Abstract
Anoctamin1 (ANO1), a calcium-activated chloride channel, is involved in the proliferation, migration, and invasion of various cancer cells including head and neck squamous cell carcinoma, lung cancer, and prostate cancer. Inhibition of ANO1 activity or downregulation of ANO1 expression in these cancer cells is known to exhibit anticancer effects. Resveratrol, a natural polyphenol abundant in wines, grapes, berries, soybeans, and peanuts, shows a wide variety of biological effects including anti-inflammatory, antioxidant, and anticancer activities. In this study, we investigated the effects of two stereoisomers of resveratrol on ANO1 activity and found that cis- and trans-resveratrol inhibited ANO1 activity with different potencies. Cis- and trans-resveratrol inhibited ANO1 channel activity with IC50 values of 10.6 and 102 μM, respectively, and had no significant effect on intracellular calcium signaling at 10 and 100 μM, respectively. In addition, cis-resveratrol downregulated mRNA and protein expression levels of ANO1 more potently than trans-resveratrol in PC-3 prostate cancer cells. Cis- and trans-resveratrol significantly reduced cell proliferation and cell migration in an ANO1-dependent manner, and both resveratrol isomers strongly increased caspase-3 activity, PARP cleavage, and apoptotic sub-G1 phase ratio in PC-3 cells. These results revealed that cis-resveratrol is a potent inhibitor of ANO1 and exhibits ANO1-dependent anticancer activity against human metastatic prostate cancer PC-3 cells.
1. Introduction
Anoctamin1 (ANO1), also known as transmembrane protein 16A (TMEM16A), has been identified as a calcium-activated chloride channel [1,2,3]. ANO1 is expressed broadly in various tissues and plays an important role in diverse physiological functions including transepithelial fluid secretion, smooth muscle contraction, neuronal excitation, and cell proliferation [4,5,6,7,8]. In particular, the ANO1 gene is located within the 11q13 amplicon which is frequently amplified in many malignant tumors and ANO1 has been reported to be highly expressed in a variety of human cancers including gastrointestinal stromal tumor, head and neck squamous cell carcinoma, prostate cancer, breast cancer, pancreatic cancer, and glioblastoma [9,10,11,12,13,14,15]. In addition, the increased expression of ANO1 is known to be associated with poor prognosis in cancer patients [16].
Even though the underlying mechanism is not clear, inhibition of ANO1 activity and downregulation of ANO1 expression show anticancer effects by inhibiting cell proliferation, migration, and invasion and inducing apoptosis in various cancers [12,17,18,19,20]. For instance, ANO1 knockdown with small hairpin RNAs (shRNAs) inhibited the proliferation, migration, and invasion of human lung cancer cells and the tumor growth was significantly reduced by ANO1 silencing in nude mice [21]. A significant reduction in proliferation, metastasis, and invasion of human metastatic prostate cancer PC-3 cells was observed after treatment with shRNA targeting human ANO1. Furthermore, tumor growth was significantly reduced by intratumoral injection of ANO1 shRNA in an orthotopic xenograft mice model using PC-3 cells [22]. To date, several compounds showing ANO1 inhibition and/or ANO1 downregulation have been reported to show anticancer effects, including T16Ainh-01 [17], idebenone [18], luteolin [23], Ani9-5f [24], Ani-D2 [25], cinobufagin [19], and diethylstilbestrol [26]. In particular, the anticancer effects of ANO1 inhibition have been well-established in human prostate cancer cells. Pharmacological blockage of ANO1 activity with idebenone reduced cell proliferation and induced apoptosis in PC-3 cells [18]. Downregulation of ANO1 expression by luteolin and Ani-D2 also showed a reduction in cell proliferation and migration and induction of apoptosis in PC-3 cells [23,25]. Upregulation of TNF-α signaling was reported to be involved in inducing apoptosis by ANO1 downregulation in PC-3 pancreatic cancer cells [27].
On the other hand, activation of the ANO1 channel was also suggested to have therapeutic potential in human diseases including salivary gland dysfunction, cystic fibrosis, dry eye syndrome, and intestinal hypomotility [28]. Since the identification of ANO1, only a few selective ANO1 activators and potentiators such as Eact and Fact have been reported [29]. Interestingly, it was recently reported that resveratrol can also activate ANO1 chloride channel [30].
Resveratrol, a trihydroxylated stilbene (3,5,4′-trihydroxystilbene), is a naturally occurring polyphenol found in significant amounts in wines, grapes, berries, soybeans, and peanuts as well as in Chinese and Japanese herbal medicines [31,32]. Resveratrol initially attracted little interest until it was postulated to be responsible for the so-called ‘French Paradox’ in 1992 [33]. Since then, resveratrol has been reported to show a wide variety of biological effects including antioxidant, anti-aging, anti-inflammatory, anticancer, cardioprotective, and anti-diabetic activities [34]. Especially, anticancer activities of resveratrol have attracted many researchers since its cancer chemopreventive activity was first reported in 1997 [35]. Resveratrol shows anticancer effects in a wide variety of tumor cells including breast, prostate, pancreatic, stomach, and colorectal cancers, head and neck squamous cell carcinoma, ovarian carcinoma, and hematological malignancies [32,36,37]. Resveratrol is known to exert its anticancer activities in various cancer states including initiation, promotion, and progression by affecting the diverse signal pathways [38].
Resveratrol is present in two isomeric forms, cis- and trans-resveratrol, with trans-isomer being more predominant and stable [39]. Exposure of trans-resveratrol to solar or ultraviolet radiation can induce cis-isomerization [40,41]. Although cis-resveratrol has never been detected in fresh grapes, a mixture of cis- and trans-resveratrol isomers exists in wines [42]. Until recently, trans-resveratrol was the main focus of investigation and most of the observed health benefits can be attributed to trans-resveratrol. Even though cis-resveratrol has not been well-explored, several studies have reported its biological activities including anti-inflammatory, antioxidant, and anticancer effects [43,44].
In this study, we investigated the effects of the two isomers of resveratrol, cis- and trans-resveratrol, on ANO1 channel activity and unexpectedly found that two resveratrol isomers inhibited ANO1. Since ANO1 inhibitors show anticancer effects, cis- and trans-resveratrol were further investigated on their anticancer activities in human prostate cancer PC-3 cells.
2. Results
2.1. Inhibitory Effect of Cis- and Trans-Resveratrol on ANO1 Channel Activity
To investigate the effect of two stereoisomers of resveratrol, cis- and trans-resveratrol, on ANO1 chloride channel activity, measurement of apical membrane currents was performed in Fisher rat thyroid (FRT) cells expressing human ANO1. As shown in Figure 1A–D, the application of cis-resveratrol and trans-resveratrol did not induce ANO1 activation, but rather inhibited the activation of ANO1 by ATP in a dose-dependent manner. Interestingly, cis-resveratrol blocked ANO1 channel activity more potently compared to trans-resveratrol. A total of 10 and 30 μM of cis-resveratrol inhibited ANO1 activity by 52 and 86%, respectively, and 100 and 300 μM of trans-resveratrol inhibited ANO1 activity by 31 and 66%, respectively. To evaluate IC50 values for cis- and trans-resveratrol, we performed YFP fluorescence quenching assays in YFP-F46L/H148Q/I152L, a halide sensor, and human ANO1-expressing FRT cells. As shown in Figure 1E–G, both cis- and trans-resveratrol significantly inhibited ANO1 activity in a dose-dependent manner, and the ATP-induced decrease in YFP fluorescence was completely blocked by Ani9, a potent and selective ANO1 inhibitor [45]. The IC50 value of cis- and trans-resveratrol was 10.6 μM and 102 μM, respectively.
