Lignin Degradation via Chlorine Dioxide at Room Temperature: Chemical Groups and Structural Characterization
Abstract
1. Introduction
2. Results and Discussion
2.1. Optimization of ClO2 Degradation-Process Conditions
2.2. Structural Characterizations of IL before and after Degradation
2.2.1. FT-IR Analysis
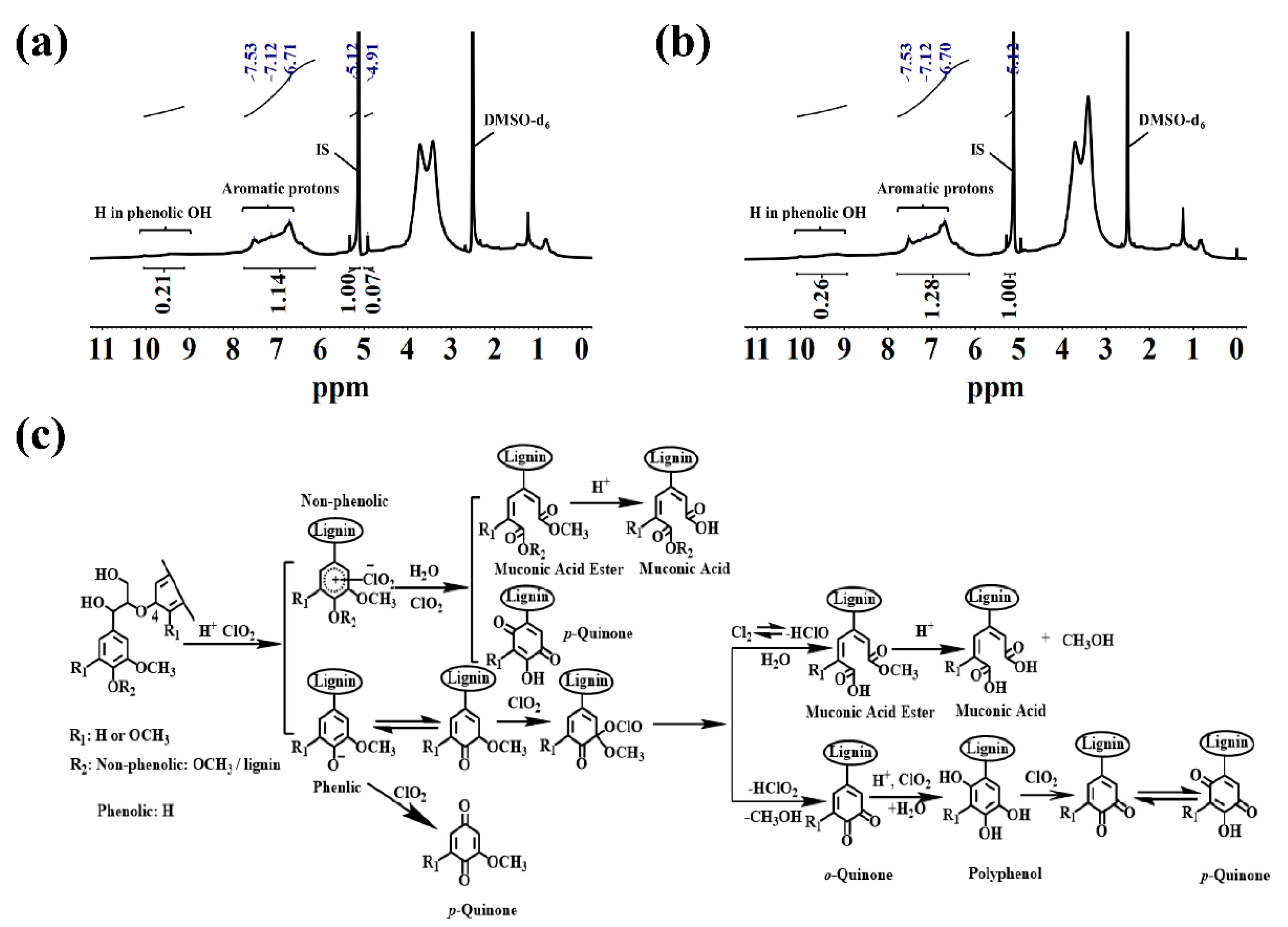
2.2.2. 1H NMR Analysis
2.2.3. 2D-HSQC Analysis
2.2.4. XPS Analysis
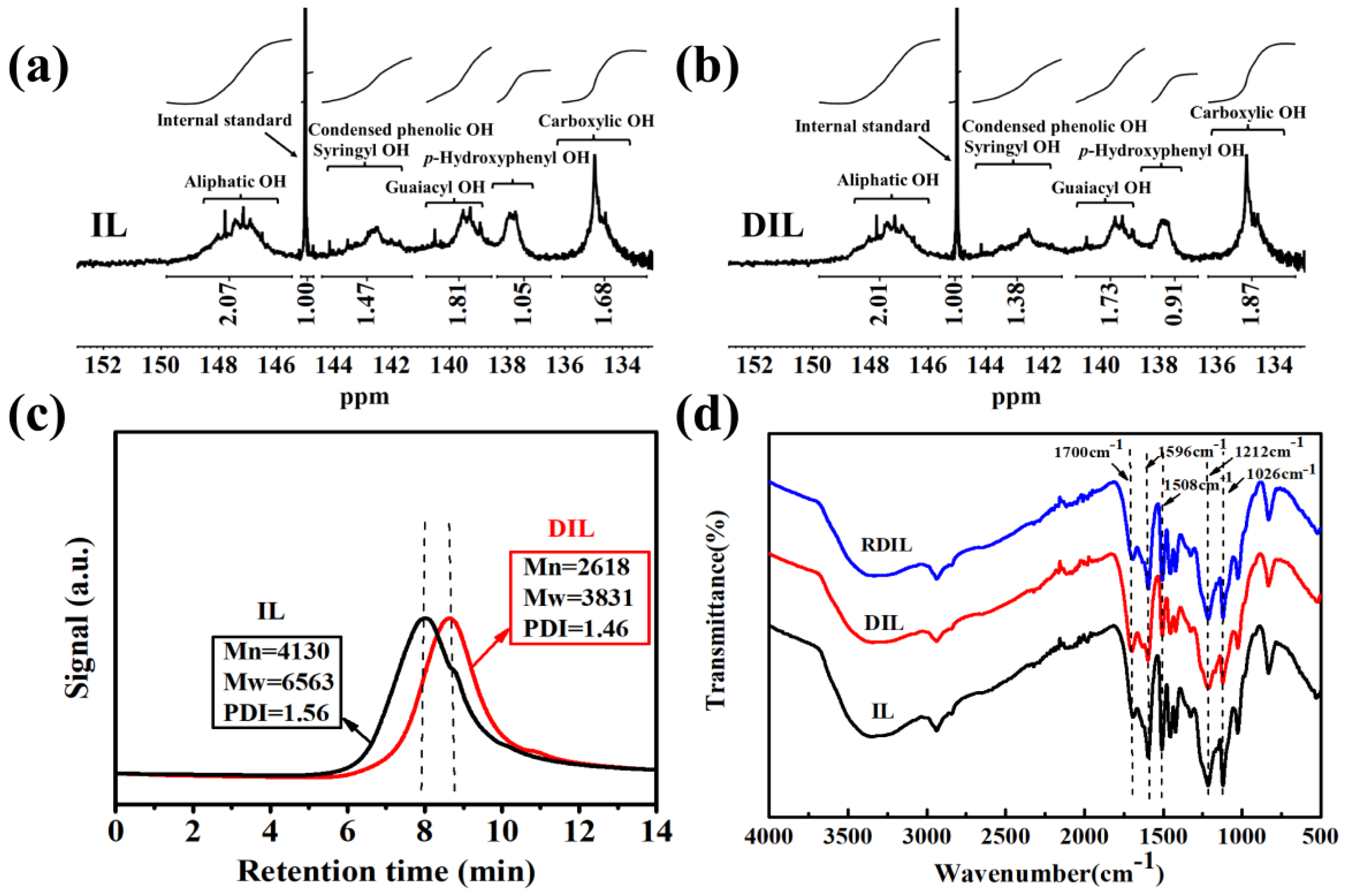
2.2.5. 31P NMR Analysis
2.2.6. GPC Analysis
2.3. Reduction Structural Analysis of DIL
2.3.1. FT-IR Analysis
2.3.2. 1H NMR Analysis
2.4. Possible Degradation Mechanism
3. Materials and Methods
3.1. Materials
3.2. Preparation of ClO2 Solution
3.3. Degradation of Lignin by ClO2
3.4. Reduction of DIL by NaBH4
3.5. Characterizations
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, C.; Zhao, X.; Wang, A.; Huber, G.W.; Zhang, T. Catalytic transformation of lignin for the production of chemicals and fuels. Chem. Rev. 2015, 115, 11559–11624. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.H.; Xia, X.; Lin, C.X.; Tonga, D.S.; Beltraminib, J. Catalytic conversion of lignocellulosic biomass to fine chemicals and fuels. Chem. Soc. Rev. 2011, 40, 5588–5617. [Google Scholar] [CrossRef] [PubMed]
- Ji, Q.; Yu, X.; Wu, P.; Yagoub, A.E.G.A.; Chen, L.; Taiye, M.A.; Zhou, C. Pretreatment of sugarcane bagasse with deep eutectic solvents affect the structure and morphology of lignin. Ind. Crop. Prod. 2021, 173, 114108. [Google Scholar] [CrossRef]
- Cotana, F.; Cavalaglio, G.; Nicolini, A.; Gelosia, M.; Coccia, V.; Petrozzi, A.; Brinchi, L. Lignin as co-product of second generation bioethanol production from ligno-cellulosic biomass. Energy Proc. 2014, 45, 52–60. [Google Scholar] [CrossRef]
