Immunologic Signatures across Molecular Subtypes and Potential Biomarkers for Sub-Stratification in Endometrial Cancer
Abstract
1. Introduction
2. Results
2.1. Characteristics of Patients
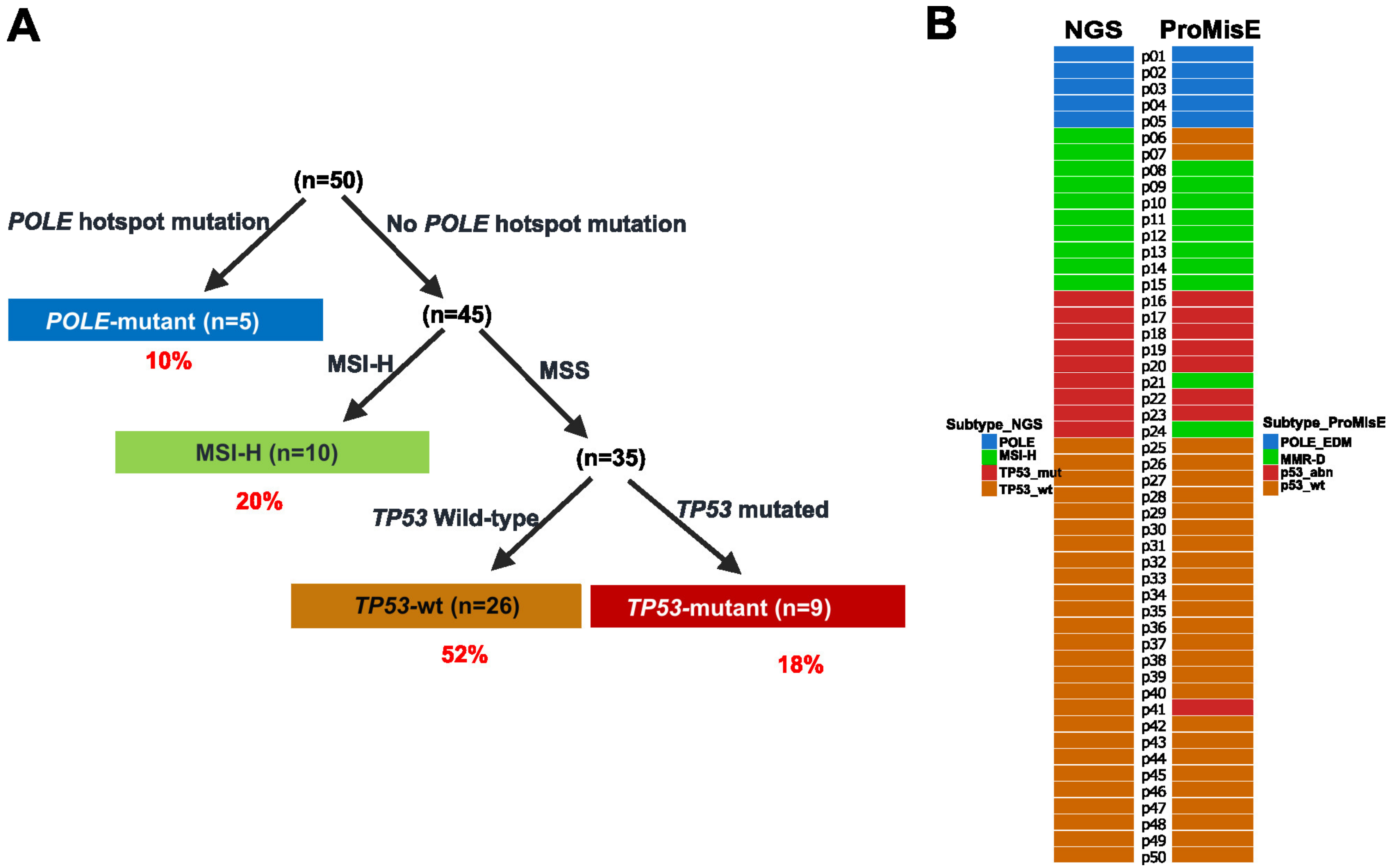
2.2. Molecular Subtyping of EC via a One-Stop Next-Generation Sequencing (NGS) Strategy
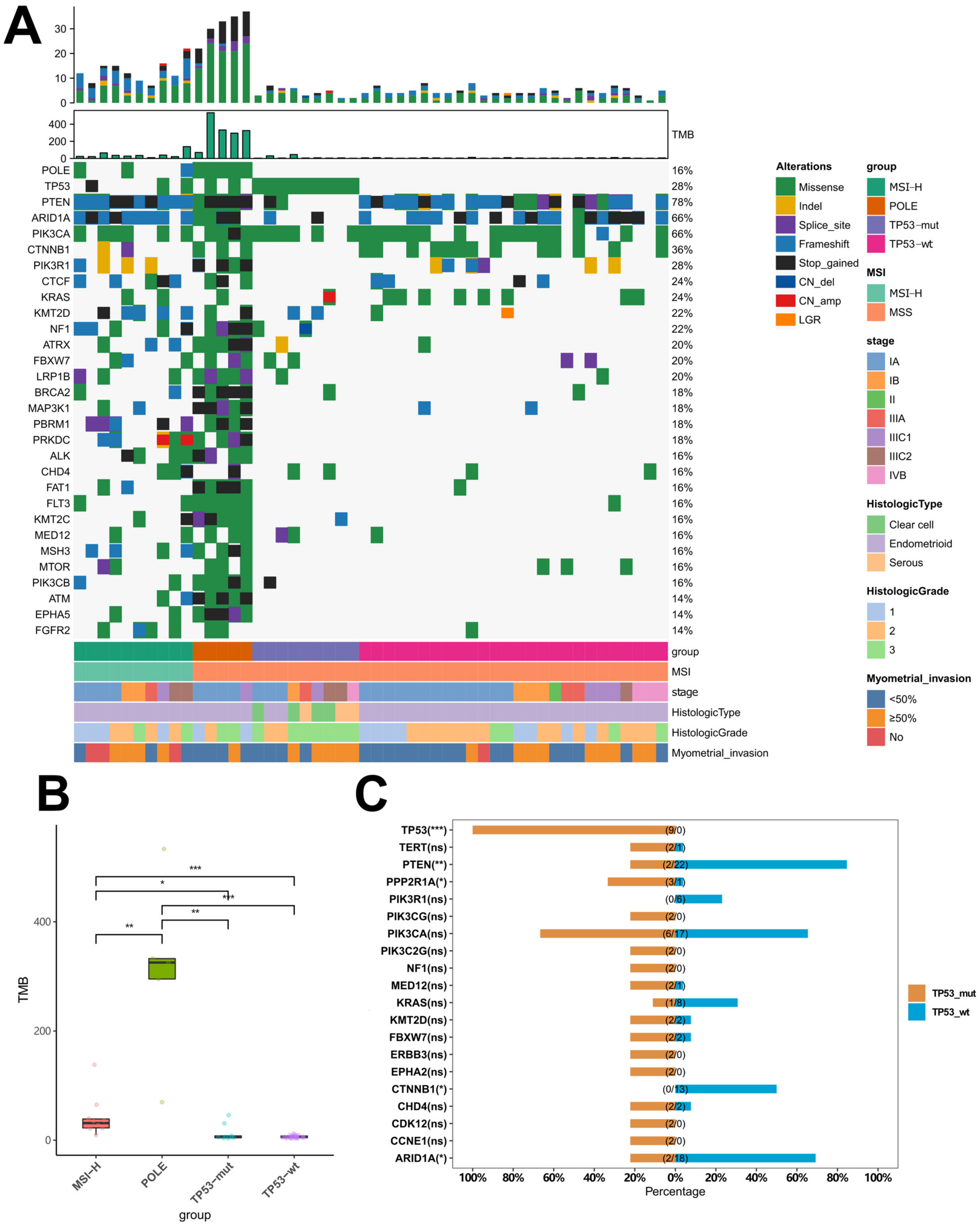
2.3. Distinctive Genomic Profiles among Different Molecular Subtypes
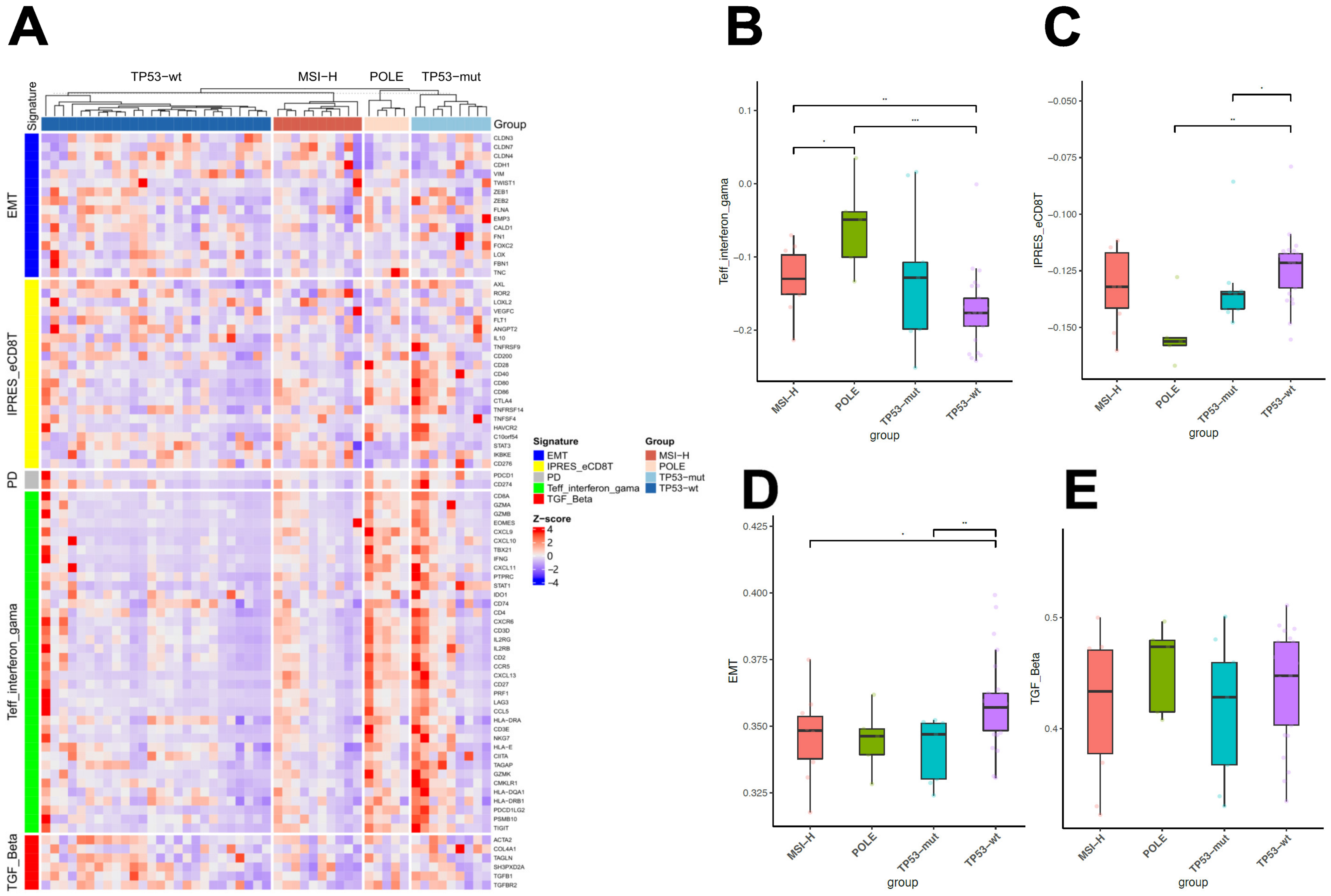
2.4. Differential Immunologic Signatures across Four Subtypes
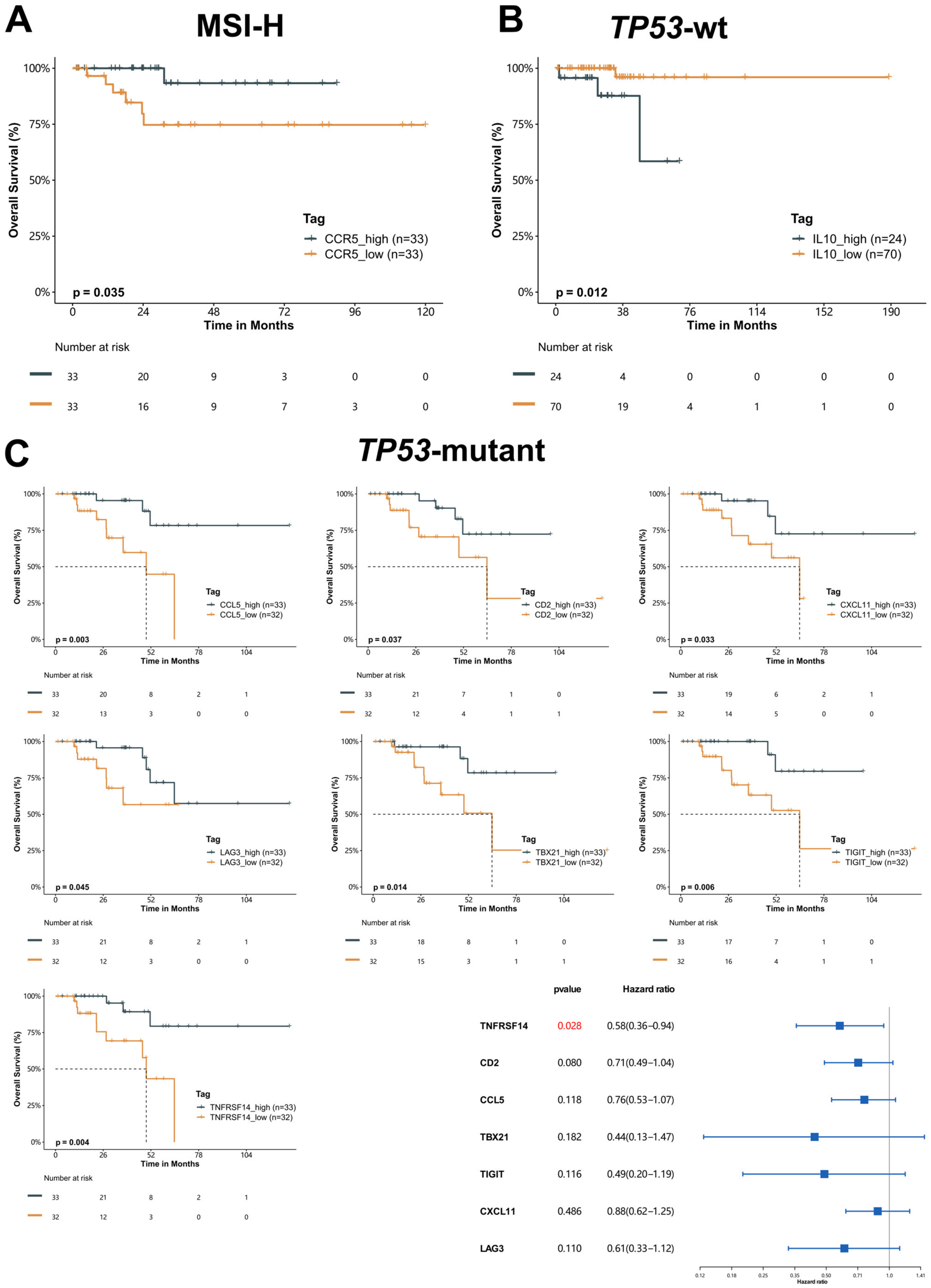
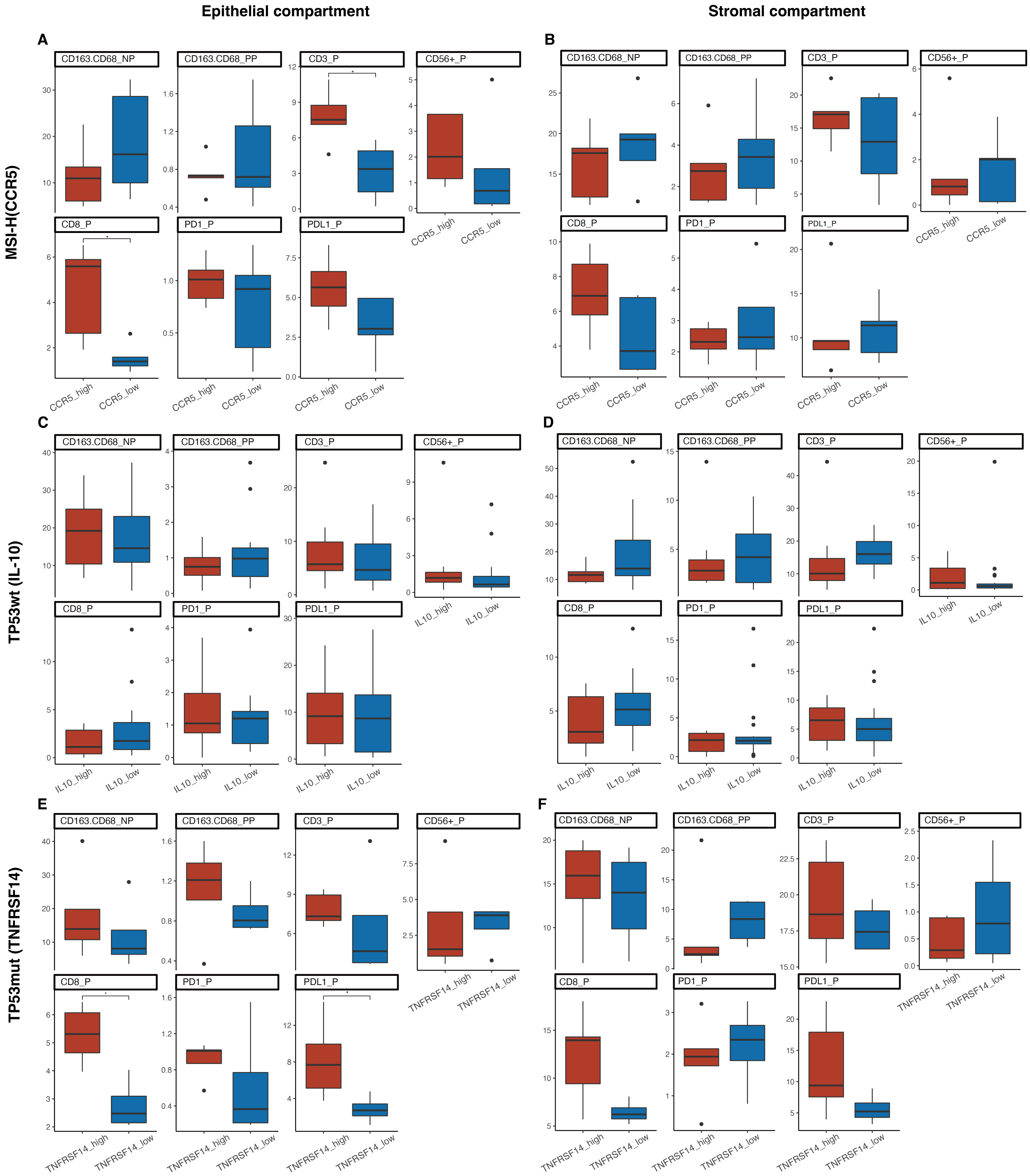
2.5. Prognostic Value of Immunomodulatory Genes
3. Discussion
4. Methods and Materials
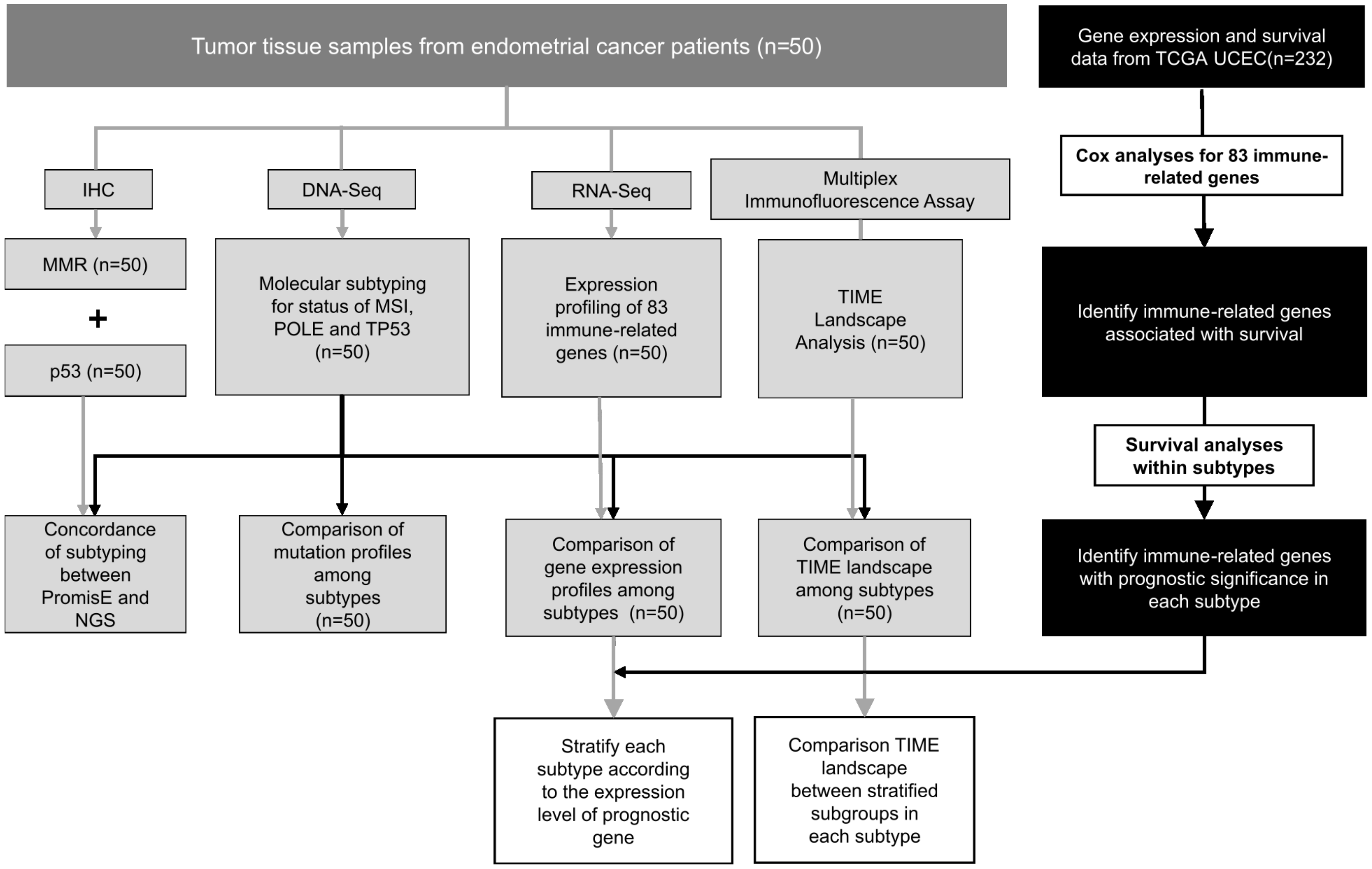
4.1. Patients and Study Design
4.2. DNA Sequencing and Molecular Classification of Patients
4.3. RNA Sequencing and Gene Expression Profiling
4.4. Multiplex Immunofluorescence Assay
4.5. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Zheng, R.; Zhang, S.; Zeng, H.; Wang, S.; Sun, K.; Chen, R.; Li, L.; Wei, W.; He, J. Cancer incidence and mortality in China, 2016. J. Natl. Cancer Center 2022, 2, 1–9. [Google Scholar] [CrossRef]
- The Cancer Genome Atlas Research Network; Levine, D.A. Integrated genomic characterization of endometrial carcinoma. Nature 2013, 497, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Stelloo, E.; Bosse, T.; Nout, R.A.; Mackay, H.J.; Church, D.N.; Nijman, H.W.; Leary, A.; Edmondson, R.J.; Powell, M.E.; Crosbie, E.J.; et al. Refining prognosis and identifying targetable pathways for high-risk endometrial cancer; a TransPORTEC initiative. Mod. Pathol. 2015, 28, 836–844. [Google Scholar] [CrossRef] [PubMed]
- Stelloo, E.; Nout, R.A.; Osse, E.M.; Jürgenliemk-Schulz, I.J.; Jobsen, J.J.; Lutgens, L.C.; van der Steen-Banasik, E.M.; Nijman, H.W.; Putter, H.; Bosse, T.; et al. Improved Risk Assessment by Integrating Molecular and Clinicopathological Factors in Early-stage Endometrial Cancer—Combined Analysis of the PORTEC Cohorts. Clin. Cancer Res. 2016, 22, 4215–4224. [Google Scholar] [CrossRef]