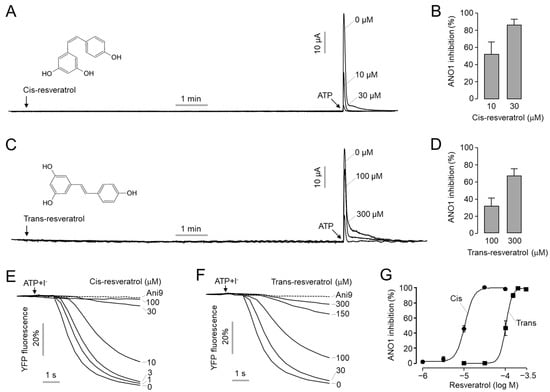
Figure 1.
Inhibition of ANO1 activity by cis- and trans-resveratrol. (A–D) Apical membrane currents were measured in human ANO1-expressing FRT cells. Indicated concentrations of cis- and trans-resveratrol were treated for 10 min before the application of 100 µM ATP. (B,D) Summary of ANO1 current inhibition (mean ± S.D., n = 4–5). (E,F) The inhibitory effect of cis- and trans-resveratrol on ANO1 activity was measured with a YFP-quenching assay in FRT cells expressing ANO1 and a halide sensor YFP. Indicated concentrations of cis- and trans-resveratrol were added 10 min before the application of iodide solution containing 100 μM ATP. (G) Summary of dose–response (mean ± S.D., n = 4).
To confirm the inhibitory effect of cis- and trans-resveratrol on ANO1 chloride channel activity, whole-cell patch clamp experiments were performed in ANO1-overexpressing HEK293T cells. As shown in Figure 2, cis- and trans-resveratrol significantly inhibited ANO1 currents. Cis-resveratrol inhibited ANO1 chloride currents by 23% and 60% at 10 and 30 μM, respectively. Trans-resveratrol inhibited ANO1 chloride currents by 47% and 80% at 100 and 300 μM, respectively, more weakly than cis-resveratrol (Figure 2C,F).
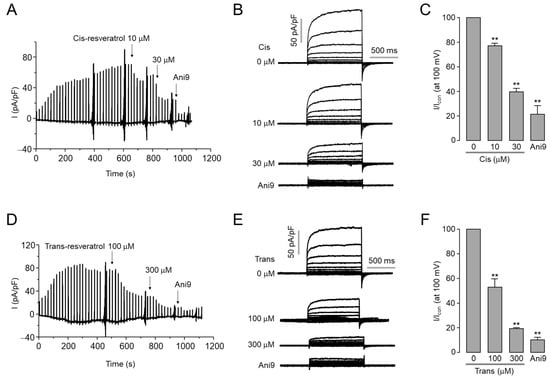
Figure 2.
Cis- and trans-resveratrol inhibit ANO1 chloride currents. (A) Representative traces of whole-cell recordings of ANO1 in HEK293T cells expressing ANO1. Indicated concentrations of cis-resveratrol and 10 μM Ani9 were treated after the activation of ANO1. (B) Whole-cell currents were recorded at a holding potential of −60 mV and pulsed to voltages between ± 100 mV in steps of 20 mV. (C) Summary of current densities measured at +100 mV (mean ± S.D., n = 3–7). (D) Traces of whole-cell recordings of ANO1. Trans-resveratrol and Ani9 were treated as indicated after the activation of ANO1. (E) Whole-cell currents were recorded at a holding potential of -60 mV and pulsed to voltages between ± 100 mV in steps of 20 mV. (F) Summary of current densities measured at +100 mV (mean ± S.D., n = 3–7). ** p < 0.01 vs. control.
2.2. Effect of Cis- and Trans-Resveratrol on Intracellular Calcium Levels
Intracellular calcium signaling plays an essential role in the regulation of ANO1 channel activity. To further investigate the mechanism of ANO1 inhibition by cis- and trans-resveratrol, the effect of both resveratrol isomers on intracellular calcium levels was measured using a fluorescent calcium indicator Fluo-4. As shown in Figure 3A,B, the application of high concentrations of cis- and trans-resveratrol did not alter the intracellular calcium levels, but ionomycin, a calcium ionophore, strongly increased intracellular calcium levels. In addition, pretreatment of 10 μM cis-resveratrol and 100 μM trans-resveratrol did not significantly affect the ionomycin-induced increase in intracellular calcium levels. However, high concentrations of cis-resveratrol (30 μM) and trans-resveratrol (300 μM) showed partial inhibition (Figure 3C,D).

Figure 3.
Effect of cis- and trans-resveratrol on intracellular calcium levels in PC-3 cells. (A,B) Intracellular calcium levels were measured using Fluo-4 NW. Cells were treated with the indicated concentrations of cis- and trans-resveratrol and stimulated with 10 μM ionomycin. (C,D) Cells were pretreated with the indicated concentrations of cis- and trans-resveratrol for 10 min, followed by stimulation with 10 μM ionomycin.
2.3. Effects of Cis- and Trans-Resveratrol on Protein and mRNA Expression Levels of ANO1
Several ANO1 inhibitors including luteolin and Ani9-5f reduce protein expression levels of ANO1 [23,45]. To investigate the effect of cis- and trans-resveratrol on protein expression levels of ANO1, Western blot analysis was performed in PC-3 cells expressing high levels of ANO1. Cis-resveratrol significantly reduced ANO1 protein expression in a dose-dependent manner, and trans-resveratrol also significantly reduced protein expression levels of ANO1 (Figure 4A–D). Notably, 30 μM cis-resveratrol and 300 μM trans-resveratrol had comparable effects on the reduction of ANO1 protein levels by 10 μM Ani9-5f. To further investigate whether cis- and trans-resveratrol affect the mRNA expression level of ANO1, real-time PCR analysis was performed in PC-3 cells. As shown in Figure 4E, 10 μM cis-resveratrol and 100 μM trans-resveratrol had no effect on ANO1 mRNA expression, whereas high concentrations of cis-resveratrol (30 μM) and trans-resveratrol (300 μM) significantly reduced mRNA expression levels of ANO1.
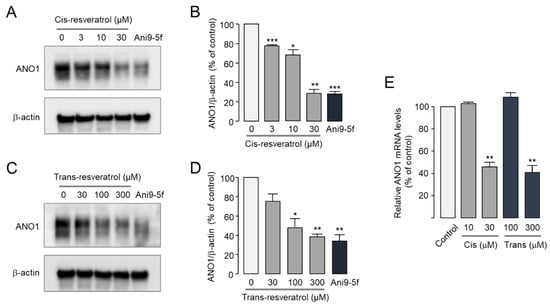
Figure 4.
