Genome-Wide Identification and Characterization of the Trehalose-6-Phosphate Synthetase Gene Family in Chinese Cabbage (Brassica rapa) and Plasmodiophora brassicae during Their Interaction
Abstract
1. Introduction
2. Results
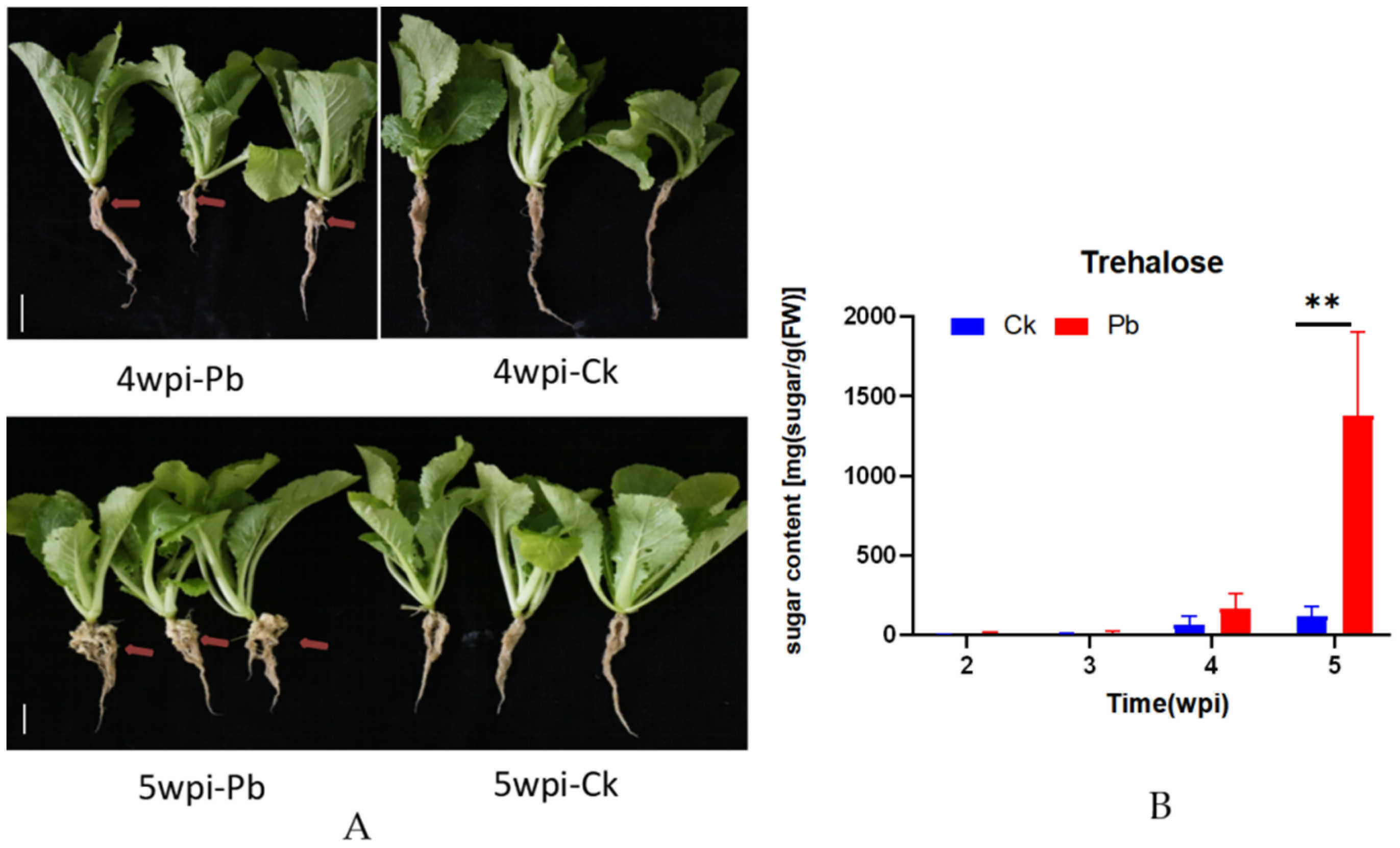
2.1. Trehalose Sugar Content in Cabbage Roots after Infection with P. brassicae
2.2. Identification of TPS Family Members in B. rapa and P. brassicae
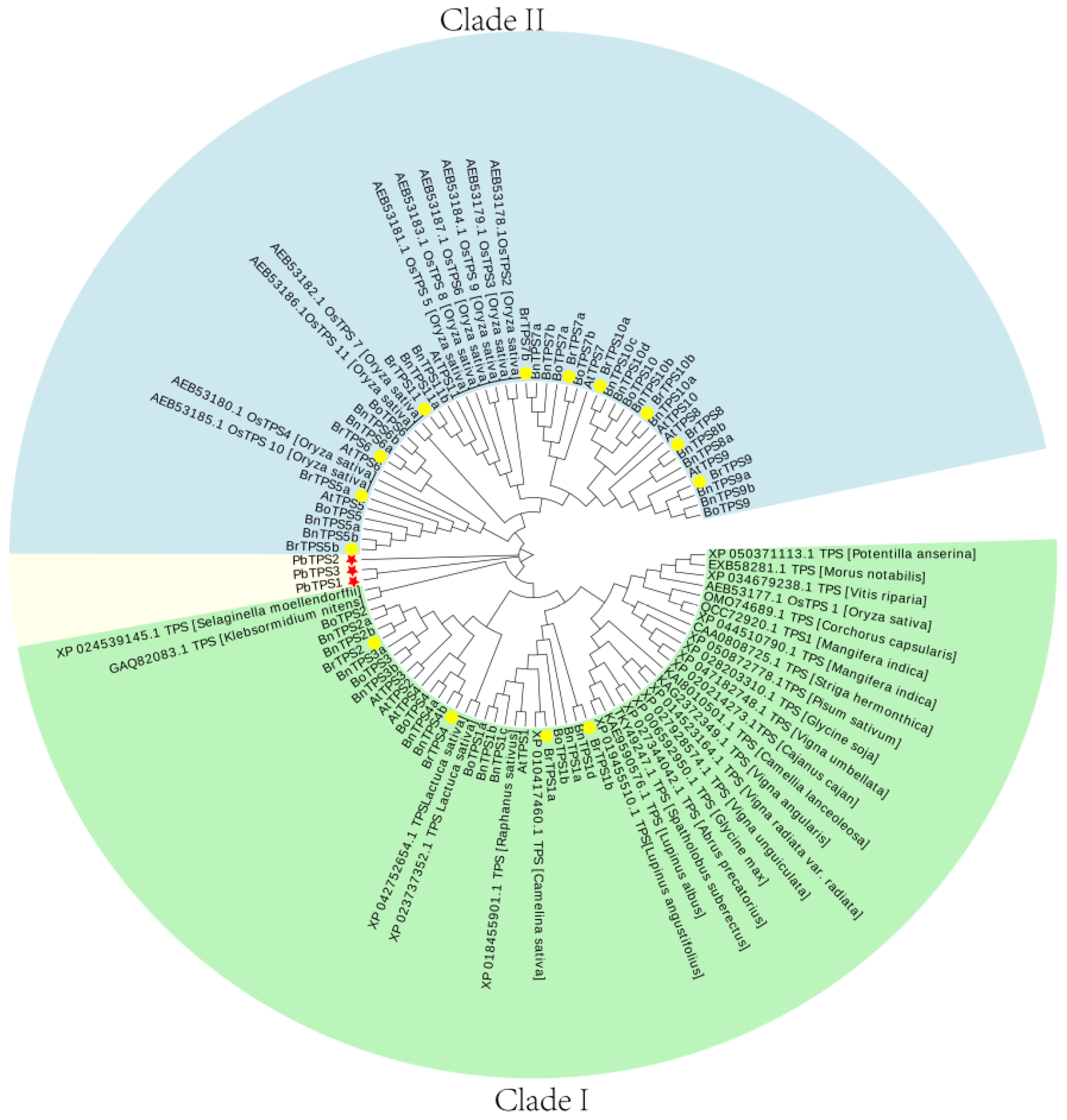
2.3. Phylogenetic Analysis of BrTPSs and PbTPSs
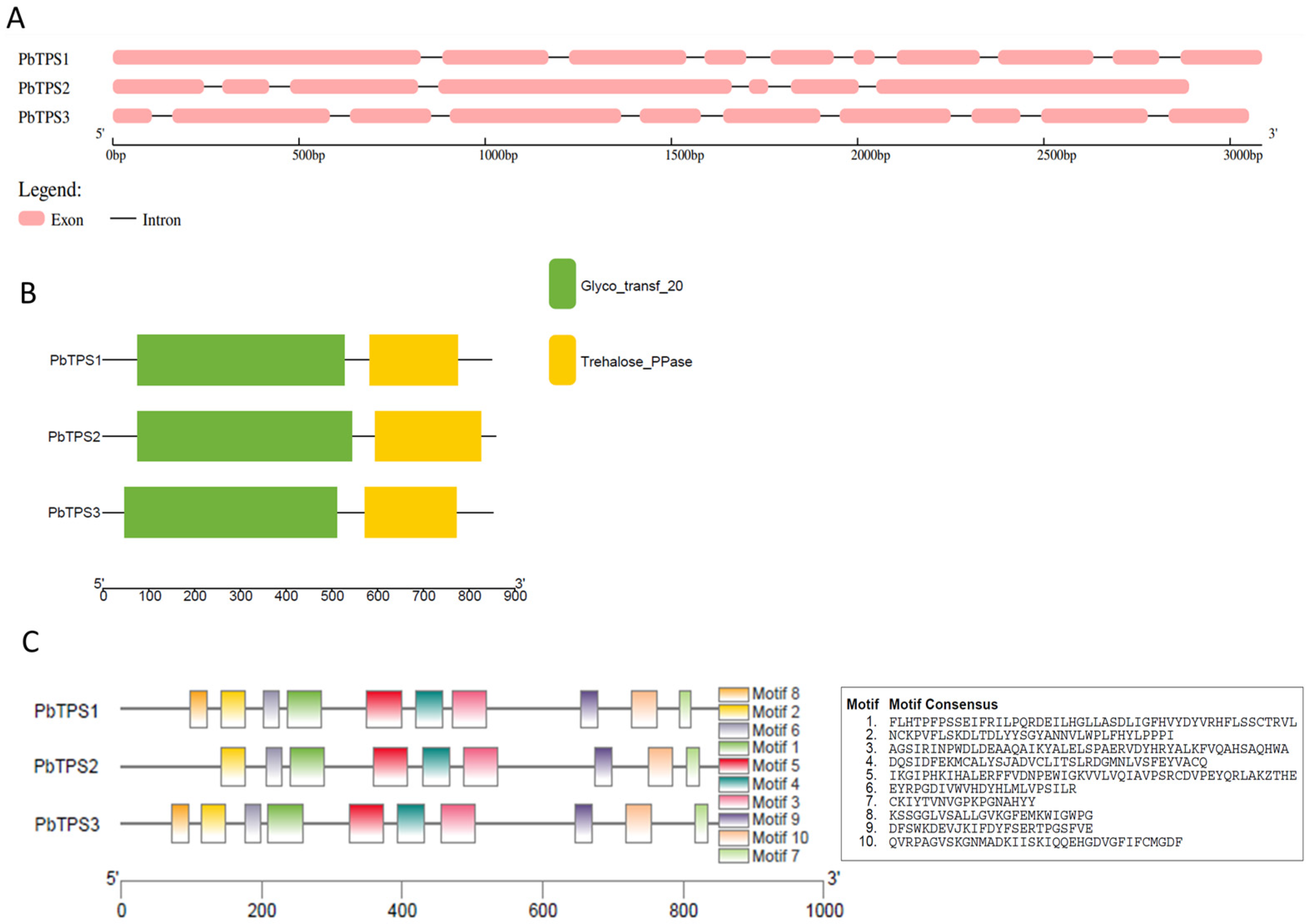
2.4. Gene Structure and Conserved Domain Analyses of BrTPSs and PbTPSs
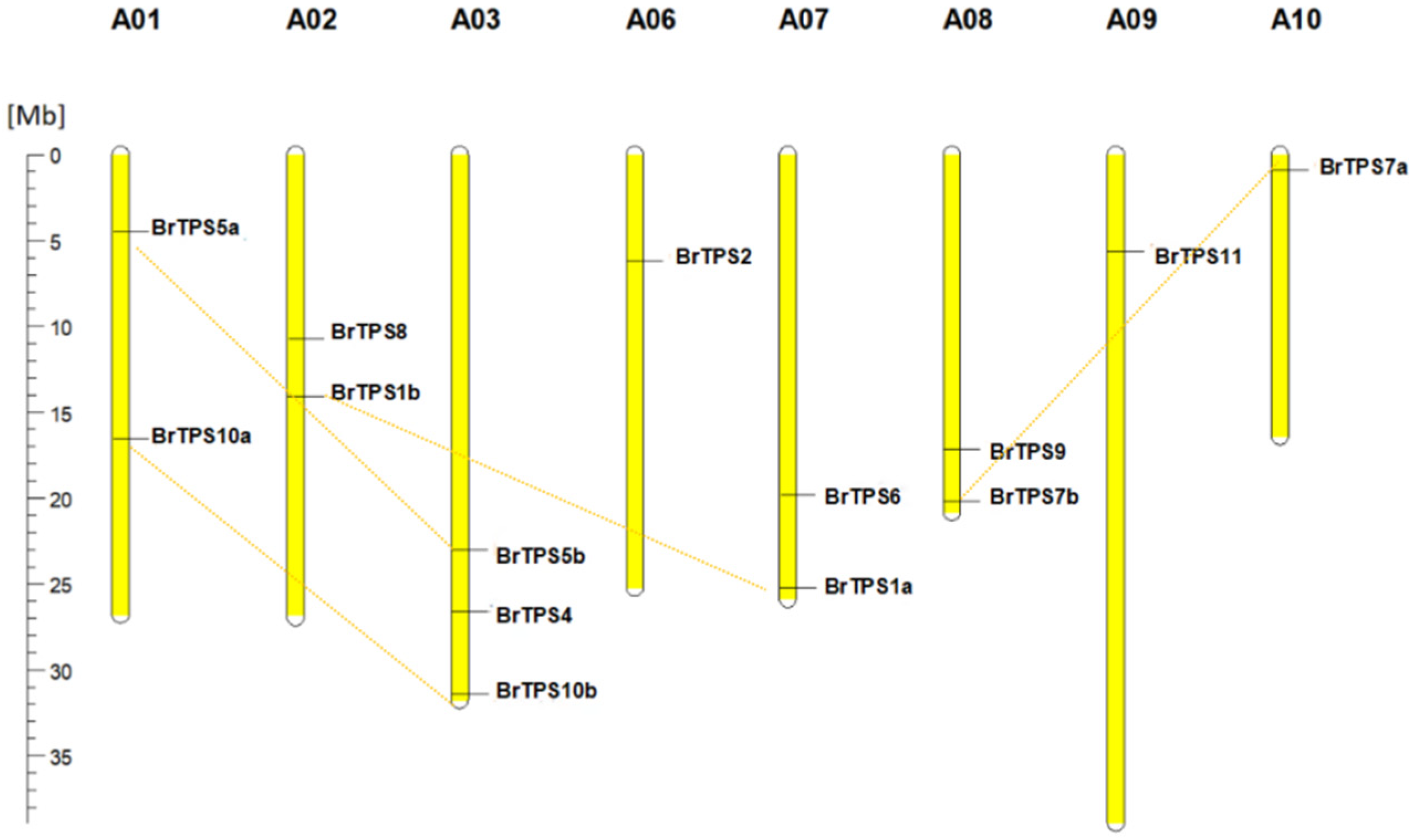
2.5. Chromosomal Location of BrTPS Genes
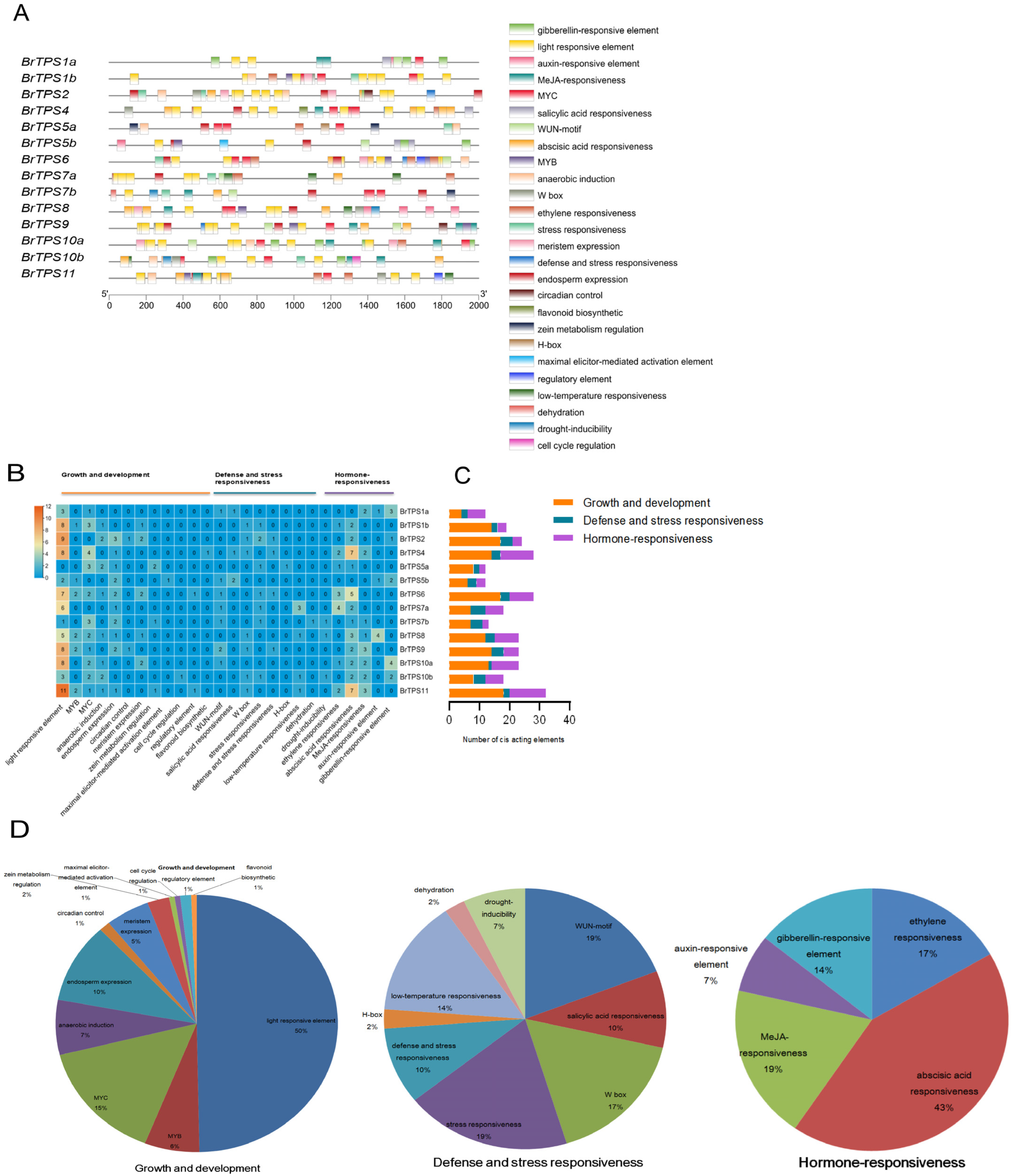
2.6. Identification of Cis-Acting Elements in the Promoter Region of BrTPS Genes
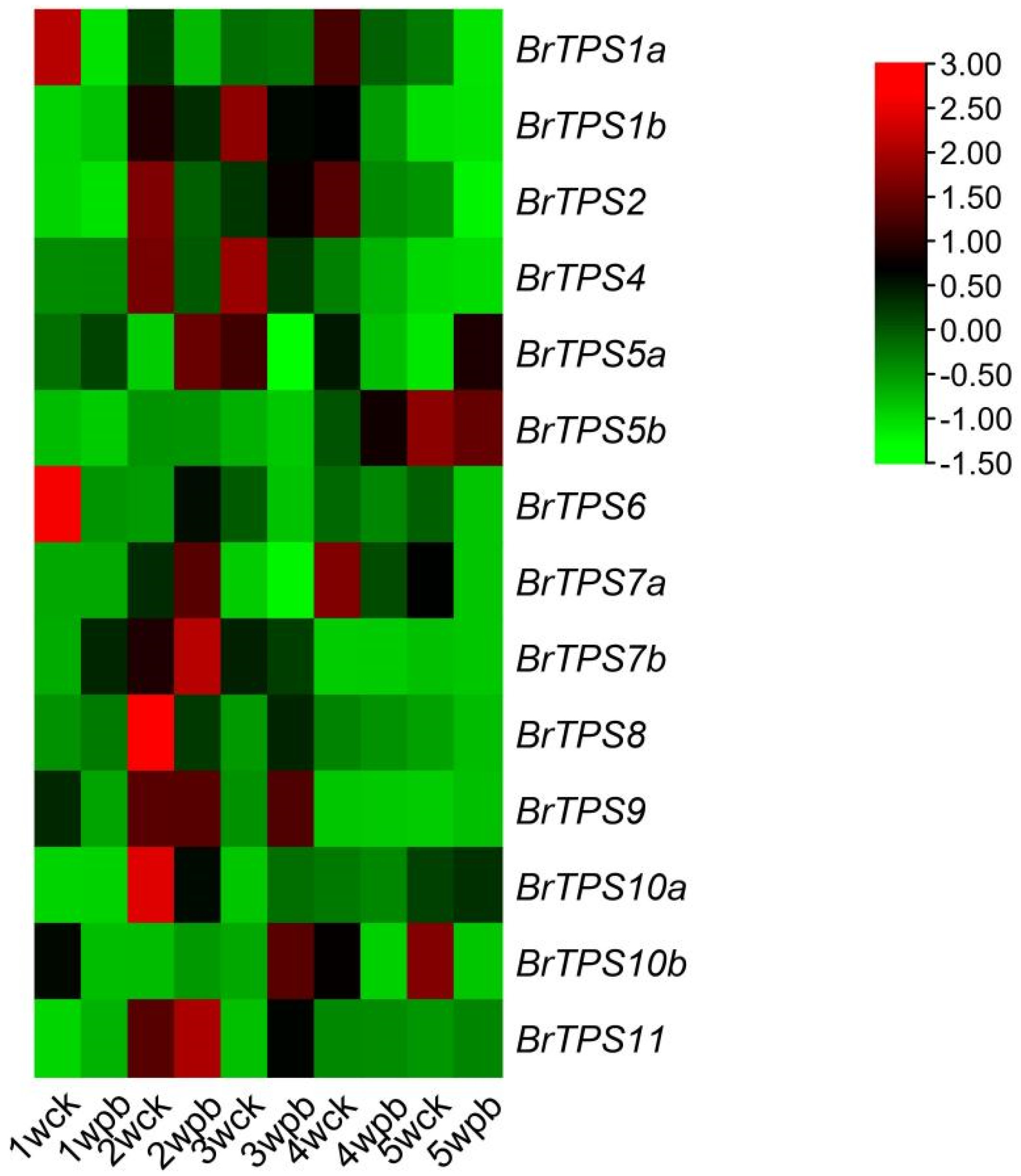
2.7. Expression of BrTPS Genes under P. brassicae Infection
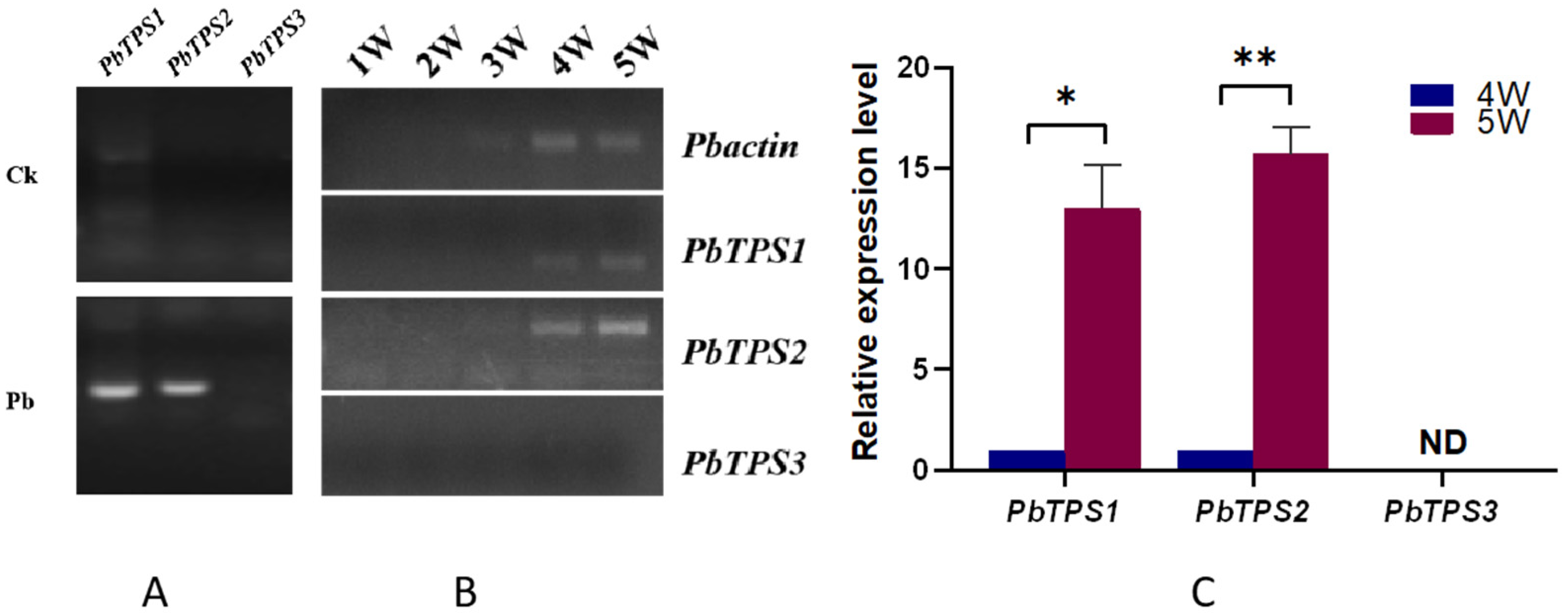
2.8. Expression Analyses of PbTPS Genes
3. Discussion
4. Materials and Methods
4.1. Plant Material and P. brassicae Inoculation
4.2. Soluble Sugar Extraction and Trehalose Content Determination
4.3. Identification of TPS Genes in B. rapa and P. brassicae
4.4. BrTPS and PbTPS Phylogenetic Evolution Analysis
4.5. Analysis of BrTPS and PbTPS Gene Structures and Conserved Motifs
4.6. Chromosomal Location and Cis-Acting Element Analysis of BrTPS Genes
4.7. Total RNA Extraction and Quantitative Real-Time PCR
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jin, Q.; Hu, X.; Li, X.; Wang, B.; Wang, Y.; Jiang, H.; Mattson, N.; Xu, Y. Genome-Wide Identification and Evolution Analysis of Trehalose-6-Phosphate Synthase Gene Family in Nelumbo nucifera. Front Plant Sci. 2016, 7, 1445. [Google Scholar] [CrossRef]
- Park, M.; Mitchell, W.J.; Rafii, F. Effect of Trehalose and Trehalose Transport on the Tolerance of Clostridium perfringens to Environmental Stress in a Wild Type Strain and Its Fluoroquinolone-Resistant Mutant. Int. J. Microbiol. 2016, 2016, 4829716. [Google Scholar] [CrossRef] [PubMed]
- Yuan, G.; Liu, J.; An, G.; Li, W.; Si, W.; Sun, D.; Zhu, Y. Genome-Wide Identification and Characterization of the Trehalose-6-phosphate Synthetase (TPS) Gene Family in Watermelon (Citrullus lanatus) and Their Transcriptional Responses to Salt Stress. Int. J. Mol. Sci. 2021, 23, 276. [Google Scholar] [CrossRef]
- Vanaporn, M.; Titball, R.W. Trehalose and bacterial virulence. Virulence 2020, 11, 1192–1202. [Google Scholar] [CrossRef] [PubMed]
