Klotho Regulated by Estrogen Plays a Key Role in Sex Differences in Stress Resilience in Rats
Abstract
1. Introduction
2. Results
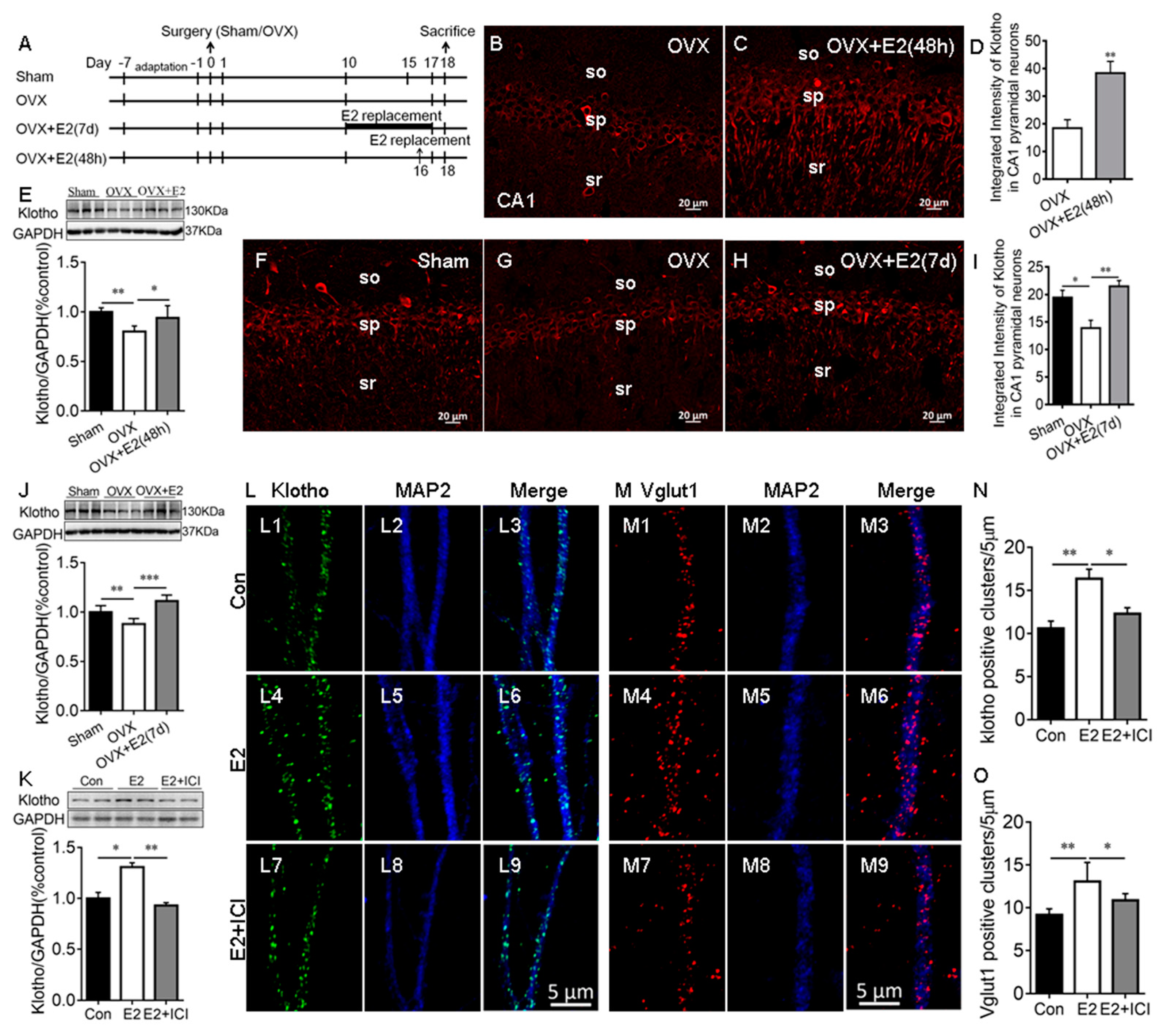
2.1. E2 Regulated the KL Protein Levels in the Hippocampal Neurons
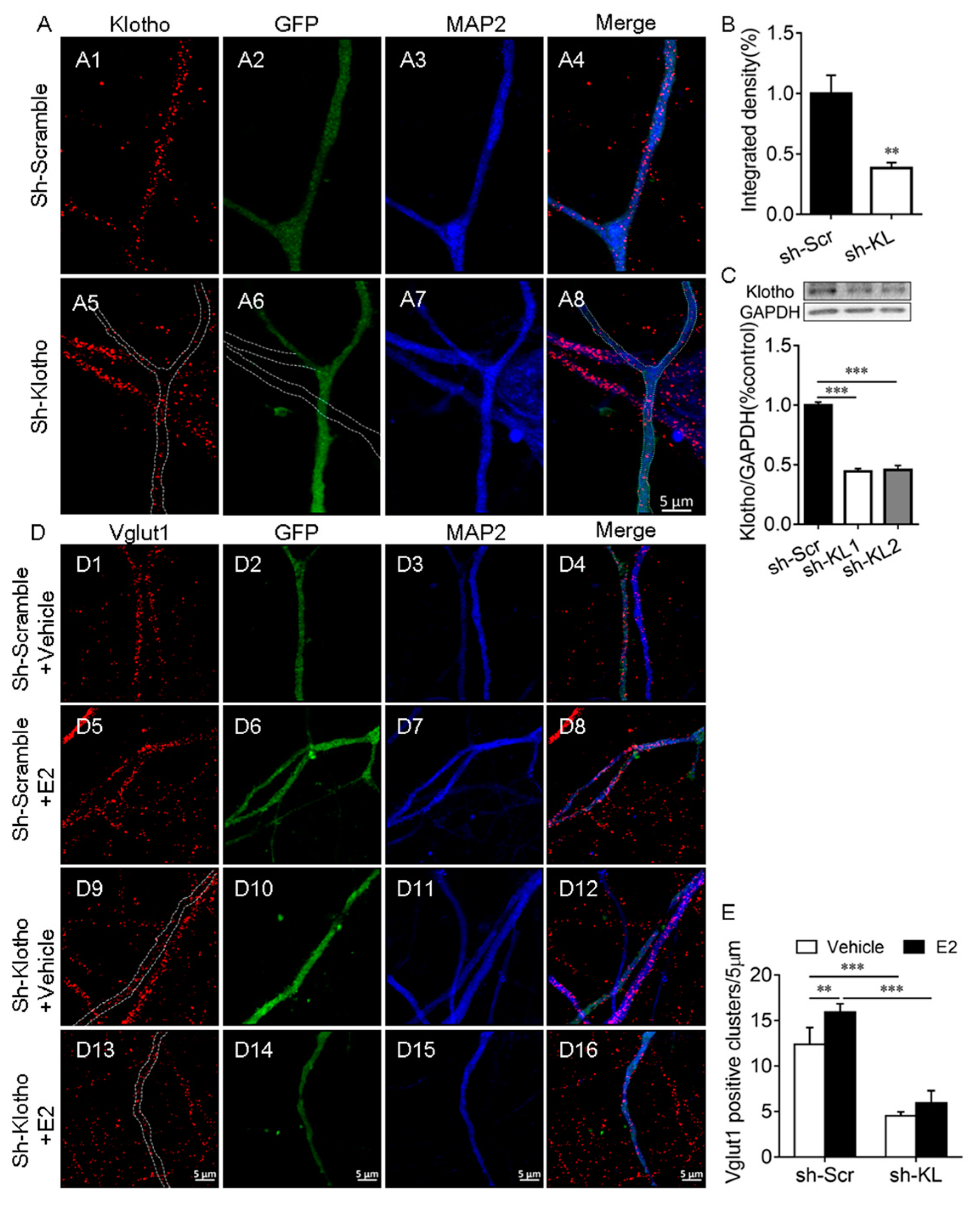
2.2. E2 Did Not Affect the Number of Vglut1-Positive Excitatory Presynaptic Terminal When the Endogenous KL Level Was Reduced in Hippocampal Neurons
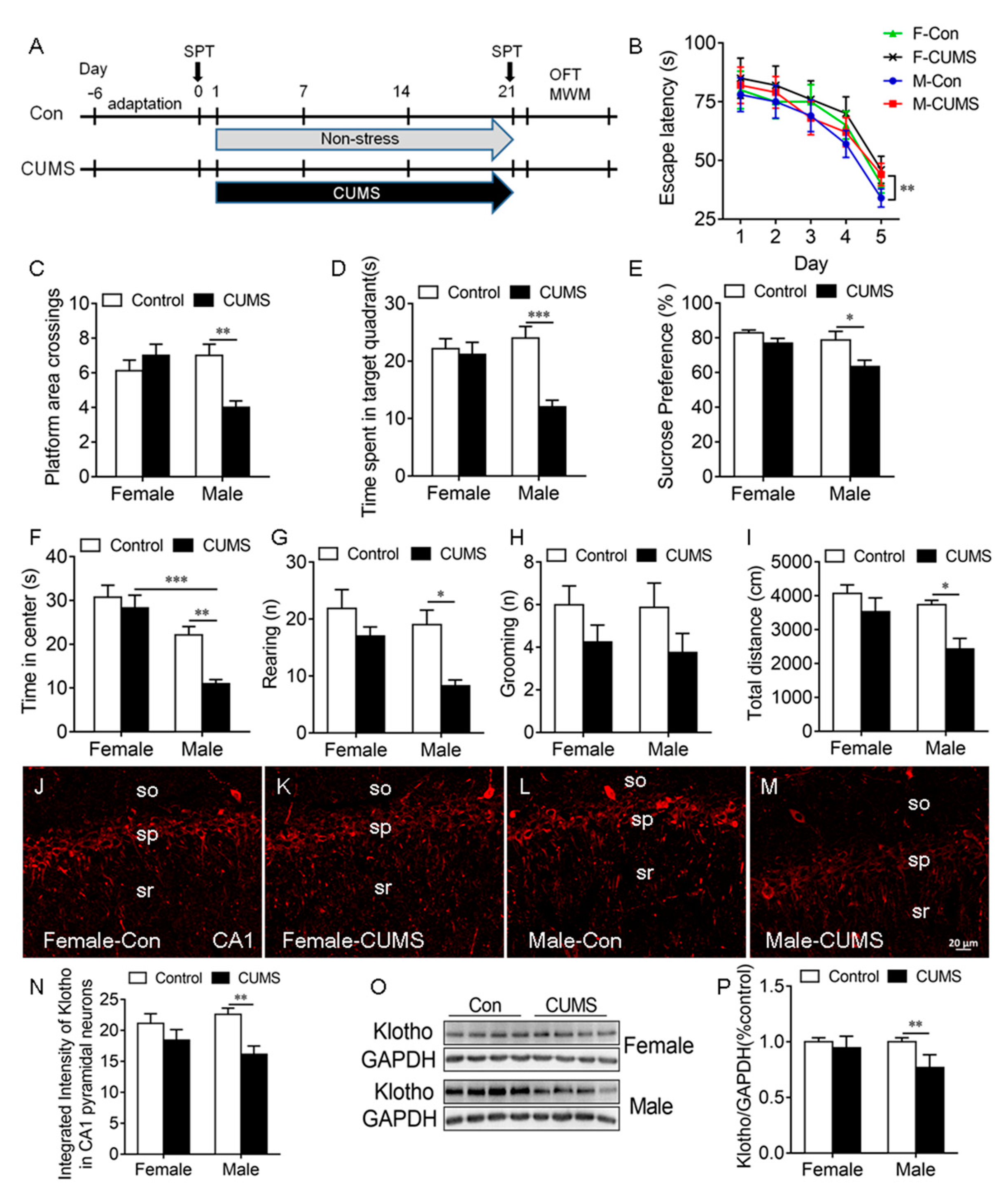
2.3. CUMS-Induced Deficit in Spatial Learning and Memory, Anhedonic-like Behaviors, and Anxiety-like Behaviors Were Accompanied by a Decrease in KL Protein Levels in Male Rats Only
2.4. Reduction in Endogenous KL in the Hippocampus Did Not Alter Spatial Learning and Memory, Anhedonic-like Behavior, and Anxiety-like Behavior in Rats of Both Sexes
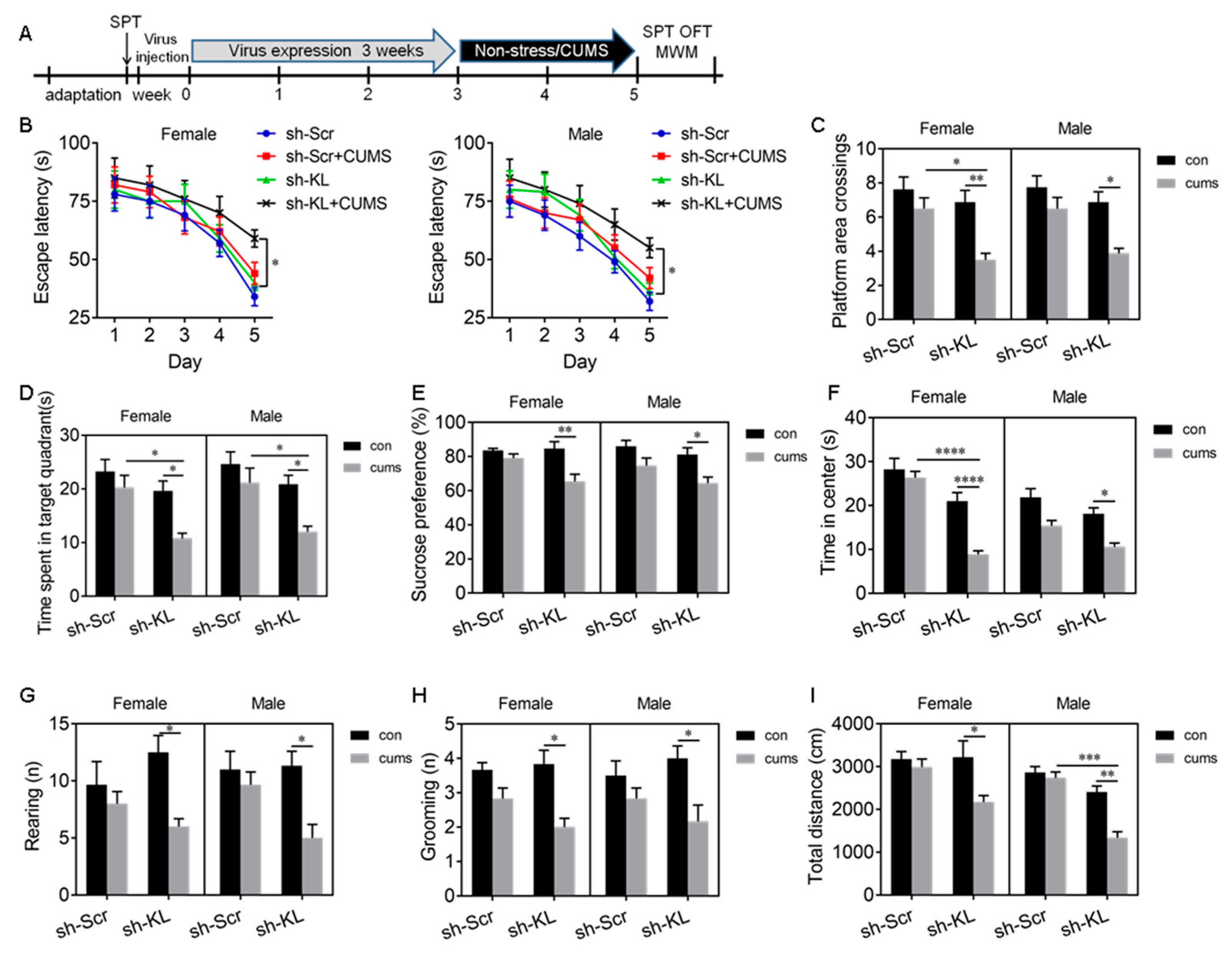
2.5. Endogenous KL Plays an Essential Role in Sex Differences in CUMS-Induced Deficit in Spatial Learning and Memory, Anhedonic-like Behaviors, and Anxiety-like Behaviors
3. Discussion
3.1. E2 Regulated KL Expression in Hippocampal Neurons
3.2. KL Plays an Essential Role in E2-Mediated Synapse Formation in Hippocampal Neurons
3.3. Sex Differences in CUMS-Induced KL Expression Is Associated with Sex Differences in Stress Resilience in Behaviors
3.4. Endogenous KL in the Hippocampus Is Essential for Sex Differences in Stress Resilience
4. Materials and Methods
4.1. Animals and Reagents
4.2. Ovariectomy (OVX) and Estradiol Replacement
4.3. Primary Rat Hippocampal Neurons, Drug Treatments, and Transfection
4.4. Western Blot
4.5. Immunohistochemistry (Brain Sections)
4.6. Immunocytochemistry of Cultured Hippocampal Neurons
4.7. Chronic Unpredictable Mild Stress (CUMS)
4.8. Stereotaxic Surgery and Adeno-Associated Virus (AAV) Microinjection
4.9. Behavioral Assessments
4.10. Data Collection and Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Frick, K.M.; Kim, J.; Tuscher, J.J.; Fortress, A.M. Sex steroid hormones matter for learning and memory: Estrogenic regulation of hippocampal function in male and female rodents. Learn. Mem. 2015, 22, 472–493. [Google Scholar] [CrossRef]
- Woolley, C.S.; McEwen, B.S. Roles of estradiol and progesterone in regulation of hippocampal dendritic spine density during the estrous cycle in the rat. J. Comp. Neurol. 1993, 336, 293–306. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.C.; McMahon, L.L. Estrogen-induced increase in the magnitude of long-term potentiation occurs only when the ratio of NMDA transmission to AMPA transmission is increased. J. Neurosci. 2005, 25, 7780–7791. [Google Scholar] [CrossRef]
- Iqbal, J.; Tan, Z.N.; Li, M.X.; Chen, H.B.; Ma, B.; Zhou, X.; Ma, X.M. Estradiol Alters Hippocampal Gene Expression during the Estrous Cycle. Endocr. Res. 2020, 45, 84–101. [Google Scholar] [CrossRef]
- Li, Q.; Vo, H.T.; Wang, J.; Fox-Quick, S.; Dobrunz, L.E.; King, G.D. Klotho regulates CA1 hippocampal synaptic plasticity. Neuroscience 2017, 347, 123–133. [Google Scholar] [CrossRef]
