Count Me in, Count Me out: Regulation of the Tooth Number via Three Directional Developmental Patterns
Abstract
1. Introduction
2. The First Direction: Initiation of Odontogenesis in the Tooth-Bearing Bones
2.1. Tooth Number Regulation at the Early Dental Placode Formation Level
2.2. Tooth Germ Arrest
2.3. Diastema in Mice
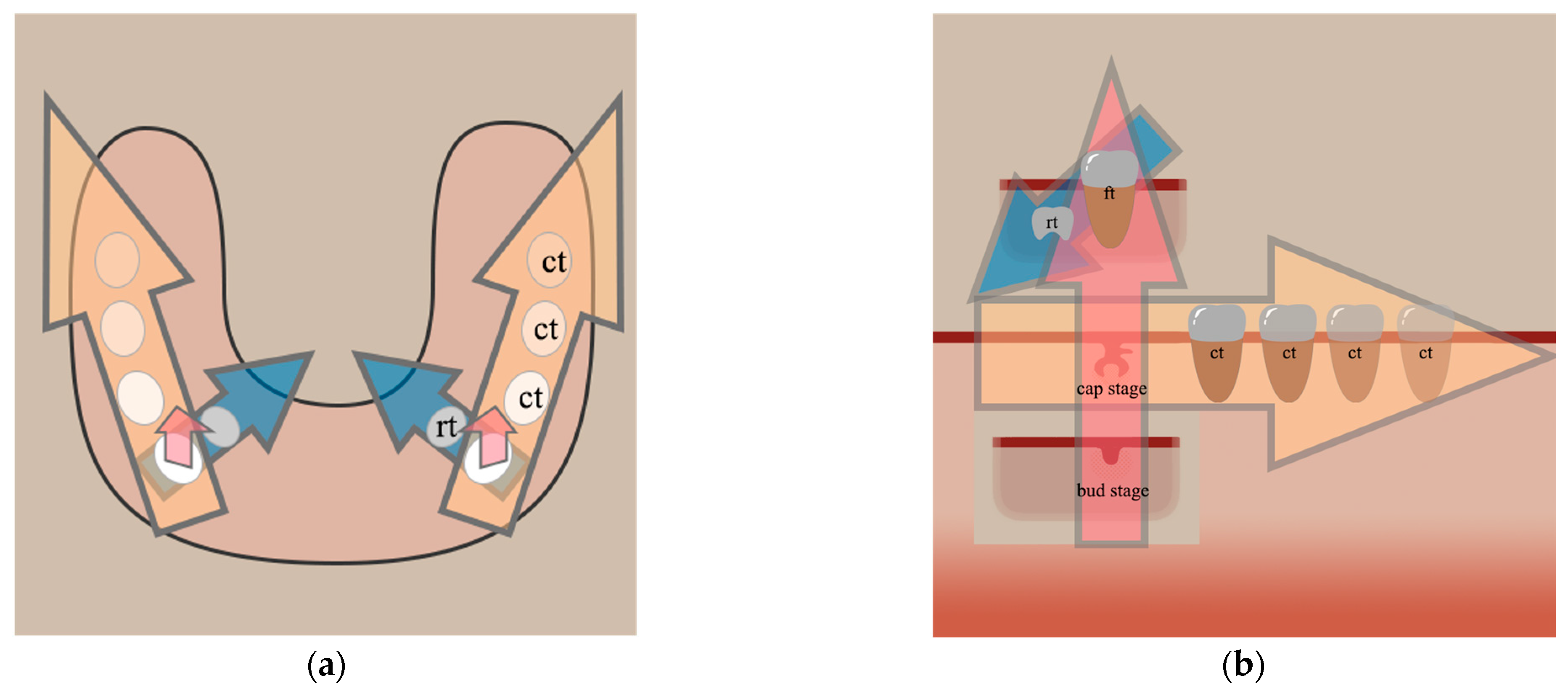
3. The Second Direction: Progression of Tooth Development in the Mesio-Distal Access
3.1. Humans
3.2. Mice
3.3. Other Models
4. The Third Direction: Replacement/Successional Tooth Development
5. Discussion and Perspective: Exploring the Dentition Development from the Perspective of the Three Directions
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shah, A.; Gill, D.S.; Tredwin, C.; Naini, F.B. Diagnosis and Management of Supernumerary Teeth. Dent. Update 2008, 35, 510–512, 514–516, 519–520. [Google Scholar] [CrossRef]
- Rajab, L.D.; Hamdan, M.A.M. Supernumerary Teeth: Review of the Literature and a Survey of 152 Cases. Int. J. Paediatr. Dent. 2002, 12, 244–254. [Google Scholar] [CrossRef]
- Davis, P.J. Hypodontia and Hyperdontia of Permanent Teeth in Hong Kong Schoolchildren. Community Dent. Oral Epidemiol. 1987, 15, 218–220. [Google Scholar] [CrossRef]
- Parolia, A.; Kundabala, M.; Dahal, M.; Mohan, M.; Thomas, M.S. Management of Supernumerary Teeth. J. Conserv. Dent. 2011, 14, 221. [Google Scholar] [CrossRef]
- Hurlen, B.; Humerfelt, D. Characteristics of Premaxillary Hyperodontia. A Radiographic Study. Acta Odontol. Scand. 1985, 43, 75–81. [Google Scholar] [CrossRef]
- Ritwik, P.; Patterson, K.K. Diagnosis of Tooth Agenesis in Childhood and Risk for Neoplasms in Adulthood. Ochsner J. 2018, 18, 345–350. [Google Scholar] [CrossRef]
- Ye, X.; Attaie, A.B. Genetic Basis of Nonsyndromic and Syndromic Tooth Agenesis. J. Pediatr. Genet. 2016, 5, 198–208. [Google Scholar] [CrossRef]
- Jonsson, L.; Magnusson, T.E.; Thordarson, A.; Jonsson, T.; Geller, F.; Feenstra, B.; Melbye, M.; Nohr, E.A.; Vucic, S.; Dhamo, B.; et al. Rare and Common Variants Conferring Risk of Tooth Agenesis. J. Dent. Res. 2018, 97, 515–522. [Google Scholar] [CrossRef]
- Ahtiainen, L.; Uski, I.; Thesleff, I.; Mikkola, M.L. Early Epithelial Signaling Center Governs Tooth Budding Morphogenesis. J. Cell Biol. 2016, 214, 753–767. [Google Scholar] [CrossRef]
- Shirokova, V.; Jussila, M.; Hytönen, M.K.; Perälä, N.; Drögemüller, C.; Leeb, T.; Lohi, H.; Sainio, K.; Thesleff, I.; Mikkola, M.L. Expression of Foxi3 Is Regulated by Ectodysplasin in Skin Appendage Placodes. Dev. Dyn. 2013, 242, 593–603. [Google Scholar] [CrossRef]
- St Amand, T.R.; Zhang, Y.; Semina, E.V.; Zhao, X.; Hu, Y.; Nguyen, L.; Murray, J.C.; Chen, Y. Antagonistic Signals between BMP4 and FGF8 Define the Expression of Pitx1 and Pitx2 in Mouse Tooth-Forming Anlage. Dev. Biol. 2000, 217, 323–332. [Google Scholar] [CrossRef]
- Juuri, E.; Jussila, M.; Seidel, K.; Holmes, S.; Wu, P.; Richman, J.; Heikinheimo, K.; Chuong, C.-M.; Arnold, K.; Hochedlinger, K.; et al. Sox2 Marks Epithelial Competence to Generate Teeth in Mammals and Reptiles. Dev. Camb. Engl. 2013, 140, 1424–1432. [Google Scholar] [CrossRef]
- Satokata, I.; Maas, R. Msx1 Deficient Mice Exhibit Cleft Palate and Abnormalities of Craniofacial and Tooth Development. Nat. Genet. 1994, 6, 348–356. [Google Scholar] [CrossRef]
- Lewington, J. Ferret Husbandry, Medicine and Surgery; Elsevier: Amsterdam, The Netherlands, 2007; ISBN 0-7020-2827-4. [Google Scholar]
- Atukorala, A.D.S.; Franz-Odendaal, T.A. Spatial and Temporal Events in Tooth Development of Astyanax Mexicanus. Mech. Dev. 2014, 134, 42–54. [Google Scholar] [CrossRef]
- Juuri, E.; Balic, A. The Biology Underlying Abnormalities of Tooth Number in Humans. J. Dent. Res. 2017, 96, 1248–1256. [Google Scholar] [CrossRef]
- Järvinen, E.; Salazar-Ciudad, I.; Birchmeier, W.; Taketo, M.M.; Jernvall, J.; Thesleff, I. Continuous Tooth Generation in Mouse Is Induced by Activated Epithelial Wnt/β-Catenin Signaling. Proc. Natl. Acad. Sci. USA 2006, 103, 18627–18632. [Google Scholar] [CrossRef]