- Branco, R.H.R.; Serafim, L.S.; Xavier, A.M.R.B. Second generation bioethanol production: On the use of pulp and paper industry wastes as feedstock. Fermentation 2018, 5, 4. [Google Scholar] [CrossRef]
- Liao, J.J.; Abd Latif, N.H.; Trache, D.; Brosse, N.; Hussin, M.H. Current advancement on the isolation, characterization and application of lignin. Int. J. Biol. Macromol. 2020, 162, 985–1024. [Google Scholar] [CrossRef]
- Lavoie, J.M.; Baré, W.; Bilodeau, M. Depolymerization of steam-treated lignin for the production of green chemicals. Bioresour. Technol. 2011, 102, 4917–4920. [Google Scholar] [CrossRef]
- Gasson, J.R.; Forchheim, D.; Sutter, T.; Hornung, U.; Kruse, A.; Barth, T. Modeling the lignin degradation kinetics in an ethanol/formic acid solvolysis approach. Part 1. Kinetic model development. Ind. Eng. Chem. Res. 2012, 51, 10595–10606. [Google Scholar] [CrossRef]
- Mahmood, N.; Yuan, Z.; Schmidt, J.; Xu, C.C. Hydrolytic depolymerization of hydrolysis lignin: Effects of catalysts and solvents. Bioresour. Technol. 2015, 190, 416–419. [Google Scholar] [CrossRef]
- Rana, M.; Nshizirungu, T.; Park, J.H. Synergistic effect of water-ethanol-formic acid for the depolymerization of industrial waste (black liquor) lignin to phenolic monomers. Biomass. Bioenerg. 2021, 153, 106204. [Google Scholar] [CrossRef]
- Gordon, G.; Rosenblatt, A.A. Chlorine dioxide: The current state of the art. Ozone-Sci. Eng. 2005, 27, 203–207. [Google Scholar] [CrossRef]
- del Río, J.C.; Gutiérrez, A.; Romero, J.; Martınez, M.J.; Martınez, A.T. Identification of residual lignin markers in eucalypt kraft pulps by Py–GC/MS. J Anal. Appl. Pyrol. 2001, 58, 425–439. [Google Scholar] [CrossRef]