- Masso, A.; Sanchez, A.; Gimenez-Llort, L.; Lizcano, J.M.; Canete, M.; Garcia, B.; Torres-Lista, V.; Puig, M.; Bosch, A.; Chillon, M. Secreted and Transmembrane alphaKlotho Isoforms Have Different Spatio-Temporal Profiles in the Brain during Aging and Alzheimer’s Disease Progression. PLoS ONE 2015, 10, e0143623. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, Y.; Aizawa, H.; Shiraki-Iida, T.; Nagai, R.; Kuro-o, M.; Nabeshima, Y. Identification of the human klotho gene and its two transcripts encoding membrane and secreted klotho protein. Biochem. Biophys. Res. Commun. 1998, 242, 626–630. [Google Scholar] [CrossRef]
- Kuro-o, M.; Matsumura, Y.; Aizawa, H.; Kawaguchi, H.; Suga, T.; Utsugi, T.; Ohyama, Y.; Kurabayashi, M.; Kaname, T.; Kume, E.; et al. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature 1997, 390, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Shiraki-Iida, T.; Aizawa, H.; Matsumura, Y.; Sekine, S.; Iida, A.; Anazawa, H.; Nagai, R.; Kuro-o, M.; Nabeshima, Y. Structure of the mouse klotho gene and its two transcripts encoding membrane and secreted protein. FEBS Lett. 1998, 424, 6–10. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Sun, Z. Molecular basis of Klotho: From gene to function in aging. Endocr. Rev. 2015, 36, 174–193. [Google Scholar] [CrossRef]
- Ohyama, Y.; Kurabayashi, M.; Masuda, H.; Nakamura, T.; Aihara, Y.; Kaname, T.; Suga, T.; Arai, M.; Aizawa, H.; Matsumura, Y.; et al. Molecular cloning of rat klotho cDNA: Markedly decreased expression of klotho by acute inflammatory stress. Biochem. Biophys. Res. Commun. 1998, 251, 920–925. [Google Scholar] [CrossRef] [PubMed]
- Kurosu, H.; Yamamoto, M.; Clark, J.D.; Pastor, J.V.; Nandi, A.; Gurnani, P.; McGuinness, O.P.; Chikuda, H.; Yamaguchi, M.; Kawaguchi, H.; et al. Suppression of aging in mice by the hormone Klotho. Science 2005, 309, 1829–1833. [Google Scholar] [CrossRef]
- Li, S.A.; Watanabe, M.; Yamada, H.; Nagai, A.; Kinuta, M.; Takei, K. Immunohistochemical localization of Klotho protein in brain, kidney, and reproductive organs of mice. Cell Struct. Funct. 2004, 29, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Nagai, T.; Yamada, K.; Kim, H.C.; Kim, Y.S.; Noda, Y.; Imura, A.; Nabeshima, Y.; Nabeshima, T. Cognition impairment in the genetic model of aging klotho gene mutant mice: A role of oxidative stress. FASEB J. 2003, 17, 50–52. [Google Scholar] [CrossRef]
- Dubal, D.B.; Yokoyama, J.S.; Zhu, L.; Broestl, L.; Worden, K.; Wang, D.; Sturm, V.E.; Kim, D.; Klein, E.; Yu, G.Q.; et al. Life extension factor klotho enhances cognition. Cell Rep. 2014, 7, 1065–1076. [Google Scholar] [CrossRef]
- Zeldich, E.; Chen, C.D.; Colvin, T.A.; Bove-Fenderson, E.A.; Liang, J.; Tucker Zhou, T.B.; Harris, D.A.; Abraham, C.R. The neuroprotective effect of Klotho is mediated via regulation of members of the redox system. J. Biol. Chem. 2014, 289, 24700–24715. [Google Scholar] [CrossRef]
