Similar Pro- and Antiangiogenic Profiles Close to Delivery in Different Clinical Presentations of Two Pregnancy Syndromes: Preeclampsia and Fetal Growth Restriction
Abstract
1. Introduction
2. Results
2.1. Patient Demographic Data and Clinical Characteristics
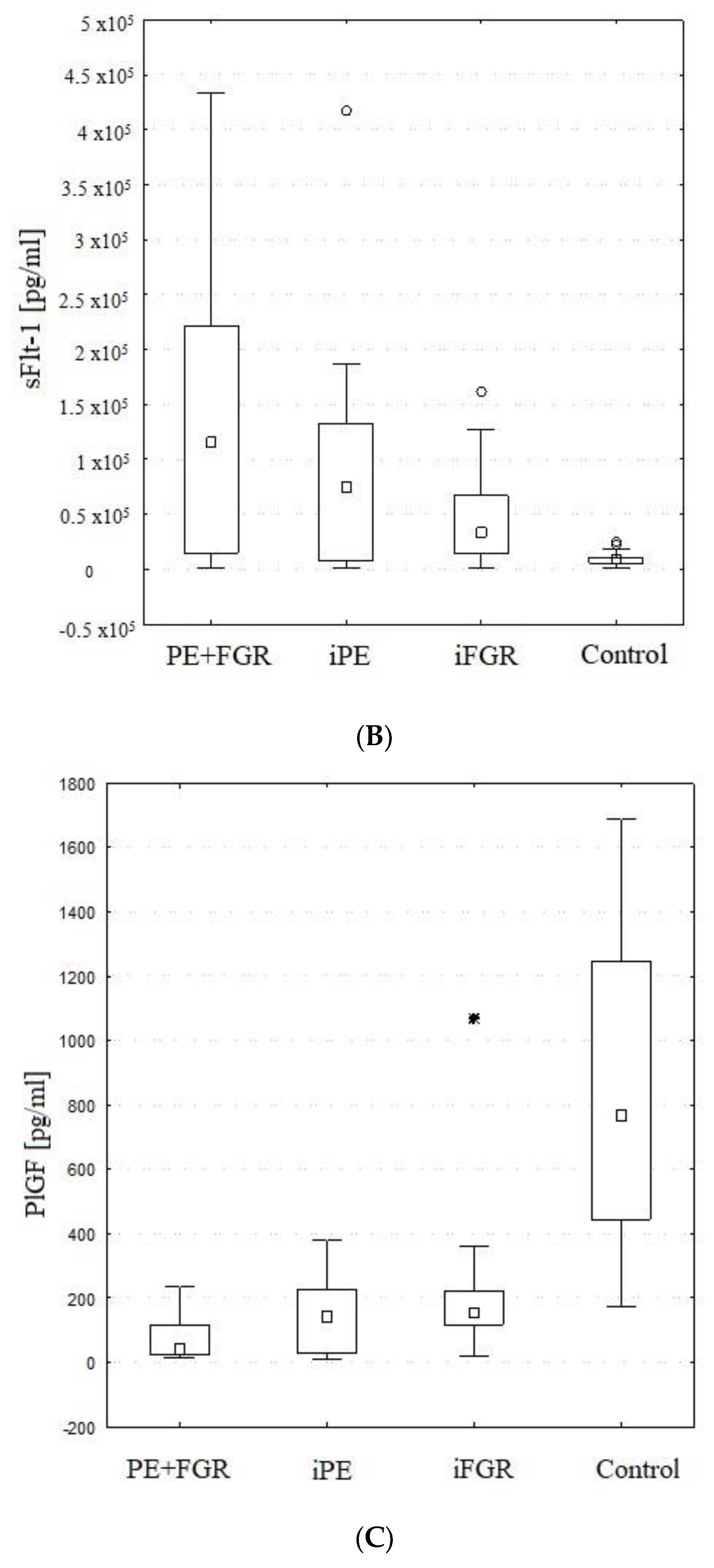
2.2. Maternal Serum PlGF
2.3. Maternal Serum sFlt-1
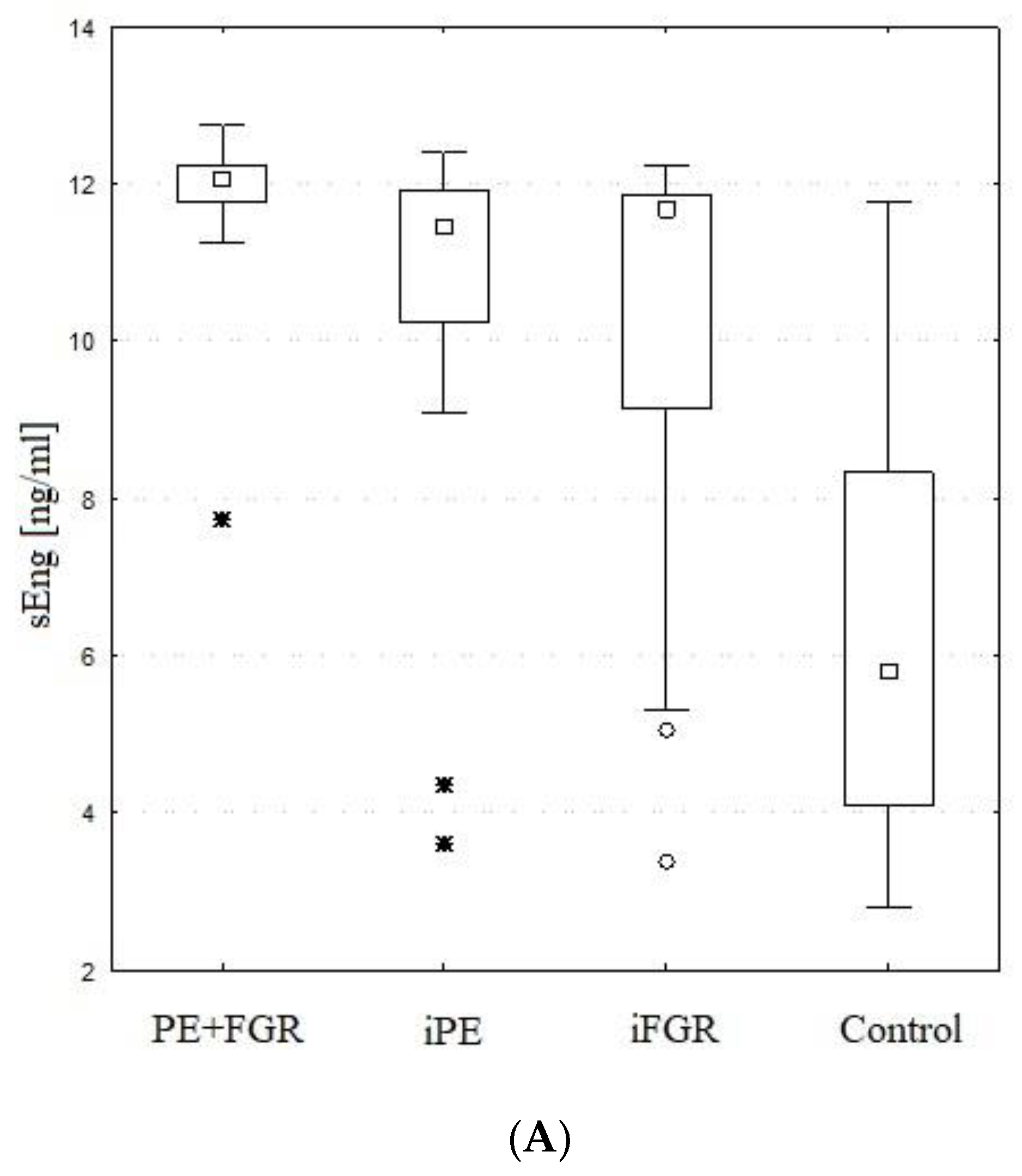
2.4. Maternal Serum sEng
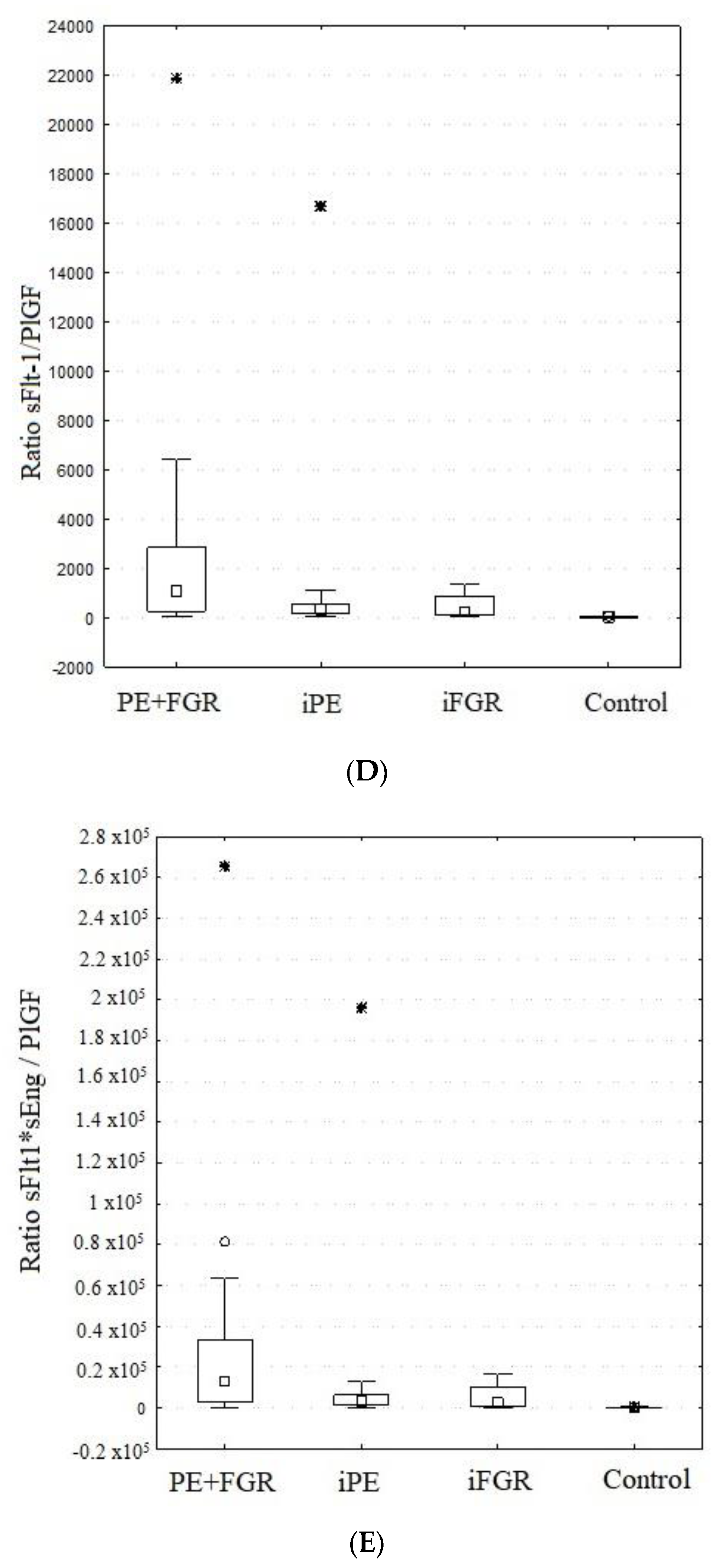
2.5. sFlt-1/PlGF and sFlt-1*sEng/PlGF Ratios
2.6. Adverse Pregnancy Outcomes
| Adverse Outcome (AO) | Total | PE + FGR | iPE | iFGR | |
|---|---|---|---|---|---|
| PATIENTS WITH AO | 23/57 | 10/22 | 11/21 | 2/14 | |
| Ratio > 655 in the AO group | 10/23 | 6/10 | 3/11 | ½ | |
| % with the ratio > 655 in AO group | 44% | 60% | 27% | 50% | |
| sFlT-1/PlGF median value in AO group | 476 | 840 | 446 | 537 | |
| sFlT-1 /PlGF mean value in AO group | 2610 | 3776 | 1926 | 537 | |
| ANY ADVERSE EVENT | 41 (100%) | 24 (59%) | 15 (37%) | 2 (4%) | |
| Maternal | |||||
| 1 | HELLP syndrome | 8 | 5 | 3 | 0 |
| 2 | Placental abruption | 3 | 2 | 0 | 1 |
| 3 | Blood product transfusion | 5 | 3 | 2 | 0 |
| 4 | Neurological symptoms (eclamptic fits, visual disturbances, intense headache) | 9 | 3 | 6 | 0 |
| 5 | Renal oliguria or AKI | 3 | 1 | 2 | 0 |
| 6 | Intravenous antihypertensive therapy with 3rd drug | 9 | 7 | 2 | 0 |
| Fetal | |||||
| 5 | Not viable fetus/ IUD | 4 | 3 | 0 | 1 |
3. Discussion
4. Materials and Methods
4.1. Patients
4.2. Methods
4.3. Statistical Analysis
5. Conclusions
6. Strengths and Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Abalos, E.; Cuesta, C.; Grosso, A.L.; Chou, D.; Say, L. Global and regional estimates of preeclampsia and eclampsia: A systematic review. Eur. J. Obstet. Gynecol. Reprod. Biol. 2013, 170, 1–7. [Google Scholar] [CrossRef]
- Lawn, J.E.; Blencowe, H.; Pattinson, R.; Cousens, S.; Kumar, R.; Ibiebele, I.; Gardosi, J.; Day, L.T.; Stanton, C. Stillbirths: Where? When? Why? How to make the data count? Lancet 2011, 377, 1448–1463. [Google Scholar] [CrossRef]
- Crovetto, F.; Crispi, F.; Scazzocchio, E.; Mercade, I.; Meler, E.; Figueras, F.; Gratacos, E. First-trimester screening for early and late small-for-gestational-age neonates using maternal serum biochemistry, blood pressure and uterine artery Doppler. Ultrasound Obstet. Gynecol. 2013, 43, 34–40. [Google Scholar] [CrossRef]
- Lees, C.C.; Marlow, N.; van Wassenaer-Leemhuis, A.; Arabin, B.; Bilardo, C.M.; Brezinka, C.; Calvert, S.; Derks, J.B.; Diemert, A.; Duvekot, J.J.; et al. 2 year neurodevelopmental and intermediate perinatal outcomes in infants with very preterm fetal growth restriction (TRUFFLE): A randomised trial. Lancet 2015, 385, 2162–2172. [Google Scholar] [CrossRef]
- Shear, R.M.; Rinfret, D.; Leduc, L. Should we offer expectant management in cases of severe preterm preeclampsia with fetal growth restriction? Am. J. Obstet. Gynecol. 2005, 192, 1119–1125. [Google Scholar] [CrossRef]
- Weiler, J.; Tong, S.; Palmer, K.R. Is Fetal Growth Restriction Associated with a More Severe Maternal Phenotype in the Setting of Early Onset Pre-Eclampsia? A Retrospective Study. PLoS ONE 2011, 6, e26937. [Google Scholar] [CrossRef]
- Churchill, D.; Perry, I.J.; Beevers, D. Ambulatory blood pressure in pregnancy and fetal growth. Lancet 1997, 349, 7–10. [Google Scholar] [CrossRef]
- McCowan, L.M.; North, R.A.; Harding, J.E. Abnormal uterine artery Doppler in small-for-gestational-age pregnancies is asso-ciated with later hypertension. Aust. N. Z. J. Obstet. Gynaecol. 2001, 41, 56–60. [Google Scholar] [CrossRef]
- Mitani, M.; Matsuda, Y.; Makino, Y.; Akizawa, Y.; Ohta, H. Clinical features of fetal growth restriction complicated later by preeclampsia. J. Obstet. Gynaecol. Res. 2009, 35, 882–887. [Google Scholar] [CrossRef]
- Figueras, F.; Gratacos, E. An integrated approach to fetal growth restriction. Best Pract. Res. Clin. Obstet. Gynaecol. 2017, 38, 48–58. [Google Scholar] [CrossRef]
- Kwiatkowski, S.; Dołęgowska, B.; Kwiatkowska, E.; Rzepka, R.; Torbè, A.; Bednarek-Jędrzejek, M. A Common Profile of Dis-ordered Angiogenic Factor Production and the Exacerbation of Inflammation in Early Preeclampsia, Late Preeclampsia, and Intrauterine Growth Restriction. PLoS ONE 2016, 11, e0165060. [Google Scholar] [CrossRef]
- Chaiworapongsa, T.; Romero, R.; Whitten, A.E.; Korzeniewski, S.J.; Chaemsaithong, P.; Hernandez-Andrade, E.; Yeo, L.; Hassan, S.S. The use of angiogenic biomarkers in maternal blood to identify which SGA fetuses will require a preterm delivery and mothers who will develop pre-eclampsia. J. Matern. Neonatal Med. 2015, 29, 1214–1228. [Google Scholar] [CrossRef]
- Triunfo, S.; Parra-Saavedra, M.; Rodriguez-Sureda, V.; Crovetto, F.; Dominguez, C.; Gratacós, E.; Figueras, F. Angiogenic Factors and Doppler Evaluation in Normally Growing Fetuses at Routine Third-Trimester Scan: Prediction of Subsequent Low Birth Weight. Fetal Diagn. Ther. 2015, 40, 13–20. [Google Scholar] [CrossRef]
