Low-Diversity Microbiota in Apical Periodontitis and High Blood Pressure Are Signatures of the Severity of Apical Lesions in Humans
Abstract
1. Introduction
2. Results
2.1. Description of the General and Clinical Characteristics of the Granuloma and Identification of a Risk Factor “High Blood Pressure”
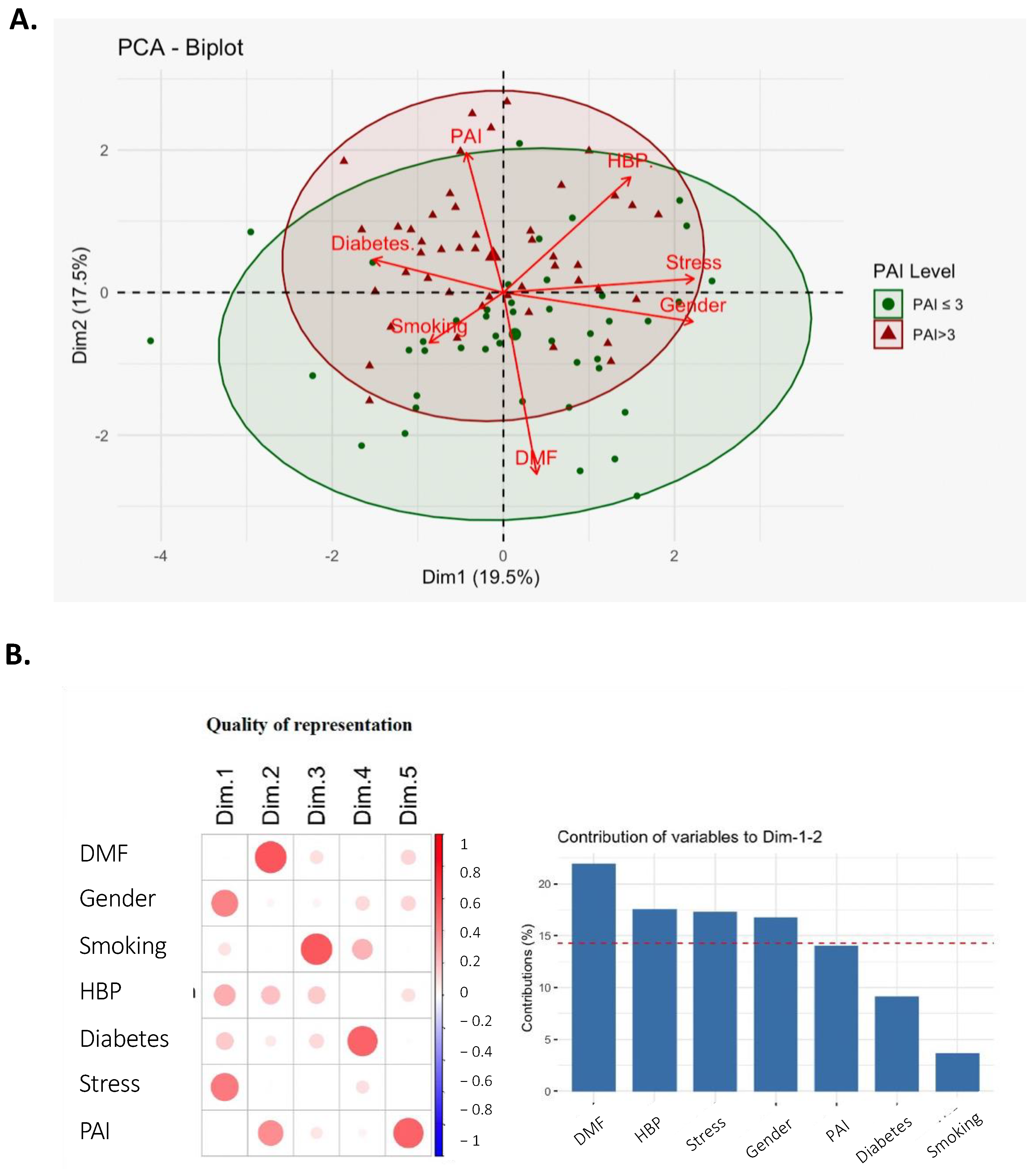
2.1.1. All Subjects
2.1.2. Epidemiologic Parameters of the Two Groups: PAI ≤ 3 and PAI > 3
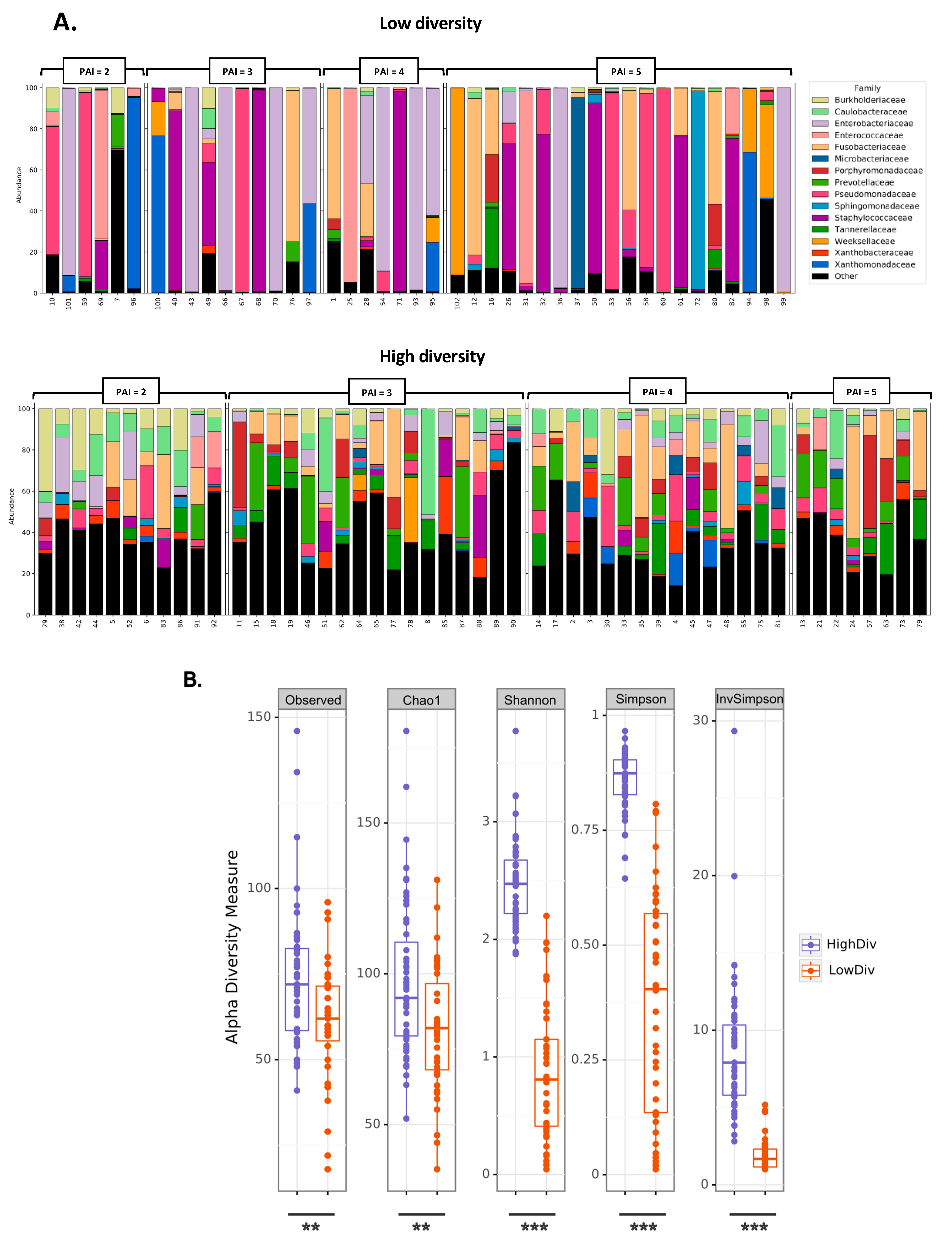
2.2. Low Diversity in Granuloma Microbiota Is Associated with the Highest Severity of AP
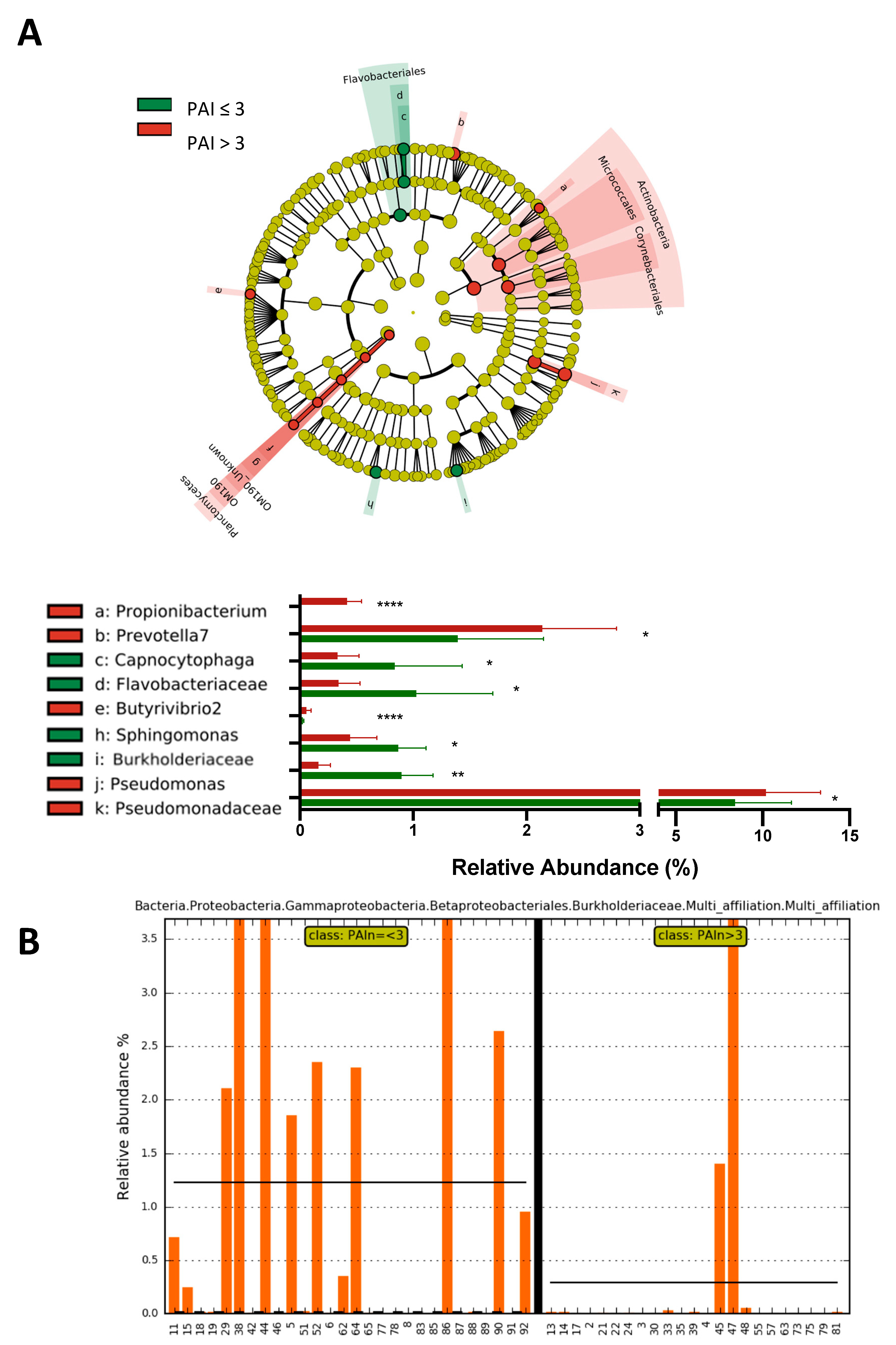
2.3. Subjects with a Periapical Index PAI > 3 Show a Significant Increase in the Abundance of Propionibacterium, Prevotella7, Pseudomonas and Pseudomonadaceae and a Significant Decrease in the Abundance of the Family Burkholderiaceae
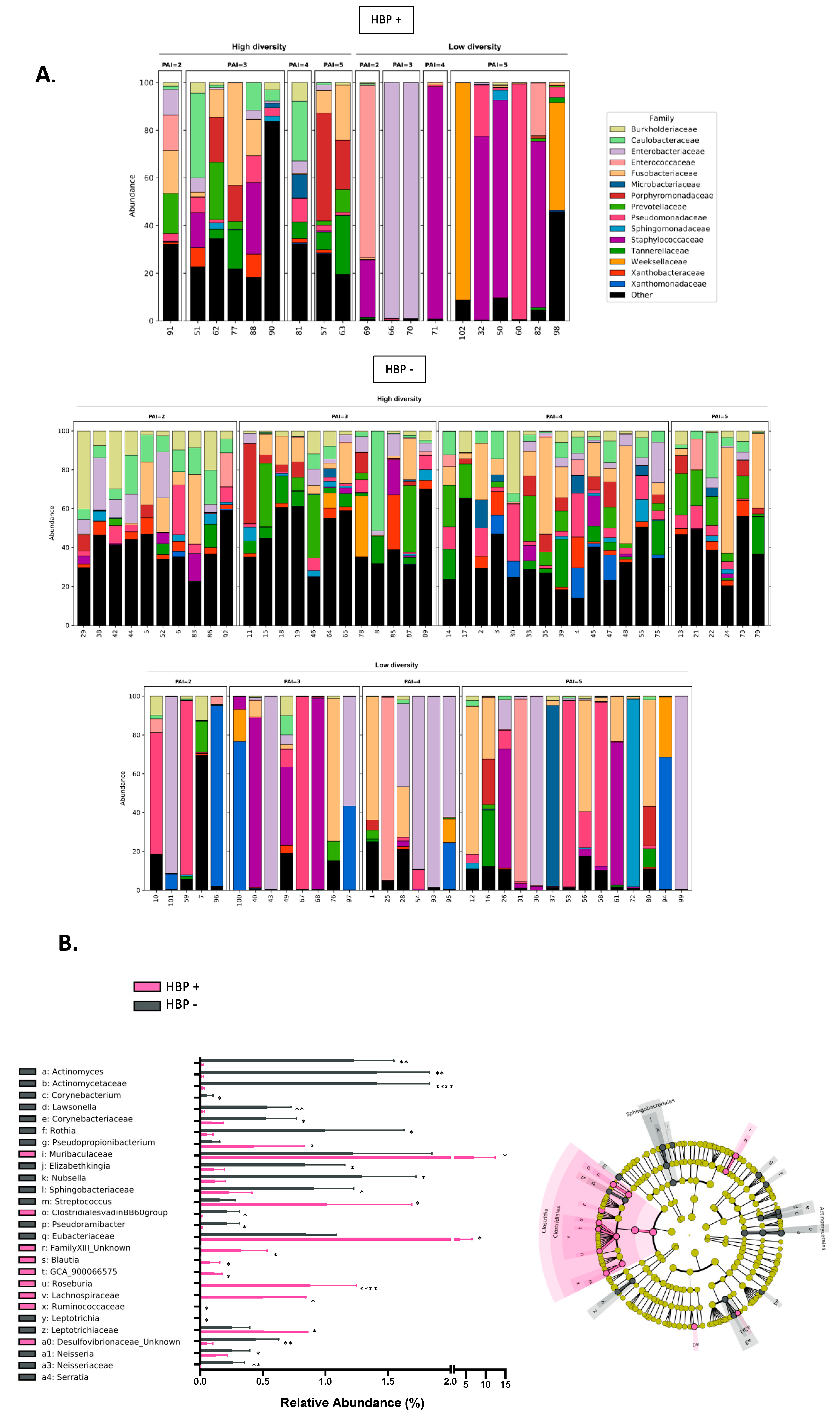
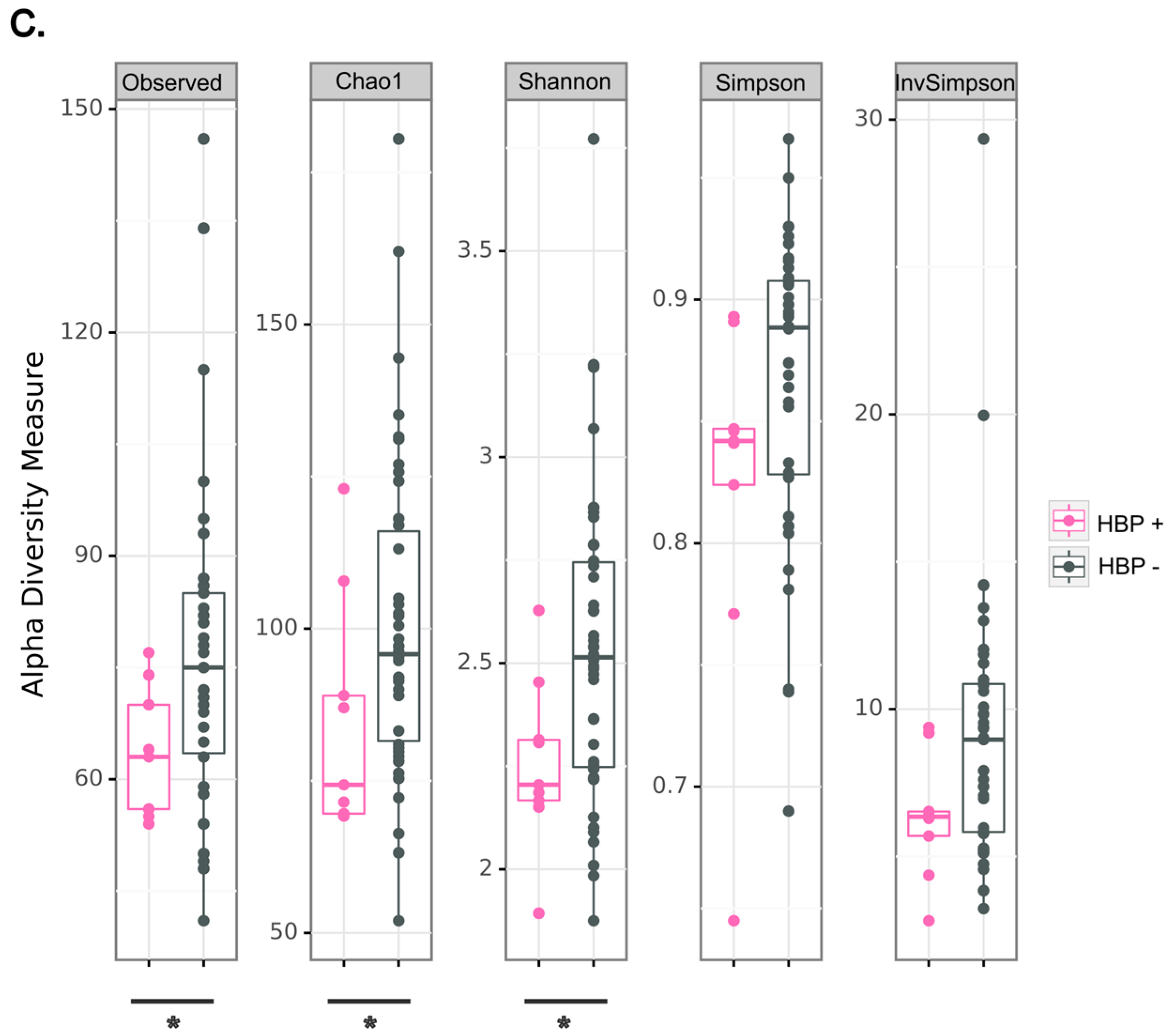
2.4. High Blood Pressure Is an Aggravating Factor for the Severity of Periapical Lesion Associated with a Decreased Diversity of Granuloma Microbiota
3. Discussion
4. Materials and Methods
4.1. Study Design and Settings
4.2. Participants
4.3. Data Collection and Variables
4.4. Microbiota Analysis
4.4.1. Bacterial 16S rRNA Gene Sequencing
4.4.2. 16S rRNA Gene Sequence Analysis
4.4.3. Statistical Analysis
4.5. Data Management and Analysis: Statistical Analyses
4.6. Cohort Low and High Diversity
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Segura-Egea, J.J.; Martín-González, J.; Cosano, L.C. Endodontic medicine: Connections between apical periodontitis and systemic diseases. Int. Endod. J. 2015, 48, 933–951. [Google Scholar] [CrossRef] [PubMed]