- Prather, A.A.; Epel, E.S.; Arenander, J.; Broestl, L.; Garay, B.I.; Wang, D.; Dubal, D.B. Longevity factor klotho and chronic psychological stress. Transl. Psychiatry 2015, 5, e585. [Google Scholar] [CrossRef]
- Hoyer, C.; Sartorius, A.; Aksay, S.S.; Bumb, J.M.; Janke, C.; Thiel, M.; Haffner, D.; Leifheit-Nestler, M.; Kranaster, L. Electroconvulsive therapy enhances the anti-ageing hormone Klotho in the cerebrospinal fluid of geriatric patients with major depression. Eur. Neuropsychopharmacol. 2018, 28, 428–435. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Sun, Z.; Ma, G.; Li, Y.; Liu, M.; Zhang, G.; Xu, H.; Gao, Y.; Zhou, J.; Deng, Q.; et al. Reduced Plasma Levels of alpha-Klotho and Their Correlation with Klotho Polymorphisms in Elderly Patients with Major Depressive Disorders. Front. Psychiatry 2021, 12, 682691. [Google Scholar] [CrossRef]
- Sathyanesan, M.; Girgenti, M.J.; Banasr, M.; Stone, K.; Bruce, C.; Guilchicek, E.; Wilczak-Havill, K.; Nairn, A.; Williams, K.; Sass, S.; et al. A molecular characterization of the choroid plexus and stress-induced gene regulation. Transl. Psychiatry 2012, 2, e139. [Google Scholar] [CrossRef]
- Wu, H.J.; Wu, W.N.; Fan, H.; Liu, L.E.; Zhan, J.Q.; Li, Y.H.; Chen, C.N.; Jiang, S.Z.; Xiong, J.W.; Yu, Z.M.; et al. Life extension factor klotho regulates behavioral responses to stress via modulation of GluN2B function in the nucleus accumbens. Neuropsychopharmacology 2022, 47, 1710–1720. [Google Scholar] [CrossRef] [PubMed]
- Pego, J.M.; Sousa, J.C.; Almeida, O.F.; Sousa, N. Stress and the neuroendocrinology of anxiety disorders. Curr. Top. Behav. Neurosci. 2010, 2, 97–117. [Google Scholar] [CrossRef] [PubMed]
- Donner, N.C.; Lowry, C.A. Sex differences in anxiety and emotional behavior. Pflug. Arch. 2013, 465, 601–626. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.W.; Kim, Y.K.; Jeon, H.J. Comorbid Anxiety and Depression: Clinical and Conceptual Consideration and Transdiagnostic Treatment. Adv. Exp. Med. Biol. 2020, 1191, 219–235. [Google Scholar] [CrossRef] [PubMed]
- Willner, P. The chronic mild stress (CMS) model of depression: History, evaluation and usage. Neurobiol. Stress 2017, 6, 78–93. [Google Scholar] [CrossRef]
- Moench, K.M.; Wellman, C.L. Differential dendritic remodeling in prelimbic cortex of male and female rats during recovery from chronic stress. Neuroscience 2017, 357, 145–159. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Yuen, E.Y.; Liu, W.; Li, X.; Zhong, P.; Karatsoreos, I.N.; McEwen, B.S.; Yan, Z. Estrogen protects against the detrimental effects of repeated stress on glutamatergic transmission and cognition. Mol. Psychiatry 2014, 19, 588–598. [Google Scholar] [CrossRef]
- Wohleb, E.S.; Terwilliger, R.; Duman, C.H.; Duman, R.S. Stress-Induced Neuronal Colony Stimulating Factor 1 Provokes Microglia-Mediated Neuronal Remodeling and Depressive-like Behavior. Biol. Psychiatry 2018, 83, 38–49. [Google Scholar] [CrossRef]
- Luine, V.; Gomez, J.; Beck, K.; Bowman, R. Sex differences in chronic stress effects on cognition in rodents. Pharmacol. Biochem. Behav. 2017, 152, 13–19. [Google Scholar] [CrossRef]
