New 3,4-seco-3,19-Dinor- and Spongian-Based Diterpenoid Lactones from the Marine Sponge Spongia sp.
Abstract
1. Introduction
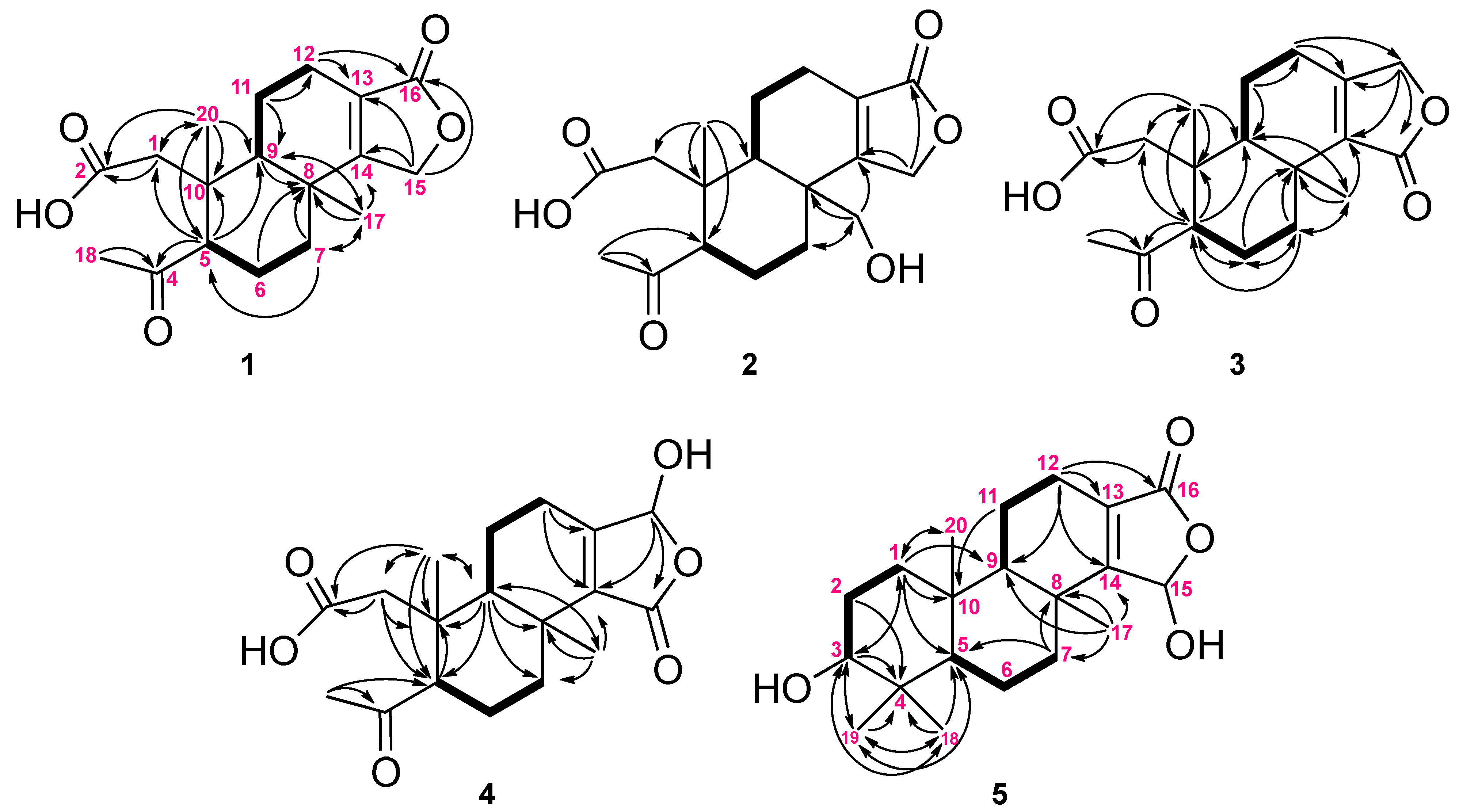
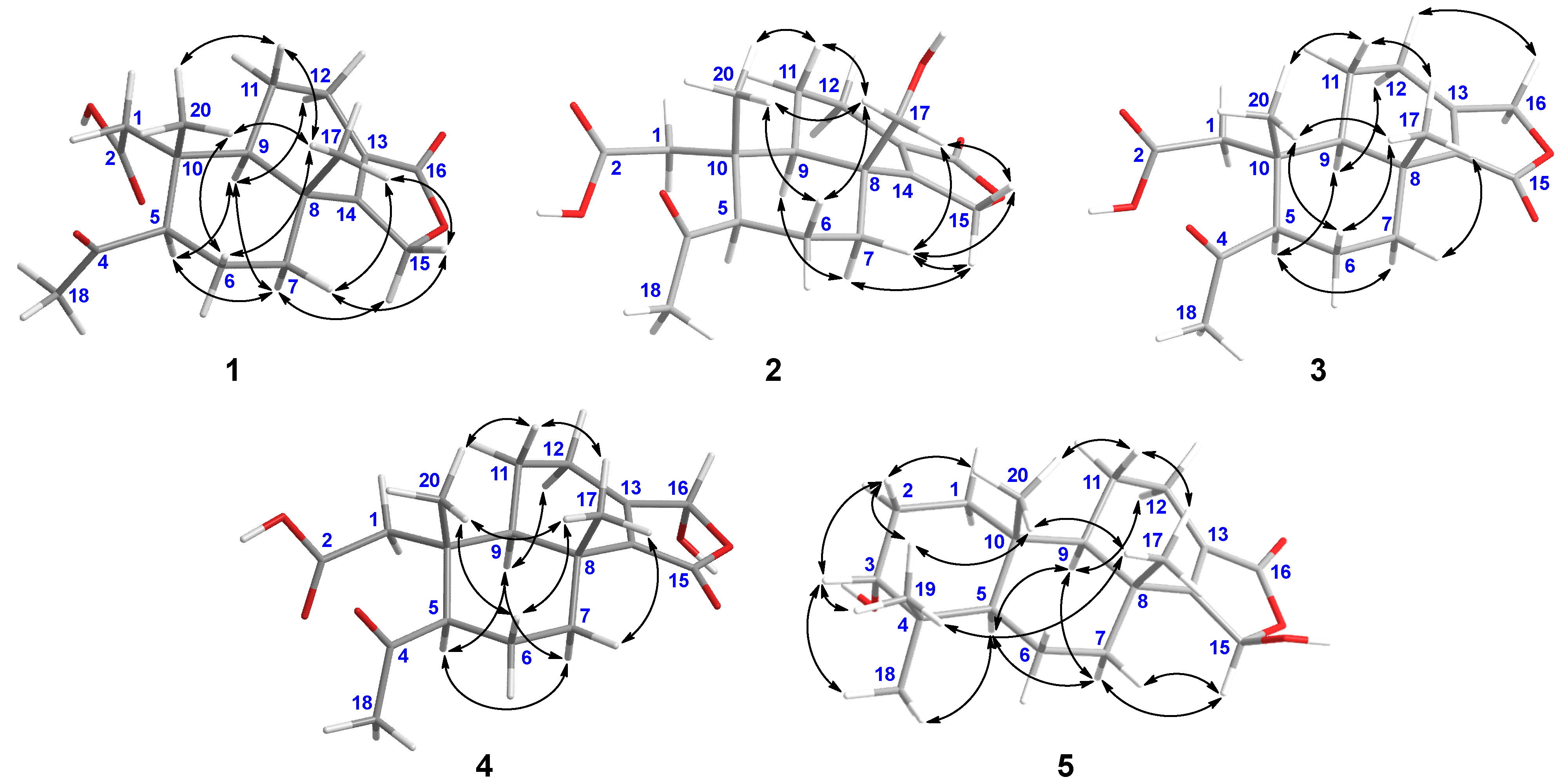
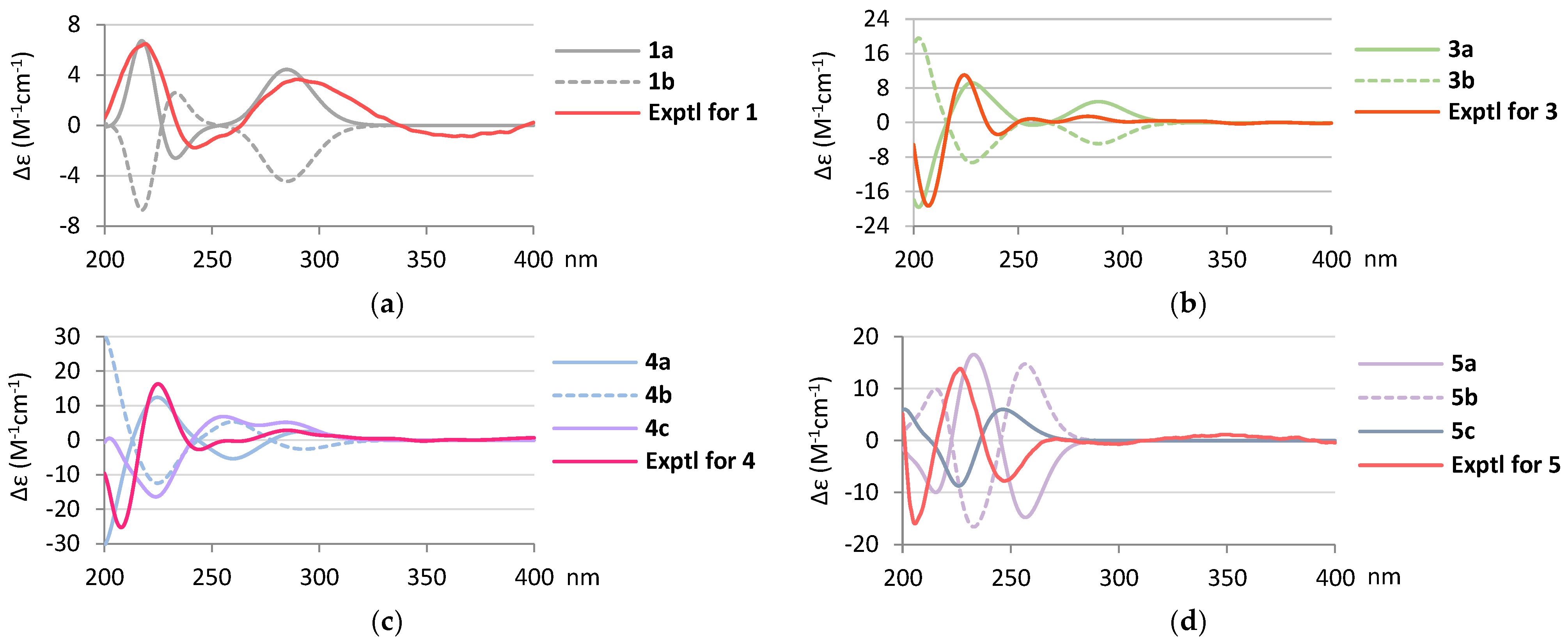
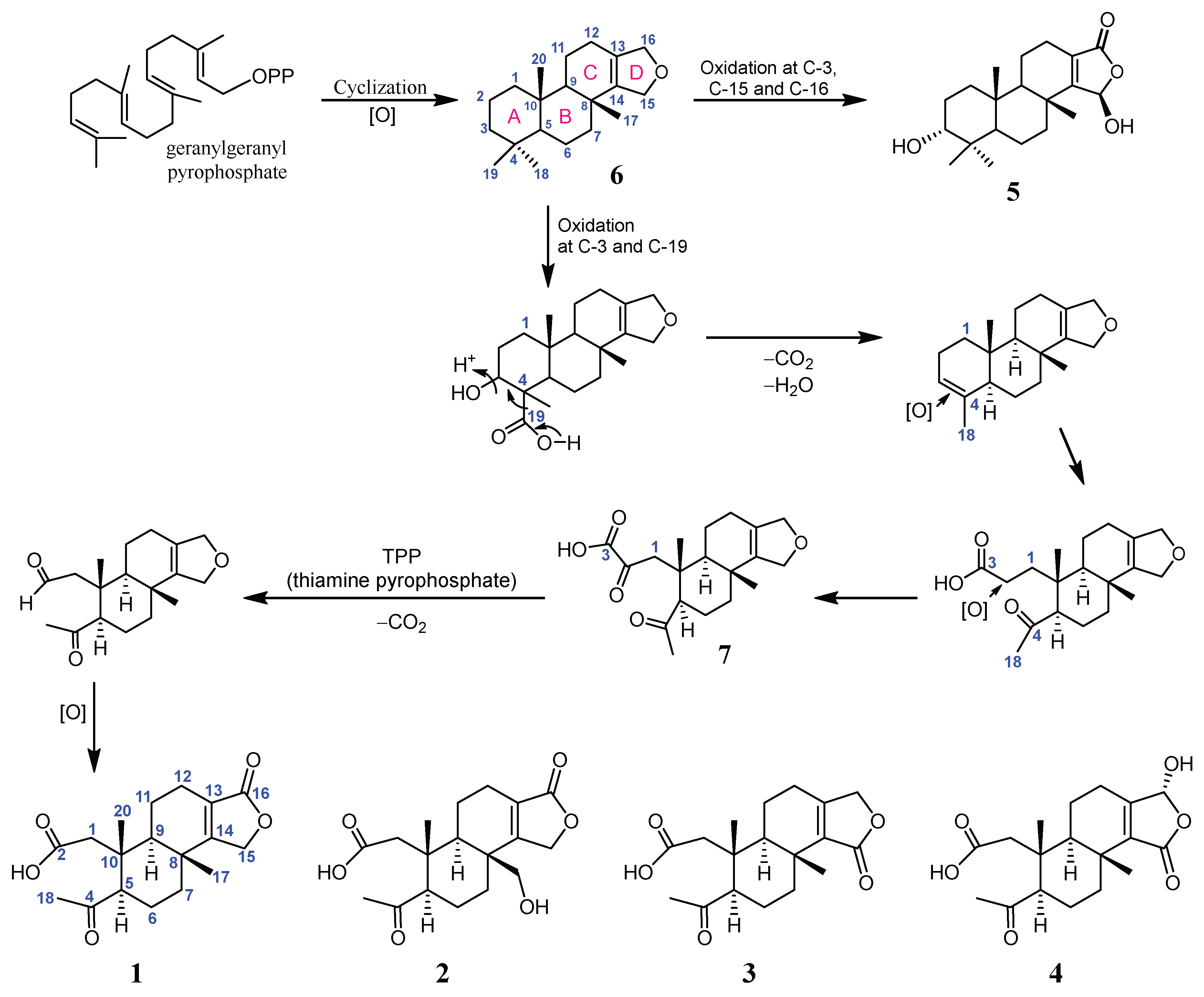
2. Results and Discussion
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Animal Material
3.3. Extraction and Separation
3.3.1. Secodinorspongin A (1)
3.3.2. Secodinorspongin B (2)
3.3.3. Secodinorspongin C (3)
3.3.4. Secodinorspongin D (4)
3.3.5. Sponginolide (5)
3.4. DFT and TD-DFT Calculations
3.5. Cytotoxicity Assay
3.6. Antibacterial Assay
3.7. Anti-Inflammatory Activity
3.8. Inhibition of Superoxide Anion Generation
3.9. Inhibition of Elastase Release
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liang, Y.; Liao, X.; Ling, L.; Yang, Y.; Zhao, B.; Xu, S. A new dinorspongian diterpene with pyridyl D-ring from the marine sponge Spongia sp. Chin. J. Org. Chem. 2022, 42, 901–904. [Google Scholar] [CrossRef]
- Liang, Y.-Q.; Liao, X.-J.; Zhao, B.-X.; Xu, S.-H. Novel 3,4-seco-3,19-dinorspongian and 5,17-epoxy-19-norspongian diterpenes from the marine sponge Spongia sp. Org. Chem. Front. 2020, 7, 3253–3261. [Google Scholar] [CrossRef]
- Jomori, T.; Setiawan, A.; Sasaoka, M.; Arai, M. Cytotoxicity of new diterpene alkaloids, ceylonamides G−I, isolated from Indonesian marine sponge of Spongia sp. Nat. Prod. Commun. 2019, 14, 1–7. [Google Scholar] [CrossRef]
