Photoperiodic Remodeling of Adiposity and Energy Metabolism in Non-Human Mammals
Abstract
1. Introduction
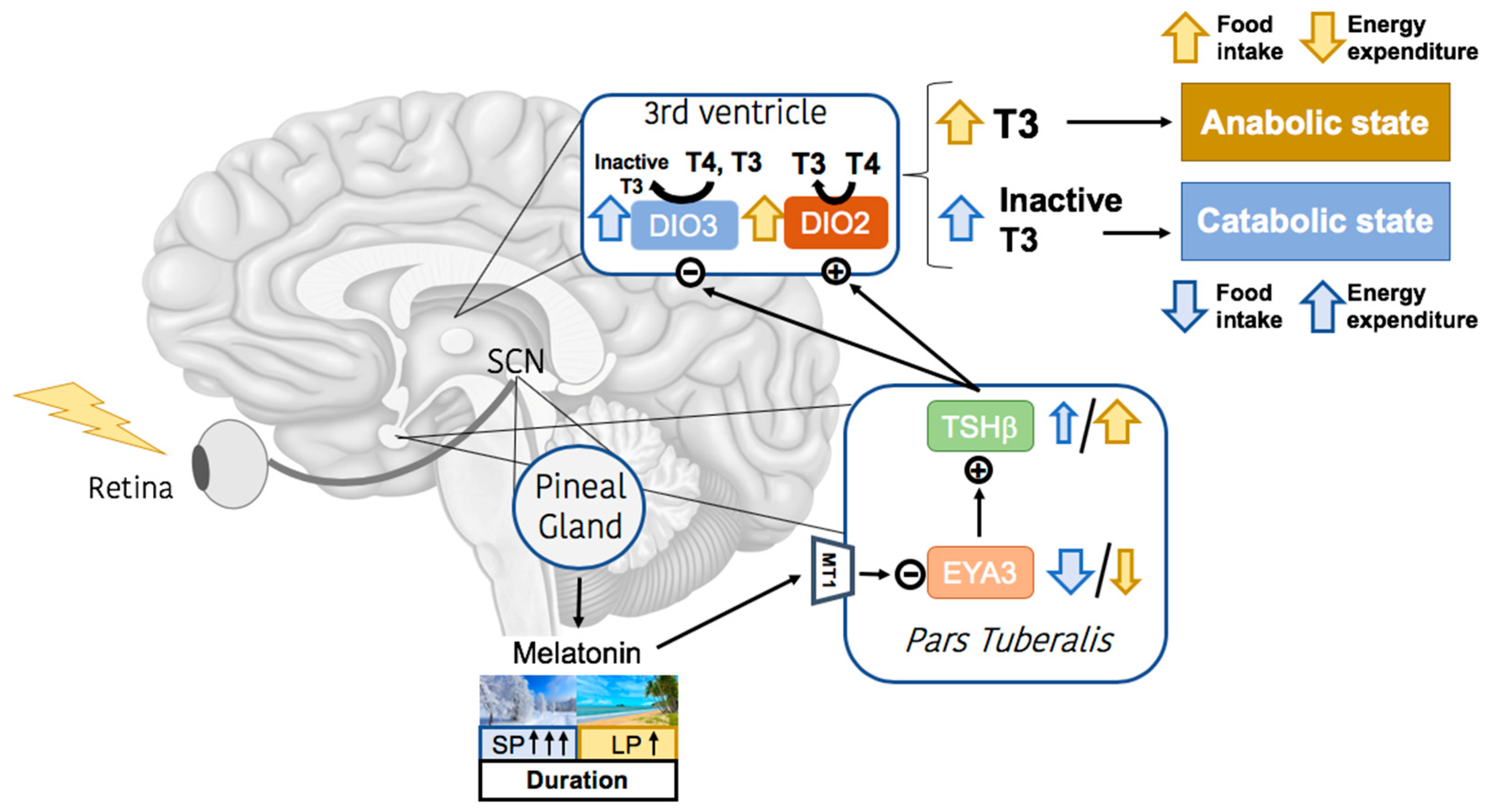
2. Melatonin Signaling and Calendar Cells
3. Effects of Photoperiod on White Adipose Tissue Metabolism
3.1. Metabolic Regulation of White Adipose Tissue
3.2. Photoperiodic Remodeling of WAT Metabolism
4. Effects of Photoperiod on Food Intake and Nutrient Choice
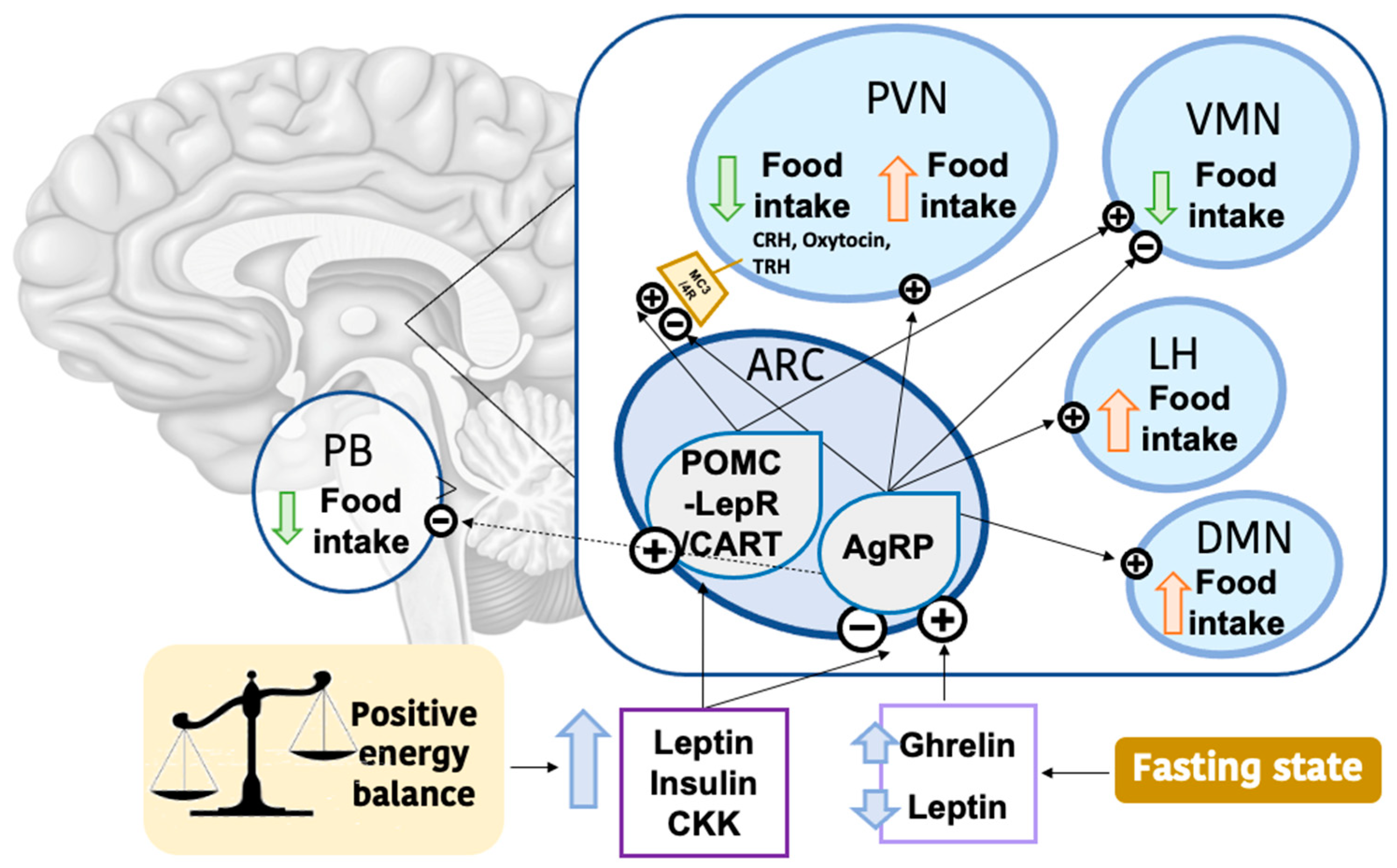
4.1. Hypothalamic Regulation of Food Intake
4.2. Photoperiodic Regulation of Food Intake
4.3. The NMU and Wnt/β-Catenin Signaling
4.4. Photoperiodic Impact in Nutrient Choice
| Species | M/F | Photoperiods (Light/Dark Hours) | Food Intake | Leptin | Hypothalamic Neuropeptides | Reference |
|---|---|---|---|---|---|---|
| F344 rats | M | 16/8–8/16 | ↓ SP | ↑ LP (after 8 h fasting) | Not measured | [17] |
| F344 rats | M | 16/8–8/16 | ↓ SP | ND | SP ↓ Agrp, ↑ Crh ↓↓ Pomc in ventral ependymal region | [46] |
| F344 rats | M | 18/6–6/18 (Control: 12/12) | ↓ Cumulative food intake of SP (from week 9 onwards) | ↑ LP | LP and SP ↑ Npy ↑ Ghrelin receptor (Ghsr) | [49,50] |
| Siberian hamsters | M | 16/8–8/16 | Not measured | ↑ LP (iWAT and rWAT mRNA) | [72] | |
| Siberian hamsters | M | 16/8–8/16 | Not measured | ↑ LP | SP ↓ Pomc, ↓ Obrb, ↓ Mc3-r, ↑ Cart LP-REST ↑ Obrb, ↓ Cart | [61] |
