Quercetin Derivatives as Potential Therapeutic Agents: An Updated Perspective on the Treatment of Nicotine-Induced Non-Small Cell Lung Cancer
Abstract
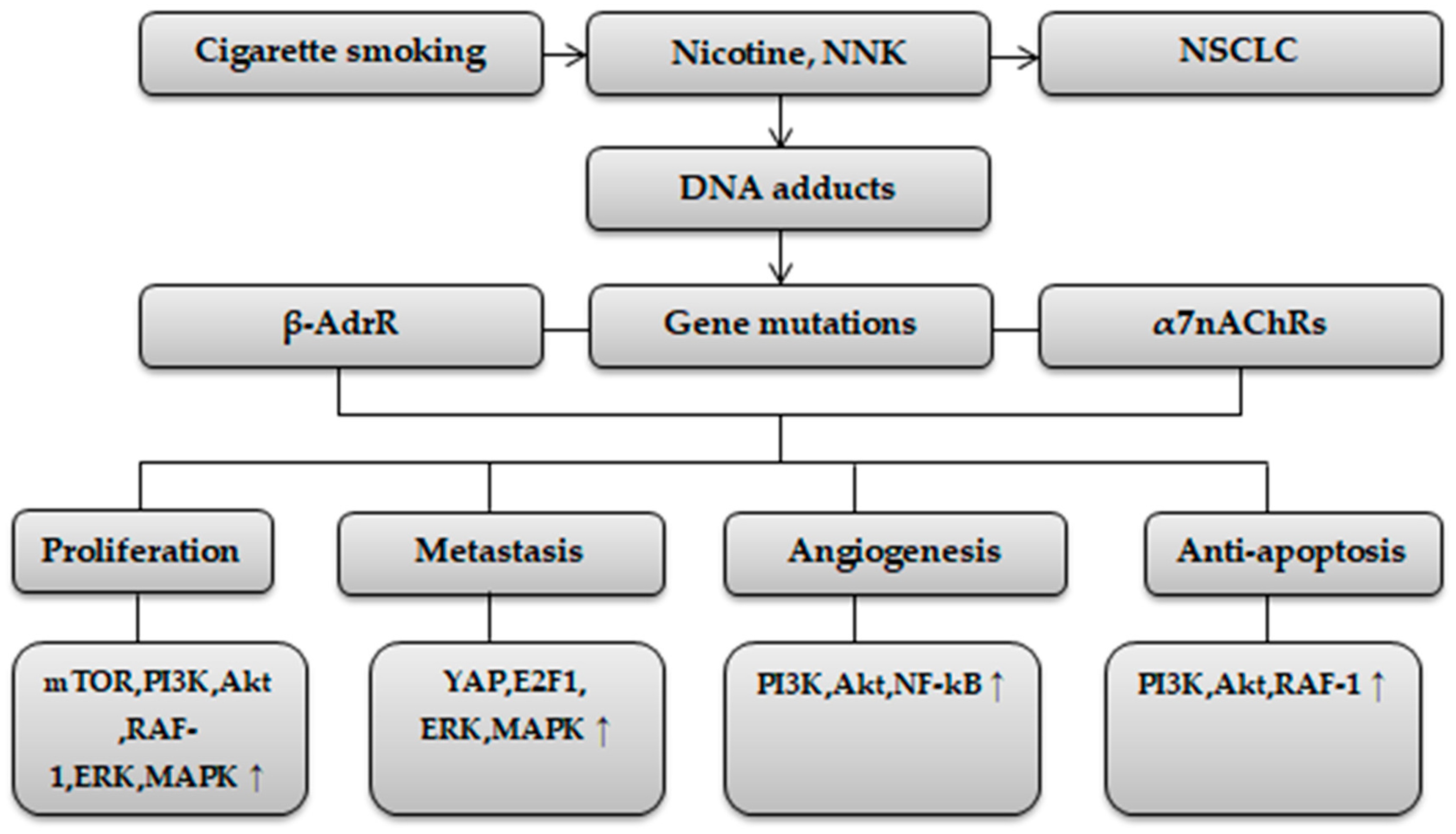
:1. Introduction
2. Methods
3. Quercetin Derivatives in Nicotine-Induced NSCLC Therapy
3.1. Rutin
3.2. Isorhamnetin
3.3. Hyperoside
3.4. Rhamnetin and Rhamnazin
3.5. Quercitrin
3.6. Tamarixetin
4. Quercetin Derivatives in Combination with Chemotherapeutics/Radiotherapy in Nicotine-Induced NSCLC Therapy
5. Limitations
6. Conclusions
7. Future Directions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Aβ | β-amyloid |
| ADAM9 | A disintegrin and a metalloprotease 9 |
| Akt | Threonine kinase |
| AMPK | AMP-activated protein kinase |
| ATP | Adenosine 5′-Triphosphate |
| β-AdrR | Beta-adrenergic receptor |
| Bax | Bcl-2-associated X protein |
| Bcl-2 | B-cell lymphoma-2 |
| β-G | β-glucosidase |
| CCAT1 | Colon cancer-associated transcript 1 |
| CCL5 | Chemokine C-C motif ligand 5 |
| CCND1 | Cyclin-dependent kinase |
| CCR4 | Chemokine receptor 4 |
| CDK | Coding sequence |
| COMT | Catechol-O-methyltransferase |
| E2F | E2F transcription factors 1 |
| 4E-BP1 | 4E-binding protein 1 |
| EGFR | Epidermal growth factor receptor |
| EMT | Epithelial-mesenchymal transition |
| ER | Endoplasmic reticulum |
| ERK | Extracellular signal-regulated kinase |
| FoxO1 | Forkhead box protein O1 |
| GADD153 | DNA damage-inducible gene 153 |
| GJB2 | Gap Junction Protein Beta 2 |
| GSH | Glutathione |
| GSK | Glycogen synthase kinase |
| GTPs | Green tea polyphenols |
| HO-1 | Heme oxygenase-1 |
| HP | Hperoside |
| IL | Interleukin |
| IQ | Isoquercitrin |
| IR | Irradiation |
| IS | Isorhamnetin |
| JNK | c-JUN NH2-terminal kinase |
| LC | Lung cancer |
| LC3-II | LC3-phospholipid conjugate |
| LDH | Lactate dehydrogenase |
| LPS | Lipopolysaccharide |
| MAPK | Mitogen-activated protein kinase |
| MLA | Methyllycaconitine |
| MMP | Metalloproteinase |
| MRPs | Multidrug resistance proteins |
| mTOR | Mammalian target of rapamycin |
| nAChR | Nicotinic acetylcholine receptor |
| NF-kB | Nuclear transcription factor-kappaB |
| NNK | 4-methylnitrosamino-1-3-pyridyl-1-butanone |
| NNN | Nitrosonornicotine |
| NO | Nitric oxide |
| NSCLC | Non-small cell lung cancer |
| OH | Hydroxyl |
| P38 | Protein 38 |
| PAM | Positive allosteric modulator |
| PARP | Poly ADP ribose polymerase |
| PCNA | Proliferating cell nuclear antigen |
| PD-1 | Anti-programmed cell death 1 |
| PD-L1 | Programmed cell death ligand 1 |
| PI3K | Phosphatidylinositol-3 kinase |
| Q | Quercetin |
| QDs | Quercetin derivatives |
| Q3G | Quercetin-3-β-D-glucoside |
| QU | Quercitrin |
| α-R | α-L-rhamnosidases |
| RAF-1 | Retinoblastoma tumor suppressor protein-proto-oncogene |
| RCTs | Randomized controlled trials |
| ROS | Reactive oxygen species |
| RT | Rhamnetin |
| RTK | Receptor tyrosine kinase |
| RU | Rutin |
| RZ | Rhamnazin |
| SCLC | Small cell lung cancer |
| TA | Tamarixetin |
| TNF | Tumor necrosis factor |
| VEGFA | Vascular endothelial growth factor A |
| VEGFR2 | Vascular endothelial growth factor receptor 2 |
| YAP | Yes-associated protein |
References
- Thandra, K.C.; Barsouk, A.; Saginala, K.; Aluru, J.S.; Barsouk, A. Epidemiology of lung cancer. Contemp. Oncol. 2021, 25, 45–52. [Google Scholar]
- Gridelli, C.; Rossi, A.; Carbone, D.P.; Guarize, J.; Karachaliou, N.; Mok, T.; Petrella, F.; Spaggiari, L.; Rosell, R. Non-small-cell lung cancer. Nat. Rev. Dis. Primers 2015, 1, 15009. [Google Scholar] [CrossRef] [PubMed]
