Application of In Vitro Models for Studying the Mechanisms Underlying the Obesogenic Action of Endocrine-Disrupting Chemicals (EDCs) as Food Contaminants—A Review
Abstract
1. Introduction
2. Adipogenesis and Obesogenic Action of Endocrine-Disrupting Chemicals (EDCs)
3. In vitro Models Used in the Study of the Obesogenic Effects of EDCs
4. Adipose Tissue Cell Models
4.1. Animal Preadipocytes
4.1.1. 3T3-L1 Cell Line
4.1.2. NIH3T3-L1 Cell Line
4.1.3. 3T3-F442A Cell Line
4.1.4. OP9 Cell Line
4.1.5. ST-13 Cell Line
4.1.6. UCP-1 Cell Line
4.2. Human Preadipocytes
4.2.1. Primary Human Preadipocytes
4.2.2. PCS-210-010
4.2.3. SGBS
4.2.4. SW 872 Cell Line
5. Mesenchymal Stem Cells (MSCs)
5.1. Animal Adipose-Derived Stem Cells (ADSCs)
5.2. Human Adipose-Derived Stem Cells (hADSCs)
6. Other In Vitro Models of Adipose Tissue and Mesenchymal Stem Cells That Can Be Used for Studying the Mechanism of Obesogenic Action of EDCs
7. Hepatic Cellular Models
7.1. Animal Hepatocytes
7.1.1. Animal Primary Hepatocytes
7.1.2. FaO Cell Line
7.1.3. BRL-3A Cell Line
7.1.4. AML12 Cell Line
7.1.5. Hepa1-6 Cell Line
7.1.6. FL83B Cell Line
7.1.7. RTL-W1 Cell Line
7.1.8. PLHC-1 and ZFL Cell Line
7.2. Human Hepatocytes
7.2.1. Human Primary Hepatocytes
7.2.2. HepG2 Cell Line
7.2.3. HepaRG Cell Line
7.2.4. HPR116 Cell Line
7.2.5. Huh-7 Cell Line
7.2.6. Huh-6 Cell Line
7.2.7. HHL-5 Cell Line
7.2.8. L02 Cell Line
8. Pancreatic Cellular Models
8.1. Animal Pancreatic Cells
8.1.1. Rat Pancreatic Islets
8.1.2. INS-1 Cell Line
8.1.3. INS-1E Cell Line
8.1.4. RIN-m5F Cell Line
8.1.5. Mouse Pancreatic Islets
8.1.6. MIN-6 Cell Line
8.1.7. β-TC-6 Cell Line
8.2. Human Pancreatic Cells
8.2.1. Human Pancreatic Islets
8.2.2. EndoC-βH1 Cell Line
8.2.3. NES2Y Cell Line
8.2.4. PANC-1 Cell Line
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 1,3-DCP | 1,3-dichloro-2-propanol |
| 11β-HSD1 | 11β-hydroxysteroid dehydrogenase type 1 |
| 4-HP | 4-hexylphenol |
| 4-NP | 4-nonylphenol |
| AACS | acetoacetyl-CoA synthetase |
| ABCA1 | ATP-binding cassette transporter A1 |
| ACACA | acetyl-CoA carboxylase α |
| ACAT3 | acetyl-coenzyme A acetyltransferase 3 |
| ACC | acetyl-CoA carboxylase |
| ACC2 | acetyl-CoA carboxylase 2 |
| ACLY | adenosine triphosphate citrate lyase |
| ACTB | actin |
| Act-D | actinomycin-D |
| ADIPOQ | adiponectin, C1Q and collagen domain containing |
| ADIPOR2 | adiponectin receptor 2 |
| ADP | adenosine diphosphate |
| ADSCs | animal adipose-derived stem cells |
| AEA | anandamide (N-arachidonoylethanolamine) |
| AKT2 | v-akt murine thymoma viral oncogene homolog 2 |
| ALT | alanine aminotransferase |
| AML12 | alpha mouse liver 12 |
| AMP | adenosine monophosphate |
| AMPK | AMP-activated protein kinase |
| AMPKα | AMP-activated protein kinase-α |
| AOX | acyl-CoA oxidase |
| AP2 | adipocyte protein 2 |
| APAF1 | apoptotic protease activating factor 1 |
| APM1 | adipocyte most abundant gene transcript-1 |
| APOA1BP | apolipoprotein A1-binding protein |
| APOA2 | apolipoprotein A2 |
| APOA4 | apolipoprotein A4 |
| APOA-I | apolipoprotein A-I |
| APOB | apolipoprotein B |
| APOE | apolipoprotein E |
| ASCs | adipose-derived stem cells |
| AST | aspartate aminotransferase |
| AT | adipose tissue |
| ATGL | adipose triglyceride lipase |
| ATP | adenosine triphosphate |
| ATP1B1 | ATPase Na+/K+ transporting subunit beta 1 |
| ATP6 | ATP synthase subunit 6 |
| ATP6v1F | ATPase H+ transporting V1 subunit F |
| BADGE | 2HCl bisphenol A bis(3-chloro-2-hydroxypropyl) ether |
| BAT | brown adipose tissue |
| BAX | BCL2 associated X apoptosis regulator |
| BBP | benzyl butyl phthalate |
| BCL-2 | apoptosis regulator Bcl-2 |
| BHPF | fluorene-9-bisphenol |
| BIEA | biliverdin reductase |
| BMI | body mass index |
| BMMSC | bone marrow-derived mesenchymal stem cells |
| BPA | bisphenol A |
| BPAF | bisphenol AF |
| BPB | bisphenol B |
| BPF | bisphenol F |
| BPS | bisphenol S |
| C/EBP | CCAAT/enhancer-binding protein |
| C/EBPα | CCAAT/enhancer-binding protein α |
| C/EBPβ | CCAAT/enhancer-binding protein β |
| C/EBPδ | CCAAT/enhancer-binding protein δ |
| CACNA1E | calcium voltage-gated channel subunit alpha1 E |
| CaMKII | Ca2+/calmodulin-dependent protein kinase II |
| cAMP | cyclic adenosine monophosphate |
| CASP3B | caspase 3B |
| CAT | catalase |
| CB1 | endocannabinoid receptor type 1 |
| CBS | cystathionine-beta-synthase |
| CCL20 | chemokine (C-C motif) ligand 20 |
| Cd | cadmium |
| CD36 | fatty acid translocase |
| CdCl2 | cadmium chloride |
| CDKN1B | cyclin-dependent kinase inhibitor 1B |
| Cer | ceramide |
| CHOP | C/EBP homologous protein |
| CIDEA | cell death-inducing DNA fragmentation factor-alpha-like effector A |
| CK18 | cytokeratin 18 |
| CK8 | cytokeratin 8 |
| CNR1 | cannabinoid receptor 1 |
| COX16 | cytochrome C oxidase assembly factor |
| CPF | chlorpyrifos |
| CPT1 | carnitine palmitoyltransferase 1 |
| CPT1A | carnitine palmitoyltransferase 1 a |
| CPT1B | carnitine palmitoyltransferase 1 b |
| CREB | cAMP response element-binding protein |
| CYP1A1 | cytochrome P450 family 1 subfamily A member 1 |
| CYP2C18 | cytochrome P450 family 2 subfamily C member 18 |
| CYP2C19 | cytochrome P450 family 2 subfamily C member 19 |
| CYP2C9 | cytochrome P450 family 2 subfamily C member 9 |
| CYP3A | cytochrome P450 family 3 subfamily A |
| CYP3A4 | cytochrome P450 family 3 subfamily A member 4 |
| CYP3A65 | cytochrome P450 family 3 subfamily A polypeptide 65 |
| CYPs | cytochromes |
| D2EFHD2 | EF-hand domain-containing protein |
| DBP | dibutyl phthalate |
| DCHP | dicyclohexyl phthalate |
| DDE | dichlorodiphenyldichloroethylene |
| DDT | dichlorodiphenyltrichloroethane |
| DEHP | di(2-ethylhexyl) phthalate |
| DEX | dexamethasone |
| DG | diacylglycerol |
| DGAT1 | diacylglycerol acyltransferase 1 |
| DGAT1A | diacylglycerol acyltransferase 1a |
| DGAT2 | diacylglycerol acyltransferase 2 |
| DIDP | diisodecyl phthalate |
| DINCH | bis(7-methyloctyl) cyclohexane-1,2-dicarboxylate |
| DINP | diisononyl phthalate |
| DLK | dual leucine zipper-bearing kinase |
| DNMT1 | DNA (cytosine-5-)-methyltransferase 1 |
| DNMT3A | DNA methyltransferase 3 alpha |
| DNMT3AA | DNA (cytosine-5-)-methyltransferase 3A |
| DNMT3B | DNA methyltransferase 3 beta |
| DPHP | bis(2-propylheptyl) phthalate |
| ECHM | enoyl-CoA hydratase |
| EDCs | endocrine-disrupting chemicals |
| EF2 | elongation factor 2 |
| EFHD2 | EF-hand domain family member D2 |
| ELOVL6 | ELOVL fatty acid elongase 6 |
| ENO1 | α-enolase |
| ER | stress endoplasmic reticulum stress |
| ERK1/2 | extracellular signal-regulated protein kinase 1/2 |
| ERRγ | estrogen-related receptor γ |
| ERs | estrogen receptors |
| ERα | estrogen receptor α |
| ERβ -/- (or BERKO) mice | estrogen receptor β knockout mice |
| ERβ | estrogen receptor β |
| ESR1 | estrogen receptor 1 |
| ESR2 | estrogen receptor 2 |
| ESRRA | estrogen related receptor alpha |
| ether-TGs | ether-triacylglycerides |
| EU | European Union |
| Eurostat | Statistical Office of the European Union |
| EZRI | ezrin |
| FA | fatty acid |
| FAAH | fatty acid amide hydrolase |
| FABP | fatty acid binding protein |
| FABP4 | fatty acid binding protein 4 |
| FABP5 | fatty acid binding protein 5 |
| FADS1 | fatty acid desaturase 1 |
| FADS2 | fatty acid desaturase |
| FAS | fatty acid synthase |
| FASN | fatty acid synthase |
| FATP1 | fatty acid transport protein 1 |
| FOS | Fos proto-oncogene, AP-1 transcription factor subunit |
| FOXO1 | forkhead box protein O1 |
| FRIL | ferritin light chain |
| FSP27 | fat-specific protein 27 |
| G3BP1 | Ras GTPase-activating protein-binding 1 |
| GCK | glucokinase |
| GDF15 | growth differentiation factor 15 |
| GLU2β | glucosidase 2 subunit beta |
| GLUT1 | glucose transporter type 1 |
| GLUT2 | glucose transporter type 2 |
| GLUT4 | glucose transporter type 4 |
| GLY | glyphosate |
| GPAT | glycerol-3-phosphate acyltransferase |
| GPAT | glycerol-3-phosphate acyltransferase |
| GPAT3 | glycerol-3-phosphate acyltransferase 3 |
| GPD1 | glycerol-3-phosphate-dehydrogenase |
| GPR109B | protein-coupled receptor 109B |
| GPR30 | G protein-coupled receptor 30 |
| GPR40 | G protein-coupled receptor 40 |
| GPR41 | G protein-coupled receptor 41 |
| GPR43 | G protein-coupled receptor 43 |
| GPX1 | glutathione peroxidase 1 |
| GPX3 | glutathione peroxidase 3 |
| GPX4 | glutathione peroxidase 4 |
| GPX8 | glutathione peroxidase 8 |
| GR | glucocorticoid receptor |
| GRP75 | 75 kDa glucose-regulated protein |
| GRP78 | 78 kDa glucose-regulated protein |
| GSH | glutathione |
| GSIS | glucose-stimulated insulin secretion |
| GSR | glutathione-disulfide reductase |
| GSSG | oxidized glutathione |
| GSTA1/2 | glutathione S-transferase alpha ½ |
| GSTA3 | glutathione S-transferase alpha 3 |
| GSTO1 | glutathione S-transferase omega-1 |
| GUSB | glucuronidase beta |
| hADSCs | human adipose-derived stem cells |
| HBCD | hexabromocyclododecane |
| HL | hepatic lipase |
| HMGCR | 3-hydroxy-3-methylglutaryl CoA reductase |
| HMOX1 | heme oxygenase 1 |
| HNF1B | hepatocyte nuclear factor 1b |
| HNF4α | hepatocyte nuclear factor 4 alpha |
| HNRH1 | heterogenous nuclear ribonucleoprotein H1 |
| HNRPF | heterogeneous nuclear ribonucleoprotein F |
| HSD11B1 | 11beta-hydroxysteroid dehydrogenase 1 |
| HSL | hormone-sensitive lipase |
| HSP27 | heat shock protein 27 |
| HSPA1A | heat shock protein family A (Hsp70) member 1A |
| HSPA8 | heat shock protein family A (Hsp70) member 8 |
| hTERT | human telomerase reverse transcriptase |
| IBMX | phosphodiesterase inhibitor 1-methyl-3-isobutyl xanthine |
| IDH3A | isocitrate dehydrogenase (NAD(+)) 3 catalytic subunit alpha |
| IFN-γ | interferon gamma |
| IGF1 | insulin-like growth factor 1 |
| IL18 | interleukin 18 |
| IL1B | (IL1β) interleukin 1 beta |
| IL1α | interleukin 1 alpha |
| IL6 | interleukin 6 |
| INS1 | insulin 1 |
| INS2 | insulin 2 |
| INSIG1 | insulin induced gene 1 |
| INSR | insulin receptor |
| IR | insulin resistance |
| IRS-1 | insulin receptor substrate 1 |
| IRS-2 | insulin receptor substrate 2 |
| IR-β | insulin receptor subunit β |
| JAK2 | Janus kinase 2 |
| JNK | c-Jun N-terminal kinase |
| K2C8 | keratin type II cytoskeletal 8 |
| KATP | channel ATP-sensitive K+ channel |
| KCNIP | potassium voltage-gated channel interacting protein |
| KCNIP1 | potassium voltage-gated channel interacting protein 1 |
| KCNMA1 | potassium calcium-activated channel subfamily M alpha 1 |
| LAP3 | leucine aminopeptidase 3 |
| LDH | lactate dehydrogenase |
| LDLR | low-density lipoprotein receptor |
| LEP | leptin |
| LEPR | leptin receptor |
| LIPE | lipase E |
| LKB1 | serine-threonine liver kinase B1 |
| LPC | lysophosphatidylcholine |
| LPE | lysophosphatidylethanolamine |
| LPL | lipoprotein lipase |
| LSD-1 | lysine-specific demethylase-1 |
| LXR | liver X receptor |
| MAPKs | mitogen-activated protein kinases |
| MAT1A2 | methionine adenosyltransferase 1A2 |
| MCP1 | monocyte chemoattractant protein-1 |
| MDA | malondialdehyde |
| MDCs | metabolism-disrupting chemicals |
| MEHP | mono-2-ethylhexyl phthalate |
| MHINP | monohydroxy isononyl phthalate |
| MINCH | 1,2-cyclohexanedicarboxylic acid mono 4-methyloctyl ester |
| MMP | mitochondrial membrane potential |
| MSCs | mesenchymal stem cells |
| mTOR | mammalian target of rapamycin |
| MTTP | microsomal triglyceride transfer protein |
| N6AMT2 | N-6 adenine-specific DNA methyltransferase 2 |
| NAFLD | non-alcoholic fatty liver disease |
| NAMPT | nicotinamide phosphoribosyltransferase |
| NANOG | nanog homeobox |
| ND4L | NADH-ubiquinone oxidoreductase subunit 4 L |
| NDRG1 | N-myc downstream regulated 1 |
| NDUFS4 | NADH: ubiquinone oxidoreductase subunit S4 |
| NDUS1 | NADH dehydrogenase [ubiquinone] iron-sulfur protein 1 |
| NDUS3 | NADH dehydrogenase [ubiquinone] iron-sulfur protein 3 |
| NF-κB | nuclear factor kappa-light-chain-enhancer of activated B cells |
| NO | nitric oxide |
| NPC2 | Niemann-Pick 2 |
| NQO1 | NAD(P)H quinone dehydrogenase 1 |
| NRF1 | nuclear respiratory factor 1 |
| NRF2 | nuclear respiratory factor 2 |
| NRs | nuclear receptors |
| OA | oleic acid |
| OCT4 | octamer-binding transcription factor 4 |
| OEA | oleoylethanolamide |
| OGDH | oxoglutarate dehydrogenase |
| OH-MPHP | 6-hydroxy monopropylheptyl phthalate |
| OPFRs | chlorinated-organophosphorus flame retardants |
| p,p’-DDE | 1,1-dichloro-2,2-bis(4-chlorophenyl)ethane |
| p,p’-DDT | 1,1,1-trichloro-2,2-bis (p-chlorophenyl)-ethane |
| pACC | phosphorylated acetyl-CoA carboxylase |
| p-AKT | phosphorylated v-akt murine thymoma viral oncogene homolog |
| p-AMPK | phosphorylated AMP-activated protein kinase |
| PARP | poly (ADP-ribose) polymerase |
| PBDC1 | polysaccharide biosynthesis domain containing 1 |
| PBT | pentabromotoluene |
| PC | prohormone convertase |
| PCB-153 | polychlorinated biphenyls-153 |
| PCK1 | phosphoenolpyruvate carboxykinase 1 |
| PCNA | proliferating cell nuclear antigen |
| PCs | phosphatidylcholines |
| PCSK1 | proprotein convertase subtilisin/kexin type 1 |
| PDK4 | pyruvate dehydrogenase kinase 4 |
| PDX1 | pancreatic and duodenal homeobox 1 |
| PE | phosphatidylethanolamine |
| PEA | palmitoylethanolamide |
| p-ERK1/2 | phosphorylated extracellular signal-regulated protein kinase ½ |
| PFOA | perfluorooctanoic acid |
| PFOS | perfluorooctanesulfonic acid |
| PG | phosphatidylglycerol |
| PHHs | primary human hepatocytes |
| PI | polyphosphoinositide |
| p-JNK | phosphorylated c-Jun N-terminal kinase |
| PKA | protein kinase A |
| PKB | protein kinase B (also known as AKT) |
| PKCε | protein kinase C epsilon type |
| PLIN1 | perilipin-1 |
| PLIN2 | perilipin 2 |
| PLIN4 | perilipin-4 |
| PNPLA2 | patatin like phospholipase domain containing 2 |
| PNPLA3 | patatin like phospholipase domain containing 3 |
| PPAP2A | phosphatidic acid phosphatase type 2A |
| PPARGC1A | (or PGC1α) PPARG coactivator 1 alpha |
| PPARα | peroxisome proliferator-activated receptor α |
| PPARβ | peroxisome proliferator-activated receptor β |
| PPARγ | peroxisome proliferator-activated receptor γ |
| PPARγ1 | peroxisome proliferator-activated receptor γ1 |
| PPARγ2 | peroxisome proliferator-activated receptor γ2 |
| PPARγC1B | peroxisome proliferator-activated receptor gamma, coactivator 1 beta |
| PPARδ | peroxisome proliferator-activated receptor δ |
| PPIA | peptidylprolyl isomerase A |
| p-PKA | phosphorylated protein kinase A |
| p-PKC | phosphorylated protein kinase C |
| PRDM16 | PR domain containing 16 |
| PREF-1 | adipocyte differentiation-associated protein |
| PTGS2 | prostaglandin-endoperoxide synthase 2 |
| PXR | pregnane X receptor |
| QpE | quizalofop-p-ethyl |
| ROS | reactive oxygen species |
| RUNX2 | runt-related transcription factor 2 |
| RXR | retinoid X receptor |
| RXRα | retinoid X receptor alpha |
| S100B | calcium binding protein B |
| SAT | subcutaneous adipose tissue |
| SCD | stearoyl-CoA desaturase |
| SCD1 | stearoyl-CoA desaturase 1 |
| SCD1A | stearoyl-CoA desaturase 1A |
| SCD1B | stearoyl-CoA desaturase 1B |
| SCN9A | sodium voltage-gated channel alpha subunit 9 |
| SCOT | succinyl-CoA-3-oxoacid CoA-transferase |
| SDHD | succinate dehydrogenase complex subunit D |
| SGBS | Simpson-Golabi-Behemel syndrome |
| SIRT1 | sirtuin 1 |
| SIRT2 | sirtuin 2 |
| SIRT3 | sirtuin 3 |
| SIRT5 | sirtuin 5 |
| SIRT6 | sirtuin 6 |
| SIRT7 | sirtuin 7 |
| SM | sphingomyelin |
| SNAP25 | synaptosome-associated protein 25 |
| SOD | superoxide dismutase |
| SOD1 | superoxide dismutase 1 |
| SOD2 | superoxide dismutase 2 |
| SOX2 | SRY-box 2 |
| SR-A1 | scavenger receptor A1 |
| SR-B1 | scavenger receptor B1 |
| SRC | spare respiratory capacity |
| SREBF1 | sterol regulatory element binding transcription factor 1 |
| SREBF2 | sterol regulatory element binding transcription factor 2 |
| SREBP1 | sterol regulatory element-binding protein 1 |
| SREBP1C | sterol regulatory element-binding protein 1c |
| SREBP2 | sterol regulatory element-binding protein 2 |
| STAT3 | signal transducer and activator of transcription 3 |
| STAT5 | signal transducer and activator of transcription |
| STAT5A | signal transducer and activator of transcription 5A |
| STAT5B | signal transducer and activator of transcription 5B |
| STS | steroid sulfatase |
| SULT1A1 | sulfotransferase family 1A member 1 |
| SULT1A3/4 | sulfotransferase family 1A member 3 |
| SUR | sulfonylurea receptor |
| SUR1 | sulfonylurea receptor 1 |
| SVF | stromal vascular fraction |
| T2DM | type 2 diabetes mellitus |
| TBB | tetrabromobenzoate |
| TBBPA | tetrabromobisphenol |
| TBT | tributyltin |
| TC | total cholesterol |
| TCBPA | tetrachlorobisphenol A |
| TCDD | 2,3,7,8-tetrachlorodibenzo-p-dioxin |
| TCEP | tris (2-chloroethyl) phosphate |
| TCP | tricresyl phosphate |
| TCPA | T-complex protein 1 subunit alpha |
| TCPP | tris (2-chloroisopropyl) phosphate |
| TCS | triclosan |
| TDCPP | tris-(2-chloro-1- (chloromethyl) ethyl) phosphate |
| TERT | telomerase reverse transcriptase |
| TF | tolylfluanid |
| TFAM | transcription factor A, mitochondrial |
| TG | triglyceride |
| THRSP | thyroid hormone responsive |
| TIP47 | perilipin 3 |
| TMBPF | tetramethyl bisphenol F |
| TNFα | tumor necrosis factor-α |
| ToxPi | toxicological Priority Index |
| TPhP | triphenyl phosphate |
| TPP | triphenylphosphate |
| TPT | terpyridine platinum(II) chloride |
| TR/RXR | thyroid-receptor/retinoid X receptor |
| TRIB3 | tribbles pseudokinase 3 |
| TSPO | translocator protein |
| UCP-1 | uncoupling protein-1 |
| UCP-2 | uncoupling protein-2 |
| UCP-3 | uncoupling protein-3 |
| UGT2B15 | UDP glucuronosyltransferase family 2 member B15 |
| UGTs | UDP-glucuronosyltransferases |
| UQCRB | ubiquinol-cytochrome C reductase binding protein |
| VAPA | VAMP associated protein A |
| VAT | visceral adipose tissue |
| VDBP | vitamin D-binding protein |
| VTG1 | Vitellogenin 1 |
| WAT | white adipose tissue |
| WHO | World Health Organization |
| WT | wild-type |
| XMEs | xenobiotic-metabolizing enzymes |
| ZFAND2A | zinc finger AN1-type containing 2A |
| α2A-AR | adrenergic receptor α2A |
| β-AR | β adrenergic receptor |
| β-TC-6 | beta-tumor cell-6 |
References
- Zorena, K.; Jachimowicz-Duda, O.; Ślęzak, D.; Robakowska, M.; Mrugacz, M. Adipokines and Obesity. Potential Link to Metabolic Disorders and Chronic Complications. Int. J. Mol. Sci 2020, 21, 3570. [Google Scholar] [CrossRef]
- Overweight and Obesity—BMI Statistics. Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Overweight_and_obesity_-_BMI_statistics#Education_level_and_overweight (accessed on 20 November 2022).
