Genome-Wide Identification of the CDPK Gene Family and Their Involvement in Taproot Cracking in Radish
Abstract
:1. Introduction
2. Results
2.1. Identification of RsCDPK Gene Members in Radish
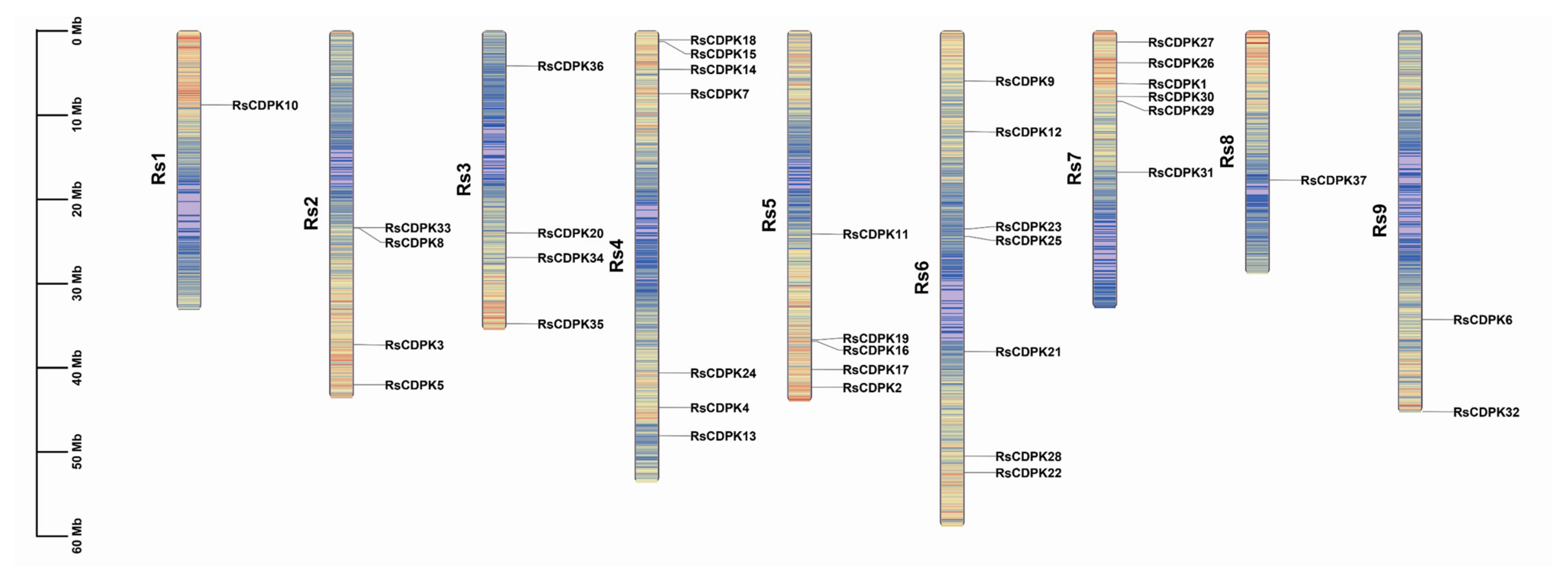
2.2. Chromosomal Position and Collinearity Analysis of RsCDPK
2.3. Analysis of Gene Structure and Conserved Motifs of RsCDPK
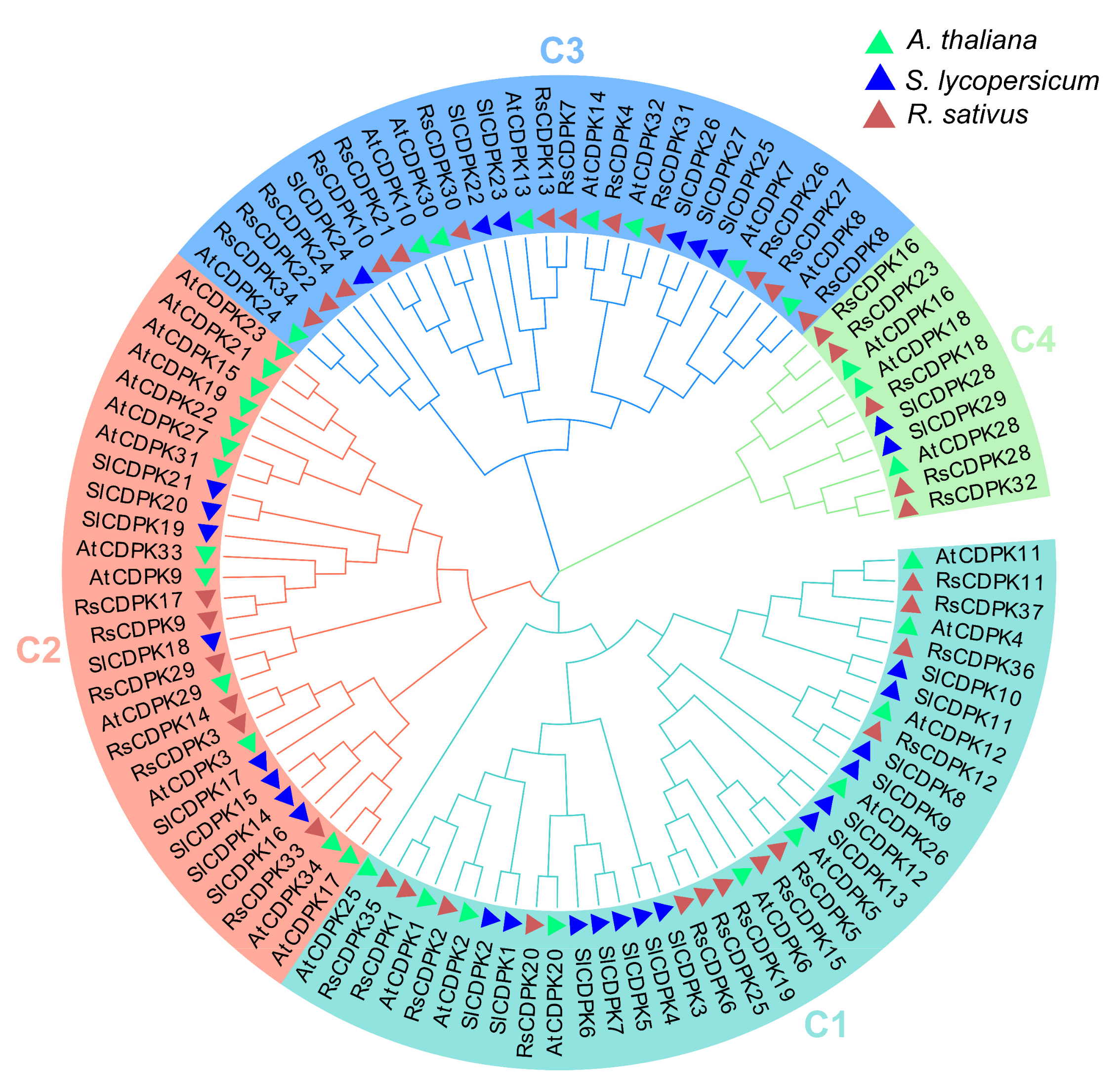
2.4. Phylogenetic and Motif Analysis of CDPK Genes
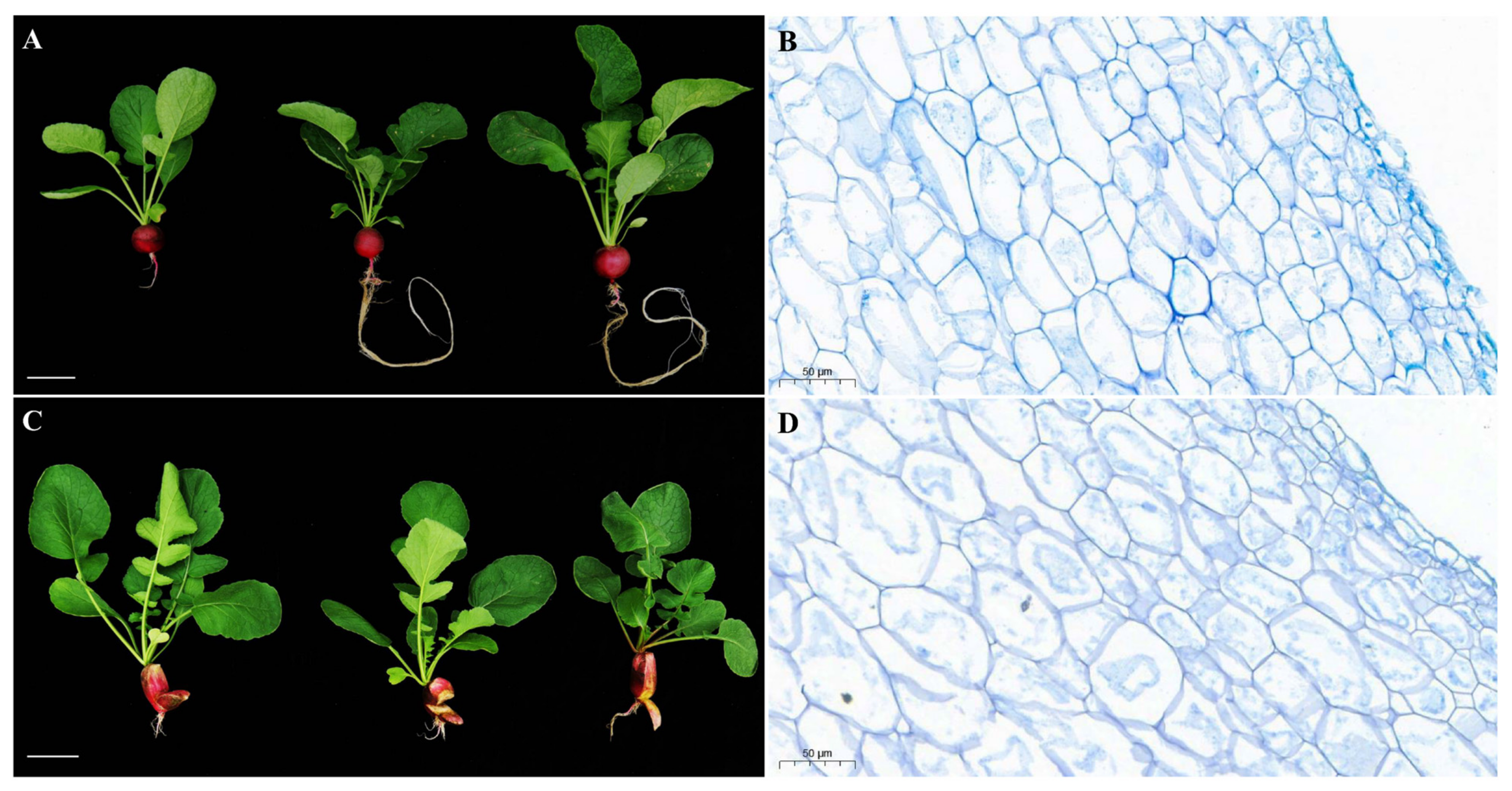
2.5. Morphological and Cytological Characterization of HongYun and 505
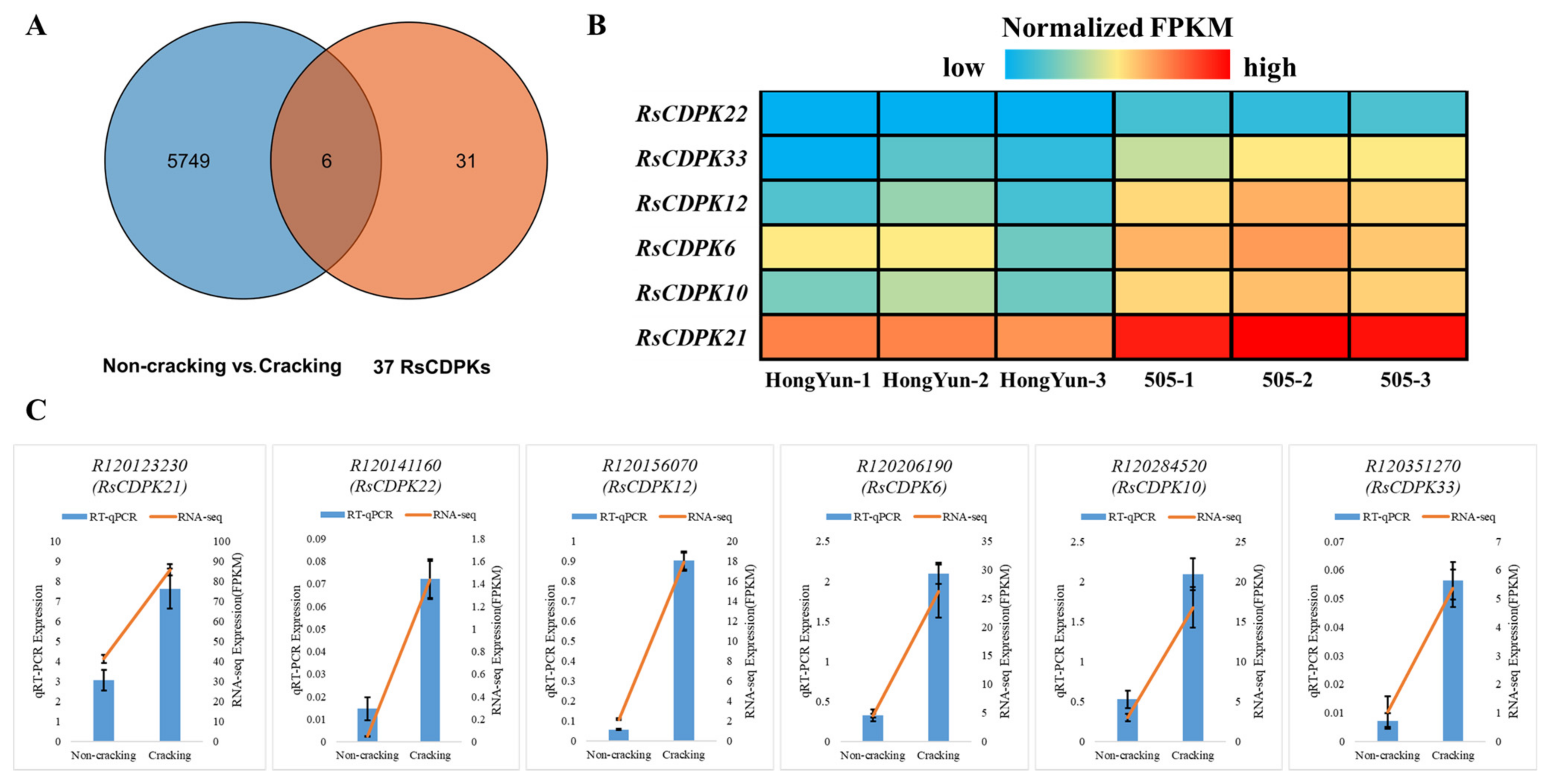
2.6. Transcriptome Data Analysis
2.7. GO and KEGG Analyses of DEGs
2.8. CDPK Genes Related to Fleshy Taproot Cracking
2.9. RsCDPK21 Significantly and Positively Regulated Root Length in Radish
3. Discussion
3.1. Taproot Cracking Formation in Radish
3.2. Cracking: A Complex Trait
3.3. CDPK Gene Members Related to Taproot Development and Cracking
4. Materials and Methods
4.1. Identification and Analysis of CDPK Family Genes in Radish
4.2. Chromosomal Position and Collinearity Analysis of RsCDPK
4.3. Gene Structure and Conserved Motif Analysis
4.4. Phylogenetic and Motif Analyses of CDPK Genes
4.5. Plant Materials and Phenotypic Observation
4.6. Transcriptomic Sequencing
4.7. Identification of DEGs and Enrichment Analyses
4.8. RT-qPCR Validation
4.9. Vector Construction and Soybean Hairy Root Transformation
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Luan, S.; Wang, C. Calcium Signaling Mechanisms Across Kingdoms. Annu. Rev. Cell Dev. Biol. 2021, 37, 311–340. [Google Scholar] [CrossRef] [PubMed]
- Sanders, D.; Pelloux, J.; Brownlee, C.; Harper, J.F. Calcium at the crossroads of signaling. Plant Cell 2002, 14 (Suppl. S1), S401–S417. [Google Scholar] [CrossRef] [PubMed]
- White, P.J.; Broadley, M.R. Calcium in plants. Ann. Bot. 2003, 92, 487–511. [Google Scholar] [CrossRef] [PubMed]
- Wymer, C.L.; Bibikova, T.N.; Gilroy, S. Cytoplasmic free calcium distributions during the development of root hairs of Arabidopsis thaliana. Plant J. 1997, 12, 427–439. [Google Scholar] [CrossRef] [PubMed]
- Demidchik, V.; Bowen, H.C.; Maathuis, F.J.; Shabala, S.N.; Tester, M.A.; White, P.J.; Davies, J.M. Arabidopsis thaliana root non-selective cation channels mediate calcium uptake and are involved in growth. Plant J. 2002, 32, 799–808. [Google Scholar] [CrossRef] [PubMed]