- Järvinen, E.; Shimomura-Kuroki, J.; Balic, A.; Jussila, M.; Thesleff, I. Mesenchymal Wnt/β-Catenin Signaling Limits Tooth Number. Dev. Camb. Engl. 2018, 145, dev158048. [Google Scholar] [CrossRef]
- Pispa, J.; Thesleff, I. Mechanisms of Ectodermal Organogenesis. Dev. Biol. 2003, 262, 195–205. [Google Scholar] [CrossRef]
- Jussila, M.; Crespo Yanez, X.; Thesleff, I. Initiation of Teeth from the Dental Lamina in the Ferret. Differ. Res. Biol. Divers. 2014, 87, 32–43. [Google Scholar] [CrossRef]
- Wu, T.; Chen, G.; Tian, F.; Liu, H.-X. Contribution of Cranial Neural Crest Cells to Mouse Skull Development. Int. J. Dev. Biol. 2017, 61, 495–503. [Google Scholar] [CrossRef]
- Morita, W.; Yano, W.; Nagaoka, T.; Abe, M.; Ohshima, H.; Nakatsukasa, M. Patterns of Morphological Variation in Enamel–Dentin Junction and Outer Enamel Surface of Human Molars. J. Anat. 2014, 224, 669–680. [Google Scholar] [CrossRef]
- Yamada, S.; Lav, R.; Li, J.; Tucker, A.S.; Green, J.B.A. Molar Bud-to-Cap Transition Is Proliferation Independent. J. Dent. Res. 2019, 98, 1253–1261. [Google Scholar] [CrossRef]
- Phillips, H.M.; Papoutsi, T.; Soenen, H.; Ybot-Gonzalez, P.; Henderson, D.J.; Chaudhry, B. Neural Crest Cell Survival Is Dependent on Rho Kinase and Is Required for Development of the Mid Face in Mouse Embryos. PLoS ONE 2012, 7, e37685. [Google Scholar] [CrossRef]
- Yu, W.; Sun, Z.; Sweat, Y.; Sweat, M.; Venugopalan, S.R.; Eliason, S.; Cao, H.; Paine, M.L.; Amendt, B.A. Pitx2-Sox2-Lef1 Interactions Specify Progenitor Oral/Dental Epithelial Cell Signaling Centers. Dev. Camb. Engl. 2020, 147, dev186023. [Google Scholar] [CrossRef]
- Mucchielli, M.L.; Mitsiadis, T.A.; Raffo, S.; Brunet, J.F.; Proust, J.P.; Goridis, C. Mouse Otlx2/RIEG Expression in the Odontogenic Epithelium Precedes Tooth Initiation and Requires Mesenchyme-Derived Signals for Its Maintenance. Dev. Biol. 1997, 189, 275–284. [Google Scholar] [CrossRef]
- Peterkova, R.; Hovorakova, M.; Peterka, M.; Lesot, H. Three-Dimensional Analysis of the Early Development of the Dentition. Aust. Dent. J. 2014, 59, 55–80. [Google Scholar] [CrossRef]
- Atukorala, A.D.S.; Franz-Odendaal, T.A. Genetic Linkage between Altered Tooth and Eye Development in Lens-Ablated Astyanax Mexicanus. Dev. Biol. 2018, 441, 235–241. [Google Scholar] [CrossRef]
- Stock, D.W.; Jackman, W.R.; Trapani, J. Developmental Genetic Mechanisms of Evolutionary Tooth Loss in Cypriniform Fishes. Dev. Camb. Engl. 2006, 133, 3127–3137. [Google Scholar] [CrossRef]
- Fraser, G.J.; Graham, A.; Smith, M.M. Conserved Deployment of Genes during Odontogenesis across Osteichthyans. Proc. R. Soc. B Biol. Sci. 2004, 271, 2311–2317. [Google Scholar] [CrossRef]
- Li, J.; Chatzeli, L.; Panousopoulou, E.; Tucker, A.S.; Green, J.B.A. Epithelial Stratification and Placode Invagination Are Separable Functions in Early Morphogenesis of the Molar Tooth. Dev. Camb. Engl. 2016, 143, 670–681. [Google Scholar] [CrossRef]
- Li, J.; Economou, A.D.; Vacca, B.; Green, J.B.A. Epithelial Invagination by a Vertical Telescoping Cell Movement in Mammalian Salivary Glands and Teeth. Nat. Commun. 2020, 11, 2366. [Google Scholar] [CrossRef]
- Guo, X.; Wang, X.-F. Signaling Cross-Talk between TGF-β/BMP and Other Pathways. Cell Res. 2009, 19, 71–88. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, L.; Zheng, Y.; Yuan, G.; Yang, G.; He, F.; Chen, Y. BMP Activity Is Required for Tooth Development from the Lamina to Bud Stage. J. Dent. Res. 2012, 91, 690–695. [Google Scholar] [CrossRef] [PubMed]
- Tucker, A.S.; Al Khamis, A.; Sharpe, P.T. Interactions between Bmp-4 and Msx-1 Act to Restrict Gene Expression to Odontogenic Mesenchyme. Dev. Dyn. Off. Publ. Am. Assoc. Anat. 1998, 212, 533–539. [Google Scholar] [CrossRef]
- Feng, X.-Y.; Wu, X.-S.; Wang, J.-S.; Zhang, C.-M.; Wang, S.-L. Homeobox Protein MSX-1 Inhibits Expression of Bone Morphogenetic Protein 2, Bone Morphogenetic Protein 4, and Lymphoid Enhancer-Binding Factor 1 via Wnt/β-Catenin Signaling to Prevent Differentiation of Dental Mesenchymal Cells during the Late Bell Stage. Eur. J. Oral Sci. 2018, 126, 1–12. [Google Scholar] [CrossRef]
