. The Formamidopyrimidines: Purine Lesions Formed in Competition With 8-Oxopurines From Oxidative Stress. Acc. Chem. Res. 2012, 45, 588–597. [Google Scholar] [CrossRef] [PubMed]
- Vieira, A.J.S.C.; Steenken, S. Pattern of OH radical reaction with N6,N6-dimethyladenosine. Production of three isomeric OH adducts and their dehydration and ring-opening reactions. J. Am. Chem. Soc. 1987, 109, 7441–7448. [Google Scholar] [CrossRef]
- Vieira, A.J.S.C.; Steenken, S. Pattern of hydroxy radical reaction with adenine and its nucleosides and nucleotides. Characterization of two types of isomeric hydroxy adduct and their unimolecular transformation reactions. J. Am. Chem. Soc. 1990, 112, 6986–6994. [Google Scholar] [CrossRef]
- Fujita, S.; Steenken, S. Pattern of hydroxyl radical addition to uracil and methyl- and carboxyl-substituted uracils. Electron transfer of hydroxyl adducts with N,N,N’,N’-tetramethyl-p-phenylenediamine and tetranitromethane. J. Am. Chem. Soc. 1981, 103, 2540–2545. [Google Scholar] [CrossRef]
- Al-Sheikhly, M.I.; Schuchmann, H.P.; von Sonntag, C. Gamma-radiolysis of N2O-saturated formate solutions. A chain reaction. Int. J. Radiat. Biol. Relat. Stud. Phys. Chem. Med. 1985, 47, 457–462. [Google Scholar] [CrossRef]
- Demirci-Cekic, S.; Özkan, G.; Avan, A.N.; Uzunboy, S.; Çapanoğlu, E.; Apak, R. Biomarkers of oxidative stress and antioxidant defense. J. Pharm. Biomed. Anal. 2022, 209, 114477. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.J.; Xia, Y.Y.; Zhao, M.W.; Da Huang, B.; Li, F. Theoretical study of the OH reaction with cytosine. J. Mol. Struct. THEOCHEM 2005, 723, 123–129. [Google Scholar] [CrossRef]
- von Sonntag, C. Free-Radical-Induced DNA Damage and Its Repair; Springer: Berlin/Heidelberg, Germany, 2006. [Google Scholar]
- Madugundu, G.S.; Cadet, J.; Wagner, J.R. Hydroxyl-radical-induced oxidation of 5-methylcytosine in isolated and cellular DNA. Nucleic Acids Res. 2014, 42, 7450–7460. [Google Scholar] [CrossRef]
- Téoule, R. Radiation-induced DNA damage and its repair. Int. J. Radiat. Biol. Relat. Stud. Phys. Chem. Med. 1987, 51, 573–589. [Google Scholar] [CrossRef]
- Dizdaroglu, M.; Holwitt, E.; Hagan, M.P.; Blakely, W.F. Formation of cytosine glycol and 5,6-dihydroxycytosine in deoxyribonucleic acid on treatment with osmium tetroxide. Biochem. J. 1986, 235, 531–536. [Google Scholar] [CrossRef]
- Gorini, F.; Scala, G.; Cooke, M.S.; Majello, B.; Amente, S. Towards a comprehensive view of 8-oxo-7,8-dihydro-2′-deoxyguanosine: Highlighting the intertwined roles of DNA damage and epigenetics in genomic instability. DNA Repair. 2021, 97, 103027. [Google Scholar] [CrossRef] [PubMed]
- Beckman, J.S.; Beckman, T.W.; Chen, J.; Marshall, P.A.; Freeman, B.A. Apparent hydroxyl radical production by peroxynitrite: Implications for endothelial injury from nitric oxide and superoxide. Proc. Natl. Acad. Sci. USA 1990, 87, 1620–1624. [Google Scholar] [CrossRef]
- Radi, R.; Peluffo, G.; Alvarez, M.N.; Naviliat, M.; Cayota, A. Unraveling peroxynitrite formation in biological systems. Free Radic. Biol. Med. 2001, 30, 463–488. [Google Scholar] [CrossRef]
- Ferrer-Sueta, G.; Radi, R. Chemical biology of peroxynitrite: Kinetics, diffusion, and radicals. ACS Chem. Biol. 2009, 4, 161–177. [Google Scholar] [CrossRef]
- Radi, R. Peroxynitrite, a stealthy biological oxidant. J. Biol. Chem. 2013, 288, 26464–26472. [Google Scholar] [CrossRef]
- Augusto, O.; Goldstein, S.; Hurst, J.K.; Lind, J.; Lymar, S.V.; Merenyi, G.; Radi, R. Carbon dioxide-catalyzed peroxynitrite reactivity–The resilience of the radical mechanism after two decades of research. Free Radic. Biol. Med. 2019, 135, 210–215. [Google Scholar] [CrossRef]
- Hiraku, Y. Formation of 8-nitroguanine, a nitrative DNA lesion, in inflammation-related carcinogenesis and its significance. Environ. Health Prev. Med. 2010, 15, 63–72. [Google Scholar] [CrossRef]
- Dedon, P.C.; Tannenbaum, S.R. Reactive nitrogen species in the chemical biology of inflammation. Arch. Biochem. Biophys. 2004, 423, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Pacher, P.; Beckman, J.S.; Liaudet, L. Nitric oxide and peroxynitrite in health and disease. Physiol. Rev. 2007, 87, 315–424. [Google Scholar] [CrossRef] [PubMed]
- Salgo, M.G.; Bermúdez, E.; Squadrito, G.L.; Pryor, W.A. Peroxynitrite causes DNA damage and oxidation of thiols in rat thymocytes [corrected]. Arch. Biochem. Biophys. 1995, 322, 500–505. [Google Scholar] [CrossRef]
- Szabó, C.; Ohshima, H. DNA damage induced by peroxynitrite: Subsequent biological effects. Nitric Oxide 1997, 1, 373–385. [Google Scholar] [CrossRef] [PubMed]
- Burney, S.; Niles, J.C.; Dedon, P.C.; Tannenbaum, S.R. DNA damage in deoxynucleosides and oligonucleotides treated with peroxynitrite. Chem. Res. Toxicol. 1999, 12, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Niles, J.C.; Wishnok, J.S.; Tannenbaum, S.R. Peroxynitrite-induced oxidation and nitration products of guanine and 8-oxoguanine: Structures and mechanisms of product formation. Nitric Oxide 2006, 14, 109–121. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, L.J.; Moore, K.; Caulfield, J.L.; Tannenbaum, S.R.; Dedon, P.C. Quantitation of 8-Oxoguanine and Strand Breaks Produced by Four Oxidizing Agents. Chem. Res. Toxicol. 1997, 10, 386–392. [Google Scholar] [CrossRef] [PubMed]