Effects of cis- and trans-resveratrol on ANO1 expression levels in PC-3 cells. (A–D) Protein expression levels of ANO1 and β-actin were measured by Western blot analysis. Cells were treated with the indicated concentrations of cis- and trans-resveratrol and 10 μM Ani9-5f for 24 h. (B,D) ANO1 band intensities were normalized to β-actin band intensities (mean ± S.D., n = 3). (E) mRNA expression levels of ANO1 were measured by real-time PCR after cells were treated with the indicated concentrations of cis- and trans-resveratrol for 24 h (mean ± S.D., n = 3). * p < 0.05, ** p < 0.01, *** p < 0.001 vs. control.
2.4. Inhibitory Effects of Cis- and Trans-Resveratrol on Cell Proliferation and Migration in PC-3 Cells
Resveratrol has been extensively researched on its anticancer activity in various tumor cells [32,36,37]. Furthermore, pharmacological inhibition and downregulation of ANO1 showed anticancer effects in metastatic prostate cancer cells [23,27]. To investigate the effects of cis- and trans-resveratrol on cell proliferation, cell viability was measured in the highly metastatic prostate cancer PC-3 cells. Cis-resveratrol significantly reduced cell viability of PC-3 cells by 17, 45, 97, and 98% at 3, 10, 30, and 100 μM, respectively (Figure 5A). Trans-resveratrol had a less potent inhibitory effect on cell proliferation. Cell viability was significantly reduced by 22, 30, and 56% at 10, 30, and 100 μM of trans-resveratrol, respectively (Figure 5B).
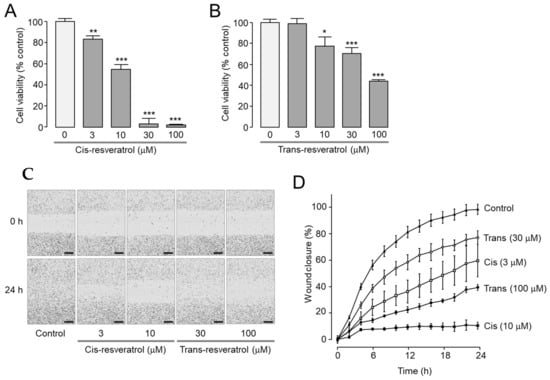
Figure 5.
Effect of cis- and trans-resveratrol on cell viability and migration in PC-3 cells. (A,B) Effect of cis- and trans-resveratrol on cell viability. Indicated concentrations of cis- and trans-resveratrol were treated for 72 h and cell viability was estimated with MTS assay (mean ± S.D., n = 5). (C,D) Effect of cis- and trans-resveratrol on cell migration. Cells were treated with indicated concentrations of cis- and trans-resveratrol and the wound closure was measured every 2 h for 24 h after wound generation (mean ± S.D., n = 4–5). Scale bars represent 300 μm. * p < 0.05, ** p < 0.01, *** p < 0.001 vs. control.
A wound healing assay was performed to observe the effects of cis- and trans-resveratrol on cell migration in PC-3 cells. As shown in Figure 5C,D, cis- and trans-resveratrol significantly reduced cell migration in a dose-dependent manner.
2.5. Cis- and Trans-Resveratrol Induce Apoptosis in PC-3 Cells
To investigate whether cis- and trans-resveratrol induce apoptosis, we observed caspase-3 activity and PARP cleavage in PC-3 cells. As shown in Figure 6A,B, cis- and trans-resveratrol significantly increased caspase-3 activity and 10 μM cis-resveratrol showed stronger caspase-3 activation compared to 100 μM trans-resveratrol. The cis-resveratrol-induced increase in caspase-3-positive cells and caspase-3 activity was completely blocked by Ac-DEVD-CHO, a synthetic tetrapeptide inhibitor of caspase-3/7. In the case of PARP cleavage, cis-resveratrol and trans-resveratrol significantly increased PARP cleavage in PC-3 cells in a dose-dependent manner (Figure 6C).

Figure 6.
Effects of cis- and trans-resveratrol on caspase-3 activity and PARP cleavage in PC-3 cells (A) Images were taken after 24 h treatment with cis- and trans-resveratrol. Cells were treated with the caspase-3 substrate (green) and Hoechst 33,342 (blue) for 20 min before image acquisition. Scale bars represent 200 μm. (B) PC-3 cells were treated with cis-resveratrol at 10 μM in the presence or absence of 10 μM Ac-DEVD-CHO and trans-resveratrol at 100 μM for 24 h. Caspase-3 activity was measured 20 min after treatment of 2 μM caspase-3 substrate (mean ± S.D., n = 3). (C) Cells were treated with indicated concentrations of cis- and trans-resveratrol for 24 h and immunoblot analysis was used to measure the expression level of PARP, cleaved-PARP, and β-actin. Cleaved-PARP protein intensities were normalized to those of β-actin (mean ± S.D., n = 3). * p < 0.05, ** p < 0.01 vs. control, ### p < 0.001 vs. cis+Ac-DEVD.
To observe the effect of cis- and trans-resveratrol on the cell cycle of PC-3 cells, flow cytometry analysis using propidium iodide was performed. As shown in Figure 7, cis- and trans-resveratrol strongly increased the ratios in the sub-G1 (apoptotic peak) phase in a dose-dependent manner. Notably, 30 μM cis-resveratrol induced a decrease in the G0/G1 phase from 84.6% to 62.7% and an increase in the sub-G1 phase from 8.2% to 28.5%. In the case of 300 μM trans-resveratrol, the G0/G1 phase was strongly decreased from 78.1% to 42.9% and the sub-G1 phase was increased from 13.0% to 44.1%. Interestingly, 30 μM cis-resveratrol did not affect the G2/M phase ratio, whereas 300 μM trans-resveratrol increased the G2/M phase ratio from 3.3% to 8.0%. These results suggest that, unlike trans-resveratrol, cis-resveratrol induces apoptosis without significantly affecting cell cycle arrest.
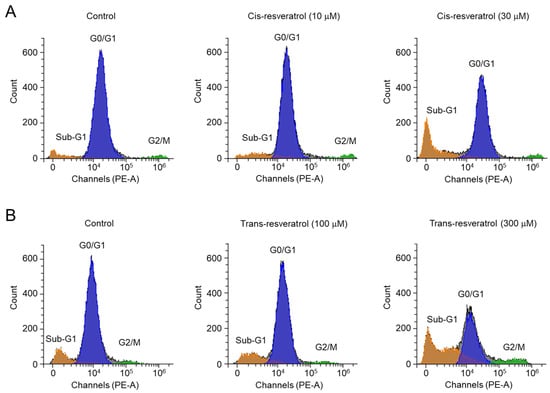
Figure 7.
Effect of cis- and trans-resveratrol on cell cycle in PC-3 cells. (A,B) Cell cycle phases were observed by propidium iodide staining followed by flow cytometric analysis after the cells were treated with the indicated concentrations of cis- and trans-resveratrol for 48 h.