- Rudin, C.M.; Brambilla, E.; Faivre-Finn, C.; Sage, J. Small-cell lung cancer. Nat. Rev. Dis. Primers 2021, 7, 3. [Google Scholar] [CrossRef]
- Li, Y.; Hecht, S.S. Carcinogenic components of tobacco and tobacco smoke: A 2022 update. Food Chem. Toxicol. 2022, 165, 113179. [Google Scholar] [CrossRef] [PubMed]
- Hecht, S.S. Lung Carcinogenesis by tobacco smoke. Int. J. Cancer 2012, 131, 2724–2732. [Google Scholar] [CrossRef]
- Warren, G.W.; Singh, A.K. Nicotine and lung cancer. J. Carcinog. 2013, 12, 1. [Google Scholar] [CrossRef]
- Xue, J.; Yang, S.; Seng, S. Mechanisms of cancer induction by tobacco-specific NNK and NNN. Cancers 2014, 6, 1138–1156. [Google Scholar] [CrossRef]
- Wang, S.; Hu, Y. α7 nicotinic acetylcholine receptors in lung cancer (Review). Oncol. Lett. 2018, 16, 1375–1382. [Google Scholar] [CrossRef] [PubMed]
- Hajiasgharzadeh, K.; Sadigh-Eteghad, S.; Mansoori, B.; Mokhtarzadeh, A.; Shanehbandi, D.; Doustvandi, M.A.; Asadzadeh, Z.; Baradaran, B. Alpha7 nicotinic acetylcholine receptors in lung inflammation and carcinogenesis: Friends or foes? J. Cell. Physiol. 2019, 234, 14666–14679. [Google Scholar] [CrossRef]
- Alsharairi, N.A. The effects of dietary supplements on asthma and lung cancer risk in smokers and non-smokers: A review of the literature. Nutrients 2019, 11, 725. [Google Scholar] [CrossRef] [PubMed]
- Alsharairi, N.A. Supplements for smoking-related lung diseases. Encyclopedia 2021, 1, 76–86. [Google Scholar] [CrossRef]
- Alsharairi, N.A. Dietary antioxidants and lung cancer risk in smokers and non-smokers. Healthcare 2022, 10, 2501. [Google Scholar] [CrossRef]
- Dafni, U.; Tsourti, Z.; Vervita, K.; Peters, S. Immune checkpoint inhibitors, alone or in combination with chemotherapy, as first-line treatment for advanced non-small cell lung cancer. A systematic review and network meta-analysis. Lung Cancer 2019, 134, 127–140. [Google Scholar] [CrossRef] [PubMed]
- Alsharairi, N.A. Scutellaria baicalensis and their natural flavone compounds as potential medicinal drugs for the treatment of nicotine-induced non-small-cell lung cancer and asthma. Int. J. Environ. Res. Public Health 2021, 18, 5243. [Google Scholar] [CrossRef] [PubMed]
- Alsharairi, N.A. Insights into the mechanisms of action of proanthocyanidins and anthocyanins in the treatment of nicotine-induced non-small cell lung cancer. Int. J. Mol. Sci. 2022, 23, 7905. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, B.E.; Bermudez, I.; Bouzat, C. Flavonoids as positive allosteric modulators of α7 nicotinic receptors. Neuropharmacology 2019, 160, 107794. [Google Scholar] [CrossRef]
- Ximenis, M.; Mulet, J.; Sala, S.; Sala, F.; Criado, M.; González-Muñiz, R.; Jesús Pérez de Vega, M. Natural polyhydroxy flavonoids, curcuminoids, and synthetic curcumin analogs as α7 nAChRs positive allosteric modulators. Int. J. Mol. Sci. 2021, 22, 973. [Google Scholar] [CrossRef]
- Baby, B.; Antony, P.; Vijayan, R. Interactions of quercetin with receptor tyrosine kinases associated with human lung carcinoma. Nat. Prod. Res. 2018, 32, 2928–2931. [Google Scholar] [CrossRef]
- Zhou, Q.; Pan, H.; Li, J. Molecular insights into potential contributions of natural polyphenols to lung cancer treatment. Cancers 2019, 11, 1565. [Google Scholar] [CrossRef]
- Zanoaga, O.; Braicu, C.; Jurj, A.; Rusu, A.; Buiga, R.; Berindan-Neagoe, I. Progress in research on the role of flavonoids in lung cancer. Int. J. Mol. Sci. 2019, 20, 4291. [Google Scholar] [CrossRef]