- Tibúrcio-Machado, C.S.; Michelon, C.; Zanatta, F.B.; Gomes, M.S.; Marin, J.A.; Bier, C.A. The global prevalence of apical periodontitis: A systematic review and meta-analysis. Int. Endod. J. 2021, 54, 712–735. [Google Scholar] [CrossRef]
- Jakovljevic, A.; Nikolic, N.; Jacimovic, J.; Pavlovic, O.; Milicic, B.; Beljic-Ivanovic, K.; Miletic, M.; Andric, M.; Milasin, J. Prevalence of Apical Periodontitis and Conventional Nonsurgical Root Canal Treatment in General Adult Population: An Updated Systematic Review and Meta-analysis of Cross-sectional Studies Published between 2012 and 2020. J. Endod. 2020, 46, 1371–1386.e8. [Google Scholar] [CrossRef]
- Azeredo, S.V.; Brasil, S.C.; Antunes, H.; Marques, F.; Pires, F.-R.; Armada, L. Distribution of macrophages and plasma cells in apical periodontitis and their relationship with clinical and image data. J. Clin. Exp. Dent. 2017, 9, e1060–e1065. [Google Scholar] [CrossRef] [PubMed]
- Bănică, A.C.; Popescu, S.M.; Mercuţ, V.; Busuioc, C.J.; Gheorghe, A.G.; Traşcă, D.M.; Brăila, A.D.; Moraru, A.I. Histological and immunohistochemical study on the apical granuloma. Rom. J. Morphol. Embryol. 2018, 59, 811–817. [Google Scholar] [PubMed]
- Blasco-Baque, V.; Garidou, L.; Pomié, C.; Escoula, Q.; Loubieres, P.; Le Gall-David, S.; Lemaitre, M.; Nicolas, S.; Klopp, P.; Waget, A.; et al. Periodontitis induced by Porphyromonas gingivalis drives periodontal microbiota dysbiosis and insulin resistance via an impaired adaptive immune response. Gut 2017, 66, 872–885. [Google Scholar] [CrossRef]