3. Discussion
Several ANO1 modulators have been reported since it was identified as a calcium-activated chloride channel in 2008 [46]. ANO1 inhibition has shown therapeutic potential as an anticancer, anti-asthmatic, and antinociceptive agent [16,46]. Potentiation of ANO1 can provide health benefits for human diseases such as salivary gland dysfunction, cystic fibrosis, dry eye syndrome, and intestinal hypomotility [29]. Even though several selective ANO1 inhibitors have been identified, only a few selective ANO1 activators have been reported [46]. Interestingly, resveratrol, a natural polyphenol found in wines, grapes, and berries, has recently been reported as an activator of ANO1 [30]. Unexpectedly, however, we found that cis-resveratrol, in particular, strongly inhibited ANO1. As shown in Figure 1A–D, both cis- and trans-resveratrol treatment did not induce an increase in ANO1 chloride currents in FRT cells expressing human ANO1. On the contrary, pretreatment of cis- and trans-resveratrol inhibited the ATP-induced increase of ANO1 chloride currents in a dose-dependent manner. The whole-cell patch clamp study also showed that both cis- and trans-resveratrol potently inhibited ANO1 chloride currents in ANO1-expressing HEK293T cells in a dose-dependent manner (Figure 2). These electrophysiological studies clearly showed that cis- and trans-resveratrol are ANO1 inhibitors rather than ANO1 activators. It is difficult to clearly explain the reason why contradictory results were observed. Interestingly, however, a previous study reported that resveratrol increased Cl− secretion in porcine ileum but significantly inhibited Ca2+-induced Cl− secretion [47]. The previous study suggested that the activation of cystic fibrosis transmembrane conductance regulator (CFTR) may be responsible for resveratrol-induced Cl− secretion, and resveratrol may inhibit Ca2+-induced Cl− secretion. Taken together, these results suggest that ANO1 inhibition by resveratrol may be involved in the inhibition of Ca2+-induced Cl− secretion by resveratrol observed in porcine ileum.
In order to investigate the mechanism of action of cis- and trans-resveratrol to inhibit ANO1, intracellular calcium signals were observed. Cis- and trans-resveratrol had minimal effects on calcium signals at concentrations up to 10 μM and 100 μM, respectively (Figure 3). On the other hand, cis- and trans-resveratrol strongly inhibited ANO1 at concentrations of 10 μM and 100 μM, respectively (Figure 1). Although we could not elucidate the reason for the different ANO1 inhibitory potency of the two stereoisomers of resveratrol, we found that they did not significantly interfere with calcium signaling and mRNA expression of ANO1 at a concentration caused a significant decrease in ANO1 channel activity and ANO1 protein levels (Figure 3 and Figure 4). These results suggest that the ANO1 inhibitory effects of cis- and trans-resveratrol may be caused by the direct binding of the two isomers of resveratrol to ANO1 with different binding modes.
ANO1 has been suggested as a therapeutic target for various cancers since it is highly amplified or overexpressed in various types of cancer and pharmacological inhibition or downregulation of ANO1 shows anticancer activities [12,13,14]. In previous studies, ANO1 inhibitors with significant inhibitory effects on channel activity as well as protein expression showed strong inhibition of cell viability and migration in PC-3 cells expressing high levels of ANO1 [18,23,24,25,26]. It was suggested that the reduction in ANO1 protein levels might be more critical in demonstrating anticancer effects than the inhibition of ANO1 channel activity [25]. In the present study, we found that cis- and trans-resveratrol directly inhibited ANO1 channel activity with a decrease in ANO1 protein levels (Figure 4). Interestingly, cis- and trans-resveratrol also showed inhibitory effects on cell proliferation and migration in PC-3 prostate cancer cells (Figure 5). In addition, cis- and trans-resveratrol significantly promoted caspase-3 activation and PARP cleavage and increased the apoptotic sub-G1 phase ratio in PC-3 cells (Figure 6 and Figure 7).
Resveratrol suppressed the growth of prostate cancer via the down-regulation of androgen receptor (AR) expression in the transgenic adenocarcinoma mouse prostate model [48]. In addition, resveratrol reduced the volume of tumors by lowering tumor-cell proliferation and neovascularization and inducing apoptosis in xenograft mice models of the AR-negative PC-3 cell [49]. Even though the majority of research on resveratrol’s anticancer effects has been focused on trans-isomer, cis-resveratrol has also been reported to show anticancer effects [50]. Trans-resveratrol has been reported to show stronger anticancer effects than the cis-isomer. For example, in human colorectal tumor SW480 cells, trans-resveratrol showed antiproliferative activities with the IC50 of 20 ± 3 μM, whereas the IC50 value of cis-resveratrol was 90 ± 12 μM [51]. Trans-resveratrol also exhibited slightly stronger cytotoxic activities than the cis-isomer in pancreatic cancer, breast cancer, lung small cell cancer, colon cancer, and prostate cancer cell lines [52]. However, in the present study, cis-resveratrol showed stronger anticancer effects than trans-resveratrol in PC-3 cells expressing high levels of ANO1, which is in correlation with their effects on the inhibition of ANO1 channel activity and the reduction in ANO1 expression. These results indicate that the anticancer mechanism of resveratrol, especially cis-resveratrol, may involve, at least in part, the inhibition and downregulation of ANO1.
In many clinical trials, resveratrol is well-tolerated and pharmacologically safe up to 5 g/day [53]. However, trans-resveratrol is rapidly metabolized mainly by sulfate and glucuronic acid conjugation in the intestine and liver and rapidly excreted [54]. After a single 500 mg oral dose in healthy volunteers, Cmax was measured as 71.2 ng/mL, 4083.9 ng/mL, and 1516.0 ng/mL for resveratrol, glucuronated resveratrol, and sulphated resveratrol, respectively, and no side effects related to resveratrol were reported during the study [55]. However, comprehensive pharmacokinetic data on cis-resveratrol are not yet available. Therefore, in order to develop cis-resveratrol as an anticancer agent, various studies including pharmacokinetic studies should be conducted in the future.
In conclusion, cis- and trans-resveratrol inhibited ANO1 channel activity and down-regulated mRNA and protein expression levels of ANO1. Notably, cis-resveratrol exhibited a stronger inhibition of ANO1 activity than trans-resveratrol and a stronger cell growth and migration inhibitory effect in PC-3 prostate cancer cells. These results suggest a new therapeutic potential for cis-resveratrol in cancers with high ANO1 expression.