- Yip Delormel, T.; Boudsocq, M. Properties and functions of calcium-dependent protein kinases and their relatives in Arabidopsis thaliana. New Phytol. 2019, 224, 585–604. [Google Scholar] [CrossRef]
- Mohanta, T.K.; Mohanta, N.; Mohanta, Y.K.; Parida, P.; Bae, H. Genome-wide identification of Calcineurin B-Like (CBL) gene family of plants reveals novel conserved motifs and evolutionary aspects in calcium signaling events. BMC Plant Biol. 2015, 15, 189. [Google Scholar] [CrossRef] [PubMed]
- Mohanta, T.K.; Kumar, P.; Bae, H. Genomics and evolutionary aspect of calcium signaling event in calmodulin and calmodulin-like proteins in plants. BMC Plant Biol. 2017, 17, 38. [Google Scholar] [CrossRef]
- Hrabak, E.M.; Chan, C.W.; Gribskov, M.; Harper, J.F.; Choi, J.H.; Halford, N.; Kudla, J.; Luan, S.; Nimmo, H.G.; Sussman, M.R.; et al. The Arabidopsis CDPK-SnRK superfamily of protein kinases. Plant Physiol. 2003, 132, 666–680. [Google Scholar] [CrossRef]
- Mohanta, T.K.; Mohanta, N.; Mohanta, Y.K.; Bae, H. Genome-Wide Identification of Calcium Dependent Protein Kinase Gene Family in Plant Lineage Shows Presence of Novel D-x-D and D-E-L Motifs in EF-Hand Domain. Front. Plant Sci. 2015, 6, 1146. [Google Scholar] [CrossRef]
- Mitra, R.M.; Gleason, C.A.; Edwards, A.; Hadfield, J.; Downie, J.A.; Oldroyd, G.E.; Long, S.R. A Ca2+/calmodulin-dependent protein kinase required for symbiotic nodule development: Gene identification by transcript-based cloning. Proc. Natl. Acad. Sci. USA 2004, 101, 4701–4705. [Google Scholar] [CrossRef]
- Wang, J.P.; Munyampundu, J.P.; Xu, Y.P.; Cai, X.Z. Phylogeny of Plant Calcium and Calmodulin-Dependent Protein Kinases (CCaMKs) and Functional Analyses of Tomato CCaMK in Disease Resistance. Front. Plant Sci. 2015, 6, 1075. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Li, S.; Asim, M.; Mao, J.; Xu, D.; Ullah, Z.; Liu, G.; Wang, Q.; Liu, H. The Arabidopsis Calcium-Dependent Protein Kinases (CDPKs) and Their Roles in Plant Growth Regulation and Abiotic Stress Responses. Int. J. Mol. Sci. 2018, 19, 1900. [Google Scholar] [CrossRef] [PubMed]
- Kudla, J.; Batistic, O.; Hashimoto, K. Calcium signals: The lead currency of plant information processing. Plant Cell 2010, 22, 541–563. [Google Scholar] [CrossRef] [PubMed]
