Cortisol Interaction with Aquaporin-2 Modulates Its Water Permeability: Perspectives for Non-Genomic Effects of Corticosteroids
Abstract
1. Introduction
2. Results
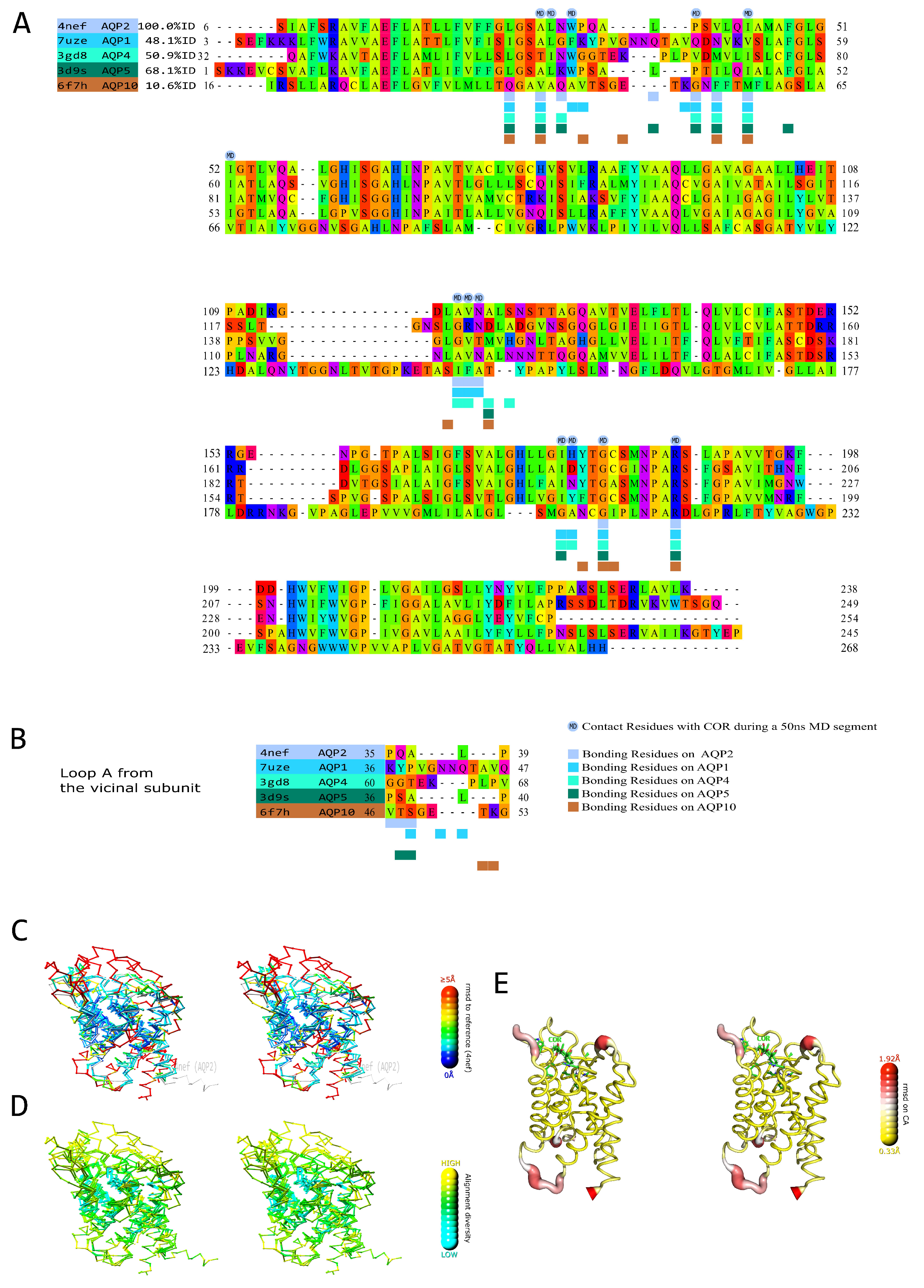
2.1. Prediction of the AQPs Corticosteroid Binding Site (ACBS) through Amino Acid Sequences and Structural Alignment
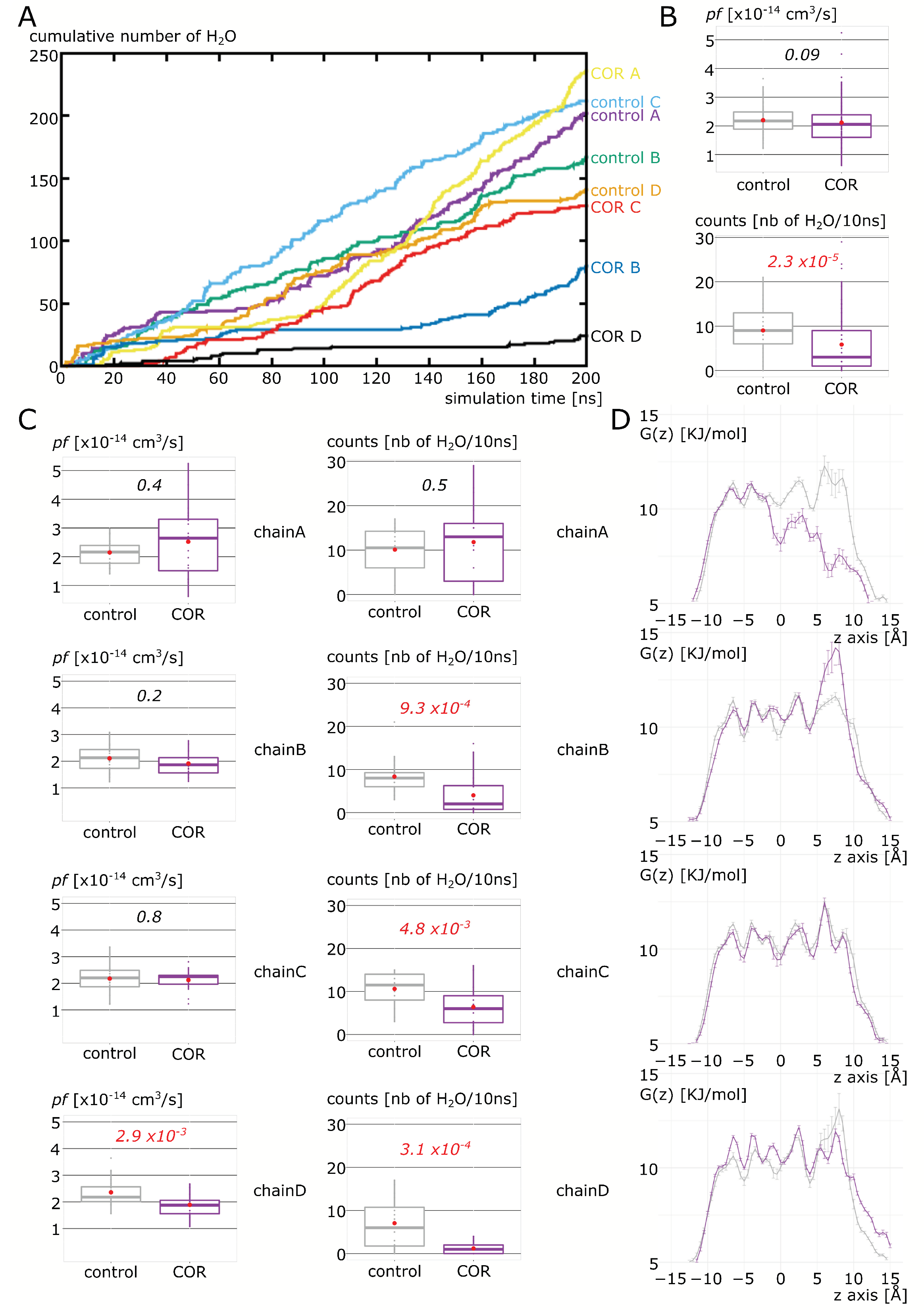
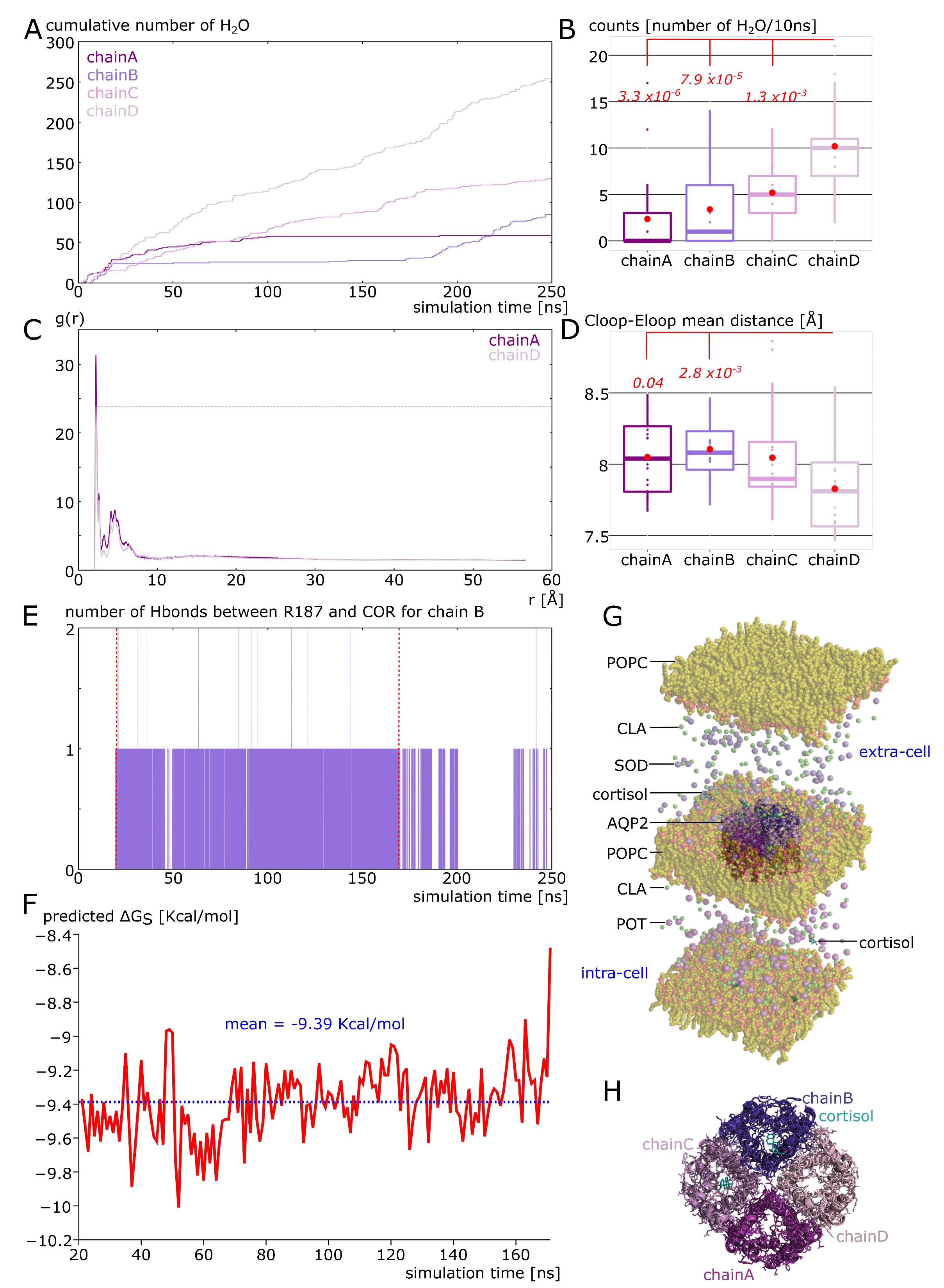
2.2. Cortisol Interaction with AQP2 Significantly Impairs Water Permeability
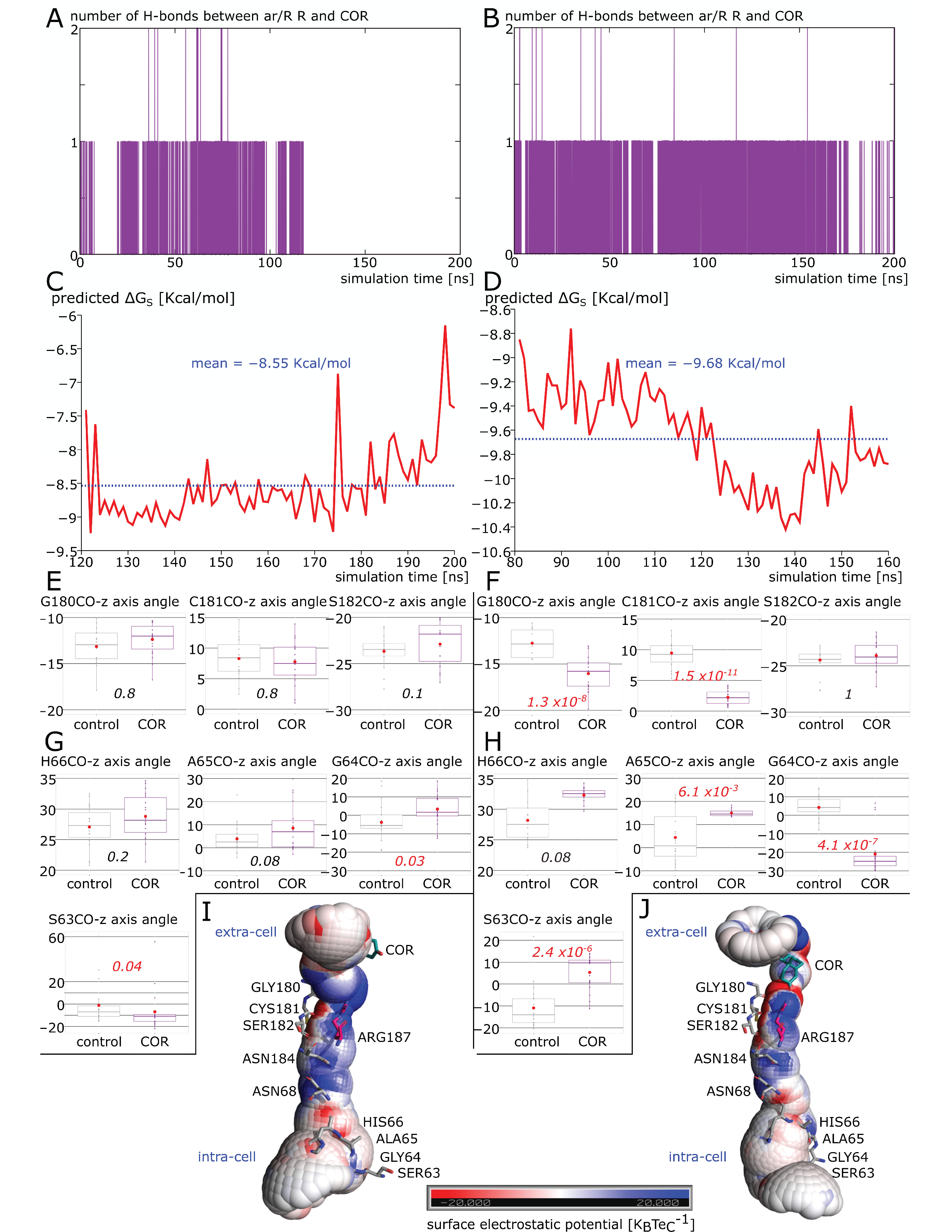
2.3. New Insights on Extra-Cellular C-Loop Regulatory Function: A Focus on Chain A
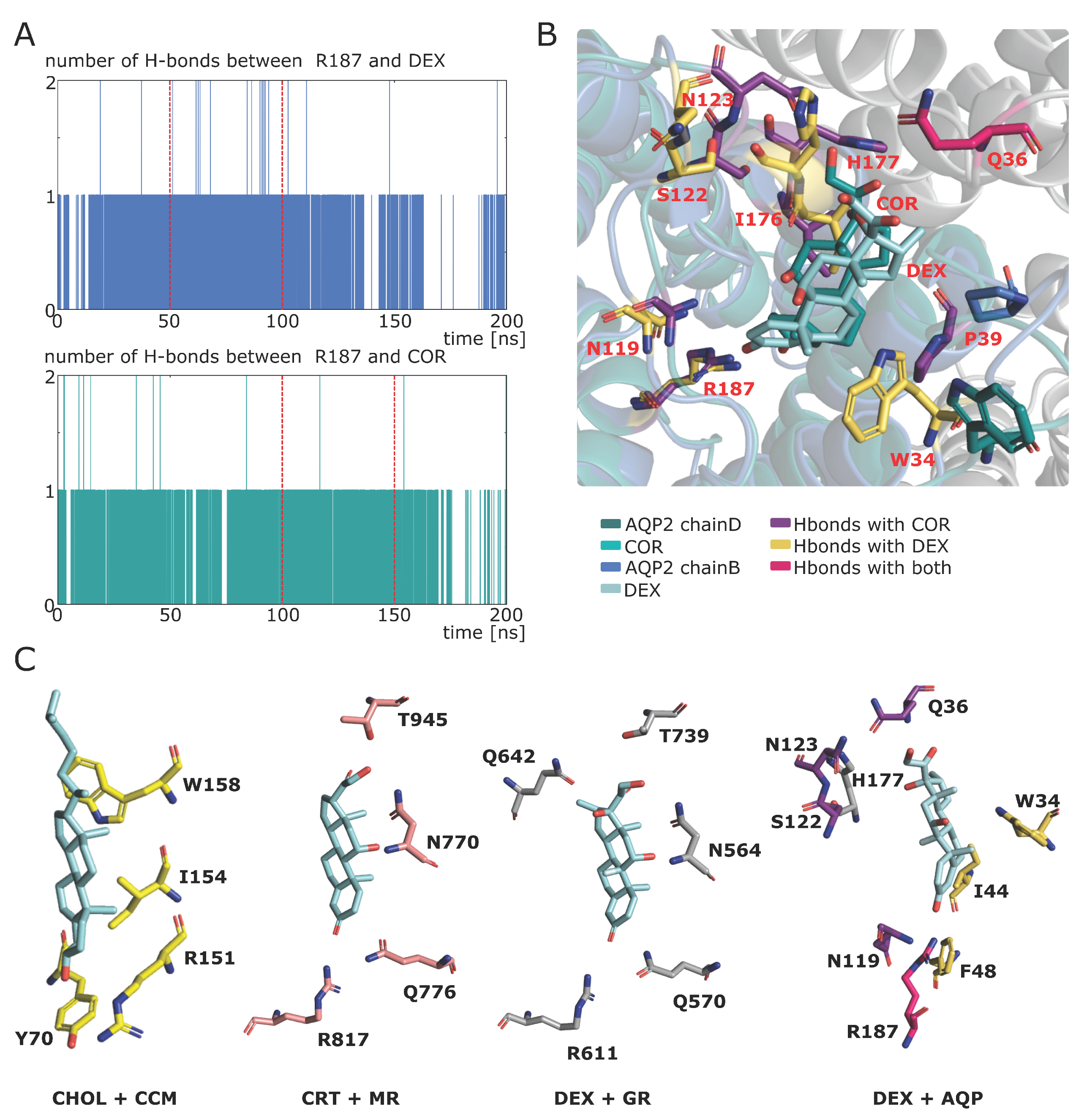
2.4. Putative Molecular Mechanism of AQP2 Water Channel Permeability Impairment by Cortisol
2.5. Spontaneous Binding of Cortisol with Human AQP2 Extra-Cellular Vestibules
2.6. Additional Insights Supporting the Regulatory Function of Ions Nature and of C-Loop
3. Discussion
3.1. Cortisol Is a Putative Ligand of AQP2
3.2. Implications for Steroid Non-Genomic Effects
4. Material and Methods
4.1. Molecular Dynamics Simulations
4.2. Analysis
4.2.1. Water Permeability
4.2.2. Free Energy Profiles
4.2.3. Binding Free Energy and Dissociation Constant
4.2.4. Other Properties
4.2.5. Statistical Analysis
4.2.6. Structural Alignment of Experimentally Solved Structures and Delineation of a Putative Conserved Binding Site to Corticoids
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Agre, P.; Sasaki, S.; Chrispeels, M.J. Aquaporins: A Family of Water Channel Proteins. Am. J. Physiol. 1993, 265, F461. [Google Scholar] [CrossRef] [PubMed]
- Preston, G.M.; Carroll, T.P.; Guggino, W.B.; Agre, P. Appearance of Water Channels in Xenopus Oocytes Expressing Red Cell CHIP28 Protein. Science 1992, 256, 385–387. [Google Scholar] [CrossRef] [PubMed]
- Beitz, E. Aquaporins; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2008; ISBN 978-3-540-79885-9. [Google Scholar]