- Alam, M.; Alam, S.; Shamsi, A.; Adnan, M.; Elasbali, A.M.; Abu Al-Soud, W.; Alreshidi, M.; Hawsawi, Y.M.; Tippana, A.; Pasupuleti, V.R.; et al. Bax/Bcl-2 cascade is regulated by the EGFR pathway: Therapeutic targeting of non-small cell lung cancer. Front. Oncol. 2022, 12, 869672. [Google Scholar] [CrossRef] [PubMed]
- Hasan, G.M.; Hassan, M.I.; Sohal, S.S.; Shamsi, A.; Alam, M. Therapeutic targeting of regulated signaling pathways of non-small cell lung carcinoma. ACS Omega 2023, 8, 26685–26698. [Google Scholar] [CrossRef] [PubMed]
- Dabeek, W.M.; Marra, M.V. Dietary quercetin and kaempferol: Bioavailability and potential cardiovascular-related bioactivity in humans. Nutrients 2019, 11, 2288. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Fang, Z.; Dou, J.; Yu, A.; Zhai, G. Bioavailability of quercetin: Problems and promises. Curr. Med. Chem. 2013, 20, 2572–2582. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.-P.; Chen, L.-J.; Fan, L.-Y.; Tang, M.-H.; Yang, G.-L.; Yang, H.-S.; Du, X.-B.; Wang, G.-Q.; Yao, W.-X.; Zhao, Q.-M.; et al. Liposomal quercetin efficiently suppresses growth of solid tumors in murine models. Clin. Cancer Res. 2006, 12, 3193–3199. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Heber, D.; Henning, S.M. Quercetin increased bioavailability and decreased methylation of green tea polyphenols in vitro and in vivo. Food Funct. 2012, 3, 635–642. [Google Scholar] [CrossRef]
- Khan, F.; Niaz, K.; Maqbool, F.; Hassan, F.I.; Abdollahi, M.; Venkata, K.C.N.; Nabavi, S.M.; Bishayee, A. Molecular targets underlying the anticancer effects of quercetin: An update. Nutrients 2016, 8, 529. [Google Scholar] [CrossRef] [PubMed]
- He, Y.-Q.; Zhou, C.-C.; Yu, L.-Y.; Wang, L.; Deng, J.-L.; Tao, Y.-L.; Zhang, F.; Chen, W.-S. Natural product derived phytochemicals in managing acute lung injury by multiple mechanisms. Pharmacol. Res. 2021, 163, 105224. [Google Scholar] [CrossRef]
- Xu, S.; Chen, S.; Xia, W.; Sui, H.; Fu, X. Hyperoside: A review of its structure, synthesis, pharmacology, pharmacokinetics and toxicity. Molecules 2022, 27, 3009. [Google Scholar] [CrossRef]
- Magar, R.T.; Sohng, J.K. A review on structure, modifications and structure-activity relation of quercetin and its derivatives. J. Microbiol. Biotechnol. 2020, 30, 11–20. [Google Scholar] [CrossRef]
- Thilakarathna, S.H.; Rupasinghe, H.P.V. Flavonoid bioavailability and attempts for bioavailability enhancement. Nutrients 2013, 5, 3367–3387. [Google Scholar] [CrossRef] [PubMed]
- Carbonaro, M.; Grant, G. Absorption of quercetin and rutin in rat small intestine. Ann. Nutr. Metab. 2005, 49, 178–182. [Google Scholar] [CrossRef]
- Lee, B.; Choi, S.; Shin, T.; Pyo, M.K.; Hwang, S.; Kim, B.; Lee, S.; Lee, J.; Kim, H.; Park, H.; et al. Quercetin enhances human α7 nicotinic acetylcholine receptor-mediated ion current through interactions with Ca(2+) binding sites. Mol. Cells 2010, 30, 245–253. [Google Scholar] [CrossRef]
- Lee, B.; Choi, S.; Kim, H.; Jung, S.; Hwang, S.; Pyo, M.; Rhim, H.; Kim, H.; Kim, H.; Lee, S.; et al. Differential effects of quercetin and quercetin glycosides on human α7 nicotinic acetylcholine receptor-mediated ion currents. Biomol. Ther. 2016, 24, 410–417. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, Y.; Su, Y.; Zhou, W.; Yang, S.; Zhang, R.; Zhao, M.; Li, Y.; Zhang, Z.; Zhan, D.; et al. Rutin inhibits β-amyloid aggregation and cytotoxicity, attenuates oxidative stress, and decreases the production of nitric oxide and proinflammatory cytokines. Neurotoxicology 2012, 33, 482–490. [Google Scholar] [CrossRef]
