Bayesian Regression Quantifies Uncertainty of Binding Parameters from Isothermal Titration Calorimetry More Accurately Than Error Propagation
Abstract
:1. Introduction
2. Results and Discussion
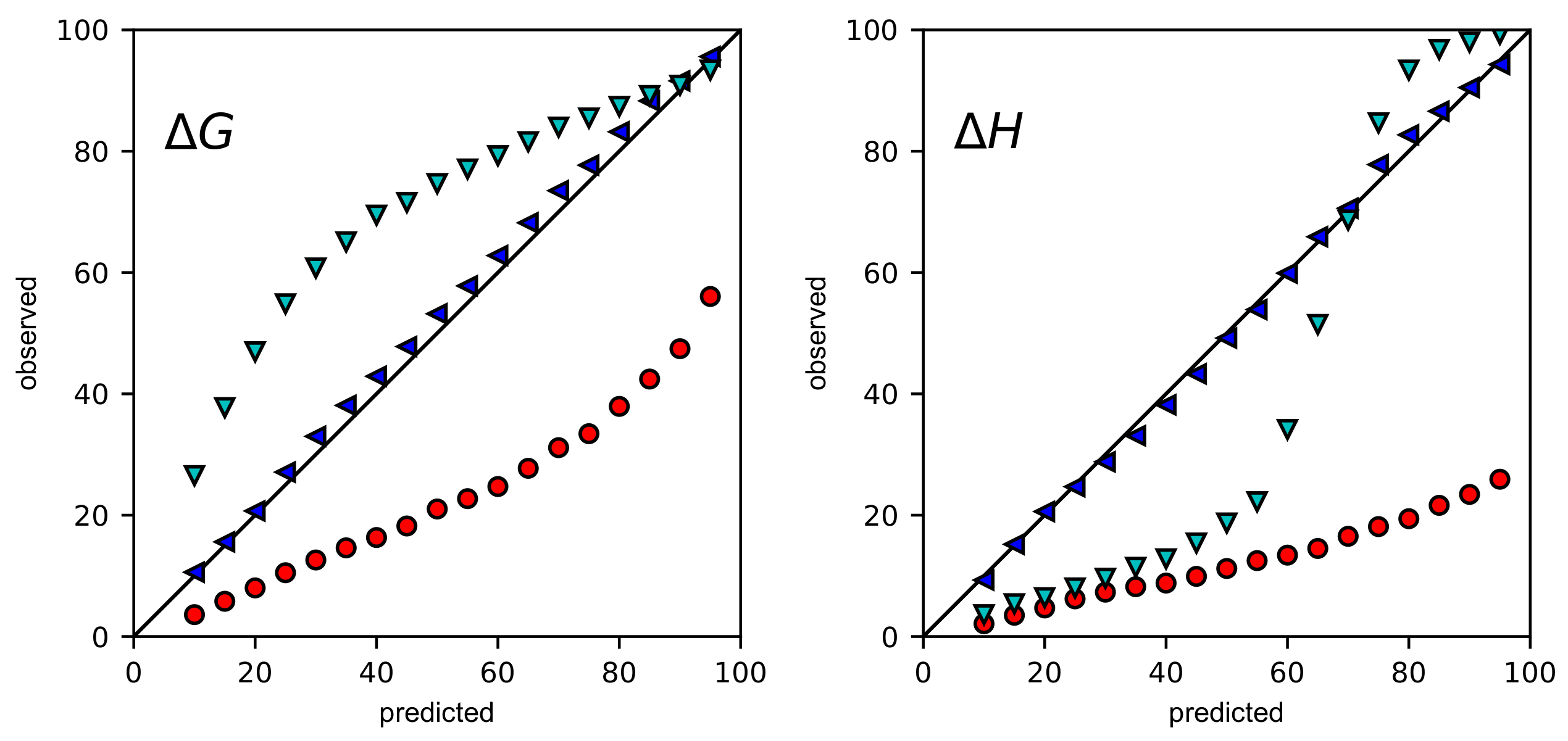
2.1. Bayesian Posteriors Are Converged
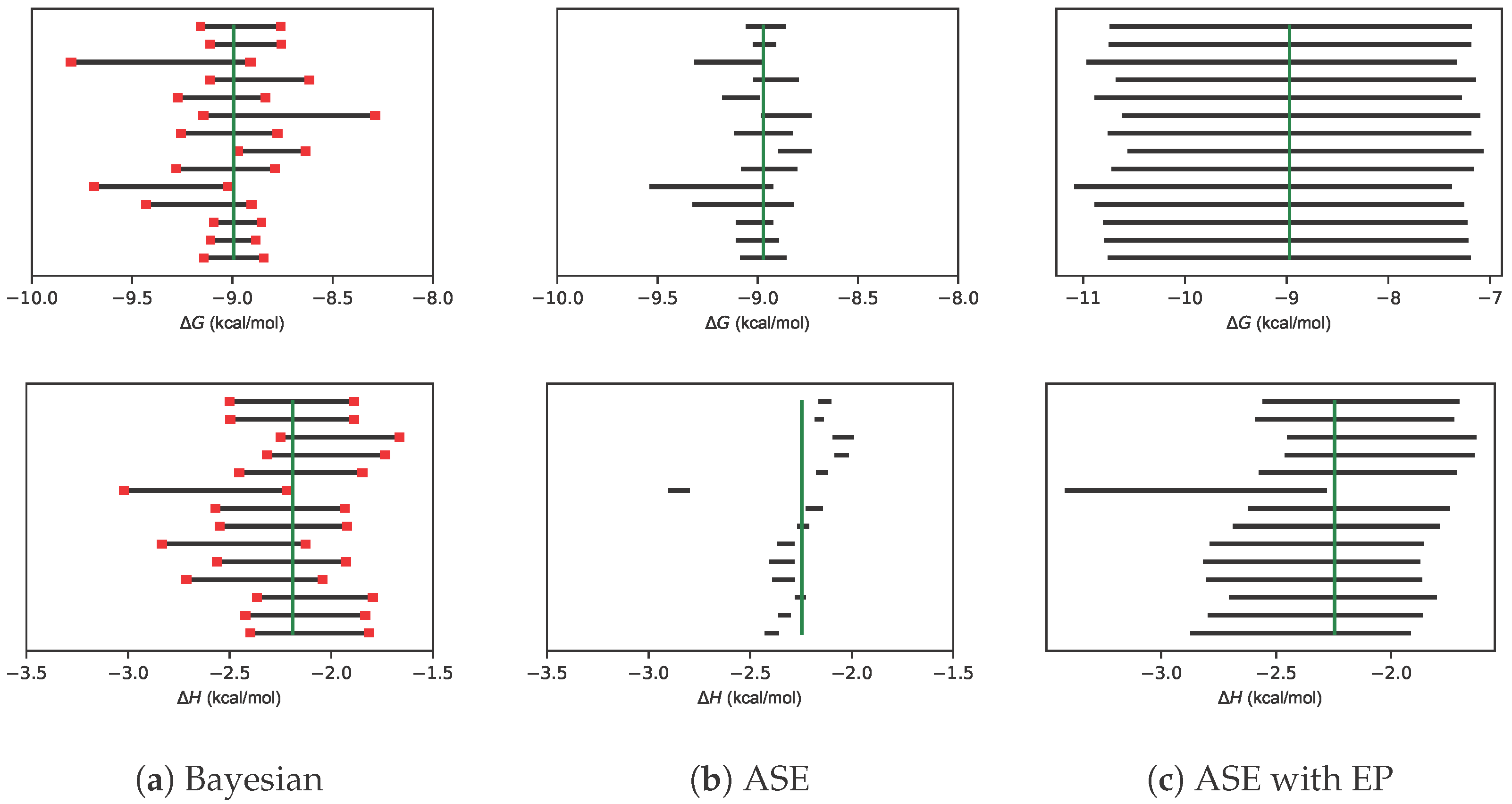
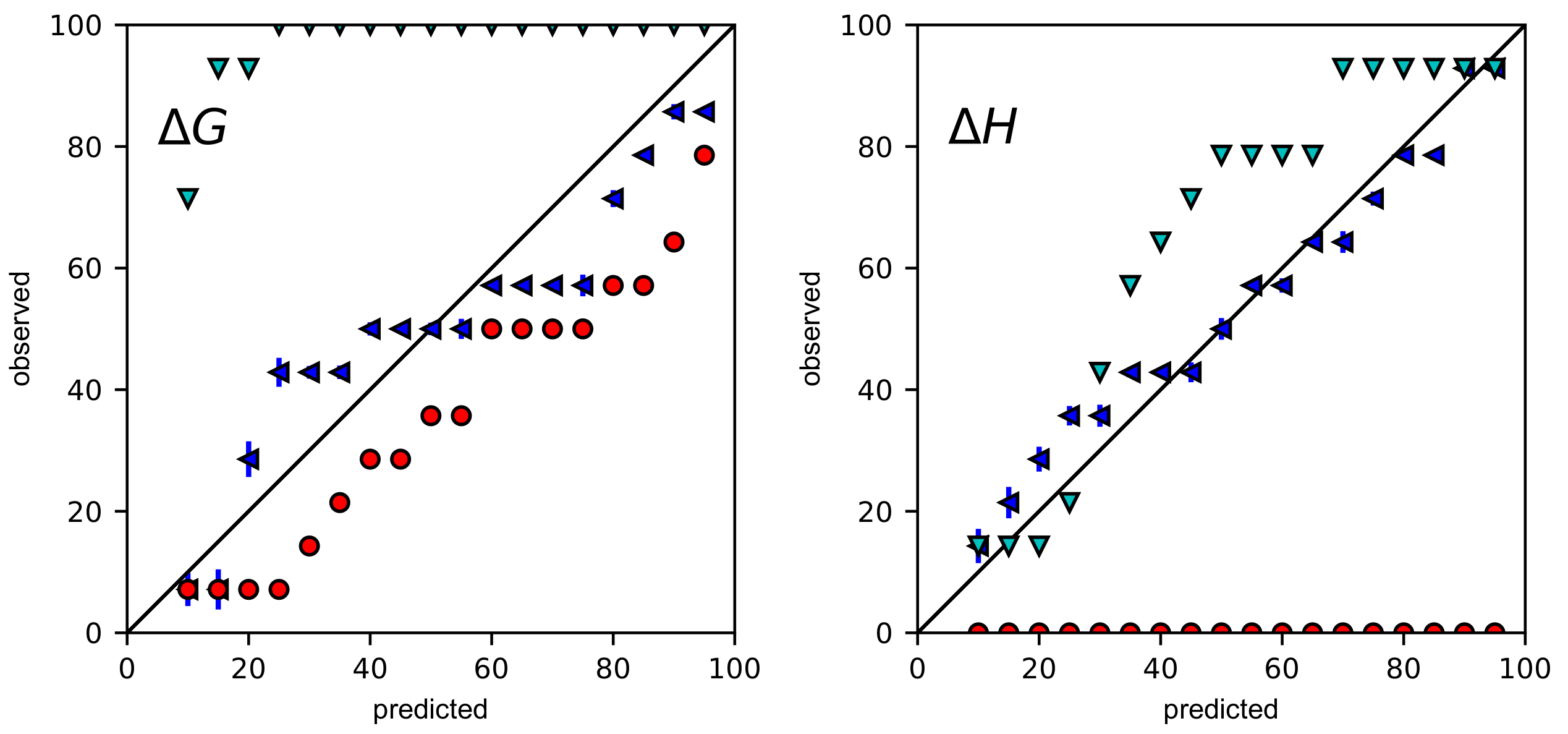
2.2. Error Propagation Expands Confidence Intervals to Be Larger Than Bayesian Credible Intervals
2.3. Even with Error Propagation, BCIs Provide More Accurate CIs Than the ASE
3. Materials and Methods
3.1. Integrated Heat Data
3.2. Regression
3.2.1. Bayesian Regression
3.2.2. Maximum Likelihood Estimation
3.2.3. Maximum Likelihood Estimation with Error Propagation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Leavitt, S.; Freire, E. Direct measurement of protein binding energetics by isothermal titration calorimetry. Curr. Opin. Struct. Biol. 2001, 11, 560–566. [Google Scholar] [CrossRef] [PubMed]
- Duff, M.R.; Grubbs, J.; Howell, E.E. Isothermal Titration Calorimetry for Measuring Macromolecule-Ligand Affinity. J. Vis. Exp. 2011, 55, e2796. [Google Scholar] [CrossRef]