- Abascal, F.; Irisarri, I.; Zardoya, R. Diversity and Evolution of Membrane Intrinsic Proteins. Biochim. Biophys. Acta BBA Gen. Subj. 2014, 1840, 1468–1481. [Google Scholar] [CrossRef] [PubMed]
- Chaumont, F.; Tyerman, S.D. Aquaporins: Highly Regulated Channels Controlling Plant Water Relations. Plant Physiol. 2014, 164, 1600–1618. [Google Scholar] [CrossRef] [PubMed]
- Takano, J.; Yoshinari, A.; Luu, D.-T. Plant Aquaporin Trafficking. In Plant Aquaporins: From Transport to Signaling; Chaumont, F., Tyerman, S.D., Eds.; Signaling and Communication in Plants; Springer International Publishing: Cham, Switzerland, 2017; pp. 47–81. ISBN 978-3-319-49395-4. [Google Scholar]
- Fenton, R.A.; Murali, S.K.; Moeller, H.B. Advances in Aquaporin-2 Trafficking Mechanisms and Their Implications for Treatment of Water Balance Disorders. Am. J. Physiol. Cell Physiol. 2020, 319, C1–C10. [Google Scholar] [CrossRef]
- Roche, J.V.; Törnroth-Horsefield, S. Aquaporin Protein-Protein Interactions. Int. J. Mol. Sci. 2017, 18, 2255. [Google Scholar] [CrossRef]
- Reichow, S.L.; Clemens, D.M.; Freites, J.A.; Németh-Cahalan, K.L.; Heyden, M.; Tobias, D.J.; Hall, J.E.; Gonen, T. Allosteric Mechanism of Water-Channel Gating by Ca2+–Calmodulin. Nat. Struct. Mol. Biol. 2013, 20, 1085–1092. [Google Scholar] [CrossRef]
- Hedfalk, K.; Törnroth-Horsefield, S.; Nyblom, M.; Johanson, U.; Kjellbom, P.; Neutze, R. Aquaporin Gating. Curr. Opin. Struct. Biol. 2006, 16, 447–456. [Google Scholar] [CrossRef]
- Törnroth-Horsefield, S.; Wang, Y.; Hedfalk, K.; Johanson, U.; Karlsson, M.; Tajkhorshid, E.; Neutze, R.; Kjellbom, P. Structural Mechanism of Plant Aquaporin Gating. Nature 2006, 439, 688–694. [Google Scholar] [CrossRef]
- Mom, R.; Muries, B.; Benoit, P.; Robert-Paganin, J.; Réty, S.; Venisse, J.-S.; Padua, A.; Label, P.; Auguin, D. Voltage-Gating of Aquaporins, a Putative Conserved Safety Mechanism during Ionic Stresses. FEBS Lett. 2021, 595, 41–57. [Google Scholar] [CrossRef]