- Boudsocq, M.; Sheen, J. CDPKs in immune and stress signaling. Trends Plant Sci. 2013, 18, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Valmonte, G.R.; Arthur, K.; Higgins, C.M.; MacDiarmid, R.M. Calcium-dependent protein kinases in plants: Evolution, expression and function. Plant Cell Physiol. 2014, 55, 551–569. [Google Scholar] [CrossRef] [PubMed]
- Schulz, P.; Herde, M.; Romeis, T. Calcium-dependent protein kinases: Hubs in plant stress signaling and development. Plant Physiol. 2013, 163, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Huang, W. Calcium-Dependent Protein Kinases in Phytohormone Signaling Pathways. Int. J. Mol. Sci. 2017, 18, 2436. [Google Scholar] [CrossRef] [PubMed]
- Takezawa, D.; Patil, S.; Bhatia, A.; Poovaiah, B.W. Calcium-dependent protein kinase genes in corn roots. J. Plant Physiol. 1996, 149, 329–335. [Google Scholar] [CrossRef]
- Dammann, C.; Ichida, A.; Hong, B.; Romanowsky, S.M.; Hrabak, E.M.; Harmon, A.C.; Pickard, B.G.; Harper, J.F. Subcellular targeting of nine calcium-dependent protein kinase isoforms from Arabidopsis. Plant Physiol. 2003, 132, 1840–1848. [Google Scholar] [CrossRef]
- Ivashuta, S.; Liu, J.; Liu, J.; Lohar, D.P.; Haridas, S.; Bucciarelli, B.; VandenBosch, K.A.; Vance, C.P.; Harrison, M.J.; Gantt, J.S. RNA interference identifies a calcium-dependent protein kinase involved in Medicago truncatula root development. Plant Cell 2005, 17, 2911–2921. [Google Scholar] [CrossRef]
- Rietz, S.; Dermendjiev, G.; Oppermann, E.; Tafesse, F.G.; Effendi, Y.; Holk, A.; Parker, J.E.; Teige, M.; Scherer, G.F. Roles of Arabidopsis patatin-related phospholipases a in root development are related to auxin responses and phosphate deficiency. Mol. Plant 2010, 3, 524–538. [Google Scholar] [CrossRef] [PubMed]
- Lanteri, M.L.; Pagnussat, G.C.; Lamattina, L. Calcium and calcium-dependent protein kinases are involved in nitric oxide- and auxin-induced adventitious root formation in cucumber. J. Exp. Bot. 2006, 57, 1341–1351. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Chen, Z.; Gao, J.; Gong, Z. Abscisic acid inhibits root growth in Arabidopsis through ethylene biosynthesis. Plant J. 2014, 79, 44–55. [Google Scholar] [CrossRef] [PubMed]