- Jin, W.; Bu, X.; Liu, Y.; Shen, L.; Zhuang, Z.; Jiao, S.; Zhu, C.; Wang, Q.; Zhou, H.; Zhang, T.; et al. Plasma amyloid-beta levels in patients with different types of cancer. Neurotox. Res. 2017, 31, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Branco de Araújo, M.E.M.; Franco, Y.E.M.; Alberto, T.G.; Sobreiro, M.A.; Conrado, M.A.; Priolli, D.G.; Frankland Sawaya, A.C.H.; Ruiz, A.L.T.G.; de Carvalho, J.E.; de Oliveira Carvalho, P. Enzymatic de-glycosylation of rutin improves its antioxidant and antiproliferative activities. Food Chem. 2013, 141, 266–273. [Google Scholar] [CrossRef]
- Nam, H.; Hong, S.; Shin, K.; Oh, D. Quercetin production from rutin by a thermostable β-rutinosidase from Pyrococcus furiosus. Biotechnol. Lett. 2012, 34, 483–489. [Google Scholar] [CrossRef]
- Suradej, B.; Pata, S.; Kasinrerk, W.; Cressey, R. Glucosidase II exhibits similarity to the p53 tumor suppressor in regards to structure and behavior in response to stress signals: A potential novel cancer biomarker. Oncol. Rep. 2013, 30, 2511–2519. [Google Scholar] [CrossRef]
- Khaodee, W.; Udomsom, S.; Kunnaja, P.; Cressey, R. Knockout of glucosidase II beta subunit inhibits growth and metastatic potential of lung cancer cells by inhibiting receptor tyrosine kinase activities. Sci. Rep. 2019, 9, 10394. [Google Scholar] [CrossRef]
- Li, B.; Peng, B.; Zhang, T.; Li, Y.; Ding, G. A spectrophotometric method for high-throughput screening of α-l-rhamnosidase activity on rutin coupled with a β-d-glucosidase assay. 3 Biotech 2019, 9, 227. [Google Scholar] [CrossRef]
- Tuan, N.N.; Thi, H.N.; Le Thi My, C.; Hai, T.X.; Trung, H.T.; Kim, A.N.T.; Tan, T.N.; Le Van, T.; Nguyen, C.Q.; De Tran, Q.; et al. Inhibition of α-glucosidase, acetylcholinesterase, and nitric oxide production by phytochemicals isolated from Millettia speciosa-In vitro and molecular docking studies. Plants 2022, 11, 388. [Google Scholar] [CrossRef] [PubMed]
- You, H.J.; Ahn, H.J.; Ji, G.E. Transformation of rutin to antiproliferative quercetin-3-glucoside by Aspergillus niger. J. Agric. Food Chem. 2010, 58, 10886–10892. [Google Scholar] [CrossRef] [PubMed]
- Paudel, K.R.; Wadhwa, R.; Tew, X.N.; Lau, N.J.X.; Madheswaran, T.; Panneerselvam, J.; Zeeshan, F.; Kumar, P.; Gupta, G.; Anand, K.; et al. Rutin loaded liquid crystalline nanoparticles inhibit non-small cell lung cancer proliferation and migration in vitro. Life Sci. 2021, 276, 119436. [Google Scholar] [CrossRef]
- Ben Sghaier, M.; Pagano, A.; Mousslim, M.; Ammari, Y.; Kovacic, H.; Luis, J. Rutin inhibits proliferation, attenuates superoxide production and decreases adhesion and migration of human cancerous cells. Biomed. Pharmacother. 2016, 84, 1972–1978. [Google Scholar] [CrossRef]
- Goda, M.S.; Nafie, M.S.; Awad, B.M.; Abdel-Kader, M.S.; Ibrahim, A.K.; Badr, J.M.; Eltamany, E.E. In vitro and in vivo studies of anti-lung cancer activity of Artemesia judaica L. crude extract combined with LC-MS/MS metabolic profiling, docking simulation and HPLC-DAD quantification. Antioxidants 2022, 11, 17. [Google Scholar] [CrossRef]
- Yeh, S.-L.; Wang, W.-Y.; Huang, C.-S.; Hu, M.-L. Flavonoids suppresses the enhancing effect of beta-carotene on DNA damage induced by 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) in A549 cells. Chem. Biol. Interact. 2006, 160, 175–182. [Google Scholar] [CrossRef]