- Ala, M.; Mohammad Jafari, R.; Hajiabbasi, A.; Dehpour, A.R. Aquaporins and Diseases Pathogenesis: From Trivial to Undeniable Involvements, a Disease-Based Point of View. J. Cell. Physiol. 2021, 236, 6115–6135. [Google Scholar] [CrossRef]
- Nielsen, S.; Kwon, T.-H.; Christensen, B.M.; Promeneur, D.; Frøkiær, J.; Marples, D. Physiology and Pathophysiology of Renal Aquaporins. JASN 1999, 10, 647–663. [Google Scholar] [CrossRef]
- Nielsen, S.; Frøkiaer, J.; Marples, D.; Kwon, T.-H.; Agre, P.; Knepper, M.A. Aquaporins in the Kidney: From Molecules to Medicine. Physiol. Rev. 2002, 82, 205–244. [Google Scholar] [CrossRef]
- Abir-Awan, M.; Kitchen, P.; Salman, M.M.; Conner, M.T.; Conner, A.C.; Bill, R.M. Inhibitors of Mammalian Aquaporin Water Channels. Int. J. Mol. Sci. 2019, 20, 1589. [Google Scholar] [CrossRef]
- Tradtrantip, L.; Jin, B.-J.; Yao, X.; Anderson, M.O.; Verkman, A.S. Aquaporin-Targeted Therapeutics: State-of-the-Field. In Aquaporins; Yang, B., Ed.; Advances in Experimental Medicine and Biology; Springer: Dordrecht, The Netherlands, 2017; pp. 239–250. ISBN 978-94-024-1057-0. [Google Scholar]
- Mom, R.; Robert-Paganin, J.; Mom, T.; Chabbert, C.; Réty, S.; Auguin, D. A Perspective for Ménière’s Disease: In Silico Investigations of Dexamethasone as a Direct Modulator of AQP2. Biomolecules 2022, 12, 511. [Google Scholar] [CrossRef]
- Williams, D.M. Clinical Pharmacology of Corticosteroids. Respir. Care 2018, 63, 655–670. [Google Scholar] [CrossRef]
- Ericson-Neilsen, W.; Kaye, A.D. Steroids: Pharmacology, Complications, and Practice Delivery Issues. Ochsner. J. 2014, 14, 203–207. [Google Scholar]
- Reul, J.M.; de Kloet, E.R. Two Receptor Systems for Corticosterone in Rat Brain: Microdistribution and Differential Occupation. Endocrinology 1985, 117, 2505–2511. [Google Scholar] [CrossRef]