- Jia, S.; Kwon, H.-J.E.; Lan, Y.; Zhou, J.; Liu, H.; Jiang, R. Bmp4-Msx1 Signaling and Osr2 Control Tooth Organogenesis through Antagonistic Regulation of Secreted Wnt Antagonists. Dev. Biol. 2016, 420, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Bei, M.; Kratochwil, K.; Maas, R.L. BMP4 Rescues a Non-Cell-Autonomous Function of Msx1 in Tooth Development. Development 2000, 127, 4711–4718. [Google Scholar] [CrossRef]
- Chen, Y.; Bei, M.; Woo, I.; Satokata, I.; Maas, R. Msx1 Controls Inductive Signaling in Mammalian Tooth Morphogenesis. Dev. Camb. Engl. 1996, 122, 3035–3044. [Google Scholar] [CrossRef]
- She, Y.; Zhang, Y.; Xiao, Z.; Yuan, G.; Yang, G. The Regulation of Msx1 by BMP4/pSmad1/5 Signaling Is Mediated by Importin7 in Dental Mesenchymal Cells. Cells Dev. 2022, 169, 203763. [Google Scholar] [CrossRef]
- Peters, H.; Neubüser, A.; Kratochwil, K.; Balling, R. Pax9-Deficient Mice Lack Pharyngeal Pouch Derivatives and Teeth and Exhibit Craniofacial and Limb Abnormalities. Genes Dev. 1998, 12, 2735–2747. [Google Scholar] [CrossRef]
- Fauzi, N.H.; Ardini, Y.D.; Zainuddin, Z.; Lestari, W. A Review on Non-Syndromic Tooth Agenesis Associated with PAX9 Mutations. Jpn. Dent. Sci. Rev. 2018, 54, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Stockton, D.W.; Das, P.; Goldenberg, M.; D’Souza, R.N.; Patel, P.I. Mutation of PAX9 Is Associated with Oligodontia. Nat. Genet. 2000, 24, 18–19. [Google Scholar] [CrossRef] [PubMed]
- Kist, R.; Watson, M.; Wang, X.; Cairns, P.; Miles, C.; Reid, D.J.; Peters, H. Reduction of Pax9 Gene Dosage in an Allelic Series of Mouse Mutants Causes Hypodontia and Oligodontia. Hum. Mol. Genet. 2005, 14, 3605–3617. [Google Scholar] [CrossRef] [PubMed]
- Nakatomi, M.; Wang, X.-P.; Key, D.; Lund, J.J.; Turbe-Doan, A.; Kist, R.; Aw, A.; Chen, Y.; Maas, R.L.; Peters, H. Genetic Interactions between Pax9 and Msx1 Regulate Lip Development and Several Stages of Tooth Morphogenesis. Dev. Biol. 2010, 340, 438–449. [Google Scholar] [CrossRef]
- Ogawa, T.; Kapadia, H.; Feng, J.Q.; Raghow, R.; Peters, H.; D’Souza, R.N. Functional Consequences of Interactions between Pax9 and Msx1 Genes in Normal and Abnormal Tooth Development. J. Biol. Chem. 2006, 281, 18363–18369. [Google Scholar] [CrossRef]
- Wang, Y.; Groppe, J.C.; Wu, J.; Ogawa, T.; Mues, G.; D’Souza, R.N.; Kapadia, H. Pathogenic Mechanisms of Tooth Agenesis Linked to Paired Domain Mutations in Human PAX9. Hum. Mol. Genet. 2009, 18, 2863–2874. [Google Scholar] [CrossRef]
- Wang, Y.; Kong, H.; Mues, G.; D’Souza, R. Msx1 Mutations: How Do They Cause Tooth Agenesis? J. Dent. Res. 2011, 90, 311–316. [Google Scholar] [CrossRef]
- Doolan, B.J.; Onoufriadis, A.; Kantaputra, P.; McGrath, J.A. WNT10A, Dermatology and Dentistry. Br. J. Dermatol. 2021, 185, 1105–1111. [Google Scholar] [CrossRef]
- Andl, T.; Reddy, S.T.; Gaddapara, T.; Millar, S.E. WNT Signals Are Required for the Initiation of Hair Follicle Development. Dev. Cell 2002, 2, 643–653. [Google Scholar] [CrossRef]
- Liu, F.; Chu, E.Y.; Watt, B.; Zhang, Y.; Gallant, N.M.; Andl, T.; Yang, S.H.; Lu, M.-M.; Piccolo, S.; Schmidt-Ullrich, R.; et al. Wnt/Beta-Catenin Signaling Directs Multiple Stages of Tooth Morphogenesis. Dev. Biol. 2008, 313, 210–224. [Google Scholar] [CrossRef]
- Lee, J.-M.; Qin, C.; Chai, O.H.; Lan, Y.; Jiang, R.; Kwon, H.-J.E. MSX1 Drives Tooth Morphogenesis Through Controlling Wnt Signaling Activity. J. Dent. Res. 2022, 101, 832–839. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Liu, Y.; Liu, H.; Wong, S.-W.; He, H.; Zhang, X.; Wang, Y.; Han, D.; Feng, H. Distinct Impacts of Bi-Allelic WNT10A Mutations on the Permanent and Primary Dentitions in Odonto-Onycho-Dermal Dysplasia. Am. J. Med. Genet. A. 2019, 179, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Li, C.-Y.; Prochazka, J.; Goodwin, A.F.; Klein, O.D. Fibroblast Growth Factor Signaling in Mammalian Tooth Development. Odontology 2014, 102, 1–13. [Google Scholar] [CrossRef]
- Porntaveetus, T.; Otsuka-Tanaka, Y.; Basson, M.A.; Moon, A.M.; Sharpe, P.T.; Ohazama, A. Expression of Fibroblast Growth Factors (Fgfs) in Murine Tooth Development. J. Anat. 2011, 218, 534–543. [Google Scholar] [CrossRef] [PubMed]