- Mazarati, A.; Jones, N.C.; Galanopoulou, A.S.; Harte-Hargrove, L.C.; Kalynchuk, L.E.; Lenck-Santini, P.P.; Medel-Matus, J.S.; Nehlig, A.; de la Prida, L.M.; Sarkisova, K.; et al. A companion to the preclinical common data elements on neurobehavioral comorbidities of epilepsy: A report of the TASK3 behavior working group of the ILAE/AES Joint Translational Task Force. Epilepsia Open 2018, 3, 24–52. [Google Scholar] [CrossRef]
- Ho, W.Y.; Navakkode, S.; Liu, F.; Soong, T.W.; Ling, S.C. Deregulated expression of a longevity gene, Klotho, in the C9orf72 deletion mice with impaired synaptic plasticity and adult hippocampal neurogenesis. Acta Neuropathol. Commun. 2020, 8, 155. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.M.; Huang, J.P.; Kim, E.J.; Zhu, Q.; Kuchel, G.A.; Mains, R.E.; Eipper, B.A. Kalirin-7, an important component of excitatory synapses, is regulated by estradiol in hippocampal neurons. Hippocampus 2011, 21, 661–677. [Google Scholar] [CrossRef] [PubMed]
- Jelks, K.B.; Wylie, R.; Floyd, C.L.; McAllister, A.K.; Wise, P. Estradiol targets synaptic proteins to induce glutamatergic synapse formation in cultured hippocampal neurons: Critical role of estrogen receptor-alpha. J. Neurosci. 2007, 27, 6903–6913. [Google Scholar] [CrossRef]
- Kretz, O.; Fester, L.; Wehrenberg, U.; Zhou, L.; Brauckmann, S.; Zhao, S.; Prange-Kiel, J.; Naumann, T.; Jarry, H.; Frotscher, M.; et al. Hippocampal synapses depend on hippocampal estrogen synthesis. J. Neurosci. 2004, 24, 5913–5921. [Google Scholar] [CrossRef]
- Xu, L.; Sun, H.; Qu, C.; Shen, J.; Qu, C.; Song, H.; Li, T.; Zheng, J.; Zhang, J. The environmental enrichment ameliorates chronic unpredictable mild stress-induced depressive-like behaviors and cognitive decline by inducing autophagy-mediated inflammation inhibition. Brain Res. Bull. 2022, 187, 98–110. [Google Scholar] [CrossRef]
- Liu, D.; Zhang, Q.; Gu, J.; Wang, X.; Xie, K.; Xian, X.; Wang, J.; Jiang, H.; Wang, Z. Resveratrol prevents impaired cognition induced by chronic unpredictable mild stress in rats. Prog. Neuropsychopharmacol Biol. Psychiatry 2014, 49, 21–29. [Google Scholar] [CrossRef]
- Gumuslu, E.; Mutlu, O.; Sunnetci, D.; Ulak, G.; Celikyurt, I.K.; Cine, N.; Akar, F. The effects of tianeptine, olanzapine and fluoxetine on the cognitive behaviors of unpredictable chronic mild stress-exposed mice. Drug Res. 2013, 63, 532–539. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Li, Y.; Qu, C.; Xu, L.; Sun, H.; Zhang, J. The enriched environment ameliorates chronic unpredictable mild stress-induced depressive-like behaviors and cognitive impairment by activating the SIRT1/miR-134 signaling pathway in hippocampus. J. Affect. Disord. 2019, 248, 81–90. [Google Scholar] [CrossRef]