- Hutchison, K.A.; Dittmar, K.D.; Pratt, W.B. All of the Factors Required for Assembly of the Glucocorticoid Receptor into a Functional Heterocomplex with Heat Shock Protein 90 Are Preassociated in a Self-Sufficient Protein Folding Structure, a “Foldosome”. J. Biol. Chem. 1994, 269, 27894–27899. [Google Scholar] [CrossRef]
- Smith, D.F.; Toft, D.O. Steroid Receptors and Their Associated Proteins. Mol. Endocrinol. 1993, 7, 4–11. [Google Scholar] [CrossRef]
- Bodwell, J.E.; Ortí, E.; Coull, J.M.; Pappin, D.J.; Smith, L.I.; Swift, F. Identification of Phosphorylated Sites in the Mouse Glucocorticoid Receptor. J. Biol. Chem. 1991, 266, 7549–7555. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, R.; Wada, H.; Ito, K.; Adcock, I.M. Effects of Glucocorticoids on Gene Transcription. Eur. J. Pharm. 2004, 500, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Zilliacus, J.; Wright, A.P.; Carlstedt-Duke, J.; Gustafsson, J.A. Structural Determinants of DNA-Binding Specificity by Steroid Receptors. Mol. Endocrinol. 1995, 9, 389–400. [Google Scholar] [CrossRef] [PubMed]
- Haller, J.; Mikics, E.; Makara, G.B. The Effects of Non-Genomic Glucocorticoid Mechanisms on Bodily Functions and the Central Neural System. A Critical Evaluation of Findings. Front. Neuroendocr. 2008, 29, 273–291. [Google Scholar] [CrossRef] [PubMed]
- Groeneweg, F.L.; Karst, H.; de Kloet, E.R.; Joëls, M. Mineralocorticoid and Glucocorticoid Receptors at the Neuronal Membrane, Regulators of Nongenomic Corticosteroid Signalling. Mol. Cell. Endocrinol. 2012, 350, 299–309. [Google Scholar] [CrossRef]
- Levin, E.R. Rapid Signaling by Steroid Receptors. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008, 295, R1425–R1430. [Google Scholar] [CrossRef]
- Li, Y.; Suino, K.; Daugherty, J.; Xu, H.E. Structural and Biochemical Mechanisms for the Specificity of Hormone Binding and Coactivator Assembly by Mineralocorticoid Receptor. Mol. Cell 2005, 19, 367–380. [Google Scholar] [CrossRef]