- Silamparasan, D.; Chang, I.F.; Jinn, T.L. Calcium-dependent protein kinase CDPK16 phosphorylates serine-856 of glutamate receptor-like GLR3.6 protein leading to salt-responsive root growth in Arabidopsis. Front. Plant Sci. 2023, 14, 1093472. [Google Scholar] [CrossRef] [PubMed]
- Manzoor, A.; Bashir, M.A.; Naveed, M.S.; Cheema, K.L.; Cardarelli, M. Role of Different Abiotic Factors in Inducing Pre-Harvest Physiological Disorders in Radish (Raphanus sativus). Plants 2021, 10, 2003. [Google Scholar] [CrossRef] [PubMed]
- Santos, M.; Egea-Cortines, M.; Goncalves, B.; Matos, M. Molecular mechanisms involved in fruit cracking: A review. Front. Plant Sci. 2023, 14, 1130857. [Google Scholar] [CrossRef] [PubMed]
- Li, W.C.; Wu, J.Y.; Zhang, H.N.; Shi, S.Y.; Liu, L.Q.; Shu, B.; Liang, Q.Z.; Xie, J.H.; Wei, Y.Z. De novo assembly and characterization of pericarp transcriptome and identification of candidate genes mediating fruit cracking in Litchi chinensis Sonn. Int. J. Mol. Sci. 2014, 15, 17667–17685. [Google Scholar] [CrossRef] [PubMed]
- Correia, S.; Santos, M.; Glinska, S.; Gapinska, M.; Matos, M.; Carnide, V.; Schouten, R.; Silva, A.P.; Goncalves, B. Effects of exogenous compound sprays on cherry cracking: Skin properties and gene expression. J. Sci. Food Agric. 2020, 100, 2911–2921. [Google Scholar] [CrossRef]
- Niu, J.; Shi, Y.; Huang, K.; Zhong, Y.; Chen, J.; Sun, Z.; Luan, M.; Chen, J. Integrative transcriptome and proteome analyses provide new insights into different stages of Akebia trifoliata fruit cracking during ripening. Biotechnol. Biofuels 2020, 13, 149. [Google Scholar] [CrossRef]
- Zhang, C.; Cui, L.; Zhang, P.; Dong, T.; Fang, J. Transcriptome and metabolite profiling reveal that spraying calcium fertilizer reduces grape berry cracking by modulating the flavonoid biosynthetic metabolic pathway. Food Chem. 2021, 2, 100025. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Zhou, X.; Qin, M.; Wang, W.; He, X.; Zhou, W. Effect of CaCl2 Sprays in Different Fruit Development Stages on Grape Berry Cracking. Front. Plant Sci. 2022, 13, 870959. [Google Scholar] [CrossRef] [PubMed]