- Gawel, K.; Gibula, E.; Marszalek-Grabska, M.; Filarowska, J.; Kotlinska, J.H. Assessment of spatial learning and memory in the Barnes maze task in rodents-methodological consideration. Naunyn Schmiedebergs Arch. Pharmacol. 2019, 392, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Russo-Savage, L.; Rao, V.K.S.; Eipper, B.A.; Mains, R.E. Role of Kalirin and Mouse Strain in Retention of Spatial Memory Training in an Alzheimer’s Disease Model Mouse Line. Neurobiol. Aging 2020, 95, 69–80. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.D.; Li, Y.; Chen, A.K.; Rudy, M.A.; Nasse, J.S.; Zeldich, E.; Polanco, T.J.; Abraham, C.R. Identification of the cleavage sites leading to the shed forms of human and mouse anti-aging and cognition-enhancing protein Klotho. PLoS ONE 2020, 15, e0226382. [Google Scholar] [CrossRef]
- Masso, A.; Sanchez, A.; Bosch, A.; Gimenez-Llort, L.; Chillon, M. Secreted alphaKlotho isoform protects against age-dependent memory deficits. Mol. Psychiatry 2018, 23, 1937–1947. [Google Scholar] [CrossRef]
- Zhou, L.; Fester, L.; Haghshenas, S.; de Vrese, X.; von Hacht, R.; Gloger, S.; Brandt, N.; Bader, M.; Vollmer, G.; Rune, G.M. Oestradiol-induced synapse formation in the female hippocampus: Roles of oestrogen receptor subtypes. J. Neuroendocrinol. 2014, 26, 439–447. [Google Scholar] [CrossRef] [PubMed]
- Murphy, D.D.; Cole, N.B.; Greenberger, V.; Segal, M. Estradiol increases dendritic spine density by reducing GABA neurotransmission in hippocampal neurons. J. Neurosci. 1998, 18, 2550–2559. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Fester, L.; von Blittersdorff, B.; Hassu, B.; Nogens, H.; Prange-Kiel, J.; Jarry, H.; Wegscheider, K.; Rune, G.M. Aromatase inhibitors induce spine synapse loss in the hippocampus of ovariectomized mice. Endocrinology 2010, 151, 1153–1160. [Google Scholar] [CrossRef]
- Winer, E.S.; Bryant, J.; Bartoszek, G.; Rojas, E.; Nadorff, M.R.; Kilgore, J. Mapping the relationship between anxiety, anhedonia, and depression. J. Affect. Disord. 2017, 221, 289–296. [Google Scholar] [CrossRef]
- Liu, J.; Dong, Q.; Lu, X.; Sun, J.; Zhang, L.; Wang, M.; Liu, B.; Ju, Y.; Wan, P.; Guo, H.; et al. Influence of comorbid anxiety symptoms on cognitive deficits in patients with major depressive disorder. J. Affect. Disord. 2020, 260, 91–96. [Google Scholar] [CrossRef]
- Zhang, Z.; Cai, X.; Yao, Z.; Wen, F.; Fu, Z.; Zhang, J.; Zhong, Z.; Huang, Y.; Qu, S. EA Ameliorated Depressive Behaviors in CUMS Rats and Was Related to Its Suppressing Autophagy in the Hippocampus. Neural Plast. 2020, 2020, 8860968. [Google Scholar] [CrossRef] [PubMed]
- Scholl, J.L.; Afzal, A.; Fox, L.C.; Watt, M.J.; Forster, G.L. Sex differences in anxiety-like behaviors in rats. Physiol. Behav. 2019, 211, 112670. [Google Scholar] [CrossRef] [PubMed]
- Knight, P.; Chellian, R.; Wilson, R.; Behnood-Rod, A.; Panunzio, S.; Bruijnzeel, A.W. Sex differences in the elevated plus-maze test and large open field test in adult Wistar rats. Pharmacol. Biochem. Behav. 2021, 204, 173168. [Google Scholar] [CrossRef] [PubMed]