4. Materials and Methods
4.1. Cell Culture
Fisher rat thyroid (FRT) cells stably expressing ANO1 were provided by Alan Verkman (University of California, San Francisco, CA, USA). ANO1-expressing FRT cells were stably transfected with the halide sensor YFP-H148Q/I152L/F46L as described in a previous study [56]. FRT cells were cultured in Ham’s F-12 Modified medium with 10% fetal bovine serum (FBS), 100 units/mL penicillin, 100 μg/mL streptomycin, and 2 mM L-glutamine. PC-3 cells were purchased from Korean Cell Line Bank (KCLB) and cultured in RPMI 1640 medium supplemented with 10% FBS, 100 units/mL penicillin, and 100 μg/mL streptomycin. FRT cells and PC-3 cells were grown at 37 °C, 5% CO2, and 95% humidity. HEK293T cells were purchased from the American Type Culture Collection and cultured in Dulbecco’s modified Eagle’s medium supplemented with 10% FBS and 1% penicillin/streptomycin at 37 °C with 10% CO2.
4.2. Materials
Cis- and trans-resveratrol were purchased from Tocris Bioscience (Bristol, UK). All other materials not described were purchased from Sigma-Aldrich (St. Louis, MO, USA).
4.3. Ussing Chamber Assay
ANO1-expressing FRT cells were cultured on snapwell inserts (1.12 cm2 surface area) until confluent. Then snapwell inserts were mounted in Ussing chambers (Physiologic Instruments, San Diego, CA, USA). The basolateral side chamber was bathed with HCO3--buffered solution containing (in mM) 120 NaCl, 5 KCl, 1 MgCl2, 1 CaCl2, 10 D-glucose, 2.5 HEPES, and 25 NaHCO3 (pH 7.4). The apical side chamber was filled with a half-Cl− solution in which 65 mM NaCl was replaced by Na-gluconate. Cells were bathed for 10 min while being aerated with 95% O2 and 5% CO2 at 37 °C. Cis- and trans-resveratrol were applied to both apical and basolateral bath solutions 10 min before ATP application to the apical bath solution to activate ANO1. Membrane currents were measured and recorded with a 4 Hz sampling rate using an EVC4000 Multi-Channel V/I Clamp (World Precision Instruments, Sarasota, FL, USA) and PowerLab 4/35 (AD Instruments, Castle Hill, Australia). Data were collected and analyzed with Lab Chart Pro 7 (AD Instruments, Castle Hill, Australia).
4.4. YFP Fluorescence Quenching Assay
FRT cells that stably express both ANO1 and YFP variant were incubated in 96-well microplates at a confluence of ~90% for 24 h and each well of the 96-well plate was washed three times with 200 μL of PBS and test compounds were treated for 10 min. YFP fluorescence of each well was measured every 400 ms with a FLUOstar Omega microplate reader (BMG Labtech, Ortenberg, Germany). After 1 s measurement for baseline, 100 μL of 70 mM iodide solution containing 100 μM ATP was added to each well to measure ANO1-mediated iodide influx. The initial iodide influx rate determined from the initial slope of fluorescence decrease was used for measuring the inhibitory effect of test compounds on ANO1 activity.
4.5. Whole-Cell Patch-Clamp
HEK293T cells were transfected with pEGFP-ready tagged ANO1 24–36 h before whole-cell patch-clamp recordings of ANO1. The bath solution contained (in mM) 150 NMDG-Cl, 1 MgCl2, 10 glucose, and 10 HEPES (pH 7.4) and the pipette solution contained (in mM) 150 NMDG-Cl, 10 EGTA, 6.6 CaCl2, 1 MgCl2, 3 MgATP, and 5 HEPES (pH 7.2). Pipettes were pulled from borosilicate glass capillaries to have an electrical resistance of 2–3 MΩ after fire polishing. The holding potential was set as −60 mV and ramp pulses were applied from −100 mV to +100 mV in steps of 20 mV over 1 s. The pulse-to-pulse interval was 20 s. All recordings were carried out at room temperature using Axopatch 700B (Molecular Devices, Sunnyvale, CA, USA) and digitalized and analyzed using Digidata 1440A (Molecular Devices) and Clampfit 10.4 (Molecular Devices). Currents were low-pass filtered at 5 kHz and sampled at 10 kHz.
4.6. Intracellular Ca2+ Measurement
PC-3 cells were cultured in 96-well black-walled microplates and loaded with Fluo-4 NW, a fluorescent Ca2+ indicator, according to the manufacturer’s manual (Invitrogen, Carlsbad, CA, USA). Briefly, PC-3 cells were incubated with 100 μL of the Fluo-4 NW loading solution containing Fluo-4 in 1X Hanks’ balanced salt solution with 2.5 mM probenecid and 20 mM HEPES. After 50 min of incubation in the dark, the 96-well plates were transferred to a microplate reader for the measurement of Fluo-4 fluorescence with a Synergy Neo2 microplate reader (BioTek Instruments, Inc., Winooski, VT, USA) equipped with syringe pumps and custom Fluo-4 excitation/emission filters (485/538 nm).
4.7. Western Blot Analysis
Preparation of the protein sample was carried out as described previously [45]. The samples were separated by 4–12% Tris-glycine precast gel (KOMA BIOTECH, Seoul, Republic of Korea) and then transferred onto a polyvinylidene Fluoride membrane (Millipore, Billerica, MA, USA). Blocking of the membrane was performed with 5% non-fat skim milk in Tris-buffered saline containing 0.1% Tween 20 (TBST) for 1 h at room temperature. The membrane was incubated overnight at 4 °C with corresponding primary antibodies, including anti-ANO1 (ab64085; Abcam, Cambridge, UK), anti-β-actin (sc-47778; Santa Cruz Biotechnology, Dallas, TX, USA), and anti-cleaved PARP (551,025; BD Biosciences, Franklin Lakes, NJ, USA) antibodies and then was washed three times with TBST followed by 1 h of incubation with HRP-conjugated anti-secondary IgG antibodies (Enzo Life Science, Farmingdale, NY, USA) at room temperature. Visualization was performed using the SuperSignal™ Western Blot Substrate (Thermo Fisher Scientific, Waltham, MA, USA).
4.8. Real-Time RT-PCR Analysis
Total mRNA was isolated from PC-3 cells using TRIzol reagent (Invitrogen, Carlsbad, CA, USA). Total mRNA was reverse transcribed with random hexamer primers, an oligo (dT) primer, and SuperScript III Reverse Transcriptase (Invitrogen, Carlsbad, CA, USA). Quantitative RT PCR was carried out using Thunderbird SYBR qPCR mix (Toyobo, Osaka, Japan) and StepOnePlus Real-Time PCR System (Applied Biosystems, Waltham, MA, USA) under thermal cycling conditions of 95 °C for 5 min, 40 cycles of 95 °C for 10 s, 60 °C for 20 s, and 72 °C for 10 s. The size of the ANO1 PCR product was 82 base pairs with the ANO1 sense primer sequence as 5′-GGAGAAGCAGCATCTATTTG-3′ and the ANO1 antisense primer sequence as 5′-GATCTCATAGACAATCGTGC-3′.
4.9. Cell Viability Assays
PC-3 cells were incubated in 96-well microplates with growth medium supplemented with 10% FBS for 24 h before treatment with cis- and trans-resveratrol. After 72 h incubation, the medium was washed out and the MTS assay was performed according to the supplier’s protocol using Cell Titer 96® AQueous One Solution Assay kit (MTS) (Promega, Madison, WI, USA). The absorbance at 490 nm was measured with an Infinite M200 microplate reader (Tecan, Grödig, Austria).