- Talhouk, A.; McConechy, M.K.; Leung, S.; Yang, W.; Lum, A.; Senz, J.; Boyd, N.; Pike, J.; Anglesio, M.; Kwon, J.S.; et al. Confirmation of ProMisE: A simple, genomics-based clinical classifier for endometrial cancer. Cancer 2017, 123, 802–813. [Google Scholar] [CrossRef]
- Santoro, A.; Angelico, G.; Travaglino, A.; Inzani, F.; Arciuolo, D.; Valente, M.; D’Alessandris, N.; Scaglione, G.; Fiorentino, V.; Raffone, A.; et al. New Pathological and Clinical Insights in Endometrial Cancer in View of the Updated ESGO/ESTRO/ESP Guidelines. Cancers 2021, 13, 2623. [Google Scholar] [CrossRef]
- Howitt, B.E.; Shukla, S.A.; Sholl, L.M.; Ritterhouse, L.L.; Watkins, J.C.; Rodig, S.J.; Stover, E.H.; Strickland, K.; D’Andrea, A.D.; Wu, C.J.; et al. Association of Polymerase e–Mutated and Microsatellite-Instable Endometrial Cancers With Neoantigen Load, Number of Tumor-Infiltrating Lymphocytes, and Expression of PD-1 and PD-L1. JAMA Oncol. 2015, 1, 1319–1323. [Google Scholar] [CrossRef]
- Bellone, S.; Bignotti, E.; Lonardi, S.; Ferrari, F.; Centritto, F.; Masserdotti, A.; Pettinella, F.; Black, J.; Menderes, G.; Altwerger, G.; et al. Polymerase ε (POLE) ultra-mutation in uterine tumors correlates with T lymphocyte infiltration and increased resistance to platinum-based chemotherapy in vitro. Gynecol. Oncol. 2016, 144, 146–152. [Google Scholar] [CrossRef]
- Van Gool, I.C.; Eggink, F.A.; Freeman-Mills, L.; Stelloo, E.; Marchi, E.; de Bruyn, M.; Palles, C.; Nout, R.A.; de Kroon, C.D.; Osse, E.M.; et al. POLE Proofreading Mutations Elicit an Antitumor Immune Response in Endometrial Cancer. Clin. Cancer Res. 2015, 21, 3347–3355. [Google Scholar] [CrossRef]
- Talhouk, A.; Derocher, H.; Schmidt, P.; Leung, S.; Milne, K.; Gilks, C.B.; Anglesio, M.S.; Nelson, B.H.; McAlpine, J.N. Molecular Subtype Not Immune Response Drives Outcomes in Endometrial Carcinoma. Clin. Cancer Res. 2019, 25, 2537–2548. [Google Scholar] [CrossRef]