- Bledsoe, R.K.; Montana, V.G.; Stanley, T.B.; Delves, C.J.; Apolito, C.J.; McKee, D.D.; Consler, T.G.; Parks, D.J.; Stewart, E.L.; Willson, T.M.; et al. Crystal Structure of the Glucocorticoid Receptor Ligand Binding Domain Reveals a Novel Mode of Receptor Dimerization and Coactivator Recognition. Cell 2002, 110, 93–105. [Google Scholar] [CrossRef]
- Hanson, M.A.; Cherezov, V.; Griffith, M.T.; Roth, C.B.; Jaakola, V.-P.; Chien, E.Y.T.; Velasquez, J.; Kuhn, P.; Stevens, R.C. A Specific Cholesterol Binding Site Is Established by the 2.8 Å Structure of the Human Β2-Adrenergic Receptor. Structure 2008, 16, 897–905. [Google Scholar] [CrossRef]
- Hadidi, H.; Kamali, R.; Binesh, A. Investigation of the Aquaporin-2 Gating Mechanism with Molecular Dynamics Simulations. Proteins Struct. Funct. Bioinform. 2021, 89, 819–831. [Google Scholar] [CrossRef]
- Hub, J.; de Groot, B.L. Mechanism of Selectivity in Aquaporins and Aquaglyceroporins. Proc. Natl. Acad. Sci. USA 2008, 105, 1198–1203. [Google Scholar] [CrossRef]
- Beitz, E.; Wu, B.; Holm, L.M.; Schultz, J.E.; Zeuthen, T. Point Mutations in the Aromatic/Arginine Region in Aquaporin 1 Allow Passage of Urea, Glycerol, Ammonia, and Protons. Proc. Natl. Acad. Sci. USA 2006, 103, 269–274. [Google Scholar] [CrossRef]
- Hub, J.S.; Aponte-Santamaría, C.; Grubmüller, H.; de Groot, B.L. Voltage-Regulated Water Flux through Aquaporin Channels In Silico. Biophys. J. 2010, 99, L97–L99. [Google Scholar] [CrossRef]
- Aponte-Santamaria, C.A. Understanding the Molecular Machinery of Aquaporins through Molecular Dynamics Simulations. Doctoral Dissertation, Georg-August-Universität Göttingen, Göttingen, Germany, 2012. [Google Scholar] [CrossRef]
- Mom, R. Functional Exploration of Aquaporins through Structural Modeling. Impact on the Leaf Hydraulic Conductance in Poplar; 2020. Available online: https://www.theses.fr/2020CLFAC050 (accessed on 7 December 2022).