- Elbein, A.D.; Pan, Y.T.; Pastuszak, I.; Carroll, D. New insights on trehalose: A multifunctional molecule. Glycobiology 2003, 13, 17r–27r. [Google Scholar] [CrossRef] [PubMed]
- Streeter, J.G.; Bhagwat, A. Biosynthesis of trehalose from maltooligosaccharides in Rhizobia. Can. J. Microbiol. 1999, 45, 716–721. [Google Scholar] [CrossRef] [PubMed]
- Wannet, W.J.; Op den Camp, H.J.; Wisselink, H.W.; van der Drift, C.; Van Griensven, L.J.; Vogels, G.D. Purification and characterization of trehalose phosphorylase from the commercial mushroom Agaricus bisporus. Biochim. Biophys. Acta 1998, 1425, 177–188. [Google Scholar] [CrossRef] [PubMed]
- Schiraldi, C.; Di Lernia, I.; De Rosa, M. Trehalose production: Exploiting novel approaches. Trends Biotechnol. 2002, 20, 420–425. [Google Scholar] [CrossRef] [PubMed]
- Qu, Q.; Lee, S.J.; Boos, W. TreT, a novel trehalose glycosyltransferring synthase of the hyperthermophilic archaeon Thermococcus litoralis. J. Biol. Chem. 2004, 279, 47890–47897. [Google Scholar] [CrossRef]
- Lunn, J.E.; Delorge, I.; Figueroa, C.M.; Van Dijck, P.; Stitt, M. Trehalose metabolism in plants. Plant J. 2014, 79, 544–567. [Google Scholar] [CrossRef]
- Mu, M.; Lu, X.K.; Wang, J.J.; Wang, D.L.; Yin, Z.J.; Wang, S.; Fan, W.-L.; Ye, W.W. Genome-wide Identification and analysis of the stress-resistance function of the TPS (Trehalose-6-Phosphate Synthase) gene family in cotton. BMC Genet. 2016, 17, 1–11. [Google Scholar]
- Tsusaki, K.; Nishimoto, T.; Nakada, T.; Kubota, M.; Chaen, H.; Sugimoto, T.; Kurimoto, M. Cloning and sequencing of trehalose synthase gene from Pimelobacter sp. R48. Biochim. Et Biophys. Acta (BBA)-Gen. Subj. 1996, 1290, 1–3. [Google Scholar] [CrossRef]
- Thammahong, A.; Puttikamonkul, S.; Perfect, J.R.; Brennan, R.G.; Cramer, R.A. Central Role of the Trehalose Biosynthesis Pathway in the Pathogenesis of Human Fungal Infections: Opportunities and Challenges for Therapeutic Development. Microbiol. Mol. Biol. Rev. 2017, 81, e00053-16. [Google Scholar] [CrossRef]
- García, M.D.; Argüelles, J.C. Trehalase inhibition by validamycin A may be a promising target to design new fungicides and insecticides. Pest Manag. Sci. 2021, 77, 3832–3835. [Google Scholar] [CrossRef]
- Tang, B.; Wang, S.; Wang, S.G.; Wang, H.J.; Zhang, J.Y.; Cui, S.Y. Invertebrate Trehalose-6-Phosphate Synthase Gene: Genetic Architecture, Biochemistry, Physiological Function, and Potential Applications. Front. Physiol. 2018, 9, 30. [Google Scholar] [CrossRef]