- Herraiz, I.; Droge, L.; Gomez-Montes, E.; Henrich, W.; Galindo, A.; Verlohren, S. Characterization of the soluble fms-like Tyro-sine Kinase-1 to Placental Growth Factor Ratio in Pregnancies Compliated by Fetal Growth Restriction. Obstet. Gynecol. 2014, 124, 265–273. [Google Scholar] [CrossRef]
- Ananth, C.V. Ischemic placental disease: A unifying concept for preeclampsia, intrauterine growth restriction, and placental abruption. Semin. Perinatol. 2014, 38, 131–132. [Google Scholar] [CrossRef]
- Kwiatkowski, S.; Kwiatkowska, E.; Rzepka, R.; Torbe, A.; Dołęgowska, B. Ischemic placental syndrome-Prediction and new disease monitoring. J. Matern. Fetal Neonatal. Med. 2016, 29, 2033–2039. [Google Scholar] [CrossRef]
- Romero, R.; Nien, J.K.; Espinoza, J.; Todem, D.; Fu, W.; Chung, H.; Kusanovic, J.P.; Gotsch, F.; Erez, O.; Mazaki-Tovi, S.; et al. A longitudinal study of angiogenic (placental growth factor) and anti-angiogenic (soluble endoglin and soluble vascular endothelial growth factor receptor-1) factors in normal pregnancy and patients destined to develop preeclampsia and deliver a small for gestational age neonate. J. Matern. Neonatal Med. 2008, 21, 9–23. [Google Scholar] [CrossRef]
- Erez, O.; Romero, R.; Espinoza, J.; Fu, W.; Todem, D.; Kusanovic, J.P.; Gotsch, F.; Edwin, S.; Nien, J.K.; Chaiworapongsa, T.; et al. The change in concentrations of angiogenic and anti-angiogenic factors in maternal plasma between the first and second trimesters in risk assessment for the subsequent development of preeclampsia and small-for-gestational age. J. Matern. Neonatal Med. 2008, 21, 279–287. [Google Scholar] [CrossRef]
- Torry, D.S.; Wang, H.-S.; Wang, T.-H.; Caudle, M.R.; Torry, R.J. Preeclampsia is associated with reduced serum levels of placenta growth factor. Am. J. Obstet. Gynecol. 1998, 179, 1539–1544. [Google Scholar] [CrossRef]
- Reuvekamp, A.; Velsing-Aarts, F.V.; Poulina, I.E.; Capello, J.J.; Duits, A.J. Selectivedeficit of angiogenic growth factors char-acterises pregnancies complicated by pre-eclampsia. Br. J. Obstet Gynaecol. 1999, 106, 1019–1022. [Google Scholar] [CrossRef]
- Tidwell, S.; Ho, H.-N.; Chiu, W.-H.; Torry, R.J.; Torry, D.S. Low maternal serum levels of placenta growth factor as an antecedent of clinical preeclampsia. Am. J. Obstet. Gynecol. 2001, 184, 1267–1272. [Google Scholar] [CrossRef]
- Taylor, R.N.; Grimwood, J.; Taylor, R.S.; McMaster, M.T.; Fisher, S.J.; North, R.A. Longitudinal serum concentrations of pla-cental growth factor: Evidence for abnormal placental angiogenesis in pathologic pregnancies. Am. J. Obstet. Gynecol. 2003, 188, 177–182. [Google Scholar] [CrossRef]
- Levine, R.J.; Maynard, S.E.; Qian, C.; Lim, K.-H.; England, L.J.; Yu, K.F.; Schisterman, E.F.; Thadhani, R.; Sachs, B.P.; Epstein, F.H.; et al. Circulating Angiogenic Factors and the Risk of Preeclampsia. N. Engl. J. Med. 2004, 350, 672–683. [Google Scholar] [CrossRef]
- Chaiworapongsa, T.; Romero, R.; Kim, Y.M.; Kim, G.J.; Kim, M.R.; Espinoza, J.; Bujold, E.; Goncalves, L.; Gómez, R.; Edwin, S.; et al. Plasma soluble vascular endothelial growth factor receptor-1 concentration is elevated prior to the clinical diagnosis of pre-eclampsia. J. Matern. Neonatal Med. 2005, 17, 3–18. [Google Scholar] [CrossRef]
- Park, C.-W.; Park, J.S.; Shim, S.-S.; Jun, J.K.; Yoon, B.H.; Romero, R. An elevated maternal plasma, but not amniotic fluid, soluble fms-like tyrosine kinase-1 (sFlt-1) at the time of mid-trimester genetic amniocentesis is a risk factor for preeclampsia. Am. J. Obstet. Gynecol. 2005, 193, 984–989. [Google Scholar] [CrossRef]
- Levine, R.J.; Lam, C.; Qian, C.; Yu, K.F.; Maynard, S.E.; Sachs, B.P.; Sibai, B.M.; Epstein, F.H.; Romero, R.; Thadhani, R.; et al. Soluble Endoglin and Other Circulating Antiangiogenic Factors in Preeclampsia. N. Engl. J. Med. 2006, 355, 992–1005. [Google Scholar] [CrossRef]
- Unal, E.R.; Robinson, C.J.; Johnson, D.D.; Chang, E.Y. Second-trimester angiogenic factors as biomarkers for future-onset preeclampsia. Am. J. Obstet. Gynecol. 2007, 197, 211.e1–211.e4. [Google Scholar] [CrossRef]
- Kusanovic, J.P.; Romero, R.; Chaiworapongsa, T.; Erez, O.; Mittal, P.; Vaisbuch, E.; Mazaki-Tovi, S.; Gotsch, F.; Edwin, S.S.; Gomez, R.; et al. A prospective cohort study of the value of maternal plasma concentrations of angiogenic and anti-angiogenic factors in early pregnancy and midtrimester in the identification of patients destined to develop preeclampsia. J. Matern. Neonatal Med. 2009, 22, 1021–1038. [Google Scholar] [CrossRef]
- Chaiworapongsa, T.; Romero, R.; Tarca, A.L.; Kusanovic, J.P.; Gotsch, F.; Mittal, P.; Kim, S.K.; Vaisbuch, E.; Mazaki-Tovi, S.; Erez, O.; et al. A decrease in maternal plasma concentrations of sVEGFR-2 precedes the clinical diagnosis of preeclampsia. Am. J. Obstet. Gynecol. 2010, 202, 550.e1–550.e10. [Google Scholar] [CrossRef]
- Verlohren, S.; Stepan, H.; Dechend, R. Angiogenic growth factors in the diagnosis and prediction of pre-eclampsia. Clin. Sci. 2011, 122, 43–52. [Google Scholar] [CrossRef]