- Stelloo, E.; Jansen, A.; Osse, E.; Nout, R.; Creutzberg, C.; Ruano, D.; Church, D.; Morreau, H.; Smit, V.; van Wezel, T.; et al. Practical guidance for mismatch repair-deficiency testing in endometrial cancer. Ann. Oncol. 2017, 28, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Smithgall, M.C.; Remotti, H.; Hsiao, S.J.; Mansukhani, M.; Liu-Jarin, X.; Fernandes, H. Investigation of discrepant mismatch repair immunohistochemistry and microsatellite instability polymerase chain reaction test results for gynecologic cancers using next-generation sequencing. Hum. Pathol. 2021, 119, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Piskorz, A.M.; Bosse, T.; Jimenez-Linan, M.; Rous, B.; Brenton, J.D.; Gilks, C.B.; Köbel, M. p53 immunohistochemistry is an accurate surrogate for TP53 mutational analysis in endometrial carcinoma biopsies. J. Pathol. 2019, 250, 336–345. [Google Scholar] [CrossRef]
- McAlpine, J.; León-Castillo, A.; Bosse, T. The rise of a novel classification system for endometrial carcinoma; integration of molecular subclasses. J. Pathol. 2018, 244, 538–549. [Google Scholar] [CrossRef]
- Ayers, M.; Lunceford, J.; Nebozhyn, M.; Murphy, E.; Loboda, A.; Kaufman, D.R.; Albright, A.; Cheng, J.D.; Kang, S.P.; Shankaran, V.; et al. IFN-γ–related mRNA profile predicts clinical response to PD-1 blockade. J. Clin. Investig. 2017, 127, 2930–2940. [Google Scholar] [CrossRef] [PubMed]
- Hugo, W.; Zaretsky, J.M.; Sun, L.; Song, C.; Moreno, B.H.; Hu-Lieskovan, S.; Berent-Maoz, B.; Pang, J.; Chmielowski, B.; Cherry, G.; et al. Genomic and Transcriptomic Features of Response to Anti-PD-1 Therapy in Metastatic Melanoma. Cell 2016, 165, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Fatima, I.; Barman, S.; Rai, R.; Thiel, K.W.; Chandra, V. Targeting Wnt Signaling in Endometrial Cancer. Cancers 2021, 13, 2351. [Google Scholar] [CrossRef]
- Mehnert, J.M.; Panda, A.; Zhong, J.; Hirshfield, K.; Damare, S.; Lane, K.; Sokol, L.; Stein, M.N.; Rodriguez-Rodriquez, L.; Kaufman, H.L.; et al. Immune activation and response to pembrolizumab in POLE-mutant endometrial cancer. J. Clin. Investig. 2016, 126, 2334–2340. [Google Scholar] [CrossRef]
- Aldinucci, D.; Borghese, C.; Casagrande, N. The CCL5/CCR5 Axis in Cancer Progression. Cancers 2020, 12, 1765. [Google Scholar] [CrossRef]