- Yang, H.L.; Liu, Y.J.; Wang, C.L.; Zeng, Q.Y. Molecular Evolution of Trehalose-6-Phosphate Synthase (TPS) Gene Family in Populus, Arabidopsis and Rice. PLoS ONE 2012, 7, e42438. [Google Scholar] [CrossRef]
- Kaasen, I.; Falkenberg, P.; Styrvold, O.B.; Strøm, A. Molecular cloning and physical mapping of the otsBA genes, which encode the osmoregulatory trehalose pathway of Escherichia coli: Evidence that transcription is activated by katF (AppR). J. Bacteriol. 1992, 174, 3422. [Google Scholar] [CrossRef]
- Vicente, R.L.; Spina, L.; Gómez, J.P.L.; Dejean, S.; Parrou, J.L.; François, J.M. Trehalose-6-phosphate promotes fermentation and glucose repression in Saccharomyces cerevisiae. Microb. Cell 2018, 5, 444–459. [Google Scholar] [CrossRef]
- Chen, Q.; Ma, E.; Behar, K.L.; Xu, T.; Haddad, G.G. Role of Trehalose Phosphate Synthase in Anoxia Tolerance and Development in Drosophila melanogaster. J. Biol. Chem. 2002, 277, 3274–3279. [Google Scholar] [CrossRef]
- Xu, J.; Bao, B.; Zhang, Z.F.; Yi, Y.Z.; Xu, W.H. Identification of a novel gene encoding the trehalose phosphate synthase in the cotton bollworm, Helicoverpa armigera. Glycobiology 2009, 19, 250–257. [Google Scholar] [CrossRef]
- Tang, B.; Chen, J.; Yao, Q.; Pan, Z.; Xu, W.; Wang, S.; Zhang, W. Characterization of a trehalose-6-phosphate synthase gene from Spodoptera exigua and its function identification through RNA interference. J. Insect Physiol. 2010, 56, 813–821. [Google Scholar] [CrossRef]
- Paul, M.J.; Primavesi, L.F.; Jhurreea, D.; Zhang, Y. Trehalose metabolism and signaling. Annu. Rev. Plant Biol. 2008, 59, 417–441. [Google Scholar] [CrossRef]
- Zhao, S.M.; Fu, F.L.; Gou, L.; Wang, H.G.; He, G.; Li, W.C. Cloning and truncation modification of trehalose-6-phosphate synthase gene from Selaginella pulvinata. Gene 2013, 512, 414–421. [Google Scholar] [CrossRef]
- Engelhardt, C.; Petereit, F.; Lechtenberg, M.; Liefländer-Wulf, U.; Hensel, A. Qualitative and quantitative phytochemical characterization of Myrothamnus flabellifolia Welw. Fitoterapia 2016, 114, 69–80. [Google Scholar] [CrossRef]
- Vandesteene, L.; López-Galvis, L.; Vanneste, K.; Feil, R.; Maere, S.; Lammens, W.; Rolland, F.; Lunn, J.E.; Avonce, N.; Beeckman, T.; et al. Expansive Evolution of the TREHALOSE-6-PHOSPHATE PHOSPHATASE Gene Family in Arabidopsis. Plant Physiol. 2012, 160, 884–896. [Google Scholar] [CrossRef]
- Zang, B.; Li, H.; Li, W.; Deng, X.W.; Wang, X. Analysis of trehalose-6-phosphate synthase (TPS) gene family suggests the formation of TPS complexes in rice. Plant Mol. Biol. 2011, 76, 507–522. [Google Scholar] [CrossRef]