- Maynard, S.E.; Min, J.-Y.; Merchan, J.; Lim, K.-H.; Li, J.; Mondal, S.; Libermann, T.A.; Morgan, J.P.; Sellke, F.W.; Stillman, I.E.; et al. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J. Clin. Investig. 2003, 111, 649–658. [Google Scholar] [CrossRef] [PubMed]
- Tsatsaris, V.; Goffin, F.; Munaut, C.; Brichant, J.-F.; Pignon, M.-R.; Noel, A.; Schaaps, J.-P.; Cabrol, D.; Frankenne, F.; Foidart, J.-M. Overexpression of the Soluble Vascular Endothelial Growth Factor Receptor in Preeclamptic Patients: Pathophysiological Consequences. J. Clin. Endocrinol. Metab. 2003, 88, 5555–5563. [Google Scholar] [CrossRef] [PubMed]
- Luttun, A.; Carmeliet, P. Soluble VEGF receptor Flt1: The elusive preeclampsia factor discovered? J. Clin. Investig. 2003, 111, 600–602. [Google Scholar] [CrossRef]
- Koga, K.; Osuga, Y.; Yoshino, O.; Hirota, Y.; Ruimeng, X.; Hirata, T.; Takeda, S.; Yano, T.; Tsutsumi, O.; Taketani, Y. Elevated Serum Soluble Vascular Endothelial Growth Factor Receptor 1 (sVEGFR-1) Levels in Women with Preeclampsia. J. Clin. Endocrinol. Metab. 2003, 88, 2348–2351. [Google Scholar] [CrossRef]
- Chaiworapongsa, T.; Romero, R.; Espinoza, J.; Bujold, E.; Kim, Y.M.; Gonçalves, L.F.; Gomez, R.; Edwin, S. Evidence supporting a role for blockade of the vascular endothelial growth factor system in the pathophysiology of preeclampsia: Young Investigator Award. Am. J. Obstet. Gynecol. 2004, 190, 1541–1547. [Google Scholar] [CrossRef]
- Bujold, E.; Romero, R.; Chaiworapongsa, T.; Kim, Y.M.; Kim, G.J.; Kim, M.R.; Espinoza, J.; Gonçalves, L.F.; Edwin, S.; Mazor, M. Evidence supporting that the excess of the sVEGFR-1 concentration in maternal plasma in preeclampsia has a uterine origin. J. Matern. Neonatal Med. 2005, 18, 9–16. [Google Scholar] [CrossRef]
- Venkatesha, S.; Toporsian, M.; Lam, C.; Hanai, J.-I.; Mammoto, T.; Kim, Y.M.; Bdolah, Y.; Lim, K.-H.; Yuan, H.-T.; Libermann, T.A.; et al. Soluble endoglin contributes to the pathogenesis of preeclampsia. Nat. Med. 2006, 12, 642–649. [Google Scholar] [CrossRef]
- Boutsikou, T.; Malamitsi-Puchner, A.; Economou, E.; Boutsikou, M.; Puchner, K.-P.; Hassiakos, D. Soluble vascular endothelial growth factor receptor-1 in intrauterine growth restricted fetuses and neonates. Early Hum. Dev. 2006, 82, 235–239. [Google Scholar] [CrossRef] [PubMed]
- Crispi, F.; Domínguez, C.; Llurba, E.; Martín-Gallán, P.; Cabero, L.; Gratacos, E. Placental angiogenic growth factors and uterine artery Doppler findings for characterization of different subsets in preeclampsia and in isolated intrauterine growth restriction. Am. J. Obstet. Gynecol. 2006, 195, 201–207. [Google Scholar] [CrossRef]
- Savvidou, M.D.; Yu, C.K.; Harland, L.C.; Hingorani, A.D.; Nicolaides, K.H. Maternal serum concentration of soluble fms-like tyrosine kinase 1 and vascular endothelial growth factor in women with abnormal uterine artery Doppler and in those with fetal growth restriction. Am. J. Obstet. Gynecol. 2006, 195, 1668–1673. [Google Scholar] [CrossRef]
- Schlembach, D.; Wallner, W.; Sengenberger, R.; Stiegler, E.; Mörtl, M.; Beckmann, M.W.; Lang, U. Angiogenic growth factor levels in maternal and fetal blood: Correlation with Doppler ultrasound parameters in pregnancies complicated by pre-eclampsia and intrauterine growth restriction. Ultrasound Obstet. Gynecol. 2007, 29, 407–413. [Google Scholar] [CrossRef]
- Wallner, W.; Sengenberger, R.; Strick, R.; Strissel, P.L.; Meurer, B.; Beckmann, M.W.; Schlembach, D. Angiogenic growth factors in maternal and fetal serum in pregnancies complicated by intrauterine growth restriction. Clin. Sci. 2006, 112, 51–57. [Google Scholar] [CrossRef]
- Chaiworapongsa, T.; Espinoza, J.; Gotsch, F.; Kim, Y.M.; Kim, G.J.; Goncalves, L.F.; Edwin, S.; Kusanovic, J.P.; Erez, O.; Than, N.G.; et al. The maternal plasma soluble vascular endothelial growth factor receptor-1 concentration is elevated in SGA and the magnitude of the increase relates to Doppler abnormalities in the maternal and fetal circulation. J. Matern. Neonatal Med. 2008, 21, 25–40. [Google Scholar] [CrossRef] [PubMed]
- Chaiworapongsa, T.; Romero, R.; Gotsch, F.; Espinoza, J.; Nien, J.K.; Goncalves, L.; Edwin, S.; Kim, Y.M.; Erez, O.; Kusanovic, J.P.; et al. Low maternal concentrations of soluble vascular endothelial growth factor receptor-2 in preeclampsia and small for gestational age. J. Matern. Neonatal Med. 2008, 21, 41–52. [Google Scholar] [CrossRef]
- Åsvold, B.O.; Vatten, L.J.; Romundstad, P.R.; Jenum, P.A.; Karumanchi, S.A.; Eskild, A. Angiogenic Factors in Maternal Circulation and the Risk of Severe Fetal Growth Restriction. Am. J. Epidemiol. 2011, 173, 630–639. [Google Scholar] [CrossRef]
- Sibiude, J.; Guibourdenche, J.; Dionne, M.-D.; Le Ray, C.; Anselem, O.; Serreau, R.; Goffinet, F.; Tsatsaris, V. Placental Growth Factor for the Prediction of Adverse Outcomes in Patients with Suspected Preeclampsia or Intrauterine Growth Restriction. PLoS ONE 2012, 7, e50208. [Google Scholar] [CrossRef]