- Jiao, X.; Velasco-Velázquez, M.A.; Wang, M.; Li, Z.; Rui, H.; Peck, A.R.; Korkola, J.E.; Chen, X.; Xu, S.; DuHadaway, J.B.; et al. CCR5 Governs DNA Damage Repair and Breast Cancer Stem Cell Expansion. Cancer Res. 2018, 78, 1657–1671. [Google Scholar] [CrossRef]
- Pervaiz, A.; Zepp, M.; Georges, R.; Bergmann, F.; Mahmood, S.; Faiza, S.; Berger, M.R.; Adwan, H. Antineoplastic effects of targeting CCR5 and its therapeutic potential for colorectal cancer liver metastasis. J. Cancer Res. Clin. Oncol. 2020, 147, 73–91. [Google Scholar] [CrossRef] [PubMed]
- Dangaj, D.; Bruand, M.; Grimm, A.J.; Ronet, C.; Barras, D.; Duttagupta, P.A.; Lanitis, E.; Duraiswamy, J.; Tanyi, J.L.; Benencia, F.; et al. Cooperation between Constitutive and Inducible Chemokines Enables T Cell Engraftment and Immune Attack in Solid Tumors. Cancer Cell 2019, 35, 885–900. [Google Scholar] [CrossRef] [PubMed]
- Huffman, A.P.; Lin, J.H.; Kim, S.I.; Byrne, K.T.; Vonderheide, R.H. CCL5 mediates CD40-driven CD4+ T cell tumor infiltration and immunity. J. Clin. Investig. 2020, 5, e137263. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, J.; Qian, Z.; Han, Y. CCR5 is Associated With Immune Cell Infiltration and Prognosis of Lung Cancer. J. Thorac. Oncol. 2019, 14, e102–e103. [Google Scholar] [CrossRef]
- Ip, W.K.E.; Hoshi, N.; Shouval, D.S.; Snapper, S.; Medzhitov, R. Anti-inflammatory effect of IL-10 mediated by metabolic reprogramming of macrophages. Science 2017, 356, 513–519. [Google Scholar] [CrossRef]
- Liu, M.; Zhao, X.; Ma, Y.; Zhou, Y.; Deng, M.; Ma, Y. Transcription factor c-Maf is essential for IL-10 gene expression in B cells. Scand. J. Immunol. 2018, 88, e12701. [Google Scholar] [CrossRef]
- Li, B.; Wang, F.; Ma, C.; Hao, T.; Geng, L.; Jiang, H. Predictive value of IL-18 and IL-10 in the prognosis of patients with colorectal cancer. Oncol. Lett. 2019, 18, 713–719. [Google Scholar] [CrossRef]
- Galizia, G.; Orditura, M.; Romano, C.; Lieto, E.; Castellano, P.; Pelosio, L.; Imperatore, V.; Catalano, G.; Pignatelli, C.; De Vita, F. Prognostic Significance of Circulating IL-10 and IL-6 Serum Levels in Colon Cancer Patients Undergoing Surgery. Clin. Immunol. 2002, 102, 169–178. [Google Scholar] [CrossRef]