- Rencoret, J.; Marques, G.; Gutiérrez, A.; Jiménez-Barbero, J.; Martínez, A.T.; del Río, J.C. Structural modifications of residual lignins from sisal and flax pulps during soda-AQ pulping and TCF/ECF bleaching. Ind. Eng. Chem. Res. 2013, 52, 4695–4703. [Google Scholar] [CrossRef]
- Wang, H.M.; Sun, Y.C.; Wang, B.; Sun, D.; Shi, Q.; Zheng, L.; Sun, R.C. Insights into the structural changes and potentials of lignin from bagasse during the integrated delignification process. ACS Sustain. Chem. Eng. 2019, 7, 13886–13897. [Google Scholar] [CrossRef]
- Wufuer, A.; Wang, Y.; Dai, L. Enhanced lignin degradation by a two-step acidic protic bio-based ionic liquid pretreatment method. Biomass. Convers. Biorefin. 2021, 1–10. [Google Scholar] [CrossRef]
- Dai, J.; Nanayakkara, S.; Lamb, T.C.; Clark, A.J.; Guo, S.X.; Zhang, J.; Saito, K. Effect of the N-based ligands in copper complexes for depolymerisation of lignin. New J. Chem. 2016, 40, 3511–3519. [Google Scholar] [CrossRef]
- Shi, L.; Ge, J.; Zhang, F.; Nie, S.; Qin, C.; Yao, S. Difference in adsorbable organic halogen formation between phenolic and non-phenolic lignin model compounds in chlorine dioxide bleaching. R. Soc. Open Sci. 2019, 6, 191202. [Google Scholar] [CrossRef]
- Qin, C.; Zeng, H.; Liu, B.; Zhu, J.; Wang, F.; Wang, S.; Yao, S. Efficient removal of residual lignin from eucalyptus pulp via high-concentration chlorine dioxide treatment and its effect on the properties of residual solids. Bioresour. Technol. 2022, 360, 127621. [Google Scholar] [CrossRef]