- Kasai, H. Analysis of a form of oxidative DNA damage, 8-hydroxy-2′-deoxyguanosine, as a marker of cellular oxidative stress during carcinogenesis. Mutat. Res./Rev. Mutat. Res. 1997, 387, 147–163. [Google Scholar] [CrossRef]
- Illés, E.; Mizrahi, A.; Marks, V.; Meyerstein, D. Carbonate-radical-anions, and not hydroxyl radicals, are the products of the Fenton reaction in neutral solutions containing bicarbonate. Free Radic. Biol. Med. 2019, 131, 1–6. [Google Scholar] [CrossRef]
- Chen, S.-N.; Cope, V.W.; Hoffman, M.Z. Behavior of carbon trioxide (-) radicals generated in the flash photolysis of carbonatoamine complexes of cobalt(III) in aqueous solution. J. Phys. Chem. 1973, 77, 1111–1116. [Google Scholar] [CrossRef]
- Meli, R.; Nauser, T.; Latal, P.; Koppenol, W.H. Reaction of peroxynitrite with carbon dioxide: Intermediates and determination of the yield of CO3•− and NO2•. JBIC J. Biol. Inorg. Chem. 2002, 7, 31–36. [Google Scholar] [CrossRef]
- Patra, S.G.; Mizrahi, A.; Meyerstein, D. The Role of Carbonate in Catalytic Oxidations. Acc. Chem. Res. 2020, 53, 2189–2200. [Google Scholar] [CrossRef]
- Ezraty, B.; Chabalier, M.; Ducret, A.; Maisonneuve, E.; Dukan, S. CO2 exacerbates oxygen toxicity. EMBO Rep. 2011, 12, 321–326. [Google Scholar] [CrossRef]
- Hoffman, A.; Goldstein, S.; Samuni, A.; Borman, J.B.; Schwalb, H. Effect of nitric oxide and nitroxide SOD-mimic on the recovery of isolated rat heart following ischemia and reperfusion. Biochem. Pharmacol. 2003, 66, 1279–1286. [Google Scholar] [CrossRef]
- Augusto, O.; Bonini, M.G.; Amanso, A.M.; Linares, E.; Santos, C.C.; De Menezes, S.L. Nitrogen dioxide and carbonate radical anion: Two emerging radicals in biology. Free Radic. Biol. Med. 2002, 32, 841–859. [Google Scholar] [CrossRef] [PubMed]
- Fleming, A.M.; Burrows, C.J. On the irrelevancy of hydroxyl radical to DNA damage from oxidative stress and implications for epigenetics. Chem. Soc. Rev. 2020, 49, 6524–6528. [Google Scholar] [CrossRef]
- Fleming, A.M.; Burrows, C.J. Iron Fenton oxidation of 2′-deoxyguanosine in physiological bicarbonate buffer yields products consistent with the reactive oxygen species carbonate radical anion not the hydroxyl radical. Chem. Commun. 2020, 56, 9779–9782. [Google Scholar] [CrossRef] [PubMed]
- Fleming, A.; Redstone, S.; Burrows, C. Oxidative DNA Damage and Repair in G-quadruplexes. In DNA Damage, DNA Repair and Disease; Royal Society of Chemistry: London, UK, 2020; pp. 61–85. [Google Scholar]
- Halliwell, B.; Adhikary, A.; Dingfelder, M.; Dizdaroglu, M. Hydroxyl radical is a significant player in oxidative DNA damage in vivo. Chem. Soc. Rev. 2021, 50, 8355–8360. [Google Scholar] [CrossRef] [PubMed]
- Buxton, G.V.; Elliot, A.J. Rate constant for reaction of hydroxyl radicals with bicarbonate ions. Int. J. Radiat. Appl. Instrum. Part C Radiat. Phys. Chem. 1986, 27, 241–243. [Google Scholar] [CrossRef]
- Joffe, A.; Geacintov, N.E.; Shafirovich, V. DNA Lesions Derived from the Site Selective Oxidation of Guanine by Carbonate Radical Anions. Chem. Res. Toxicol. 2003, 16, 1528–1538. [Google Scholar] [CrossRef]
- Halliwell, B.; Gutteridge, J.M.C. Free Radicals in Biology and Medicine; Oxford University Press: Oxford, UK, 2015. [Google Scholar]
- Chatgilialoglu, C.; Ferreri, C.; Krokidis, M.G.; Masi, A.; Terzidis, M.A. On the relevance of hydroxyl radical to purine DNA damage. Free Radic. Res. 2021, 55, 384–404. [Google Scholar] [CrossRef]
- Maynard, S.; Schurman, S.H.; Harboe, C.; de Souza-Pinto, N.C.; Bohr, V.A. Base excision repair of oxidative DNA damage and association with cancer and aging. Carcinogenesis 2009, 30, 2–10. [Google Scholar] [CrossRef]
- Mehta, A.; Haber, J.E. Sources of DNA double-strand breaks and models of recombinational DNA repair. Cold Spring Harb. Perspect. Biol. 2014, 6, a016428. [Google Scholar] [CrossRef]
- Dizdaroglu, M.; Jaruga, P. Mechanisms of free radical-induced damage to DNA. Free Radic. Res. 2012, 46, 382–419. [Google Scholar] [CrossRef]
- Miaskiewicz, K.; Osman, R. Theoretical study on the deoxyribose radicals formed by hydrogen abstraction. J. Am. Chem. Soc. 1994, 116, 232–238. [Google Scholar] [CrossRef]
- Pogozelski, W.K.; Tullius, T.D. Oxidative Strand Scission of Nucleic Acids: Routes Initiated by Hydrogen Abstraction from the Sugar Moiety. Chem. Rev. 1998, 98, 1089–1108. [Google Scholar] [CrossRef] [PubMed]
- von Sonntag, C.; Neuwald, K.; Dizdaroglu, M. Radiation chemistry of DNA model compounds. III. Gamma-radiolysis of 2-deoxy-D-ribose in the crystalline state. Conversion of 2-deoxy-D-ribose into 2,5-dideoxy-D-erythro-pentonic acid via a chain reaction. Radiat. Res. 1974, 58, 1–8. [Google Scholar] [CrossRef]
- Saunthwal, R.K.; Schwarz, M.; Mallick, R.K.; Terry-Wright, W.; Clayden, J. Enantioselective Intramolecular α-Arylation of Benzylamine Derivatives: Synthesis of a Precursor to Levocetirizine. Angew. Chem. Int. Ed. 2023, 62, e202216758. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Sheppard, T.L. Half-life and DNA strand scission products of 2-deoxyribonolactone oxidative DNA damage lesions. Chem. Res. Toxicol. 2004, 17, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Dedon, P.C. The chemical toxicology of 2-deoxyribose oxidation in DNA. Chem. Res. Toxicol. 2008, 21, 206–219. [Google Scholar] [CrossRef]