- OECD/EU. Health at a Glance: Europe 2018: State of Health in the EU Cycle; OECD Publishing: Paris, France, 2018; p. 124. [Google Scholar]
- Nappi, F.; Barrea, L.; Di Somma, C.; Savanelli, M.C.; Muscogiuri, G.; Orio, F.; Savastano, S. Endocrine Aspects of Environmental “Obesogen” Pollutants. Int. J. Res. Public Health 2016, 13, 765. [Google Scholar] [CrossRef]
- Peña-Romero, A.C.; Navas-Carrillo, D.; Marín, F.; Orenes-Piñero, E. The future of nutrition: Nutrigenomics and nutrigenetics in obesity and cardiovascular diseases. Crit. Rev. Food Sci. Nutr. 2018, 58, 3030–3041. [Google Scholar] [CrossRef] [PubMed]
- Engin, A.B. MicroRNA and Adipogenesis. Adv. Exp. Med. Biol. 2017, 960, 489–509. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.-I.; Kwon, H.-Y.; Han, X.; Men, X.; Choi, Y.-E.; Jang, G.-W.; Park, K.-T.; Han, J.; Lee, O.-H. Environmental obesogens (bisphenols, phthalates and parabens) and their impacts on adipogenic transcription factors in the absence of dexamethasone in 3T3-L1 cells. J. Steroid Biochem. Mol. Biol. 2021, 214, 105994. [Google Scholar] [CrossRef]
- Schaffert, A.; Krieg, L.; Weiner, J.; Schlichting, R.; Ueberham, E.; Karkossa, I.; Bauer, M.; Landgraf, K.; Junge, K.M.; Wabitsch, M.; et al. Alternatives for the worse: Molecular insights into adverse effects of bisphenol a and substitutes during human adipocyte differentiation. Environ. Int. 2021, 156, 106730. [Google Scholar] [CrossRef] [PubMed]
- Heindel, J.J.; Blumberg, B. Environmental Obesogens: Mechanisms and Controversies. Annu. Rev. Pharmacol. Toxicol. 2019, 6, 89–106. [Google Scholar] [CrossRef]
- Kladnicka, I.; Bludovska, M.; Plavinowa, I.; Muller, L.; Mullerova, D. Obesogenes in food. Biomolecules 2022, 12, 680. [Google Scholar] [CrossRef]
- Diamanti-Kandarakis, E.; Bourguignon, J.-P.; Giudice, L.C.; Hauser, R.; Prins, G.S.; Soto, A.M.; Zoeller, R.T.; Gore, A.C. Endocrine-disrupting chemicals: An Endocrine Society scientific statement. Endocr. Rev. 2009, 30, 293–342. [Google Scholar] [CrossRef]
- Kumar, M.; Sarma, D.K.; Shubham, S.; Kumawat, M.; Verma, V.; Prakash, A.; Tiwari, R. Environmental Endocrine-Disrupting Chemical Exposure: Role in Non-Communicable Diseases. Front. Public Health 2020, 8, 553850. [Google Scholar] [CrossRef] [PubMed]
- Janesick, A.; Blumberg, B. Obesogens, Stem Cells and the Developmental Programming of Obesity. Int. J. Androl. 2012, 35, 437–448. [Google Scholar] [CrossRef] [PubMed]
- Legler, J.; Zalko, D.; Jourdan, F.; Jacobs, M.; Fromenty, B.; Balaguer, P.; Bourguet, W.; Kos, V.M.; Nadal, A.; Beausoleil, C.; et al. The GOLIATH Project: Towards an Internationally Harmonised Approach for Testing Metabolism Disrupting Compounds. Int. J. Mol. Sci. 2020, 21, 3480. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Lu, H.; Wu, K.; Zhang, Z.; Shuk-Wa Lau, E.; Ge, W. Genetic evidence for estrogenicity of bisphenol A in zebrafish gonadal differentiation and its signalling mechanism. J. Hazard. Mater. 2020, 386, 121886. [Google Scholar] [CrossRef] [PubMed]
- Mattiske, D.M.; Pask, A.J. Endocrine disrupting chemicals in the pathogenesis of hypospadias; developmental and toxicological perspectives. Curr. Res. Toxicol. 2021, 2, 179–191. [Google Scholar] [CrossRef]
- Thambirajah, A.A.; Wade, M.G.; Verreault, J.; Buisine, N.; Alves, V.A.; Langlois, V.S.; Helbing, C.C. Disruption by stealth—Interference of endocrine disrupting chemicals on hormonal crosstalk with thyroid axis function in humans and other animals. Environ. Res. 2022, 203, 111906. [Google Scholar] [CrossRef]
- Gupta, R.; Kumar, P.; Fahmi, N.; Garg, B.; Dutta, S.; Sachar, S.; Matharu, A.S.; Vimaleswaran, K.S. Endocrine disruption and obesity: A current review on environmental obesogens. CRGSC 2020, 3, 100009. [Google Scholar] [CrossRef]
- Mohajer, N.; Du, C.Y.; Checkcinco, C.; Blumberg, B. Obesogens: How They Are Identified and Molecular Mechanisms Underlying Their Action. Front. Endocrinol. 2021, 12, 780888. [Google Scholar] [CrossRef]
- Heindel, J.J.; Blumberg, B.; Cave, M.; Machtinger, R.; Mantovani, A.; Mendez, M.A.; Nadal, A.; Palanza, P.; Panzica, G.; Sargis, R.; et al. Metabolism disrupting chemicals and metabolic disorders. Reprod. Toxicol. 2017, 68, 3–33. [Google Scholar] [CrossRef]
- Sokal, A.; Jarmakiewicz-Czaja, S.; Tabarkiewicz, J.; Filip, R. Dietary Intake of Endocrine Disrupting Substances Presents in Environment and Their Impact on Thyroid Function. Nutrients 2021, 13, 867. [Google Scholar] [CrossRef]
- Naomi, R.; Yazid, M.D.; Bahari, H.; Keong, T.T.; Rajandram, R.; Embomg, H.; Teoh, S.H.; Halim, S.; Othman, F. Bisphenol A (BPA) leading to obesity and cardiovascular complication: A complication of current in vivo study. Int. J. Mol. Sci. 2022, 23, 2969. [Google Scholar] [CrossRef]
- Dos Santos, R.S.; Medina-Gali, R.M.; Babiloni-Chust, I.; Marroqui, L.; Nadal, A. In vitro Assays to Identify Metabolism-Disrupting Chemicals with Diabetogenic Activity in a Human Pancreatic β-Cell Model. Int. J. Mol. Sci. 2022, 23, 5040. [Google Scholar] [CrossRef] [PubMed]
- Norgren, K.; Tuck, A.; Vieira Silva, A.; Burkhardt, P.; Öberg, M.; Munic Kos, V. High throughput screening of bisphenols and their mixtures under conditions of low-intensity adipogenesis of human mesenchymal stem cells (hMSCs). Food Chem. Toxicol. 2022, 161, 112842. [Google Scholar] [CrossRef]
- Hines, E.P.; White, S.S.; Stanko, J.P.; Gibbs-Flournoy, E.A.; Lau, H.; Fenton, S.E. Phenotypic dichotomy following developmental exposure to perfluorooctanoic acid (PFOA) in female CD-1 mice: Low doses induce elevated serum leptin and insulin, and overweight in mid-life. Mol. Cell Endocrinol. 2009, 304, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Bokobza, E.; Hinault, C.; Tiroille, V.; Clavel, S.; Bost, F.; Chevalier, N. The adipose tissue at the crosstalk between EDCs and cancer development. Front. Endocrinol. 2021, 12, 691658. [Google Scholar] [CrossRef]
- Ramskov Tetzlaff, C.N.; Svingen, T.; Vinggaard, A.M.; Rosenmai, A.K.; Taxvig, C. Bisphenols B, E, F, and S and 4-cumylphenol induce lipid accumulation in mouse adipocytes similarly to bisphenol A. Environ. Toxicol. 2020, 35, 543–552. [Google Scholar] [CrossRef]
- Haq, M.E.U.; Akash, M.S.H.; Rehman, K.; Mahmood, M.H. Chronic exposure of bisphenol A impairs carbohydrate and lipid metabolism by altering corresponding enzymatic and metabolic pathways. Environ. Toxicol. Pharmacol. 2020, 78, 103387. [Google Scholar] [CrossRef]
- Wang, B.; Tsakiridis, E.E.; Zhang, S.; Llanos, A.; Desjardins, E.M.; Yabut, J.M.; Green, A.E.; Day, E.A.; Smith, B.K.; Lally, J.S.V.; et al. The pesticide chlorpyrifos promotes obesity by inhibiting diet-induced thermogenesis in brown adipose tissue. Nat. Commun. 2021, 12, 1. [Google Scholar] [CrossRef]
- Zhou, L.; Chen, H.; Xu, Q.; Han, X.; Zhao, Y.; Song, X.; Zhao, T.; Ye, L. The effect of di-2-ethylhexyl phthalate on inflammation and lipid metabolic disorder in rats. Ecotoxicol. Environ. Saf. 2019, 170, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Amato, A.A.; Wheeler, H.B.; Blumberg, B. Obesity and endocrine-disrupting chemicals. Endocr. Connect. 2021, 10, R87–R105. [Google Scholar] [CrossRef] [PubMed]
- Callaghan, M.A.; Alatorre-Hinojosa, S.; Connors, L.T.; Singh, R.D.; Thompson, J.A. Plasticizers and Cardiovascular Health: Role of Adipose Tissue Dysfunction. Front. Pharmacol. 2021, 11, 626448. [Google Scholar] [CrossRef]
- Giuliani, A.; Zuccarini, M.; Cichelli, A.; Khan, H.; Reale, M. Critical Review on the Presence of Phthalates in Food and Evidence of Their Biological Impact. Int. J. Environ. Res. Public Health 2020, 17, 5655. [Google Scholar] [CrossRef] [PubMed]
- Almeida, S.; Raposo, A.; Almeida-González, M.; Carrascosa, C. Bisphenol A: Food Exposure and Impact on Human Health. CRFSFS 2018, 17, 1503–1517. [Google Scholar] [CrossRef] [PubMed]
- Mnif, W.; Hassine, A.I.; Bouaziz, A.; Bartegi, A.; Thomas, O.; Roig, B. Effect of endocrine disruptor pesticides: A review. Int. J. Environ. Res. Public Health 2011, 8, 2265–2303. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.M.; Kuo, Y.; Blumberg, B. Agrochemicals and obesity. Mol. Cell. Endocrinol. 2020, 515, 110926. [Google Scholar] [CrossRef]
- Mukherjee, R.; Pandya, P.; Baxi, D.; Ramachandran, A.V. Endocrine Disruptors-‘Food’ for Thought. Proc. Zool. Soc. 2021, 74, 432–442. [Google Scholar] [CrossRef]
- Garí, M.; Moos, R.; Bury, D.; Kasper-Sonnenberg, M.; Jankowska, A.; Andysz, A.; Hanke, W.; Nowak, D.; Bose-O’Reilly, S.; Koch, H.M.; et al. Human-biomonitoring derived exposure and daily intakes of Bisphenol A and their associations with neurodevelopmental outcomes among children of the Polish Mother and Child Cohort Study. Environ. Health 2021, 20, 95. [Google Scholar] [CrossRef]
- Kassotis, C.D.; Masse, L.; Kim, S.; Schlezinger, J.J.; Webster, T.F.; Stapleton, H.M. Characterization of Adipogenic Chemicals in Three Different Cell Culture Systems: Implications for Reproducibility Based on Cell Source and Handling. Sci. Rep. 2017, 7, 1. [Google Scholar] [CrossRef]
- Grün, F.; Blumberg, B. Environmental obesogens: Organotins and endocrine disruption via nuclear receptor signaling. Endocrinology 2006, 147 (Suppl. 6), S50–S55. [Google Scholar] [CrossRef]
- Rubin, B.S. Bisphenol A: An endocrine disruptor with widespread exposure and multiple effects. J. Steroid Biochem. Mol. Biol. 2011, 127, 27–34. [Google Scholar] [CrossRef]
- Ernst, J.; Grabiec, U.; Falk, K.; Dehghani, F.; Schaedlich, K. The endocrine disruptor DEHP and the ECS: Analysis of a possible crosstalk. Endocr. Connect. 2020, 9, 101–110. [Google Scholar] [CrossRef]
- Lincho, J.; Martins, R.C.; Gomes, J. Paraben Compounds—Part I: An Overview of Their Characteristics, Detection, and Impacts. Appl. Sci. 2021, 1, 2307. [Google Scholar] [CrossRef]
- Metcalfe, C.D.; Bayen, S.; Desrosiers, M.; Muñoz, G.; Sauvé, S.; Yargeau, V. An introduction to the sources, fate, occurrence and effects of endocrine disrupting chemicals released into the environment. Environ. Res. 2022, 207, 112658. [Google Scholar] [CrossRef] [PubMed]
- Wan, H.T.; Leung, P.Y.; Zhao, Y.G.; Wei, X.; Wong, M.H.; Wong, C.K. Blood plasma concentrations of endocrine disrupting chemicals in Hong Kong populations. J. Hazard. Mater. 2013, 261, 763–769. [Google Scholar] [CrossRef]
- Arbuckle, T.E.; Davis, K.; Marro, L.; Fisher, M.; Legrand, M.; LeBlanc, A.; Gaudreau, E.; Foster, W.G.; Choeurng, V.; Fraser, W.D. MIREC Study Group. Phthalate and bisphenol A exposure among pregnant women in Canada—Results from the MIREC study. Environ. Int. 2014, 68, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Wong, L.Y.; Kramer, J.; Zhou, X.; Jia, T.; Calafat, A.M. Urinary Concentrations of Bisphenol A and Three Other Bisphenols in Convenience Samples of U.S. Adults during 2000–2014. Environ. Sci. Technol. 2015, 49, 11834–11839. [Google Scholar] [CrossRef] [PubMed]
- Desalegn, A.A.; Iszatt, N.; Stigum, H.; Jensen, T.K.; Eggesbø, M. A case-cohort study of perinatal exposure to potential endocrine disrupters and the risk of cryptorchidism in the Norwegian HUMIS study. Environ. Int. 2021, 157, 106815. [Google Scholar] [CrossRef]
- Cariou, R.; Antignac, J.P.; Zalko, D.; Berrebi, A.; Cravedi, J.P.; Maume, D.; Marchand, P.; Monteau, F.; Riu, A.; Andre, F.; et al. Exposure assessment of French women and their newborns to tetrabromobisphenol-A: Occurrence measurements in maternal adipose tissue, serum, breast milk and cord serum. Chemosphere 2008, 73, 1036–1041. [Google Scholar] [CrossRef]
- Mangum, L.H.; Howell, G.E., 3rd; Chambers, J.E. Exposure to p,p’-DDE enhances differentiation of 3T3-L1 preadipocytes in a model of sub-optimal differentiation. Toxicol. Lett. 2015, 238, 65–71. [Google Scholar] [CrossRef]
- Garí, M.; Koch, H.M.; Pälmke, C.; Jankowska, A.; Wesołowska, E.; Hanke, W.; Nowak, D.; Bose-O’Reilly, S.; Polańska, K. Determinants of phthalate exposure and risk assessment in children from Poland. Environ. Int. 2019, 127, 742–753. [Google Scholar] [CrossRef]
- Ravichandran, G.; Lakshmanan, D.K.; Arunachalam, A.; Thilagar, S. Food obesogens as emerging metabolic disruptors; A toxicological insight. J. Steroid Biochem. Mol. Biol. 2022, 217, 106042. [Google Scholar] [CrossRef]
- Lowell Center for Sustainable Production at the University of Massachusetts Lowell. Phthalates and Their Alternatives: Health and Environmental Concerns; University of Massachusetts: Lowell, MA, USA, 2011; pp. 1–24. [Google Scholar]
- Wang, Y.; Qian, H. Phthalates and Their Impacts on Human Health. Healthcare 2021, 9, 603. [Google Scholar] [CrossRef] [PubMed]
- Cohen, I.C.; Cohenour, E.R.; Harnett, K.G.; Schuh, S.M. BPA, BPAF and TMBPF Alter Adipogenesis and Fat Accumulation in Human Mesenchymal Stem Cells, with Implications for Obesity. Int. J. Mol. Sci. 2021, 22, 5363. [Google Scholar] [CrossRef] [PubMed]
- Harnett, K.G.; Chin, A.; Schuh, S.M. BPA and BPA alternatives BPS, BPAF, and TMBPF, induce cytotoxicity and apoptosis in rat and human stem cells. Ecotoxicol. Environ. Saf. 2021, 216, 112210. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, A.L.; Silva, M.J.; Louro, H. In vitro exposure to the next-generation plasticizer diisononyl cyclohexane-1,2-dicarboxylate (DINCH): Cytotoxicity and genotoxicity assessment in human cells. J. Toxicol. Environ. Health A 2019, 82, 526–536. [Google Scholar] [CrossRef]
- Andújar, N.; Gálvez-Ontiveros, Y.; Zafra-Gómez, A.; Rodrigo, L.; Álvarez-Cubero, M.J.; Aguilera, M.; Monteagudo, C.; Rivas, A.A. Bisphenol A Analogues in Food and Their Hormonal and Obesogenic Effects: A Review. Nutrients 2019, 11, 2136. [Google Scholar] [CrossRef]
- Zughaibi, T.A.; Sheikh, I.A.; Beg, M.A. Insights into the Endocrine Disrupting Activity of Emerging Non-Phthalate Alternate Plasticizers against Thyroid Hormone Receptor: A Structural Perspective. Toxics 2022, 10, 263. [Google Scholar] [CrossRef]
- Schaffert, A.; Arnold, J.; Karkossa, I.; Blühe, M.; von Bergen, M.; Schubert, K. The Emerging Plasticizer Alternative DINCH and Its Metabolite MINCH Induce Oxidative Stress and Enhance Inflammatory Responses in Human THP-1 Macrophages. Cells 2021, 10, 2367. [Google Scholar] [CrossRef]
- Campioli, E.; Lau, M.; Papadopoulos, V. Effect of subacute and prenatal DINCH plasticizer exposure on rat dams and male offspring hepatic function: The role of PPAR-α. Environ. Res. 2019, 179 Pt A, 108773. [Google Scholar] [CrossRef]
- Schaffert, A.; Karkossa, I.; Ueberham, E.; Schlichting, R.; Walter, K.; Arnold, J.; Blüher, M.; Heiker, J.T.; Lehmann, J.; Wabitsch, M.; et al. Di-(2-ethylhexyl) phthalate substitutes accelerate human adipogenesis through PPARγ activation and cause oxidative stress and impaired metabolic homeostasis in mature adipocytes. Environ. Int. 2022, 164, 107279. [Google Scholar] [CrossRef]
- Doke, S.K.; Dhawale, S.C. Alternatives to animal testing: A review. Saudi Pharm. J. 2015, 23, 223–229. [Google Scholar] [CrossRef]
- Porro, S.; Genchi, V.A.; Cignarelli, A.; Natalicchio, A.; Laviola, L.; Giorgino, F.; Perrini, S. Dysmetabolic adipose tissue in obesity: Morphological and functional characteristics of adipose stem cells and mature adipocytes in healthy and unhealthy obese subjects. J. Endocrinol. Investig. 2021, 44, 921–941. [Google Scholar] [CrossRef]
- Jung, U.J.; Choi, M.S. Obesity and Its Metabolic Complications: The Role of Adipokines and the Relationship between Obesity, Inflammation, Insulin Resistance, Dyslipidemia and Nonalcoholic Fatty Liver Disease. Int. J. Mol. Sci. 2014, 15, 6184–6223. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, B.; Serpell, C.J.; Fong, I.L.; Wong, E.H. Molecular Mechanisms of Adipogenesis: The Anti-adipogenic Role of AMP-Activated Protein Kinase. Front. Mol. Biosci. 2020, 7, 76. [Google Scholar] [CrossRef]
- Mentor, A.; Brunström, B.; Mattsson, A.; Jönsson, M. Developmental exposure to a human relevant mixture of endocrine disruptors alters metabolism and adipogenesis in zebrafish (Danio rerio). Chemosphere 2020, 238, 124584. [Google Scholar] [CrossRef] [PubMed]
- Longo, M.; Zatterale, F.; Naderi, J.; Parrillo, L.; Formisano, P.; Raciti, G.A.; Beguinot, F.; Miele, C. Adipose Tissue Dysfunction as Determinant of Obesity-Associated Metabolic Complications. Int. J. Mol. Sci. 2019, 20, 2358. [Google Scholar] [CrossRef] [PubMed]
- González-Casanova, J.E.; Pertuz-Cruz, S.L.; Caicedo-Ortega, N.H.; Rojas-Gomez, D.M. Adipogenesis Regulation and Endocrine Disruptors: Emerging Insights in Obesity. Biomed. Res. Int. 2020, 2020, 7453786. [Google Scholar] [CrossRef]
- Boucher, J.G.; Husain, M.; Rowan-Carroll, A.; Williams, A.; Yauk, C.L.; Atlas, E. Identification of mechanisms of action of bisphenol a-induced human preadipocyte differentiation by transcriptional profiling. Obesity 2014, 22, 2333–2343. [Google Scholar] [CrossRef]
- Baker, A.H.; Watt, J.; Huang, C.K.; Gerstenfeld, L.C.; Schlezinger, J.J. Tributyltin engages multiple nuclear receptor pathways and suppresses osteogenesis in bone marrow multipotent stromal cells. Chem. Res. Toxicol. 2015, 28, 1156–1166. [Google Scholar] [CrossRef]
- Ahmed, S.; Atlas, E. Bisphenol S-and bisphenol A-induced adipogenesis of murine preadipocytes occurs through direct peroxisome proliferator-activated receptor gamma activation. Int. J. Obes. 2016, 40, 1566. [Google Scholar] [CrossRef]
- Feige, J.N.; Gelman, L.; Rossi, D.; Zoete, V.; Metivier, R.; Tudor, C.; Anghel, S.I.; Grosdidier, A.; Lathion, C.; Engelborghs, Y.; et al. The endocrine disruptor monoethyl-hexyl-phthalate is a selective peroxisome proliferator-activated receptor gamma modulator that promotes adipogenesis. J. Biol. Chem. 2007, 282, 19152–19166. [Google Scholar] [CrossRef]
- Stark, J.M.; Coquet, J.M.; Tibbitt, C.A. The Role of PPAR-γ in Allergic Disease. Curr. Allergy Asthma Rep. 2021, 21, 45. [Google Scholar] [CrossRef]