- Blasco-Baque, V.; Serino, M.; Vergnes, J.-N.; Riant, E.; Loubieres, P.; Arnal, J.F.; Gourdy, P.; Sixou, M.; Burcelin, R.; Kemoun, P. High-Fat Diet Induces Periodontitis in Mice through Lipopolysaccharides (LPS) Receptor Signaling: Protective Action of Estrogens. PLoS ONE 2012, 7, e48220. [Google Scholar] [CrossRef]
- Palsson-McDermott, E.M.; O’Neill, L. Signal transduction by the lipopolysaccharide receptor, Toll-like receptor-4. Immunology 2004, 113, 153–162. [Google Scholar] [CrossRef]
- Filho, E.M.M.; Calisto, A.M.; Tavarez, R.R.D.J.; Segato, R.A.B.; da Silva, L.A.B. Correlation between the Periapical Index and Lesion Volume in Cone-Beam Computed Tomography Images. Iran. Endod. J. 2018, 13, 155–158. [Google Scholar] [CrossRef]
- Aysal, Z.; Kocasarac, H.D.; Orhan, K.; Helvacioglu-Yigit, D. Radiological Assessment of Prevalance and Quality of Periapical Status of Endodontic Treatments. Med. Sci. Monit. 2022, 28, e936569-1–e936569-11. [Google Scholar] [CrossRef]
- Karamifar, K.; Saghiri, M.A.; Tondari, A. Endodontic Periapical Lesion: An Overview on the Etiology, Diagnosis and Current Treatment Modalities. Eur. Endod. J. 2020, 5, 54–67. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Yang, Z.; Nie, Y.; Hou, B. Microbial Communities in the Extraradicular and Intraradicular Infections Associated With Persistent Apical Periodontitis. Front. Cell. Infect. Microbiol. 2022, 11, 798367. [Google Scholar] [CrossRef] [PubMed]
- Berlin-Broner, Y.; Febbraio, M.; Levin, L. Association between apical periodontitis and cardiovascular diseases: A systematic review of the literature. Int. Endod. J. 2017, 50, 847–859. [Google Scholar] [CrossRef]