- Hosokawa, R.; Deng, X.; Takamori, K.; Xu, X.; Urata, M.; Bringas, P.; Chai, Y. Epithelial-Specific Requirement of FGFR2 Signaling during Tooth and Palate Development. J. Exp. Zool. Part B Mol. Dev. Evol. 2009, 312B, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-P.; Suomalainen, M.; Felszeghy, S.; Zelarayan, L.C.; Alonso, M.T.; Plikus, M.V.; Maas, R.L.; Chuong, C.-M.; Schimmang, T.; Thesleff, I. An Integrated Gene Regulatory Network Controls Stem Cell Proliferation in Teeth. PLoS Biol. 2007, 5, e159. [Google Scholar] [CrossRef]
- Kratochwil, K.; Galceran, J.; Tontsch, S.; Roth, W.; Grosschedl, R. FGF4, a Direct Target of LEF1 and Wnt Signaling, Can Rescue the Arrest of Tooth Organogenesis in Lef1−/− Mice. Genes Dev. 2002, 16, 3173–3185. [Google Scholar] [CrossRef]
- Wang, X.-P.; O’Connell, D.J.; Lund, J.J.; Saadi, I.; Kuraguchi, M.; Turbe-Doan, A.; Cavallesco, R.; Kim, H.; Park, P.J.; Harada, H.; et al. Apc Inhibition of Wnt Signaling Regulates Supernumerary Tooth Formation during Embryogenesis and throughout Adulthood. Dev. Camb. Engl. 2009, 136, 1939–1949. [Google Scholar] [CrossRef]
- Cobourne, M.T.; Hardcastle, Z.; Sharpe, P.T. Sonic Hedgehog Regulates Epithelial Proliferation and Cell Survival in the Developing Tooth Germ. J. Dent. Res. 2001, 80, 1974–1979. [Google Scholar] [CrossRef]
- Horakova, L.; Dalecka, L.; Zahradnicek, O.; Lochovska, K.; Lesot, H.; Peterkova, R.; Tucker, A.S.; Hovorakova, M. Eda Controls the Size of the Enamel Knot during Incisor Development. Front. Physiol. 2023, 13, 2713. [Google Scholar] [CrossRef]
- Mustonen, T.; Ilmonen, M.; Pummila, M.; Kangas, A.T.; Laurikkala, J.; Jaatinen, R.; Pispa, J.; Gaide, O.; Schneider, P.; Thesleff, I.; et al. Ectodysplasin A1 Promotes Placodal Cell Fate during Early Morphogenesis of Ectodermal Appendages. Development 2004, 131, 4907–4919. [Google Scholar] [CrossRef] [PubMed]
- Thesleff, I. Current Understanding of the Process of Tooth Formation: Transfer from the Laboratory to the Clinic. Aust. Dent. J. 2014, 59, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Jiang, X.; Wei, Z.; Long, H.; Lai, W. The EDA/EDAR/NF-κB Pathway in Non-Syndromic Tooth Agenesis: A Genetic Perspective. Front. Genet. 2023, 14, 1168538. [Google Scholar] [CrossRef] [PubMed]
- Andreoni, F.; Sgattoni, C.; Bencardino, D.; Simonetti, O.; Forabosco, A.; Magnani, M. Missense Mutations in EDA and EDAR Genes Cause Dominant Syndromic Tooth Agenesis. Mol. Genet. Genom. Med. 2021, 9, e1555. [Google Scholar] [CrossRef]
- Pispa, J.; Jung, H.S.; Jernvall, J.; Kettunen, P.; Mustonen, T.; Tabata, M.J.; Kere, J.; Thesleff, I. Cusp Patterning Defect in Tabby Mouse Teeth and Its Partial Rescue by FGF. Dev. Biol. 1999, 216, 521–534. [Google Scholar] [CrossRef]
- Jia, S.; Oliver, J.D.; Turner, E.C.; Renouard, M.; Bei, M.; Wright, J.T.; D’Souza, R.N. Pax9’s Interaction With the Ectodysplasin Signaling Pathway During the Patterning of Dentition. Front. Physiol. 2020, 11, 581843. [Google Scholar] [CrossRef]
- Nikopensius, T.; Annilo, T.; Jagomägi, T.; Gilissen, C.; Kals, M.; Krjutškov, K.; Mägi, R.; Eelmets, M.; Gerst-Talas, U.; Remm, M.; et al. Non-Syndromic Tooth Agenesis Associated with a Nonsense Mutation in Ectodysplasin-A (EDA). J. Dent. Res. 2013, 92, 507–511. [Google Scholar] [CrossRef]
- Lan, R.; Wu, Y.; Dai, Q.; Wang, F. Gene Mutations and Chromosomal Abnormalities in Syndromes with Tooth Agenesis. Oral Dis. 2023, 29, 2401–2408. [Google Scholar] [CrossRef]
- Yamamoto, H.; Cho, S.-W.; Song, S.-J.; Hwang, H.-J.; Lee, M.-J.; Kim, J.-Y.; Jung, H.-S. Characteristic Tissue Interaction of the Diastema Region in Mice. Arch. Oral Biol. 2005, 50, 189–198. [Google Scholar] [CrossRef]
- Ahn, Y.; Sanderson, B.W.; Klein, O.D.; Krumlauf, R. Inhibition of Wnt Signaling by Wise (Sostdc1) and Negative Feedback from Shh Controls Tooth Number and Patterning. Development 2010, 137, 3221–3231. [Google Scholar] [CrossRef]
- Yuan, G.H.; Zhang, L.; Zhang, Y.D.; Fan, M.W.; Bian, Z.; Chen, Z. Mesenchyme Is Responsible for Tooth Suppression in the Mouse Lower Diastema. J. Dent. Res. 2008, 87, 386–390. [Google Scholar] [CrossRef] [PubMed]