- Feig, A.L. Chapter 19—Studying RNA–RNA and RNA–Protein Interactions by Isothermal Titration Calorimetry. In Methods in Enzymology; Biophysical, Chemical, and Functional Probes of RNA Structure, Interactions and Folding: Part A; Academic Press: Cambridge, MA, USA, 2009; Volume 468, pp. 409–422. [Google Scholar] [CrossRef]
- Malecek, K.; Ruthenburg, A. Chapter Nine—Validation of Histone-Binding Partners by Peptide Pull-Downs and Isothermal Titration Calorimetry. In Methods in Enzymology; Nucleosomes, Histones & Chromatin Part A; Academic Press: Cambridge, MA, USA, 2012; Volume 512, pp. 187–220. [Google Scholar] [CrossRef]
- Swamy, M.J.; Sankhala, R.S.; Singh, B.P. Thermodynamic Analysis of Protein-Lipid Interactions by Isothermal Titration Calorimetry. In Methods in Molecular Biology; Humana: New York, NY, USA, 2019; Volume 2003, pp. 71–89. [Google Scholar] [CrossRef]
- Myszka, D.G.; Abdiche, Y.N.; Arisaka, F.; Byron, O.; Eisenstein, E.; Hensley, P.; Thomson, J.A.; Lombardo, C.R.; Schwarz, F.; Stafford, W.; et al. The ABRF-MIRG’02 Study: Assembly State, Thermodynamic, and Kinetic Analysis of an Enzyme/Inhibitor Interaction. J. Biomol. Tech. 2003, 14, 247–269. [Google Scholar]
- Estelle, A.B.; George, A.; Barbar, E.J.; Zuckerman, D.M. Quantifying cooperative multisite binding in the hub protein LC8 through Bayesian inference. PLoS Comput. Biol. 2023, 19, e1011059. [Google Scholar] [CrossRef]