- Chen, Q.; Mao, Q.; Bao, M.; Mou, Y.; Fang, C.; Zhao, M.; Jiang, W.; Yu, X.; Wang, C.; Dai, L.; et al. Spongian diterpenes including one with a rearranged skeleton from the marine sponge Spongia officinalis. J. Nat. Prod. 2019, 82, 1714–1718. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.-Q.; Liao, X.-J.; Lin, J.-L.; Xu, W.; Chen, G.-D.; Zhao, B.-X.; Xu, S.-H. Spongiains A−C: Three new spongian diterpenes with ring A rearrangement from the marine sponge Spongia sp. Tetrahedron 2019, 75, 3802–3808. [Google Scholar] [CrossRef]
- Maximo, P.; Ferreira, L.M.; Branco, P.; Lima, P.; Lourenco, A. The role of Spongia sp. in the discovery of marine lead compounds. Mar. Drugs 2016, 14, 139. [Google Scholar] [CrossRef] [PubMed]
- Ponomarenko, L.P.; Terent’eva, N.A.; Krasokhin, V.B.; Kalinovsky, A.I.; Rasskazov, V.A. Terpenoid metabolites from Spongia spp. and their effects on nucleic acid biosynthesis in sea urchin eggs. Nat. Prod. Commun. 2011, 6, 773–776. [Google Scholar] [CrossRef]
- Carroll, A.R.; Lamb, J.; Moni, R.; Hooper, J.N.A.; Quinn, R.J. Spongian diterpenes with thyrotropin releasing hormone receptor 2 binding affinity from Spongia sp. J. Nat. Prod. 2008, 71, 884–886. [Google Scholar] [CrossRef]
- Mori, D.; Kimura, Y.; Kitamura, S.; Sakagami, Y.; Yoshioka, Y.; Shintani, T.; Okamoto, T.; Ojika, M. Spongolactams, farnesyl transferase inhibitors from a marine sponge: Isolation through an LC/MS-Guided assay, structures, and semisyntheses. J. Org. Chem. 2007, 72, 7190–7198. [Google Scholar] [CrossRef] [PubMed]
- Gunasekera, S.P.; Schmitz, F.J. New spongian diterpenoids from a Great Barrier Reef sponge, Spongia sp. J. Org. Chem. 1991, 56, 1250–1253. [Google Scholar] [CrossRef]