- Magee, L.A.; von Dadelszen, O.; Rey, E. and the CHIPS Study Group. Less-tight versus tight control of hypertension in preg-nancy. N. Eng. J. Med. 2015, 372, 407–417. [Google Scholar] [CrossRef]
- Spencer, R.N.; Carr, D.J.; David, A.L. Treatment of poor placentation and the prevention of associated adverse outcomes—What does the future hold? Prenat Diagn. 2014, 34, 677–684. [Google Scholar] [CrossRef]
- Marlow, N.; Wolke, D.; Bracewell, M.A.; Samara, M. Neurologic and developmental disability at six years of age after ex-tremely preterm birth. N. Engl. J. Med. 2005, 352, 9–19. [Google Scholar] [CrossRef]
- Brodszki, J.; Morsing, E.; Malcus, P.; Thuring, A.; Ley, D.; Maršál, K. Early intervention in management of very preterm growth-restricted fetuses: 2-year outcome of infants delivered on fetal indication before 30 gestational weeks. Ultrasound Obstet. Gynecol. 2009, 34, 288–296. [Google Scholar] [CrossRef]
- Morsing, E.; Åsard, M.; Ley, D.; Stjernqvist, K.; Maršál, K. Cognitive Function After Intrauterine Growth Restriction and Very Preterm Birth. Pediatrics 2011, 127, e874–e882. [Google Scholar] [CrossRef] [PubMed]
- Brosens, I.; Pijnenborg, R.; Vercruysse, L.; Romero, R. The “Great Obstetrical Syndromes” are associated with disorders of deep placentation. Am. J. Obstet. Gynecol. 2011, 204, 193–201. [Google Scholar] [CrossRef]
- Hadlock, F.P.; Harrist, R.B.; Martinez-Poyer, J. In utero analysis of fetal growth: A sonographic weight standard. Radiology 1991, 181, 129–133. [Google Scholar] [CrossRef] [PubMed]
- Nicolaides, K.; Wright, D.; Syngelaki, A.; Wright, A.; Akolekar, R. Fetal Medicine Foundation fetal and neonatal population weight charts. Ultrasound Obstet. Gynecol. 2018, 52, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Stepan, H.; Herraiz, I.; Schlembach, D.; Verlohren, S.; Brennecke, S.; Chantraine, F.; Klein, E.; Lapaire, O.; Llurba, E.; Ramoni, A.; et al. Implementation of the sFlt-1/PlGF ratio for prediction and diagnosis of pre-eclampsia in singleton pregnancy: Implications for clinical practice. Ultrasound Obstet. Gynecol. 2015, 45, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Herraiz, I.; Llurba, E.; Verlohren, S.; Galindo, A.; on behalf of the Spanish Group for the Study of Angiogenic Markers in Preeclampsia. Update on the Diagnosis and Prognosis of Preeclampsia with the Aid of the sFlt-1/PlGF Ratio in Singleton Pregnancies. Fetal Diagn. Ther. 2017, 43, 81–89. [Google Scholar] [CrossRef]
- Nanjo, S.; Minami, S.; Mizoguchi, M.; Yamamoto, M.; Yahata, T.; Toujima, S.; Shiro, M.; Kobayashi, A.; Muragaki, Y.; Ino, K. Levels of serum-circulating angiogenic factors within 1 week prior to delivery are closely related to conditions of pregnant women with pre-eclampsia, gestational hypertension, and/or fetal growth restriction. J. Obstet. Gynaecol. Res. 2017, 43, 1805–1814. [Google Scholar] [CrossRef]
- Shibata, E.; Rajakumar, A.; Powers, R.W.; Larkin, R.W.; Gilmour, C.; Bodnar, L.M.; Crombleholme, W.R.; Ness, R.B.; Roberts, J.M.; Hubel, C.A. Soluble fms-Like Tyrosine Kinase 1 Is Increased in Preeclampsia But Not in Normotensive Pregnancies with Small-for-Gestational-Age Neonates: Relationship to Circulating Placental Growth Factor. J. Clin. Endocrinol. Metab. 2005, 90, 4895–4903. [Google Scholar] [CrossRef]
- Alahakoon, T.I.; Zhang, W.; Trudinger, B.J.; Lee, V.W. Discordant clinical presentations of preeclampsia and intrauterine fetal growth restriction with similar pro- and anti-angiogenic profiles. J. Matern. Neonatal Med. 2014, 27, 1854–1859. [Google Scholar] [CrossRef]
- Stepan, H.; Krämer, T.; Faber, R. Maternal Plasma Concentrations of Soluble Endoglin in Pregnancies with Intrauterine Growth Restriction. J. Clin. Endocrinol. Metab. 2007, 92, 2831–2834. [Google Scholar] [CrossRef]
- Stepan, H.; Jank, A. Angiogenic factors and their role in pathogenesis and prediction of preeclampsia. Z. Geburtshilfe Neonatol. 2009, 213, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Laskowska, M.; Laskowska, K.; Oleszczuk, J. Endoglin in pregnancy complicated by fetal IUGR in normotensive and preeclamptic pregnant women: A comparison between preeclamptic patients with appropriate-for-gestational-age weight in-fants and healthy pregnant women. J. Matern. Fetal Neonatal. Med. 2012, 25, 806–811. [Google Scholar] [CrossRef] [PubMed]
- Perry, H.; Khalil, A.; Thilaganathan, B. Preeclampsia and the cardiovascular system: An update. Trends Cardiovasc. Med. 2018, 28, 505–513. [Google Scholar] [CrossRef] [PubMed]
- Thilaganathan, B. Pre-eclampsia and the cardiovascular-placental axis. Ultrasound Obstet. Gynecol. 2018, 51, 714–717. [Google Scholar] [CrossRef] [PubMed]
- Craici, I.; Wagner, S.; Garovic, V.D. Review: Preeclampsia and future cardiovascular risk: Formal risk factor or failed stress test? Ther. Adv. Cardiovasc. Dis. 2008, 2, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Duckitt, K.; Harrington, D. Risk factors for pre-eclampsia at antenatal booking: Systematic review of controlled studies. BMJ 2005, 330, 565. [Google Scholar] [CrossRef]
