Montelukast Increased IL-25, IL-33, and TSLP via Epigenetic Regulation in Airway Epithelial Cells
Abstract
1. Introduction
2. Results
2.1. LTRAs Increased IL-25, IL-33, and TSLP Expression in Human Airway Epithelial Cells and ALI Cultures
2.2. LTRAs/ICS Cotreatment Suppressed IL-25, IL-33, and TSLP Expression in A549 Cells and ALI Cultures
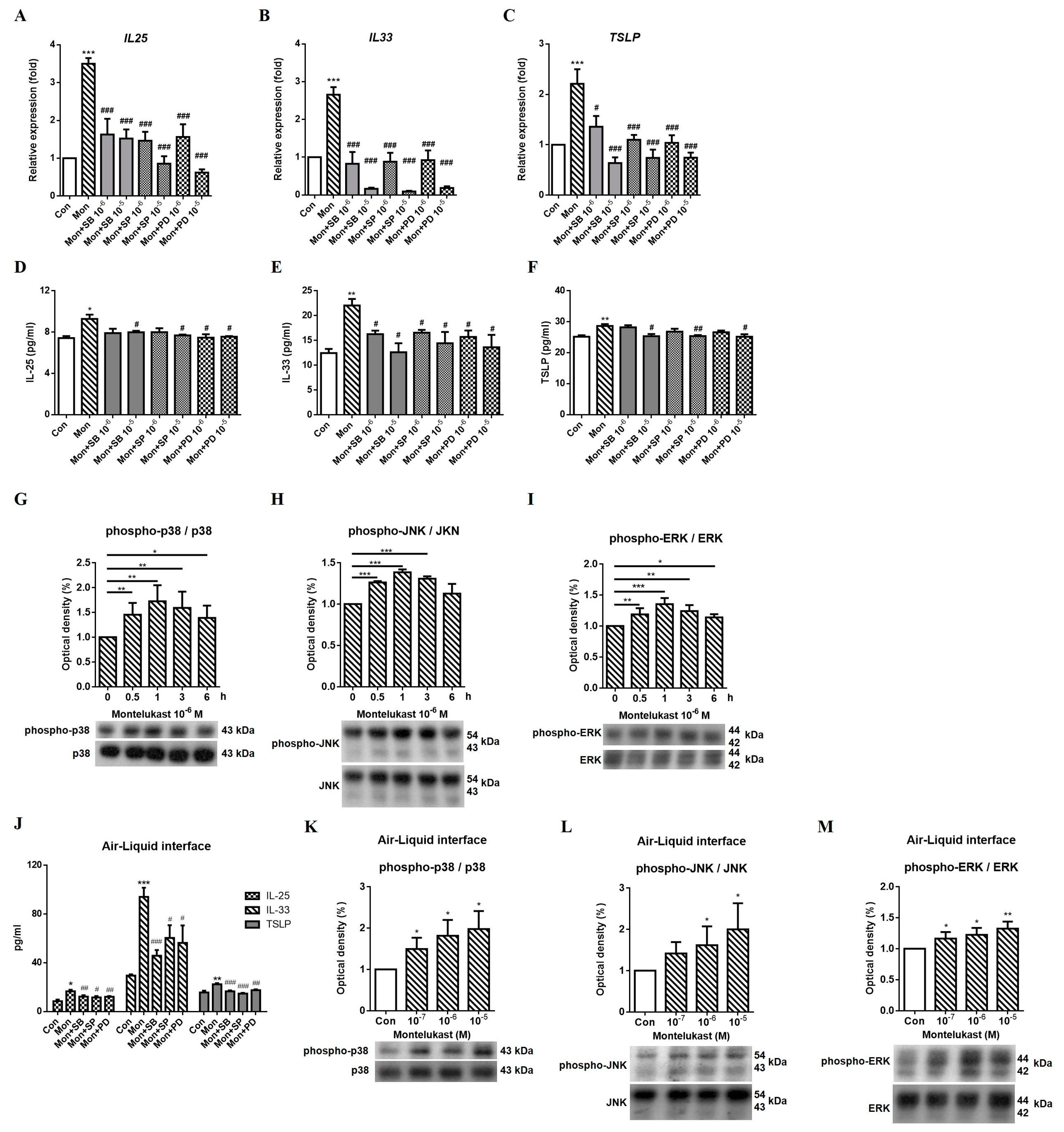
2.3. Montelukast Increased IL-25, IL-33, and TSLP Expression via the MAPK Pathway
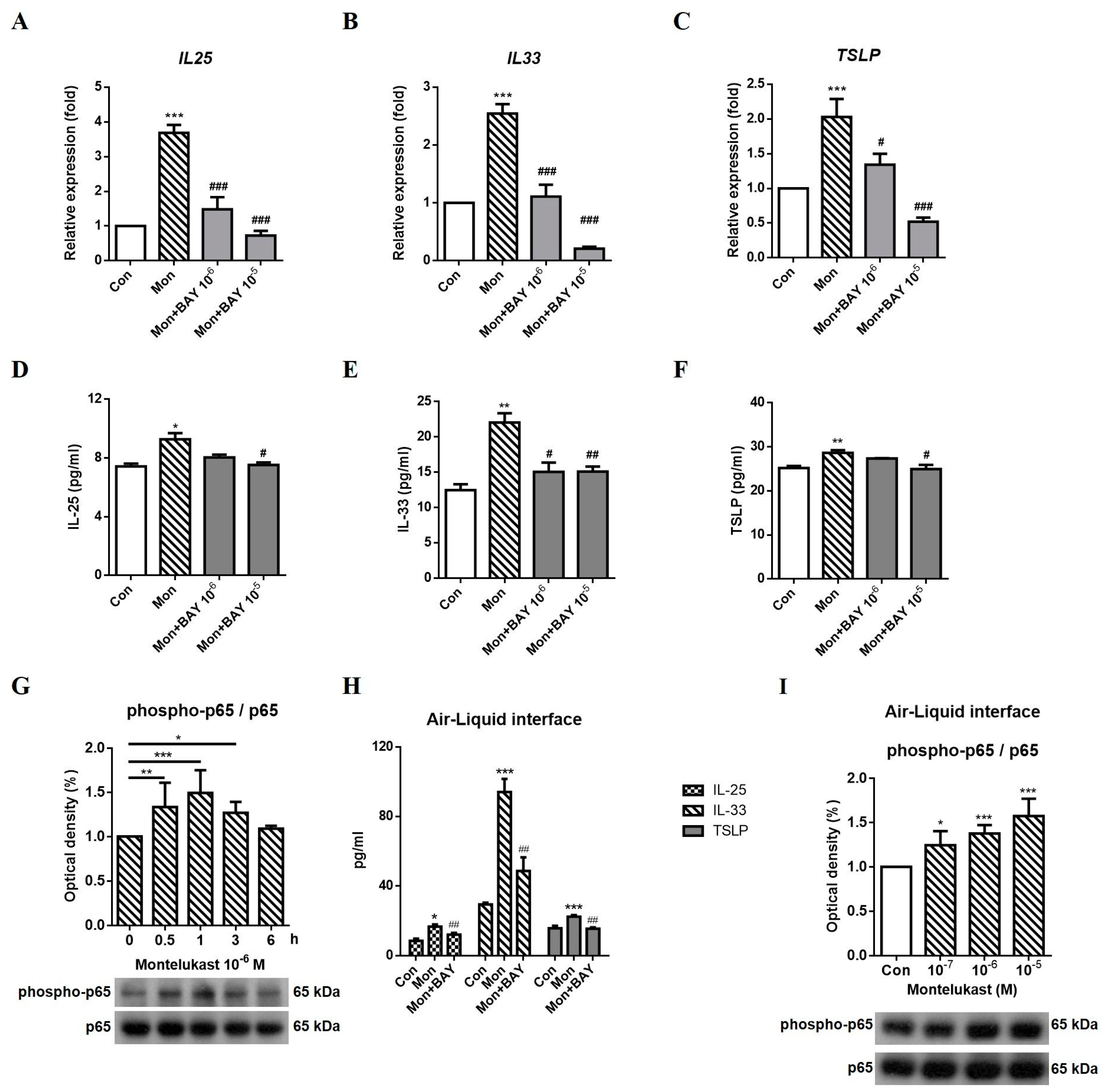
2.4. Montelukast Increased IL-25, IL-33, and TSLP Expression via the NFκB Pathway
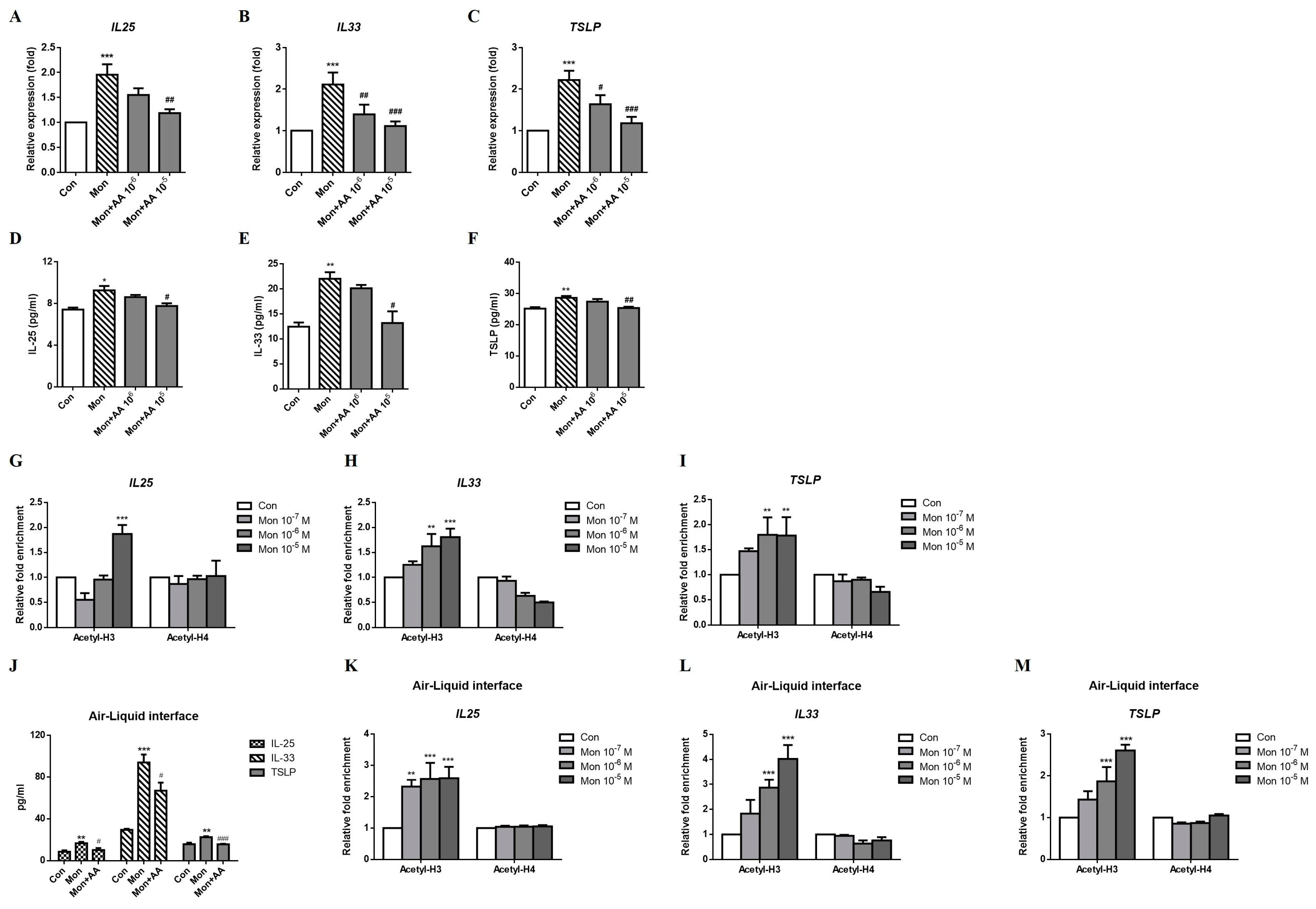
2.5. Montelukast Increased IL-25, IL-33, and TSLP Expression via Histone Modification
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Quantitative Real-Time PCR (qRT–PCR)
4.3. Enzyme-Linked Immunosorbent Assay (ELISA)
4.4. Western Blotting Analysis
4.5. Chromatin Immunoprecipitation Assay (ChIP)
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hung, C.H. Role of leukotriene receptor antagonists in asthma. Pediatr. Neonatol. 2012, 53, 219–220. [Google Scholar] [CrossRef]
- Mitchell, P.D.; O’Byrne, P.M. Epithelial-Derived Cytokines in Asthma. Chest 2017, 151, 1338–1344. [Google Scholar] [CrossRef]
- Tamachi, T.; Maezawa, Y.; Ikeda, K.; Kagami, S.; Hatano, M.; Seto, Y.; Suto, A.; Suzuki, K.; Watanabe, N.; Saito, Y.; et al. IL-25 enhances allergic airway inflammation by amplifying a TH2 cell-dependent pathway in mice. J. Allergy Clin. Immunol. 2006, 118, 606–614. [Google Scholar] [CrossRef]
- Prefontaine, D.; Lajoie-Kadoch, S.; Foley, S.; Audusseau, S.; Olivenstein, R.; Halayko, A.J.; Lemiere, C.; Martin, J.G.; Hamid, Q. Increased expression of IL-33 in severe asthma: Evidence of expression by airway smooth muscle cells. J. Immunol. 2009, 183, 5094–5103. [Google Scholar] [CrossRef]