- Kohmoto, S.; Mcconnell, O.J.; Wright, A.; Cross, S. Isospongiadiol, a cytotoxic and antiviral diterpene from a Caribbean deep-water marine sponge, Spongia sp. Chem. Lett. 1987, 16, 1687–1690. [Google Scholar] [CrossRef]
- Tai, C.-J.; Ahmed, A.F.; Chao, C.-H.; Yen, C.-H.; Hwang, T.-L.; Chang, F.-R.; Huang, Y.M.; Sheu, J.-H. Spongenolactones A−C, bioactive 5,5,6,6,5-pentacyclic spongian diterpenes from the Red Sea sponge Spongia sp. Mar. Drugs 2022, 20, 498. [Google Scholar] [CrossRef] [PubMed]
- Tai, C.-J.; Huang, C.-Y.; Ahmed, A.-F.; Orfali, R.-S.; Alarif, W.-M.; Huang, Y.M.; Wang, Y.-H.; Hwang, T.-L.; Sheu, J.-H. An anti-inflammatory 2,4-cyclized-3,4-secospongian diterpenoid and furanoterpene-related metabolites of a marine sponge Spongia sp. from the Red Sea. Mar. Drugs 2021, 19, 38. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.-M.; Guan, Z.; Su, J.-Y.; Feng, X.-L.; Cai, J.-W. Two new spongian diterpene lactones. Acta Chim. Sin. 2001, 59, 1675–1679. [Google Scholar]
- Li, C.-J.; Schmitz, F.J.; Kelly-Borges, M. Six new spongian diterpenes from the sponge Spongia matamata. J. Nat. Prod. 1999, 62, 287–290. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Wang, J.-S.; Gu, Y.-C.; Kong, L.-Y. Triterpenoids from Aglaia abbreviata and their cytotoxic activities. J. Nat. Prod. 2010, 73, 2042–2046. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, R.; Matsuda, M.; Matsunaga, S. 3β-Hydroxyhexanordammaran-20-one from Euphorbia supina. Phytochemistry 1987, 26, 3365–3366. [Google Scholar] [CrossRef]