4.10. Wound Healing Assay
PC-3 cells were cultured in a 96-well plate to reach ~80% confluence as a monolayer. The wound was formed on a cell layer using a 96-Well WoundMaker (Essen BioScience, Ann Arbor, MI, USA) and each well was washed twice with serum-free medium. The cells were incubated with serum-free medium and IncuCyte ZOOM (Essen BioScience, Ann Arbor, MI, USA) was used to take images of the wounds. The percentage of wound closure was analyzed with IncuCyte software 2018A.
4.11. Caspase-3 Activity Assay
PC-3 cells were cultured in 96-well plates to reach 30% confluence before treatment of each well with cis-resveratrol, trans-resveratrol, and Ac-DEVD-CHO, a caspase-3 inhibitor. After 24 h incubation, each well was washed with PBS and incubated at room temperature with 100 μL of PBS containing 1 μM of NucView 488 caspase-3 substrate for 30 min followed by the staining of cells with 1 μM Hoechst 33,342. The fluorescence measurement of NucView 488 and Hoechst 33,342 was performed using FLUOstar Omega microplate reader (BMG Labtech) and Lionheart FX Automated Microscope (BioTek, Winooski, VT, USA) was used to take multicolor images.
4.12. Flow Cytometry Analysis
PC-3 cells were grown to ~50% confluence in a 6-well plate and then cis- and trans-resveratrol were treated for 48 h, then PC-3 cells were washed twice with PBS and centrifuged at 1000 RPM for 2 min. The cells were stained with propidium iodide (PI) for 15 min and then cell cycle phases were determined by using fluorescence activated cell sorting (Beckman Coulter, Fullerton, CA, USA).
4.13. Statistical Analysis
All experiments were performed independently a minimum of three times. The results for multiple trials were presented as the mean ± standard deviation (S.D.). GraphPad Prism 9.0 (GraphPad Software Inc., San Diego, CA, USA) was used for statistical analysis of the Student’s t-test or the one-way analysis of variance coupled with Dunnett’s T3 post-hoc test, as appropriate. p values less than 0.05 were regarded as statistically significant. GraphPad Prism Software was used for plotting the dose–response curve and calculating IC50 values.
Author Contributions
Conceptualization, D.J., M.J. and W.N.; methodology, D.J. and M.J.; software, D.J. and M.J.; validation, D.J. and M.J.; formal analysis, D.J. and M.J.; investigation, D.J., M.J., Y.L., S.-H.P., H.T.L.P. and J.H.N.; resources, W.N.; data curation, D.J., M.J. and W.N.; writing—original draft preparation, D.J. and M.J.; writing—review and editing, D.J., M.J. and W.N.; supervision, W.N.; project administration, W.N.; funding acquisition, W.N. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by a Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2018R1A6A1A03023718).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| ANO1 | Anoctamin1 |
| AR | Androgen receptor |
| CaCC | Calcium-activated chloride channels |
| CFTR | Cystic fibrosis transmembrane conductance regulator |
| FRT | Fisher rat thyroid |
| HTS | High-throughput screening |
| shRNA | Short hairpin RNA |
| TMEM16A | Transmembrane protein 16A |
| TNF-α | Tumor Necrosis Factor-α |
| YFP | Yellow fluorescent protein |
References
- Yang, Y.D.; Cho, H.; Koo, J.Y.; Tak, M.H.; Cho, Y.; Shim, W.S.; Park, S.P.; Lee, J.; Lee, B.; Kim, B.M.; et al. TMEM16A confers receptor-activated calcium-dependent chloride conductance. Nature 2008, 455, 1210–1215. [Google Scholar] [CrossRef]
- Caputo, A.; Caci, E.; Ferrera, L.; Pedemonte, N.; Barsanti, C.; Sondo, E.; Pfeffer, U.; Ravazzolo, R.; Zegarra-Moran, O.; Galietta, L.J. TMEM16A, a membrane protein associated with calcium-dependent chloride channel activity. Science 2008, 322, 590–594. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, B.C.; Cheng, T.; Jan, Y.N.; Jan, L.Y. Expression cloning of TMEM16A as a calcium-activated chloride channel subunit. Cell 2008, 134, 1019–1029. [Google Scholar] [CrossRef]
- Catalán, M.A.; Kondo, Y.; Peña-Munzenmayer, G.; Jaramillo, Y.; Liu, F.; Choi, S.; Crandall, E.; Borok, Z.; Flodby, P.; Shull, G.E.; et al. A fluid secretion pathway unmasked by acinar-specific Tmem16A gene ablation in the adult mouse salivary gland. Proc. Natl. Acad. Sci. USA 2015, 112, 2263–2268. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.J.; Blair, P.J.; Britton, F.C.; O’Driscoll, K.E.; Hennig, G.; Bayguinov, Y.R.; Rock, J.R.; Harfe, B.D.; Sanders, K.M.; Ward, S.M. Expression of anoctamin 1/TMEM16A by interstitial cells of Cajal is fundamental for slow wave activity in gastrointestinal muscles. J. Physiol. 2009, 587, 4887–4904. [Google Scholar] [CrossRef]
- Namkung, W.; Phuan, P.W.; Verkman, A.S. TMEM16A inhibitors reveal TMEM16A as a minor component of calcium-activated chloride channel conductance in airway and intestinal epithelial cells. J. Biol. Chem. 2011, 286, 2365–2374. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.; Yang, Y.D.; Lee, J.; Lee, B.; Kim, T.; Jang, Y.; Back, S.K.; Na, H.S.; Harfe, B.D.; Wang, F.; et al. The calcium-activated chloride channel anoctamin 1 acts as a heat sensor in nociceptive neurons. Nat. Neurosci. 2012, 15, 1015–1021. [Google Scholar] [CrossRef] [PubMed]
- Kunzelmann, K.; Ousingsawat, J.; Benedetto, R.; Cabrita, I.; Schreiber, R. Contribution of Anoctamins to Cell Survival and Cell Death. Cancers 2019, 11, 382. [Google Scholar] [CrossRef]
- Akervall, J.A.; Jin, Y.; Wennerberg, J.P.; Zätterström, U.K.; Kjellén, E.; Mertens, F.; Willén, R.; Mandahl, N.; Heim, S.; Mitelman, F. Chromosomal abnormalities involving 11q13 are associated with poor prognosis in patients with squamous cell carcinoma of the head and neck. Cancer 1995, 76, 853–859. [Google Scholar] [CrossRef] [PubMed]