- Shen, Q.; Fu, Z.; Li, R.; Wu, Y. A study on the pyrolysis mechanism of a β-O-4 lignin dimer model compound using DFT combined with Py-GC/MS. J. Therm. Anal. Calorim. 2021, 146, 1751–1761. [Google Scholar] [CrossRef]
- Chio, C.; Sain, M.; Qin, W. Lignin utilization: A review of lignin depolymerization from various aspects. Renew. Sustain. Energy Rev. 2019, 107, 232–249. [Google Scholar] [CrossRef]
- Amit, T.A.; Roy, R.; Raynie, D.E. Thermal and structural characterization of two commercially available technical lignins for potential depolymerization via hydrothermal liquefaction. Curr. Res. Green Sustain. Chem. 2021, 4, 100106. [Google Scholar] [CrossRef]
- Li, S.; Lundquist, K. A new method for the analysis of phenolic groups in lignins by ʹH NMR spectrometry. Nord. Pulp. Pap. Res. J. 1994, 9, 191–195. [Google Scholar] [CrossRef]
- Lundquist, K. NMR studies of lignins, 3: 1H NMR spectroscopic data for lignin model compounds. Acta Chem. Scand B 1979, 33, 418–420. [Google Scholar] [CrossRef]
- Yuan, T.Q.; Sun, S.N.; Xu, F.; Sun, R.C. Characterization of lignin structures and lignin–carbohydrate complex (LCC) linkages by quantitative 13C and 2D HSQC NMR spectroscopy. J. Agric. Food. Chem. 2011, 59, 10604–10614. [Google Scholar] [CrossRef] [PubMed]
- Pikovskoi, I.I.; Kosyakov, D.S.; Faleva, A.V.; Shavrina, I.S.; Kozhevnikov, A.Y.; Ul’yanovskii, N.V. Study of the sedge (Cárex) lignin by high-resolution mass spectrometry and NMR spectroscopy. Russ. Chem. Bull. 2020, 69, 2004–2012. [Google Scholar] [CrossRef]
- Sun, S.C.; Sun, D.; Li, H.Y.; Cao, X.F.; Sun, S.N.; Wen, J.L. Revealing the topochemical and structural changes of poplar lignin during a two-step hydrothermal pretreatment combined with alkali extraction. Ind. Crop. Prod. 2021, 168, 113588. [Google Scholar] [CrossRef]