- West, E.E.; Kashyap, M.; Leonard, W.J. TSLP: A Key Regulator of Asthma Pathogenesis. Drug Discov. Today Dis. Mech. 2012, 9, e83–e88. [Google Scholar] [CrossRef] [PubMed]
- Su, R.C.; Becker, A.B.; Kozyrskyj, A.L.; Hayglass, K.T. Altered epigenetic regulation and increasing severity of bronchial hyperresponsiveness in atopic asthmatic children. J. Allergy Clin. Immunol. 2009, 124, 1116–1118. [Google Scholar] [CrossRef]
- Seumois, G.; Chavez, L.; Gerasimova, A.; Lienhard, M.; Omran, N.; Kalinke, L.; Vedanayagam, M.; Ganesan, A.P.; Chawla, A.; Djukanovic, R.; et al. Epigenomic analysis of primary human T cells reveals enhancers associated with TH2 memory cell differentiation and asthma susceptibility. Nat. Immunol. 2014, 15, 777–788. [Google Scholar] [CrossRef]
- Wei, G.; Wei, L.; Zhu, J.; Zang, C.; Hu-Li, J.; Yao, Z.; Cui, K.; Kanno, Y.; Roh, T.Y.; Watford, W.T.; et al. Global mapping of H3K4me3 and H3K27me3 reveals specificity and plasticity in lineage fate determination of differentiating CD4+ T cells. Immunity 2009, 30, 155–167. [Google Scholar] [CrossRef] [PubMed]
- Ho, S.M. Environmental epigenetics of asthma: An update. J. Allergy Clin. Immunol. 2010, 126, 453–465. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Cao, X. Epigenetic regulation of the innate immune response to infection. Nat. Rev. Immunol. 2019, 19, 417–432. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Smith, S.G.; Beaudin, S.; Dua, B.; Howie, K.; Gauvreau, G.; O’Byrne, P.M. IL-25 and IL-25 receptor expression on eosinophils from subjects with allergic asthma. Int. Arch. Allergy Immunol. 2014, 163, 5–10. [Google Scholar] [CrossRef] [PubMed]
- Cheng, D.; Xue, Z.; Yi, L.; Shi, H.; Zhang, K.; Huo, X.; Bonser, L.R.; Zhao, J.; Xu, Y.; Erle, D.J.; et al. Epithelial interleukin-25 is a key mediator in Th2-high, corticosteroid-responsive asthma. Am. J. Respir. Crit. Care Med. 2014, 190, 639–648. [Google Scholar] [CrossRef] [PubMed]