- Boyce, S.E.; Tellinghuisen, J.; Chodera, J.D. Avoiding accuracy-limiting pitfalls in the study of protein-ligand interactions with isothermal titration calorimetry. bioRxiv 2015. [Google Scholar] [CrossRef]
- Nguyen, T.H.; Rustenburg, A.S.; Krimmer, S.G.; Zhang, H.; Clark, J.D.; Novick, P.A.; Branson, K.; Pande, V.S.; Chodera, J.D.; Minh, D.D.L. Bayesian analysis of isothermal titration calorimetry for binding thermodynamics. PLoS ONE 2018, 13, e0203224. [Google Scholar] [CrossRef] [PubMed]
- Jeffreys, H. An invariant form for the prior probability in estimation problems. Proc. R. Soc. Lond. Ser. A Math. Phys. Sci. 1946, 186, 453–461. [Google Scholar] [CrossRef]
- Bingham, E.; Chen, J.P.; Jankowiak, M.; Obermeyer, F.; Pradhan, N.; Karaletsos, T.; Singh, R.; Szerlip, P.; Horsfall, P.; Goodman, N.D. Pyro: Deep universal probabilistic programming. J. Mach. Learn. Res. 2019, 20, 973–978. [Google Scholar] [CrossRef]
- Phan, D.; Pradhan, N.; Jankowiak, M. Composable Effects for Flexible and Accelerated Probabilistic Programming in NumPyro. arXiv 2019. [Google Scholar] [CrossRef]