- Kroeger, K.M.; Jiang, Y.L.; Kow, Y.W.; Goodman, M.F.; Greenberg, M.M. Mutagenic effects of 2-deoxyribonolactone in Escherichia coli. An abasic lesion that disobeys the A-rule. Biochemistry 2004, 43, 6723–6733. [Google Scholar] [CrossRef]
- Faure, V.; Constant, J.F.; Dumy, P.; Saparbaev, M. 2′-deoxyribonolactone lesion produces G->A transitions in Escherichia coli. Nucleic Acids Res. 2004, 32, 2937–2946. [Google Scholar] [CrossRef]
- Dizdaroglu, M.; Schulte-Frohlinde, D.; von Sonntag, C. gamma-Radiolyses of DNA in oxygenated aqueous solution. Structure of an alkali-labile site. Z. Für. Naturforschung C 1977, 32, 1021–1022. [Google Scholar] [CrossRef]
- Dizdaroglu, M.; Von Sonntag, C.; Schulte-Frohlinde, D. Strand breaks and sugar release by.gamma.-irradiation of DNA in aqueous solution. J. Am. Chem. Soc. 1975, 97, 2277–2278. [Google Scholar] [CrossRef]
- Beesk, F.; Dizdaroglu, M.; Schulte-Frohlinde, D.; von Sonntag, C. Radiation-induced DNA strand breaks in deoxygenated aqueous solutions. The formation of altered sugars as end groups. Int. J. Radiat. Biol. Relat. Stud. Phys. Chem. Med. 1979, 36, 565–576. [Google Scholar] [CrossRef]
- Ma, J.; Marignier, J.-L.; Pernot, P.; Houée-Levin, C.; Kumar, A.; Sevilla, M.D.; Adhikary, A.; Mostafavi, M. Direct observation of the oxidation of DNA bases by phosphate radicals formed under radiation: A model of the backbone-to-base hole transfer. Phys. Chem. Chem. Phys. 2018, 20, 14927–14937. [Google Scholar] [CrossRef]
- Chatgilialoglu, C.; Ferreri, C.; Geacintov, N.E.; Krokidis, M.G.; Liu, Y.; Masi, A.; Shafirovich, V.; Terzidis, M.A.; Tsegay, P.S. 5′,8-Cyclopurine Lesions in DNA Damage: Chemical, Analytical, Biological, and Diagnostic Significance. Cells 2019, 8, 513. [Google Scholar] [CrossRef]
- Chatgilialoglu, C.; Guerra, M.; Mulazzani, Q.G. Model studies of DNA C5′ radicals. Selective generation and reactivity of 2′-deoxyadenosin-5′-yl radical. J. Am. Chem. Soc. 2003, 125, 3839–3848. [Google Scholar] [CrossRef] [PubMed]
- Manetto, A.; Georganakis, D.; Leondiadis, L.; Gimisis, T.; Mayer, P.; Carell, T.; Chatgilialoglu, C. Independent generation of C5′-nucleosidyl radicals in thymidine and 2′-deoxyguanosine. J. Org. Chem. 2007, 72, 3659–3666. [Google Scholar] [CrossRef] [PubMed]
- Chatgilialoglu, C.; Eriksson, L.A.; Krokidis, M.G.; Masi, A.; Wang, S.; Zhang, R. Oxygen Dependent Purine Lesions in Double-Stranded Oligodeoxynucleotides: Kinetic and Computational Studies Highlight the Mechanism for 5′,8-Cyclopurine Formation. J. Am. Chem. Soc. 2020, 142, 5825–5833. [Google Scholar] [CrossRef]
- Box, H.C.; Budzinski, E.E.; Dawidzik, J.B.; Gobey, J.S.; Freund, H.G. Free radical-induced tandem base damage in DNA oligomers. Free Radic. Biol. Med. 1997, 23, 1021–1030. [Google Scholar] [CrossRef]
- Box, H.C.; Budzinski, E.E.; Dawidzik, J.B.; Wallace, J.C.; Iijima, H. Tandem lesions and other products in X-irradiated DNA oligomers. Radiat. Res. 1998, 149, 433–439. [Google Scholar] [CrossRef] [PubMed]
- Box, H.C.; Patrzyc, H.B.; Dawidzik, J.B.; Wallace, J.C.; Freund, H.G.; Iijima, H.; Budzinski, E.E. Double base lesions in DNA X-irradiated in the presence or absence of oxygen. Radiat. Res. 2000, 153, 442–446. [Google Scholar] [CrossRef] [PubMed]
- Romieu, A.; Bellon, S.; Gasparutto, D.; Cadet, J. Synthesis and UV photolysis of oligodeoxynucleotides that contain 5-(phenylthiomethyl)-2′-deoxyuridine: A specific photolabile precursor of 5-(2′-deoxyuridilyl)methyl radical. Org. Lett. 2000, 2, 1085–1088. [Google Scholar] [CrossRef]
- Bellon, S.; Ravanat, J.L.; Gasparutto, D.; Cadet, J. Cross-linked thymine-purine base tandem lesions: Synthesis, characterization, and measurement in gamma-irradiated isolated DNA. Chem. Res. Toxicol. 2002, 15, 598–606. [Google Scholar] [CrossRef]
- Hong, H.; Cao, H.; Wang, Y.; Wang, Y. Identification and quantification of a guanine-thymine intrastrand cross-link lesion induced by Cu(II)/H2O2/ascorbate. Chem. Res. Toxicol. 2006, 19, 614–621. [Google Scholar] [CrossRef]
- Bellon, S.; Gasparutto, D.; Saint-Pierre, C.; Cadet, J. Guanine–thymine intrastrand cross-linked lesion containing oligonucleotides: From chemical synthesis to in vitro enzymatic replication. Org. Biomol. Chem. 2006, 4, 3831–3837. [Google Scholar] [CrossRef]
- Labet, V.; Morell, C.; Grand, A.; Cadet, J.; Cimino, P.; Barone, V. Formation of cross-linked adducts between guanine and thymine mediated by hydroxyl radical and one-electron oxidation: A theoretical study. Org. Biomol. Chem. 2008, 6, 3300–3305. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y. Bulky DNA lesions induced by reactive oxygen species. Chem. Res. Toxicol. 2008, 21, 276–281. [Google Scholar] [CrossRef] [PubMed]
- Xerri, B.; Morell, C.; Grand, A.; Cadet, J.; Cimino, P.; Barone, V. Radiation-induced formation of DNA intrastrand crosslinks between thymine and adenine bases: A theoretical approach. Org. Biomol. Chem. 2006, 4, 3986–3992. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Hong, H.; Cao, H.; Wang, Y. In vivo formation and in vitro replication of a guanine-thymine intrastrand cross-link lesion. Biochemistry 2007, 46, 12757–12763. [Google Scholar] [CrossRef]