- Kokras, N.; Dalla, C. Sex differences in animal models of psychiatric disorders. Br. J. Pharmacol. 2014, 171, 4595–4619. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Wu, X.Y.; Zhu, Q.B.; Li, J.; Shi, L.G.; Wu, J.L.; Zhang, Q.J.; Huang, M.L.; Bao, A.M. Sex differences in the stress response in SD rats. Behav. Brain Res. 2015, 284, 231–237. [Google Scholar] [CrossRef]
- Grippo, A.J.; Sullivan, N.R.; Damjanoska, K.J.; Crane, J.W.; Carrasco, G.A.; Shi, J.; Chen, Z.; Garcia, F.; Muma, N.A.; Van de Kar, L.D. Chronic mild stress induces behavioral and physiological changes, and may alter serotonin 1A receptor function, in male and cycling female rats. Psychopharmacology 2005, 179, 769–780. [Google Scholar] [CrossRef] [PubMed]
- Dalla, C.; Antoniou, K.; Kokras, N.; Drossopoulou, G.; Papathanasiou, G.; Bekris, S.; Daskas, S.; Papadopoulou-Daifoti, Z. Sex differences in the effects of two stress paradigms on dopaminergic neurotransmission. Physiol. Behav. 2008, 93, 595–605. [Google Scholar] [CrossRef] [PubMed]
- Kalueff, A.V.; Stewart, A.M.; Song, C.; Berridge, K.C.; Graybiel, A.M.; Fentress, J.C. Neurobiology of rodent self-grooming and its value for translational neuroscience. Nat. Rev. Neurosci. 2016, 17, 45–59. [Google Scholar] [CrossRef]
- Sturman, O.; Germain, P.L.; Bohacek, J. Exploratory rearing: A context- and stress-sensitive behavior recorded in the open-field test. Stress 2018, 21, 443–452. [Google Scholar] [CrossRef]
- Bouwknecht, J.A.; Spiga, F.; Staub, D.R.; Hale, M.W.; Shekhar, A.; Lowry, C.A. Differential effects of exposure to low-light or high-light open-field on anxiety-related behaviors: Relationship to c-Fos expression in serotonergic and non-serotonergic neurons in the dorsal raphe nucleus. Brain Res. Bull. 2007, 72, 32–43. [Google Scholar] [CrossRef]
- Jianguo, L.; Xueyang, J.; Cui, W.; Changxin, W.; Xuemei, Q. Altered gut metabolome contributes to depression-like behaviors in rats exposed to chronic unpredictable mild stress. Transl. Psychiatry 2019, 9, 40. [Google Scholar] [CrossRef]
- Wang, Y.L.; Wu, H.R.; Zhang, S.S.; Xiao, H.L.; Yu, J.; Ma, Y.Y.; Zhang, Y.D.; Liu, Q. Catalpol ameliorates depressive-like behaviors in CUMS mice via oxidative stress-mediated NLRP3 inflammasome and neuroinflammation. Transl. Psychiatry 2021, 11, 353. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Li, J.; Gaurav, C.; Muhammad, U.; Chen, Y.; Li, X.; Chen, J.; Wang, Z. CUMS and dexamethasone induce depression-like phenotypes in mice by differentially altering gut microbiota and triggering macroglia activation. Gen. Psychiatry 2021, 34, e100529. [Google Scholar] [CrossRef]
- Fu, X.Y.; Chen, H.H.; Zhang, N.; Ding, M.X.; Qiu, Y.E.; Pan, X.M.; Fang, Y.S.; Lin, Y.P.; Zheng, Q.; Wang, W.Q. Effects of chronic unpredictable mild stress on ovarian reserve in female rats: Feasibility analysis of a rat model of premature ovarian failure. Mol. Med. Rep. 2018, 18, 532–540. [Google Scholar] [CrossRef]
- Casillas, F.; Betancourt, M.; Juarez-Rojas, L.; Ducolomb, Y.; Lopez, A.; Avila-Quintero, A.; Zamora, J.; Ommati, M.M.; Retana-Marquez, S. Chronic Stress Detrimentally Affects In Vivo Maturation in Rat Oocytes and Oocyte Viability at All Phases of the Estrous Cycle. Animals 2021, 11, 2478. [Google Scholar] [CrossRef]