- Boucher, J.G.; Boudreau, A.; Atlas, E. Bisphenol A induces differentiation of human preadipocytes in the absence of glucocorticoid and is inhibited by an estrogen-receptor antagonist. Nutr. Diabetes 2014, 4, e102. [Google Scholar] [CrossRef]
- Wang, J.; Sun, B.; Hou, M.; Li, X. The environmental obesogen bisphenol A promotes adipogenesis by increasing the amount of 11β-hydroxysteroid dehydrogenase type 1 in the adipose tissue of children. Int. J. Obes. 2013, 37, 999–1005. [Google Scholar] [CrossRef] [PubMed]
- Pesta, M.; Cedikova, M.; Dvorak, P.; Dvorakova, J.; Kulda, V.; Srbecka, K.; Muller, L.; Bouchalova, V.; Kralickova, M.; Babuska, V.; et al. Trends in gene expression changes during adipogenesis in human adipose derived mesenchymal stem cells under dichlorodiphenyldichloroethylene exposure. Mol. Cell. Toxicol. 2018, 14, 369–379. [Google Scholar] [CrossRef]
- Kirchner, S.; Kieu, T.; Chow, C.; Casey, S.; Blumberg, B. Prenatal exposure to the environmental obesogen tributyltin predisposes multipotent stem cells to become adipocytes. Mol. Endocrinol. 2010, 24, 526–539. [Google Scholar] [CrossRef] [PubMed]
- Reina-Pérez, I.; Olivas-Martínez, A.; Mustieles, V.; Ruiz-Ojeda, F.J.; Molina-Molina, J.M.; Olea, N.; Fernández, M.F. Bisphenol F and bisphenol S promote lipid accumulation and adipogenesis in human adipose-derived stem cells. Food Chem. Toxicol. 2021, 152, 112216. [Google Scholar] [CrossRef]
- Howell, G., 3rd; Mangum, L. Exposure to bioaccumulative organochlorine compounds alters adipogenesis, fatty acid uptake, and adipokine production in NIH3T3-L1 cells. Toxicol. Vitr. 2011, 25, 394–402. [Google Scholar] [CrossRef]
- Sakurai, K.; Kawazuma, M.; Adachi, T.; Harigaya, T.; Saito, Y.; Hashimoto, N.; Mori, C. Bisphenol A affects glucose transport in mouse 3T3-F442A adipocytes. Br. J. Pharmacol. 2004, 141, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Yamasaki, M.; Hasegawa, S.; Imai, M.; Fukui, T.; Takahashi, N. Browning Effect of Brominated Flame Retardant, TBBP-A, on Undifferentiated Adipocytes. BPB Rep. 2021, 4, 41–46. [Google Scholar] [CrossRef]
- El-Atta, H.M.; Ahmed, E.R. Study of the In-vitro Epigenetic Toxicity Effects of Malaoxon, Malathion Dicarboxylic Acid, Cadmium Chloride and Bisphenol-A on PPAR γ, PPIA and aP2 gene Expressions. J. Clin. Toxicol. 2018, 8, 3. [Google Scholar] [CrossRef]
- Schaedlich, K.; Gebauer, S.; Hunger, L.; Beier, L.S.; Koch, H.M.; Wabitsch, M.; Fischer, B.; Ernst, J. DEHP deregulates adipokine levels and impairs fatty acid storage in human SGBS-adipocytes. Sci. Rep. 2018, 8, 3447. [Google Scholar] [CrossRef] [PubMed]
- Xue, P.; Hou, Y.; Zhang, Q.; Woods, C.G.; Yarborough, K.; Liu, H.; Sun, G.; Andersen, M.E.; Pi, J. Prolonged inorganic arsenite exposure suppresses insulin-stimulated AKT S473 phosphorylation and glucose uptake in 3T3-L1 adipocytes: Involvement of the adaptive antioxidant response. Biochem. Biophys. Res. Commun. 2011, 407, 360–365. [Google Scholar] [CrossRef]
- Jin, Y.; Lin, X.; Miao, W.; Wu, T.; Shen, H.; Chen, S.; Li, Y.; Pan, Q.; Fu, Z. Chronic exposure of mice to environmental endocrine-disrupting chemicals disturbs their energy metabolism. Toxicol. Lett. 2014, 225, 392–400. [Google Scholar] [CrossRef]
- Al-Suhaimi, E.A.; Shehzad, A. Leptin, resistin and visfatin: The missing link between endocrine metabolic disorders and immunity. Eur. J. Med. Res. 2013, 18, 12. [Google Scholar] [CrossRef]
- Graudejus, O.; Ponce Wong, R.D.; Varghese, N.; Wagner, S.; Morrison, B. Bridging the gap between in vivo and in vitro research: Reproducing in vitro the mechanical and electrical environment of cells in vivo. In Proceedings of the MEA Meeting 2018|11th International Meeting on Substrate Integrated Microelectrode Arrays, Reutlingen, Germany, 4–6 July 2018. [Google Scholar] [CrossRef]
- Griffin, M.; Pereira, S.R.; DeBari, M.K.; Abbott, R.D. Mechanisms of action, chemical characteristics, and model systems of obesogens. BMC Biomed. Eng. 2020, 2, 6. [Google Scholar] [CrossRef] [PubMed]
- Forgacs, A.L.; Dere, E.; Angrish, M.M.; Zacharewski, T.R. Comparative analysis of temporal and dose-dependent TCDD-elicited gene expression in human, mouse, and rat primary hepatocytes. Toxicol. Sci. 2013, 133, 54–66. [Google Scholar] [CrossRef] [PubMed]
- Janesick, A.S.; Blumberg, B. Obesogens: An emerging threat to public health. Am. J. Obstet. Gynecol. 2016, 214, 559–565. [Google Scholar] [CrossRef]
- Morrison, S.; McGee, S.L. 3T3-L1 adipocytes display phenotypic characteristics of multiple adipocyte lineages. Adipocyte 2015, 4, 295–302. [Google Scholar] [CrossRef]
- Sun, Z.; Cao, H.; Liu, Q.S.; Liang, Y.; Fiedler, H.; Zhang, J.; Zhou, Q.; Jiang, G. 4-Hexylphenol influences adipogenic differentiation and hepatic lipid accumulation in vitro. Environ. Pollut. 2021, 268, 115635. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Ojeda, F.J.; Rupérez, A.I.; Gomez-Llorente, C.; Gil, A.; Aguilera, C.M. Cell Models and Their Application for Studying Adipogenic Differentiation in Relation to Obesity: A Review. Int. J. Mol. Sci. 2016, 17, 1040. [Google Scholar] [CrossRef] [PubMed]
- Vernochet, C.; Peres, S.B.; Davis, K.E.; McDonald, M.E.; Qiang, L.; Wang, H.; Scherer, P.E.; Farmer, S.R. C/EBPalpha and the corepressors CtBP1 and CtBP2 regulate repression of select visceral white adipose genes during induction of the brown phenotype in white adipocytes by peroxisome proliferator-activated receptor gamma agonists. Mol. Cell. Biol. 2009, 29, 4714–4728. [Google Scholar] [CrossRef] [PubMed]
- De Filippis, E.; Li, T.; Rosen, E.D. Exposure of adipocytes to bisphenol-A in vitro interferes with insulin action without enhancing adipogenesis. PLoS ONE 2018, 13, e0201122. [Google Scholar] [CrossRef]
- Sargis, R.M.; Johnson, D.N.; Choudhury, R.A.; Brady, M.J. Environmental endocrine disruptors promote adipogenesis in the 3T3-L1 cell line through glucocorticoid receptor activation. Obesity 2010, 18, 1283–1288. [Google Scholar] [CrossRef] [PubMed]
- Meruvu, S.; Zhang, J.; Choudhury, M. Butyl Benzyl Phthalate Promotes Adipogenesis in 3T3-L1 Cells via the miRNA-34a-5p Signaling Pathway in the Absence of Exogenous Adipogenic Stimuli. Chem. Res. Toxicol. 2021, 34, 2251–2260. [Google Scholar] [CrossRef] [PubMed]
- Biserni, M.; Mesnage, R.; Ferro, R.; Wozniak, E.; Xenakis, T.; Mein, C.A.; Antoniou, M.N. Quizalofop-p-Ethyl Induces Adipogenesis in 3T3-L1 Adipocytes. Toxicol. Sci. 2019, 170, 452–461. [Google Scholar] [CrossRef]
- Kim, J.; Sun, Q.; Yue, Y.; Yoon, K.S.; Whang, K.Y.; Marshall Clark, J.; Park, Y. 4,4′-Dichlorodiphenyltrichloroethane (DDT) and 4,4′-dichlorodiphenyldichloroethylene (DDE) promote adipogenesis in 3T3-L1 adipocyte cell culture. Pestic. Biochem. Physiol. 2016, 131, 40–45. [Google Scholar] [CrossRef]
- Smith, A.; Yu, X.; Yin, L. Diazinon exposure activated transcriptional factors CCAAT-enhancer-binding proteins α (C/EBPα) and peroxisome proliferator-activated receptor γ (PPARγ) and induced adipogenesis in 3T3-L1 preadipocytes. Pestic. Biochem. Physiol. 2018, 150, 48–58. [Google Scholar] [CrossRef]
- Blanco, J.; Guardia-Escote, L.; Mulero, M.; Basaure, P.; Biosca-Brull, J.; Cabré, M.; Colomina, M.T.; Domingo, J.L.; Sánchez, D.J. Obesogenic effects of chlorpyrifos and its metabolites during the differentiation of 3T3-L1 preadipocytes. Food Chem. Toxicol. 2020, 137, 111171. [Google Scholar] [CrossRef]
- Regnier, S.M.; El-Hashani, E.; Kamau, W.; Zhang, X.; Massad, N.L.; Sargis, R.M. Tributyltin differentially promotes development of a phenotypically distinct adipocyte. Obesity 2015, 23, 1864–1871. [Google Scholar] [CrossRef]
- Janesick, A.S.; Dimastrogiovanni, G.; Vanek, L.; Boulos, C.; Chamorro-García, R.; Tang, W.; Blumberg, B. On the Utility of ToxCast™ and ToxPi as Methods for Identifying New Obesogens. Environ. Health Perspect. 2016, 124, 1214–1226. [Google Scholar] [CrossRef]
- Carchia, E.; Porreca, I.; Almeida, P.J.; D’Angelo, F.; Cuomo, D.; Ceccarelli, M.; De Felice, M.; Mallardo, M.; Ambrosino, C. Evaluation of low doses BPA-induced perturbation of glycemia by toxicogenomics points to a primary role of pancreatic islets and to the mechanism of toxicity. Cell Death Dis. 2015, 6, e1959. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-W.; Lan, K.-C.; Tsai, J.-R.; Weng, T.-I.; Yang, C.-Y.; Liu, S.-H. Tributyltin exposure at noncytotoxic doses dysregulates pancreatic β-cell function in vitro and in vivo. Arch. Toxicol. 2017, 91, 3135–3144. [Google Scholar] [CrossRef]
- Pavlikova, N.; Sramek, J.; Jelinek, M.; Halada, P.; Kovar, J. Markers of acute toxicity of DDT exposure in pancreatic beta-cells determined by a proteomic approach. PLoS ONE 2020, 15, e0229430. [Google Scholar] [CrossRef]
- NIH3T3. NIH3T3 General Information. Available online: https://www.nih3t3.com/ (accessed on 20 November 2022).
- Riu, A.; Grimaldi, M.; le Maire, A.; Bey, G.; Phillips, K.; Boulahtouf, A.; Perdu, E.; Zalko, D.; Bourguet, W.; Balaguer, P. Peroxisome proliferator-activated receptor γ is a target for halogenated analogs of bisphenol A. Environ. Health Perspect. 2011, 119, 1227–1232. [Google Scholar] [CrossRef] [PubMed]
- Klein, J.; Fasshauer, M.; Klein, H.H.; Benito, M.; Kahn, C.R. Novel adipocyte lines from brown fat: A model system for the study of differentiation, energy metabolism, and insulin action. Bioessays 2002, 24, 382–388. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Aliaga, M.J.; Matsumura, F. Effects of 1,1,1-trichloro-2,2-bis(p-chlorophenyl)-ethane (p,p′-DDT) on 3T3-L1 and 3T3-F442A adipocyte differentiation. Biochem. Pharmacol. 2002, 63, 997–1007. [Google Scholar] [CrossRef] [PubMed]
- Rylander, L.; Nilsson-Ehle, P.; Hagmar, L. A simplified precise method for adjusting serum levels of persistent organohalogen pollutants to total serum lipids. Chemosphere 2006, 62, 333–336. [Google Scholar] [CrossRef]
- Azzouz, A.; Hausler, R.; El-Akhrass, M. Pesticides and removal approaches. In Sorbents Materials for Controlling Environmental Pollution, Current State and Trends; Núñez-Delgado, A., Ed.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 435–462. [Google Scholar]
- DDT—A Brief History and Status. Available online: https://www.epa.gov/ingredients-used-pesticide-products/ddt-brief-history-and-status (accessed on 21 November 2022).
- Tawar, N.; Banerjee, B.D.; Mishra, B.K.; Sharma, T.; Tyagi, S.; Madhu, S.V.; Agarwal, V.; Gupta, S. Adipose Tissue Levels of DDT as Risk Factor for Obesity and Type 2 Diabetes Mellitus. Indian J. Endocrinol. Metab. 2021, 25, 160–165. [Google Scholar] [CrossRef]
- La Merrill, M.A.; Johnson, C.L.; Smith, M.T.; Kandula, N.R.; Macherone, A.; Pennell, K.D.; Kanaya, A.M. Exposure to Persistent Organic Pollutants (POPs) and Their Relationship to Hepatic Fat and Insulin Insensitivity among Asian Indian Immigrants in the United States. Environ. Sci. Technol. 2019, 53, 13906–13918. [Google Scholar] [CrossRef]
- Tyagi, S.; Mishra, B.K.; Sharma, T.; Tawar, N.; Urfi, A.J.; Banerjee, B.D.; Madhu, S.V. Level of Organochlorine Pesticide in Prediabetic and Newly Diagnosed Diabetes Mellitus Patients with Varying Degree of Glucose Intolerance and Insulin Resistance among North Indian Population. Diabetes Metab. J. 2021, 45, 558–568. [Google Scholar] [CrossRef]
- Bahmad, H.F.; Daouk, R.; Azar, J.; Sapudom, J.; Teo, J.C.M.; Abou-Kheir, W.; Al-Sayegh, M. Modeling Adipogenesis: Current and Future Perspective. Cells 2020, 9, 2326. [Google Scholar] [CrossRef]
- Lane, J.M.; Doyle, J.R.; Fortin, J.P.; Kopin, A.S.; Ordovás, J.M. Development of an OP9 derived cell line as a robust model to rapidly study adipocyte differentiation. PLoS ONE 2014, 9, e112123. [Google Scholar] [CrossRef]
- Andrews, F.V.; Kim, S.M.; Edwards, L.; Schlezinger, J.J. Identifying adipogenic chemicals: Disparate effects in 3T3-L1, OP9 and primary mesenchymal multipotent cell models. Toxicol. Vitr. 2020, 67, 104904. [Google Scholar] [CrossRef]
- Yajima, Y.; Sato, M.; Sumida, M.; Kawashima, S. Mechanism of adult primitive mesenchymal ST-13 preadipocyte differentiation. Endocrinology 2003, 144, 2559–2565. [Google Scholar] [CrossRef]
- Wabitsch, M.; Brenner, R.E.; Melzner, I.; Braun, M.; Möller, P.; Heinze, E.; Debatin, K.M.; Hauner, H. Characterization of a human preadipocyte cell strain with high capacity for adipose differentiation. Int. J. Obes. Relat. Metab. Disord. 2001, 25, 8–15. [Google Scholar] [CrossRef]
- Fischer-Posovszky, P.; Newell, F.S.; Wabitsch, M.; Tornqvist, H.E. Human SGBS cells—A unique tool for studies of human fat cell biology. Obes. Facts 2008, 1, 184–189. [Google Scholar] [CrossRef]
- Menale, C.; Piccolo, M.T.; Cirillo, G.; Calogero, R.A.; Papparella, A.; Mita, L.; Del Giudice, E.M.; Diano, N.; Crispi, S.; Mita, D.G. Bisphenol A effects on gene expression in adipocytes from children: Association with metabolic disorders. J. Mol. Endocrinol. 2015, 54, 289–303. [Google Scholar] [CrossRef]
- Primary Subcutaneous Pre-Adipocytes; Normal, Human PCS-210-010™. Available online: https://www.atcc.org/products/pcs-210-010 (accessed on 20 November 2022).
- Yeo, C.R.; Agrawal, M.; Hoon, S.; Shabbir, A.; Shrivastava, M.K.; Huang, S.; Khoo, C.M.; Chhay, V.; Yassin, M.S.; Tai, E.S.; et al. SGBS cells as a model of human adipocyte browning: A comprehensive comparative study with primary human white subcutaneous adipocytes. Sci. Rep. 2017, 7, 1. [Google Scholar] [CrossRef]
- Wassef, H.; Bernier, L.; Davignon, J.; Cohn, J.S. Synthesis and secretion of apoC-I and apoE during maturation of human SW872 liposarcoma cells. J. Nutr. 2004, 134, 2935–2941. [Google Scholar] [CrossRef][Green Version]
- Carmel, J.F.; Tarnus, E.; Cohn, J.S.; Bourdon, E.; Davignon, J.; Bernier, L. High expression of apolipoprotein E impairs lipid storage and promotes cell proliferation in human adipocytes. J. Cell. Biochem. 2009, 106, 608–617. [Google Scholar] [CrossRef] [PubMed]
- Campioli, E.; Batarseh, A.; Li, J.; Papadopoulos, V. The endocrine disruptor mono-(2-ethylhexyl) phthalate affects the differentiation of human liposarcoma cells (SW 872). PLoS ONE 2011, 6, e28750. [Google Scholar] [CrossRef]
- Hu, P.; Overby, H.; Heal, E.; Wang, S.; Chen, J.; Shen, C.-L.; Zhao, L. Methylparaben and butylparaben alter multipotent mesenchymal stem cell fates towards adipocyte lineage. Toxicol. Appl. Pharmacol. 2017, 329, 48–57. [Google Scholar] [CrossRef]
- Bateman, M.E.; Strong, A.L.; McLachlan, J.A.; Burow, M.E.; Bunnell, B.A. The Effects of Endocrine Disruptors on Adipogenesis and Osteogenesis in Mesenchymal Stem Cells: A Review. Front. Endocrinol. 2017, 7, 171. [Google Scholar] [CrossRef]
- Casteilla, L.; Planat-Benard, V.; Laharrague, P.; Cousin, B. Adipose-derived stromal cells: Their identity and uses in clinical trials, an update. World J. Stem Cells 2011, 3, 25–33. [Google Scholar] [CrossRef]
- Zhao, L.; Li, G.; Chan, K.-M.; Wang, Y.; Tang, P.-F. Comparison of multipotent differentiation potentials of murine primary bone marrow stromal cells and mesenchymal stem cell line C3H10T1/2. Calcif. Tissue Int. 2009, 84, 56–64. [Google Scholar] [CrossRef]
- Reznikoff, C.A.; Bertram, J.S.; Brankow, D.W.; Heidelberger, C. Quantitative and qualitative studies of chemical transformation of cloned C3H mouse embryo cells sensitive to postconfluence inhibition of cell division. Cancer Res. 1973, 33, 3239–3249. [Google Scholar]
- Lee, N.; Kim, I.; Park, S.; Han, D.; Ha, S.; Kwon, M. Creatine inhibits adipogenesis by downregulating insulin-induced activation of the phosphatidylinositol 3-kinase signaling pathway. Stem Cells Dev. 2015, 24, 983–994. [Google Scholar] [CrossRef]
- Beg, M.; Chauhan, P.; Varshney, S.; Shankar, K.; Rajan, S.; Saini, D. A withanolide coagulin-L inhibits adipogenesis modulating Wnt/β-catenin pathway and cell cycle in mitotic clonal expansion. Phytomedicine 2014, 21, 406–414. [Google Scholar] [CrossRef]
- Zhang, J.; Choudhury, M. Benzyl Butyl Phthalate Induced Early lncRNA H19 Regulation in C3H10T1/2 Stem Cell Line. Chem. Res. Toxicol. 2021, 34, 54–62. [Google Scholar] [CrossRef]
- Zhang, J.; Choudhury, M. The plasticizer BBP selectively inhibits epigenetic regulator sirtuin during differentiation of C3H10T1/2 stem cell line. Toxicol. Vitr. 2017, 39, 75–83. [Google Scholar] [CrossRef]
- Biemann, R.; Navarrete Santos, A.; Navarrete Santos, A.; Riemann, D.; Knelangen, J.; Blüher, M.; Koch, H.; Fischer, B. Endocrine disrupting chemicals affect the adipogenic differentiation of mesenchymal stem cells in distinct ontogenetic windows. Biochem. Biophys. Res. Commun. 2012, 417, 747–752. [Google Scholar] [CrossRef] [PubMed]
- Biemann, R.; Fischer, B.; Navarrete Santos, A. Adipogenic effects of a combination of the endocrine-disrupting compounds bisphenol A, diethylhexylphthalate, and tributyltin. Obes. Facts 2014, 7, 48–56. [Google Scholar] [CrossRef]
- Bukowska, J.; Szóstek-Mioduchowska, A.Z.; Kopcewicz, M.; Walendzik, K.; Machcińska, S.; Gawrońska-Kozak, B. Adipose-Derived Stromal/Stem Cells from Large Animal Models: From Basic to Applied Science. SCRR 2021, 17, 719–738. [Google Scholar] [CrossRef] [PubMed]
- Gigante, P.; Berni, M.; Bussolati, S.; Grasselli, F.; Grolli, S.; Ramoni, R.; Basini, G. Glyphosate affects swine ovarian and adipose stromal cell functions. Anim. Reprod. Sci. 2018, 195, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Berni, M.; Gigante, P.; Bussolati, S.; Grasselli, F.; Grolli, S.; Ramoni, R.; Basini, G. Bisphenol S, a Bisphenol A alternative, impairs swine ovarian and adipose cell functions. Domest. Anim. Endocrinol. 2019, 66, 48–56. [Google Scholar] [CrossRef]
- Dubois, S.G.; Floyd, E.Z.; Zvonic, S.; Kilroy, G.; Wu, X.; Carling, S.; Halvorsen, Y.D.; Ravussin, E.; Gimble, J.M. Isolation of human adipose-derived stem cells from biopsies and liposuction specimens. Methods Mol. Biol. 2008, 449, 69–79. [Google Scholar] [CrossRef]
- Human Adipose Derived Stem Cells (ADSCs, Type 1 Diabetes). Available online: https://www.ixcellsbiotech.com/human-primary-cells/human-adipose-derived-stem-cells-adscs-type-1-diabetes (accessed on 20 November 2022).