- Vandesteene, L.; Ramon, M.; Roy, K.L.; Dijck, P.V.; Rolland, F. A single active trehalose-6-P synthase (TPS) and a family of putative regulatory TPS-like proteins in Arabidopsis. Mol. Plant 2010, 3, 406–419. [Google Scholar] [CrossRef]
- Morabito, C.; Secchi, F.; Schubert, A. Grapevine TPS (trehalose-6-phosphate synthase) family genes are differentially regulated during development, upon sugar treatment and drought stress. Plant Physiol. Biochem. 2021, 164, 54–62. [Google Scholar] [CrossRef]
- Tian, L.; Xie, Z.; Lu, C.; Hao, X.; Wu, S.; Huang, Y.; Li, D.; Chen, L. The trehalose-6-phosphate synthase TPS5 negatively regulates ABA signaling in Arabidopsis thaliana. Plant Cell Rep. 2019, 38, 869–882. [Google Scholar] [CrossRef]
- Eastmond, P.J.; van Dijken, A.J.; Spielman, M.; Kerr, A.; Tissier, A.F.; Dickinson, H.G.; Jones, J.D.; Smeekens, S.C.; Graham, I.A. Trehalose-6-phosphate synthase 1, which catalyses the first step in trehalose synthesis, is essential for Arabidopsis embryo maturation. Plant J. 2002, 29, 225–235. [Google Scholar] [CrossRef]
- Wang, S.; Ouyang, K.; Wang, K. Genome-Wide Identification, Evolution, and Expression Analysis of TPS and TPP Gene Families in Brachypodium distachyon. Plants 2019, 8, 362. [Google Scholar] [CrossRef] [PubMed]
- Avonce, N.; Mendoza-Vargas, A.; Morett, E.; Iturriaga, G. Insights on the evolution of trehalose biosynthesis. BMC Evol. Biol. 2006, 6, 109. [Google Scholar] [CrossRef]
- Wegener, G.; Macho, C.; Schlöder, P.; Kamp, G.; Ando, O. Long-term effects of the trehalase inhibitor trehazolin on trehalase activity in locust flight muscle. J. Exp. Biol. 2010, 213, 3852–3857. [Google Scholar] [CrossRef]
- Pampurova, S.; Verschooten, K.; Avonce, N.; Van Dijck, P. Functional screening of a cDNA library from the desiccation-tolerant plant Selaginella lepidophylla in yeast mutants identifies trehalose biosynthesis genes of plant and microbial origin. J. Plant Res. 2014, 127, 803–813. [Google Scholar] [CrossRef]
- Schluepmann, H.; van Dijken, A.; Aghdasi, M.; Wobbes, B.; Paul, M.; Smeekens, S. Trehalose mediated growth inhibition of Arabidopsis seedlings is due to trehalose-6-phosphate accumulation. Plant Physiol. 2004, 135, 879–890. [Google Scholar] [CrossRef]
- O’Hara, L.E.; Paul, M.J.; Wingler, A. How do sugars regulate plant growth and development? New insight into the role of trehalose-6-phosphate. Mol. Plant 2013, 6, 261–274. [Google Scholar] [CrossRef]