- Roberts, J.M.; Catov, J.M. Preeclampsia more than 1 disease: Or is it? Hypertension 2008, 51, 989–990. [Google Scholar] [CrossRef]
- Friedman, A.M.; Cleary, K.L. Prediction and prevention of ischemic placental disease. Semin. Perinatol. 2014, 38, 177–182. [Google Scholar] [CrossRef]
- Figueras, F.; Gratacos, E. Stage-based approach to the management of fetal growth restriction. Prenat. Diagn. 2014, 34, 655–659. [Google Scholar] [CrossRef]
- Triunfo, S.; Lonmaier, S.; Parra-Saavedra, M.; Crovetto, F.; Nadal, A.; Gratscos, E.; Figureas, F. Angiogenic factors at late-onset small for gestational age and histological placental under perfusion. Placenta 2014, 35, 398e40. [Google Scholar] [CrossRef]
- Gaccioli, F.; Sovio, U.; Cook, E. Screening for fetal growth restriction using ultrasound and the sFLT1/PlGF ratio in nulliparous women: A prospective cohort study. Lancet Child Adolesc. Health 2018, 2, 569–581. [Google Scholar] [CrossRef] [PubMed]
- Quezada, M.S.; Rodríguez-Calvo, J.; Villalaín, C. sFlt-1/PlGF ratio and timing of delivery in early-onset fetal growth restriction with antegrade umbilical artery flow. Ultrasound Obstet. Gynecol. 2020, 56, 549–556. [Google Scholar] [CrossRef]
- Mendoza, M.; Hurtado, I.; Bonacina, E.; Garcia-Manau, P.; Serrano, B.; Tur, H.; Rodo, C.; Maiz, N.; Carreras, E. Individual risk assessment for prenatal counseling in early-onset growth-restricted and small-for-gestational-age fetuses. Acta Obstet. et Gynecol. Scand. 2020, 100, 504–512. [Google Scholar] [CrossRef] [PubMed]
- Di Martino, D.; Cetin, I.; Frusca, T.; Ferrazzi, E.; Fuse, F.; Gervasi, M.T.; Plerbani, M.; Todros, T. Italian advisory board: sFlt-1/ PlGF ratio and preeclampsia, state of the art and developments in diagnostic, therapeutic and clinical management. Eur. J. Obstet. Gynecol. Reprod. Biol. 2016, 206, 70–73. [Google Scholar] [CrossRef] [PubMed]
- Verlohren, S.; Galindo, A.; Schlembach, D.; Zeisler, H.; Herraiz, I.; Moertl, M.G.; Pape, J.; Dudenhausen, J.W.; Denk, B.; Stepan, H. An automated method for the determination of the sFlt-1/PIGF ratio in the assessment of preeclampsia. Am. J. Obstet. Gynecol. 2010, 202, 161. [Google Scholar] [CrossRef]
- Brownbill, P.; McKeeman, G.C.; Brockelsby, J.C.; Crocker, I.P.; Sibley, C.P. Vasoactive and Permeability Effects of Vascular Endothelial Growth Factor-165 in the Term in Vitro Dually Perfused Human Placental Lobule. Endocrinology 2007, 148, 4734–4744. [Google Scholar] [CrossRef]
- Jones, C.J.P.; Fox, H. Ultrastructure of the placenta in prolonged pregnancy. J. Pathol. 1978, 126, 173–179. [Google Scholar] [CrossRef]
- Caughey, A.B.; Stotland, N.E.; Escobar, G.J. What is the best measure of maternal complications of term pregnancy: Ongoing pregnancies or pregnancies delivered? Am. J. Obstet. Gynecol. 2003, 189, 1047e52. [Google Scholar] [CrossRef]
- Verlohren, S.; Herraiz, I.; Lapaire, O.; Schlembach, D.; Moertl, M.; Zeisler, H.; Calda, P.; Holzgreve, W.; Galindo, A.; Engels, T.; et al. The sFlt-1/PlGF ratio in different types of hypertensive pregnancy disorders and its prognostic potential in preeclamptic patients. Am. J. Obstet. Gynecol. 2012, 206, 58. [Google Scholar] [CrossRef]
- Kenneth, L.; Hall, D.R.; Gebhardt, S.; Grové, D. Late Onset Preeclampsia is not an Innocuous Condition. Hypertens. Pregnancy 2010, 29, 262–270. [Google Scholar] [CrossRef]
- Schoofs, K.; Grittner, U.; Engels, T.; Pape, J.; Denk, B.; Henrich, W.; Verlohren, S. The importance of repeated measurements of the sFlt-1/PlGF ratio for the prediction of preeclampsia and intrauterine growth restriction. J. Perinat. Med. 2014, 42, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.A.; Magee, L.A.; Kenny, L.C.; Karumanchi, S.A.; McCarthy, F.P.; Saito, S.; Hall, D.R.; Warren, C.E.; Adoyi, G.; Ishaku, S. The hypertensive disorders of pregnancy: ISSHP classification, diagnosis & management recommendations for international practice. Pregnancy Hypertens. 2018, 13, 291–310. [Google Scholar] [PubMed]
- Gordijn, S.J.; Beune, I.M.; Thilaganathan, B.; Papageorghiou, A.; Baschat, A.A.; Baker, P.N.; Silver, R.M.; Wynia, K.; Ganzevoort, W. Consensus definition of fetal growth restriction: A Delphi procedure. Ultrasound Obstet. Gynecol. 2016, 48, 333–339. [Google Scholar] [CrossRef]
- Melamed, N.; Baschat, A.; Yinon, Y.; Athanasiadis, A.; Mecacci, F.; Figueras, F.; Berghella, V.; Nazareth, A.; Tahlak, M.; McIntyre, H.D.; et al. FIGO (International Federation of Gynecology and Obstetrics) initiative on fetal growth: Best practice advice for screening, diagnosis, and management of fetal growth restriction. Int. J. Gynecol. Obstet. 2021, 152, 3–57. [Google Scholar] [CrossRef]
- Magee, L.A.; von Dadelszen, P.; Singer, J.; Lee, T.; Rey, E.; Ross, S.; Asztalos, E.; Murphy, K.E.; Menzies, J.; Sanchez, J.; et al. The CHIPS Randomized Controlled Trial (Control of Hypertension in Pregnancy Study): Is Severe Hypertension Just an Elevated Blood Pressure? Hypertension 2016, 68, 1153–1159. [Google Scholar] [CrossRef] [PubMed]