- Huai, B.; Wu, Y.; Liang, C.; Tu, P.; Mei, T.; Guan, A.; Yao, Q.; Li, J.; Chen, J. Effects of calcium on cell wall metabolism enzymes and expression of related genes associated with peel creasing in Citrus fruits. PeerJ 2022, 10, e14574. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Yu, J.; Wang, R.; Zeng, Y.; Kang, L.; Chen, Z. Nano-calcium alleviates the cracking of nectarine fruit and improves fruit quality. Plant Physiol. Biochem. 2023, 196, 370–380. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Choi, S.R.; Chhapekar, S.S.; Lu, L.; Ma, Y.; Lee, J.Y.; Hong, S.; Kim, Y.Y.; Oh, S.H.; Lim, Y.P. Genetic and physiological analyses of root cracking in radish (Raphanus sativus L.). Theor. Appl. Genet. 2019, 132, 3425–3437. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Cui, L.; Chen, W.; Liu, Z.; Yuan, W.; Zhu, F.; Jiao, Z.; Zhang, Z.; Deng, X.; Wang, L.; et al. Comprehensive analysis of NAC transcription factors and their expressions during taproot coloration in radish (Raphanus sativus L.). Sci. Hortic. 2022, 299, 111047. [Google Scholar] [CrossRef]
- Wu, P.; Wang, W.; Duan, W.; Li, Y.; Hou, X. Comprehensive Analysis of the CDPK-SnRK Superfamily Genes in Chinese Cabbage and Its Evolutionary Implications in Plants. Front. Plant Sci. 2017, 8, 162. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Tang, H.; Debarry, J.D.; Tan, X.; Li, J.; Wang, X.; Lee, T.H.; Jin, H.; Marler, B.; Guo, H.; et al. MCScanX: A toolkit for detection and evolutionary analysis of gene synteny and collinearity. Nucleic Acids Res. 2012, 40, e49. [Google Scholar] [CrossRef]
- Cannon, S.B.; Mitra, A.; Baumgarten, A.; Young, N.D.; May, G. The roles of segmental and tandem gene duplication in the evolution of large gene families in Arabidopsis thaliana. BMC Plant Biol. 2004, 4, 10. [Google Scholar] [CrossRef]
- Schulze, S.K.; Kanwar, R.; Golzenleuchter, M.; Therneau, T.M.; Beutler, A.S. SERE: Single-parameter quality control and sample comparison for RNA-Seq. BMC Genom. 2012, 13, 524. [Google Scholar] [CrossRef]
- Khadivi-Khub, A. Physiological and genetic factors influencing fruit cracking. Acta Physiol. Plant. 2014, 37, 1718. [Google Scholar] [CrossRef]
- Capel, C.; Yuste-Lisbona, F.J.; Lopez-Casado, G.; Angosto, T.; Cuartero, J.; Lozano, R.; Capel, J. Multi-environment QTL mapping reveals genetic architecture of fruit cracking in a tomato RIL Solanum lycopersicum × S. pimpinellifolium population. Theor. Appl. Genet. 2017, 130, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Hoque, A.; Jewel, K.; Mian, M. Seed yield of radish as affected by uprooting time and root cutting. Azarian J. Agric. 2015, 2, 91–98. [Google Scholar]