| Siberian hamsters | M | 16/8–8/16 Acute leptin treatment | ↓ SP compared with LP ↓ SP + leptin compared to SP ↓↓ LP + leptin compared with LP | SP ↓ Pomc ↓ Npy (8 weeks) ND in Orexin or Npy (12 weeks) | [63] |
5. Effects of Photoperiod on Energy Expenditure
5.1. Photoperiodic Remodeling of BAT Metabolism
| Species | M/F | Photoperiods (Light/Dark Hours) | BAT Activity | Reference |
|---|---|---|---|---|
| F344 rats | M | 18/6–6/18 | SP ↓ β-oxidation-related genes ↓ FA transport ↓ Thermogenesis (not significant) ↑ Adipogenesis | [18] |
| C57BL/6 mice | M | 12/12–16/8 (No SP group) | LP ↓ Nutrient uptake | [44] |
| Siberian hamsters | M | 16/8–8/16 | SP ↑ Thermogenesis | [9] |
| Siberian hamsters | M | 16/8–8/16 | SP ↑ Thermogenesis | [72] |
| Collared lemmings | M | 16/8–8/16 | SP ↓ Thermogenesis | [84] |
| Syrian Hamsters | F | 16/8–8/16 | SP ↑ BAT weight | [85] |
5.2. Photoperiodic Regulation of Other Metabolic Tissues
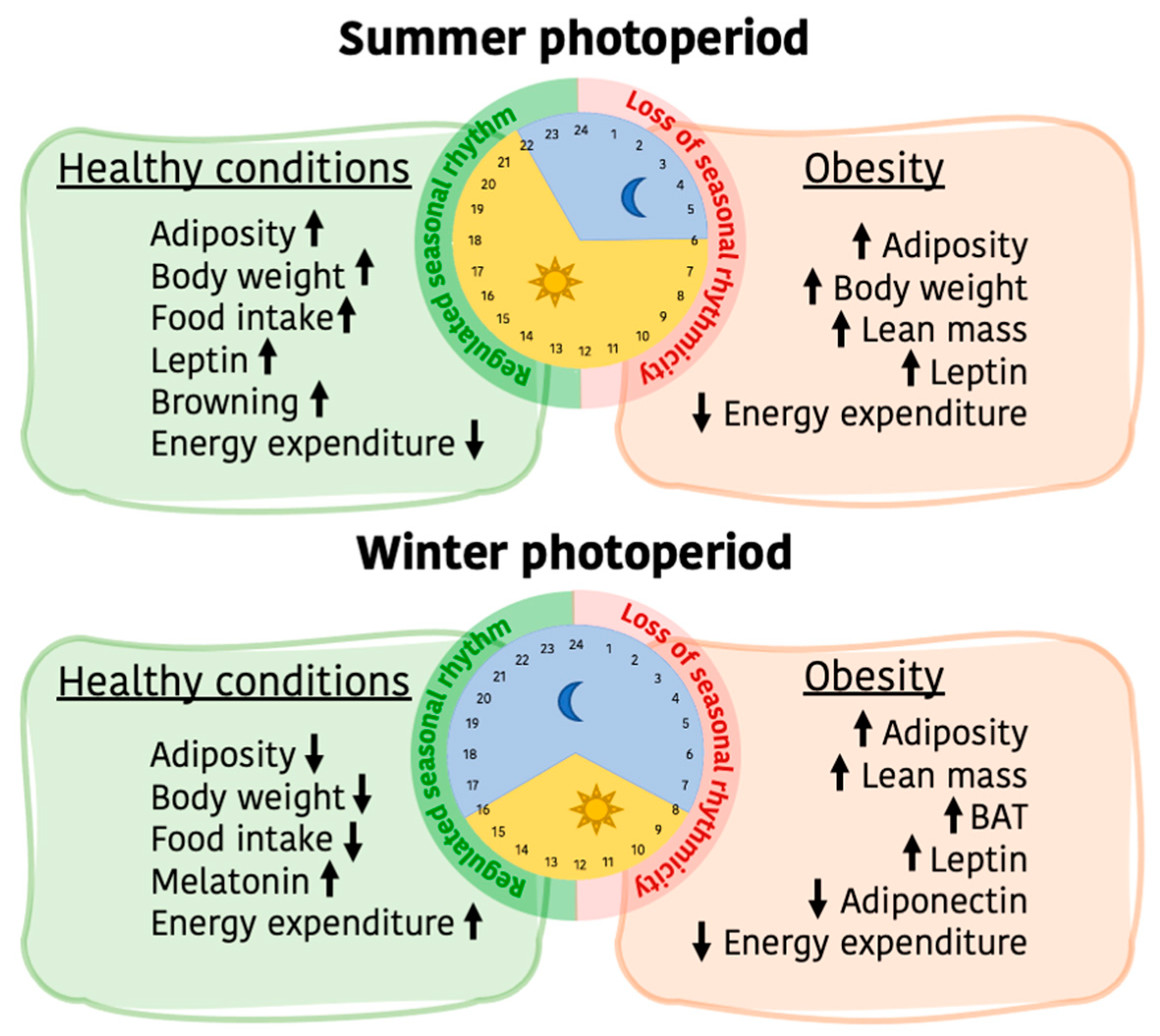
6. Impact of Obesity in the Photoperiodic Remodeling of Adiposity and Energy Metabolism
7. Concluding Remarks