- Chen, Y.-S.; Chang, H.-S.; Hsiao, H.-H.; Chen, Y.-F.; Kuo, Y.-P.; Yen, F.-L.; Yen, C.-H. Identification of beilschmiedia tsangii root extract as a liver cancer cell-normal keratinocyte dual-selective NRF2 regulator. Antioxidants 2021, 10, 544. [Google Scholar] [CrossRef]
- Kao, Y.-T.; Chen, Y.-S.; Tang, K.-W.; Lee, J.-C.; Tseng, C.-H.; Tzeng, C.-C.; Yen, C.-H.; Chen, Y.-L. Discovery of 4-anilinoquinolinylchalcone derivatives as potential NRF2 activators. Molecules 2020, 25, 3133. [Google Scholar] [CrossRef]
- Yu, H.-P.; Hsieh, P.-W.; Chang, Y.-J.; Chung, P.-J.; Kuo, L.-M.; Hwang, T.-L. 2-(2-Fluorobenzamido)benzoate ethyl ester (EFB-1) inhibits superoxide production by human neutrophils and attenuates hemorrhagic shock-induced organ dysfunction in rats. Free Radic. Biol. Med. 2011, 50, 1737–1748. [Google Scholar] [CrossRef]
- Yang, S.-C.; Chung, P.-J.; Ho, C.-M.; Kuo, C.-Y.; Hung, M.-F.; Huang, Y.-T.; Chang, W.-Y.; Chang, Y.-W.; Chan, K.-H.; Hwang, T.-L. Propofol inhibits superoxide production, elastase release, and chemotaxis in formyl peptide-activated human neutrophils by blocking formyl peptide receptor 1. J. Immunol. 2013, 190, 6511–6519. [Google Scholar] [CrossRef]
- Hwang, T.-L.; Su, Y.-C.; Chang, H.-L.; Leu, Y.-L.; Chung, P.-J.; Kuo, L.-M.; Chang, Y.-J. Suppression of superoxide anion and elastase release by C18 unsaturated fatty acids in human neutrophils. J. Lipid Res. 2009, 50, 1395–1408. [Google Scholar] [CrossRef]