- Moffatt, M.F.; Gut, I.G.; Demenais, F.; Strachan, D.P.; Bouzigon, E.; Heath, S.; von Mutius, E.; Farrall, M.; Lathrop, M.; Cookson, W.; et al. A large-scale, consortium-based genomewide association study of asthma. N. Engl. J. Med. 2010, 363, 1211–1221. [Google Scholar] [CrossRef]
- Traister, R.S.; Uvalle, C.E.; Hawkins, G.A.; Meyers, D.A.; Bleecker, E.R.; Wenzel, S.E. Phenotypic and genotypic association of epithelial IL1RL1 to human TH2-like asthma. J. Allergy Clin. Immunol. 2015, 135, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Corren, J.; Parnes, J.R.; Wang, L.; Mo, M.; Roseti, S.L.; Griffiths, J.M.; van der Merwe, R. Tezepelumab in Adults with Uncontrolled Asthma. N. Engl. J. Med. 2017, 377, 936–946. [Google Scholar] [CrossRef]
- Vivier, E.; Artis, D.; Colonna, M.; Diefenbach, A.; Di Santo, J.P.; Eberl, G.; Koyasu, S.; Locksley, R.M.; McKenzie, A.N.J.; Mebius, R.E.; et al. Innate Lymphoid Cells: 10 Years On. Cell 2018, 174, 1054–1066. [Google Scholar] [CrossRef]
- Bartemes, K.R.; Kephart, G.M.; Fox, S.J.; Kita, H. Enhanced innate type 2 immune response in peripheral blood from patients with asthma. J. Allergy Clin. Immunol. 2014, 134, 671–678 e4. [Google Scholar] [CrossRef]
- Smith, S.G.; Chen, R.; Kjarsgaard, M.; Huang, C.; Oliveria, J.P.; O’Byrne, P.M.; Gauvreau, G.M.; Boulet, L.P.; Lemiere, C.; Martin, J.; et al. Increased numbers of activated group 2 innate lymphoid cells in the airways of patients with severe asthma and persistent airway eosinophilia. J. Allergy Clin. Immunol. 2016, 137, 75–86.e8. [Google Scholar] [CrossRef]
- Columbo, M. Asthma in the elderly: A double-blind, placebo-controlled study of the effect of montelukast. Asthma Res. Pract. 2017, 3, 3. [Google Scholar] [CrossRef]