- Hong, H.; Cao, H.; Wang, Y. Formation and genotoxicity of a guanine-cytosine intrastrand cross-link lesion in vivo. Nucleic Acids Res. 2007, 35, 7118–7127. [Google Scholar] [CrossRef]
- Hong, I.S.; Greenberg, M.M. Efficient DNA Interstrand Cross-Link Formation from a Nucleotide Radical. J. Am. Chem. Soc. 2005, 127, 3692–3693. [Google Scholar] [CrossRef]
- Hong, I.S.; Ding, H.; Greenberg, M.M. Oxygen Independent DNA Interstrand Cross-Link Formation by a Nucleotide Radical. J. Am. Chem. Soc. 2006, 128, 485–491. [Google Scholar] [CrossRef]
- Ding, H.; Greenberg, M.M. Gamma-radiolysis and hydroxyl radical produce interstrand cross-links in DNA involving thymidine. Chem. Res. Toxicol. 2007, 20, 1623–1628. [Google Scholar] [CrossRef]
- Ding, H.; Majumdar, A.; Tolman, J.R.; Greenberg, M.M. Multinuclear NMR and kinetic analysis of DNA interstrand cross-link formation. J. Am. Chem. Soc. 2008, 130, 17981–17987. [Google Scholar] [CrossRef][Green Version]
- Chen, N.-Y.; Li, C.-P.; Huang, H.-F. Synthesis, antitumor evaluation and computational study of thiazolidinone derivatives of dehydroabietic acid-based B ring-fused-thiazole. Mol. Divers. 2023, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Karwowski, B.T. The Influence of (5′R)- and (5′S)-5′,8-Cyclo-2′-Deoxyadenosine on UDG and hAPE1 Activity. Tandem Lesions are the Base Excision Repair System’s Nightmare. Cells 2019, 8, 1303. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Das, R.S.; Basu, A.K.; Stone, M.P. Structure of (5′S)-8,5′-Cyclo-2′-deoxyguanosine in DNA. J. Am. Chem. Soc. 2011, 133, 20357–20368. [Google Scholar] [CrossRef]
- Shibutani, S.; Takeshita, M.; Grollman, A.P. Insertion of specific bases during DNA synthesis past the oxidation-damaged base 8-oxodG. Nature 1991, 349, 431–434. [Google Scholar] [CrossRef] [PubMed]
- Burak, M.J.; Guja, K.E.; Garcia-Diaz, M. Nucleotide binding interactions modulate dNTP selectivity and facilitate 8-oxo-dGTP incorporation by DNA polymerase lambda. Nucleic Acids Res. 2015, 43, 8089–8099. [Google Scholar] [CrossRef]
- Kasai, H.; Nishimura, S. Hydroxylation of deoxyguanosine at the C-8 position by ascorbic acid and other reducing agents. Nucleic Acids Res. 1984, 12, 2137–2145. [Google Scholar] [CrossRef]
- Sies, H.; Berndt, C.; Jones, D.P. Oxidative Stress. Annu. Rev. Biochem. 2017, 86, 715–748. [Google Scholar] [CrossRef]
- Ock, C.Y.; Kim, E.H.; Choi, D.J.; Lee, H.J.; Hahm, K.B.; Chung, M.H. 8-Hydroxydeoxyguanosine: Not mere biomarker for oxidative stress, but remedy for oxidative stress-implicated gastrointestinal diseases. World J. Gastroenterol. 2012, 18, 302–308. [Google Scholar] [CrossRef]
- Cheng, K.C.; Cahill, D.S.; Kasai, H.; Nishimura, S.; Loeb, L.A. 8-Hydroxyguanine, an abundant form of oxidative DNA damage, causes G----T and A----C substitutions. J. Biol. Chem. 1992, 267, 166–172. [Google Scholar] [CrossRef]
- Nakabeppu, Y.; Sakumi, K.; Sakamoto, K.; Tsuchimoto, D.; Tsuzuki, T.; Nakatsu, Y. Mutagenesis and carcinogenesis caused by the oxidation of nucleic acids. Biol. Chem. 2006, 387, 373–379. [Google Scholar] [CrossRef]
- Hsu, G.W.; Ober, M.; Carell, T.; Beese, L.S. Error-prone replication of oxidatively damaged DNA by a high-fidelity DNA polymerase. Nature 2004, 431, 217–221. [Google Scholar] [CrossRef]
- Nair, D.T.; Johnson, R.E.; Prakash, S.; Prakash, L.; Aggarwal, A.K. Replication by human DNA polymerase-iota occurs by Hoogsteen base-pairing. Nature 2004, 430, 377–380. [Google Scholar] [CrossRef]
- Suzuki, T.; Kamiya, H. Mutations induced by 8-hydroxyguanine (8-oxo-7,8-dihydroguanine), a representative oxidized base, in mammalian cells. Genes Environ. 2017, 39, 2. [Google Scholar] [CrossRef]
- Kino, K.; Sugasawa, K.; Mizuno, T.; Bando, T.; Sugiyama, H.; Akita, M.; Miyazawa, H.; Hanaoka, F. Eukaryotic DNA Polymerases α, β and ε Incorporate Guanine Opposite 2,2,4-Triamino-5(2H)-oxazolone. ChemBioChem 2009, 10, 2613–2616. [Google Scholar] [CrossRef]
- Henderson, P.T.; Delaney, J.C.; Gu, F.; Tannenbaum, S.R.; Essigmann, J.M. Oxidation of 7,8-Dihydro-8-oxoguanine Affords Lesions That Are Potent Sources of Replication Errors in Vivo. Biochemistry 2002, 41, 914–921. [Google Scholar] [CrossRef]
- Kino, K.; Hirao-Suzuki, M.; Morikawa, M.; Sakaga, A.; Miyazawa, H. Generation, repair and replication of guanine oxidation products. Genes Environ. 2017, 39, 21. [Google Scholar] [CrossRef]
- Chen, B.; Zhou, X.; Taghizadeh, K.; Chen, J.; Stubbe, J.; Dedon, P.C. GC/MS Methods To Quantify the 2-Deoxypentos-4-ulose and 3′-Phosphoglycolate Pathways of 4′ Oxidation of 2-Deoxyribose in DNA: Application to DNA Damage Produced by γ Radiation and Bleomycin. Chem. Res. Toxicol. 2007, 20, 1701–1708. [Google Scholar] [CrossRef]
- Hegde, M.L.; Hazra, T.K.; Mitra, S. Early steps in the DNA base excision/single-strand interruption repair pathway in mammalian cells. Cell Res. 2008, 18, 27–47. [Google Scholar] [CrossRef]
- Heeres, J.T.; Hergenrother, P.J. Poly(ADP-ribose) makes a date with death. Curr. Opin. Chem. Biol. 2007, 11, 644–653. [Google Scholar] [CrossRef] [PubMed]
- Jurkovicova, D.; Neophytou, C.M.; Gašparović, A.Č.; Gonçalves, A.C. DNA Damage Response in Cancer Therapy and Resistance: Challenges and Opportunities. Int. J. Mol. Sci. 2022, 23, 14672. [Google Scholar] [CrossRef] [PubMed]