- Research on seasonal rhythms and associated physiological responses goes back to the past century, but there is still a wide gap in the current understanding of its impact on humans. Important advances in knowledge have been achieved to date, such as describing and establishing the basic mechanisms by which seasonal changes are received and expanded through the organism. Animal-based studies have shown that the main seasonal cue is daylength, and metabolic adaptations are developed to guarantee species survival.
- These metabolic adaptations naturally involve changes in body weight, which are mainly based on adiposity. Here, we have highlighted the seasonal modifications reported in WAT, showing that the impact of photoperiod in this tissue is noticeable. However, the specific metabolic pathways still need to be drawn in order to understand the impact of seasonality on WAT functionality. WAT is an important metabolic tissue whose function is highly affected by obesity. In the situation of obesity, seasonal adaptations in adipose are not possible and the organism is less protected against environmental adversities.
- Changes in food intake are also related to photoperiod, involving both the amount of energy consumed and the type of nutrients ingested. Evidence suggests that seasonal food intake regulation is set in different regions of the hypothalamus, where molecules other than the already established ones could have an important role. In this context, further studies that explore the role of these specific hypothalamic regions and molecules would help decipher the full mechanisms by which food intake is seasonally regulated. Considering that seasonal regulation of food intake is altered by obesity, as well as by disturbed environmental cues which can lead to wrong responses in food consumption, it is important to maintain seasonal patterns in feeding behavior.
- BAT activity and browning are deeply involved in regulating energy expenditure, and are induced by melatonin signaling that follows changes in photoperiod. Knowing that human BAT also follows this pattern, it would be interesting to promote this feedback mechanism through favoring already known methods or new strategies that boost melatonin production.
- Overall, this review has demonstrated that changes in photoperiod have an important regulatory role in energy balance and metabolism. Molecular changes in the main metabolic tissues have been observed and are described here, providing new perspectives in the regulation of energy metabolism. Applied research in seasonal animals and humans will possibly help understanding the long-term regulation of food intake and body weight.