- Jakovljevic, A.; Duncan, H.F.; Nagendrababu, V.; Jacimovic, J.; Milasin, J.; Dummer, P.M.H. Association between cardiovascular diseases and apical periodontitis: An umbrella review. Int. Endod. J. 2020, 53, 1374–1386. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.; Minty, M.; Vinel, A.; Canceill, T.; Loubières, P.; Burcelin, R.; Kaddech, M.; Blasco-Baque, V.; Laurencin-Dalicieux, S. Oral Microbiota: A Major Player in the Diagnosis of Systemic Diseases. Diagnostics 2021, 11, 1376. [Google Scholar] [CrossRef]
- Dash, G.; Mishra, L.; Singh, N.R.; Behera, R.; Misra, S.R.; Kumar, M.; Sokolowski, K.; Agarwal, K.; Behera, S.K.; Mishra, S.; et al. Prevalence and Quality of Endodontic Treatment in Patients with Cardiovascular Disease and Associated Risk Factors. J. Clin. Med. 2022, 11, 6046. [Google Scholar] [CrossRef]
- González-Navarro, B.; Segura-Egea, J.J.; Estrugo-Devesa, A.; Pintó-Sala, X.; Jane-Salas, E.; Jiménez-Sánchez, M.C.; Cabanillas-Balsera, D.; López-López, J. Relationship between Apical Periodontitis and Metabolic Syndrome and Cardiovascular Events: A Cross-Sectional Study. J. Clin. Med. 2020, 9, 3205. [Google Scholar] [CrossRef]
- Paxton, E.S.; Green, A.; Krueger, V.S. Periprosthetic Infections of the Shoulder. J. Am. Acad. Orthop. Surg. 2019, 27, e935–e944. [Google Scholar] [CrossRef]
- Meirinhos, J.; Martins, J.N.R.; Pereira, B.; Baruwa, A.; Gouveia, J.; Quaresma, S.A.; Monroe, A.; Ginjeira, A. Prevalence of apical periodontitis and its association with previous root canal treatment, root canal filling length and type of coronal restoration—A cross-sectional study. Int. Endod. J. 2020, 53, 573–584. [Google Scholar] [CrossRef]