- Harper, J.W.; Elledge, S.J. The DNA damage response: Ten years after. Mol. Cell 2007, 28, 739–745. [Google Scholar] [CrossRef]
- Liu, Y.; Prasad, R.; Beard, W.A.; Kedar, P.S.; Hou, E.W.; Shock, D.D.; Wilson, S.H. Coordination of steps in single-nucleotide base excision repair mediated by apurinic/apyrimidinic endonuclease 1 and DNA polymerase beta. J. Biol. Chem. 2007, 282, 13532–13541. [Google Scholar] [CrossRef] [PubMed]
- Krokan, H.E.; Bjørås, M. Base excision repair. Cold Spring Harb. Perspect. Biol. 2013, 5, a012583. [Google Scholar] [CrossRef]
- Svilar, D.; Goellner, E.M.; Almeida, K.H.; Sobol, R.W. Base excision repair and lesion-dependent subpathways for repair of oxidative DNA damage. Antioxid. Redox Signal. 2011, 14, 2491–2507. [Google Scholar] [CrossRef] [PubMed]
- Chaudhuri, J.; Alt, F.W. Class-switch recombination: Interplay of transcription, DNA deamination and DNA repair. Nat. Rev. Immunol. 2004, 4, 541–552. [Google Scholar] [CrossRef]
- Farrington, S.M.; Tenesa, A.; Barnetson, R.; Wiltshire, A.; Prendergast, J.; Porteous, M.; Campbell, H.; Dunlop, M.G. Germline susceptibility to colorectal cancer due to base-excision repair gene defects. Am. J. Hum. Genet. 2005, 77, 112–119. [Google Scholar] [CrossRef]
- Cleaver, J.E.; Karplus, K.; Kashani-Sabet, M.; Limoli, C.L. Nucleotide excision repair “a legacy of creativity”. Mutat. Res. 2001, 485, 23–36. [Google Scholar] [CrossRef]
- Wang, M.; Gu, D.; Zhang, Z.; Zhou, J.; Zhang, Z. XPD polymorphisms, cigarette smoking, and bladder cancer risk: A meta-analysis. J. Toxicol. Environ. Health A 2009, 72, 698–705. [Google Scholar] [CrossRef] [PubMed]
- Mittal, R.D.; Mandal, R.K. Genetic variation in nucleotide excision repair pathway genes influence prostate and bladder cancer susceptibility in North Indian population. Indian J. Hum. Genet. 2012, 18, 47–55. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Qiao, Y.; Spitz, M.R.; Guo, Z.; Hadeyati, M.; Grossman, L.; Kraemer, K.H.; Wei, Q. Rapid assessment of repair of ultraviolet DNA damage with a modified host-cell reactivation assay using a luciferase reporter gene and correlation with polymorphisms of DNA repair genes in normal human lymphocytes. Mutat. Res. 2002, 509, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Goukassian, D.; Gad, F.; Yaar, M.; Eller, M.S.; Nehal, U.S.; Gilchrest, B.A. Mechanisms and implications of the age-associated decrease in DNA repair capacity. FASEB J. 2000, 14, 1325–1334. [Google Scholar] [CrossRef]
- Jiricny, J. The multifaceted mismatch-repair system. Nat. Rev. Mol. Cell Biol. 2006, 7, 335–346. [Google Scholar] [CrossRef]
- Arana, M.E.; Kunkel, T.A. Mutator phenotypes due to DNA replication infidelity. Semin. Cancer Biol. 2010, 20, 304–311. [Google Scholar] [CrossRef]
- Rossetti, G.; Dans, P.D.; Gomez-Pinto, I.; Ivani, I.; Gonzalez, C.; Orozco, M. The structural impact of DNA mismatches. Nucleic Acids Res. 2015, 43, 4309–4321. [Google Scholar] [CrossRef]
- Chatterjee, N.; Lin, Y.; Wilson, J.H. Mismatch repair enhances convergent transcription-induced cell death at trinucleotide repeats by activating ATR. DNA Repair. 2016, 42, 26–32. [Google Scholar] [CrossRef]
- Peltomäki, P. DNA mismatch repair and cancer. Mutat. Res. 2001, 488, 77–85. [Google Scholar] [CrossRef]
- Deans, A.J.; West, S.C. DNA interstrand crosslink repair and cancer. Nat. Rev. Cancer 2011, 11, 467–480. [Google Scholar] [CrossRef]
- Clauson, C.; Schärer, O.D.; Niedernhofer, L. Advances in understanding the complex mechanisms of DNA interstrand cross-link repair. Cold Spring Harb. Perspect. Biol. 2013, 5, a012732. [Google Scholar] [CrossRef] [PubMed]
- Amna, A.; Javaria, Z.; Naureen, E.; Qurat Ul, A.; Mahnoor, T.; Abdul, H. Interstrand Crosslink Repair. In DNA; Payam, B., Ed.; IntechOpen: Rijeka, Croatia, 2021; p. 121. [Google Scholar]
- Schneider, M.; Chandler, K.; Tischkowitz, M.; Meyer, S. Fanconi anaemia: Genetics, molecular biology, and cancer—Implications for clinical management in children and adults. Clin. Genet. 2015, 88, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Pfeiffer, P.; Goedecke, W.; Obe, G. Mechanisms of DNA double-strand break repair and their potential to induce chromosomal aberrations. Mutagenesis 2000, 15, 289–302. [Google Scholar] [CrossRef] [PubMed]
- Khanna, K.K.; Jackson, S.P. DNA double-strand breaks: Signaling, repair and the cancer connection. Nat. Genet. 2001, 27, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Moore, J.K.; Haber, J.E. Cell cycle and genetic requirements of two pathways of nonhomologous end-joining repair of double-strand breaks in Saccharomyces cerevisiae. Mol. Cell. Biol. 1996, 16, 2164–2173. [Google Scholar] [CrossRef] [PubMed]
- Pannunzio, N.R.; Li, S.; Watanabe, G.; Lieber, M.R. Non-homologous end joining often uses microhomology: Implications for alternative end joining. DNA Repair. 2014, 17, 74–80. [Google Scholar] [CrossRef]
- Muraki, K.; Han, L.; Miller, D.; Murnane, J.P. The Role of ATM in the Deficiency in Nonhomologous End-Joining near Telomeres in a Human Cancer Cell Line. PLoS Genet. 2013, 9, e1003386. [Google Scholar] [CrossRef]
- Rodgers, K.; McVey, M. Error-Prone Repair of DNA Double-Strand Breaks. J. Cell. Physiol. 2016, 231, 15–24. [Google Scholar] [CrossRef]