- Argüelles, J.C. Physiological roles of trehalose in bacteria and yeasts: A comparative analysis. Arch. Microbiol. 2000, 174, 217–224. [Google Scholar] [CrossRef]
- Djonović, S.; Urbach, J.M.; Drenkard, E.; Bush, J.; Feinbaum, R.; Ausubel, J.L.; Traficante, D.; Risech, M.; Kocks, C.; Fischbach, M.A.; et al. Trehalose biosynthesis promotes Pseudomonas aeruginosa pathogenicity in plants. PLoS Pathog. 2013, 9, e1003217. [Google Scholar] [CrossRef]
- MacIntyre, A.M.; Barth, J.X.; Pellitteri Hahn, M.C.; Scarlett, C.O.; Genin, S.; Allen, C. Trehalose Synthesis Contributes to Osmotic Stress Tolerance and Virulence of the Bacterial Wilt Pathogen Ralstonia solanacearum. Mol. Plant Microbe. Interact. 2020, 33, 462–473. [Google Scholar] [CrossRef]
- Dixon, G.R. The biology of Plasmodiophora brassicae Wor.—A review of recent advances. Acta Hortic. 2006, 706, 271–282. [Google Scholar] [CrossRef]
- Brodmann, A.; Schuller, A.; Ludwig-Müller, J.; Aeschbacher, R.A.; Wiemken, A.; Boller, T.; Wingler, A. Induction of trehalase in Arabidopsis plants infected with the trehalose-producing pathogen Plasmodiophora brassicae. Mol Plant Microbe Interact. 2002, 15, 693–700. [Google Scholar] [CrossRef]
- Yuan, M.; Wang, S. Rice MtN3/saliva/SWEET family genes and their homologs in cellular organisms. Mol. Plant 2013, 6, 665–674. [Google Scholar] [CrossRef]
- Chen, L.Q.; Cheung, L.S.; Feng, L.; Tanner, W.; Frommer, W.B. Transport of sugars. Annu. Rev. Biochem. 2015, 84, 865–894. [Google Scholar] [CrossRef]
- Doidy, J.; Grace, E.; Kühn, C.; Simon-Plas, F.; Casieri, L.; Wipf, D. Sugar transporters in plants and in their interactions with fungi. Trends Plant Sci. 2012, 17, 413–422. [Google Scholar] [CrossRef]
- Li, H.; Li, X.; Xuan, Y.; Jiang, J.; Wei, Y.; Piao, Z. Genome Wide Identification and Expression Profiling of SWEET Genes Family Reveals Its Role during Plasmodiophora brassicae-Induced Formation of Clubroot in Brassica rapa. Front. Plant Sci. 2018, 9, 207. [Google Scholar] [CrossRef]
- Dan, Y.; Niu, Y.; Wang, C.; Yan, M.; Liao, W. Genome-wide identification and expression analysis of the trehalose-6-phosphate synthase (TPS) gene family in cucumber (Cucumis sativus L.). PeerJ 2021, 9, e11398. [Google Scholar] [CrossRef]
- Chen, Z.; Lou, J. Identification and expression of the trehalose-6-phosphate synthase gene family members in tomato exposed to different light spectra. Arch. Biol. Sci. 2017, 69, 93–101. [Google Scholar] [CrossRef]