- Stankiewicz, A.M.; Goscik, J.; Majewska, A.; Swiergiel, A.H.; Juszczak, G.R. The Effect of Acute and Chronic Social Stress on the Hippocampal Transcriptome in Mice. PLoS ONE 2015, 10, e0142195. [Google Scholar] [CrossRef] [PubMed]
- Laszczyk, A.M.; Fox-Quick, S.; Vo, H.T.; Nettles, D.; Pugh, P.C.; Overstreet-Wadiche, L.; King, G.D. Klotho regulates postnatal neurogenesis and protects against age-related spatial memory loss. Neurobiol. Aging 2017, 59, 41–54. [Google Scholar] [CrossRef]
- Zhou, H.J.; Zeng, C.Y.; Yang, T.T.; Long, F.Y.; Kuang, X.; Du, J.R. Lentivirus-mediated klotho up-regulation improves aging-related memory deficits and oxidative stress in senescence-accelerated mouse prone-8 mice. Life Sci. 2018, 200, 56–62. [Google Scholar] [CrossRef]
- Salech, F.; Varela-Nallar, L.; Arredondo, S.B.; Bustamante, D.B.; Andaur, G.A.; Cisneros, R.; Ponce, D.P.; Ayala, P.; Inestrosa, N.C.; Valdes, J.L.; et al. Local Klotho enhances neuronal progenitor proliferation in the adult hippocampus. J. Gerontol. A Biol. Sci. Med. Sci. 2017, 74, 1043–1051. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Jing, D.; Liu, Z.; Chen, Y.; Huang, F.; Behnisch, T. Enhanced Expression of Secreted alpha-Klotho in the Hippocampus Alters Nesting Behavior and Memory Formation in Mice. Front. Cell. Neurosci. 2019, 13, 133. [Google Scholar] [CrossRef]
- Qiao, H.; Li, M.X.; Xu, C.; Chen, H.B.; An, S.C.; Ma, X.M. Dendritic Spines in Depression: What We Learned from Animal Models. Neural Plast. 2016, 2016, 8056370. [Google Scholar] [CrossRef]
- Mahmmoud, R.R.; Sase, S.; Aher, Y.D.; Sase, A.; Groger, M.; Mokhtar, M.; Hoger, H.; Lubec, G. Spatial and Working Memory Is Linked to Spine Density and Mushroom Spines. PLoS ONE 2015, 10, e0139739. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Rex, C.S.; Rice, C.J.; Dube, C.M.; Gall, C.M.; Lynch, G.; Baram, T.Z. Correlated memory defects and hippocampal dendritic spine loss after acute stress involve corticotropin-releasing hormone signaling. Proc. Natl. Acad. Sci. USA 2010, 107, 13123–13128. [Google Scholar] [CrossRef]
- Qu, Y.; Yang, C.; Ren, Q.; Ma, M.; Dong, C.; Hashimoto, K. Regional differences in dendritic spine density confer resilience to chronic social defeat stress. Acta Neuropsychiatr. 2018, 30, 117–122. [Google Scholar] [CrossRef]
- Walker, C.K.; Herskowitz, J.H. Dendritic Spines: Mediators of Cognitive Resilience in Aging and Alzheimer’s Disease. Neuroscientist 2021, 27, 487–505. [Google Scholar] [CrossRef]
- Holmes, S.E.; Scheinost, D.; Finnema, S.J.; Naganawa, M.; Davis, M.T.; DellaGioia, N.; Nabulsi, N.; Matuskey, D.; Angarita, G.A.; Pietrzak, R.H.; et al. Lower synaptic density is associated with depression severity and network alterations. Nat. Commun. 2019, 10, 1529. [Google Scholar] [CrossRef]