- Kang, Y.; Wan, S. Effect of soil water potential on radish (Raphanus sativus L.) growth and water use under drip irrigation. Sci. Hortic. 2005, 106, 275–292. [Google Scholar] [CrossRef]
- Wan, S.; Kang, Y. Effect of drip irrigation frequency on radish (Raphanus sativus L.) growth and water use. Irrig. Sci. 2005, 24, 161–174. [Google Scholar] [CrossRef]
- Pang, W.; Li, X.; Choi, S.R.; Nguyen, V.D.; Dhandapani, V.; Kim, Y.-Y.; Ramchiary, N.; Kim, J.G.; Edwards, D.; Batley, J.; et al. Mapping QTLs of resistance to head splitting in cabbage (Brassica oleracea L.var. capitata L.). Mol. Breed. 2015, 35, 126. [Google Scholar] [CrossRef]
- Su, Y.; Liu, Y.; Li, Z.; Fang, Z.; Yang, L.; Zhuang, M.; Zhang, Y. QTL Analysis of Head Splitting Resistance in Cabbage (Brassica oleracea L. var. capitata) Using SSR and InDel Makers Based on Whole-Genome Re-Sequencing. PLoS ONE 2015, 10, e0138073. [Google Scholar] [CrossRef]
- Vogg, G.; Fischer, S.; Leide, J.; Emmanuel, E.; Jetter, R.; Levy, A.A.; Riederer, M. Tomato fruit cuticular waxes and their effects on transpiration barrier properties: Functional characterization of a mutant deficient in a very-long-chain fatty acid beta-ketoacyl-CoA synthase. J. Exp. Bot. 2004, 55, 1401–1410. [Google Scholar] [CrossRef]
- Moctezuma, E.; Smith, D.L.; Gross, K.C. Antisense suppression of a beta-galactosidase gene (TB G6) in tomato increases fruit cracking. J. Exp. Bot. 2003, 54, 2025–2033. [Google Scholar] [CrossRef]
- Kasai, S.; Hayama, H.; Kashimura, Y.; Kudo, S.; Osanai, Y. Relationship between fruit cracking and expression of the expansin gene MdEXPA3 in ‘Fuji’ apples (Malus domestica Borkh.). Sci. Hortic. 2008, 116, 194–198. [Google Scholar] [CrossRef]
- Yong, W.; Wangjin, L.; Jianguo, L.; Yueming, J. Differential Expression of Two Expansin Genes in Developing Fruit of Cracking-susceptible and -resistant Litchi Cultivars. J. Am. Soc. Hortic. Sci. 2006, 131, 118–121. [Google Scholar] [CrossRef]
- Jiang, H.; Tian, H.; Yan, C.; Jia, L.; Wang, Y.; Wang, M.; Jiang, C.; Li, Y.; Jiang, J.; Fang, L.; et al. RNA-seq analysis of watermelon (Citrullus lanatus) to identify genes involved in fruit cracking. Sci. Hortic. 2019, 248, 248–255. [Google Scholar] [CrossRef]