- Wittke, F.; Hoffmann, R.; Buer, J.; Dallmann, I.; Oevermann, K.; Sel, S.; Wandert, T.; Ganser, A.; Atzpodien, J. Interleukin 10 (IL-10): An immunosuppressive factor and independent predictor in patients with metastatic renal cell carcinoma. Br. J. Cancer 1999, 79, 1182–1184. [Google Scholar] [CrossRef]
- Zheng, M.; Hu, Y.; Gou, R.; Li, S.; Nie, X.; Li, X.; Lin, B. Development of a seven-gene tumor immune microenvironment prognostic signature for high-risk grade III endometrial cancer. Mol. Ther. - Oncolytics 2021, 22, 294–306. [Google Scholar] [CrossRef] [PubMed]
- Šedý, J.R.; Ramezani-Rad, P. Chapter Five—HVEM network signaling in cancer. In Advances in Cancer Research; Tew, K.D., Fisher, P.B., Eds.; Academic Press: Cambridge, MA, USA, 2019; Volume 142, pp. 145–186. [Google Scholar]
- Zhu, Y.; Lu, M. Increased expression of TNFRSF14 indicates good prognosis and inhibits bladder cancer proliferation by promoting apoptosis. Mol. Med. Rep. 2018, 18, 3403–3410. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Dong, C.; Chen, J.; Gao, X.; Xie, X.; Zhang, X. Identification of an immune checkpoint gene signature that accurately predicts prognosis and immunotherapy response in endometrial carcinoma. Aging 2021, 13, 16696–16712. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.; Dong, Y.; Tan, Q.; Kong, J.; Yu, B. Tissue Infiltrating Immune Cells as Prognostic Biomarkers in Endometrial Cancer: A Meta-Analysis. Dis. Markers 2020, 2020, 1805764. [Google Scholar] [CrossRef]
- Lu, L.; Li, Y.; Luo, R.; Xu, J.; Feng, J.; Wang, M. Prognostic and Clinicopathological Role of PD-L1 in Endometrial Cancer: A Meta-Analysis. Front. Oncol. 2020, 10, 632. [Google Scholar] [CrossRef]
- Kim, J.; Kim, S.; Lee, H.S.; Yang, W.; Cho, H.; Chay, D.B.; Cho, S.J.; Hong, S.; Kim, J.-H. Prognostic implication of programmed cell death 1 protein and its ligand expressions in endometrial cancer. Gynecol. Oncol. 2018, 149, 381–387. [Google Scholar] [CrossRef]