- Allareddy, V.; Lin, C.Y.; Shah, A.; Lee, M.K.; Nalliah, R.; Elangovan, S.; Allareddy, V.; Karimbux, N.Y. Outcomes in patients hospitalized for periapical abscess in the United States: An analysis involving the use of a nationwide inpatient sample. J. Am. Dent. Assoc. 2010, 141, 1107–1116. [Google Scholar] [CrossRef]
- Tsioufis, C.; Kasiakogias, A.; Thomopoulos, C.; Stefanadis, C. Periodontitis and blood pressure: The concept of dental hypertension. Atherosclerosis 2011, 219, 1–9. [Google Scholar] [CrossRef]
- Wiersinga, W.J.; Virk, H.S.; Torres, A.G.; Currie, B.J.; Peacock, S.J.; Dance, D.A.B.; Limmathurotsakul, D. Melioidosis. Nat. Rev. Dis. Prim. 2018, 4, 17107. [Google Scholar] [CrossRef] [PubMed]
- Rhodes, K.A.; Schweizer, H.P. Antibiotic resistance in Burkholderia species. Drug Resist. Updat. 2016, 28, 82–90. [Google Scholar] [CrossRef]
- Limmathurotsakul, D.; Golding, N.; Dance, D.A.B.; Messina, J.P.; Pigott, D.; Moyes, C.L.; Rolim, D.B.; Bertherat, E.; Day, N.P.J.; Peacock, S.J.; et al. Predicted global distribution of Burkholderia pseudomallei and burden of melioidosis. Nat. Microbiol. 2016, 1, 15008. [Google Scholar] [CrossRef]
- Jenjaroen, K.; Chumseng, S.; Sumonwiriya, M.; Ariyaprasert, P.; Chantratita, N.; Sunyakumthorn, P.; Hongsuwan, M.; Wuthiekanun, V.; Fletcher, H.A.; Teparrukkul, P.; et al. T-Cell Responses Are Associated with Survival in Acute Melioidosis Patients. PLoS Negl. Trop. Dis. 2015, 9, e0004152. [Google Scholar] [CrossRef]
- Fåk, F.; Tremaroli, V.; Bergström, G.; Bäckhed, F. Oral microbiota in patients with atherosclerosis. Atherosclerosis 2015, 243, 573–578. [Google Scholar] [CrossRef]