- Chang, M.; Wang, X.; Lin, Q.; Li, R.; Zhao, L.; Ren, J.; Zhang, F. Formic acid–hydrogen peroxide treatment of furfural residue for production of nanocellulose, lignin, and nano-scale lignin. Green Chem. 2022, 24, 6232–6240. [Google Scholar] [CrossRef]
- Bober, P.; Gavrilov, N.; Kovalcik, A.; Mičušík, M.; Unterweger, C.; Pašti, I.A.; Stejskal, J. Electrochemical properties of lignin/polypyrrole composites and their carbonized analogues. Mater. Chem. Phys. 2018, 213, 352–361. [Google Scholar] [CrossRef]
- Xue, B.; Yang, Y.; Sun, Y.; Fan, J.; Li, X.; Zhang, Z. Photoluminescent lignin hybridized carbon quantum dots composites for bioimaging applications. Int. J. Biol. Macromol. 2019, 122, 954–961. [Google Scholar] [CrossRef]
- Lv, D.; Li, Y.; Wang, L. Carbon aerogels derived from sodium lignin sulfonate embedded in carrageenan skeleton for methylene-blue removal. Int. J. Biol. Macromol. 2020, 148, 979–987. [Google Scholar] [CrossRef]
- Meng, X.; Crestini, C.; Ben, H.; Hao, N.; Pu, Y.; Ragauskas, A.J.; Argyropoulos, D.S. Determination of hydroxyl groups in biorefinery resources via quantitative 31P NMR spectroscopy. Nat. Protoc. 2019, 14, 2627–2647. [Google Scholar] [CrossRef] [PubMed]
- Stücker, A.; Podschun, J.; Saake, B.; Lehnen, R. A novel quantitative 31 P NMR spectroscopic analysis of hydroxyl groups in lignosulfonic acids. Anal Methods UK 2018, 10, 3481–3488. [Google Scholar]
- Schendorf, T.M.; Del Vecchio, R.; Koech, K.; Blough, N.V. A standard protocol for NaBH4 reduction of CDOM and HS. Limnol. Oceanogr. Meth. 2016, 14, 414–423. [Google Scholar] [CrossRef]