- Virtanen, P.; Gommers, R.; Oliphant, T.E.; Haberland, M.; Reddy, T.; Cournapeau, D.; Burovski, E.; Peterson, P.; Weckesser, W.; Bright, J.; et al. SciPy 1.0: Fundamental algorithms for scientific computing in Python. Nat. Methods 2020, 17, 261–272. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
La, V.N.T.; Minh, D.D.L. Bayesian Regression Quantifies Uncertainty of Binding Parameters from Isothermal Titration Calorimetry More Accurately Than Error Propagation. Int. J. Mol. Sci. 2023, 24, 15074. https://doi.org/10.3390/ijms242015074
La VNT, Minh DDL. Bayesian Regression Quantifies Uncertainty of Binding Parameters from Isothermal Titration Calorimetry More Accurately Than Error Propagation. International Journal of Molecular Sciences. 2023; 24(20):15074. https://doi.org/10.3390/ijms242015074
Chicago/Turabian StyleLa, Van N. T., and David D. L. Minh. 2023. "Bayesian Regression Quantifies Uncertainty of Binding Parameters from Isothermal Titration Calorimetry More Accurately Than Error Propagation" International Journal of Molecular Sciences 24, no. 20: 15074. https://doi.org/10.3390/ijms242015074
APA StyleLa, V. N. T., & Minh, D. D. L. (2023). Bayesian Regression Quantifies Uncertainty of Binding Parameters from Isothermal Titration Calorimetry More Accurately Than Error Propagation. International Journal of Molecular Sciences, 24(20), 15074. https://doi.org/10.3390/ijms242015074