- Castro-Rodriguez, J.A.; Rodriguez-Martinez, C.E.; Ducharme, F.M. Daily inhaled corticosteroids or montelukast for preschoolers with asthma or recurrent wheezing: A systematic review. Pediatr. Pulmonol. 2018, 53, 1670–1677. [Google Scholar] [CrossRef]
- Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention; GINA: Fontana, WI, USA, 2022. [Google Scholar]
- Ramires, R.; Caiaffa, M.F.; Tursi, A.; Haeggstrom, J.Z.; Macchia, L. Novel inhibitory effect on 5-lipoxygenase activity by the anti-asthma drug montelukast. Biochem. Biophys. Res. Commun. 2004, 324, 815–821. [Google Scholar] [CrossRef] [PubMed]
- Ware, L.B. Modeling human lung disease in animals. Am. J. Physiol. Lung Cell Mol. Physiol. 2008, 294, L149–L150. [Google Scholar] [CrossRef] [PubMed]
- Blume, C.; Davies, D.E. In vitro and ex vivo models of human asthma. Eur. J. Pharm. Biopharm. 2013, 84, 394–400. [Google Scholar] [CrossRef] [PubMed]
- Baldassi, D.; Gabold, B.; Merkel, O. Air-liquid interface cultures of the healthy and diseased human respiratory tract: Promises, challenges and future directions. Adv. Nanobiomed. Res. 2021, 1, 2000111. [Google Scholar] [CrossRef]
- Pezzulo, A.A.; Starner, T.D.; Scheetz, T.E.; Traver, G.L.; Tilley, A.E.; Harvey, B.G.; Crystal, R.G.; McCray, P.B., Jr.; Zabner, J. The air-liquid interface and use of primary cell cultures are important to recapitulate the transcriptional profile of in vivo airway epithelia. Am. J. Physiol. Lung Cell Mol. Physiol. 2011, 300, L25–L31. [Google Scholar] [CrossRef] [PubMed]
- Morrison, D.K. MAP kinase pathways. Cold Spring Harb. Perspect. Biol. 2012, 4, a011254. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-kappaB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef]
- Yu, H.S.; Angkasekwinai, P.; Chang, S.H.; Chung, Y.; Dong, C. Protease allergens induce the expression of IL-25 via Erk and p38 MAPK pathway. J. Korean Med. Sci. 2010, 25, 829–834. [Google Scholar] [CrossRef] [PubMed]