- Mari, P.O.; Florea, B.I.; Persengiev, S.P.; Verkaik, N.S.; Brüggenwirth, H.T.; Modesti, M.; Giglia-Mari, G.; Bezstarosti, K.; Demmers, J.A.; Luider, T.M.; et al. Dynamic assembly of end-joining complexes requires interaction between Ku70/80 and XRCC4. Proc. Natl. Acad. Sci. USA 2006, 103, 18597–18602. [Google Scholar] [CrossRef]
- Panier, S.; Boulton, S.J. Double-strand break repair: 53BP1 comes into focus. Nat. Rev. Mol. Cell Biol. 2014, 15, 7–18. [Google Scholar] [CrossRef]
- Galhardo, R.S.; Hastings, P.J.; Rosenberg, S.M. Mutation as a stress response and the regulation of evolvability. Crit. Rev. Biochem. Mol. Biol. 2007, 42, 399–435. [Google Scholar] [CrossRef]
- Volkova, N.V.; Meier, B.; González-Huici, V.; Bertolini, S.; Gonzalez, S.; Vöhringer, H.; Abascal, F.; Martincorena, I.; Campbell, P.J.; Gartner, A.; et al. Mutational signatures are jointly shaped by DNA damage and repair. Nat. Commun. 2020, 11, 2169. [Google Scholar] [CrossRef]
- Chang, D.J.; Cimprich, K.A. DNA damage tolerance: When it’s OK to make mistakes. Nat. Chem. Biol. 2009, 5, 82–90. [Google Scholar] [CrossRef]
- Bartek, J.; Bartkova, J.; Lukas, J. DNA damage signalling guards against activated oncogenes and tumour progression. Oncogene 2007, 26, 7773–7779. [Google Scholar] [CrossRef]
- Bi, X. Mechanism of DNA damage tolerance. World J. Biol. Chem. 2015, 6, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Yamanaka, K.; Chatterjee, N.; Hemann, M.T.; Walker, G.C. Inhibition of mutagenic translesion synthesis: A possible strategy for improving chemotherapy? PLoS Genet. 2017, 13, e1006842. [Google Scholar] [CrossRef]
- Friedberg, E.C.; Lehmann, A.R.; Fuchs, R.P. Trading places: How do DNA polymerases switch during translesion DNA synthesis? Mol. Cell 2005, 18, 499–505. [Google Scholar] [CrossRef]
- Waters, L.S.; Minesinger, B.K.; Wiltrout, M.E.; D’Souza, S.; Woodruff, R.V.; Walker, G.C. Eukaryotic translesion polymerases and their roles and regulation in DNA damage tolerance. Microbiol. Mol. Biol. Rev. 2009, 73, 134–154. [Google Scholar] [CrossRef]
- Zhu, H.; Fan, Y.; Shen, J.; Qi, H.; Shao, J. Characterization of human DNA polymerase κ promoter in response to benzo[a]pyrene diol epoxide. Environ. Toxicol. Pharmacol. 2012, 33, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Xie, K.; Doles, J.; Hemann, M.T.; Walker, G.C. Error-prone translesion synthesis mediates acquired chemoresistance. Proc. Natl. Acad. Sci. USA 2010, 107, 20792–20797. [Google Scholar] [CrossRef] [PubMed]
- Litwin, I.; Bakowski, T.; Szakal, B.; Pilarczyk, E.; Maciaszczyk-Dziubinska, E.; Branzei, D.; Wysocki, R. Error-free DNA damage tolerance pathway is facilitated by the Irc5 translocase through cohesin. EMBO J. 2018, 37, e98732. [Google Scholar] [CrossRef] [PubMed]
- Mönttinen, H.A.M.; Löytynoja, A. Template switching in DNA replication can create and maintain RNA hairpins. Proc. Natl. Acad. Sci. USA 2022, 119, e2107005119. [Google Scholar] [CrossRef] [PubMed]
- Chinemerem Nwobodo, D.; Ugwu, M.C.; Oliseloke Anie, C.; Al-Ouqaili, M.T.; Chinedu Ikem, J.; Victor Chigozie, U.; Saki, M. Antibiotic resistance: The challenges and some emerging strategies for tackling a global menace. J. Clin. Lab. Anal. 2022, 36, e24655. [Google Scholar] [CrossRef]
- Pang, X.; Li, D.; Zhu, J.; Cheng, J.; Liu, G. Beyond antibiotics: Photo/sonodynamic approaches for bacterial theranostics. Nano-Micro Lett. 2020, 12, 1–23. [Google Scholar] [CrossRef]
- Kohanski, M.A.; Dwyer, D.J.; Hayete, B.; Lawrence, C.A.; Collins, J.J. A common mechanism of cellular death induced by bactericidal antibiotics. Cell 2007, 130, 797–810. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, X. Contribution of oxidative damage to antimicrobial lethality. Antimicrob. Agents Chemother. 2009, 53, 1395–1402. [Google Scholar] [CrossRef]
- Oexle, H.; Gnaiger, E.; Weiss, G. Iron-dependent changes in cellular energy metabolism: Influence on citric acid cycle and oxidative phosphorylation. Biochim. Biophys. Acta (BBA)-Bioenerg. 1999, 1413, 99–107. [Google Scholar] [CrossRef]
- Hong, Y.; Zeng, J.; Wang, X.; Drlica, K.; Zhao, X. Post-stress bacterial cell death mediated by reactive oxygen species. Proc. Natl. Acad. Sci. USA 2019, 116, 10064–10071. [Google Scholar] [CrossRef]
- Boto, A.; Pérez de la Lastra, J.M.; González, C.C. The road from host-defense peptides to a new generation of antimicrobial drugs. Molecules 2018, 23, 311. [Google Scholar] [CrossRef]
- Dijksteel, G.S.; Ulrich, M.M.; Middelkoop, E.; Boekema, B.K. Lessons learned from clinical trials using antimicrobial peptides (AMPs). Front. Microbiol. 2021, 12, 616979. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wang, Y.; Xue, Z.; Jia, Y.; Li, R.; He, C.; Chen, H. The structure-mechanism relationship and mode of actions of antimicrobial peptides: A review. Trends Food Sci. Technol. 2021, 109, 103–115. [Google Scholar] [CrossRef]
- Kashef, N.; Hamblin, M.R. Can microbial cells develop resistance to oxidative stress in antimicrobial photodynamic inactivation? Drug Resist. Updates 2017, 31, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Oyinloye, B.E.; Adenowo, A.F.; Kappo, A.P. Reactive oxygen species, apoptosis, antimicrobial peptides and human inflammatory diseases. Pharmaceuticals 2015, 8, 151–175. [Google Scholar] [CrossRef]