- Yoshinaga, K.; Yasue, A.; Mitsui, S.N.; Minegishi, Y.; Oyadomari, S.; Imoto, I.; Tanaka, E. Effects of Wnt10a and Wnt10b Double Mutations on Tooth Development. Genes 2023, 14, 340. [Google Scholar] [CrossRef] [PubMed]
- Klein, O.D.; Minowada, G.; Peterkova, R.; Kangas, A.; Yu, B.D.; Lesot, H.; Peterka, M.; Jernvall, J.; Martin, G.R. Sprouty Genes Control Diastema Tooth Development via Bidirectional Antagonism of Epithelial-Mesenchymal FGF Signaling. Dev. Cell 2006, 11, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Munne, P.M.; Tummers, M.; Järvinen, E.; Thesleff, I.; Jernvall, J. Tinkering with the Inductive Mesenchyme: Sostdc1 Uncovers the Role of Dental Mesenchyme in Limiting Tooth Induction. Dev. Camb. Engl. 2009, 136, 393–402. [Google Scholar] [CrossRef]
- Ooë, T. Development of Human First and Second Permanent Molar, with Special Reference to the Distal Protion of the Dental Lamina. Anat. Embryol. 1979, 155, 221–240. [Google Scholar] [CrossRef]
- Wang, F.; Li, Y.; Wu, X.; Yang, M.; Cong, W.; Fan, Z.; Wang, J.; Zhang, C.; Du, J.; Wang, S. Transcriptome Analysis of Coding and Long Non-Coding RNAs Highlights the Regulatory Network of Cascade Initiation of Permanent Molars in Miniature Pigs. BMC Genom. 2017, 18, 148. [Google Scholar] [CrossRef]
- Wang, F.; Xiao, J.; Cong, W.; Li, A.; Song, T.; Wei, F.; Xu, J.; Zhang, C.; Fan, Z.; Wang, S. Morphology and Chronology of Diphyodont Dentition in Miniature Pigs, Sus Scrofa. Oral Dis. 2014, 20, 367–379. [Google Scholar] [CrossRef]
- Williams, K.L.; Evans, K.M.; Simons, A.M. Tooth Replacement and Attachment Morphology in the Pacific Leaping Blenny, Alticus Arnoldorum (Blenniiformes: Blenniidae: Salariini) with a Discussion on Tooth Function. Anat. Rec. 2022, 305, 1787–1803. [Google Scholar] [CrossRef]
- Gómez-Gil, D.F.; Orjuela-Vásquez, M.C.; Pino-Duque, M.; Pino-Araujo, A.; Sánchez-Garzón, J.; Gómez-Gil, D.F.; Orjuela-Vásquez, M.C.; Pino-Duque, M.; Pino-Araujo, A.; Sánchez-Garzón, J. Historic Background and Current Perspectives in Dental Crown Formation. In Embryology Update; IntechOpen: London, UK, 2022; ISBN 978-1-80356-291-9. [Google Scholar]
- Ko, D.; Kelly, T.; Thompson, L.; Uppal, J.K.; Rostampour, N.; Webb, M.A.; Zhu, N.; Belev, G.; Mondal, P.; Cooper, D.M.L.; et al. Timing of Mouse Molar Formation Is Independent of Jaw Length Including Retromolar Space. J. Dev. Biol. 2021, 9, 8. [Google Scholar] [CrossRef]
- Kozawa, Y.; Mishima, H.; Suzuki, K.; Ferguson, M. Dental Formula of Elephant by the Development of Tooth Germ. In Proceedings of the World of Elephants: Rome, First International Congress, Rome, Italy, 6–20 October 2001; pp. 639–642. [Google Scholar]
- Gaete, M.; Fons, J.M.; Popa, E.M.; Chatzeli, L.; Tucker, A.S. Epithelial Topography for Repetitive Tooth Formation. Biol. Open 2015, 4, 1625–1634. [Google Scholar] [CrossRef]
- Nasrullah, Q.; Renfree, M.; Evans, A.R. From Embryo to Adult: The Complete Development and Unusual Replacement of the Dentition of the Tammar Wallaby (Macropus Eugenii). J. Mamm. Evol. 2022, 29, 515–529. [Google Scholar] [CrossRef]
- Sánchez, N.; González-Ramírez, M.C.; Contreras, E.G.; Ubilla, A.; Li, J.; Valencia, A.; Wilson, A.; Green, J.B.A.; Tucker, A.S.; Gaete, M. Balance Between Tooth Size and Tooth Number Is Controlled by Hyaluronan. Front. Physiol. 2020, 11, 996. [Google Scholar] [CrossRef] [PubMed]
- Tucker, A.S.; Fraser, G.J. Evolution and Developmental Diversity of Tooth Regeneration. Semin. Cell Dev. Biol. 2014, 25–26, 71–80. [Google Scholar] [CrossRef]
- Thiery, A.P.; Shono, T.; Kurokawa, D.; Britz, R.; Johanson, Z.; Fraser, G.J. Spatially Restricted Dental Regeneration Drives Pufferfish Beak Development. Proc. Natl. Acad. Sci. USA 2017, 114, E4425–E4434. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.-J.; Jung, S.-Y.; Wu, Z.; Zhang, S.; Jung, H.-S. Sox2 Maintains Epithelial Cell Proliferation in the Successional Dental Lamina. Cell Prolif. 2020, 53, e12729. [Google Scholar] [CrossRef] [PubMed]