- Xu, G.; Guo, C.; Shan, H.; Kong, H. Divergence of duplicate genes in exon-intron structure. Proc. Natl. Acad. Sci. USA 2012, 109, 1187–1192. [Google Scholar] [CrossRef] [PubMed]
- Yuan, G.; He, S.; Bian, S.; Han, X.; Liu, K.; Cong, P.; Zhang, C. Genome-wide identification and expression analysis of major latex protein (MLP) family genes in the apple (Malus domestica Borkh.) genome. Gene 2020, 733, 144275. [Google Scholar] [CrossRef] [PubMed]
- Koralewski, T.E.; Krutovsky, K.V. Evolution of exon-intron structure and alternative splicing. PLoS ONE 2011, 6, e18055. [Google Scholar] [CrossRef]
- Heidari, P.; Puresmaeli, F.; Mora-Poblete, F. Genome-Wide Identification and Molecular Evolution of the Magnesium Transporter (MGT) Gene Family in Citrullus lanatus and Cucumis sativus. Agronomy 2022, 12, 2253. [Google Scholar] [CrossRef]
- van Dijken, A.J.; Schluepmann, H.; Smeekens, S.C. Arabidopsis trehalose-6-phosphate synthase 1 is essential for normal vegetative growth and transition to flowering. Plant Physiol. 2004, 135, 969–977. [Google Scholar] [CrossRef] [PubMed]
- Vishal, B.; Krishnamurthy, P.; Ramamoorthy, R.; Kumar, P.P. OsTPS8 controls yield-related traits and confers salt stress tolerance in rice by enhancing suberin deposition. New Phytol. 2019, 221, 1369–1386. [Google Scholar] [CrossRef] [PubMed]
- Foster, A.J.; Jenkinson, J.M.; Talbot, N.J. Trehalose synthesis and metabolism are required at different stages of plant infection by Magnaporthe grisea. EMBO J. 2003, 22, 225–235. [Google Scholar] [CrossRef]
- Carlos, G.; Carmen-Lisset, F. The importance of a functional trehalose biosynthetic pathway for the life of yeasts and fungi. FEMS Yeast Res. 2010, 4, 351–359. [Google Scholar]
- Clegg, J.S.; Filosa, M.F. Trehalose in the Cellular Slime Mould Dictyostelium mucoroides. Nature 1961, 192, 1077–1078. [Google Scholar] [CrossRef]
- Sussman, A.S.; Lingappa, B.T. Role of Trehalose in Ascospores of Neurospora Tetrasperma. Science 1959, 130, 1343. [Google Scholar] [CrossRef]
- Hwang, S.F.; Strelkov, S.E.; Feng, J.; Gossen, B.D.; Howard, R.J. Plasmodiophora brassicae: A review of an emerging pathogen of the Canadian canola (Brassica napus) crop. Mol. Plant Pathol. 2012, 13, 105–113. [Google Scholar] [CrossRef]
- Sedaghatkish, A.; Gossen, B.D.; Yu, F.; Torkamaneh, D.; Mcdonald, M.R. Whole-genome DNA similarity and population structure of Plasmodiophora brassicae strains from Canada. BMC Genom. 2019, 20, 744. [Google Scholar] [CrossRef]