- Mao, X.; Zhang, Z.; Zheng, X.; Xie, F.; Duan, F.; Jiang, L.; Chuai, S.; Han-Zhang, H.; Han, B.; Sun, J. Capture-Based Targeted Ultradeep Sequencing in Paired Tissue and Plasma Samples Demonstrates Differential Subclonal ctDNA-Releasing Capability in Advanced Lung Cancer. J. Thorac. Oncol. 2016, 12, 663–672. [Google Scholar] [CrossRef]
- Li, Y.; Jiang, B.; Yang, J.; Zhang, X.; Zhang, Z.; Ye, J.; Zhong, W.; Tu, H.; Chen, H.; Wang, Z.; et al. Unique genetic profiles from cerebrospinal fluid cell-free DNA in leptomeningeal metastases of EGFR-mutant non-small-cell lung cancer: A new medium of liquid biopsy. Ann. Oncol. 2018, 29, 945–952. [Google Scholar] [CrossRef]
- Wang, M.; Chen, X.; Dai, Y.; Wu, D.; Liu, F.; Yang, Z.; Song, B.; Xie, L.; Yang, L.; Zhao, W.; et al. Concordance Study of a 520-Gene Next-Generation Sequencing-Based Genomic Profiling Assay of Tissue and Plasma Samples. Mol. Diagn. Ther. 2022, 26, 309–322. [Google Scholar] [CrossRef]
- Xiang, C.; Guo, L.; Zhao, R.; Teng, H.; Wang, Y.; Xiong, L.; Han, Y. Identification and Validation of Noncanonical RET Fusions in Non–Small-Cell Lung Cancer through DNA and RNA Sequencing. J. Mol. Diagn. 2022, 24, 374–385. [Google Scholar] [CrossRef]
- Mariathasan, S.; Turley, S.J.; Nickles, D.; Castiglioni, A.; Yuen, K.; Wang, Y.; Kadel, E.E., III; Koeppen, H.; Astarita, J.L.; Cubas, R.; et al. TGFβ attenuates tumour response to PD-L1 blockade by contributing to exclusion of T cells. Nature 2018, 554, 544–548. [Google Scholar] [CrossRef] [PubMed]
- Fehrenbacher, L.; Spira, A.; Ballinger, M.; Kowanetz, M.; Vansteenkiste, J.; Mazieres, J.; Park, K.; Smith, D.; Artal-Cortes, A.; Lewanski, C.; et al. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): A multicentre, open-label, phase 2 randomised controlled trial. Lancet 2016, 387, 1837–1846. [Google Scholar] [CrossRef] [PubMed]
- Hänzelmann, S.; Castelo, R.; Guinney, J. GSVA: Gene set variation analysis for microarray and RNA-Seq data. BMC Bioinform. 2013, 14, 7. [Google Scholar] [CrossRef] [PubMed]
| Overall (n = 50) | MSI-H Subgroup (n = 10) | POLE-Mutant Subgroup (n = 5) | TP53-Mutant Subgroup (n = 9) | TP53-wt Subgroup (n = 26) | p | |