- Ejaz, A.; Hatzmann, F.M.; Hammerle, S.; Ritthammer, H.; Mattesich, M.; Zwierzina, M.; Waldegger, P.; Zwerschke, W. Fibroblast feeder layer supports adipogenic differentiation of human adipose stromal/progenitor cells. Adipocyte 2019, 8, 178–189. [Google Scholar] [CrossRef]
- Valentino, R.; D’Esposito, V.; Passaretti, F.; Liotti, A.; Cabaro, S.; Longo, M.; Perruolo, G.; Oriente, F.; Beguinot, F.; Formisano, P. Bisphenol-A impairs insulin action and up-regulates inflammatory pathways in human subcutaneous adipocytes and 3T3-L1 cells. PLoS ONE 2013, 8, e82099. [Google Scholar] [CrossRef]
- Ohlstein, J.F.; Strong, A.L.; McLachlan, J.A.; Gimble, J.M.; Burow, M.E.; Bunnell, B.A. Bisphenol A enhances adipogenic differentiation of human adipose stromal/stem cells. J. Mol. Endocrinol. 2014, 53, 345–353. [Google Scholar] [CrossRef]
- Markussen, L.K.; Isidor, M.S.; Breining, P.; Andersen, E.S.; Rasmussen, N.E.; Petersen, L.I.; Pedersen, S.B.; Richelsen, B.; Hansen, J.B. Characterization of immortalized human brown and white pre-adipocyte cell models from a single donor. PLoS ONE 2017, 12, e0185624. [Google Scholar] [CrossRef]
- Wabitsch, M.; Brüderlein, S.; Melzner, I.; Braun, M.; Mechtersheimer, G.; Möller, P. LiSa-2, a novel human liposarcoma cell line with a high capacity for terminal adipose differentiation. Int. J. Cancer 2000, 88, 889–894. [Google Scholar] [CrossRef] [PubMed]
- Hugo, E.R.; Brandebourg, T.D.; Comstock, C.E.; Gersin, K.S.; Sussman, J.J.; Ben-Jonathan, N. LS14: A novel human adipocyte cell line that produces prolactin. Endocrinology 2006, 147, 306–313. [Google Scholar] [CrossRef] [PubMed]
- Darimont, C.; Zbinden, I.; Avanti, O.; Leone-Vautravers, P.; Giusti, V.; Burckhardt, P.; Pfeifer, A.M.; Macé, K. Reconstitution of telomerase activity combined with HPV-E7 expression allow human preadipocytes to preserve their differentiation capacity after immortalization. Cell Death Differ. 2003, 10, 1025–1031. [Google Scholar] [CrossRef] [PubMed]
- Zilberfarb, V.; Piétri-Rouxel, F.; Jockers, R.; Krief, S.; Delouis, C.; Issad, T.; Strosberg, A.D. Human immortalized brown adipocytes express functional beta3-adrenoceptor coupled to lipolysis. J. Cell Sci. 1997, 110 Pt 7, 801–807. [Google Scholar] [CrossRef]
- Dufau, J.; Shen, J.X.; Couchet, M.; De Castro Barbosa, T.; Mejhert, N.; Massier, L.; Griseti, E.; Mouisel, E.; Amri, E.Z.; Lauschke, V.M.; et al. In vitro and ex vivo models of adipocytes. Am. J. Physiol. Cell Physiol. 2021, 320, C822–C841. [Google Scholar] [CrossRef]
- Alam, M.T.; Ott, S.; Kumar, S.; Saravanan, P. Low vitamin b12 in pregnancy is associated with adipose-derived circulating miRs targeting PPARgamma and insulin resistance. J. Clin. Endocrinol. Metab. 2017, 102, 4200–4209. [Google Scholar] [CrossRef]
- Jackisch, L.; Murphy, A.M.; Kumar, S.; Randeva, H.; Tripathi, G.; McTernan, P.G. Tunicamycin-Induced Endoplasmic Reticulum Stress Mediates Mitochondrial Dysfunction in Human Adipocytes. J. Clin. Endocrinol. Metab. 2020, 105, dgaa258. [Google Scholar] [CrossRef]
- Pan, S.; Cui, Y.; Fu, Z.; Zhang, L.; Xing, H. MicroRNA-128 is involved in dexamethasone-induced lipid accumulation via repressing SIRT1 expression in cultured pig preadipocytes. J. Steroid Biochem. Mol. Biol. 2018, 186, 185–195. [Google Scholar] [CrossRef]
- Riedel, J.; Badewien-Rentzsch, B.; Kohn, B.; Hoeke, L.; Einspanier, R. Characterization of key genes of the renin–angiotensin system in mature feline adipocytes and during in vitro adipogenesis. J. Anim. Physiol. Anim. Nutr. 2016, 100, 1139–1148. [Google Scholar] [CrossRef]
- Pu, Y.; Veiga-Lopez, A. PPARγ agonist through the terminal differentiation phase is essential for adipogenic differentiation of fetal ovine preadipocytes. Cell. Mol. Biol. Lett. 2017, 22, 1. [Google Scholar] [CrossRef]
- Jetter, A.; Kullak-Ublick, G.A. Drugs and hepatic transporters: A review. Pharmacol. Res. 2020, 154, 104234. [Google Scholar] [CrossRef] [PubMed]
- Cano, R.; Pérez, J.L.; Dávila, L.A.; Dávila, L.A.; Ortega, Á.; Gómez, Y.; Valero-Cedeño, N.J.; Parra, H.; Manzano, A.; Véliz Castro, T.I.; et al. Role of Endocrine-Disrupting Chemicals in the Pathogenesis of Non-Alcoholic Fatty Liver Disease: A Comprehensive Review. Int. J. Mol. Sci. 2021, 22, 4807. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Jan, J.; Hardesty, J.E.; Falkner, K.C.; Prough, R.A.; Balamurugan, A.N.; Mokshagundam, S.P.; Chari, S.T.; Cave, M.C. Polychlorinated biphenyl exposures differentially regulate hepatic metabolism and pancreatic function: Implications for nonalcoholic steatohepatitis and diabetes. Toxicol. Appl. Pharmacol. 2019, 363, 22–33. [Google Scholar] [CrossRef] [PubMed]
- Neel, B.A.; Brady, M.J.; Sargis, R.M. The Endocrine Disrupting Chemical Tolylfluanid Alters Adipocyte Metabolism via Glucocorticoid Receptor Activation. Mol. Endocrinol. 2013, 27, 394–406. [Google Scholar] [CrossRef]
- Bucher, S.; Jalili, P.; Le Guillou, D.; Begriche, K.; Rondel, K.; Martinais, S.; Zalko, D.; Corlu, A.; Robin, M.-A.; Fromenty, B. Bisphenol A induces steatosis in HepaRG cells using a model of perinatal exposure. Environ. Toxicol. 2017, 32, 1024–1036. [Google Scholar] [CrossRef]
- Yang, L.; Guo, X.; Mao, X.; Jia, X.; Zhou, Y.; Hu, Y.; Sun, L.; Guo, J.; Xiao, H.; Zhang, Z. Hepatic toxicity of fluorene-9-bisphenol (BHPF) on CD-1 mice. Ecotoxicol. Environ. Saf. 2021, 219, 112298. [Google Scholar] [CrossRef]
- Eweda, S.M.; Newairy, A.S.A.; Abdou, H.M.; Gaber, A.S. Bisphenol A-induced oxidative damage in the hepatic and cardiac tissues of rats: The modulatory role of sesame lignans. Exp. Ther. Med. 2020, 19, 33–44. [Google Scholar] [CrossRef]
- Meng, Z.; Wang, D.; Liu, W.; Li, R.; Yan, S.; Jia, M.; Zhang, L.; Zhou, Z.; Zhu, W. Perinatal exposure to Bisphenol S (BPS) promotes obesity development by interfering with lipid and glucose metabolism in male mouse offspring. Environ. Res. 2019, 173, 189–198. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, X.; Zhou, Y.; Zhang, J.; Cui, W.; Wang, E.; Du, J.; Wei, B.; Xu, X. Protective effect of metformin on BPA-induced liver toxicity in rats through upregulation of cystathionine β synthase and cystathionine γ lyase expression. Sci. Total Environ. 2021, 750, 141685. [Google Scholar] [CrossRef]
- Cocci, P.; Mosconi, G.; Arukwe, A.; Mozzicafreddo, M.; Angeletti, M.; Aretusi, G.; Palermo, F.A. Effects of Diisodecyl Phthalate on PPAR:RXR-Dependent Gene Expression Pathways in Sea Bream Hepatocytes. Chem. Res. Toxicol. 2015, 28, 935–947. [Google Scholar] [CrossRef]
- Olsvik, P.A.; Søfteland, L. Metabolic effects of p,p′-DDE on Atlantic salmon hepatocytes. J. Appl. Toxicol. 2018, 38, 489–503. [Google Scholar] [CrossRef]
- Grasselli, E.; Cortese, K.; Voci, A.; Vergani, L.; Fabbri, R.; Barmo, C.; Gallo, G.; Canesi, L. Direct effects of Bisphenol A on lipid homeostasis in rat hepatoma cells. Chemosphere 2013, 91, 1123–1129. [Google Scholar] [CrossRef] [PubMed]
- Boess, F.; Kamber, M.; Romer, S.; Gasser, R.; Muller, D.; Albertini, S.; Suter, L. Gene expression in two hepatic cell lines, cultured primary hepatocytes, and liver slices compared to the in vivo liver gene expression in rats: Possible implications for toxicogenomics use of in vitro systems. Toxicol. Sci. 2003, 73, 386–402. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, S.; Zhao, T.; Yang, L.; Guo, S.; Shi, Y.; Zhang, X.; Zhou, L.; Ye, L. Mono-2-ethylhexyl phthalate (MEHP) promoted lipid accumulation via JAK2/STAT5 and aggravated oxidative stress in BRL-3A cells. Ecotoxicol. Environ. Saf. 2019, 184, 109611. [Google Scholar] [CrossRef] [PubMed]
- Sefried, S.; Häring, H.U.; Weigert, C.; Eckstein, S.S. Suitability of hepatocyte cell lines HepG2, AML12 and THLE-2 for investigation of insulin signalling and hepatokine gene expression. Open Biol. 2018, 8, 180147. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Yu, W.; Meng, F.; Mi, J.; Peng, J.; Liu, J.; Zhang, X.; Hai, C.; Wang, X. Polychlorinated biphenyls-153 induces metabolic dysfunction through activation of ROS/NF-κB signaling via downregulation of HNF1b. Redox Biol. 2017, 12, 300–310. [Google Scholar] [CrossRef]
- Le, Y.; Shen, H.; Yang, Z.; Lu, D.; Wang, C. Comprehensive analysis of organophosphorus flame retardant-induced mitochondrial abnormalities: Potential role in lipid accumulation. Environ. Pollut. 2021, 274, 116541. [Google Scholar] [CrossRef]
- Hepa 1-6: A Murine Model of Hepatocellular Carcinoma. Available online: https://drugdevelopment.labcorp.com/industry-solutions/oncology/preclinical/tumor-spotlights/hepa-1-6-a-murine-model-of-hepatocellular-carcinoma.html (accessed on 20 November 2022).
- Ke, Z.-H.; Pan, J.-X.; Jin, L.-Y.; Xu, H.-Y.; Yu, T.-T.; Ullah, K.; Rahman, T.U.; Ren, J.; Cheng, Y.; Dong, X.-Y.; et al. Bisphenol A Exposure May Induce Hepatic Lipid Accumulation via Reprogramming the DNA Methylation Patterns of Genes Involved in Lipid Metabolism. Sci. Rep. 2016, 6, 31331. [Google Scholar] [CrossRef]
- Breslow, J.L.; Sloan, H.R.; Ferrans, V.J.; Anderson, J.L.; Levy, R.I. Characterization of the mouse liver cell line FL83B. Exp. Cell Res. 1973, 78, 441–453. [Google Scholar] [CrossRef]
- Liu, X.H.; Pan, J.P.; Bauman, W.A.; Cardozo, C.P. AdipoRon prevents myostatin-induced upregulation of fatty acid synthesis and downregulation of insulin activity in a mouse hepatocyte line. Physiol. Rep. 2019, 7, e14152. [Google Scholar] [CrossRef]
- ATCC. FL83B CRL-2390TM. Available online: https://www.atcc.org/products/crl-2390 (accessed on 20 November 2022).
- Chang, Y.-H.; Chen, Y.-L.; Huang, W.-C.; Liou, C.-J. Fucoxanthin attenuates fatty acid-induced lipid accumulation in FL83B hepatocytes through regulated Sirt1/AMPK signaling pathway. Biochem. Biophys. Res. Commun. 2018, 495, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.W.; Chen, T.Y.; Yang, P.M. Differential effect of herbal tea extracts on free fatty acids-, ethanol- and acetaminophen-induced hepatotoxicity in FL83B hepatocytes. Drug Chem. Toxicol. 2022, 45, 347–352. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Ji, K.; Wang, Y.; Li, Y.; Tang, Y.; Gu, J.; Cai, L. Exposure to low dose cadmium enhances FL83B cells proliferation through down-regulation of caspase-8 by DNA hypermethylation. Toxicol. Res. 2015, 4, 248–259. [Google Scholar] [CrossRef]
- Lo, D.; Wang, Y.T.; Wu, M.C. Hepatoprotective effect of silymarin on di(2-ethylhexyl)phthalate (DEHP) induced injury in liver FL83B cells. Environ. Toxicol. Pharmacol. 2014, 38, 112–118. [Google Scholar] [CrossRef]
- Dimastrogiovanni, G.; Córdoba, M.; Navarro, I.; Jáuregui, O.; Porte, C. Alteration of cellular lipids and lipid metabolism markers in RTL-W1 cells exposed to model endocrine disrupters. Aquat. Toxicol. 2015, 165, 277–285. [Google Scholar] [CrossRef]
- Malhão, F.; Urbatzka, R.; Navas, J.M.; Cruzeiro, C.; Monteiro, R.A.; Rocha, E. Cytological, immunocytochemical, ultrastructural and growth characterization of the rainbow trout liver cell line RTL-W1. Tissue Cell 2013, 45, 159–174. [Google Scholar] [CrossRef] [PubMed]
- Marqueño, A.; Flores, C.; Casado, M.; Porte, C. Dysregulation of lipid metabolism in PLHC-1 and ZFL cells exposed to tributyltin an all-trans retinoic acid. Aquat. Toxicol. 2021, 231, 105733. [Google Scholar] [CrossRef]
- Fernandes, D.; Pujol, S.; Pérez-Albaladejo, E.; Tauler, R.; Bebianno, M.J.; Porte, C. Characterization of the environmental quality of sediments from two estuarine systems based on different in-vitro bioassays. Mar. Environ. Res. 2014, 96, 127–135. [Google Scholar] [CrossRef]
- Marqueño, A.; Pérez-Albaladejo, E.; Denslow, N.D.; Bowden, J.A.; Porte, C. Untargeted lipidomics reveals the toxicity of bisphenol A bis(3-chloro-2- hydroxypropyl) ether and bisphenols A and F in zebrafish liver cells. Ecotoxicol. Environ. Saf. 2021, 219, 112311. [Google Scholar] [CrossRef]
- Pérez-Albaladejo, E.; Solís, A.; Bani, I.; Porte, C. PLHC-1 topminnow liver cells: An alternative model to investigate the toxicity of plastic additives in the aquatic environment. Ecotoxicol. Environ. Saf. 2021, 208, 111746. [Google Scholar] [CrossRef]
- Gomez-Lechon, M.; Donato, M.; Castell, J.; Jover, R. Human Hepatocytes as a Tool for Studying Toxicity and Drug Metabolism. Curr. Drug Metab. 2003, 4, 292–312. [Google Scholar] [CrossRef]
- Clayton, R.F.; Rinaldi, A.; Kandyba, E.E.; Edward, M.; Willberg, C.; Klenerman, P.; Patel, A.H. Liver cell lines for the study of hepatocyte functions and immunological response. Liver Int. 2005, 25, 389–402. [Google Scholar] [CrossRef]
- Tolosa, L.; Gómez-Lechón, M.J.; López, S.; Guzmán, C.; Castell, J.V.; Donato, M.T.; Jover, R. Human Upcyte Hepatocytes: Characterization of the Hepatic Phenotype and Evaluation for Acute and Long-Term Hepatotoxicity Routine Testing. Toxicol. Sci. 2016, 152, 214–229. [Google Scholar] [CrossRef]
- Baquerizo, A.; Bañares, R.; Saliba, F. Current Clinical Status of the Extracorporeal Liver Support Devices. Transplant. Liver 2015, 107, 1463–1487. [Google Scholar] [CrossRef]
- HepG2 Cell Line. Available online: https://encyclopedia.pub/entry/17273 (accessed on 20 November 2022).
- Kammerer, S.; Küpper, J.-H. Human hepatocyte systems for in vitro toxicology analysis. J. Cell. Biotechnol. 2018, 3, 85–93. [Google Scholar] [CrossRef]
- Arzumanian, V.A.; Kiseleva, O.I.; Poverennaya, E.V. The Curious Case of the HepG2 Cell Line: 40 Years of Expertise. Int. J. Mol. Sci. 2021, 22, 13135. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Fang, B.; Zheng, Y.; Yu, X.; Huang, G.; Wang, Z.; Deng, X.; Guan, S. 1,3-dichloro-2-propanol induced lipid accumulation in HepG2 cells through cAMP/protein kinase A and AMP-activated protein kinase pathways via Gi/o-coupled receptors. Environ. Toxicol. Pharmacol. 2017, 55, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Maia, M.L.; Sousa, S.; Pestana, D.; Faria, A.; Teixeira, D.; Delerue-Matos, C.; Domingues, V.F.; Calhau, C. Impact of brominated flame retardants on lipid metabolism: An in vitro approach. Environ. Pollut. 2022, 294, 118639. [Google Scholar] [CrossRef]
- ThermoFisher Scientific. HepaRG™ Cells, Cryopreserved. Available online: https://www.thermofisher.com/order/catalog/product/HPRGC10 (accessed on 20 November 2022).
- Stossi, F.; Dandekar, R.D.; Johnson, H.; Lavere, P.; Foulds, C.E.; Mancini, M.G.; Mancini, M.A. Tributyltin chloride (TBT) induces RXRA down-regulation and lipid accumulation in human liver cells. PLoS ONE 2019, 14, e0224405. [Google Scholar] [CrossRef] [PubMed]
- Differentiated HepaRG Cells—HPR116. Available online: https://www.heparg.com/rubrique-differentiated-heparg-cells-hpr116 (accessed on 20 November 2022).
- Huh-7 Cell Line Origins and Characteristics. Available online: https://huh7.com/ (accessed on 20 November 2022).
- Wada, K.; Sakamoto, H.; Nishikawa, K.; Sakuma, S.; Nakajima, A.; Fujimoto, Y.; Kamisaki, Y. Life style-related diseases of the digestive system: Endocrine disruptors stimulate lipid accumulation in target cells related to metabolic syndrome. J. Pharmacol. Sci. 2007, 105, 133–137. [Google Scholar] [CrossRef]
- Lee, J.-L.; Wang, Y.-C.; Hsu, Y.-A.; Chen, C.-S.; Weng, R.-C.; Lu, Y.-P.; Chuang, C.-Y.; Wan, L. Bisphenol A Coupled with a High-Fat Diet Promotes Hepatosteatosis through Reactive-Oxygen-Species-Induced CD36 Overexpression. Toxics 2022, 10, 208. [Google Scholar] [CrossRef]
- Lorenzetti, S.; Marcoccia, D.; Mantovani, A. Biomarkers of effect in endocrine disruption: How to link a functional assay to an adverse outcome pathway. Ann. Ist. Super. Sanita 2015, 51, 167–171. [Google Scholar] [CrossRef]
- La Rocca, C.; Tait, S.; Mantovani, A. Use of a combinedin vitroassay for effect-directed assessment of infant formulas. Int. J. Food Sci. 2014, 50, 77–83. [Google Scholar] [CrossRef]
- Štampar, M.; Breznik, B.; Filipič, M.; Žegura, B. Characterization of In vitro 3D Cell Model Developed from Human Hepatocellular Carcinoma (HepG2) Cell Line. Cells 2020, 9, 2557. [Google Scholar] [CrossRef] [PubMed]
- Martella, A.; Silvestri, C.; Maradonna, F.; Gioacchini, G.; Allarà, M.; Radaelli, G.; Overby, D.R.; Di Marzo, V.; Carnevali, O. Bisphenol A Induces Fatty Liver by an Endocannabinoid-Mediated Positive Feedback Loop. Endocrinology 2016, 157, 1751–1763. [Google Scholar] [CrossRef] [PubMed]
- Mardonna, F.; Carnevali, O. Lipid Metabolism Alteration by Endocrine Disruptors in Animal Models: An Overview. Front. Endocrinol. 2018, 9, 654. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Xu, G.; Gao, Q.; Tao, X. LKB1 expression reverses the tumorigenicity of L02 cells. Oncol. Rep. 2016, 36, 1055–1061. [Google Scholar] [CrossRef][Green Version]
- Zhang, H.; Shao, X.; Zhao, H.; Li, X.; Wei, J.; Yang, C.; Cai, Z. Integration of Metabolomics and Lipidomics Reveals Metabolic Mechanisms of Triclosan-Induced Toxicity in Human Hepatocytes. Environ. Sci. Technol. 2019, 53, 5406–5415. [Google Scholar] [CrossRef]
- Alonso-Magdalena, P.; Laribi, O.; Ropero, A.B.; Fuentes, E.; Ripoll, C.; Soria, B.; Nadal, A. Low doses of bisphenol A and diethylstilbestrol impair Ca2+ signals in pancreatic alpha-cells through a nonclassical membrane estrogen receptor within intact islets of Langerhans. Environ. Health Perspect. 2005, 113, 969–977. [Google Scholar] [CrossRef]
- Stojanoska, M.M.; Milosevic, N.; Milic, N.; Abenavoli, L. The influence of phthalates and bisphenol A on the obesity development and glucose metabolism disorders. Endocrine 2017, 55, 666–681. [Google Scholar] [CrossRef]
- Adachi, T.; Yasuda, K.; Mori, C.; Yoshinaga, M.; Aoki, N.; Tsujimoto, G.; Tsuda, K. Promoting insulin secretion in pancreatic islets by means of bisphenol A and nonylphenol via intracellular estrogen receptors. Food Chem. Toxicol. 2005, 43, 713–719. [Google Scholar] [CrossRef] [PubMed]
- Ghaemmaleki, F.; Mohammadi, P.; Baeeri, M.; Navaei-Nigjeh, M.; Abdollahi, M.; Mostafalou, S. Estrogens counteract tributyltin-induced toxicity in the rat islets of Langerhans. Heliyon 2020, 6, e03562. [Google Scholar] [CrossRef] [PubMed]
- Acosta-Montalvo, A.; Saponaro, C.; Kerr-Conte, J.; Prehn, J.H.M.; Pattou, F.; Bonner, C. Proglucagon-Derived Peptides Expression and Secretion in Rat Insulinoma INS-1 Cells. Front. Cell Dev. Biol. 2020, 8, 590763. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Sun, X.; Qiu, L.; Wei, J.; Huang, Q.; Fang, C.; Ye, T.; Kang, M.; Shen, H.; Dong, S. Exposure to bisphenol A induces dysfunction of insulin secretion and apoptosis through the damage of mitochondria in rat insulinoma (INS-1) cells. Cell Death Dis. 2013, 4, e460. [Google Scholar] [CrossRef]
- Pavlíková, N.; Daniel, P.; Šrámek, J.; Jelínek, M.; Šrámková, V.; Němcová, V.; Balušíková, K.; Halada, P.; Kovář, J. Upregulation of vitamin D-binding protein is associated with changes in insulin production in pancreatic beta-cells exposed to p,p′-DDT and p,p′-DDE. Sci. Rep. 2019, 9, 18026. [Google Scholar] [CrossRef]
- Huang, C.-F.; Yang, C.-Y.; Tsai, J.-R.; Wu, C.-T.; Liu, S.-H.; Lan, K.-C. Low-dose tributyltin exposure induces an oxidative stress-triggered JNK-related pancreatic β-cell apoptosis and a reversible hypoinsulinemic hyperglycemia in mice. Sci. Rep. 2018, 8, 5734. [Google Scholar] [CrossRef]
- Suh, K.S.; Choi, E.M.; Kim, Y.J.; Hong, S.M.; Park, S.Y.; Rhee, S.Y.; Oh, S.; Kim, S.W.; Pak, Y.K.; Choe, W.; et al. Perfluorooctanoic acid induces oxidative damage and mitochondrial dysfunction in pancreatic β-cells. Mol. Med. Rep. 2017, 15, 3871–3878. [Google Scholar] [CrossRef]
- Soriano, S.; Alonso-Magdalena, P.; García-Arévalo, M.; Novials, A.; Muhammed, S.J.; Salehi, A.; Gustafsson, J.A.; Quesada, I.; Nadal, A. Rapid insulinotropic action of low doses of bisphenol-A on mouse and human islets of Langerhans: Role of estrogen receptor β. PLoS ONE 2012, 7, e31109. [Google Scholar] [CrossRef]
- Marroqui, L.; Martinez-Pinna, J.; Castellano-Muñoz, M.; Dos Santos, R.S.; Medina-Gali, R.M.; Soriano, S.; Quesada, I.; Gustafsson, J.-A.; Encinar, J.A.; Nadal, A. Bisphenol-S and Bisphenol-F alter mouse pancreatic β-cell ion channel expression and activity and insulin release through an estrogen receptor ERβ mediated pathway. Chemosphere 2021, 265, 129051. [Google Scholar] [CrossRef]
- Nakashima, K.; Kanda, Y.; Hirokawa, Y.; Kawasaki, F.; Matsuki, M.; Kaku, K. MIN6 is not a pure beta cell line but a mixed cell line with other pancreatic endocrine hormones. Endocr. J. 2009, 56, 45–53. [Google Scholar] [CrossRef]
- Yamato, E.; Tashiro, F.; Miyazaki, J. Microarray analysis of novel candidate genes responsible for glucose-stimulated insulin secretion in mouse pancreatic β cell line MIN6. PLoS ONE 2013, 8, e61211. [Google Scholar] [CrossRef]
- Skelin, M.; Rupnik, M.; Cencic, A. Pancreatic beta cell lines and their applications in diabetes mellitus research. ALTEX 2010, 27, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, S.; Tashiro, F.; Tsuchiya, T.; Sasaki, K.; Miyazaki, J.I. Establishment of a long-term stable β-cell line and its application to analyze the effect of Gcg expression on insulin secretion. Sci. Rep. 2021, 11, 477. [Google Scholar] [CrossRef] [PubMed]
- Al-Abdulla, R.; Ferrero, H.; Soriano, S.; Boronat-Belda, T.; Alonso-Magdalena, P. Screening of Relevant Metabolism-Disrupting Chemicals on Pancreatic β-Cells: Evaluation of Murine and Human In vitro Models. Int. J. Mol. Sci. 2022, 23, 4182. [Google Scholar] [CrossRef] [PubMed]
- ATCC. Beta-TC-6. Available online: https://www.atcc.org/products/crl-11506 (accessed on 12 January 2022).