- Tajkhorshid, E.; Nollert, P.; Jensen, M.Ø.; Miercke, L.J.W.; O’Connell, J.; Stroud, R.M.; Schulten, K. Control of the Selectivity of the Aquaporin Water Channel Family by Global Orientational Tuning. Science 2002, 296, 525–530. [Google Scholar] [CrossRef]
- Walz, T.; Fujiyoshi, Y.; Engel, A. The AQP Structure and Functional Implications. In Handbook of Experimental Pharmacology; Springer: Berlin/Heidelberg, Germany, 2009; pp. 31–56. [Google Scholar] [CrossRef]
- De Groot, B.L.; Frigato, T.; Helms, V.; Grubmüller, H. The Mechanism of Proton Exclusion in the Aquaporin-1 Water Channel. J. Mol. Biol. 2003, 333, 279–293. [Google Scholar] [CrossRef]
- Ma, W.; Yang, L.; He, L. Overview of the Detection Methods for Equilibrium Dissociation Constant KD of Drug-Receptor Interaction. J. Pharm. Anal. 2018, 8, 147–152. [Google Scholar] [CrossRef]
- Horner, A.; Siligan, C.; Cornean, A.; Pohl, P. Positively Charged Residues at the Channel Mouth Boost Single-File Water Flow. Faraday Discuss. 2018, 209, 55–65. [Google Scholar] [CrossRef]
- Gössweiner-Mohr, N.; Siligan, C.; Pluhackova, K.; Umlandt, L.; Köfler, S.; Trajkovska, N.; Horner, A. The Hidden Intricacies of Aquaporins: Remarkable Details in a Common Structural Scaffold. Small 2022, 18, 2202056. [Google Scholar] [CrossRef]
- Atkovska, K.; Klingler, J.; Oberwinkler, J.; Keller, S.; Hub, J.S. Rationalizing Steroid Interactions with Lipid Membranes: Conformations, Partitioning, and Kinetics. ACS Cent. Sci. 2018, 4, 1155–1165. [Google Scholar] [CrossRef]
- Boldyreff, B.; Wehling, M. Rapid Aldosterone Actions: From the Membrane to Signaling Cascades to Gene Transcription and Physiological Effects. J. Steroid Biochem. Mol. Biol. 2003, 85, 375–381. [Google Scholar] [CrossRef]
- Grossmann, C.; Gekle, M. New Aspects of Rapid Aldosterone Signaling. Mol. Cell. Endocrinol. 2009, 308, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Vinson, G.P.; Coghlan, J.P. Expanding View of Aldosterone Action, with an Emphasis on Rapid Action. Clin. Exp. Pharm. Physiol. 2010, 37, 410–416. [Google Scholar] [CrossRef] [PubMed]
- Karst, H.; den Boon, F.S.; Vervoort, N.; Adrian, M.; Kapitein, L.C.; Joëls, M. Non-Genomic Steroid Signaling through the Mineralocorticoid Receptor: Involvement of a Membrane-Associated Receptor? Mol. Cell. Endocrinol. 2022, 541, 111501. [Google Scholar] [CrossRef] [PubMed]
- Nilius, B.; Voets, T. A TRP Channel-Steroid Marriage. Nat. Cell Biol. 2008, 10, 1383–1384. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Kumari, S.; Majhi, R.K.; Swain, N.; Yadav, M.; Goswami, C. Regulation of TRP Channels by Steroids: Implications in Physiology and Diseases. Gen. Comp. Endocrinol. 2015, 220, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Méndez-Reséndiz, K.A.; Enciso-Pablo, Ó.; González-Ramírez, R.; Juárez-Contreras, R.; Rosenbaum, T.; Morales-Lázaro, S.L. Steroids and TRP Channels: A Close Relationship. Int. J. Mol. Sci. 2020, 21, 3819. [Google Scholar] [CrossRef]
- Liedtke, W. Molecular Mechanisms of TRPV4-Mediated Neural Signaling. Ann. N. Y. Acad. Sci. 2008, 1144, 42–52. [Google Scholar] [CrossRef]
- Mutai, H.; Heller, S. Vertebrate and Invertebrate TRPV-like Mechanoreceptors. Cell Calcium 2003, 33, 471–478. [Google Scholar] [CrossRef]
- Asuthkar, S.; Elustondo, P.A.; Demirkhanyan, L.; Sun, X.; Baskaran, P.; Velpula, K.K.; Thyagarajan, B.; Pavlov, E.V.; Zakharian, E. The TRPM8 Protein Is a Testosterone Receptor: I. Biochemical Evidence for Direct TRPM8-Testosterone Interactions. J. Biol. Chem. 2015, 290, 2659–2669. [Google Scholar] [CrossRef]
- Benfenati, V.; Caprini, M.; Dovizio, M.; Mylonakou, M.N.; Ferroni, S.; Ottersen, O.P.; Amiry-Moghaddam, M. An Aquaporin-4/Transient Receptor Potential Vanilloid 4 (AQP4/TRPV4) Complex Is Essential for Cell-Volume Control in Astrocytes. Proc. Natl. Acad. Sci. USA 2011, 108, 2563–2568. [Google Scholar] [CrossRef]
- Galizia, L.; Pizzoni, A.; Fernandez, J.; Rivarola, V.; Capurro, C.; Ford, P. Functional Interaction between AQP2 and TRPV4 in Renal Cells. J. Cell. Biochem. 2012, 113, 580–589. [Google Scholar] [CrossRef]
- Liu, X.; Bandyopadhyay, B.C.; Bandyopadhyay, B.; Nakamoto, T.; Singh, B.; Liedtke, W.; Melvin, J.E.; Ambudkar, I. A Role for AQP5 in Activation of TRPV4 by Hypotonicity: Concerted Involvement of AQP5 and TRPV4 in Regulation of Cell Volume Recovery. J. Biol. Chem. 2006, 281, 15485–15495. [Google Scholar] [CrossRef]