|---|---|---|---|---|---|---|
| Age | 0.038 c | |||||
| Median [IQR] | 57.0 (52.0, 66.0] | 61.0 (57.5, 67.5] | 54.0 (47.0, 55.0] | 67.0 (58.0, 71.0] | 56.0 (46.8, 62.3] | |
| FIGO stage, n (%) | 0.961 a | |||||
| IA | 24 (48.0) | 4 (40.0) | 4 (80.0) | 3 (33.3) | 13 (50.0) | |
| IB | 6 (12.0) | 2 (20.0) | 0 (0.0) | 1 (11.1) | 3 (11.5) | |
| II | 1 (2.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (3.9) | |
| IIIA | 4 (8.0) | 1 (10.0) | 0 (0.0) | 1 (11.1) | 2 (7.7) | |
| IIIC1 | 6 (12.0) | 1 (10.0) | 1 (20.0) | 1 (11.1) | 3 (11.5) | |
| IIIC2 | 5 (10.0) | 2 (20.0) | 0 (0.0) | 2 (22.2) | 1 (3.9) | |
| IVB | 4 (8.0) | 0 (0.0) | 0 (0.0) | 1 (11.1) | 3 (11.5) | |
| Histologic Type, n (%) | <0.001 b | |||||
| Endometrioid | 43 (86.0) | 10 (100.0) | 5 (100.0) | 2 (22.2) | 26 (100.0) | |
| Serous | 3 (6.0) | 0 (0.0) | 0 (0.0) | 3 (33.3) | 0 (0.0) | |
| Clear cell | 4 (8.0) | 0 (0.0) | 0 (0.0) | 4 (44.44%) | 0 (0.0) | |
| Histologic Grade, n (%) | 0.021 a | |||||
| G1 | 14 (28.0) | 4 (40.0) | 2 (40.0) | 0 (0.0) | 8 (30.8) | |
| G2 | 21 (42.0) | 4 (40.0) | 1 (20.0) | 2 (22.2) | 14 (53.9) | |
| G3 | 15 (30.0) | 2 (20.0) | 2 (40.0) | 7 (77.8) | 4 (15.4) | |
| LVSI, n (%) | 0.048 b | |||||
| No | 29 (58.0) | 7 (70.0) | 0 (0.0) | 6 (66.7) | 16 (61.5) | |
| Yes | 21 (42.0) | 3 (30.0) | 5 (100.0) | 3 (33.3) | 10 (38.5) | |
| Myometrial invasion, n (%) | 0.078 a | |||||
| No | 4 (8.0) | 3 (30.0) | 0 (0.0) | 0 (0.0) | 1 (3.9) | |
| <50% | 27 (54.0) | 3 (30.0) | 4 (80.0) | 4 (44.4) | 16 (61.5) | |
| ≥50% | 19 (38.0) | 4 (40.0) | 1 (20.0) | 5 (55.6) | 9 (34.6) | |
| Lymph node metastasis, n (%) | 0.955 b | |||||
| No | 37 (74.0) | 7 (70.0) | 4 (80.0) | 6 (66.7) | 20 (76.9) | |
| Yes | 12 (24.0) | 3 (30.0) | 1 (20.0) | 2 (22.2) | 6 (23.1) | |
| ND | 1 (2.0) | 0 (0.0) | 0 (0.0) | 1 (11.1) | 0 (0.0) | |
| ER, n (%) | 0.006 a | |||||
| Negative | 10 (20.0) | 1 (10.0) | 1 (20.0) | 6 (66.7) | 2 (7.7) | |
| Positive (>95%) | 28 (56.0) | 5 (50.0) | 3 (60.0) | 1 (11.1) | 19 (73.1) | |
| Partial positive (≤95%) | 12 (24.0) | 4 (40.0) | 1 (20.0) | 2 (22.2) | 5 (19.2) | |