- Ma, H.; Yang, L.; Tian, Z.; Zhu, L.; Peng, J.; Fu, P.; Xiu, J.; Guo, G. Antimicrobial peptide AMP-17 exerts anti—Candida albicans effects through ROS-mediated apoptosis and necrosis. Int. Microbiol. 2023, 26, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Rather, I.A.; Sabir, J.S.; Asseri, A.H.; Ali, S. Antifungal activity of human cathelicidin LL-37, a membrane disrupting peptide, by triggering oxidative stress and cell cycle arrest in Candida auris. J. Fungi 2022, 8, 204. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Lee, D.G. Role of calcium in reactive oxygen species-induced apoptosis in Candida albicans: An antifungal mechanism of antimicrobial peptide, PMAP-23. Free Radic. Res. 2019, 53, 8–17. [Google Scholar] [CrossRef]
- Peng, C.; Liu, Y.; Shui, L.; Zhao, Z.; Mao, X.; Liu, Z. Mechanisms of Action of the Antimicrobial Peptide Cecropin in the Killing of Candida albicans. Life 2022, 12, 1581. [Google Scholar] [CrossRef]
- Cho, J.; Lee, D.G. Oxidative stress by antimicrobial peptide pleurocidin triggers apoptosis in Candida albicans. Biochimie 2011, 93, 1873–1879. [Google Scholar] [CrossRef]
- Ostling, O.; Johanson, K.J. Microelectrophoretic study of radiation-induced DNA damages in individual mammalian cells. Biochem. Biophys. Res. Commun. 1984, 123, 291–298. [Google Scholar] [CrossRef]
- Freeman, S.E.; Blackett, A.D.; Monteleone, D.C.; Setlow, R.B.; Sutherland, B.M.; Sutherland, J.C. Quantitation of radiation-, chemical-, or enzyme-induced single strand breaks in nonradioactive DNA by alkaline gel electrophoresis: Application to pyrimidine dimers. Anal. Biochem. 1986, 158, 119–129. [Google Scholar] [CrossRef]
- Klee, W.A. Conformation of ribonuclease S-peptide. Biochemistry 1968, 7, 2731–2736. [Google Scholar] [CrossRef] [PubMed]
- Wani, A.A.; D’Ambrosio, S.M.; Alvi, N.K. Quantitation of pyrimidine dimers by immunoslot blot following sublethal UV-irradiation of human cells. Photochem. Photobiol. 1987, 46, 477–482. [Google Scholar] [CrossRef]
- Saiki, R.K.; Scharf, S.; Faloona, F.; Mullis, K.B.; Horn, G.T.; Erlich, H.A.; Arnheim, N. Enzymatic amplification of beta-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science 1985, 230, 1350–1354. [Google Scholar] [CrossRef]
- Kalinowski, D.P.; Illenye, S.; Van Houten, B. Analysis of DNA damage and repair in murine leukemia L1210 cells using a quantitative polymerase chain reaction assay. Nucleic Acids Res. 1992, 20, 3485–3494. [Google Scholar] [CrossRef]
- Pfeifer, G.P.; Drouin, R.; Riggs, A.D.; Holmquist, G.P. In vivo mapping of a DNA adduct at nucleotide resolution: Detection of pyrimidine (6-4) pyrimidone photoproducts by ligation-mediated polymerase chain reaction. Proc. Natl. Acad. Sci. USA 1991, 88, 1374–1378. [Google Scholar] [CrossRef] [PubMed]
- Gavrieli, Y.; Sherman, Y.; Ben-Sasson, S.A. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J. Cell Biol. 1992, 119, 493–501. [Google Scholar] [CrossRef] [PubMed]
- Teng, Y.; Bennett, M.; Evans, K.E.; Zhuang-Jackson, H.; Higgs, A.; Reed, S.H.; Waters, R. A novel method for the genome-wide high resolution analysis of DNA damage. Nucleic Acids Res. 2011, 39, e10. [Google Scholar] [CrossRef]
- Zavala, A.G.; Morris, R.T.; Wyrick, J.J.; Smerdon, M.J. High-resolution characterization of CPD hotspot formation in human fibroblasts. Nucleic Acids Res. 2014, 42, 893–905. [Google Scholar] [CrossRef]
- Sloan, D.B.; Broz, A.K.; Sharbrough, J.; Wu, Z. Detecting Rare Mutations and DNA Damage with Sequencing-Based Methods. Trends Biotechnol. 2018, 36, 729–740. [Google Scholar] [CrossRef]
- Zatopek, K.M.; Potapov, V.; Maduzia, L.L.; Alpaslan, E.; Chen, L.; Evans, T.C., Jr.; Ong, J.L.; Ettwiller, L.M.; Gardner, A.F. RADAR-seq: A RAre DAmage and Repair sequencing method for detecting DNA damage on a genome-wide scale. DNA Repair. 2019, 80, 36–44. [Google Scholar] [CrossRef]
- McMaster, G.K.; Carmichael, G.G. Analysis of single- and double-stranded nucleic acids on polyacrylamide and agarose gels by using glyoxal and acridine orange. Proc. Natl. Acad. Sci. USA 1977, 74, 4835–4838. [Google Scholar] [CrossRef]
- McKelvey-Martin, V.J.; Ho, E.T.S.; McKeown, S.R.; Johnston, S.R.; McCarthy, P.J.; Rajab, N.F.; Downes, C.S. Emerging applications of the single cell gel electrophoresis (Comet) assay. I. Management of invasive transitional cell human bladder carcinoma. II. Fluorescent in situ hybridization Comets for the identification of damaged and repaired DNA sequences in individual cells. Mutagenesis 1998, 13, 1–8. [Google Scholar] [CrossRef][Green Version]
- Wood, D.K.; Weingeist, D.M.; Bhatia, S.N.; Engelward, B.P. Single cell trapping and DNA damage analysis using microwell arrays. Proc. Natl. Acad. Sci. USA 2010, 107, 10008–10013. [Google Scholar] [CrossRef]
- Sykora, P.; Witt, K.L.; Revanna, P.; Smith-Roe, S.L.; Dismukes, J.; Lloyd, D.G.; Engelward, B.P.; Sobol, R.W. Next generation high throughput DNA damage detection platform for genotoxic compound screening. Sci. Rep. 2018, 8, 2771. [Google Scholar] [CrossRef]
- Ngo, L.P.; Owiti, N.A.; Swartz, C.; Winters, J.; Su, Y.; Ge, J.; Xiong, A.; Han, J.; Recio, L.; Samson, L.D.; et al. Sensitive CometChip assay for screening potentially carcinogenic DNA adducts by trapping DNA repair intermediates. Nucleic Acids Res. 2020, 48, e13. [Google Scholar] [CrossRef] [PubMed]