- Gong, G.; Guan, Y.-Y.; Zhang, Z.-L.; Rahman, K.; Wang, S.-J.; Zhou, S.; Luan, X.; Zhang, H. Isorhamnetin: A review of pharmacological effects. Biomed. Pharmacother. 2020, 128, 110301. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Luo, H.; Duan, J.; Hong, C.; Ma, P.; Li, G.; Zhang, T.; Wu, T.; Ji, G. Phytic acid enhances the oral absorption of isorhamnetin, quercetin, and kaempferol in total flavones of Hippophae rhamnoides L. Fitoterapia 2014, 93, 216–225. [Google Scholar] [CrossRef]
- Chen, Z.-P.; Sun, J.; Chen, H.-X.; Xiao, Y.-Y.; Liu, D.; Chen, J.; Cai, H.; Cai, B.-C. Comparative pharmacokinetics and bioavailability studies of quercetin, kaempferol and isorhamnetin after oral administration of Ginkgo biloba extracts, Ginkgo biloba extract phospholipid complexes and Ginkgo biloba extract solid dispersions in rats. Fitoterapia 2010, 81, 1045–1052. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Dong, Y.; Xie, Y.; Wang, L.; Wang, J.; Liu, Y.; Zhao, L.; Cao, F. Effects of β-glucosidase and α-rhamnosidase on the contents of flavonoids, ginkgolides, and aroma components in ginkgo tea drink. Molecules 2019, 24, 2009. [Google Scholar] [CrossRef] [PubMed]
- Ruan, Y.; Hu, K.; Chen, H. Autophagy inhibition enhances isorhamnetin-induced mitochondria-dependent apoptosis in non-small cell lung cancer cells. Mol. Med. Rep. 2015, 12, 5796–5806. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Liu, Q.; Jiang, N.; Li, M.; Shi, L. Isorhamnetin inhibited migration and invasion via suppression of Akt/ERK-mediated epithelial-to-mesenchymal transition (EMT) in A549 human non-small-cell lung cancer cells. Biosci. Rep. 2019, 39, BSR20190159. [Google Scholar] [CrossRef]
- Wang, Q.; Wei, H.-C.; Zhou, S.-J.; Li, Y.; Zheng, T.-T.; Zhou, C.-Z.; Wan, X.-H. Hyperoside: A review on its sources, biological activities, and molecular mechanisms. Phytother. Res. 2022, 36, 2779–2802. [Google Scholar] [CrossRef]
- Li, Z.; Meng, F.; Zhang, Y.; Sun, L.; Yu, L.; Zhang, Z.; Peng, S.; Guo, J. Simultaneous quantification of hyperin, reynoutrin and guaijaverin in mice plasma by LC-MS/MS: Application to a pharmacokinetic study. Biomed. Chromatogr. 2016, 30, 1124–1130. [Google Scholar] [CrossRef]
- Yuan, W.; Wang, J.; An, X.; Dai, M.; Jiang, Z.; Zhang, L.; Yu, S.; Huang, X. UPLC-MS/MS method for the determination of hyperoside and application to pharmacokinetics study in rat after different administration routes. Chromatographia 2021, 84, 249–256. [Google Scholar] [CrossRef]
- Hu, Z.; Zhao, P.; Xu, H. Hyperoside exhibits anticancer activity in non-small cell lung cancer cells with T790M mutations by upregulating FoxO1 via CCAT1. Oncol. Rep. 2020, 43, 617–624. [Google Scholar] [CrossRef]
- Yang, Y.; Sun, Y.; Guo, X.; Zhong, C.; Li, Z. Hyperoside inhibited the migration and invasion of lung cancer cells through the upregulation of PI3K/AKT and p38 MAPK pathways. Int. J. Clin. Exp. Pathol. 2017, 10, 9382–9390. [Google Scholar]
- Yang, Y.; Tantai, J.; Sun, Y.; Zhong, C.; Li, Z. Effect of hyperoside on the apoptosis of A549 human non-small cell lung cancer cells and the underlying mechanism. Mol. Med. Rep. 2017, 16, 6483–6488. [Google Scholar] [CrossRef]
- Fu, T.; Wang, L.; Jin, X.-N.; Sui, H.-J.; Liu, Z.; Jin, Y. Hyperoside induces both autophagy and apoptosis in non-small cell lung cancer cells in vitro. Acta Pharmacol. Sin. 2016, 37, 505–518. [Google Scholar] [CrossRef] [PubMed]