- Salman, M.M.; Kitchen, P.; Woodroofe, M.N.; Brown, J.E.; Bill, R.M.; Conner, A.C.; Conner, M.T. Hypothermia Increases Aquaporin 4 (AQP4) Plasma Membrane Abundance in Human Primary Cortical Astrocytes via a Calcium/Transient Receptor Potential Vanilloid 4 (TRPV4)- and Calmodulin-Mediated Mechanism. Eur. J. Neurosci. 2017, 46, 2542–2547. [Google Scholar] [CrossRef]
- Taguchi, D.; Takeda, T.; Kakigi, A.; Takumida, M.; Nishioka, R.; Kitano, H. Expressions of Aquaporin-2, Vasopressin Type 2 Receptor, Transient Receptor Potential Channel Vanilloid (TRPV)1, and TRPV4 in the Human Endolymphatic Sac. Laryngoscope 2007, 117, 695–698. [Google Scholar] [CrossRef]
- Day, R.E.; Kitchen, P.; Owen, D.S.; Bland, C.; Marshall, L.; Conner, A.C.; Bill, R.M.; Conner, M.T. Human Aquaporins: Regulators of Transcellular Water Flow. Biochim. Biophys. Acta BBA Gen. Subj. 2014, 1840, 1492–1506. [Google Scholar] [CrossRef]
- Fushimi, K.; Uchida, S.; Harat, Y.; Hirata, Y.; Marumo, F.; Sasaki, S. Cloning and Expression of Apical Membrane Water Channel of Rat Kidney Collecting Tubule. Nature 1993, 361, 549–552. [Google Scholar] [CrossRef]
- Kwon, T.-H.; Nielsen, J.; Møller, H.B.; Fenton, R.A.; Nielsen, S.; Frøkiaer, J. Aquaporins in the Kidney. In Handbook of Experimental Pharmacology; Springer: Berlin/Heidelberg, Germany, 2009; pp. 95–132. [Google Scholar] [CrossRef]
- Briet, M.; Schiffrin, E.L. Aldosterone: Effects on the Kidney and Cardiovascular System. Nat. Rev. Nephrol. 2010, 6, 261–273. [Google Scholar] [CrossRef]
- Arlt, W.; Stewart, P.M. Adrenal Corticosteroid Biosynthesis, Metabolism, and Action. Endocrinol. Metab. Clin. N. Am. 2005, 34, 293–313. [Google Scholar] [CrossRef]
- Marver, D. Evidence of Corticosteroid Action along the Nephron. Am. J. Physiol. 1984, 246, F111–F123. [Google Scholar] [CrossRef]
- Raff, H. Glucocorticoid Inhibition of Neurohypophysial Vasopressin Secretion. Am. J. Physiol. 1987, 252, R635–R644. [Google Scholar] [CrossRef]
- Grossmann, C.; Benesic, A.; Krug, A.W.; Freudinger, R.; Mildenberger, S.; Gassner, B.; Gekle, M. Human Mineralocorticoid Receptor Expression Renders Cells Responsive for Nongenotropic Aldosterone Actions. Mol. Endocrinol. 2005, 19, 1697–1710. [Google Scholar] [CrossRef] [PubMed]
- Maier, C.; Rünzler, D.; Schindelar, J.; Grabner, G.; Waldhäusl, W.; Köhler, G.; Luger, A. G-Protein-Coupled Glucocorticoid Receptors on the Pituitary Cell Membrane. J. Cell Sci. 2005, 118, 3353–3361. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, K.L.; Malisch, J.L.; Breuner, C.W.; Soma, K.K. Corticosterone and Cortisol Binding Sites in Plasma, Immune Organs and Brain of Developing Zebra Finches: Intracellular and Membrane-Associated Receptors. Brain Behav. Immun. 2010, 24, 908–918. [Google Scholar] [CrossRef] [PubMed]
- Breuner, C.W.; Orchinik, M. Pharmacological Characterization of Intracellular, Membrane, and Plasma Binding Sites for Corticosterone in House Sparrows. Gen. Comp. Endocrinol. 2009, 163, 214–224. [Google Scholar] [CrossRef] [PubMed]
- Orchinik, M.; Murray, T.F.; Moore, F.L. A Corticosteroid Receptor in Neuronal Membranes. Science 1991, 252, 1848–1851. [Google Scholar] [CrossRef]
- Orchinik, M.; Murray, T.F.; Franklin, P.H.; Moore, F.L. Guanyl Nucleotides Modulate Binding to Steroid Receptors in Neuronal Membranes. Proc. Natl. Acad. Sci. USA 1992, 89, 3830–3834. [Google Scholar] [CrossRef]
- Orchinik, M.; Hastings, N.; Witt, D.; McEwen, B.S. High-Affinity Binding of Corticosterone to Mammalian Neuronal Membranes: Possible Role of Corticosteroid Binding Globulin. J. Steroid Biochem. Mol. Biol. 1997, 60, 229–236. [Google Scholar] [CrossRef]
- Orchinik, M.; Matthews, L.; Gasser, P.J. Distinct Specificity for Corticosteroid Binding Sites in Amphibian Cytosol, Neuronal Membranes, and Plasma. Gen. Comp. Endocrinol. 2000, 118, 284–301. [Google Scholar] [CrossRef]
- Guo, Z.; Chen, Y.Z.; Xu, R.B.; Fu, H. Binding Characteristics of Glucocorticoid Receptor in Synaptic Plasma Membrane from Rat Brain. Funct. Neurol. 1995, 10, 183–194. [Google Scholar]
- Vázquez-Juárez, E.; Ramos-Mandujano, G.; Hernández-Benítez, R.; Pasantes-Morales, H. On the Role of G-Protein Coupled Receptors in Cell Volume Regulation. CPB 2008, 21, 001–014. [Google Scholar] [CrossRef]