- Qi, F.; Bai, S.; Wang, D.; Xu, L.; Hu, H.; Zeng, S.; Chai, R.; Liu, B. Macrophages produce IL-33 by activating MAPK signaling pathway during RSV infection. Mol. Immunol. 2017, 87, 284–292. [Google Scholar] [CrossRef]
- Beale, J.; Jayaraman, A.; Jackson, D.J.; Macintyre, J.D.R.; Edwards, M.R.; Walton, R.P.; Zhu, J.; Man Ching, Y.; Shamji, B.; Edwards, M.; et al. Rhinovirus-induced IL-25 in asthma exacerbation drives type 2 immunity and allergic pulmonary inflammation. Sci. Transl. Med. 2014, 6, 256ra134. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, J.L.; Koziol-White, C.J.; Jeffus, S.; Rettiganti, M.R.; Fisher, P.; Kurten, M.; Eze, A.; House, S.; Sikes, J.D.; Askew, E.; et al. Effects of rhinovirus 39 infection on airway hyperresponsiveness to carbachol in human airways precision cut lung slices. J. Allergy Clin. Immunol. 2018, 141, 1887–1890.e1. [Google Scholar] [CrossRef]
- Kanno, Y.; Vahedi, G.; Hirahara, K.; Singleton, K.; O’Shea, J.J. Transcriptional and epigenetic control of T helper cell specification: Molecular mechanisms underlying commitment and plasticity. Annu. Rev. Immunol. 2012, 30, 707–731. [Google Scholar] [CrossRef] [PubMed]
- Hew, M.; Bhavsar, P.; Torrego, A.; Meah, S.; Khorasani, N.; Barnes, P.J.; Adcock, I.; Chung, K.F. Relative corticosteroid insensitivity of peripheral blood mononuclear cells in severe asthma. Am. J. Respir. Crit. Care Med. 2006, 174, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Kuo, C.H.; Yang, S.N.; Kuo, H.F.; Lee, M.S.; Huang, M.Y.; Huang, S.K.; Lin, Y.C.; Hsieh, C.C.; Hung, C.H. Cysteinyl leukotriene receptor antagonist epigenetically modulates cytokine expression and maturation of human myeloid dendritic cells. Pulm. Pharmacol. Ther. 2016, 39, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Weng, C.M.; Wang, C.H.; Lee, M.J.; He, J.R.; Huang, H.Y.; Chao, M.W.; Chung, K.F.; Kuo, H.P. Aryl hydrocarbon receptor activation by diesel exhaust particles mediates epithelium-derived cytokines expression in severe allergic asthma. Allergy 2018, 73, 2192–2204. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.C.; Lin, Y.C.; Huang, M.Y.; Kuo, P.L.; Wu, C.C.; Lee, M.S.; Hsieh, C.C.; Kuo, H.F.; Kuo, C.H.; Tsai, W.C.; et al. Tumor necrosis factor-alpha inhibitors suppress CCL2 chemokine in monocytes via epigenetic modification. Mol. Immunol. 2017, 83, 82–91. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsai, M.-L.; Tsai, M.-K.; Tsai, Y.-G.; Lin, Y.-C.; Hsu, Y.-L.; Chen, Y.-T.; Lin, Y.-C.; Hung, C.-H. Montelukast Increased IL-25, IL-33, and TSLP via Epigenetic Regulation in Airway Epithelial Cells. Int. J. Mol. Sci. 2023, 24, 1227. https://doi.org/10.3390/ijms24021227
Tsai M-L, Tsai M-K, Tsai Y-G, Lin Y-C, Hsu Y-L, Chen Y-T, Lin Y-C, Hung C-H. Montelukast Increased IL-25, IL-33, and TSLP via Epigenetic Regulation in Airway Epithelial Cells. International Journal of Molecular Sciences. 2023; 24(2):1227. https://doi.org/10.3390/ijms24021227
Chicago/Turabian StyleTsai, Mei-Lan, Ming-Kai Tsai, Yi-Giien Tsai, Yu-Chih Lin, Ya-Ling Hsu, Yi-Ting Chen, Yi-Ching Lin, and Chih-Hsing Hung. 2023. "Montelukast Increased IL-25, IL-33, and TSLP via Epigenetic Regulation in Airway Epithelial Cells" International Journal of Molecular Sciences 24, no. 2: 1227. https://doi.org/10.3390/ijms24021227
APA StyleTsai, M.-L., Tsai, M.-K., Tsai, Y.-G., Lin, Y.-C., Hsu, Y.-L., Chen, Y.-T., Lin, Y.-C., & Hung, C.-H. (2023). Montelukast Increased IL-25, IL-33, and TSLP via Epigenetic Regulation in Airway Epithelial Cells. International Journal of Molecular Sciences, 24(2), 1227. https://doi.org/10.3390/ijms24021227