- von Dadelszen, P.; Menzies, J.M.; Payne, B.; Magee, L.A.; PIERS (Pre-eclampsia Integrated Estimate of RiSk) Study Group. Predicting adverse outcomes in women with severe pre-eclampsia. Semin. Perinatol. 2009, 33, 152–157. [Google Scholar] [CrossRef] [PubMed]
- Thangaratinam, S.; Allotey, J.; Marlin, N.; Dodds, J.; Cheong-See, F.; von Dadelszen, P.; Ganzevoort, W.; Akkermans, J.; Kerry, S.; Mol, B.W.; et al. PREP Collaborative Network. Prediction of complications in early-onset pre-eclampsia (PREP): Development and external multinational validation of prognostic models. BMC Med. 2017, 15, 68. [Google Scholar] [CrossRef]
- Cheong-See, F.; Allotey, J.; Marlin, N.; Mol, B.; Schuit, E.; Riet, G.; Riley, R.; Moons, K.; Khan, K.; Thangaratinam, S. Prediction models in obstetrics: Understanding the treatment paradox and potential solutions to the threat it poses. BJOG Int. J. Obstet. Gynaecol. 2015, 123, 1060–1064. [Google Scholar] [CrossRef]
- Gómez, O.; Figueras, F.; Fernández, S.; Bennasar, M.; Martínez, J.M.; Puerto, B.; Gratacos, E. Reference ranges for uterine artery mean pulsatility index at 11-41 weeks of gestation. Ultrasound Obstet. Gynecol. 2008, 32, 128–132. [Google Scholar] [CrossRef]
- Ciobanu, A.; Wright, A.; Syngelaki, A.; Wright, D.; Akolekar, R.; Nicolaides, K.H. Fetal Medicine Foundation reference ranges for umbilical artery and middle cerebral artery pulsatility index and cerebroplacental ratio. Ultrasound Obstet. Gynecol. 2019, 53, 465–472. [Google Scholar] [CrossRef]
| PE + FGR | iPE | iFGR | Control | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| I | II | III | IV | |||||||
| Parameter | Median | Q1–Q3 | Median | Q1–Q3 | Median | Q1–Q3 | Median | Q1–Q3 | p-Value | Differences |
| Gravidity | 1 | 1–2 | 1 | 1–2 | 2 | 2–3 | 2 | 1.5–3 | ns | - |
| Parity | 1 | 1–2 | 1 | 1–2 | 2 | 1–2 | 2 | 1–2 | ns | - |
| Gestation age (weeks *) | 32 | 28–34 | 35 | 33–37 | 35 | 33–37 | 34 | 31–37 | ns | I < II |
| Age (years) | 29 | 27–35 | 30 | 27–34 | 33 | 30–37 | 29 | 28–37 | ns | - |
| Height (cm) | 167 | 160–170 | 164 | 160–168 | 167 | 164–171 | 165 | 164–168 | ns | - |
| Weight (kg) | 72 | 66–89 | 80 | 72–92 | 70 | 67–79 | 78 | 68–89 | ns | - |
| I trimester | ||||||||||
| Weight (kg) | 63 | 56–76 | 67 | 61–75 | 60 | 58–69 | 71 | 65–75 | ns | - |
| BMI | 24 | 20–26 | 25 | 23–28 | 23 | 21–26 | 28 | 24–28 | ns | - |
| MAP | 95 | 88–97 | 93 | 83–96 | 88 | 87–92 | 79 | 75–91 | <0.05 | I, I + II > IV |
| DBP (mmHg) | 80 | 76–82 | 77 | 68–80 | 75 | 70–78 | 67 | 62–76 | <0.05 | I, I + II > IV |
| SBP (mmHg) | 120 | 110–130 | 126 | 115–129 | 119 | 116–124 | 111 | 100–120 | <0.05 | I + II > IV |
| PE + FGR | iPE | iFGR | Control | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| I | II | III | IV | |||||||
| Parameter | Median | Q1–Q3 | Median | Q1–Q3 | Median | Q1–Q3 | Median | Q1–Q3 | p-Value | Groups |
| SBP max | 170 | 156–178 | 156 | 150–165 | 127 | 115–134 | 114 | 104–122 | <0.005 | I > II > III > IV |
| DBP max | 104 | 102–111 | 98 | 95–105 | 81.5 | 76–84 | 66 | 62–75 | <0.00005 | I,II > IV |
| MAP | 128 | 121–131 | 117.3 | 114–127 | 95.7 | 92–98.7 | 84 | 76–89 | <0.00005 | I > II > III > IV |
| Proteinuria (mg/24 h) | 1438 | 547–3483 | 668 | 295–1981 | 170 | 138–192 | 0 | 0 | <0.00005 | I,II > IV |
| Total protein (g/dL) | 6.05 | 5.8–6.3 | 5.8 | 5.6–6.3 | 6.35 | 6–6.7 | 6.0 | 5.5–6.2 | ns | - |
| Fibrinogen (g/L) | 4.4 | 3.7–5 | 5.2 | 4.3–5.6 | 4.7 | 4–5.2 | 3.85 | 3.5–4.2 | <0.05 | II > IV |
| INR | 0.9 | 0.86–0.93 | 0.9 | 0.9–0.98 | 0.92 | 0.9–0.97 | 1.0 | 1–1 | <0.00005 | I,II,III < IV |
| PT Index(%) | 109 | 105–114 | 106 | 100–110 | 105.3 | 102–110 | 98 | 96–100 | <0.005 | I,II,III > IV |
| PT (s) | 10 | 10.4–9.6 | 10.3 | 10.9–9.9 | 10.4 | 10.7–9.9 | 11.1 | 1.1–10.9 | <0.005 | I,II,III < IV |
| APTT (s) | 28.8 | 26.6–29.6 | 26.5 | 25.6–27.3 | 27.4 | 26.2–29.6 | 26.9 | 25.9–29.4 | ns | - |
| D-dimers (ng/mL) | 1277 | 1050–1800 | 1362 | 1140–1731 | 1306 | 951–1670 | 1359 | 923–2389 | ns | - |
| WBC (×109/L) | 10.3 | 8.8–11.4 | 9.8 | 9–12.4 | 9.4 | 7.5–11.4 | 9.05 | 8.4–10.9 | ns | - |
| RBC (×1012/L) | 4.16 | 3.95–4.34 | 4.0 | 3.8–4.3 | 4.2 | 4–4.3 | 4.0 | 3.7–4.2 | ns | - |
| Hb | 12.5 | 11.8–13.5 | 12.2 | 11.4–13.2 | 12.6 | 12–13.1 | 12.25 | 11.5–12.7 | ns | - |
| HCT (%) | 37 | 34.3–38.8 | 34.9 | 33.7–38.5 | 36.8 | 35.6–37.9 | 35.3 | 33.9–37.2 | ns | - |
| PLT (×109/L) | 192 | 147–220 | 189 | 144–213 | 207 | 178–253 | 220 | 179–263 | ns | - |
| ALT (U/L) | 28.5 | 21–76 | 24.5 | 18–46 | 16 | 14–28 | 17 | 12–18 | <0.005 | I,II > IV |
| AST (U/L) | 42 | 30–69 | 30 | 25–41 | 23 | 20–28 | 20 | 15–21 | <0.00005 | I,II > IV |
| Creatinine (mg/dL) | 0.7 | 0.6–0.8 | 0.7 | 0.6–0.8 | 0.65 | 0.5–0.7 | 0.5 | 0.5–0.6 | <0.05 | I, I + II > IV |
| UA (mg/dL) | 7.2 | 6.7–8.2 | 6.5 | 5.5–7.2 | 5.5 | 3.95–6.6 | 4.2 | 3.2–4.6 | <0.00005 | I,II > IV |