- Pescitelli, G.; Bruhn, T. Good computational practice in the assignment of absolute configurations by TDDFT calculations of ECD spectra. Chirality 2016, 28, 466–474. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision D.01; Gaussian Inc.: Wallingford, CT, USA, 2013.
- Tai, C.-J.; Ahmed, A.F.; Chao, C.-H.; Yen, C.-H.; Hwang, T.-L.; Chang, F.-R.; Huang, Y.M.; Sheu, J.-H. The chemically highly diversified metabolites from the Red Sea marine sponge Spongia sp. Mar. Drugs 2022, 20, 241. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Watkins, D.; Jin, Y.; Gong, C.; King, A.; Washington, A.Z.; Green, K.D.; Garneau-Tsodikova, S.; Oyelere, A.K.; Arya, D.P. Rapid synthesis, RNA binding, and antibacterial screening of a peptidic-aminosugar (PA) library. ACS Chem. Biol. 2015, 10, 1278–1289. [Google Scholar] [CrossRef] [PubMed]
- Betancur-Galvis, L.; Zuluaga, C.; Arno, M.; Gonzalez, M.A.; Zaragoza, R.J. Cytotoxic effect (on tumor cells) and in vitro antiviral activity against herpes simplex virus of synthetic spongiane diterpenes. J. Nat. Prod. 2002, 65, 189–192. [Google Scholar] [CrossRef] [PubMed]


| 1 | 2 | 3 | 4 | |||||
|---|---|---|---|---|---|---|---|---|
| Position | δH | δC | δH | δC | δH | δC | δH | δC |
| 1 | 2.30, d (14.5) a | 44.2, CH2 | 2.29, m | 44.5, CH2 | 2.30, d (14.5) | 44.4, CH2 | 2.29, d (14.5) | 44.3, CH2 |
| 2.57, d (14.5) | 2.54, d (13.0) | 2.55, d (14.5) | 2.54, d (14.5) | |||||
| 2 | − | 172.8, C | − | 174.8, C | − | 172.8, C | − | 172.9, C |
| 4 | − | 211.6, C | − | 211.9 C | − | 211.9, C | − | 211.9, C |