- Qin, W.-P.; Cao, L.-Y.; Li, C.-H.; Guo, L.-H.; Colbourne, J.; Ren, X.-M. Perfluoroalkyl Substances Stimulate Insulin Secretion by Islet β Cells via G Protein-Coupled Receptor 40. Environ. Sci. Technol. 2020, 54, 3428–3436. [Google Scholar] [CrossRef]
- Ward, A.B.; Dail, M.B.; Chambers, J.E. In vitro effect of DDE exposure on the regulation of B-TC-6 pancreatic beta cell insulin secretion: A potential role in beta cell dysfunction and type 2 diabetes mellitus. Toxicol. Mech. Methods 2021, 31, 667–673. [Google Scholar] [CrossRef] [PubMed]
- Chandiramani, N.; Wang, X.; Margeta, M. Molecular basis for vulnerability to mitochondrial and oxidative stress in a neuroendocrine CRI-G1 cell line. PLoS ONE 2011, 6, e14485. [Google Scholar] [CrossRef]
- Pavlikova, N.; Smetana, P.; Halada, P.; Kovar, J. Effect of prolonged exposure to sublethal concentrations of DDT and DDE on protein expression in human pancreatic beta cells. Environ. Res. 2015, 142, 257–263. [Google Scholar] [CrossRef]
- Lieber, M.; Mazzetta, J.; Nelson-Rees, W.; Kaplan, M.; Todaro, G. Establishment of a continuous tumor-cell line (panc-1) from a human carcinoma of the exocrine pancreas. Int. J. Cancer 1975, 15, 741–747. [Google Scholar] [CrossRef]
- Hamil, L.; Benghuzzi, H.; Tucci, M. Evaluation of insulin secretion by pancreatic cells in response to increasing amounts of glucose. Biomed. Sci. Instrum. 2008, 44, 441–446. [Google Scholar]

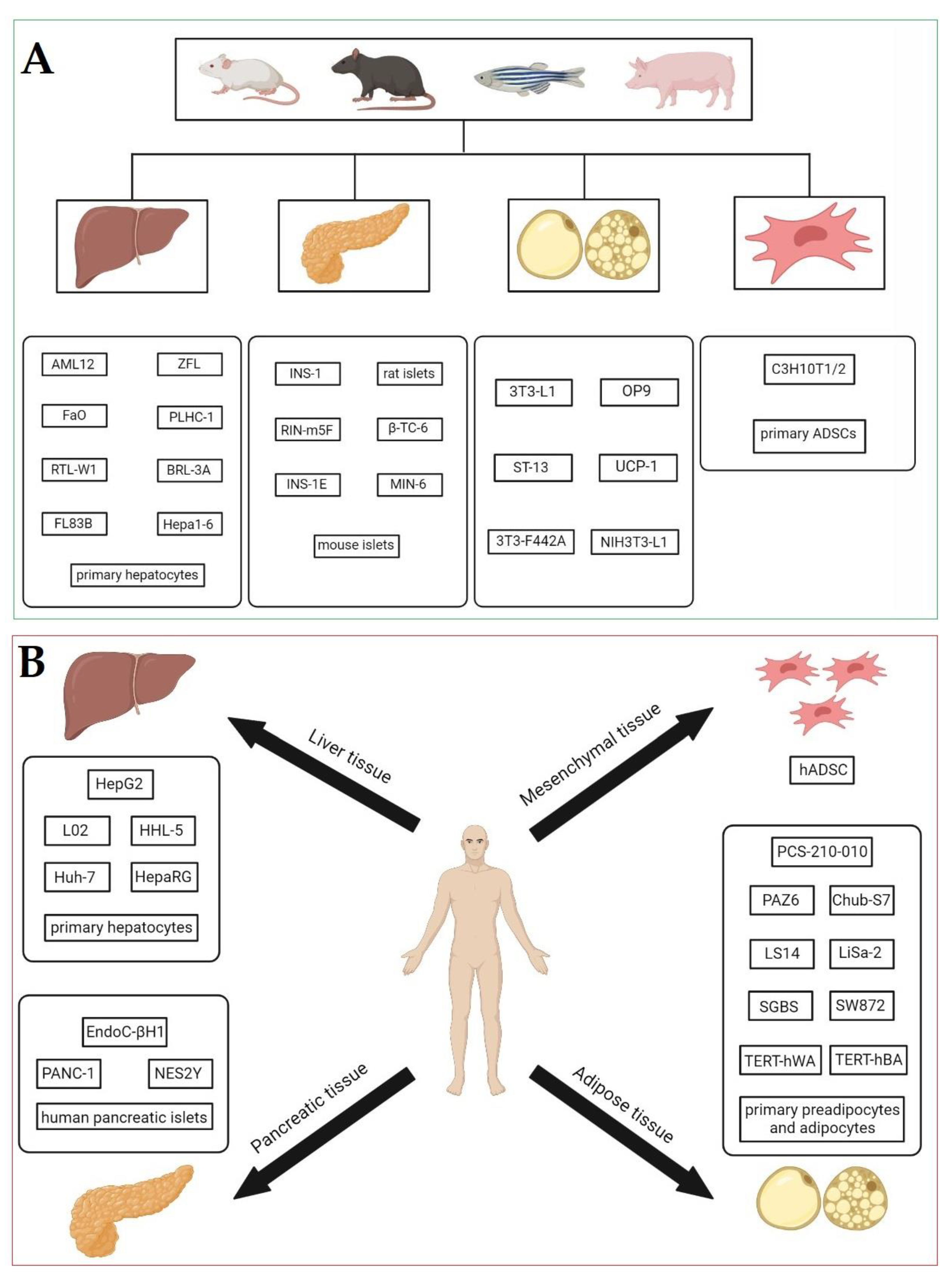
| Cell Type | Organism | In vitro Model | EDCs | Mechanism of Action | Concentration * | References |
|---|---|---|---|---|---|---|
| Preadipocytes | Animal | 3T3-L1 cell line | 4-HP | adipogenic differentiation ↑ intracellular lipid accumulation ↑ (10 µM, 20 µM) mRNA level of PPARγ, FABP4, CD36, perilipin, adiponectin ↑ (10 µM, 20 µM); C/EBPα (−) (10 µM, 20 µM) | 10 µM, 20 µM | Sun et al. [93] |
| BPA | lipid accumulation ↑ mRNA and protein levels of PPARγ, C/EBPα, and AP2 ↑ | 20 µM | Choi et al. [7] | |||
| adipogenic differentiation (–) (1 nM, 10 nM), ↓ (100 nM) mRNA level of PPARγ, FABP4, FASN (–) (1 nM, 10 nM), ↓ (100 nM); TNFα, IL6 ↑ (1 nM, 3 nM) insulin-stimulated glucose uptake ↓ (1 nM) | 1 nM, 3 nM, 10 nM, 100 nM | De Filippis et al. [96] | ||||
| adipogenic differentiation ↑ lipid accumulation ↑ (100 nM) glucocorticoid-like activity ↑ (1 µM) PPARγ activity ↑ (however, less activation of PPARγ than GR) (1 µM) protein expression of IR-β (insulin receptor subunit β) ↑; C/EBPα ↑ (1 µM–100 pM), adiponectin ↑ (10 nM, 100 nM) | 100 pM, 1 nM, 10 nM, 100 nM, 1 µM | Sargis et al. [97] | ||||
| BPS | lipid accumulation ↑ mRNA and protein levels of PPARγ, C/EBPα, and AP2 ↑ | 20 µM | Choi et al. [7] | |||
| BPF | lipid accumulation ↑ mRNA and protein levels of PPARγ, C/EBPα, and AP2 ↑ | 20 µM | Choi et al. [7] | |||
| TBBPA | Adipogenesis ↑ lipid accumulation ↑ (20 µM) PPARγ activity ↑ (10, 20 µM) | 20 µM | Andrews et al. [120] | |||
| BBP | adipogenic differention ↑ lipid accumulation ↑ (1 µM, 10 µM, 50 µM), (–) (0.001 µM, 0.01 µM, 0.1 µM) miR-34a-5p expression level ↑ (1 µM, 10 µM, 50 µM), (−) (0.01 µM, 0.1 µM) mRNA level of PPARγ ↑ (10 µM, 50 µM), (–) (0.01 µM, 0.1 µM, 1 µM); AP2 ↑ (50 µM), (–) (0.01 µM, 0.1 µM, 1 µM); NAMPT, SIRT1, SIRT3 ↓ (0.01 µM, 0.1 µM, 1 µM, 10 µM, 50 µM) protein level of NAMPT ↓, SIRT1, SIRT3 (–) (0.1 µM, 50 µM) NAD/NADH ratio ↓ (0.1 µM, 50 µM) | 0.01 µM, 0.1 µM, 1 µM, 10 µM, 50 µM | Meruvu et al. [98] | |||
| DCHP | adipogenic differentiation ↑ lipid accumulation ↑ (100 nM) glucocorticoid-like activity ↑ (1 µM) PPARγ activity ↑ (however, less activation of PPARγ than GR) (1 µM) protein expression of IR-β ↑, C/EBPα ↑ (1 µM–100 pM), adiponectin ↑ (1 µM–100 pM) | 100 pM, 1 nM, 10 nM, 100 nM, 1 µM | Sargis et al. [97] | |||
| endrin | adipogenic differentiation ↑ lipid accumulation ↑ (100 nM) glucocorticoid-like activity ↑ (1 µM) PPARγ activity ↑ (however, less activation of PPARγ than GR) (1 µM) protein expression of IR-β ↑, C/EBPα ↑ (1 µM–100 pM), adiponectin ↑ (100 nM–100 pM) | 100 pM, 1 nM, 10 nM, 100 nM, 1 µM | Sargis et al. [97] | |||
| TF | adipogenic adifferentiation ↑ lipid accumulation ↑ (100 nM) glucocorticoid-like activity ↑ (1 µM) PPARγ activity ↑ (however, less activation of PPARγ than GR) (1 µM) protein expression of IR-β ↑, C/EBPα ↑ (1 µM–100 pM), adiponectin ↑ (1 µM–100 pM) | 100 pM, 1 nM, 10 nM, 100 nM, 1 µM | Sargis et al. [97] | |||
| QpE | adipogenesis ↑ lipid accumulation ↑ (5–100 µM) PPARγ activity ↑ (100 µM) | 5–100 µM | Biserni et al. [99] | |||
| p,p’-DDT | adipogenesis ↑ lipid accumulation ↑ (20 µM) mRNA level of C/EBPα, PPARγ, FAS, ACC, ATGL, HSL, LEP ↑ (10 µM, 20 µM) C/EBPα, PPARγ, AMPKα, ACC protein expression ↑ (10 µM, 20 µM) phosphorylated forms of AMPKα, ACC protein expression ↓ (10 µM, 20 µM) | 10 µM, 20 µM | Kim et al [100] | |||
| adipogenesis ↑ lipid accumulation ↑ (10 µM, 20 µM, 30 µM, 50 µM) protein level of C/EBPβ (–), C/EBPα ↑ (20 µM); PPARγ1, PPARγ2 ↑ (10 µM, 20 µM, 30 µM) | 10 µM, 20 µM, 30 µM, 50 µM | Moreno-Aliaga and Matsumura [111] | ||||
| p,p’-DDE | adipogenesis ↑ lipid accumulation ↑ (10 µM, 20 µM) mRNA level of C/EBPα, PPARγ, FAS, ACC, ATGL, HSL, LEP (10 µM, 20 µM), Lpl (20 µM) ↑ C/EBPα, PPARγ, AMPKα, Acc protein expression ↑ (10 µM, 20 µM) phosphorylated forms of AMPKα, ACC protein expression ↓ (10 µM, 20 µM) | 10 µM, 20 µM | Kim et al. [100] | |||
| adipogenesis ↑ lipid accumulation ↑ (10 µM, 20 µM) mRNA level of SREBF1, FASN, PPARγ, LEP, FABP4 ↑ (2.5 µM, 10 µM, 20 µM) | 2.5 µM, 10 µM, 20 µM | Mangum et al. [51] | ||||
| diazinon | adipogenesis ↑ lipid accumulation ↑ (1 µM, 10 µM, 25 µM, 50 µM, 100 µM) mRNA level of C/EBPα, PPARγ, FASN ↑ (10 µM) C/EBPα, PPARγ, FASN, ACC, adiponectin, perilipin protein expression ↑ (10 µM, 100 µM) | 1 µM, 10 µM, 25 µM, 50 µM, 100 µM | Smith, Yu, Yin [101] | |||
| CPF | adipogenesis ↑ lipid accumulation ↑ (10 µM, 50 µM) mRNA level of C/EBPα, PPARγ, FABP4 ↑ (50 µM) protein expression of C/EBPα, PPARγ, FABP4 ↑ (50 µM) | 10 µM, 50 µM | Blanco et al. [102] | |||
| TBT | adipogenesis ↑ lipid accumulation ↑ (50 nM) PPARγ activity ↑ (5 nM, 50 nM, 100 nM) basal glucose uptake (50 nM) | 5 nM, 50 nM, 100 nM | Regnier et al. [103] | |||
| zoxamide | adipogenesis ↑ lipid accumulation ↑ (0.02 µM) PPARγ activity ↑ (EC50 = 1.31 µM, EC10 = 0.31 µM) mRNA level of FABP4 ↑ (2 µM) | 0.02 µM, 0.31 µM, 1.31 µM, 2 µM | Janesick et al. [104] | |||
| spirodiclofen | adipogenesis ↑ lipid accumulation ↑ (0.02 µM, 0.2 µM, 2 µM, 10 µM, 20 µM) PPARγ activity ↑ (EC50 = 12.76 µM, EC10 = 7.27 µM) mRNA level of LPL ↑ (0.02 µM, 2 µM, 10 µM, 20 µM) | 0.02 µM, 0.2 µM, 2 µM, 7.27 µM, 10 µM, 12.76 µM, 20 µM | Janesick et al. [104] | |||
| flusilazole | adipogenesis ↑ lipid accumulation ↑ (0.02 µM, 0.2 µM, 2 µM) mRNA level of FABP4 ↑ (0.02 µM), FSP27 ↑ (0.02 µM, 0.2 µM), LPL ↑ (0.02 µM) | 0.02 µM, 0.2 µM, 2 µM | Janesick et al. [104] | |||
| acetamiprid | adipogenesis ↑ lipid accumulation ↑ (0.2 µM) mRNA level of FABP4, FSP27, LPL ↑ (0.2 µM) | 0.2 µM | Janesick et al. [104] | |||
| NIH3T3-L1 cell line | TBBPA | adipogenesis ↑ lipid accumulation ↑ ERα, ERβ, PPARγ activity ↑ mRNA level of APOA2/FABP4 (AP2), PPARγ ↑ | 10 µM | Riu et al. [109] | ||
| TCBPA | adipogenesis ↑ lipid accumulation ↑ ERα, ERβ, PPARγ activity ↑ mRNA level of APOA2/FABP4 (AP2), PPARγ ↑ | 10 µM | Riu et al. [109] | |||
| p,p’-DDE | adipogenesis (−) lipid accumulation (−) (2 µM, 20 µM) basal fatty acid uptake ↑ (2 µM) insulin-stimulated fatty acid uptake (−) (2 µM, 20 µM) lipolysis (−) (2 µM, 20 µM) leptin, resistin, adiponectin release ↑ (2 µM, 20 µM) mRNA level of adiponectin, resistin ↑ (20 µM) | 2 µM, 20 µM | Howell et al. [80] | |||
| oxychlordane | adipogenesis (−) lipid accumulation (−) (2 µM, 20 µM) basal fatty acid uptake ↑ (20 µM) insulin-stimulated fatty acid uptake (−) (2 µM, 20 µM) lipolysis (−) (2 µM, 20 µM) | 20 µM | Howell et al. [80] | |||
| dieldrin | adipogenesis (−) (2 µM), ↓ (20 µM) lipid accumulation (−) (2 µM), ↓ (20 µM) basal fatty acid uptake ↑ (2 µM, 20 µM) insulin-stimulated fatty acid uptake (−) (2 µM, 20 µM) lipolysis (−) (2 µM, 20 µM) adiponectin release ↑ (2 µM) | 2 µM, 20 µM | Howell et al [80] | |||
| 3T3-F442A cell line | BPA | basal glucose uptake ↑ (10−4 M) insulin-stimulated glucose uptake ↑ (10−4 M, 10−6 M) GLUT4 protein expression ↑ (10−4 M, 10−6 M) | 10−4 M, 10−6 M | Sakurai et al. [81] | ||
| p,p’-DDT | adipogenesis ↓ (most cells did not differentiate completely) C/EBPα protein level ↓ | 20 µM | Moreno-Aliaga and Matsumura [111] | |||
| OP9 cell line | TBT | preadipocyte differentiation ↑ triglyceride accumulation ↑ | 100 nM | Kassotis et al. [39] | ||
| TBBPA | preadipocyte differentiation ↑ triglyceride accumulation ↑ | 10 µM | Kassotis et al. [39] | |||
| adipogenesis ↑ lipid accumulation ↑ (20 µM) PPARγ activity ↑ (10, 20 µM) | 20 µM | Andrews et al. [120] | ||||
| TCBPA | preadipocyte differentiation ↑ triglyceride accumulation ↑ | 10 µM | Kassotis et al. [39] | |||
| ST-13 cell line | TBBPA | undifferentiated cells: lipid accumulation (−) (0.5 µM, 1 µM) mRNA level of AACS, PLIN1, FAS, CIDEA, LSD-1 ↑ (0.5 µM, 1 µM); UCP-1, UCP-3, PRDM16 ↑ (1 µM); SCOT, PPARγ (−) (0.5 µM, 1 µM) mature adipocytes: lipid accumulation (−) (0.5 µM, 1 µM) mRNA level of AACS, SCOT (−) (0.5 µM, 1 µM) | 0.5 µM, 1 µM | Yamasaki et al. [82] | ||
| UCP-1 cell line | CPF | mRNA level of UCP1, CPT1A, CPT1B, ACAT3, COX16 ↓ mRNA level of PPARγ, PPARGC1A, PRDM16 (−) mitochondrial respiration ↓ | 1 pM | Wang et al. [29] | ||
| Human | Primary preadipocytes | BPA | adipogenesis ↑ lipid accumulation ↑ (25 µM, 50 µM) mRNA level of AP2, C/EBPα ↑ (25 µM, 50 µM), adipsin, PPARγ, C/EBPβ ↑ (50 µM) protein level of AP2 ↑ (25 µM) probable adipogenic action via a non-classical estrogen-receptor (ER) pathway rather than through the glucocorticoid-receptor (GR) activation | 25 µM, 50 µM | Boucher, Boudreau, and Atlas [75] | |
| preadipocyte differentiation ↑ expression of genes ACACA, APOA1BP, PLIN2, FADS1, NPC2, PPAP2A ↑ mRNA level of SREBF1, LDLR, LPL, INSIG1, GDF15 ↑ probable mechanism of action via mTOR signaling and TR/RXR activation | 50 µM | Boucher et al. [70] | ||||
| adipogenesis ↑ lipid accumulation ↑ (10 nM, 1 µM, 80 µM) mRNA level of 11β-HSD1, PPARγ ↑ (10 nM, 1 µM, 80 µM), LPL ↑ (10 nM, 80 µM) probable mechanism of action through GR pathway | 10 nM, 1 µM, 80 µM | Wang et al [76] | ||||
| adipogenesis ↑ lipid accumulation ↑ (1 nM, 10 nM) mRNA level of ERα ↑ (10 nM, 100 nM), ERRγ ↑ (10 nM), LEP ↑ (10 nM, 100 nM), ERβ (−) (1 nM, 10 nM, 100 nM), GPR30 ↓ (10 nM) the expression of CD36, FABP4 ↑ (1 nM, 10 nM) secretion of IL1B, IL18, CCL20 ↑ (10 nM) | 1 nM, 10 nM, 100 nM | Menale et al. [124] | ||||
| PCS-210-010 cells | BPA | adipogenesis ↑ mRNA level of PPARγ, AP2, PPIA ↑ adiponectin release ↑ | 0.059 µM | El-Atta et al. [83] | ||
| SGBS cells | BPA | lipid accumulation ↓ (10 nM, 100 nM, 1 µM, 10 µM) binding to PPARγ ↑ (50 µM) PPARγ activity (−) (10 nM, 100 nM, 1 µM, 10 µM) protein level of FABP4 ↓ (10 nM, 100 nM, 1 µM), GPD1 ↓ (10 nM, 100 nM, 1 µM), LPL ↓ (100 nM, 1 µM), APOE ↓ (10 nM, 100 nM, 1 µM, 10 µM) protein level of LIPE ↑ (10 nM, 100 nM, 1 µM, 10 µM), PNPLA2 ↑ (10 nM, 100 nM, 10 µM), CD36 ↑ (10 nM, 100 nM, 1 µM, 10 µM), ADIPOQ ↑ (10 nM, 100 nM, 10 µM) release of MCP1 ↑ (1 µM), leptin ↑ (10 nM) proteins related to oxidative stress level of CAT ↓ (100 nM, 1 µM), SOD2 ↓ (10 nM, 100 nM, 1 µM, 10 µM) cellular ROS level ↓ (10 nM, 100 nM, 1 µM, 10 µM) insulin sensitivity, pAKT/AKT ratio ↓ (1 µM) | 10 nM, 100 nM, 1 µM, 10 µM, 50 µM | Schaffert et al. [8] | ||
| BPS | lipid accumulation ↓ (10 nM, 100 nM, 1 µM, 10 µM) binding to PPARγ ↑ (50 µM) PPARγ activity (−) (10 nM, 100 nM, 1 µM, 10 µM) protein level of FABP4 ↓ (100 nM, 1 µM), GPD1 ↓ (10 nM, 100 nM, 1 µM, 10 µM), LPL ↓ (100 nM, 1 µM, 10 µM), APOE ↓ (10 nM, 100 nM, 1 µM, 10 µM) protein level of LIPE ↑ (10 nM, 1 µM, 10 µM), PNPLA2 ↑ (10 nM, 100 nM, 1 µM, 10 µM), CD36 ↑ (10 nM, 1 µM, 10 µM), ADIPOQ ↑ (10 nM, 1 µM, 10 µM) release of adiponectin ↓ (1 µM), LEP ↑ (10 nM) proteins related to oxidative stress level of CAT ↓ (10 nM, 100 nM), SOD1 ↓ (10 µM), SOD2 ↓ (10 nM, 100 nM, 1 µM, 10 µM) cellular ROS level ↓ (10 nM, 100 nM, 1 µM, 10 µM) insulin sensitivity, pAKT/AKT ratio ↓ (1 µM) | 10 nM, 100 nM, 1 µM, 10 µM, 50 µM | Schaffert et al. [8] | |||
| BPB | lipid accumulation ↓ (10 nM, 100 nM, 1 µM, 10 µM) binding to PPARγ ↑ (50 µM) PPARγ activity (−) (10 nM, 100 nM, 1 µM, 10 µM) protein level of FABP4, GPD1, LPL, APOE, ADIPOQ ↓ (10 nM, 100 nM, 1 µM, 10 µM) protein level of PNPLA2 ↑ (10 nM), CD36 ↑ (10 nM, 10 µM) release of adiponectin ↓, MCP1 ↑, leptin ↓ (1 µM) proteins related to oxidative stress level of CAT ↓ (10 nM, 100 nM, 1 µM, 10 µM), SOD1 ↓ (10 nM, 100 nM, 1 µM, 10 µM), SOD2 ↓ (10 nM, 100 nM, 1 µM) cellular ROS level ↓ (10 nM, 100 nM, 1 µM, 10 µM) insulin sensitivity, pAKT/AKT ratio ↓ (1 µM) | 10 nM, 100 nM, 1 µM, 10 µM, 50 µM | Schaffert et al. [8] | |||
| BPF | lipid accumulation ↓ (10 nM, 100 nM, 1 µM, 10 µM) binding to PPARγ ↑ (50 µM) PPARγ activity (−) (10 nM, 100 nM, 1 µM, 10 µM) protein level of FABP4, GPD1, LPL, APOE ↓ (10 nM, 100 nM, 1 µM, 10 µM) protein level of LIPE ↑ (1 µM, 10 µM), PNPLA2 ↑ (10 µM), CD36 ↑ (10 nM, 100 nM, 1 µM, 10 µM), ADIPOQ ↑ (10 nM, 10 µM) release of adiponectin ↓ (1 µM), MCP1 ↑ (1 µM), leptin ↑ (10 nM, 1 µM) proteins related to oxidative stress level of CAT ↓ (10 nM, 100 nM, 1 µM, 10 µM), SOD1 ↓ (10 nM, 100 nM, 10 µM), SOD2 ↓ (100 nM, 1 µM, 10 µM) cellular ROS level ↓ (10 nM, 100 nM, 1 µM, 10 µM) | 10 nM, 100 nM, 1 µM, 10 µM 50 µM | Schaffert et al. [8] | |||
| BPAF | lipid accumulation ↓ (10 nM, 100 nM, 1 µM) binding to PPARγ ↑ (50 µM) PPARγ activity (−) (10 nM, 100 nM, 1 µM, 10 µM) protein level of FABP4, GPD1, LPL, APOE ↓ (10 nM, 100 nM, 1 µM) protein level of LIPE ↑ (10 nM, 100 nM), CD36 ↑ (10 nM, 1 µM), ADIPOQ ↑ (1 µM) release of adiponectin ↓ (1 µM), leptin ↑ (10 nM) proteins related to oxidative stress level of CAT ↓ (100 nM), SOD1 ↓ (10 nM, 100 nM, 1 µM), SOD2 ↓ 10 nM, 100 nM, 1 µM) cellular ROS level ↓ (10 nM, 100 nM, 1 µM) | 10 nM, 100 nM, 1 µM, 50 µM | Schaffert et al. [8] | |||
| DEHP | triglyceride content ↓ mRNA level of ADIPOR2, GLUT4 (−), LEPR, CD36, FABP4, LPL, LIPE, ATGL ↓ protein level of PPARα, PPARγ, SOD2, GPX1 (−) secretion of adiponectin ↓, LEP ↑ ratio of pAMPK/AMPK, pSTAT3/STAT3 (−), pACC2/ACC2 ↑ phosphorylation of ERK1, ERK2 ↓ lipolysis ↑, level of free glycerol ↑ ROS level ↑ | 50 µg/mL | Schaedlich et al. [84] | |||
| DINCH | binding to PPARγ ↑ (−) (6.25 µM, 12.5 µM, 25 µM, 50 µM, 100 µM, 200 µM, 400 µM) PPARγ activation (−) undifferentiated cells: lipid accumulation (−) (10 nM, 1 µM, 10 µM, 25 µM, 50 µM, 100 µM) secretion of adipsin ↑ (10 nM), MCP-1 ↓ (10 µM) mature adipocytes: lipid accumulation ↓ (10 µM, 25 µM, 50 µM, 100 µM) secretion of LEP ↑ (10 nM, 10 µM), adipsin ↑ (10 nM, 10 µM), MCP-1 ↑ (10 nM, 10 µM), adiponectin ↓ (10 nM, 10 µM) protein level of GPX1 ↑ (10 µM), GPX4 ↑ (10 µM), GSTO1 ↑ (10 nM, 10 µM), LAP3 ↑ (10 µM) | 10 nM, 10 µM, 25 µM, 50 µM, 100 µM | Schaffert et al. [62] | |||
| DINP | binding to PPARγ ↑ (−) (6.25 µM, 12.5 µM, 25 µM, 50 µM, 100 µM, 200 µM, 400 µM, PPARγ activation (−) undifferentiated cells: lipid accumulation (−) (10 nM, 1 µM, 10 µM, 25 µM, 50 µM, 100 µM), secretion of adipsin ↑ (10 nM) mature adipocytes: lipid accumulation (−) (10 nM, 1 µM, 10 µM, 25 µM, 50 µM, 100 µM), secretion of LEP ↑ (10 nM, 10 µM), adipsin ↑ (10 µM), MCP-1 ↑ (10 µM), adiponectin ↓ (10 nM, 10 µM) protein level of GPX1 ↑ (10 µM), GPX4 ↑ (10 nM, 10 µM), GPX8 ↑ (10 nM), GSR ↑ (10 nM), GSTO1 ↑ (10 nM, 10 µM), LAP3 ↑ (10 µM) | 10 nM, 10 µM | Schaffert et al. [62] | |||