- Nie, S.; Liu, X.; Wu, Z.; Zhan, L.; Yin, G.; Yao, S.; Wang, S. Kinetics study of oxidation of the lignin model compounds by chlorine dioxide. Chem. Eng. J. 2014, 241, 410–417. [Google Scholar] [CrossRef]
- Ni, Y.; Shen, X.; Van Heiningen, A.R.P. Studies on the reactions of phenolic and non-phenolic lignin model compounds with chlorine dioxide. J. Wood. Chem. Technol. 1994, 14, 243–262. [Google Scholar] [CrossRef]
- Brogdon, B.N.; Mancosky, D.G.; Lucia, L.A. New insights into lignin modification during chlorine dioxide bleaching sequences (I): Chlorine dioxide delignification. J. Wood. Chem. Technol. 2005, 24, 201–219. [Google Scholar] [CrossRef]
- Mark, H.F.; Othmer, D.F.; Overberger, C.G.; Seaborg, G.T. Castor oil to chlorosulfuric acid. Kirk Othmer Encycl. Chem. Technol. 1993, 5, 81–97. [Google Scholar]
- Chis, D.; Bogatu, C.; Vlaicu, I.; Iovi, A.; Botau, D.; Ursoiu, I. Chlorine dioxide synthesis and its analysis by chemical methods. J. Environ. Prot. Ecol. 2006, 7, 36–42. [Google Scholar]
- Roy, R.; Jadhav, B.; Rahman, M.S.; Raynie, D.E. Characterization of residue from catalytic hydrothermal depolymerization of lignin. Curr. Res. Green Sustain. Chem. 2021, 4, 100052. [Google Scholar] [CrossRef]
- Sun, S.-N.; Cao, X.F.; Xu, F.; Sun, R.C.; Jones, G.L. Structural features and antioxidant activities of lignins from steam-exploded bamboo (Phyllostachys pubescens). J. Agric. Food. Chem. 2014, 62, 5939–5947. [Google Scholar] [CrossRef]
- Wen, J.L.; Xue, B.L.; Xu, F.; Sun, R.C. Unveiling the structural heterogeneity of bamboo lignin by in situ HSQC NMR technique. Bioenergy Res. 2012, 5, 886–903. [Google Scholar] [CrossRef]