| 5 | 3.15, dd (13.0, 3.5) | 56.3, CH | 3.20, br d (11.5) | 56.2, CH | 3.11, dd (12.5, 3.5) | 56.5, CH | 3.11, dd (12.5, 3.5) | 56.4, CH |
| 6α | 1.78, ddd (13.5, 7.0, 3.5) | 22.1, CH2 | 1.73, m | 21.9, CH2 | 1.75, ddd (13.5, 7.0, 3.5) | 22.1, CH2 | 1.74, ddd (13.5, 7.0, 3.5) | 22.1, CH2 |
| 6β | 1.90, dd (13.5, 3.5) | 1.87, m | 1.82, td (13.5, 3.5) | 1.81, dd (13.5, 3.5) | ||||
| 7α | 1.54, td (13.5, 3.5) | 36.1, CH2 | 1.33, m | 30.9, CH2 | 1.22, td (13.5, 3.5) | 35.2, CH2 | 1.25, td (13.5, 3.5) | 35.5, CH2 |
| 7β | 1.83, dt (13.5, 3.5) | 2.27, m | 2.61, dt (13.5, 3.5) | 2.57, dt (13.5, 3.5) | ||||
| 8 | − | 37.6, C | − | 43.7, C | − | 35.9, C | − | 35.9, C |
| 9 | 1.67, m | 48.7, CH | 1.77, m | 48.9, CH | 1.63, m | 49.6, CH | 1.59, m | 49.3, CH |
| 10 | − | 40.2, C | − | 40.1, C | − | 40.2, C | − | 40.2, C |
| 11α | 2.06, m | 18.6, CH2 | 2.05, m | 18.1, CH2 | 2.07, m | 18.7, CH2 | 2.06, m | 18.7, CH2 |
| 11β | 1.65, m | 1.77, m | 1.63, m | 1.59, m | ||||
| 12α | 2.05, m | 22.2, CH2 | 2.11, m | 22.1, CH2 | 2.34, m | 25.6, CH2 | 2.37, m | 24.7, CH2 |
| 12β | 2.29, m | 2.29, m | 2.48, m | 2.53, m | ||||
| 13 | − | 123.9, C | − | 125.1, C | − | 161.2, C | − | 160.1, C |
| 14 | − | 171.2, C | − | 169.8, C | − | 135.1, C | − | 138.2, C |
| 15α | 4.72, dd (17.0, 2.5) | 68.8, CH2 | 4.71, dd (17.0) | 72.3, CH2 | − | 172.5, C | − | 169.7, C |
| 15β | 4.86, dt (17.0, 2.5) | 5.00, dt (17.0) | ||||||
| 16α | − | 174.3, C | − | 174.8, C | 4.67, d (17.5) | 71.3, C | − | 97.5, CH |
| 16β | 4.61, d (17.5) | 5.88, d (4.0) | ||||||