- Lü, P. Inhibitory effects of hyperoside on lung cancer by inducing apoptosis and suppressing inflammatory response via caspase-3 and NF-κB signaling pathway. Biomed. Pharmacother. 2016, 82, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Li, J.-P.; Liao, X.-H.; Xiang, Y.; Yao, A.; Song, R.-H.; Zhang, Z.-J.; Huang, F.; Dai, Z.-T.; Zhang, T.-C. Hyperoside and let-7a-5p synergistically inhibits lung cancer cell proliferation via inducing G1/S phase arrest. Gene 2018, 679, 232–240. [Google Scholar] [CrossRef]
- Liu, Y.-H.; Liu, G.-H.; Mei, J.-J.; Wang, J. The preventive effects of hyperoside on lung cancer in vitro by inducing apoptosis and inhibiting proliferation through Caspase-3 and P53 signaling pathway. Biomed. Pharmacother. 2016, 83, 381–391. [Google Scholar] [CrossRef]
- Liu, F.; Zhao, Y.; Lu, J.; Chen, S.; Zhang, X.; Mao, W. Hyperoside inhibits proinflammatory cytokines in human lung epithelial cells infected with Mycoplasma pneumonia. Mol. Cell. Biochem. 2019, 453, 179–186. [Google Scholar] [CrossRef]
- Chen, D.; Wu, Y.-X.; Qiu, Y.-B.; Wan, B.-B.; Liu, G.; Chen, G.-L.; Lu, M.-D.; Pang, Q.-F. Hyperoside suppresses hypoxia-induced A549 survival and proliferation through ferrous accumulation via AMPK/HO-1 axis. Phytomedicine 2020, 67, 153138. [Google Scholar] [CrossRef]
- Medeiros, D.L.; Lima, E.T.G.; Silva, J.C.; Medeiros, M.A.; Pinheiro, E.B.F. Rhamnetin: A review of its pharmacology and toxicity. J. Pharm. Pharmacol. 2022, 74, 793–799. [Google Scholar] [CrossRef] [PubMed]
- Heim, K.E.; Tagliaferro, A.R.; Bobilya, D.J. Flavonoid antioxidants: Chemistry, metabolism and structure-activity relationships. J. Nutr. Biochem. 2002, 13, 572–584. [Google Scholar] [CrossRef] [PubMed]
- Lutz, J.A.; Kulshrestha, M.; Rogers, D.T.; Littleton, J.M. A nicotinic receptor-mediated anti-inflammatory effect of the flavonoid rhamnetin in BV2 microglia. Fitoterapia 2014, 98, 11–21. [Google Scholar] [CrossRef]
- Lutz, J.A.; Carter, M.; Fields, L.; Barron, S.; Littleton, J.M. The dietary flavonoid rhamnetin inhibits both inflammation and excitotoxicity during ethanol withdrawal in rat organotypic hippocampal slice cultures. Alcohol. Clin. Exp. Res. 2015, 39, 2345–2353. [Google Scholar] [CrossRef]
- Wang, S.S.; Liu, Y.; Zhang, X.T.; Yu, D.Q. Rhamnazin enhanced anti-tumor efficacy of anti-PD-1 therapy for lung cancer in mice through inhibition of PD-L1 expression. Tohoku J. Exp. Med. 2023, 260, 63–73. [Google Scholar] [CrossRef]
- Cincin, Z.P.; Unlu, M.; Kiran, B.; Bireller, E.S.; Baran, Y.; Cakmakoglu, B. Molecular mechanisms of quercitrin-induced apoptosis in non-small cell lung cancer. Arch. Med. Res. 2014, 45, 445–454. [Google Scholar] [CrossRef]
- Li, D.Y.; Yue, L.X.; Wang, S.G.; Wang, T.X. Quercitrin restrains the growth and invasion of lung adenocarcinoma cells by regulating gap junction protein beta 2. Bioengineered 2022, 13, 6126–6135. [Google Scholar] [CrossRef] [PubMed]
- Sak, K.; Lust, H.; Kase, M.; Jaal, J. Cytotoxic action of methylquercetins in human lung adenocarcinoma cells. Oncol. Lett. 2018, 15, 1973–1978. [Google Scholar] [CrossRef]
- D'Abrosca, B.; Ciaramella, V.; Graziani, V.; Papaccio, F.; Corte, C.M.D.; Potenza, N.; Fiorentino, A.; Ciardiello, F.; Morgillo, F. Urtica dioica L. inhibits proliferation and enhances cisplatin cytotoxicity in NSCLC cells via Endoplasmic Reticulum-stress mediated apoptosis. Sci. Rep. 2019, 9, 4986. [Google Scholar] [CrossRef]