- Haasch, R.T. X-ray photoelectron spectroscopy (XPS) and auger electron spectroscopy (AES). In Practical Materials Characterization; Springer: New York, NY, USA, 2014; pp. 93–132. [Google Scholar]
- Kim, K.H.; Dutta, T.; Walter, E.D.; Isern, N.G.; Cort, J.R.; Simmons, B.A.; Singh, S. Chemoselective methylation of phenolic hydroxyl group prevents quinone methide formation and repolymerization during lignin depolymerization. ACS Sustain. Chem. Eng. 2017, 5, 3913–3919. [Google Scholar] [CrossRef]
- Zhao, C.; Qiao, X.; Shao, Q.; Hassan, M.; Ma, Z. Evolution of the lignin chemical structure during the bioethanol production process and its inhibition to enzymatic hydrolysis. Energy Fuels 2020, 34, 5938–5947. [Google Scholar] [CrossRef]

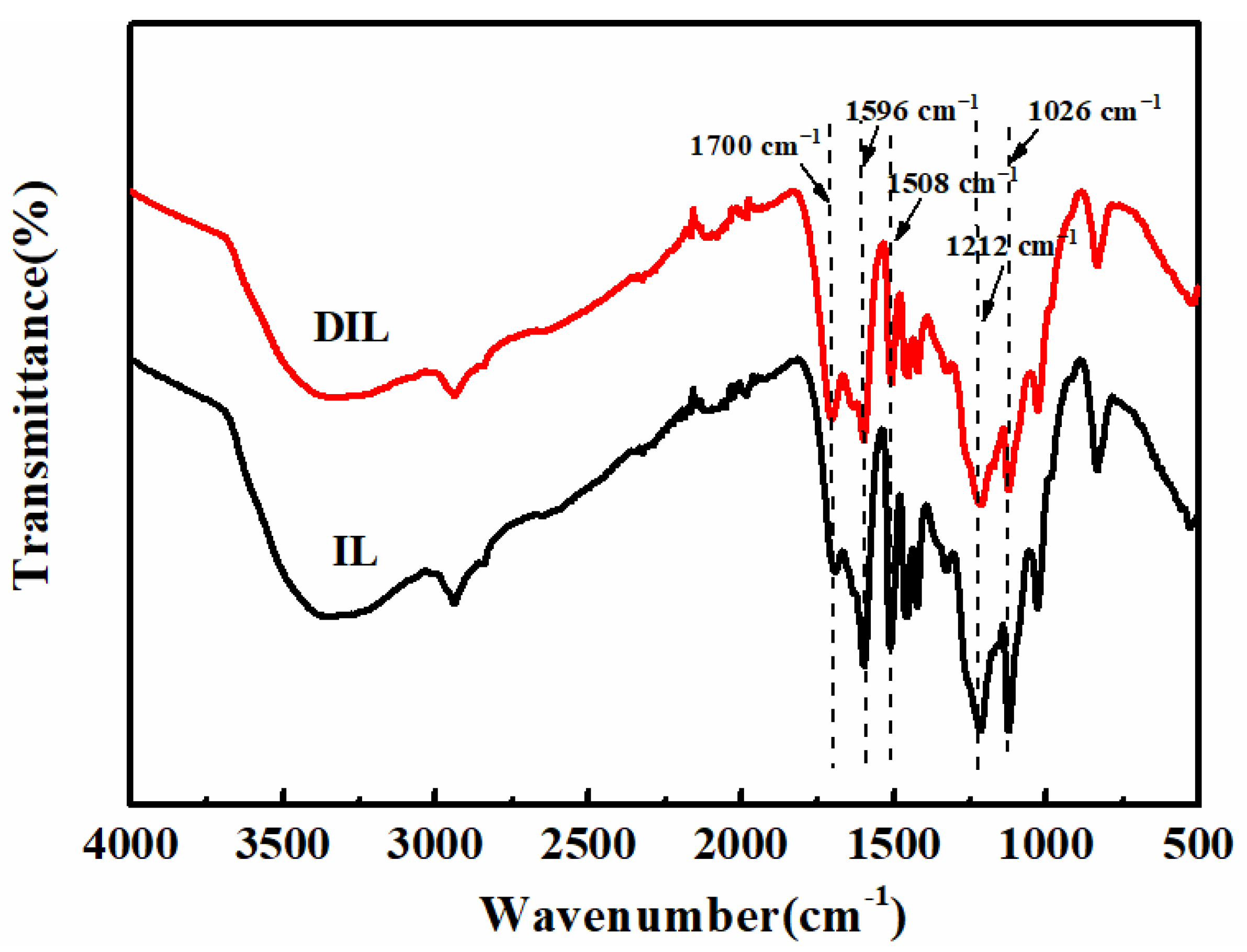
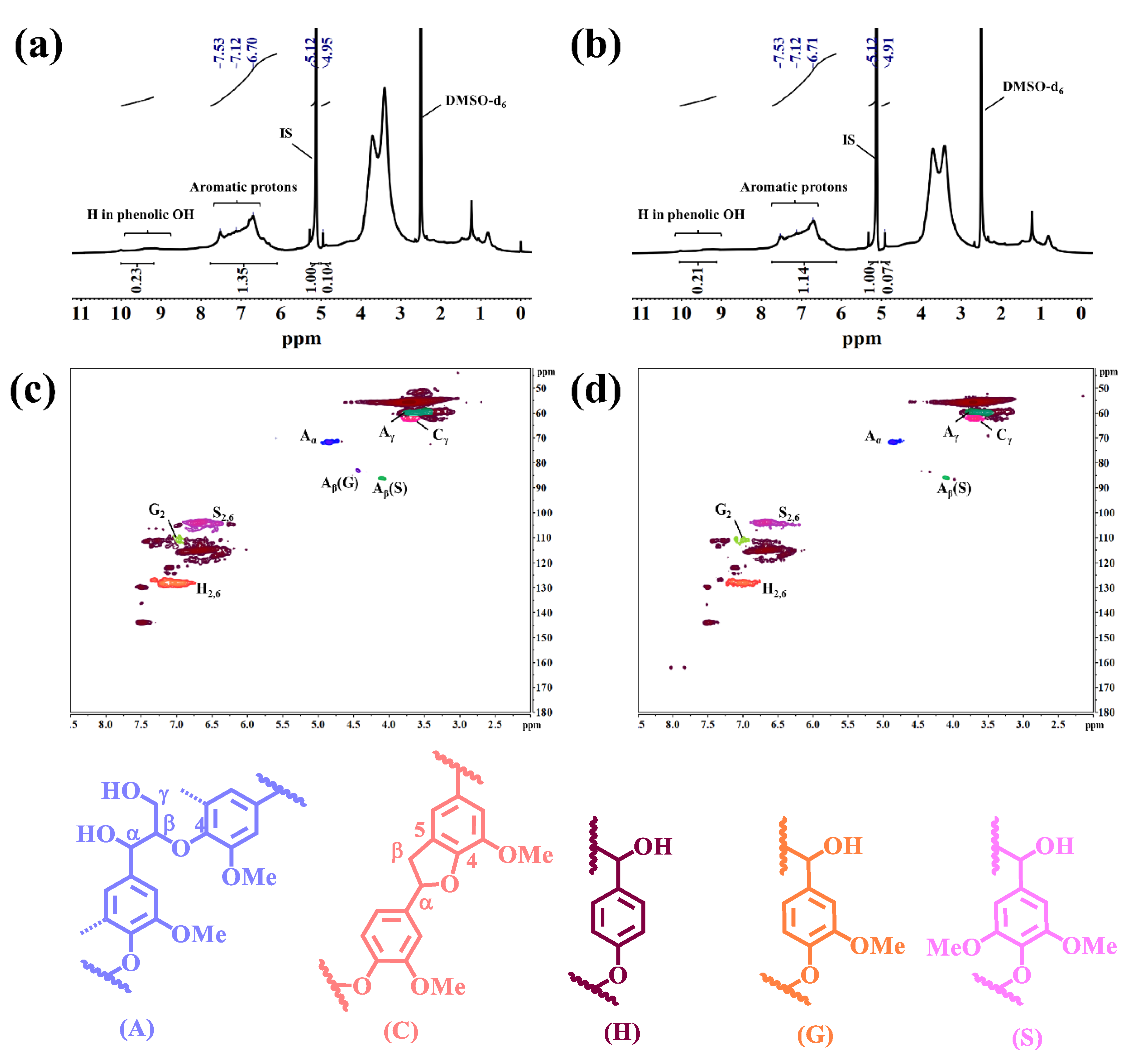
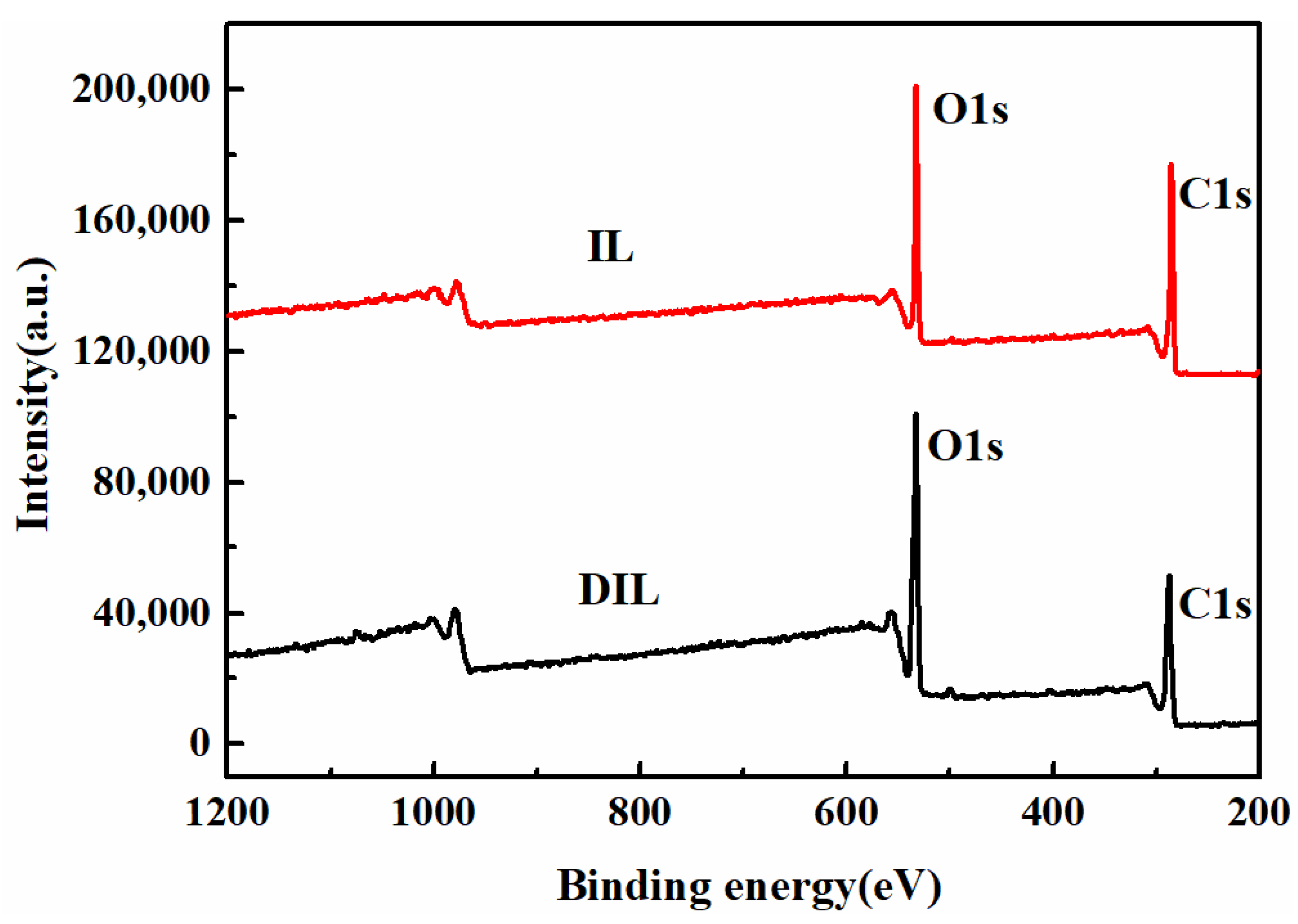
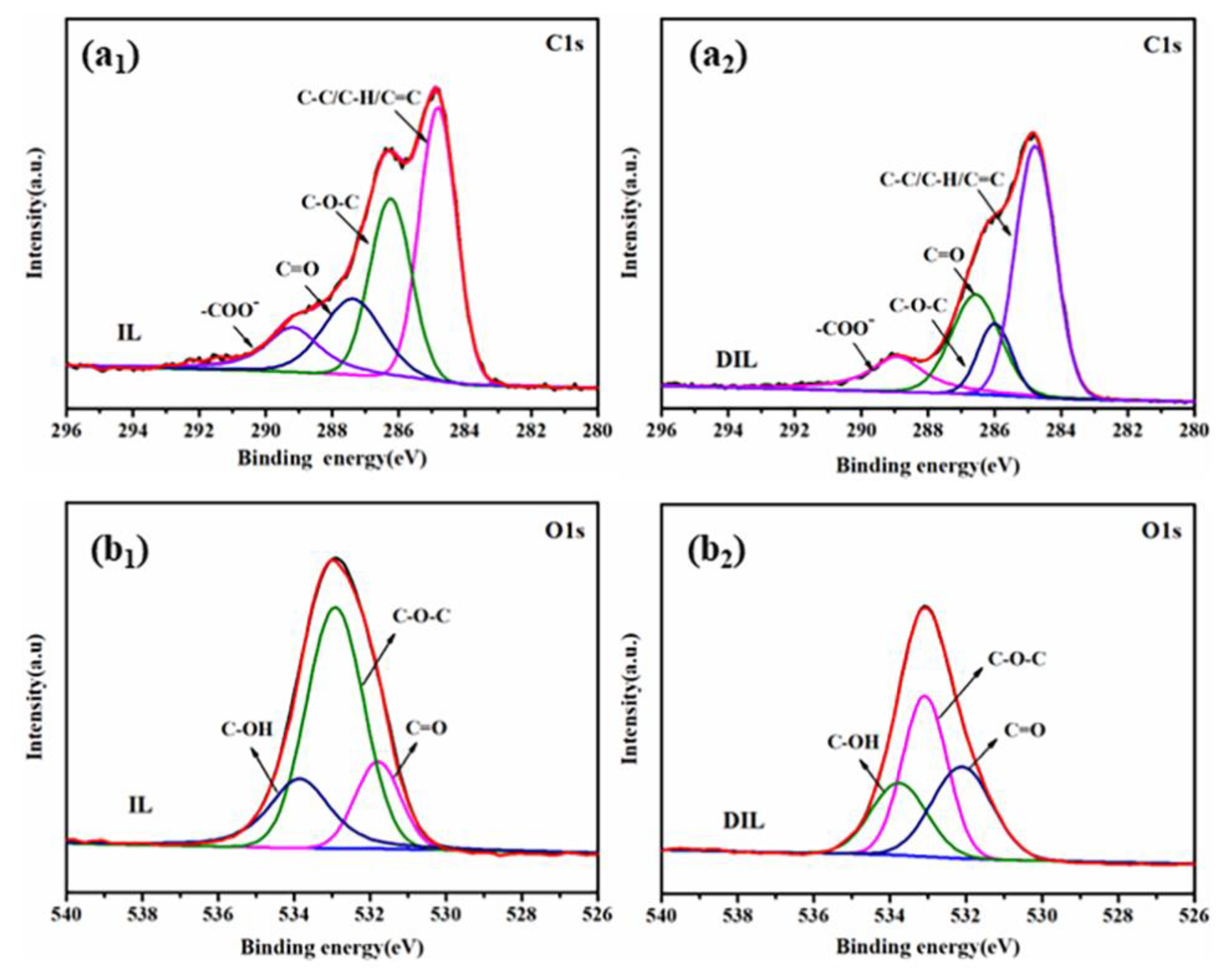
| Functional Groups | Wavenumber (cm−1) |
|---|---|
| -OH | 3253 |
| -CH2, -CH3 | 2934 |
| C-H in -OCH3 | 2840 |
| C=O | 1674 |
| Aromatic ring vibrations | 1511 |
| 1592 | |
| C-H in -OCH3 | 1451 |
| C-O in phenolic OH | 1211 |
| Ether bond (C-O-C) | 1030 |
| C-H in p-hydroxyphenyl units | 816 |
| Chemical Shift (ppm) | Functional Groups | IL (mmol·g−1) | DIL (mmol·g−1) |
|---|---|---|---|
| 2.54 | DMSO-d6 | - | - |
| 4.93 | β-O-4 bond | 1.05 | 0.74 |
| 5.12 | 1,3,5-trioxane | - | - |
| 7.78–7.41 | Aromatic H in H unit | 0.58 | 0.48 |
| 7.41–6.85 | Aromatic H in G unit | 1.80 | 1.58 |
| 6.85–6.10 | Aromatic H in S unit | 3.27 | 2.69 |
| 9.21–9.98 | Proton in phenolic OH | 2.43 | 2.22 |
| Characteristic Groups | Relative Content Change |
|---|---|
| C-OH | −14.3% |