- Rolfe, S.A.; Strelkov, S.E.; Links, M.G.; Clarke, W.E.; Robinson, S.J.; Djavaheri, M.; Malinowski, R.; Haddadi, P.; Kagale, S.; Parkin, I.A.; et al. The compact genome of the plant pathogen Plasmodiophora brassicae is adapted to intracellular interactions with host Brassica spp. BMC Genom. 2016, 17, 272. [Google Scholar] [CrossRef]
- Bi, K.; He, Z.; Gao, Z.; Zhao, Y.; Fu, Y.; Cheng, J.; Xie, J.; Jiang, D.; Chen, T. Integrated omics study of lipid droplets from Plasmodiophora brassicae. Sci. Rep. 2016, 6, 36965. [Google Scholar] [CrossRef] [PubMed]
- Schwelm, A.; Fogelqvist, J.; Knaust, A.; Jülke, S.; Lilja, T.; Bonilla-Rosso, G.; Karlsson, M.; Shevchenko, A.; Dhandapani, V.; Choi, S.R.; et al. The Plasmodiophora brassicae genome reveals insights in its life cycle and ancestry of chitin synthases. Sci. Rep. 2015, 5, 11153. [Google Scholar] [CrossRef] [PubMed]
- Larkin, M.A.; Blackshields, G.; Brown, N.P.; Chenna, R.; Mcgettigan, P.A.; Mcwilliam, H.; Valentin, F.; Wallace, I.M.; Wilm, A.; Lopez, R. Clustal W and Clustal X version 2.0. Bioinformatics 2007, 23, 2947–2948. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef]
- Hu, B.; Jin, J.; Guo, A.Y.; Zhang, H.; Luo, J.; Gao, G. GSDS 2.0: An upgraded gene feature visualization server. Bioinformatics 2015, 31, 1296–1297. [Google Scholar] [CrossRef]
- Chen, C.; Rui, X.; Hao, C.; He, Y. TBtools, a Toolkit for Biologists integrating various HTS-data handling tools with a user-friendly interface. bioRxiv 2018. [Google Scholar] [CrossRef]
- Voorrips, R.E. MapChart: Software for the Graphical Presentation of Linkage Maps and QTLs. J. Hered. 2002, 93, 77–78. [Google Scholar] [CrossRef]
- Kenneth, J.L.; Thomas, D.S. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2002, 25, 402–408. [Google Scholar]

| Gene Name | Gene Accession Number | Arabidopsis homologous Genes | CDS Length (bp) | Amino Acids Length (aa) | pI 1 | Molecular Weight (KDa) | Predicted Subcellular Localization |
|---|---|---|---|---|---|---|---|
| BrTPS1a | Bra035049 | AT1G78580 | 2853 | 950 | 6.63 | 106.9 | Cell wall/cytoplasm/vacuole |
| BrTPS1b | Bra008366 | AT1G78580 | 2787 | 928 | 6.87 | 104.5 | Vacuole |
| BrTPS2 | Bra026011 | AT1G16980 | 2535 | 844 | 6.04 | 95.7 | Chloroplast/vacuole |
| BrTPS4 | Bra019043 | AT4G27550 | 2361 | 786 | 6.11 | 88.6 | Chloroplast/vacuole |
| BrTPS5a | Bra040180 | AT4G17770 | 390 | 129 | 9.48 | 14.7 | Nucleus/vacuole |
| BrTPS5b | Bra012642 | AT4G17770 | 2574 | 857 | 5.92 | 96.9 | Vacuole |
| BrTPS6 | Bra004054 | AT1G68020 | 2592 | 863 | 6.03 | 98 | Cytoplasm/Vacuole |