| Urea | 31 | 26.3–42.1 | 24.5 | 20–35 | 19 | 19–26 | 15.6 | 13.7–17 | <0.00005 | I,II > IV |
| PE + FGR | iPE | iFGR | Control | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| I | II | III | IV | |||||||
| Parameter | Median | Q1–Q3 | Median | Q1–Q3 | Median | Q1–Q3 | Median | Q1–Q3 | p | Differences |
| UtPI mean | 1.6 | 1.4–2.0 | 1.0 | 1–1 | 1.6 | 1.3–2 | 0.7 | 0.65–0.75 | <0.0005 | I,III > IV |
| Ut PI pc | 100 | 99–100 | 91 | 85–96 | 100 | 100–100 | 52 | 42–62 | <0.0005 | I,III > IV |
| UA PI | 1.3 | 1–2.42 | 1.0 | 0.9–1 | 1.2 | 1–1.7 | 0.8 | 0.65–0.87 | <0.00005 | I,II,III > IV |
| UA PI pc | 84 | 53–100 | 73 | 59–82 | 96 | 70–100 | 11 | 3–46 | <0.00005 | I,II,III > IV |
| UA RI | 0.8 | 0.6–1.0 | 0.6 | 0.6–0.7 | 0.7 | 0.6–0.8 | 0.6 | 0.49–0.58 | <0.0001 | I,III > IV |
| MCA PI | 1.2 | 1.1–1.7 | 1.6 | 1.5–2 | 1.3 | 1.3–1.5 | 1.6 | 1.2–1.7 | <0.05 | I < II |
| MCA PI pc | 1 | 1–23 | 32 | 12–46 | 3 | 1–15 | 21 | 8–35 | <0.05 | I < II |
| CPR | 1.2 | 0.6–1.6 | 1.6 | 1.5–1.8 | 1 | 0.7–1.6 | 2.0 | 1.7–2.2 | <0.0005 | I,III < IV |
| CPR pc | 1 | 1–16 | 19 | 6–35 | 1 | 1–13 | 51 | 30–83 | <0.0005 | I,III < IV |
| AFI | 6.5 | 3–10 | 10 | 8–14 | 10 | 7.5–11 | 11 | 9–14 | <0.05 | I < II |
| EFW | 1326 | 708–1714 | 2760 | 2167–3173 | 1915 | 1464–2255 | 2607 | 1773–3351 | <0.00005 | I < IV |
| EFW pc | 1 | 1–2 | 56 | 33–83 | 2 | 1–5 | 64 | 43–87 | <0.00005 | I,III < IV |
| AC | 242 | 217–260 | 323 | 299–336 | 281 | 245–286 | 304 | 260–342 | <0.0005 | I < IV |
| AC pc | 1 | 1–5 | 58 | 48–74 | 4 | 1–7 | 57 | 40–81 | <0.00005 | I,III < IV |
| PE + FGR | iPE | iFGR | Control | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| I | II | III | IV | |||||||
| Parameter | Median | Q1–Q3 | Median | Q1–Q3 | Median | Q1–Q3 | Median | Q1–Q3 | p | Differences |
| Gestational age at birth (weeks) | 32 | 28–34 | 35 | 33–37 | 35 | 33–37 | 34 | 37–39 | ns | - |
| Birth weight (g) | 1370 | 680–1700 | 2500 | 1980–2980 | 1985 | 1480–2320 | 3340 | 3170–3520 | <0.00005 | I,II,III < IV |
| Birth percentile Hadlock * 1991 | 1 | 1–2 | 33 | 18–81 | 3.5 | 1–8 | 61 | 37–77 | <0.00005 | I,III < IV |
| Birth percentile Akolekar ** 2018 | 1 | 1–1 | 46 | 22–86 | 3 | 1–7 | 70 | 47–87 | <0.00005 | I,III < IV |
| Apgar 1 minute | 7 | 6–8 | 8 | 7–10 | 8 | 7–10 | 10 | 9–10 | <0.0005 | I,II < IV |
| Apgar 5 min | 7.5 | 6–9 | 9 | 8–10 | 8 | 8–10 | 10 | 9.5–10 | <0.00005 | I < IV |
| Group | M | Me | Q1 | Q3 | SD | H, p | |
|---|---|---|---|---|---|---|---|
| sEng [ng/mL] | (I) PE + FGR | 11.9 | 12.1 | 11.8 | 12.2 | 1.0 | H = 34.598 |
| (II) iPE | 10.5 | 11.5 | 10.2 | 11.9 | 2.4 | p < 0.001 | |
| (III) iFGR | 9.9 | 11.7 | 9.2 | 11.9 | 3.0 | I > IV, II > IV, III > IV | |
| (IV) Control | 6.4 | 5.8 | 4.1 | 8.3 | 2.9 | ||
| PIGF [pg/mL] | (I) PE + FGR | 72 | 42 | 22 | 113 | 62 | H = 42.603 |
| (II) iPE | 149 | 142 | 27 | 227 | 118 | p < 0.001 | |
| (III) iFGR | 216 | 154 | 117 | 221 | 261 | I > IV, II > IV, III > IV | |
| (IV) Control | 851 | 769 | 444 | 1248 | 480 | ||
| sFlt-1 [pg/mL] | (I) PE + FGR | 129,263 | 115,702 | 14,981 | 221,278 | 123,234 | H = 14.510 |
| (II) iPE | 87,234 | 76,345 | 8614 | 133,888 | 99,327 | p = 0.002 | |
| (III) iFGR | 51,193 | 33,590 | 13,871 | 66,994 | 49,647 | I > IV, II > IV | |
| (IV) Control | 9787 | 8878 | 5574 | 10,809 | 6416 | ||
| RATIO sFlt-1/PlGF | (I) PE + FGR | 2577 | 1072 | 250 | 2833 | 4638 | H = 46.100 |
| (II) iPE | 1181 | 314 | 143 | 547 | 3567 | p < 0.001 | |
| (III) iFGR | 408 | 219 | 81 | 846 | 438 | I > IV, II > IV, III > IV | |
| (IV) Control | 18 | 10 | 5 | 24 | 17 | ||
| RATIO sFlt-1*sEng/PlGF | (I) PE + FGR | 31,549 | 12,859 | 3013 | 33,345 | 56,617 | H = 44.965 |
| (II) iPE | 13,832 | 3251 | 1492 | 6418 | 41,999 | p < 0.001 | |
| (III) iFGR | 4727 | 2587 | 646 | 10,109 | 5350 | I > IV, II > IV, III > IV | |
| (IV) Control | 142 | 53 | 24 | 187 | 191 |
| Ratio sFlt-1/PlGF | PE + FGR (n = 22) | iPE (n = 21) | iFGR (n = 14) | Control (n = 20) | |
|---|---|---|---|---|---|
| Mean/Median | 2577/1072 | 1181/314 | 408/218 | 18/10 | |
| Low | 5% | 0 | 0 | 85% | |
| <38 | 1 (5%) | 0 | 1 (7%) | 17 (85%) | |
| Intermediate | 9% | 24% | 29% | 15% | |
| 38–85 | <34 w | 2 (9%) | 1 (5%) | 0 | 0 |
| 38–110 | >34 w | 0 | 4 (19%) | 4 (29%) | 3 (15%) |
| High | 14% | 14% | 0 | 0 | |
| >85 | <34 w | 3 (14%) | 3 (14%) | 0 | 0 |
| >110 | >34 w | 0 | 0 | 0 | 0 |
| Very high | 16 (73%) | 13 (65%) | 9 (64%) | 0 | |
| >655 | <34 w | 10 (45%) | 3 (14%) | 4 (29%) | 0 |
| >201 | >34 w | 6 (27%) | 10 (48%) | 5 (36%) | 0 |
| Mean | Median | Q1 | Q3 | SD | ||
|---|---|---|---|---|---|---|
| sEng [ng/mL] | adverse | 11.6 | 11.8 | 11.3 | 12.2 | 1 |
| non adverse | 10.4 | 11.8 | 9.8 | 12.1 | 2.8 | |
| PIGF [pg/mL] | adverse | 107 | 58 | 25 | 186 | 100 |
| non adverse | 156 | 123 | 26 | 190 | 189 | |
| sFlt-1 [pg/mL] | adverse | 108,727 | 77,932 | 6201 | 156,292 | 124,261 |
| non adverse | 82,512 | 48,260 | 13,052 | 135,395 | 87,355 | |
| Ratio sFlt-1/PlGF | adverse | 2610 | 476 | 130 | 1032 | 5544 |
| non adverse | 800 | 322 | 143 | 1090 | 982 | |