- Harper, J.F.; Breton, G.; Harmon, A. Decoding Ca2+ signals through plant protein kinases. Annu. Rev. Plant Biol. 2004, 55, 263–288. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wu, X.F.; Tang, Y.; Li, J.G.; Zhao, M.L. RNA-Seq Provides New Insights into the Molecular Events Involved in “Ball-Skin versus Bladder Effect” on Fruit Cracking in Litchi. Int. J. Mol. Sci. 2021, 22, 454. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.L. Gene family evolution in green plants with emphasis on the origination and evolution of Arabidopsis thaliana genes. Plant J. 2013, 73, 941–951. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.H.; Hettenhausen, C.; Baldwin, I.T.; Wu, J. Silencing Nicotiana attenuata calcium-dependent protein kinases, CDPK4 and CDPK5, strongly up-regulates wound- and herbivory-induced jasmonic acid accumulations. Plant Physiol. 2012, 159, 1591–1607. [Google Scholar] [CrossRef] [PubMed]
- Hettenhausen, C.; Yang, D.H.; Baldwin, I.T.; Wu, J. Calcium-dependent protein kinases, CDPK4 and CDPK5, affect early steps of jasmonic acid biosynthesis in Nicotiana attenuata. Plant Signal. Behav. 2013, 8, e22784. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.G.; Gao, X.M.; Ma, Z.L.; Chen, J.; Liu, Y.N.; Shi, W.Q. Metabolomic and transcriptomic profiling of three types of litchi pericarps reveals that changes in the hormone balance constitute the molecular basis of the fruit cracking susceptibility of Litchi chinensis cv. Baitangying. Mol. Biol. Rep. 2019, 46, 5295–5308. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, P.; Geng, Y.; Xie, X.; Wen, P. Cracking of jujube fruits is associated with differential expression of metabolic genes. FEBS Open Bio 2020, 10, 1765–1773. [Google Scholar] [CrossRef]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An Integrative Toolkit Developed for Interactive Analyses of Big Biological Data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef]
- Yang, Q.; Nong, X.; Xu, J.; Huang, F.; Wang, F.; Wu, J.; Zhang, C.; Liu, C. Unraveling the Genetic Basis of Fertility Restoration for Cytoplasmic Male Sterile Line WNJ01A Originated from Brassica juncea in Brassica napus. Front. Plant Sci. 2021, 12, 721980. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed]