| C-O-C or -OCH3 | −34.9% |
| C=O | +16.5% |
| -COOH | +12.0% |
| Chemical Shift (ppm) | Functional Groups | IL (mmol·g−1) | DIL (mmol·g−1) |
|---|---|---|---|
| 149.7–145.5 | Aliphatic OH | 1.16 | 1.13 |
| 145.1–144.7 | Cyclohexanol | - | - |
| 144.3–141.1 | S-OH and condensed phenolic OH | 0.82 | 0.77 |
| 140.7–138.5 | G-OH | 1.01 | 0.97 |
| 138.3–136.4 | H-OH | 0.59 | 0.51 |
| 136.0–133.1 | -COOH | 0.94 | 1.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weng, S.; Zhang, G.; Hu, Y.; Bo, C.; Song, F.; Feng, G.; Hu, L.; Zhou, Y.; Jia, P. Lignin Degradation via Chlorine Dioxide at Room Temperature: Chemical Groups and Structural Characterization. Int. J. Mol. Sci. 2023, 24, 1479. https://doi.org/10.3390/ijms24021479
Weng S, Zhang G, Hu Y, Bo C, Song F, Feng G, Hu L, Zhou Y, Jia P. Lignin Degradation via Chlorine Dioxide at Room Temperature: Chemical Groups and Structural Characterization. International Journal of Molecular Sciences. 2023; 24(2):1479. https://doi.org/10.3390/ijms24021479
Chicago/Turabian StyleWeng, Shuxian, Guixin Zhang, Yun Hu, Caiying Bo, Fei Song, Guodong Feng, Lihong Hu, Yonghong Zhou, and Puyou Jia. 2023. "Lignin Degradation via Chlorine Dioxide at Room Temperature: Chemical Groups and Structural Characterization" International Journal of Molecular Sciences 24, no. 2: 1479. https://doi.org/10.3390/ijms24021479
APA StyleWeng, S., Zhang, G., Hu, Y., Bo, C., Song, F., Feng, G., Hu, L., Zhou, Y., & Jia, P. (2023). Lignin Degradation via Chlorine Dioxide at Room Temperature: Chemical Groups and Structural Characterization. International Journal of Molecular Sciences, 24(2), 1479. https://doi.org/10.3390/ijms24021479