- Silvestri, A.R.; Singh, I. The Unresolved Problem of the Third Molar: Would People Be Better off without It? J. Am. Dent. Assoc. 2003, 134, 450–455. [Google Scholar] [CrossRef]
- Nagahama, S. A Histological Study of the Development of Human Dental Laminae. Okajimas Folia Anat. Jpn. 1984, 61, 59–67. [Google Scholar] [CrossRef][Green Version]
- Shahzad, K.M.; Roth, L.E. Prevalence and Management of Fourth Molars: A Retrospective Study and Literature Review. J. Oral Maxillofac. Surg. 2012, 70, 272–275. [Google Scholar] [CrossRef]
- Mosqueyra, V.M.V.; Meléndez, M.T.E.; Flores, F.H. Presence of the fourth molar. Literature review. Rev. Odontol. Mex. 2018, 22, 104–118. [Google Scholar]
- Juuri, E.; Isaksson, S.; Jussila, M.; Heikinheimo, K.; Thesleff, I. Expression of the Stem Cell Marker, SOX2, in Ameloblastoma and Dental Epithelium. Eur. J. Oral Sci. 2013, 121, 509–516. [Google Scholar] [CrossRef]
- Chu, K.-Y.; Wang, Y.-L.; Chen, J.-T.; Lin, C.-H.; Yao, C.-C.J.; Chen, Y.-J.; Chen, H.-W.; Simmer, J.P.; Hu, J.C.-C.; Wang, S.-K. PAX9 Mutations and Genetic Synergism in Familial Tooth Agenesis. Ann. N. Y. Acad. Sci. 2023, 1524, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Peterkova, R.; Lesot, H.; Peterka, M. Phylogenetic Memory of Developing Mammalian Dentition. J. Exp. Zool. B Mol. Dev. Evol. 2006, 306, 234–250. [Google Scholar] [CrossRef] [PubMed]
- Viriot, L.; Lesot, H.; Vonesch, J.L.; Ruch, J.V.; Peterka, M.; Peterková, R. The Presence of Rudimentary Odontogenic Structures in the Mouse Embryonic Mandible Requires Reinterpretation of Developmental Control of First Lower Molar Histomorphogenesis. Int. J. Dev. Biol. 2000, 44, 233–240. [Google Scholar] [PubMed]
- Viriot, L.; Peterková, R.; Peterka, M.; Lesot, H. Evolutionary Implications of the Occurrence of Two Vestigial Tooth Germs During Early Odontogenesis in the Mouse Lower Jaw. Connect. Tissue Res. 2002, 43, 129–133. [Google Scholar] [CrossRef]
- Chlastakova, I.; Lungova, V.; Wells, K.; Tucker, A.S.; Radlanski, R.J.; Misek, I.; Matalova, E. Morphogenesis and Bone Integration of the Mouse Mandibular Third Molar. Eur. J. Oral Sci. 2011, 119, 265–274. [Google Scholar] [CrossRef]
- Xu, M.; Horrell, J.; Snitow, M.; Cui, J.; Gochnauer, H.; Syrett, C.M.; Kallish, S.; Seykora, J.T.; Liu, F.; Gaillard, D.; et al. WNT10A Mutation Causes Ectodermal Dysplasia by Impairing Progenitor Cell Proliferation and KLF4-Mediated Differentiation. Nat. Commun. 2017, 8, 15397. [Google Scholar] [CrossRef]
- Plikus, M.V.; Zeichner-David, M.; Mayer, J.-A.; Reyna, J.; Bringas, P.; Thewissen, J.G.M.; Snead, M.L.; Chai, Y.; Chuong, C.-M. Morphoregulation of Teeth: Modulating the Number, Size, Shape and Differentiation by Tuning Bmp Activity. Evol. Dev. 2005, 7, 440–457. [Google Scholar] [CrossRef]
- Bertonnier-Brouty, L. Dental Development and Replacement in Lagomorpha. Ph.D. Thesis, Université de Lyon, Lyon, France, 2019. [Google Scholar]
- Bertonnier-Brouty, L.; Viriot, L.; Joly, T.; Charles, C. Morphological Features of Tooth Development and Replacement in the Rabbit Oryctolagus Cuniculus. Arch. Oral Biol. 2020, 109, 104576. [Google Scholar] [CrossRef]
- Järvinen, E.; Tummers, M.; Thesleff, I. The Role of the Dental Lamina in Mammalian Tooth Replacement. J. Exp. Zool. B Mol. Dev. Evol. 2009, 312B, 281–291. [Google Scholar] [CrossRef]
- Yang, G.; Jiang, B.; Cai, W.; Liu, S.; Zhao, S. Hyaluronan and Hyaluronan Synthases Expression and Localization in Embryonic Mouse Molars. J. Mol. Histol. 2016, 47, 413–420. [Google Scholar] [CrossRef]
- Dosedělová, H.; Dumková, J.; Lesot, H.; Glocová, K.; Kunová, M.; Tucker, A.S.; Veselá, I.; Krejčí, P.; Tichý, F.; Hampl, A.; et al. Fate of the Molar Dental Lamina in the Monophyodont Mouse. PLoS ONE 2015, 10, e0127543. [Google Scholar] [CrossRef] [PubMed]
- Zahradnicek, O.; Horacek, I.; Tucker, A.S. Tooth Development in a Model Reptile: Functional and Null Generation Teeth in the Gecko Paroedura Picta. J. Anat. 2012, 221, 195–208. [Google Scholar] [CrossRef] [PubMed]