| PR, n (%) | 0.002 a | |||||
| Negative | 9 (18.0) | 1 (10.0) | 1 (20.0) | 6 (66.7) | 1 (3.9) | |
| Positive (>95%) | 28 (56.0) | 5 (50.0) | 3 (60.0) | 1 (11.1) | 19 (73.1) | |
| Partial positive (≤95%) | 12 (24.0) | 4 (40.0) | 1 (20.0) | 2 (22.2) | 5 (19.2) | |
| ND | 1 (2.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (3.9) | |
| Ascitic fluid Cytology, n (%) | 0.395 b | |||||
| Negative | 40 (80.0) | 7 (70.0) | 5 (100.0) | 5 (55.6) | 23 (88.5) | |
| Positive | 5 (10.0) | 0 (0.0) | 0 (0.0) | 2 (22.2) | 3 (11.5) | |
| ND | 5 (10.0) | 3 (30.0) | 0 (0.0) | 2 (22.2) | 0 (0.0) | |
| TMB, mutations/Mb | <0.001 c | |||||
| Median [IQR] | 7.5 (4.8,26.8] | 31.4 (22.6,38.9] | 325.4 (295.2,332.5] | 5.6 (4.8,7.9] | 5.96 (4.8,7.8] | |
| Ki67 expression, % | 0.001 c | |||||
| Median [IQR] | 70.0 (40.0,80.0] | 72.5 (70.0,80.0] | 70.0 (60.0,80.0] | 80.0 (70.0,80.0] | 50.0 (20.0,68.8] | |
| ND, n (%) | 3 (6.00) | 1 (10.00) | 0 (0.00) | 0 (0.00) | 2 (7.69) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, F.; Jiang, S.; Cao, D.; Mao, M.; Xiang, Y. Immunologic Signatures across Molecular Subtypes and Potential Biomarkers for Sub-Stratification in Endometrial Cancer. Int. J. Mol. Sci. 2023, 24, 1791. https://doi.org/10.3390/ijms24021791
Jiang F, Jiang S, Cao D, Mao M, Xiang Y. Immunologic Signatures across Molecular Subtypes and Potential Biomarkers for Sub-Stratification in Endometrial Cancer. International Journal of Molecular Sciences. 2023; 24(2):1791. https://doi.org/10.3390/ijms24021791
Chicago/Turabian StyleJiang, Fang, Shiyang Jiang, Dongyan Cao, Mingyi Mao, and Yang Xiang. 2023. "Immunologic Signatures across Molecular Subtypes and Potential Biomarkers for Sub-Stratification in Endometrial Cancer" International Journal of Molecular Sciences 24, no. 2: 1791. https://doi.org/10.3390/ijms24021791
APA StyleJiang, F., Jiang, S., Cao, D., Mao, M., & Xiang, Y. (2023). Immunologic Signatures across Molecular Subtypes and Potential Biomarkers for Sub-Stratification in Endometrial Cancer. International Journal of Molecular Sciences, 24(2), 1791. https://doi.org/10.3390/ijms24021791