| DPHP | binding to PPARγ ↑ (−) (6.25 µM, 12.5 µM, 25 µM, 50 µM, 100 µM, 200 µM, 400 µM), PPARγ activation (−) undifferentiated cells: lipid accumulation (−) (10 nM, 1 µM, 10 µM, 25 µM, 50 µM, 100 µM), secretion of MCP-1 ↓ (10 nM, 10 µM) mature adipocytes: lipid accumulation ↓ (10 µM, 25 µM, 50 µM, 100 µM) secretion of LEP ↑ (10 µM), adipsin ↑ (10 µM), MCP-1 ↑ (10 µM), adiponectin ↓ (10 nM, 10 µM) protein level of GPX1 ↑ (10 nM, 10 µM), GPX4 ↑ (10 µM), GPX8 ↑ (10 µM), GSR ↑ (10 nM), GSTO1 ↑ (10 nM, 10 µM), LAP3 ↑ (10 µM) | 10 nM, 10 µM, 25 µM, 50 µM, 100 µM | Schaffert et al. [62] | |||
| MINCH | binding to PPARγ ↑ (25 µM, 50 µM, 100 µM, 200 µM, 400 µM) PPARγ activation (−) undifferentiated cells: preadipocyte differentiation ↑ lipid accumulation ↑ (10 µM, 25 µM, 50 µM) secretion of LEP ↑ (10 µM), adipsin ↑ (10 nM), MCP-1 ↓ (10 nM, 10 µM) protein level of FABP4, FASN, FABP5, GPD1, PLIN1 ↑ (10 µM) mature adipocytes: lipid accumulation ↓ (10 µM, 25 µM, 50 µM) secretion of LEP ↑ (10 nM, 10 µM), adipsin ↑ (10 µM), MCP-1 ↑ (10 nM, 10 µM), adiponectin ↓ (10 nM, 10 µM) protein level of GPX1 ↑ (10 µM), GPX4 ↑ (10 µM), GPX8 ↑ (10 nM, 10 µM), GSTO1 ↑ (10 nM, 10 µM), LAP3 ↑ (10 µM) | 10 nM, 10 µM, 25 µM, 50 µM, 100 µM, 200 µM, 400 µM | Schaffert et al. [62] | |||
| MHINP | binding to PPARγ ↑ (100 µM, 200 µM, 400 µM) PPARγ activation ↑ (1 µM) undifferentiated cells: preadipocyte differentiation ↑ lipid accumulation ↑ (10 µM, 25 µM, 50 µM, 100 µM) secretion of LEP ↑ (10 µM), adipsin ↑ (10 nM, 10 µM), MCP-1 ↓ (10 nM) protein level of FABP4, FASN, FABP5, GPD1, PLIN1 ↑ (10 µM) mature adipocytes: lipid accumulation ↑ (1 µM) secretion of LEP ↑ (10 nM, 10 µM), adipsin ↑ (10 µM), MCP-1 ↑ (10 nM, 10 µM), adiponectin ↓ (10 nM, 10 µM) protein level of GPX1 ↑ (10 nM, 10 µM), GPX4 ↑ (10 nM, 10 µM), GPX8 ↑ (10 nM), GSR ↑ (10 nM), GSTO1 ↑ (10 nM, 10 µM), LAP3 ↑ (10 µM) | 10 nM, 10 µM, 25 µM, 50 µM, 100 µM, 200 µM, 400 µM | Schaffert et al. [62] | |||
| OH-MPHP | binding to PPARγ ↑ (200 µM, 400 µM) PPARγ activation ↑ (10 µM) undifferentiated cells: preadipocyte differentiation ↑ lipid accumulation ↑ (10 µM, 25 µM, 50 µM) secretion of LEP ↑ (10 µM) protein level of FABP4, FASN, FABP5, GPD1 ↑ (10 µM) mature adipocytes: lipid accumulation ↓ (10 nM, 10 µM, 25 µM, 50 µM, 100 µM) secretion of LEP ↑ (10 nM, 10 µM), adipsin ↑ (10 µM), MCP-1 ↑ (10 nM, 10 µM), adiponectin ↓ (10 nM, 10 µM) protein level of GPX1 ↑ (10 µM), GPX4 ↑ (10 µM), GPX8 ↑ (10 µM), GSR ↑ (10 nM, 10 µM), GSTO1 ↑ (10 nM, 10 µM), LAP3 ↑ (10 µM) | 10 nM, 10 µM, 25 µM, 50 µM, 100 µM, 200 µM, 400 µM | Schaffert et al. [62] | |||
| SW 872 cell line | MEHP | differentiating effect ↑ lipid accumulation (−) mRNA level of TSPO, PKCε, PPARα, ACACA, ACLY, GLUT1, GLUT4, S100B ↑, PPARγ ↓, PPARβ/δ (−) protein level of TSPO ↓ | 10 µM | Campioli et al. [129] |
| Cell Type | Organism | In vitro Model | EDCs | Mechanism of Action | Concentration * | References |
|---|---|---|---|---|---|---|
| Adipose-derived mesenchymal stem cells (ADSCs) | Animal | C3H10T1/2 cell line | BPA | adipogenic differentiation (− (1 nM, 3 nM) lipid accumulation (−) (1 nM, 3 nM) mRNA level of PPARγ, C/EBPα and FABP4 (−) (1 nM, 3 nM) | 1 nM, 3 nM | De Filippis et al. [96] |
| whole period of adipogenic differentiation: amount of adipocytes ↓, triglyceride content ↓, mRNA level of FABP4, PPARγ, LPL, adiponectin ↓ (10 µM); amount of adipocytes (−), triglyceride content (−) (10 nM) selective treatment during undifferentiated growth: amount of adipocytes ↓, triglyceride content ↓ (10 µM); hormonal induction: amount of adipocytes (−), triglyceride content (−) (10 µM); terminal differentiation: amount of adipocytes (−), triglyceride content (−) (10 µM) | 10 µM | Biemann et al. [139] | ||||
| DEHP | whole period of adipogenic differentiation: amount of adipocytes ↑, triglyceride content ↑ (100 µM); amount of adipocytes (−), triglyceride content (−) (100 nM) selective treatment during undifferentiated growth: amount of adipocytes (−), triglyceride content (−) (100 µM); hormonal induction: amount of adipocytes ↑, triglyceride content ↑ (100 µM); terminal differentiation: amount of adipocytes (−), triglyceride content (−) (100 µM) | 100 µM | Biemann et al. [139] | |||
| BBP | adipogenesis ↑ lipid accumulation ↑ mRNA level of AP2, PPARγ ↑, SIRT1, SIRT7, PGC1α, NRF1, NRF2, TFAM ↓; SIRT2, SIRT3, SIRT4, SIRT5, SIRT6 (−) protein level of FOXO1 ↑, SIRT1, SIRT3 ↓, SIRT7, β-catenin (−) acetylation of FOXO1, β-catenin ↑ | 50 µM | Zhang and Choudhury [138] | |||
| expression of miR-103/107, miR-let7 (a, b, c, d, f, g) ↑, lncRNA H19 ↓, miR-let7e (−) at day 2 of differentiation mRNA level of IRS-1 ↓, IR, IRS-2 (−) at day 2, 4, 6, 8 of differentiation protein expression of phospho-Akt ↓ at day 4 of differentiation | 50 µM | Zhang and Choudhury [137] | ||||
| TBT | whole period of adipogenic differentiation: amount of adipocytes ↑, triglyceride content ↑ (100 nM); amount of adipocytes (−), triglyceride content (−) (1 nM) selective treatment during undifferentiated growth: amount of adipocytes ↑, triglyceride content ↑ (100 nM); hormonal induction: amount of adipocytes ↑, triglyceride content ↑ (100 nM); terminal differentiation: amount of adipocytes ↑, triglyceride content ↑ (100 nM) | 100 nM | Biemann et al. [139] | |||
| butylparaben | adipogenic differentiation ↑ lipid accumulation ↑ PPARγ activity ↑ GR activity (−) mRNA level of PPARγ, C/EBPα, FABP4 ↑, RUNX2 ↓ | 100 µM | Hu et al. [130] | |||
| mixture of BPA, DEHP, TBT | BPA (10 nM), DEHP (100 nM) and TBT (1 nM) whole period of adipogenic differentiation, undifferentiated growth, hormonal induction and terminal differentiation: amount of adipocytes (−), triglyceride content (−), mRNA level of FABP4, adiponectin, LPL, PPARγ2 (−) BPA (10 µM), DEHP (100 µM) and TBT (100 nM) whole period of adipogenic differentiation: amount of adipocytes ↑, triglyceride content (−), mRNA level of FABP4, adiponectin ↑, LPL, PPARγ2 (−); undifferentiated growth: amount of adipocytes ↑, triglyceride content (−), mRNA level of FABP4 ↑, adiponectin, LPL, PPARγ2 (−); hormonal induction: amount of adipocytes ↑, triglyceride content (−), mRNA level of FABP4, adiponectin, LPL, PPARγ2 ↑; terminal differentiation: amount of adipocytes ↑, triglyceride content (−); mRNA level of FABP4 ↑, adiponectin, Lpl, PPARγ2 (−) | 1 nM, 10 nM, 100 nM, 10 µM, 100 µM | Biemann, Fisher and Navarrete Santos [140] | |||
| Primary mouse ADSCs | TBT | adipogenesis ↑ lipid accumulation ↑ mRNA level of FABP4, PPARΓ2, LEP ↑, PREF-1 ↓, ADIPOQ (−) | 50 nM | Kirchner et al. [78] | ||
| Primary porcine ADSCs | BPS | viability of cells ↓ lipid accumulation ↓ | 1 µM | Berni et al. [143] | ||
| GLY | viability of cells ↓ adipogenic differentiation ↓ | 4 µg/mL | Gigante et al. [142] | |||
| Human | Primary hADSCs | BPA | adipogenic differentiation (−) (1 nM, 3 nM) lipid accumulation (−) (1 nM, 3 nM) mRNA level of PPARγ, C/EBPα and FABP4 (−) (1 nM, 3 nM) | 1 nM, 3 nM | De Filippis et al. [96] | |
| mRNA level of PPARγ, GLUT4 (−) insulin-stimulated glucose utilization ↓ insulin-activated insulin receptor (IR) tyrosine phosphorylation ↓, ERK1/2 phosphorylation ↓, PKB/Akt phosphorylation ↓ release of IL6, IFN-γ ↑ JAK/STAT, JNK, NFkB pathways activity ↑ | 1 nM | Valentino et al. [147] | ||||
| adipogenesis ↑ lipid accumulation ↑ mRNA level of ERα ERβ, IGF1, PPARγ, LPL, C/EBPα, DLK ↑, AP2, SREBP1c, C/EBPβ (−) protein level of LPL ↑ probable mechanism of action through ER-dependent pathway | 1 µM | Ohlstein et al. [148] | ||||
| adipogenesis ↑ (0.1 µM), ↓ (1 µM) lipid production ↑ (0.1 µM), ↓ (1 µM) | 0.1 µM, 1 µM | Cohen et al. [55] | ||||
| cell viability ↓ (1 µM, 10 µM) apoptosis ↑ (1 µM) caspase-6 activity (1 µM) | 1 µM, 10 µM | Harnett et al. [56] | ||||
| BPF | adipogenesis ↑ lipid accumulation ↑ (10 µM, 25 µM) mRNA level of PPARγ, LPL, FABP4 ↑ (10 µM, 25 µM); C/EBPα ↑ (1 µM, 10 µM, 25 µM) protein level of PPARγ, C/EBPα, LPL ↑ (10 µM, 25 µM) probable mechanism of action via an ER-dependent pathway | 1 µM, 10 µM, 25 µM | Reina-Pérez et al. [79] | |||
| BPS | adipogenesis ↑ lipid accumulation ↑ (1 µM, 10 µM, 25 µM) mRNA level of PPARγ, C/EBPα, LPL ↑ (1 µM, 10 µM, 25 µM); FABP4 ↑ (10 µM, 25 µM) protein level of PPARγ ↑ (10 µM, 25 µM); C/EBPα, LPL ↓ (10 µM, 25 µM) | 1 µM, 10 µM, 25 µM | Reina-Pérez et al. [79] | |||
| BPAF | adipogenesis ↑ (0.1 nM), (−) (1 nM), ↓ (10 nM) lipid production ↑ (0.1 nM), (−) (1 nM), ↓ (10 nM) | 0.1 nM, 10 nM | Cohen et al. [55] | |||
| cell viability ↓ (0.0003 µM, 0.003 µM, 0.03 µM, 0.3 µM) apoptosis ↑ (0.003 µM) caspase-6 activity (0.003 µM) | 0.0003 µM, 0.003 µM, 0.03 µM, 0.3 µM | Harnett et al. [56] | ||||
| TMBPF | adipogenesis ↓ lipid production ↓ | 0.01 µM, 0.1 µM | Cohen et al. [55] | |||
| cell viability ↓ (0.01 µM, 0.1 µM, 1 µM, 10 µM, 50 µM) apoptosis ↑ (1 µM) caspase-6 activity (1 µM) | 0.01 µM, 0.1 µM, 1 µM, 10 µM, 50 µM | Harnett et al. [56] | ||||
| TBT | adipogenesis ↑ lipid accumulation ↑ mRNA level of FABP4, PPARγ2, LEP ↑, PREF-1 ↓, ADIPOQ (−) | 50 nM | Kirchner et al. [78] | |||
| p,p’-DDE | maintenance of cells in an undifferentiated state ↑ mRNA level of OCT4, PPARγ, SREBP1, FASN, INSR, AKT2, UCP-3 ↑ (0.1 µM, 1 µM, 10 µM); SOX2 ↑ (1 µM, 10 µM); PPARγC1B ↑ (0.1 µM, 1 µM); NANOG ↓ (0.1 µM, 1 µM, 10 µM) | 0.1 µM, 1 µM, 10 µM | Pesta et al. [77] |
| Cell Type | Organism | In vitro Model | EDC | Mechanism of Action | Concentration * | References |
|---|---|---|---|---|---|---|
| Hepatocytes | Animal | Primary mouse hepatocytes | BHPF | cell viability ↓ LDH activity ↑ | 1 µM, 10 µM | Yang et al. [165] |
| Primary gilthead seabream hepatocytes | DIDP | binding to PPARγ, PPARα, RXRα ↑ mRNA level of PPARA, PPARΒ, PPARγ, CPT1A, FADS2, SCD1A, SCD1B, LPL, HL, FABP, APOA-I, SREBP1 ↑ (0.1 µM, 1 µM), (−) (10 µM), CPT1B ↑ (1 µM), (−) (10 µM) probable mechanism of action via PPAR:RXR signaling | 0.1 µM, 1 µM | Cocci et al. [169] | ||
| Primary Atlantic salmon hepatocytes | p,p’-DDE | viability of cells ↓ (100 μM), (−) (0.1 μM, 1 μM, 10 μM) global DNA methylation (−) (0.1 μM, 1 μM, 10 μM, 100 μM) mRNA level of stress-responsive genes such as estrogenic markers ESR1 ↑ (10 μM), (−) (0.1 μM, 1 μM, 100 μM); ESR2 (−) (0.1 μM, 1 μM, 10 μM, 100 μM); VTG1 ↑ (1 μM, 10 μM), (−) (0.1 μM, 100 μM); markers of detoxification CYP1A1, CYP3A (−) (0.1 μM, 1 μM, 10 μM, 100 μM); genes whose protein products are associated with cell death HSPA8, FOS ↑ (100 μM), (−) (0.1 μM, 1 μM, 10 μM); CASP3B, PTGS2 ↓ (100 μM), (−) (0.1 μM, 1 μM, 10 μM); CDKN1B, INSIG1 (−) (0.1 μM, 1 μM, 10 μM, 100 μM) mRNA level of DNA methylation-relevant genes DNMT3AA, CBS, N6AMT2, MAT1A2 ↓ (100 μM), (−) (0.1 μM, 1 μM, 10 μM); DNMT1 (−) (0.1 μM, 1 μM, 10 μM, 100 μM) bile acid metabolism (level of glycocholate, glycochenodeoxycholate, taurocholate, taurochenodeoxycholate, deoxycholate, glycodeoxycholate, taurolithocholate ↑ (100 μM)) glucose metabolism (level of glycolytic intermediates such as 3-phosphoglycerate, phosphoenolpyruvate, glucose-6-phosphate, glucose ↓, pyruvate ↑, pentose sugars such as arabonate/xylonite, arabitol/xylitol, sedoheptulose ↓, pentose phosphate pathway intermediate such as 5-phosphogluconate ↓, glycogen hydrolysis products such as maltotriose, maltotetraose, maltose ↓ (100 μM)) amino acids metabolism (level of glutamine, N-acetylglutamine, glutamate, N-acetylglutamate ↓, gamma-aminobutyrate ↑, (100 μM)) lipid metabolism (level of monoacylglycerols such as 1-mirystoylglycerol ↑, diacylglycerols such as 1-palmitoyl-2-arachidononyl-GPE, phosphatidylethanolamine species such as oleoyl-oleoyl-glycerol ↓ (100 μM)) | 1 μM, 10 μM, 100 μM | Olsvik and Søfteland [170] | ||
| BRL-3A cell line | MEHP | lipid accumulation ↑ (100 µM, 200 µM) mRNA level of FAS, PDK4, aP2 ↑ (10 µM, 50 µM, 100 µM, 200 µM); AOX, PPARγ ↑ (50 µM, 100 µM, 200 µM); JAK2, STAT5A ↓ (50 µM, 100 µM, 200 µM), STAT5B ↓ (10 µM, 50 µM, 100 µM, 200 µM) protein level of AOX, PDK4, FAS ↑ (100 µM, 200 µM); aP2 ↑ (50 µM, 200 µM; PPARγ ↑ (200 µM); JAK2, STAT5A, STAT5B ↓ (10 µM, 50 µM, 100 µM, 200 µM); JAK2/STAT5 signalling ↓ level of indicators of oxidative stress: SOD ↓, MDA ↑ (10 µM, 50 µM, 100 µM, 200 µM) level of indicators of damage status of liver cells: ALT ↑ (50 µM, 100 µM, 200 µM), AST ↑ (10 µM, 50 µM, 100 µM, 200 µM) | 10 µM, 50 µM, 100 µM, 200 µM | Zhang et al. [173] | ||
| FaO cell line | BPA | intracellular lipid content ↑ (30 ng/mL, 300 ng/mL) mRNA level of PPARα, PPARβ, PPARδ, PPARγ, AOX, CPT1, APOB ↓, FAS, GPAT (−) (300 ng/mL) | 30 ng/mL and 300 ng/mL corresponding to 0.1 µM and 1 µM | Grasselli et al. [171] | ||
| AML12 cell line | PCB-153 | lipid accumulation ↑ mRNA level of p65 subunit of NFkB, IL1α, IL6 ↑, HNF1B, GPX1 ↓ nuclear protein expression of p65 subunit of NFkB ↑, cytoplasmic protein expression of p65 subunit of NFkB ↓ protein level of HNF1b, GPX1 ↓ ROS level ↑ ratio of glutathione (GSH)/ /oxidized GSH (GSSG) ↓ ratio of NADP+/NADPH ↑ insulin-stimulated glucose uptake ↓ | 0.5 µM, 1 µM | Wu et al. [175] | ||
| TCEP | lipid accumulation ↑ (10 µM) disturbance of mitochondrial structure ↑ (1 µM, 10 µM) mitoATP rate/glycoATP rate ↓ (1 µM, 10 µM) | 1 µM, 10 µM | Le et al [176] | |||
| TCPP | lipid accumulation ↑ (10 µM) disturbance of mitochondrial structure ↑ (1 µM, 10 µM) mitochondrial membrane potential (MMP) ↓ (10 µM) mitoATP rate/glycoATP rate ↓ (10 µM) | 1 µM, 10 µM | Le et al. [176] | |||
| TDCPP | lipid accumulation ↑ (1 µM, 10 µM) disturbance of mitochondrial structure ↑ (1 µM, 10 µM) mitochondrial ROS production ↑ (1 µM, 10 µM) MMP ↓ (10 µM) mitochondrial basal respiration ↓ (10 µM) proton leak ↓ (10 µM) mitoATP production rate ↓ (10 µM) mitoATP rate/glycoATP rate ↓ (10 µM) | 1 µM, 10 µM | Le et al. [176] | |||
| TPhP | lipid accumulation ↑ (1 µM, 10 µM) disturbance of mitochondrial structure ↑ (1 µM, 10 µM) mitochondrial ROS production ↑ (1 µM, 10 µM) MMP ↓ (1 µM, 10 µM) mitochondrial basal respiration ↓ (10 µM) proton leak ↓ (10 µM) spare respiratory capacity (SRC) ↑ (10 µM) mitoATP production rate ↓ (10 µM) mitoATP rate/glycoATP rate ↓ (10 µM) | 1 µM, 10 µM | Le et al. [176] | |||
| TCP | lipid accumulation ↑ (0.1 µM, 1 µM, 10 µM) disturbance of mitochondrial structure ↑ (1 µM, 10 µM) mitochondrial ROS production ↑ (1 µM, 10 µM) MMP ↓ (1 µM, 10 µM) mitochondrial basal respiration ↓ (10 µM) proton leak ↓ (10 µM) SRC ↑ (10 µM) mitoATP production rate ↓ (10 µM) mitoATP rate/glycoATP rate ↓ (10 µM) | 0.1 µM, 1 µM, 10 µM | Le et al. [176] | |||
| Hepa1-6 cell line | BPA | mRNA level of DNA methyltransferases: DNMT1, DNMT3A ↓ (0.001 µM, 0.01 µM); DNMT3B ↓ (0.001 µM) mRNA level of SREBF1, SREBF2, FASN, HMGCR ↑ (0.001 µM, 0.01 µM) | 0.001 µM, 0.01 µM | Ke et al. [178] | ||
| FL83B cell line | DEHP | cell viability ↓ (250 µM, 500 µM, 1000 µM) LDH release ↑ (125 µM, 250 µM, 500 µM, 1000 µM), ALT release ↑ (500 µM, 1000 µM) cell populations of sub-G1 and S phase ↑ (250 µM, 500 µM, 1000 µM) | 125 µM, 250 µM, 500 µM, 1000 µM | Lo et al. [185] | ||
| RTL-W1 cell line | BPA | lipid accumulation ↑ alteration of membrane lipids (phosphatidylcholines (PCs), plasmalogen PCs) mRNA level of ABCA1, CD36, FATP1, FAS, LPL, PPARα, PPARβ ↑ | 10 µM | Dimastrogiovanni et al. [186] | ||
| TBT | alteration of membrane lipids (phosphatidylcholines (PCs), plasmalogen PCs) mRNA level of ABCA1, CD36, FAS, LPL ↑ | 100 nM | Dimastrogiovanni et al. [186] | |||