- Wu, F.; Chen, J.; Fan, L.-M.; Liu, K.; Zhang, N.; Li, S.-W.; Zhu, H.; Gao, H.-C. Analysis of the effect of rutin on GSK-3β and TNF-α expression in lung cancer. Exp. Ther. Med. 2017, 14, 127–130. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.-Y.; Wang, Y.-M.; Gong, H.; Zhao, H.; Lv, X.-Y.; Yuan, G.-H.; Han, S.-R. Isorhamnetin flavonoid synergistically enhances the anticancer activity and apoptosis induction by cisplatin and carboplatin in non-small cell lung carcinoma (NSCLC). Int. J. Clin. Exp. Pathol. 2015, 8, 25–37. [Google Scholar] [PubMed]
- Li, Q.; Ren, F.-Q.; Yang, C.-L.; Zhou, L.-M.; Liu, Y.-Y.; Xiao, J.; Zhu, L.; Wang, Z.-G. Anti-proliferation effects of isorhamnetin on lung cancer cells in vitro and in vivo. Asian Pac. J. Cancer Prev. 2015, 16, 3035–3042. [Google Scholar] [CrossRef]
- Du, Y.; Jia, C.; Liu, Y.; Li, Y.; Wang, J.; Sun, K. Isorhamnetin enhances the radiosensitivity of A549 Cells through interleukin-13 and the NF-κB signaling pathway. Front. Pharmacol. 2020, 11, 610772. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Kim, E.; Kim, W.; Seong, K.M.; Youn, H.; Kim, J.W.; Kim, J.; Youn, B. Rhamnetin and cirsiliol induce radiosensitization and inhibition of epithelial-mesenchymal transition (EMT) by miR-34a-mediated suppression of Notch-1 expression in non-small cell lung cancer cell lines. J. Biol. Chem. 2013, 288, 27343–27357. [Google Scholar] [CrossRef] [PubMed]
| Study Design | QDs | Cell Line | Dosage | Activity | Target Molecular Genes | Target Mechanisms | Ref. |
|---|---|---|---|---|---|---|---|
| In vitro | RU | A549 | 0, 50, 100, 150, 200, 250 μM | Anti-proliferative | NA | NA | [43] |
| In vitro | RU | A549 | 2.5, 5, 10, 20 μM | Anti-proliferative, anti-migration, apoptosis | MMP-9 ↓ | NA | [44] |
| In vitro | RU | A549 | 31.25, 62.5, 125, 250, 500, 1000 μM | Anti-proliferative, anti-migration, anti-adhesion | ROS ↓ | NA | [45] |
| In vitro/vivo | RU | A549 | 0.1, 1, 10, 100 μg/mL (in vitro) 100 mg/kg BW/day of A. judaica L. (in vivo) | Apoptosis, G2/M phase cell cycle arrest | Caspase-3/8/9, P53 Bax ↑ Bcl-2 ↓ | NA | [46] |
| In vitro/vivo | IS | A549 | 0–16 μM (in vitro) 0.5 mg/kg BW/day (in vivo) | Anti-proliferative, apoptosis, autophagy | Caspase-3/9, cytochrome C, Bax, P53, c-PARP, Beclin1, LC3-II ↑ | Mitochondria-dependent caspase ↑ | [52] |
| In vitro | IS | A549 | 2.5, 5, 10 μM | Anti-migration, anti-adhesion, anti-invasion | E-cadherin ↑ MMP2/9, snail, vimentin, N-cadherin ↓ | Akt/ERK1/2 ↓ | [53] |
| In vitro/vivo | HP | H1975 | 0–150 μM (in vitro) 25 mg/kg BW/day (in vivo) | Anti-proliferative, apoptosis | FoxO1 ↑ | CCAT1 ↓ | [57] |
| In vitro | HP | A549 | 1, 2, 5 μM | Anti-migration, anti-invasion | MMP-2/9 TIMP-2 ↓ | PI3K/Akt, p38 MAPK ↓ | [58] |
| In vitro | HP | A549 | 10, 50, 100 μM | Apoptosis, anti-viability | Caspase- 3/9, cytochrome c, apoptosis-inducing factor ↑ | p38 MAPK, JNK ↑ | [59] |
| In vitro | HP | A549 | 0.5, 1, 2 mmol/L | Apoptosis, autophagy | Caspase- 3, c-PARP, LC3-II ↑ | Akt, mTOR, p70S6K. 4E-BP1 ↓ ERK1/2 ↑ | [60] |
| In vivo | HP | A549 | 15, 20, 25 mg/kg BW/for a month | Anti-proliferative, anti-migration, anti-invasion, anti-inflammatory, apoptosis | Caspase- 3, Bax ↑ MMP-2/9, Bcl-2, IL-6, IL-18, IL-1β, TNF-α ↓ | NF-kB ↓ | [61] |
| In vitro | HP | A549 | 0, 10, 20, 50, 100, 200, 400 μg/mL | Anti-proliferative, apoptosis, G1/S phase cell cycle arrest | CCND1, CDK-4/6 ↓ | NA | [62] |