- Ormandy, C.J.; Musgrove, E.A.; Hui, R.; Daly, R.J.; Sutherland, R.L. Cyclin D1, EMS1 and 11q13 amplification in breast cancer. Breast Cancer Res. Treat. 2003, 78, 323–335. [Google Scholar] [CrossRef] [PubMed]
- Guan, L.; Song, Y.; Gao, J.; Gao, J.; Wang, K. Inhibition of calcium-activated chloride channel ANO1 suppresses proliferation and induces apoptosis of epithelium originated cancer cells. Oncotarget 2016, 7, 78619–78630. [Google Scholar] [CrossRef] [PubMed]
- Duvvuri, U.; Shiwarski, D.J.; Xiao, D.; Bertrand, C.; Huang, X.; Edinger, R.S.; Rock, J.R.; Harfe, B.D.; Henson, B.J.; Kunzelmann, K.; et al. TMEM16A induces MAPK and contributes directly to tumorigenesis and cancer progression. Cancer Res. 2012, 72, 3270–3281. [Google Scholar] [CrossRef] [PubMed]
- West, R.B.; Corless, C.L.; Chen, X.; Rubin, B.P.; Subramanian, S.; Montgomery, K.; Zhu, S.; Ball, C.A.; Nielsen, T.O.; Patel, R.; et al. The novel marker, DOG1, is expressed ubiquitously in gastrointestinal stromal tumors irrespective of KIT or PDGFRA mutation status. Am. J. Pathol. 2004, 165, 107–113. [Google Scholar] [CrossRef]
- Ayoub, C.; Wasylyk, C.; Li, Y.; Thomas, E.; Marisa, L.; Robé, A.; Roux, M.; Abecassis, J.; de Reyniès, A.; Wasylyk, B. ANO1 amplification and expression in HNSCC with a high propensity for future distant metastasis and its functions in HNSCC cell lines. Br. J. Cancer 2010, 103, 715–726. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liu, Y.; Ren, Y.; Kang, L.; Zhang, L. Transmembrane protein with unknown function 16A overexpression promotes glioma formation through the nuclear factor-κB signaling pathway. Mol. Med. Rep. 2014, 9, 1068–1074. [Google Scholar] [CrossRef]
- Guo, S.; Zhang, L.; Li, N. ANO1: More Than Just Calcium-Activated Chloride Channel in Cancer. Front. Oncol. 2022, 12, 922838. [Google Scholar] [CrossRef]
- Mazzone, A.; Eisenman, S.T.; Strege, P.R.; Yao, Z.; Ordog, T.; Gibbons, S.J.; Farrugia, G. Inhibition of cell proliferation by a selective inhibitor of the Ca2+-activated Cl− channel, Ano1. Biochem. Biophys. Res. Commun. 2012, 427, 248–253. [Google Scholar] [CrossRef]
- Seo, Y.; Park, J.; Kim, M.; Lee, H.K.; Kim, J.H.; Jeong, J.H.; Namkung, W. Inhibition of ANO1/TMEM16A Chloride Channel by Idebenone and Its Cytotoxicity to Cancer Cell Lines. PLoS ONE 2015, 10, e0133656. [Google Scholar] [CrossRef]
- Jo, S.; Yang, E.; Lee, Y.; Jeon, D.; Namkung, W. Cinobufagin Exerts Anticancer Activity in Oral Squamous Cell Carcinoma Cells through Downregulation of ANO1. Int. J. Mol. Sci. 2021, 22, 12037. [Google Scholar] [CrossRef]
- Britschgi, A.; Bill, A.; Brinkhaus, H.; Rothwell, C.; Clay, I.; Duss, S.; Rebhan, M.; Raman, P.; Guy, C.T.; Wetzel, K.; et al. Calcium-activated chloride channel ANO1 promotes breast cancer progression by activating EGFR and CAMK signaling. Proc. Natl. Acad. Sci. USA 2013, 110, E1026–E1034. [Google Scholar] [CrossRef]
- Jia, L.; Liu, W.; Guan, L.; Lu, M.; Wang, K. Inhibition of Calcium-Activated Chloride Channel ANO1/TMEM16A Suppresses Tumor Growth and Invasion in Human Lung Cancer. PLoS ONE 2015, 10, e0136584. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Lu, M.; Liu, B.; Huang, Y.; Wang, K. Inhibition of Ca2+-activated Cl− channel ANO1/TMEM16A expression suppresses tumor growth and invasiveness in human prostate carcinoma. Cancer Lett. 2012, 326, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Seo, Y.; Ryu, K.; Park, J.; Jeon, D.K.; Jo, S.; Lee, H.K.; Namkung, W. Inhibition of ANO1 by luteolin and its cytotoxicity in human prostate cancer PC-3 cells. PLoS ONE 2017, 12, e0174935. [Google Scholar] [CrossRef]
- Seo, Y.; Kim, J.; Chang, J.; Kim, S.S.; Namkung, W.; Kim, I. Synthesis and biological evaluation of novel Ani9 derivatives as potent and selective ANO1 inhibitors. Eur. J. Med. Chem. 2018, 160, 245–255. [Google Scholar] [CrossRef]
- Seo, Y.; Anh, N.H.; Heo, Y.; Park, S.H.; Kiem, P.V.; Lee, Y.; Yen, D.T.H.; Jo, S.; Jeon, D.; Tai, B.H.; et al. Novel ANO1 Inhibitor from Mallotus apelta Extract Exerts Anticancer Activity through Downregulation of ANO1. Int. J. Mol. Sci. 2020, 21, 6470. [Google Scholar] [CrossRef] [PubMed]
- Seo, Y.; Jeong, S.B.; Woo, J.H.; Kwon, O.B.; Lee, S.; Oh, H.I.; Jo, S.; Park, S.J.; Namkung, W.; Moon, U.Y.; et al. Diethylstilbestrol, a Novel ANO1 Inhibitor, Exerts an Anticancer Effect on Non-Small Cell Lung Cancer via Inhibition of ANO1. Int. J. Mol. Sci. 2021, 22, 7100. [Google Scholar] [CrossRef]
- Song, Y.; Gao, J.; Guan, L.; Chen, X.; Gao, J.; Wang, K. Inhibition of ANO1/TMEM16A induces apoptosis in human prostate carcinoma cells by activating TNF-α signaling. Cell Death Dis. 2018, 9, 703. [Google Scholar] [CrossRef]
- Verkman, A.S.; Galietta, L.J. Chloride channels as drug targets. Nat. Rev. Drug Discov. 2009, 8, 153–171. [Google Scholar] [CrossRef]
- Namkung, W.; Yao, Z.; Finkbeiner, W.E.; Verkman, A.S. Small-molecule activators of TMEM16A, a calcium-activated chloride channel, stimulate epithelial chloride secretion and intestinal contraction. FASEB. J. 2011, 25, 4048–4062. [Google Scholar] [CrossRef]
- Chai, R.; Chen, Y.; Yuan, H.; Wang, X.; Guo, S.; Qi, J.; Zhang, H.; Zhan, Y.; An, H. Identification of Resveratrol, an Herbal Compound, as an Activator of the Calcium-Activated Chloride Channel, TMEM16A. J. Membr. Biol. 2017, 250, 483–492. [Google Scholar] [CrossRef]
- Burns, J.; Yokota, T.; Ashihara, H.; Lean, M.E.; Crozier, A. Plant foods and herbal sources of resveratrol. J. Agric. Food Chem. 2002, 50, 3337–3340. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Bhardwaj, A.; Aggarwal, R.S.; Seeram, N.P.; Shishodia, S.; Takada, Y. Role of resveratrol in prevention and therapy of cancer: Preclinical and clinical studies. Anticancer Res. 2004, 24, 2783–2840. [Google Scholar] [PubMed]