| TPT | mRNA level of ABCA1, FATP1, FAS ↑ | 100 nM | Dimastrogiovanni et al. [186] | |||
| DEHP | lipid accumulation ↑ alteration of membrane lipids (phosphatidylcholines (PCs), plasmalogen PCs) mRNA level of CD36, FAS, LPL ↓ | 5 µM | Dimastrogiovanni et al. [186] | |||
| 4-NP | alteration of membrane lipids (phosphatidylcholines (PCs), plasmalogen PCs) mRNA level of ABCA1 ↑, CD36, FAS, LPL, PPARβ ↓ | 20 µM | Dimastrogiovanni et al. [186] | |||
| PLHC-1 cell line | DBP | triacylglyceride accumulation ↑ (20 µM), (−) (5 µM) ROS generation ↑ (5 µM, 20 µM, 50 µM, 100 µM) | 5 µM, 20 µM, 50 µM, 100 µM | Pérez-Albaladejo et al. [191] | ||
| DEHP | triacylglyceride accumulation ↑ (5 µM, 10 µM) ROS generation ↑ (100 µM) | 5 µM, 10 µM, 100 µM | Pérez-Albaladejo et al. [191] | |||
| BADGE·2HCl | triacylglyceride accumulation ↓ (1 µM, 5 µM) ROS generation ↑ (100 µM) | 1 µM, 5 µM, 100 µM | Pérez-Albaladejo et al. [191] | |||
| TBT | intracellular accumulation of triglycerides and diglycerides ↑ | 100 nM, 200 nM | Marqueño et al. [188] | |||
| ZFL cell line | BPA | lipid accumulation: dihydroceramides ↑ (50 µM), ether-triacylglycerides (ether-TGs), saturated and lower unsaturated TGs ↑ (5 µM, 50 µM) mRNA level of SCD, ELOVL6 ↑, LXR, FASN, GPAT3, DGAT1A (−) (20 µM) ROS production ↑ (20 µM, 50 µM, 70 µM, 100 µM, 150 µM, 200 µM) | 5 µM, 20 µM, 50 µM, 70 µM, 100 µM, 150 µM, 200 µM | Marqueño et al. [190] | ||
| BPF | lipid accumulation: dihydroceramides, ether-triacylglycerides (ether-TGs) ↑ (50 µM), TGs containing polyunsaturated fatty acids (PUFAs) ↓ (50 µM) mRNA level of SCD, ELOVl6, ABCA1B, CYP3A65 ↑, PPARα ↓, LXR, FASN, GPAT3, DGAT1a (−) (20 µM) ROS production ↑ (5 µM, 20 µM, 50 µM, 100 µM, 150 µM, 200 µM, 500 µM) | 5 µM, 20 µM, 50 µM, 100 µM, 150 µM, 200 µM, 500 µM | Marqueño et al. [190] | |||
| BADGE·2HCl | lipid accumulation: dihydroceramides, ether-TGs) ↑ (10 µM), saturated and lower unsaturated TGs ↑ (5 µM) mRNA level of ABCA1b, PPARα ↓, LXR, FASN, GPAT3, DGAT1A (−) (5 µM) ROS production (−) (20 µM, 50 µM, 60 µM, 70 µM, 80 µM, 100 µM) | 5 µM, 10 µM | Marqueño et al. [190] | |||
| Human | Primary hepatocytes | TCDD | fatty acids accumulation ↑ | 10 nM | Forgacs et al. [90] | |
| HepG2 cell line | 4-HP | lipid deposition in OA (oleic acid)-treated cells ↑ de novo lipogenesis ↓ fatty acid oxidation ↓ mRNA level of CD36 ↑ mRNA level of PPARα, SREBP1c, CPT1A, ACC ↓, PPARγ (−) | 20 µM | Sun et al. [93] | ||
| 1,3-DCP | cell viability ↓ (250 µg/mL, 500 µg/mL), (−) (0.1 µg/mL, 0.5 µg/mL, 1 µg/mL, 5 µg/mL, 25 µg/mL, 50 µg/mL) intracellular TG and TC (total cholesterol) content ↑ (0.5 µg/mL, 1 µg/mL, 2 µg/mL) level of cAMP, AMP, ADP ↓, ATP (−) (0.5 µg/mL, 1 µg/mL, 2 µg/mL) intracellular calcium level (−) (0.5 µg/mL, 1 µg/mL, 2 µg/mL) mRNA level of LDLR, SREBP2, HMGCR ↑ (0.5 µg/mL, 1 µg/mL, 2 µg/mL) protein level of SREBP1c, SCD1, FAS, CD36, HMGCR, GPAT ↑, CREB, LKB1, HSL, p-AMPK, p-PKA, pACC, PGC1α, PPARα, SIRT1, CPT1, HNF4α ↓ (0.5 µg/mL, 1 µg/mL, 2 µg/mL), GPR41, GPR43 ↓, GPR109B (−) (2 µg/mL), Caldumolin 1, Calpain 1, Calpain 2, CaMKII, p-CaMKII (−) (0.5 µg/mL, 1 µg/mL, 2 µg/mL) Gi/o expression ↑ (2 µg/mL) probable mechanism of action through cAMP/PKA and AMPK signaling pathways via Gi/o-coupled receptor | 0.5 µg/mL, 1 µg/mL, 2 µg/mL, 250 µg/mL, 500 µg/mL | Lu et al. [199] | |||
| TCS | level of diacylglycerol (DG) ↑ (1 µM, 10 µM), phosphatidylcholine (PC), sphingomyelin (SM), phosphatidylethanolamine (PE) ↑ (10 µM), ceramide (Cer) ↓ (1 µM, 10 µM), triglyceride (TG), polyphosphoinositide (PI), lysophosphatidylethanolamine (LPE), phosphatidylglycerol (PG), lysophosphatidylcholine (LPC) ↓ (10 µM) ROS generation ↑ (10 µM), (−) (1 µM) MDA content (−) (1 µM, 10 µM) SOD activity ↑ (10 µM), (−) (1 µM) CAT activity ↑ (10 µM), (−) (1 µM) level of GSH ↑ (10 µM), (−) (1 µM), taurine (−) (1 µM, 10 µM) | 1 µM, 10 µM | Zhang et al. [213] | |||
| DINCH | cell viability (−) (1 µg/mL, 5 µg/mL, 10 µg/mL, 100 µg/mL, 250 µg/mL, 500 µg/mL) oxidative DNA damage at 3 h exposure ↑ (1 µg/mL, 10 µg/mL, 100 µg/mL, 250 µg/mL, 500 µg/mL), at 24 h exposure ↑ (100 µg/mL) | 1 µg/mL, 10 µg/mL, 100 µg/mL, 250 µg/mL, 500 µg/mL | Vasconcelosa, Silva and Louroa [57] | |||
| HepaRG cell line | BPA | triglycerides and neutral lipids accumulation ↑ (2 nM) mRNA level of lipid-responsive genes such as APOA4 ↑, PLIN2 (ADFP or ADRP), TIP47 (−) (0.2 nM, 2 nM, 20 nM, 200 nM, 2000 nM) mRNA level of genes involved in lipid and carbohydrate homeostasis FASN, ACLY, ACACA, HMGCR, PPARγ, PNPLA3, THRSP (SPOT14), PDK4, APOB, GLUT2 (SLC2A2), MTTP, CPT1A (−) (0.2 nM, 2 nM, 20 nM, 200 nM, 2000 nM) mRNA level of genes involved in oxidative stress NFKB1, HMOX1, GSTA1/2, GSTA3, NQO1, TRIB3, HSPA1A (HSP70-1A) (−) (0.2 nM, 2 nM, 20 nM, 200 nM, 2000 nM) mRNA level of ERRγ target genes SDHD, PCK1, ESRRA (−) (0.2 nM, 2 nM, 20 nM, 200 nM, 2000 nM) mRNA level of PXR target genes CD36, CYP2C9, CYP3A4 (−) (0.2 nM, 2 nM, 20 nM, 200 nM, 2000 nM) mRNA level of genes involved in BPA biotransformation CYP2C19, CYP2C18, SULT1A1, SULT1A3/4, UGT2B15, STS, GUSB (−) (0.2 nM, 2 nM, 20 nM, 200 nM, 2000 nM) | 2 nM | Bucher et al. [164] | ||
| TBT | lipid accumulation ↑ (5 nM, 10 nM, 50 nM) mRNA level of SREBF1, FASN ↑ (50 nM) protein level of RXRα ↓ (50 nM) | 5 nM, 10 nM, 50 nM | Stossi et al. [202] | |||
| Huh-7 cell line | BPA | cell viability ↓ (200 µM, 400 µM), (−) (10 µM, 100 µM) lipid accumulation ↑ (10 µM, 50 µM, 100 µM, 200 µM), (−) (1 µM) fatty acid uptake ↑ (10 µM, 50 µM, 100 µM), (−) (1 µM) mRNA level of CD36 ↑ (50 µM, 100 µM), SR-A1, SR-B1 (−) (10 µM, 50 µM, 100 µM) protein level of CD36 ↑ (50 µM, 100 µM), SR-A1, SR-B1 (−) (10 µM, 50 µM, 100 µM) intracellular ROS generation ↑ (10 µM, 50 µM, 100 µM, 200 µM), (−) (1 µM) | 10 µM, 50 µM, 100 µM, 200 µM, 400 µM | Lee et al. [206] | ||
| HHL-5 cell line | BPA | lipid accumulation ↑ (10 nM, 100 nM, 1000 nM, 22.5 µM, 45 µM) mRNA level of SREBP1c, ACACA, FASN ↑ (22.5 µM), CNR1 ↑ (45 µM) level of AEA ↑, PEA, OEA ↓ (45 µM) CB1 activity ↑ (45 µM) FAAH activity ↓ (45 µM, 90 µM) probable mechanism of action by endocannabinoid action at CB1 receptors | 10 nM, 100 nM, 1000 nM, 22.5 µM, 45 µM, 90 µM | Martella et al. [210] | ||
| L02 cell line | TCS | level of TG, PG, LPC, LPE ↑ (1 µM, 2.5 µM), DG, PE, PC, SM, Cer ↑ (2.5 µM), PI ↓ (1 µM, 2.5 µM) ROS generation ↑ (1 µM, 2.5 µM) MDA content ↑ (1 µM, 2.5 µM) SOD activity ↑ (2.5 µM), (−) (1 µM) CAT activity ↑ (1 µM, 2.5 µM) level of GSH ↓ (2.5 µM), (−) (1 µM), taurine ↓ (1 µM, 2.5 µM) | 1 µM, 2.5 µM | Zhang et al. [213] |
| Cell Type | Organism | In Vitro Model | EDCs | Mechanism of Action | Concentration * | References |
|---|---|---|---|---|---|---|
| Pancreatic cells/islets | Animal | Rat pancreatic islets | BPA | acute exposure (60 min) insulin secretion with glucose (8.3 mM or 16.7 mM) stimulation (−) (0.1 µg/L, 1 µg/L, 10 µg/L, 100 µg/L, 1000 µg/L) long-term exposure (24 h) insulin secretion with glucose (16.7 mM) stimulation ↑ (10 µg/L, 100 µg/L) co-incubation of BPA (10 µg/L) with actinomycin-D (Act-D) significantly suppressed insulin secretion probable mechanism of action via cytosolic/nuclear estrogen receptors | 10 µg/L, 100 µg/L | Adachi et al. [216] |
| NP | acute exposure (60 min) insulin secretion with glucose (8.3 mM or 16.7 mM) stimulation (−) (0.1 µg/L, 1 µg/L, 10 µg/L, 100 µg/L, 1000 µg/L) long-term exposure (24 h) insulin secretion with glucose (16.7 mM) stimulation ↑ (0.1 µg/L, 1 µg/L, 10 µg/L, 100 µg/L) co-incubation of NP (10 µg/L) with Act-D significantly suppressed insulin secretion probable mechanism of action via cytosolic/nuclear estrogen receptors | 0.1 µg/L, 1 µg/L, 10 µg/L, 100 µg/L | Adachi et al. [216] | |||
| TBT | viability of cells ↓ (0.01 µM, 0.1 µM, 1 µM, 10 µM, 100 µM) ↑ (10 µM) insulin secretion at both basal (2.8 mM) and stimulatory (16.7 mM) concentrations ROS generation ↑ (10 µM) | 0.01 µM, 0.1 µM, 1 µM, 10 µM, 100 µM | Ghaemmaleki et al. [217] | |||
| INS-1 cell line | BPA | viability of cells after 48 h exposure ↓ (0.002 µM, 0.02 µM, 0.2 µM, 2 µM) insulin secretion with basal glucose concentration (5.6 mM) ↑ (0.02 µM), (−) (0.002 µM, 0.2 µM, 2 µM) insulin secretion with stimulatory glucose concentration (16.7 mM) ↑ (0.002 µM), ↓ (0.2 µM, 2 µM), (−) (0.02 µM) mRNA level of insulin ↓ (0.02 µM, 0.2 µM, 2 µM), (−) (0.002 µM) protein level of insulin ↓ (0.02 µM, 0.2 µM, 2 µM) mRNA level of genes involved in GSIS pathway: GLUT2, GCK ↓ (0.2 µM, 2 µM), (−) (0.002 µM, 0.02 µM; KIR6.2, SUR ↑ (0.002 µM), ↓ (0.02 µM, 0.2 µM, 2 µM) changes in mitochondrial morphology and mass ↑ (0.02 µM, 0.2 µM, 2 µM) cellular ATP level ↓ (0.02 µM, 0.2 µM, 2 µM), (−) (0.002 µM) mitochondrial potential ↓ (0.2 µM, 2 µM), (−) (0.002 µM, 0.02 µM) mRNA level of genes involved in mitochondrial metabolism and function: UCP2 ↑ (0.02 µM, 0.2 µM, 2 µM), (−) (0.002 µM); ATP6, Citrate synthase ↓ (0.02 µM, 0.2 µM, 2 µM), (−) (0.002 µM); TFAM ↓ (0.2 µM, 2 µM), (−) (0.002 µM, 0.02 µM); OGDH ↓ (0.002 µM, 0.02 µM, 0.2 µM, 2 µM); ND4L (−) (0.002 µM, 0.02 µM, 0.2 µM, 2 µM) apoptosis ↑ (0.2 µM, 2 µM) release of cytochrome c from the mitochondria to the cytosol ↑ (0.02 µM, 0.2 µM, 2 µM), (−) (0.002 µM) protein level of pro-apoptotic protein BAX, APAF1, 17-kDa cleaved form of caspase-9 ↑ (0.02 µM, 0.2 µM, 2 µM), (−) (0.002 µM), 17-kDa form of cleaved caspase-3 ↑ (0.02 µM, 0.2 µM, 2 µM), (−) (0.002 µM), 19-kDa form of cleaved caspase-3 ↑ (0.002 µM, 0.02 µM, 0.2 µM, 2 µM), anti-apoptotic protein Bcl-2 ↓ (0.02 µM, 0.2 µM, 2 µM), (−) (0.002 µM), 40-kDa cleaved form of caspase-9 (−) (0.002 µM, 0.02 µM, 0.2 µM, 2 µM) | 0.002 µM, 0.02 µM, 0.2 µM, 2 µM | Lin et al. [219] | ||
| INS-1E cell line | BPA | cell viability after 48 h exposure ↓ (10 pM, 1 nM, 1 µM), (−) (0.1 pM, 1 pM, 100 pM) β-cell apoptosis ↑ (0.1 pM, 1 pM, 10 pM, 100 pM, 1 nM, 1 µM) ERα and ERβ involved in BPA-induced apoptosis ROS generation ↑ (1 nM, 1 µM) mRNA level of MAFA, PDX1, INS1, INS2, GLUT2, GCK (−) (0.1 pM, 1 pM, 10 pM, 100 pM, 1 nM, 1 µM) | 0.1 pM, 1 pM, 10 pM, 100 pM, 1 nM, 1 µM | Dos Santos et al. [23] | ||
| TBT | cell viability after 48 h exposure ↓ (20 nM, 50 nM, 100 nM, 200 nM), (−) (1 nM, 10 nM) β-cell apoptosis ↑ (10 nM, 20 nM, 50 nM, 100 nM, 200 nM), (−) (1 nM) PPARγ involved in TBT-induced apoptosis ROS generation ↑ (20 nM, 200 nM) mRNA level of MAFA, PDX1 ↓ (200 nM), (−) (1 nM, 10 nM, 20 nM, 50 nM, 100 nM), INS1, INS2, GLUT2, GCK (−) (1 nM, 10 nM, 20 nM, 50 nM, 100 nM, 200 nM) | 10 nM, 20 nM, 50 nM, 100 nM, 200 nM | Dos Santos et al. [23] | |||
| PFOA | cell viability after 48 h exposure (−) (10 pM, 100 pM, 1 nM, 10 nM, 100 nM, 1 µM) β-cell apoptosisthe ↑ (10 µM, 20 µM, 50 µM, 10the 0 µM, 200 µM), (−) (1 nM, 1 µM) ROS generation (−) (1 nM, 1 µM) mRNA level of MAFA, PDX1, INS1, INS2, GLUT2, GCK (−) (1 nM, 1 µM) | 10 µM, 20 µM, the 50 µM, 100 µM, 200 µM | Dos Santos et al. [23] | |||
| TPP | cell viability after 48 h exposure (−) (10 pM, 100 the pM, 1 nM, 10 nM, 100 nM, 1 µM) β-cell apoptosis (−) (10 pM, 100 pM, 1 nM, 10 nM, 100 nM, 1 µM) mRNAthe level of MAFA, PDX1, INS1, INS2, GLUT2, GCK (−) (1 nM, 1 µM) | none of the the doses tested resulted in a biological effect | Dos Santos et al. [23] | |||
| TCS | cell viability after 48 h exposure (−) (10 pM, 100 pM, 1 nM, 10 nM, 100 nM, 1 µM) β-cell apoptosis (−) (10 pM, 100 pM, 1 nM, 10 nM, 100 nM, 1 µM) mRNA level of MAFA, PDX1, INS1, INS2, GLUT2, GCK (−) (1 nM, 1 µM) | none of the doses tested resulted in a biological effect | Dos Santos et al. [23] | |||
| DDE | cell viability after 48 h exposure (−) (10 pM, 100 pM, 1 nM, 10 nM, 100 nM, 1 µM) β-cell apoptosis (−) (10 pM, 100 pM, 1 nM, 10 nM, 100 nM, 1 µM) mRNA level of MAFA, PDX1, INS1, INS2, GLUT2, GCK (−) (1 nM, 1 µM) | none of the doses tested resulted in a biological effect | Dos Santos et al. [23] | |||
| p,p’-DDE | protein expression of vitamin D-binding protein (VDBP) ↑, glucosidase 2 subunit beta precursor ↓ intracellular protein level of proinsulin, insulin monomer ↓, hexameric insulin (−) mRNA level of INS1, INS2 ↓, VDBP (−) | 10 µM | Pavlíková et al. [220] | |||
| p,p’-DDT | protein expression of tubulin beta-5 chain, annexin A4, vitamin D-binding protein (VDBP) ↑, actin, mortalin/GRP75 ↓ intracellular protein level of proinsulin, hexameric insulin, insulin monomer ↓ mRNA level of VDBP ↑, INS1, INS2 ↓ | 10 µM | Pavlíková et al. [220] | |||
| RIN-m5F cell line | TBT | viability of cells (−) (0.05 µM, 0.1 µM, 0.2 µM) GSIS (20 mM glucose) ↑ (0.1 µM, 0.2 µM), (−) (0.05 µM) insulin secretion ↑ (0.1 µM) intracellular calcium level ↑ (0.1 µM, 0.2 µM) protein level of p-PKC, p-ERK1/2 ↑, p-Akt (−) (0.1 µM, 0.2 µM) level of intracellular ROS ↑ (0.05 µM, 0.1 µM, 0.2 µM) | 0.05 µM, 0.1 µM, 0.2 µM | Chen et al. [106] | ||
| viability of cells ↓ (0.5 µM, 1 µM), (−) (0.1 µM, 0.2 µM) GSIS (after 24 h exposure) ↓ (0.5 µM, 1 µM) apoptosis ↑ (0.5 µM) cleavage of PARP ↑ (0.5 µM, 1 µM) level of p-JNK, p-ERK1/2 ↑, p-38 (−) (0.5 µM, 1 µM) intracellular ROS generation ↑ (0.2 µM, 0.5 µM, 1 µM) caspase-3 activity ↑ (0.5 µM) | 0.2 µM, 0.5 µM, 1 µM | Huang et al. [221] | ||||
| PFOA | viability of cells ↓ (100 µM, 200 µM, 300 µM, 500 µM), (−) (1 µM) apoptosis ↑ (100 µM, 300 µM, 500 µM), (−) (1 µM) ROS generation ↑ (200 µM, 300 µM, 400 µM, 500 µM), (−) (1 µM, 10 µM, 50 µM, 100 µM) mitochondrial superoxide accumulation ↑ (200 µM, 300 µM, 500 µM), (−) (1 µM, 10 µM, 50 µM, 100 µM) NO (nitric oxide) production ↑ (50 µM, 100 µM, 200 µM, 300 µM, 400 µM, 500 µM), (−) (1 µM, 10 µM) cytosolic level of TNFα ↑ (100 µM, 150 µM, 200 µM, 300 µM, 500 µM), (−) (10 µM), Il1β ↑ (200 µM, 300 µM, 500 µM), (−) (10 µM, 100 µM, 150 µM) MMP collapse ↑ (300 µM, 500 µM), (−) (1 µM, 10 µM, 50 µM, 100 µM, 200 µM) intracellular ATP level ↑ (10 µM, 50 µM), ↓ (200 µM, 300 µM, 500 µM), (−) (1 µM, 100 µM) cytochrome c release ↑ (200 µM, 300 µM, 500 µM), (−) (10 µM, 100 µM) cardiolipin peroxidation ↑ (200 µM, 300 µM, 500 µM), (−) (10 µM, 100 µM) | 10 µM, 50 µM, 100 µM, 150 µM, 200 µM, 300 µM, 400 µM, 500 µM | Suh et al. [222] | |||
| Primary β-cells from wild type mouse | BPA | β-cell apoptosis ↑ (1 nM, 1 µM) | 1 nM, 1 µM | Dos Santos et al. [23] | ||
| insulin secretion in the presence of 8 mM glucose ↑ KATP channel activity ↓ frequency of [Ca2+]i oscillations ↑ | 1 nM | Soriano et al. [223] | ||||
| viability of cells (after 48 h exposure) ↓ (0.001 µM, 1 µM, 100 µM) number of apoptotic cells (after 48 h exposure) ↑ (0.001 µM, 1 µM, 100 µM) mRNA level of genes encoding components of respiratory chain complexes: NDUFS4, UQCRB, genes involved in ATP production and/or in insulin exocytosis process: VAPA, ATP1B1, ATP6V1F, genes involved in detoxification: SOD2, GPX3, ZFAND2A, anti-apoptotic gene BCL-2 ↓ (0.001 µM), pro-apoptotic gene Bax ↑ (0.001 µM) ROS level ↑ (0.001 µM) MMP ↓ (0.001 µM) GSIS (16 mM glucose) ↓ (0.001 µM, 100 µM) probable mechanism of action via activation of NF-kB pathway | 0.001 µM, 1 µM, 100 µM | Carchia et al. [105] | ||||
| BPS | insulin secretion in response to 16.7 mM concentration (during 1 h treatment of BPS) ↑ (1 nM, 1 µM) insulin secretion in response to 8.3 mM concentration (during 48 h treatment of BPS) ↑ (1 nM, 1 µM) insulin secretion in response to 16.7 mM concentration (during 48 h treatment of BPS) ↑ (1 nM), (−) (1 µM) KATP channel activity ↓ (1 nM) mRNA level of (after 48 h treatment of BPS) CACNA1E, KCNMa1, SCN9A ↓ (1 nM), (−) (100 nM, 1 µM), KCNIP ↑ (1 nM), (−) (100 nM, 1 µM) Ca2+ currents ↓ (1 nM), (−) (100 nM, 1 µM) | 1 nM, 1 µM | Marroqui et al. [224] | |||