| In vitro/vivo | HP | A549, H1975 | 0, 20, 40, 60, 80, 100 μg/mL (in vitro) 20, 40, 80 μg/mL (in vivo) | Anti-proliferative, anti-migration, anti- invasion, anti-angiogenic, apoptosis, S phase cell cycle arrest | Caspase- 3/9, c-PARP, Bax, Bad, Bak, Cyto-c, Apaf-1, p53 ↑ MMP-2/7, c-Myc, Cyclin-D1, CDK1, Akt, Bcl-2, Bcl-xl ↓ | NF-kB ↓ | [63] |
| In vitro | HP | A549 | 0, 25, 50, 100, 200, 400, 1000 μg/ml | Anti-inflammatory | IL-8, TNF-α, CCR4, CCL5, p-NF-κB-p65 ↓ | NF-kB ↓ | [64] |
| In vitro | HP | A549 | 10, 50, 100 μg/ml | Anti-proliferative, anti-viability | HO-1, ROS ↑ GSH ↓ | AMPK ↑ | [65] |
| In vivo | RZ | H1975 | 200 mg/kg BW/day | anti-angiogenic | VEGFA, VEGFR2 ↓ | PD-1/PD-L1 ↓ | [70] |
| In vitro | QU | A549, H358 | 5, 10, 25, 50 μM | Anti-proliferative, apoptosis | Caspase-3, loss of mitochondrial membrane potential ↑ | Phosphatidylinositol signaling system, leukocyte transendothelial migration, cell adhesion ↓ | [71] |
| In vitro | QU | H1299, H1650 | 2, 5, 10 μM | Anti-migration, anti-invasion | GJB2 ↓ | NA | [72] |
| In vitro | TA, IS | A549, HCC-44 | 15–26 μM | Anti-proliferative, apoptosis | Caspase- 3/9 ↑ | Intrinsic and extrinsic apoptotic pathway- caspase cascades ↑ | [73] |
| Study Design | QDs | Cell Line | Dosage | Activity | Target Molecular Genes | Target Mechanisms | Ref. |
|---|---|---|---|---|---|---|---|
| In vitro | RU | A549, H1299, H460, H322 | 10–100 μg/mL (Urtica dioica L.) | Anti-proliferative, apoptosis | Caspase- 3/8, c-PARP ↑ | GADD153 ↑ | [74] |
| In vitro | RU | A549 | 1 × 10−8 (low group), 2 × 10−8 (medium group) or 4 × 10−8 mol/L (high group) | Apoptosis | TNF-α, GSK-3β ↑ | NA | [75] |
| In vitro | IS | A549 | 0, 2.5, 5, 25, 50, 100 μM | Anti-proliferative, apoptosis, G2/M phase cell cycle arrest | Caspase-3/9, PARP ↑ | Mitochondria-mediated caspase ↑ | [76] |
| In vitro/vivo | IS | A549 | 20, 40, 80 μg/mL (in vitro) 50 mg/kg/day (in vivo) | Anti-proliferative, apoptosis, S and G0/G1 phase cell cycle arrest | Caspase-3, Bax, P53 ↑ Bcl-2, cyclinD1, PCNA ↓ | NA | [77] |
| In vitro | IS | A549, H460 | 5, 10, 20, 40, 60, 80 μM | Anti-proliferative, apoptosis | Bax ↑ Bcl2, p-IκBα, p-NF-κBp65 ↓ | NF-κB ↓ IL-13 ↑ | [78] |
| In vivo | RT | H1299, H460 | 200 μg/mL /kg body weight | Anti-proliferative, apoptosis | miR-34a, p53, E-cadherin ↑ NF-kB, p65, Hes-1, Hey-1, vimentin, fibronectin ↓ | NF-κB, Notch-1 ↓ | [79] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alsharairi, N.A. Quercetin Derivatives as Potential Therapeutic Agents: An Updated Perspective on the Treatment of Nicotine-Induced Non-Small Cell Lung Cancer. Int. J. Mol. Sci. 2023, 24, 15208. https://doi.org/10.3390/ijms242015208
Alsharairi NA. Quercetin Derivatives as Potential Therapeutic Agents: An Updated Perspective on the Treatment of Nicotine-Induced Non-Small Cell Lung Cancer. International Journal of Molecular Sciences. 2023; 24(20):15208. https://doi.org/10.3390/ijms242015208
Chicago/Turabian StyleAlsharairi, Naser A. 2023. "Quercetin Derivatives as Potential Therapeutic Agents: An Updated Perspective on the Treatment of Nicotine-Induced Non-Small Cell Lung Cancer" International Journal of Molecular Sciences 24, no. 20: 15208. https://doi.org/10.3390/ijms242015208
APA StyleAlsharairi, N. A. (2023). Quercetin Derivatives as Potential Therapeutic Agents: An Updated Perspective on the Treatment of Nicotine-Induced Non-Small Cell Lung Cancer. International Journal of Molecular Sciences, 24(20), 15208. https://doi.org/10.3390/ijms242015208