| BrTPS7a | Bra015497 | AT1G06410 | 1125 | 374 | 4.7 | 42.9 | Cytoplasm/vacuole |
| BrTPS7b | Bra030651 | AT1G06410 | 2559 | 852 | 5.54 | 96.9 | Vacuole |
| BrTPS8 | Bra007906 | AT1G70290 | 4380 | 1459 | 6.81 | 165.8 | Chloroplast/vacuole |
| BrTPS9 | Bra016328 | AT1G23870 | 2607 | 868 | 5.85 | 98.6 | Vacuole |
| BrTPS10a | Bra031526 | AT1G60140 | 2574 | 857 | 6.04 | 97 | Chloroplast/vacuole |
| BrPS10b | Bra017888 | AT1G60140 | 2586 | 861 | 6.01 | 96.9 | Chloroplast/vacuole |
| BrTPS11 | Bra038548 | AT2G18700 | 2595 | 864 | 6.16 | 97.8 | Chloroplast/vacuole |
| Gene Name | GenBank ID | CDS Length (bp) | Amino Acids Length (aa) | pI 1 | Molecular Weight (KDa) | Predicted Subcellular Localization |
|---|---|---|---|---|---|---|
| PbTPS1 | CAL69928.1 | 2853 | 860 | 6.31 | 96.49 | Cytoplasm |
| PbTPS2 | CEP00348.1 | 2577 | 858 | 6.93 | 95.87 | Cell membrane/cytoplasm |
| PbTPS3 | CEO95489.1 | 2562 | 853 | 6.18 | 95.50 | Cytoplasm |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kong, L.; Liu, J.; Zhang, W.; Li, X.; Zhang, Y.; Chen, X.; Zhan, Z.; Piao, Z. Genome-Wide Identification and Characterization of the Trehalose-6-Phosphate Synthetase Gene Family in Chinese Cabbage (Brassica rapa) and Plasmodiophora brassicae during Their Interaction. Int. J. Mol. Sci. 2023, 24, 929. https://doi.org/10.3390/ijms24020929
Kong L, Liu J, Zhang W, Li X, Zhang Y, Chen X, Zhan Z, Piao Z. Genome-Wide Identification and Characterization of the Trehalose-6-Phosphate Synthetase Gene Family in Chinese Cabbage (Brassica rapa) and Plasmodiophora brassicae during Their Interaction. International Journal of Molecular Sciences. 2023; 24(2):929. https://doi.org/10.3390/ijms24020929
Chicago/Turabian StyleKong, Liyan, Jiaxiu Liu, Wenjun Zhang, Xiaonan Li, Yuting Zhang, Xueyu Chen, Zongxiang Zhan, and Zhongyun Piao. 2023. "Genome-Wide Identification and Characterization of the Trehalose-6-Phosphate Synthetase Gene Family in Chinese Cabbage (Brassica rapa) and Plasmodiophora brassicae during Their Interaction" International Journal of Molecular Sciences 24, no. 2: 929. https://doi.org/10.3390/ijms24020929
APA StyleKong, L., Liu, J., Zhang, W., Li, X., Zhang, Y., Chen, X., Zhan, Z., & Piao, Z. (2023). Genome-Wide Identification and Characterization of the Trehalose-6-Phosphate Synthetase Gene Family in Chinese Cabbage (Brassica rapa) and Plasmodiophora brassicae during Their Interaction. International Journal of Molecular Sciences, 24(2), 929. https://doi.org/10.3390/ijms24020929