| Ratio sFlt*sEng/PlGF | adverse | 31,599 | 5876 | 1560 | 12,714 | 66,852 |
| non adverse | 9527 | 3423 | 1493 | 12,996 | 12,084 | |
| Gestational age [weeks] | adverse | 31 | 32 | 27 | 35 | 4.8 |
| non adverse | 35 | 35 | 33 | 37 | 2.7 |
| Early PE <34 Weeks | Late PE ≥34 Weeks | p Value | |||
|---|---|---|---|---|---|
| n = 22 | n = 21 | ||||
| Median | Q1–Q3 | Median | Q1–Q3 | ||
| Gestational age [weeks] | 31.5 | 27–32 | 36 | 35–37 | 0.0000001 |
| PlGF pg/mL [pg/mL] | 33 | 21–125 | 124 | 36–238 | 0.01 |
| sFlt-1 [pg/mL] | 104,310 | 3152–172,024 | 74,759 | 14,981–135,395 | NS |
| sEng [ng/mL] | 12 | 11.6–12.2 | 11.7 | 10.2–12.1 | NS |
| sFlt-1/PlGF | 973 | 228–2247 | 364 | 228–960 | NS |
| Early FGR <34 Weeks | Late FGR ≥34 Weeks | p Value | |||
|---|---|---|---|---|---|
| n = 5 | n = 9 | ||||
| Median | Q1–Q3 | Median | Q1–Q3 | ||
| Gestational age [weeks] | 31 | 27–32 | 36 | 35–37 | 0.0000001 |
| PlGF [pg/mL] | 119 | 117–150 | 172 | 143–221 | 0.01 |
| sFlt-1 [pg/mL] | 115,490 | 56,238–127,053 | 29,183 | 13,051–36,878 | <0.05 |
| sEng [ng/mL] | 11.7 | 11.6–12 | 10.9 | 9.2–11.8 | NS |
| sFlt-1/PlGF | 972 | 845–984 | 212 | 80–222 | 0.01 |
| Preeclampsia |
| Preeclampsia is gestational hypertension accompanied by ≥1 of the following new-onset conditions at or after 20 weeks’ gestation: |
| Proteinuria |
| Other maternal organ dysfunction, including: |
| AKI (creatinine ≥ 90 umol/L; 1 mg/dL) |
| Liver involvement (elevated transaminases, e.g., alanine aminotransferase or aspartate aminotransferase >40 IU/L) with or without right upper quadrant or epigastric abdominal pain |
| Neurological complications (examples include eclampsia, altered mental status, blindness, stroke, clonus, severe headaches, and persistent visual scotomata) |
| Hematological complications (thrombocytopenia–platelet count <150,000/μL, disseminated intravascular coagulation, hemolysis) |
| Uteroplacental dysfunction (such as fetal growth restriction, abnormal umbilical artery [UA] Doppler wave form analysis, or stillbirth) |
| Early FGR: GA <32 weeks, in absence of congenital anomalies | Late FGR: GA ≥32 weeks, in absence of congenital anomalies |
| AC/EFW < 3rd centile or UA-AEDF Or 1. AC/EFW < 10th centile combined with 2. UtA-PI > 95th centile and/or 3. UA-PI >95th centile | AC/EFW < 3rd centile Or at least two out of three of the following 1. AC/EFW < 10th centile 2. AC/EFW crossing centiles >2 quartiles on growth centiles * 3. CPR < 5th centile or UA-PI > 95th centile |
| Ratio sFlt-1/PlGF (EP/LP) | Interpretation | Time to Delivery (ep) | What Should Be Done? |
|---|---|---|---|
| LOW: <38 | Rule out PE: 1 week: NPV ≈ 99% 4 weeks: NPV ≈ 95% | Unmodified | Reassuring the patient. No further determinations are needed unless new suspicion arises. |
| Intermediate: 38–85/38–110 | Rule in PE: 4 weeks PPV ≈ 40% | 20% remain pregnant after 1 month | Follow up visit and retest in 1–2 weeks. Maternal education about signs and symptoms of PE |
| High: >85/>110 | Diagnosis of PE (or PD-related disorder) is highly likely | 15% remain pregnant after 2 weeks | Follow up visit and retest in 2–4 days. EP: consider referral to higher-level center. LP: consider lowering the threshold for labour induction. |
| Very high: >655/>201 | Short-term complications and need to deliver are highly likely | 30% remain pregnant after 2 days | Close surveillance. EP: corticosteroids to the mother for fetal lung maturation |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dymara-Konopka, W.; Laskowska, M.; Grywalska, E.; Hymos, A.; Błażewicz, A.; Leszczyńska-Gorzelak, B. Similar Pro- and Antiangiogenic Profiles Close to Delivery in Different Clinical Presentations of Two Pregnancy Syndromes: Preeclampsia and Fetal Growth Restriction. Int. J. Mol. Sci. 2023, 24, 972. https://doi.org/10.3390/ijms24020972
Dymara-Konopka W, Laskowska M, Grywalska E, Hymos A, Błażewicz A, Leszczyńska-Gorzelak B. Similar Pro- and Antiangiogenic Profiles Close to Delivery in Different Clinical Presentations of Two Pregnancy Syndromes: Preeclampsia and Fetal Growth Restriction. International Journal of Molecular Sciences. 2023; 24(2):972. https://doi.org/10.3390/ijms24020972
Chicago/Turabian StyleDymara-Konopka, Weronika, Marzena Laskowska, Ewelina Grywalska, Anna Hymos, Anna Błażewicz, and Bożena Leszczyńska-Gorzelak. 2023. "Similar Pro- and Antiangiogenic Profiles Close to Delivery in Different Clinical Presentations of Two Pregnancy Syndromes: Preeclampsia and Fetal Growth Restriction" International Journal of Molecular Sciences 24, no. 2: 972. https://doi.org/10.3390/ijms24020972
APA StyleDymara-Konopka, W., Laskowska, M., Grywalska, E., Hymos, A., Błażewicz, A., & Leszczyńska-Gorzelak, B. (2023). Similar Pro- and Antiangiogenic Profiles Close to Delivery in Different Clinical Presentations of Two Pregnancy Syndromes: Preeclampsia and Fetal Growth Restriction. International Journal of Molecular Sciences, 24(2), 972. https://doi.org/10.3390/ijms24020972