- Borrego-Soto, G.; Ortiz-López, R.; Rojas-Martínez, A. Ionizing radiation-induced DNA injury and damage detection in patients with breast cancer. Genet. Mol. Biol. 2015, 38, 420–432. [Google Scholar] [CrossRef]
- Thomas, D.C.; Morton, A.G.; Bohr, V.A.; Sancar, A. General method for quantifying base adducts in specific mammalian genes. Proc. Natl. Acad. Sci. USA 1988, 85, 3723–3727. [Google Scholar] [CrossRef] [PubMed]
- Wellinger, R.-E.; Thoma, F. Taq DNA Polymerase Blockage at Pyrimidine Dimers. Nucleic Acids Res. 1996, 24, 1578–1579. [Google Scholar] [CrossRef]
- Wellinger, R.E.; Thoma, F. Nucleosome structure and positioning modulate nucleotide excision repair in the non-transcribed strand of an active gene. EMBO J. 1997, 16, 5046–5056. [Google Scholar] [CrossRef]
- Leung, C.H.; Zhong, H.J.; Lu, L.; Chan, D.S.; Ma, D.L. Luminescent and colorimetric strategies for the label-free DNA-based detection of enzyme activity. Brief. Funct. Genom. 2013, 12, 525–535. [Google Scholar] [CrossRef] [PubMed]
- Wilson, D.L.; Kool, E.T. Fluorescent Probes of DNA Repair. ACS Chem. Biol. 2018, 13, 1721–1733. [Google Scholar] [CrossRef]
- Li, W.; Sancar, A. Methodologies for detecting environmentally induced DNA damage and repair. Environ. Mol. Mutagen. 2020, 61, 664–679. [Google Scholar] [CrossRef]
- Hemeryck, L.Y.; Moore, S.A.; Vanhaecke, L. Mass Spectrometric Mapping of the DNA Adductome as a Means to Study Genotoxin Exposure, Metabolism, and Effect. Anal. Chem. 2016, 88, 7436–7446. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.J.; Cooke, M.S.; Hu, C.W.; Chao, M.R. Novel approach to integrated DNA adductomics for the assessment of in vitro and in vivo environmental exposures. Arch. Toxicol. 2018, 92, 2665–2680. [Google Scholar] [CrossRef] [PubMed]
- Slatko, B.E.; Gardner, A.F.; Ausubel, F.M. Overview of Next-Generation Sequencing Technologies. Curr. Protoc. Mol. Biol. 2018, 122, e59. [Google Scholar] [CrossRef]
- Mingard, C.; Wu, J.; McKeague, M.; Sturla, S.J. Next-generation DNA damage sequencing. Chem. Soc. Rev. 2020, 49, 7354–7377. [Google Scholar] [CrossRef] [PubMed]
- Vitelli, V.; Galbiati, A.; Iannelli, F.; Pessina, F.; Sharma, S.; d’Adda di Fagagna, F. Recent Advancements in DNA Damage-Transcription Crosstalk and High-Resolution Mapping of DNA Breaks. Annu. Rev. Genom. Hum. Genet. 2017, 18, 87–113. [Google Scholar] [CrossRef] [PubMed]
- Crosetto, N.; Mitra, A.; Silva, M.J.; Bienko, M.; Dojer, N.; Wang, Q.; Karaca, E.; Chiarle, R.; Skrzypczak, M.; Ginalski, K.; et al. Nucleotide-resolution DNA double-strand break mapping by next-generation sequencing. Nat. Methods 2013, 10, 361–365. [Google Scholar] [CrossRef]
- Canela, A.; Sridharan, S.; Sciascia, N.; Tubbs, A.; Meltzer, P.; Sleckman, B.P.; Nussenzweig, A. DNA Breaks and End Resection Measured Genome-wide by End Sequencing. Mol. Cell 2016, 63, 898–911. [Google Scholar] [CrossRef]
- Yan, W.X.; Mirzazadeh, R.; Garnerone, S.; Scott, D.; Schneider, M.W.; Kallas, T.; Custodio, J.; Wernersson, E.; Li, Y.; Gao, L.; et al. BLISS is a versatile and quantitative method for genome-wide profiling of DNA double-strand breaks. Nat. Commun. 2017, 8, 15058. [Google Scholar] [CrossRef] [PubMed]
- Biernacka, A.; Zhu, Y.; Skrzypczak, M.; Forey, R.; Pardo, B.; Grzelak, M.; Nde, J.; Mitra, A.; Kudlicki, A.; Crosetto, N.; et al. i-BLESS is an ultra-sensitive method for detection of DNA double-strand breaks. Commun. Biol. 2018, 1, 181. [Google Scholar] [CrossRef] [PubMed]
- Baranello, L.; Kouzine, F.; Wojtowicz, D.; Cui, K.; Przytycka, T.M.; Zhao, K.; Levens, D. DNA Break Mapping Reveals Topoisomerase II Activity Genome-Wide. Int. J. Mol. Sci. 2014, 15, 13111–13122. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Salazar-García, L.; Gao, F.; Wahlestedt, T.; Wu, C.-L.; Han, X.; Cai, Y.; Xu, D.; Wang, F.; Tang, L.; et al. Novel approach reveals genomic landscapes of single-strand DNA breaks with nucleotide resolution in human cells. Nat. Commun. 2019, 10, 5799. [Google Scholar] [CrossRef] [PubMed]







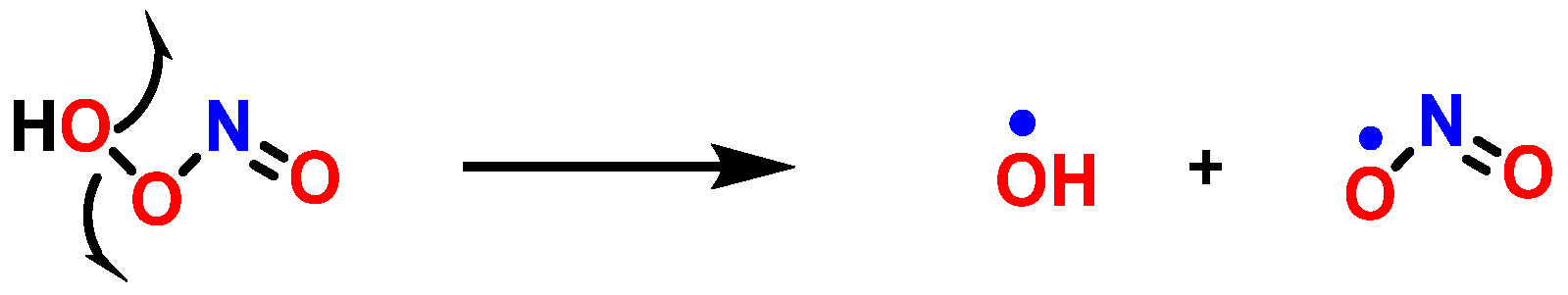
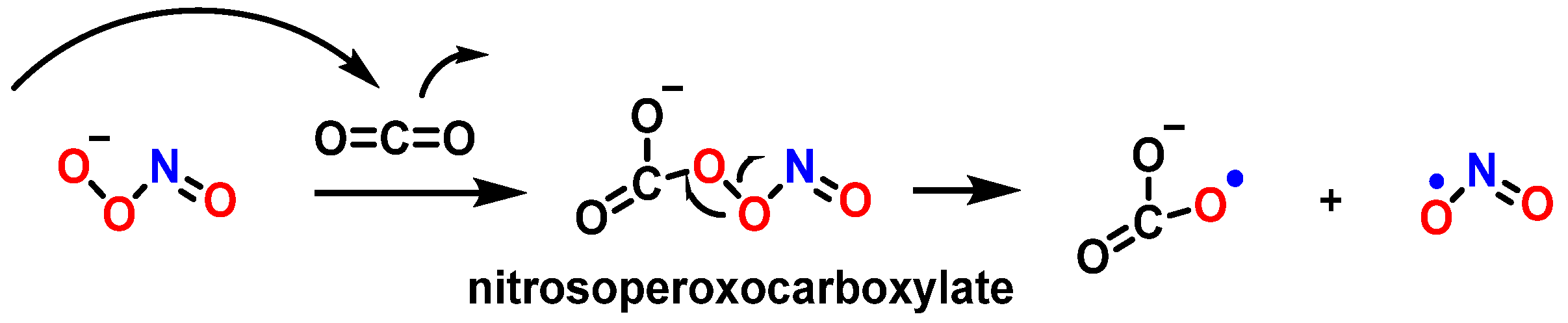






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andrés, C.M.C.; Lastra, J.M.P.d.l.; Juan, C.A.; Plou, F.J.; Pérez-Lebeña, E. Chemical Insights into Oxidative and Nitrative Modifications of DNA. Int. J. Mol. Sci. 2023, 24, 15240. https://doi.org/10.3390/ijms242015240
Andrés CMC, Lastra JMPdl, Juan CA, Plou FJ, Pérez-Lebeña E. Chemical Insights into Oxidative and Nitrative Modifications of DNA. International Journal of Molecular Sciences. 2023; 24(20):15240. https://doi.org/10.3390/ijms242015240
Chicago/Turabian StyleAndrés, Celia María Curieses, José Manuel Pérez de la Lastra, Celia Andrés Juan, Francisco J. Plou, and Eduardo Pérez-Lebeña. 2023. "Chemical Insights into Oxidative and Nitrative Modifications of DNA" International Journal of Molecular Sciences 24, no. 20: 15240. https://doi.org/10.3390/ijms242015240
APA StyleAndrés, C. M. C., Lastra, J. M. P. d. l., Juan, C. A., Plou, F. J., & Pérez-Lebeña, E. (2023). Chemical Insights into Oxidative and Nitrative Modifications of DNA. International Journal of Molecular Sciences, 24(20), 15240. https://doi.org/10.3390/ijms242015240