- Human Oral, Gut, and Plaque Microbiota in Patients with Atherosclerosis. Available online: https://www.pnas.org/doi/10.1073/pnas.1011383107 (accessed on 14 December 2022).
- Desvarieux, M.; Demmer, R.; Jacobs, D.R., Jr.; Rundek, T.; Boden-Albala, B.; Sacco, R.L.; Papapanou, P.N. Periodontal bacteria and hypertension: The oral infections and vascular disease epidemiology study (INVEST). J. Hypertens. 2010, 28, 1413–1421. [Google Scholar] [CrossRef]
- Echen, H.; Ejiang, W. Application of high-throughput sequencing in understanding human oral microbiome related with health and disease. Front. Microbiol. 2014, 5, 508. [Google Scholar] [CrossRef]
- Lluch, J.; Servant, F.; Païssé, S.; Valle, C.; Valière, S.; Kuchly, C.; Vilchez, G.; Donnadieu, C.; Courtney, M.; Burcelin, R.; et al. The Characterization of Novel Tissue Microbiota Using an Optimized 16S Metagenomic Sequencing Pipeline. PLoS ONE 2015, 10, e0142334. [Google Scholar] [CrossRef] [PubMed]
- Escudié, F.; Auer, L.; Bernard, M.; Mariadassou, M.; Cauquil, L.; Vidal, K.; Maman, S.; Hernandez-Raquet, G.; Combes, S.; Pascal, G. FROGS: Find, Rapidly, OTUs with Galaxy Solution. Bioinformatics 2018, 34, 1287–1294. [Google Scholar] [CrossRef] [PubMed]
- Segata, N.; Huttenhower, C. Toward an Efficient Method of Identifying Core Genes for Evolutionary and Functional Microbial Phylogenies. PLoS ONE 2011, 6, e24704. [Google Scholar] [CrossRef] [PubMed]
| All Subjects (n = 94) | PAI ≤ 3 (n = 44) | PAI > 3 (n = 50) | p Value | |
|---|---|---|---|---|
| Age (years) | 54.53 ± 14.22 | 55.40 ± 14.48 | 53.76 ± 13.54 | p = 0.36 |
| Weight (kg) | 71.39 ± 13.89 | 73.52 ± 15.63 | 69.31 ± 11.99 | p = 0.12 |