| 17 | 1.26, s | 21.4, CH3 | 3.71, d (10.0) | 64.3, CH2 | 1.18, s | 20.8, CH3 | 1.17, s | 21.4, CH3 |
| 4.15, d (10.0) | ||||||||
| 18 | 2.23, s | 31.8, CH3 | 2.23, s | 31.9, CH3 | 2.22, s | 31.7, CH3 | 2.22, s | 31.7, CH3 |
| 20 | 1.07, s | 19.5, CH3 | 1.01, s | 20.0, CH3 | 1.05, s | 19.5, CH3 | 1.04, s | 19.5, CH3 |
| 5 | |||||
|---|---|---|---|---|---|
| Position | δH | δC | Position | δH | δC |
| 1 | 1.35, m | 32.9, CH2 | 10 | − | 37.5, C |
| 1.51, m | 11 | 1.52, m | 16.8, CH2 | ||
| 2α | 1.61, m | 24.9, CH2 | 12α | 2.13, m | 21.4, CH2 |
| 2β | 2.00, t (3.0) a | 12β | 2.41, dd (18.6, 6.6) | ||
| 3 | 3.44, t (3.0) | 75.9, CH | 13 | − | 127.7, C |
| 4 | − | 37.6, C | 14 | − | 167.6, C |
| 5 | 1.41, m | 49.2, CH | 15 | 6.06, br s | 96.8, CH |
| 6 | 1.57, m | 17.7, CH2 | 16 | − | 173.5, C |
| 7α | 1.50, m | 36.8, CH2 | 17 | 1.25, s | 20.6, CH3 |
| 7β | 2.04, m | 18 | 0.96, s | 28.2, CH3 | |
| 8 | − | 37.6, C | 19 | 0.87, s | 21.8, CH3 |
| 9 | 1.18, m | 55.6, CH | 20 | 0.93, s | 16.4, CH3 |
| Compound | Growth of S. aureus | ||
|---|---|---|---|
| 50 µM (%, Mean ± SD) | 100 µM (%, Mean ± SD) | 200 µM (%, Mean ± SD) | |
| 1 | 57.2 ± 6.4 | 46.4 ± 22.3 | 25.0 ± 20.5 |
| 2 | 96.7 ± 3.1 | 96.3 ± 4.7 | 96.2 ± 4.8 |
| 3 | 101.2 ± 2.4 | 100.4 ± 2.3 | 100.1 ± 3.5 |
| 4 | 100.4 ± 9.9 | 98.4 ± 2.3 | 95.7 ± 7.8 |
| 5 | 102.2 ± 6.9 | 103.2 ± 8.1 | 101.5 ± 7.7 |
| Tetracycline a | 0.9 ± 0.3 | ||
| Compound | Superoxide Anion | Elastase Release | ||
|---|---|---|---|---|
| IC50 (μM) a | Inh% b | IC50 (μM) | Inh% | |
| 1 | >20 | 7.1 ± 5.3 | >20 | 12.2 ± 2.4 ** |