- Strock, J.; Diversé-Pierluissi, M.A. Ca2+ Channels as Integrators of G Protein-Mediated Signaling in Neurons. Mol. Pharm. 2004, 66, 1071–1076. [Google Scholar] [CrossRef]
- Abraham, M.J.; Murtola, T.; Schulz, R.; Páll, S.; Smith, J.C.; Hess, B.; Lindahl, E. GROMACS: High Performance Molecular Simulations through Multi-Level Parallelism from Laptops to Supercomputers. SoftwareX 2015, 1–2, 19–25. [Google Scholar] [CrossRef]
- Huang, J.; Rauscher, S.; Nawrocki, G.; Ran, T.; Feig, M.; de Groot, B.L.; Grubmüller, H.; MacKerell, A.D. CHARMM36m: An Improved Force Field for Folded and Intrinsically Disordered Proteins. Nat. Methods 2017, 14, 71–73. [Google Scholar] [CrossRef]
- Lee, J.; Cheng, X.; Swails, J.M.; Yeom, M.S.; Eastman, P.K.; Lemkul, J.A.; Wei, S.; Buckner, J.; Jeong, J.C.; Qi, Y.; et al. CHARMM-GUI Input Generator for NAMD, GROMACS, AMBER, OpenMM, and CHARMM/OpenMM Simulations Using the CHARMM36 Additive Force Field. J. Chem. Theory Comput. 2016, 12, 405–413. [Google Scholar] [CrossRef]
- Wu, E.L.; Cheng, X.; Jo, S.; Rui, H.; Song, K.C.; Dávila-Contreras, E.M.; Qi, Y.; Lee, J.; Monje-Galvan, V.; Venable, R.M.; et al. CHARMM-GUI Membrane Builder toward Realistic Biological Membrane Simulations. J. Comput. Chem. 2014, 35, 1997–2004. [Google Scholar] [CrossRef]
- Gowers, R.J.; Linke, M.; Barnoud, J.; Reddy, T.J.E.; Melo, M.N.; Seyler, S.L.; Domański, J.; Dotson, D.L.; Buchoux, S.; Kenney, I.M.; et al. MDAnalysis: A Python Package for the Rapid Analysis of Molecular Dynamics Simulations. In Proceedings of the 15th Python in Science Conference, Austin, TX, USA, 11–17 July 2016; pp. 98–105. [Google Scholar] [CrossRef]
- Michaud-Agrawal, N.; Denning, E.J.; Woolf, T.B.; Beckstein, O. MDAnalysis: A Toolkit for the Analysis of Molecular Dynamics Simulations. J. Comput. Chem. 2011, 32, 2319–2327. [Google Scholar] [CrossRef]
- Zhu, F.; Tajkhorshid, E.; Schulten, K. Collective Diffusion Model for Water Permeation through Microscopic Channels. Phys. Rev. Lett. 2004, 93, 224501. [Google Scholar] [CrossRef]
- Vangone, A.; Schaarschmidt, J.; Koukos, P.; Geng, C.; Citro, N.; Trellet, M.E.; Xue, L.C.; Bonvin, A.M.J.J. Large-Scale Prediction of Binding Affinity in Protein-Small Ligand Complexes: The PRODIGY-LIG Web Server. Bioinformatics 2019, 35, 1585–1587. [Google Scholar] [CrossRef]
- Kurkcuoglu, Z.; Koukos, P.I.; Citro, N.; Trellet, M.E.; Rodrigues, J.P.G.L.M.; Moreira, I.S.; Roel-Touris, J.; Melquiond, A.S.J.; Geng, C.; Schaarschmidt, J.; et al. Performance of HADDOCK and a Simple Contact-Based Protein-Ligand Binding Affinity Predictor in the D3R Grand Challenge 2. J. Comput. Aided Mol. Des. 2018, 32, 175–185. [Google Scholar] [CrossRef]
- Smart, O.S.; Neduvelil, J.G.; Wang, X.; Wallace, B.A.; Sansom, M.S.P. HOLE: A Program for the Analysis of the Pore Dimensions of Ion Channel Structural Models. J. Mol. Graph. 1996, 14, 354–360. [Google Scholar] [CrossRef]
- Guex, N.; Peitsch, M.C. SWISS-MODEL and the Swiss-PdbViewer: An Environment for Comparative Protein Modeling. Electrophoresis 1997, 18, 2714–2723. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mom, R.; Réty, S.; Auguin, D. Cortisol Interaction with Aquaporin-2 Modulates Its Water Permeability: Perspectives for Non-Genomic Effects of Corticosteroids. Int. J. Mol. Sci. 2023, 24, 1499. https://doi.org/10.3390/ijms24021499
Mom R, Réty S, Auguin D. Cortisol Interaction with Aquaporin-2 Modulates Its Water Permeability: Perspectives for Non-Genomic Effects of Corticosteroids. International Journal of Molecular Sciences. 2023; 24(2):1499. https://doi.org/10.3390/ijms24021499
Chicago/Turabian StyleMom, Robin, Stéphane Réty, and Daniel Auguin. 2023. "Cortisol Interaction with Aquaporin-2 Modulates Its Water Permeability: Perspectives for Non-Genomic Effects of Corticosteroids" International Journal of Molecular Sciences 24, no. 2: 1499. https://doi.org/10.3390/ijms24021499
APA StyleMom, R., Réty, S., & Auguin, D. (2023). Cortisol Interaction with Aquaporin-2 Modulates Its Water Permeability: Perspectives for Non-Genomic Effects of Corticosteroids. International Journal of Molecular Sciences, 24(2), 1499. https://doi.org/10.3390/ijms24021499