| Height (cm) | 169.67 ± 13.22 | 171.13 ± 07.92 | 169.35 ± 16.36 | p = 0.4 |
| BMI (kg/m2) | 25.66 ± 13.16 | 24.92 ± 4.03 | 23.71 ± 2.84 | p = 0.20 |
| Stress Score (Scale 0 to 10) | 4.68 ± 2.68 | 4.71 ± 2.85 | 4.56 ± 2.52 | p = 0.55 |
| DMF index (decayed, missing and filled) | 14.63 ± 5.38 | 15.25 ± 5.83 | 14.10 ± 4.95 | p = 0.31 |
| Number of decayed teeth (D) | 0.51 ± 1.00 | 0.60 ± 1.10 | 0.42 ± 0.90 | p = 0.36 |
| Number of missing teeth (M) | 5.62 ± 3.86 | 6.18 ± 4.37 | 8.96 ± 3.48 | p = 0.18 |
| Number of filled teeth (F) | 8.80 ± 4.02 | 8.63 ± 4.60 | 5.14 ± 3.32 | p = 0.63 |
| Number of dental brushings/day | 2.01 ± 0.59 | 2.045 ± 0,52 | 1.98 ± 0,65 | p = 0.60 |
| PAI score | 3.64 ± 1.09 | 2.63 ± 0.48 | 4.56 ± 0.50 | p < 0.0001 |
| HBP− (n = 75) | HBP+ (n = 19) | p Value | |
|---|---|---|---|
| Age (years) | 53.66 ± 13.96 | 52.5 ± 13.95 | p = 0.77 |
| Weight (kg) | 71.49 ± 14.41 | 69.00 ± 06.63 | p = 0.50 |
| Height (cm) | 169.09 ± 15.29 | 168.94 ± 5.22 | p = 0.97 |
| BMI (kg/m2) | 26.29 ± 15.77 | 24.23 ± 2.62 | p = 0.60 |
| Stress Score (scale 0 to 10) | 4.66 ± 2.59 | 4.65 ± 2.65 | p = 0.72 |
| DMF index (decayed, missing and filled) | 14.74 ± 5.26 | 13.56 ± 6.03 | p = 0.44 |
| Number of decayed teeth (D) | 0.59 ± 1.08 | 0.38 ± 0.89 | p = 0.47 |
| Number of missing teeth (M) | 5.62 ± 3.77 | 650 ± 4.83 | p = 3.77 |
| Number of filled teeth (F) | 8.75 ± 4.07 | 7.31 ± 3.55 | p = 0.19 |
| Number of dental brushings/day | 1.98 ± 0,58 | 2.10 ± 0.65 | p = 0.4437 |
| PAI score | 3.47 ± 1.05 | 4.05 ± 0.96 | p = 0.0416 |