- Bemis, K.E.; Burke, S.M.; St. John, C.A.; Hilton, E.J.; Bemis, W.E. Tooth Development and Replacement in the Atlantic Cutlassfish, Trichiurus Lepturus, with Comparisons to Other Scombroidei. J. Morphol. 2019, 280, 78–94. [Google Scholar] [CrossRef] [PubMed]
- Huysseune, A. Formation of a Successional Dental Lamina in the Zebrafish (Danio Rerio): Support for a Local Control of Replacement Tooth Initiation. Int. J. Dev. Biol. 2004, 50, 637–643. [Google Scholar] [CrossRef]
- Popa, E.M.; Buchtova, M.; Tucker, A.S. Revitalising the Rudimentary Replacement Dentition in the Mouse. Development 2019, 146, dev171363. [Google Scholar] [CrossRef]
- Solares, R.; Romero, M.I. Supernumerary Premolars: A Literature Review. Pediatr. Dent. 2004, 26, 450–458. [Google Scholar]
- Whitlock, J.A.; Richman, J.M. Biology of Tooth Replacement in Amniotes. Int. J. Oral Sci. 2013, 5, 66–70. [Google Scholar] [CrossRef]
- Handrigan, G.R.; Richman, J.M. A Network of Wnt, Hedgehog and BMP Signaling Pathways Regulates Tooth Replacement in Snakes. Dev. Biol. 2010, 348, 130–141. [Google Scholar] [CrossRef]
- Li, G.; Li, Q.; Shen, Z.; Lin, X.; Li, X.; Wang, J.; Zhao, B.; Feng, Y.; Feng, L.; Guo, W.; et al. Fibulin-1 Regulates Initiation of Successional Dental Lamina. J. Dent. Res. 2023, 102, 220345231182052. [Google Scholar] [CrossRef]
- Mao, C.; Lai, Y.; Liao, C.; Chen, J.; Hong, Y.; Ren, C.; Wang, C.; Lu, M.; Chen, W. Revitalizing Mouse Diphyodontic Dentition Formation by Inhibiting the Sonic Hedgehog Signaling Pathway. Dev. Dyn. 2022, 251, 759–776. [Google Scholar] [CrossRef]
- Shim, J.S.; Kim, B.; Park, H.C.; Ryu, J.J. Temporal Control of WNT Activity Regulates Tooth Number in Fish. J. Dent. Res. 2019, 98, 339–346. [Google Scholar] [CrossRef]
- Kiso, H.; Takahashi, K.; Mishima, S.; Murashima-Suginami, A.; Kakeno, A.; Yamazaki, T.; Asai, K.; Tokita, Y.; Uozumi, R.; Sugai, M.; et al. Third Dentition Is the Main Cause of Premolar Supernumerary Tooth Formation. J. Dent. Res. 2019, 98, 968–974. [Google Scholar] [CrossRef]
- Townsend, G.; Harris, E.F.; Lesot, H.; Clauss, F.; Brook, A. Morphogenetic Fields within the Human Dentition: A New, Clinically Relevant Synthesis of an Old Concept. Arch. Oral Biol. 2009, 54, S34–S44. [Google Scholar] [CrossRef] [PubMed]
- Bateson, W. Materials for the Study of Variation: Treated with Especial Regard to Discontinuity in the Origin of Species; Macmillan and Company: London, UK, 1894. [Google Scholar]
- Butler, P.M. Studies of the Mammalian Dentition.–Differentiation of the Post-Canine Dentition. Proc. Zool. Soc. Lond. 1939, B109, 1–36. [Google Scholar] [CrossRef]
- Dahlberg, A.A. The Changing Dentition of Man. J. Am. Dent. Assoc. 1945, 32, 676–690. [Google Scholar] [CrossRef]
- Dahlberg, A.A. The Dentition of the American Indian. Pap. Phys. Anthropol. Am. Indians 1949, 138–176. [Google Scholar]
- Osborn, J.W. Morphogenetic Gradients: Field versus Clones. Dev. Funct. Evol. Teeth 1978, 171–202. [Google Scholar]
- Zhou, M.; Zhang, H.; Camhi, H.; Seymen, F.; Koruyucu, M.; Kasimoglu, Y.; Kim, J.-W.; Kim-Berman, H.; Yuson, N.M.R.; Benke, P.J.; et al. Analyses of Oligodontia Phenotypes and Genetic Etiologies. Int. J. Oral Sci. 2021, 13, 32. [Google Scholar] [CrossRef]
- Fournier, B.P.; Bruneau, M.H.; Toupenay, S.; Kerner, S.; Berdal, A.; Cormier-Daire, V.; Hadj-Rabia, S.; Coudert, A.E.; de La Dure-Molla, M. Patterns of Dental Agenesis Highlight the Nature of the Causative Mutated Genes. J. Dent. Res. 2018, 97, 1306–1316. [Google Scholar] [CrossRef]
- Thiery, A.P.; Standing, A.S.; Cooper, R.L.; Fraser, G.J. An Epithelial Signalling Centre in Sharks Supports Homology of Tooth Morphogenesis in Vertebrates. eLife 2022, 11, e73173. [Google Scholar] [CrossRef]
- Li, J.; Xu, J.; Cui, Y.; Wang, L.; Wang, B.; Wang, Q.; Zhang, X.; Qiu, M.; Zhang, Z. Mesenchymal Sufu Regulates Development of Mandibular Molars via Shh Signaling. J. Dent. Res. 2019, 98, 1348–1356. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Zhang, S.; Chen, G.; Lin, C.; Huang, Z.; Chen, Y.; Zhang, Y. Expression of SHH Signaling Molecules in the Developing Human Primary Dentition. BMC Dev. Biol. 2013, 13, 11. [Google Scholar] [CrossRef] [PubMed]