- Varet, H.; Brillet-Gueguen, L.; Coppee, J.Y.; Dillies, M.A. SARTools: A DESeq2- and EdgeR-Based R Pipeline for Comprehensive Differential Analysis of RNA-Seq Data. PLoS ONE 2016, 11, e0157022. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Feng, Z.; Wang, X.; Wang, X.; Zhang, X. DEGseq: An R package for identifying differentially expressed genes from RNA-seq data. Bioinformatics 2010, 26, 136–138. [Google Scholar] [CrossRef] [PubMed]
- Young, M.D.; Wakefield, M.J.; Smyth, G.K.; Oshlack, A. Gene ontology analysis for RNA-seq: Accounting for selection bias. Genome Biol. 2010, 11, R14. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.Z.; Cai, T.; Olyarchuk, J.G.; Wei, L.P. Automated genome annotation and pathway identification using the KEGG Orthology (KO) as a controlled vocabulary. Bioinformatics 2005, 21, 3787–3793. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Kereszt, A.; Li, D.; Indrasumunar, A.; Nguyen, C.D.; Nontachaiyapoom, S.; Kinkema, M.; Gresshoff, P.M. Agrobacterium rhizogenes-mediated transformation of soybean to study root biology. Nat. Protoc. 2007, 2, 948–952. [Google Scholar] [CrossRef]
- Jian, B.; Hou, W.; Wu, C.; Liu, B.; Liu, W.; Song, S.; Bi, Y.; Han, T. Agrobacterium rhizogenes-mediated transformation of Superroot-derived Lotus corniculatus plants: A valuable tool for functional genomics. BMC Plant Biol. 2009, 9, 78. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Q.; Huang, Y.; Cui, L.; Gan, C.; Qiu, Z.; Yan, C.; Deng, X. Genome-Wide Identification of the CDPK Gene Family and Their Involvement in Taproot Cracking in Radish. Int. J. Mol. Sci. 2023, 24, 15059. https://doi.org/10.3390/ijms242015059
Yang Q, Huang Y, Cui L, Gan C, Qiu Z, Yan C, Deng X. Genome-Wide Identification of the CDPK Gene Family and Their Involvement in Taproot Cracking in Radish. International Journal of Molecular Sciences. 2023; 24(20):15059. https://doi.org/10.3390/ijms242015059
Chicago/Turabian StyleYang, Qian, Yan Huang, Lei Cui, Caixia Gan, Zhengming Qiu, Chenghuan Yan, and Xiaohui Deng. 2023. "Genome-Wide Identification of the CDPK Gene Family and Their Involvement in Taproot Cracking in Radish" International Journal of Molecular Sciences 24, no. 20: 15059. https://doi.org/10.3390/ijms242015059
APA StyleYang, Q., Huang, Y., Cui, L., Gan, C., Qiu, Z., Yan, C., & Deng, X. (2023). Genome-Wide Identification of the CDPK Gene Family and Their Involvement in Taproot Cracking in Radish. International Journal of Molecular Sciences, 24(20), 15059. https://doi.org/10.3390/ijms242015059