| BPF | insulin secretion in response to 16.7 mM concentration (during 1 h treatment of BPF) ↑ (1 nM, 1 µM) insulin secretion in response to 16.7 mM concentration (during 48 h treatment of BPF) ↑ (1 µM) KATP channel activity ↓ (10 nM), (−) (1 nM) mRNA level of (after 48 h treatment of BPF) CACNA1E ↓ (100 nM, 1 µM), (−) (1 nM), KCNMA1, SCN9A, KCNIP1 ↓ (1 µM), (−) (1 nM, 100 nM) Ca2+ currents ↓ (1 µM), (−) (1 nM, 100 nM) | 1 nM, 10 nM, 100 nM, 1 µM | Marroqui et al. [224] | |||
| TBT | β-cell apoptosis ↑ (20 nM, 200 nM) | 20 nM, 200 nM | Dos Santos et al. [23] | |||
| GSIS (20 mM glucose) ↑ ROS generation ↑ | 0.1 µM, 0.2 µM | Chen et al. [106] | ||||
| PFOA | β-cell apoptosis (−) (1 nM, 1 µM) | none of the doses tested resulted in a biological effect | Dos Santos et al. [23] | |||
| TPP | β-cell apoptosis (−) (1 nM, 1 µM) | none of the doses tested resulted in a biological effect | Dos Santos et al. [23] | |||
| TCS | β-cell apoptosis (−) (1 nM, 1 µM) | none of the doses tested resulted in a biological effect | Dos Santos et al. [23] | |||
| DDE | β-cell apoptosis (−) (1 nM, 1 µM) | none of the doses tested resulted in a biological effect | Dos Santos et al. [23] | |||
| Primary β-cells from ERβ-/- mouse (estrogen receptor β (ERβ) knockout (BERKO) mouse) | BPA | insulin secretion in the presence of 8 mM glucose (−) KATP channel activity (−) frequency of [Ca2+]i oscillations ↓ | 1 nM | Soriano et al. [223] | ||
| BPS | KATP channel activity (−) (1 nM) Ca2+ currents (−) (1 nM, 100 nM, 1 µM) mRNA level of (after 48 h treatment of BPS) CACNA1E, KCNMA1, SCN9A (−) (1 nM) | none of the doses tested resulted in a biological effect | Marroqui et al. [224] | |||
| BPF | KATP channel activity (−) (1 nM, 10 nM) Ca2+ currents (−) (1 nM, 100 nM, 1 µM) mRNA level of (after 48 h treatment of BPF) CACNA1E ↓, KCNMA1, SCN9A (−) (1 µM) | 1 µM | Marroqui et al. [224] | |||
| MIN-6 cell line | BPA | viability of cells (after 24 h exposure) ↓ (100 pM, 1 nM, 10 nM, 100 nM, 1 µM), (−) (10 µM) mRNA level of PDX1 ↑ (100 pM, 10 nM, 1 µM), (−) (1 nM, 100 nM, 10 µM); HNF4α ↑ (100 pM, 10 nM, 1 µM, 10 µM), (−) (1 nM, 100 nM); KIR6.2 ↑ (10 nM), (−) (100 pM, 1 nM, 100 nM, 1 µM, 10 µM); MAFA ↑ (100 nM), (−) (100 pM, 1 nM, 10 nM, 1 µM, 10 µM); INS, SUR1, GLUT2, GCK (−) (100 pM, 1 nM, 10 nM, 100 nM, 1 µM, 10 µM) GSIS (20 mM glucose) ↑ (100 nM), (−) (100 pM, 1 nM, 10 nM, 1 µM, 10 µM) insulin secretion in response to low glucose concentration (1.67 mM) ↑ (100 nM, 10 µM), (−) (100 pM, 1 nM, 10 nM, 1 µM) insulin content ↑ (10 µM), (−) (100 pM, 1 nM, 10 nM, 100 nM, 1 µM) | 100 pM, 1 nM, 10 nM, 100 nM, 1 µM, 10 µM | Al-Abdulla et al. [229] | ||
| BPS | viability of cells (after 24 h exposure) ↓ (100 pM, 1 nM, 10 nM, 100 nM, 1 µM, 10 µM) mRNA level of MAFA ↑ (10 nM, 100 nM), (−) (100 pM, 1 nM, 1 µM, 10 µM); KIR6.2 ↑ (10 nM, 100 nM, 10 µM), (−) (100 pM, 1 nM, 1 µM); HNF4α ↓ (100 pM), (−) (1 nM, 10 nM, 100 nM, 1 µM, 10 µM); GlUT2 ↓ (1 nM, 10 nM, 100 nM, 1 µM), (−) (100 pM, 10 µM), INS, PDX1, SUR1, GCK (−) (100 pM, 1 nM, 10 nM, 100 nM, 1 µM, 10 µM) GSIS (20 mM) ↓ (100 pM, 10 nM, 1 µM, 10 µM), (−) (1 nM, 100 nM) insulin secretion in response to low glucose concentration (1.67 mM) (−) (100 pM, 1 nM, 10 nM, 100 nM, 1 µM, 10 µM) insulin content ↓ (10 nM, 100 nM, 1 µM, 10 µM), (−) (100 pM, 1 nM) | 100 pM, 1 nM, 10 nM, 100 nM, 1 µM, 10 µM | Al-Abdulla et al. [229] | |||
| DEHP | viability of cells (after 24 h exposure) ↓ (1 µM), (−) (100 pM, 1 nM, 10 nM, 100 nM, 10 µM) mRNA level of SUR1, GLUT2 ↑ (10 µM), (−) (100 pM, 1 nM, 10 nM, 100 nM, 1 µM); INS, PDX1, HNF4α, MAFA, KIR6.2, GCK (−) (100 pM, 1 nM, 10 nM, 100 nM, 1 µM, 10 µM) GSIS (20 mM glucose) ↓ (100 pM, 1 nM, 10 nM, 100 nM, 1 µM, 10 µM) insulin secretion in response to low glucose concentration (1.67 mM) (−) (100 pM, 1 nM, 10 nM, 100 nM, 1 µM, 10 µM) insulin content ↓ (1 µM), (−) (100 pM, 1 nM, 10 nM, 100 nM, 10 µM) | 100 pM, 1 nM, 10 nM, 100 nM, 1 µM, 10 µM | Al-Abdulla et al. [229] | |||
| PFOS | viability of cells (after 24 h exposure) ↓ (100 pM, 1 nM, 10 nM, 100 nM, 1 µM, 10 µM) mRNA level of INS ↑ (1 nM), (−) (100 pM, 10 nM, 100 nM, 1 µM, 10 µM), HNF4α ↑ (10 nM), (−) (100 pM, 1 nM, 100 nM, 1 µM, 10 µM), MAFA ↓ (1 µM), (−) (100 pM, 1 nM, 10 nM, 100 nM, 10 µM), GLUT2 ↓ (10 nM, 100 nM, 1 µM, 10 µM), (−) (100 pM, 1 nM), PDX1, KIR6.2, SUR1, GCK (−) (100 pM, 1 nM, 10 nM, 100 nM, 1 µM, 10 µM) GSIS (20 mM) ↓ (100 pM, 100 nM, 10 µM), (−) (1 nM, 10 nM, 1 µM) insulin secretion in response to low glucose concentration (1.67 mM) (−) (100 pM, 1 nM, 10 nM, 100 nM, 1 µM, 10 µM) insulin content (−) (100 pM, 1 nM, 10 nM, 100 nM, 1 µM, 10 µM) | 100 pM, 1 nM, 10 nM, 100 nM, 1 µM, 10 µM | Al-Abdulla et al. [229] | |||
| CdCl2 | viability of cells (after 24 h exposure) ↓ (100 pM, 1 nM, 10 nM, 100 nM, 1 µM, 10 µM) mRNA level of INS ↑ (100 pM, 100 nM), (−) (1 nM, 10 nM); MAFA ↑ (100 nM), (−) (100 pM, 1 nM, 10 nM); PDX1, HNF4α, KIR6.2, SUR1, GLUT2, GCK (−) (100 pM, 1 nM, 10 nM, 100 nM) GSIS (20 mM glucose) (−) (100 pM, 1 nM, 10 nM, 100 nM, 1 µM) insulin secretion in response to low glucose concentration (1.67 mM) ↓ (100 pM), (−) (1 nM, 10 nM, 100 nM, 1 µM) insulin content (−) (100 pM, 1 nM, 10 nM, 100 nM, 1 µM) | 100 pM, 1 nM, 10 nM, 100 nM, 1 µM, 10 µM | Al-Abdulla et al. [229] | |||
| DDE | viability of cells (after 24 h exposure) ↓ (10 nM), (−) (100 pM, 1 nM, 100 nM, 1 µM, 10 µM) mRNA level of SUR1 ↑ (100 pM), (−) (1 nM, 10 nM, 100 nM, 1 µM, 10 µM); INS ↓ (1 nM, 1 µM, 10 µM), (−) (100 pM, 10 nM, 100 nM); MAFA ↓ (1 nM), (−) (1 nM, 10 nM, 100 nM, 1 µM, 10 µM); PDX1; HNF4α, KIR6.2, GLUT2, GCK (−) (100 pM, 1 nM, 10 nM, 100 nM, 1 µM, 10 µM) GSIS (20 mM) (−) (100 pM, 1 nM, 10 nM, 100 nM, 1 µM, 10 µM) insulin secretion in response to low glucose concentration (1.67 mM) (−) (100 pM, 1 nM, 10 nM, 100 nM, 1 µM, 10 µM) insulin content (−) (100 pM, 1 nM, 10 nM, 100 nM, 1 µM, 10 µM) | 100 pM, 1 nM, 10 nM, 1 µM, 10 µM | Al-Abdulla et al. [229] | |||
| β-TC-6 cell line | PFOS | GSIS (1.4 mM glucose) ↑ (50 µM, 100 µM) insulin secretion in the absence of glucose ↑ (50 µM) intracellular calcium level ↑ (5 µM, 10 µM, 50 µM, 100 µM) probable mechanism of action via GPR40 activation | 5 µM, 10 µM, 50 µM, 100 µM | Qin et al. [231] | ||
| p,p’-DDE | basal insulin secretion ↑ (10 µM) GSIS (5 mM) ↑ (10 µM) ROS level (−) (10 µM) intracellular protein level of PDX1 (−) (1 µM, 10 µM, 100 µM) PC enzyme activity ↑ (10 µM), (−) (100 µM) | 10 µM | Ward et al. [232] | |||
| Human | Pancreatic islets | BPA | insulin secretion in the presence of 8 mM glucose ↑ KATP channel activity ↓ | 1 nM | Soriano et al. [223] | |
| TBT | GSIS (20 mM glucose) ↑ | 0.1 µM | Chen et al. [106] | |||
| EndoC-βH1 cell line | BPA | cell viability after 48 h exposure ↓ (1 pM, 10 pM, 100 pM, 1 nM, 1 µM), (−) (0.1 pM) β-cell apoptosis ↑ (1 pM, 10 pM, 100 pM, 1 nM, 1 µM), (−) (0.1 pM) caspase 3/7 activity ↑ (10 nM, 1 µM) Erα and Erβ involved in BPA-induced apoptosis ROS generation ↑ (1 nM, 1 µM) GSIS (20 mM glucose) (−) (0.1 pM, 1 pM, 10 pM, 100 pM, 1 nM, 1 µM) insulin content (−) (0.1 pM, 1 pM, 10 pM, 100 pM, 1 nM, 1 µM) mRNA level of MAFA, PDX1, INS, GLUT2, GCK (−) (0.1 pM, 1 pM, 10 pM, 100 pM, 1 nM, 1 µM) | 1 pM, 10 pM, 100 pM, 1 nM, 10 nM, 1 µM | Dos Santos et al. [23] | ||
| cell viability after 72 h exposure (−) (1 nM, 10 nM, 100 nM, 1 µM) mRNA level of MAFB ↑ (10 nM), (−) (1 nM, 100 nM), SNAP25 ↑ (1 nM), (−) (10 nM, 100 nM), INS, PDX1, HNF4α, MAFA, KIR6.2, SUR1, GLUT1, GCK (−) (1 nM, 10 nM, 100 nM, 1 µM) GSIS (20 mM glucose) ↑ (10 nM, 100 nM, 1 µM), (−) (1 nM) basal insulin secretion ↑ (1 µM), (−) (1 nM, 10 nM, 100 nM) insulin content ↑ (1 nM, 10 nM, 100 nM, 1 µM) | 1 nM, 10 nM, 100 nM, 1 µM | Al-Abdulla et al. [229] | ||||
| BPS | cell viability after 48 h exposure ↓ (1 µM), (−) (1 nM, 10 nM, 100 nM) mRNA level of HNF4α ↑ (1 nM), (−) (10 nM, 100 nM), MAFA ↓ (100 nM), (−) (1 nM, 10 nM), MAFB, GLUT1 ↓ (10 nM, 100 nM), (−) (1 nM), KIR6.2 ↓ (10 nM), (−) (1 nM, 100 nM), SNAP25 ↓ (1 nM), (−) (10 nM, 100 nM), INS, PDX1, SUR1, GCK (−) (1 nM, 10 nM, 100 nM) GSIS (20 mM) ↓ (1 nM, 1 µM), (−) (10 nM, 100 nM) basal insulin secretion (−) (1 nM, 10 nM, 100 nM, 1 µM) insulin content (−) (1 nM, 10 nM, 100 nM, 1 µM) | 1 nM, 10 nM, 100 nM, 1 µM | Al-Abdulla et al. [229] | |||
| BPF | cell viability after 72 h exposure ↓ (1 µM), (−) (1 nM, 10 nM, 100 nM) GSIS (20 mM glucose) (−) (1 nM, 10 nM, 100 nM, 1 µM) insulin secretion in response to low glucose concentration (2.8 mM) (−) (1 nM, 10 nM, 100 nM, 1 µM) | 1 µM | Al-Abdulla et al. [229] | |||
| DEHP | cell viability after 7 days of exposure ↓ (1 nM, 10 nM, 100 nM), (−) (1 µM) mRNA level of INS, PDX1, HNF4α, MAFA, MAFB, KIR6.2, SUR1, SNAP25, GLUT1, GCK (−) (1 nM, 10 nM, 100 nM) GSIS (20 mM) ↑ (1 µM), ↓ (1 nM, 10 nM, 100 nM) insulin secretion in response to low glucose concentration (2.8 mM) (−) (1 nM, 10 nM, 100 nM, 1 µM) insulin content ↑ (1 µM), ↓ (1 nM, 10 nM, 100 nM) | 1 nM, 10 nM, 100 nM, 1 µM | Al-Abdulla et al. [229] | |||
| TBT | cell viability after 48 h exposure ↓ (20 nM, 50 nM, 100 nM, 200 nM), (−) (1 nM, 10 nM) β-cell apoptosis ↑ (1 nM, 20 nM, 50 nM, 100 nM, 200 nM), (−) (10 nM) caspase 3/7 activity ↑ (20 nM, 200 nM) PPARγ involved in TBT-induced apoptosis ROS generation ↑ (20 nM, 200 nM) GSIS ↑ (20 mM glucose), (−) (1 nM, 10 nM, 20 nM, 50 nM, 100 nM) insulin content ↑ (20 nM), ↓ (200 nM), (−) (1 nM, 10 nM, 50 nM, 100 nM) mRNA level of GLUT2 ↑ (200 nM), (−) (1 nM, 10 nM, 20 nM, 50 nM, 100 nM), MAFA ↓ (10 nM, 20 nM, 50 nM, 100 nM, 200 nM), (−) (1 nM), PDX1, INS, GCK (−) (1 nM, 10 nM, 20 nM, 50 nM, 100 nM, 200 nM) | 1 nM, 20 nM, 50 nM, 100 nM, 200 nM | Dos Santos et al. [23] | |||
| PFOA | cell viability after 48 h exposure (−) (10 pM, 100 pM, 1 nM, 10 nM, 100 nM, 1 µM) β-cell apoptosis ↑ (20 µM, 50 µM, 100 µM, 200 µM), (−) (1 nM, 1 µM) caspase 3/7 activity (−) (1 µM) ROS generation (−) (1 nM, 1 µM) GSIS (20 mM glucose) ↓ (10 pM, 1 nM, 10 nM, 100 nM), (−) (100 pM, 1 µM) insulin secretion at low glucose concentration (0 mM) ↓ (1 nM), (−) (10 pM, 100 pM, 10 nM, 100 nM, 1 µM) insulin content (−) (10 pM, 100 pM, 1 nM, 10 nM, 100 nM, 1 µM) mRNA level of MAFA, PDX1, INS, GLUT2, GCK (−) (1 nM, 1 µM) | 10 pM, 1 nM, 10 nM, 100 nM, 20 µM, 50 µM, 100 µM, 200 µM | Dos Santos et al. [23] | |||
| PFOS | cell viability after 72 h exposure (−) (1 nM, 10 nM, 100 nM, 1 µM) mRNA level of INS, PDX1, HNF4α, MAFA, MAFB, KIR6.2, SUR1, SNAP25, GLUT1, GCK (−) (10 nM, 100 nM) GSIS (20 mM glucose) ↑ (10 nM, 100 nM), (−) (1 nM, 1 µM) insulin secretion in response to low glucose concentration (2.8 mM) ↑ (10 nM, 1 µM), (−) (1 nM, 100 nM) insulin content ↑ (1 nM, 10 nM, 1 µM), (−) (100 nM) | 1 nM, 10 nM, 100 nM, 1 µM | Al-Abdulla et al. [229] | |||
| TPP | cell viability after 48 h exposure (−) (10 pM, 100 pM, 1 nM, 10 nM, 100 nM, 1 µM) β-cell apoptosis (−) (1 nM, 1 µM) caspase 3/7 activity (−) (1 µM) GSIS (20 mM glucose) ↑ (1 µM), (−) (10 pM, 100 pM, 1 nM, 10 nM, 100 nM) insulin secretion at low glucose concentration (0 mM) ↑ (100 pM) insulin content (−) (10 pM, 100 pM, 1 nM, 10 nM, 100 nM, 1 µM) mRNA level of MAFA, PDX1, INS, GLUT2, GCK (−) (1 nM, 1 µM) | 100 pM, 1 µM | Dos Santos et al. [23] | |||
| TCS | cell viability after 48 h exposure (−) (10 pM, 100 pM, 1 nM, 10 nM, 100 nM, 1 µM) β-cell apoptosis (−) (1 nM, 1 µM) caspase 3/7 activity (−) (1 µM) GSIS (−) (10 pM, 100 pM, 1 nM, 10 nM, 100 nM, 1 µM) insulin content (−) (10 pM, 100 pM, 1 nM, 10 nM, 100 nM, 1 µM) mRNA level of MAFA, PDX1, INS, GLUT2, GCK (−) (1 nM, 1 µM) | none of the doses tested resulted in a biological effect | Dos Santos et al. [23] | |||
| CdCl2 | cell viability after 72 h exposure ↓ (1 nM, 10 nM, 100 nM), (−) (1 µM) mRNA level of HNF4α, SNAP25 ↓ (100 nM), (−) (10 nM), INS, PDX1, MAFA, MAFB, KIR6.2, SUR1, GLUT1, GCK (−) (10 nM, 100 nM) GSIS (20 mM glucose) ↑ (10 nM), (−) (1 nM, 100 nM, 1 µM) insulin secretion in response to low glucose concentration (2.8 mM) (−) (1 nM, 10 nM, 100 nM, 1 µM) insulin content (−) (1 nM, 10 nM, 100 nM, 1 µM) | 1 nM, 10 nM, 100 nM | Al-Abdulla et al. [229] | |||
| DDE | cell viability after 48 h exposure (−) (10 pM, 100 pM, 1 nM, 10 nM, 100 nM, 1 µM) β-cell apoptosis (−) (1 nM, 1 µM) caspase 3/7 activity (−) (1 µM) GSIS (20 mM glucose) ↑ (10 nM, 1 µM), (−) (10 pM, 100 pM, 1 nM, 100 nM) insulin content (−) (10 pM, 100 pM, 1 nM, 10 nM, 100 nM, 1 µM) mRNA level of MAFA, PDX1, INS, GLUT2, GCK (−) (1 nM, 1 µM) | 10 nM, 1 µM | Dos Santos et al. [23] | |||
| cell viability after 7 days of exposure ↓ (1 nM, 10 nM, 100 nM, 1 µM) mRNA level of INS, PDX1, HNF4α, MAFA, MAFB, KIR6.2, SUR1, SNAP25, GLUT1, GCK (−) (1 nM, 10 nM, 100 nM) GSIS (20 mM glucose) (−) (1 nM, 10 nM, 100 nM, 1 µM) insulin secretion in response to low glucose concentration (2.8 mM) (−) (1 nM, 10 nM, 100 nM, 1 µM) insulin content (−) (1 nM, 10 nM, 100 nM, 1 µM) | 1 nM, 10 nM, 100 nM, 1 µM | Al-Abdulla et al. [229] | ||||
| NES2Y cell line | p,p’-DDT | viability of cells after 24 h and 48 h exposure ↓ (100 µM), (−) (0.1 nM, 1 nM, 10 nM, 0.1 µM, 1 µM, 10 µM) protein expression of cytokeratin 8, cytokeratin 18, alpha-enolase, actin ↓ (10 µM) | 10 µM, 100 µM | Pavlikova et al. [234] | ||
| viability of cells after 24 h exposure ↓ (150 µM, 175 µM, 200 µM), (−) (100 µM, 125 µM) protein expression of cleaved caspase -6, -7, -8, -9, cleaved PARP, CHOP, GRP78 (BiP), GRP75 (mortalin), NDRG1, EFHD2 ↑, ECHM, vimentin, HSP27, IDH3A, K2C8, HNRPF, BIEA, EF2, EZRI, FRIL, G3BP1, HNRH1, NDUS3, NDUS1, PBDC1, PCNA, TCPA ↓ (150 µM) | 150 µM, 175 µM, 200 µM | Pavlikova et al. [107] | ||||
| p,p’-DDE | viability of cells after 24 h and 48 h exposure ↓ (100 µM), (−) (0.1 nM, 1 nM, 10 nM, 0.1 µM, 1 µM, 10 µM) protein expression of cytokeratin 18, HNRH1 ↓ (10 µM) | 10 µM, 100 µM | Pavlikova et al. [234] | |||
| PANC-1 cell line | BPA | mRNA level of PCSK1 ↓, insulin secretion ↓ | 10 nM | Menale et al. [124] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kowalczyk, M.; Piwowarski, J.P.; Wardaszka, A.; Średnicka, P.; Wójcicki, M.; Juszczuk-Kubiak, E. Application of In Vitro Models for Studying the Mechanisms Underlying the Obesogenic Action of Endocrine-Disrupting Chemicals (EDCs) as Food Contaminants—A Review. Int. J. Mol. Sci. 2023, 24, 1083. https://doi.org/10.3390/ijms24021083
Kowalczyk M, Piwowarski JP, Wardaszka A, Średnicka P, Wójcicki M, Juszczuk-Kubiak E. Application of In Vitro Models for Studying the Mechanisms Underlying the Obesogenic Action of Endocrine-Disrupting Chemicals (EDCs) as Food Contaminants—A Review. International Journal of Molecular Sciences. 2023; 24(2):1083. https://doi.org/10.3390/ijms24021083
Chicago/Turabian StyleKowalczyk, Monika, Jakub P. Piwowarski, Artur Wardaszka, Paulina Średnicka, Michał Wójcicki, and Edyta Juszczuk-Kubiak. 2023. "Application of In Vitro Models for Studying the Mechanisms Underlying the Obesogenic Action of Endocrine-Disrupting Chemicals (EDCs) as Food Contaminants—A Review" International Journal of Molecular Sciences 24, no. 2: 1083. https://doi.org/10.3390/ijms24021083
APA StyleKowalczyk, M., Piwowarski, J. P., Wardaszka, A., Średnicka, P., Wójcicki, M., & Juszczuk-Kubiak, E. (2023). Application of In Vitro Models for Studying the Mechanisms Underlying the Obesogenic Action of Endocrine-Disrupting Chemicals (EDCs) as Food Contaminants—A Review. International Journal of Molecular Sciences, 24(2), 1083. https://doi.org/10.3390/ijms24021083