- Ravi, V.; Murashima-Suginami, A.; Kiso, H.; Tokita, Y.; Huang, C.L.; Bessho, K.; Takagi, J.; Sugai, M.; Tabata, Y.; Takahashi, K. Advances in Tooth Agenesis and Tooth Regeneration. Regen. Ther. 2023, 22, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Lan, Y.; Chai, Y.; Jiang, R. Antagonistic Actions of Msx1 and Osr2 Pattern Mammalian Teeth into a Single Row. Science 2009, 323, 1232–1234. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Wang, S.-K.; Choi, M.; Reid, B.M.; Hu, Y.; Lee, Y.-L.; Herzog, C.R.; Kim-Berman, H.; Lee, M.; Benke, P.J.; et al. Taurodontism, Variations in Tooth Number, and Misshapened Crowns in Wnt10a Null Mice and Human Kindreds. Mol. Genet. Genom. Med. 2015, 3, 40–58. [Google Scholar] [CrossRef] [PubMed]
- Edmund, A.G.; Museum, R.O. Tooth Replacement Phenomena in the Lower Vertebrates; Royal Ontario Museum: Toronto, ON, Canada, 1960; pp. 1–202. [Google Scholar]
- Sadier, A.; Jackman, W.R.; Laudet, V.; Gibert, Y. The Vertebrate Tooth Row: Is It Initiated by a Single Organizing Tooth? BioEssays 2020, 42, 1900229. [Google Scholar] [CrossRef]
- Phattarataratip, E.; Panitkul, T.; Khodkaew, W.; Anupuntanun, P.; Jaroonvechatam, J.; Pitarangsikul, S. Expression of SOX2 and OCT4 in Odontogenic Cysts and Tumors. Head Face Med. 2021, 17, 29. [Google Scholar] [CrossRef]
- Domning, D.P.; Hayek, L.-A. Horizontal Tooth Replacement in the Amazonian Manatee (Trichechus inunguis). Mammalia 1984, 48, 105–128. [Google Scholar] [CrossRef]
- Woodard, N. Are Florida Manatees (Trichechus Manatus Latirostris) Wearing Their Teeth Beyond Functionality? Interspecific and Intraspecific Mesowear in Manatees. Honors Thesis, Andrews University, Berrien Springs, MI, USA, 2020. [Google Scholar] [CrossRef]

| Type of Dental Lamina | Continual Dental Lamina | Successional Dental Lamina |
|---|---|---|
| Direction | The Second Direction | The Third Direction |
| Definition | The dental lamina extends parallel to the dentition and gives rise to teeth of same dentition. | The dental lamina gives rise to the replacement tooth lingually. |
| Previous descriptions of the dental lamina and relevant tooth development pattern | Additional [77,78,79] Continual [80] Continuous [81] Distal [82] Primary [12] Successional [83,84,85] | Distal [86,87] Successional [84,88] |
| The First Direction | The Second Direction | The Third Direction | |
|---|---|---|---|
| Main role | The basis of single tooth development | Gives rise to several teeth within one dentition | Maintaining a consistent tooth number in general, and taking part in adding/reducing tooth in some cases |
| Mechanism of adding a tooth | The first direction itself is the process of adding a tooth | The continual lamina extends posteriorly by adding an additional tooth to the hind dentition | The successional lamina may give rise to an extra pathological replacement tooth |
| Mechanism of reducing a tooth | Tooth germ arrest reduces the tooth number | Arrest of continual lamina extension prevents the formation of extra teeth | Arrest of the successional lamina reduces the tooth number of the next dentition |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fang, Z.; Atukorallaya, D. Count Me in, Count Me out: Regulation of the Tooth Number via Three Directional Developmental Patterns. Int. J. Mol. Sci. 2023, 24, 15061. https://doi.org/10.3390/ijms242015061
Fang Z, Atukorallaya D. Count Me in, Count Me out: Regulation of the Tooth Number via Three Directional Developmental Patterns. International Journal of Molecular Sciences. 2023; 24(20):15061. https://doi.org/10.3390/ijms242015061
Chicago/Turabian StyleFang, Zheng, and Devi Atukorallaya. 2023. "Count Me in, Count Me out: Regulation of the Tooth Number via Three Directional Developmental Patterns" International Journal of Molecular Sciences 24, no. 20: 15061. https://doi.org/10.3390/ijms242015061
APA StyleFang, Z., & Atukorallaya, D. (2023). Count Me in, Count Me out: Regulation of the Tooth Number via Three Directional Developmental Patterns. International Journal of Molecular Sciences, 24(20), 15061. https://doi.org/10.3390/ijms242015061