- Renaud, S.; de Lorgeril, M. Wine, alcohol, platelets, and the French paradox for coronary heart disease. Lancet 1992, 339, 1523–1526. [Google Scholar] [CrossRef]
- Wu, J.M.; Wang, Z.R.; Hsieh, T.C.; Bruder, J.L.; Zou, J.G.; Huang, Y.Z. Mechanism of cardioprotection by resveratrol, a phenolic antioxidant present in red wine (Review). Int. J. Mol. Med. 2001, 8, 3–17. [Google Scholar] [CrossRef]
- Jang, M.; Cai, L.; Udeani, G.O.; Slowing, K.V.; Thomas, C.F.; Beecher, C.W.; Fong, H.H.; Farnsworth, N.R.; Kinghorn, A.D.; Mehta, R.G.; et al. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science 1997, 275, 218–220. [Google Scholar] [CrossRef]
- Carter, L.G.; D’Orazio, J.A.; Pearson, K.J. Resveratrol and cancer: Focus on in vivo evidence. Endocr. Relat. Cancer 2014, 21, R209–R225. [Google Scholar] [CrossRef]
- Espinoza, J.L.; Kurokawa, Y.; Takami, A. Rationale for assessing the therapeutic potential of resveratrol in hematological malignancies. Blood Rev. 2019, 33, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Ko, J.H.; Sethi, G.; Um, J.Y.; Shanmugam, M.K.; Arfuso, F.; Kumar, A.P.; Bishayee, A.; Ahn, K.S. The Role of Resveratrol in Cancer Therapy. Int. J. Mol. Sci. 2017, 18, 2589. [Google Scholar] [CrossRef]
- Wang, F.; Chatterjee, S. Dominant Carbons in trans- and cis-Resveratrol Isomerization. J. Phys. Chem. B 2017, 121, 4745–4755. [Google Scholar] [CrossRef]
- Merino, E.; Ribagorda, M. Control over molecular motion using the cis-trans photoisomerization of the azo group. Beilstein J. Org. Chem. 2012, 8, 1071–1090. [Google Scholar] [CrossRef] [PubMed]
- Figueiras, T.S.; Neves-Petersen, M.T.; Petersen, S.B. Activation energy of light induced isomerization of resveratrol. J. Fluoresc. 2011, 21, 1897–1906. [Google Scholar] [CrossRef]
- Cvejic, J.M.; Djekic, S.V.; Petrovic, A.V.; Atanackovic, M.T.; Jovic, S.M.; Brceski, I.D.; Gojkovic-Bukarica, L.C. Determination of trans- and cis-resveratrol in Serbian commercial wines. J. Chromatogr. Sci. 2010, 48, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Leiro, J.; Alvarez, E.; Arranz, J.A.; Laguna, R.; Uriarte, E.; Orallo, F. Effects of cis-resveratrol on inflammatory murine macrophages: Antioxidant activity and down-regulation of inflammatory genes. J. Leukoc. Biol. 2004, 75, 1156–1165. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.T.; Lai, H.C.; Chen, Y.B.; Chen, L.G.; Wu, Y.H.; Ko, Y.F.; Lu, C.C.; Chang, C.J.; Wu, C.Y.; Martel, J.; et al. cis-Resveratrol produces anti-inflammatory effects by inhibiting canonical and non-canonical inflammasomes in macrophages. Innate Immun. 2014, 20, 735–750. [Google Scholar] [CrossRef]
- Seo, Y.; Lee, H.K.; Park, J.; Jeon, D.K.; Jo, S.; Jo, M.; Namkung, W. Ani9, A Novel Potent Small-Molecule ANO1 Inhibitor with Negligible Effect on ANO2. PLoS ONE 2016, 11, e0155771. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, Z.; Wang, K. The Ca2+-activated chloride channel ANO1/TMEM16A: An emerging therapeutic target for epithelium-originated diseases? Acta Pharm. Sin. B 2021, 11, 1412–1433. [Google Scholar] [CrossRef]
- Hoppe, S.; Breves, G.; Klinger, S. Calcium-induced chloride secretion is decreased by Resveratrol in ileal porcine tissue. BMC Res. Notes 2018, 11, 719. [Google Scholar] [CrossRef]
- Harper, C.E.; Patel, B.B.; Wang, J.; Arabshahi, A.; Eltoum, I.A.; Lamartiniere, C.A. Resveratrol suppresses prostate cancer progression in transgenic mice. Carcinogenesis 2007, 28, 1946–1953. [Google Scholar] [CrossRef] [PubMed]
- Ganapathy, S.; Chen, Q.; Singh, K.P.; Shankar, S.; Srivastava, R.K. Resveratrol enhances antitumor activity of TRAIL in prostate cancer xenografts through activation of FOXO transcription factor. PLoS ONE 2010, 5, e15627. [Google Scholar] [CrossRef] [PubMed]
- Jhanji, M.; Rao, C.N.; Sajish, M. Towards resolving the enigma of the dichotomy of resveratrol: Cis- and trans-resveratrol have opposite effects on TyrRS-regulated PARP1 activation. Geroscience 2021, 43, 1171–1200. [Google Scholar] [CrossRef]
- Mazué, F.; Colin, D.; Gobbo, J.; Wegner, M.; Rescifina, A.; Spatafora, C.; Fasseur, D.; Delmas, D.; Meunier, P.; Tringali, C.; et al. Structural determinants of resveratrol for cell proliferation inhibition potency: Experimental and docking studies of new analogs. Eur. J. Med. Chem. 2010, 45, 2972–2980. [Google Scholar] [CrossRef] [PubMed]
- Pettit, G.R.; Grealish, M.P.; Jung, M.K.; Hamel, E.; Pettit, R.K.; Chapuis, J.C.; Schmidt, J.M. Antineoplastic agents. 465. Structural modification of resveratrol: Sodium resverastatin phosphate. J. Med. Chem. 2002, 45, 2534–2542. [Google Scholar] [CrossRef]
- Patel, K.R.; Scott, E.; Brown, V.A.; Gescher, A.J.; Steward, W.P.; Brown, K. Clinical trials of resveratrol. Ann. N. Y. Acad. Sci. 2011, 1215, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, E.; Somoza, V. Metabolism and bioavailability of trans-resveratrol. Mol. Nutr. Food Res. 2005, 49, 472–481. [Google Scholar] [CrossRef] [PubMed]
- Sergides, C.; Chirilă, M.; Silvestro, L.; Pitta, D.; Pittas, A. Bioavailability and safety study of resveratrol 500 mg tablets in healthy male and female volunteers. Exp. Ther. Med. 2016, 11, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Namkung, W.; Thiagarajah, J.R.; Phuan, P.W.; Verkman, A.S. Inhibition of Ca2+-activated Cl− channels by gallotannins as a possible molecular basis for health benefits of red wine and green tea. FASEB J. 2010, 24, 4178–4186. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).