| 2 | >20 | 7.2 ± 2.4 * | >20 | 18.5 ± 1.7 *** |
| 3 | >20 | 3.7 ± 2.7 | >20 | 17.6 ± 2.3 ** |
| 4 | >20 | 20.4 ± 4.5 ** | >20 | 30.8 ± 2.6 *** |
| 5 | >20 | 22.0 ± 4.6 ** | >20 | 22.5 ± 4.2 ** |
| LY294002 | 1.9 ± 0.8 *** | 88.7 ± 1.5 *** | 2.9 ± 0.1 *** | 79.5 ± 2.0 *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tai, C.-J.; Chao, C.-H.; Ahmed, A.F.; Yen, C.-H.; Hwang, T.-L.; Chang, F.-R.; Huang, Y.M.; Sheu, J.-H. New 3,4-seco-3,19-Dinor- and Spongian-Based Diterpenoid Lactones from the Marine Sponge Spongia sp. Int. J. Mol. Sci. 2023, 24, 1252. https://doi.org/10.3390/ijms24021252
Tai C-J, Chao C-H, Ahmed AF, Yen C-H, Hwang T-L, Chang F-R, Huang YM, Sheu J-H. New 3,4-seco-3,19-Dinor- and Spongian-Based Diterpenoid Lactones from the Marine Sponge Spongia sp. International Journal of Molecular Sciences. 2023; 24(2):1252. https://doi.org/10.3390/ijms24021252
Chicago/Turabian StyleTai, Chi-Jen, Chih-Hua Chao, Atallah F. Ahmed, Chia-Hung Yen, Tsong-Long Hwang, Fang-Rong Chang, Yusheng M. Huang, and Jyh-Horng Sheu. 2023. "New 3,4-seco-3,19-Dinor- and Spongian-Based Diterpenoid Lactones from the Marine Sponge Spongia sp." International Journal of Molecular Sciences 24, no. 2: 1252. https://doi.org/10.3390/ijms24021252
APA StyleTai, C.-J., Chao, C.-H., Ahmed, A. F., Yen, C.-H., Hwang, T.-L., Chang, F.-R., Huang, Y. M., & Sheu, J.-H. (2023). New 3,4-seco-3,19-Dinor- and Spongian-Based Diterpenoid Lactones from the Marine Sponge Spongia sp. International Journal of Molecular Sciences, 24(2), 1252. https://doi.org/10.3390/ijms24021252