| Diagnostic of severe periapical diseases (PAI = 5) | 27 % (n = 20) | 42% (n = 8) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Minty, M.; Lê, S.; Canceill, T.; Thomas, C.; Azalbert, V.; Loubieres, P.; Sun, J.; Sillam, J.; Terce, F.; Servant, F.; et al. Low-Diversity Microbiota in Apical Periodontitis and High Blood Pressure Are Signatures of the Severity of Apical Lesions in Humans. Int. J. Mol. Sci. 2023, 24, 1589. https://doi.org/10.3390/ijms24021589
Minty M, Lê S, Canceill T, Thomas C, Azalbert V, Loubieres P, Sun J, Sillam J, Terce F, Servant F, et al. Low-Diversity Microbiota in Apical Periodontitis and High Blood Pressure Are Signatures of the Severity of Apical Lesions in Humans. International Journal of Molecular Sciences. 2023; 24(2):1589. https://doi.org/10.3390/ijms24021589
Chicago/Turabian StyleMinty, Matthieu, Sylvie Lê, Thibault Canceill, Charlotte Thomas, Vincent Azalbert, Pascale Loubieres, Jiuwen Sun, Jonathan Sillam, François Terce, Florence Servant, and et al. 2023. "Low-Diversity Microbiota in Apical Periodontitis and High Blood Pressure Are Signatures of the Severity of Apical Lesions in Humans" International Journal of Molecular Sciences 24, no. 2: 1589. https://doi.org/10.3390/ijms24021589
APA StyleMinty, M., Lê, S., Canceill, T., Thomas, C., Azalbert, V., Loubieres, P., Sun, J., Sillam, J., Terce, F., Servant, F., Roulet, A., Ribiere, C., Ardouin, M., Mallet, J.-P., Burcelin, R., Diemer, F., Georgelin-Gurgel, M., & Blasco-Baque, V. (2023). Low-Diversity Microbiota in Apical Periodontitis and High Blood Pressure Are Signatures of the Severity of Apical Lesions in Humans. International Journal of Molecular Sciences, 24(2), 1589. https://doi.org/10.3390/ijms24021589