- Wang, X.; Sun, Z. RNAi silencing of brain klotho potentiates cold-induced elevation of blood pressure via the endothelin pathway. Physiol. Genom. 2010, 41, 120–126. [Google Scholar] [CrossRef]
- Ma, X.M.; Kiraly, D.D.; Gaier, E.D.; Wang, Y.; Kim, E.J.; Levine, E.S.; Eipper, B.A.; Mains, R.E. Kalirin-7 is required for synaptic structure and function. J. Neurosci. 2008, 28, 12368–12382. [Google Scholar] [CrossRef]
- Qiao, H.; An, S.C.; Ren, W.; Ma, X.M. Progressive alterations of hippocampal CA3–CA1 synapses in an animal model of depression. Behav. Brain Res. 2014, 275, 191–200. [Google Scholar] [CrossRef]
- Zhou, M.H.; Sun, F.F.; Xu, C.; Chen, H.B.; Qiao, H.; Cai, X.; Ma, X.M.; An, S.C. Modulation of Kalirin-7 Expression by Hippocampal CA1 5-HT1B Receptors in Spatial Memory Consolidation. Behav. Brain Res. 2019, 356, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Ma, X.M.; Chen, H.B.; Zhou, M.H.; Qiao, H.; An, S.C. Orbitofrontal cortex 5-HT2A receptor mediates chronic stress-induced depressive-like behaviors and alterations of spine density and Kalirin7. Neuropharmacology 2016, 109, 7–17. [Google Scholar] [CrossRef]
- Macedo, P.T.; Aquino, A.C.Q.; Meurer, Y.S.R.; Brandao, L.E.M.; Campelo, C.L.C.; Lima, R.H.; Costa, M.R.; Ribeiro, A.M.; Silva, R.H. Subtle Alterations in Spatial Memory Induced by Amyloid Peptides Infusion in Rats. Front. Aging Neurosci. 2018, 10, 18. [Google Scholar] [CrossRef] [PubMed]
- Powers, K.G.; Ma, X.M.; Eipper, B.A.; Mains, R.E. Cell-type specific knockout of peptidylglycine alpha-amidating monooxygenase reveals specific behavioral roles in excitatory forebrain neurons and cardiomyocytes. Genes Brain Behav. 2021, 20, e12699. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.M.; Wang, Y.; Ferraro, F.; Mains, R.E.; Eipper, B.A. Kalirin-7 is an essential component of both shaft and spine excitatory synapses in hippocampal interneurons. J. Neurosci. 2008, 28, 711–724. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, Z.; Li, Y.; Guan, Y.; Iqbal, J.; Wang, C.; Yan, R.; Ma, X.-M. Klotho Regulated by Estrogen Plays a Key Role in Sex Differences in Stress Resilience in Rats. Int. J. Mol. Sci. 2023, 24, 1206. https://doi.org/10.3390/ijms24021206
Tan Z, Li Y, Guan Y, Iqbal J, Wang C, Yan R, Ma X-M. Klotho Regulated by Estrogen Plays a Key Role in Sex Differences in Stress Resilience in Rats. International Journal of Molecular Sciences. 2023; 24(2):1206. https://doi.org/10.3390/ijms24021206
Chicago/Turabian StyleTan, Zhinei, Yongxia Li, Yinzheng Guan, Javed Iqbal, Chenyue Wang, Riqiang Yan, and Xin-Ming Ma. 2023. "Klotho Regulated by Estrogen Plays a Key Role in Sex Differences in Stress Resilience in Rats" International Journal of Molecular Sciences 24, no. 2: 1206. https://doi.org/10.3390/ijms24021206
APA StyleTan, Z., Li, Y., Guan, Y., Iqbal, J., Wang, C., Yan, R., & Ma, X.-M. (2023). Klotho Regulated by Estrogen Plays a Key Role in Sex Differences in Stress Resilience in Rats. International Journal of Molecular Sciences, 24(2), 1206. https://doi.org/10.3390/ijms24021206