Author Contributions
Funding
Conflicts of Interest
References
- Dardente, H.; Wood, S.; Ebling, F.; Sáenz de Miera, C. An Integrative View of Mammalian Seasonal Neuroendocrinology. J. Neuroendocrinol. 2019, 31, e12729. [Google Scholar] [CrossRef] [PubMed]
- Helfer, G.; Barrett, P.; Morgan, P.J. A Unifying Hypothesis for Control of Body Weight and Reproduction in Seasonally Breeding Mammals. J. Neuroendocrinol. 2019, 31, e12680. [Google Scholar] [CrossRef] [PubMed]
- Moreno, J.P.; Crowley, S.J.; Alfano, C.A.; Thompson, D. Physiological Mechanisms Underlying Children’s Circannual Growth Patterns and Their Contributions to the Obesity Epidemic in Elementary School Age Children. Obes. Rev. 2020, 21, e12973. [Google Scholar] [CrossRef] [PubMed]
- Yokoya, M.; Higuchi, Y. Day Length May Make Geographical Difference in Body Size and Proportions: An Ecological Analysis of Japanese Children and Adolescents. PLoS ONE 2019, 14, e0210265. [Google Scholar] [CrossRef] [PubMed]
- Ebling, F.J.P. On the Value of Seasonal Mammals for Identifying Mechanisms Underlying the Control of Food Intake and Body Weight. Horm. Behav. 2014, 66, 56–65. [Google Scholar] [CrossRef]
- Bartness, T.J.; Demas, G.E.; Kay Song, C. Seasonal Changes in Adiposity: The Roles of the Photoperiod, Melatonin and Other Hormones, and Sympathetic Nervous System. Soc. Exp. Biol. Med. 2002, 227, 363–376. [Google Scholar] [CrossRef]
- Lincoln, G.A.; Rhind, S.M.; Pompolo, S.; Clarke, I.J. Hypothalamic Control of Photoperiod-Induced Cycles in Food Intake, Body Weight, and Metabolic Hormones in Rams. Am. J. Physiol.–Regul. Integr. Comp. Physiol. 2002, 281, 76–90. [Google Scholar] [CrossRef]
- Lincoln, G.A.; Anderson, H.; Loudon, A. Clock Genes in Calendar Cells as the Basis of Annual Timekeeping in Mammals–A Unifying Hypothesis. J. Endocrinol. 2003, 179, 1–13. [Google Scholar] [CrossRef]
- Ryu, V.; Zarebidaki, E.; Albers, E.H.; Xue, B.; Bartness, T.J. Short Photoperiod Reverses Obesity in Siberian Hamsters via Sympathetically Induced Lipolysis and Browning in Adipose Tissue. Physiol. Behav. 2018, 190, 11–20. [Google Scholar] [CrossRef]
- Bartness, T.J.; Goldman, B.D. Peak Duration of Serum Melatonin and Short-Day Responses in Adult Siberian Hamsters. Am. J. Physiol.–Regul. Integr. Comp. Physiol. 1988, 255, R812–R822. [Google Scholar] [CrossRef]
- Bartness, T.J.; Powers, J.B.; Hastings, M.H.; Bittman, E.L.; Goldman, B.D. The Timed Infusion Paradigm for Melatonin Delivery: What Has It Taught Us about the Melatonin Signal, Its Reception, and the Photoperiodic Control of Seasonal Responses? J. Pineal Res. 1993, 15, 161–190. [Google Scholar] [CrossRef] [PubMed]
- Helfer, G.; Dumbell, R. Endocrine Drivers of Photoperiod Response. Curr. Opin. Endocr. Metab. Res. 2020, 11, 49–54. [Google Scholar] [CrossRef]
- Gorman, M.R. Temporal Organization of Pineal Melatonin Signaling in Mammals. Mol. Cell Endocrinol. 2020, 503, 110687. [Google Scholar] [CrossRef] [PubMed]
- Bao, R.; Onishi, K.G.; Tolla, E.; Ebling, F.J.P.; Lewis, J.E.; Anderson, R.L.; Barrett, P.; Prendergast, B.J.; Stevenson, T.J. Genome Sequencing and Transcriptome Analyses of the Siberian Hamster Hypothalamus Identify Mechanisms for Seasonal Energy Balance. Proc. Natl. Acad. Sci. USA 2019, 116, 13116–13121. [Google Scholar] [CrossRef]
- Zhang, Z.; Boelen, A.; Bisschop, P.H.; Kalsbeek, A.; Fliers, E. Hypothalamic Effects of Thyroid Hormone. Mol. Cell Endocrinol. 2017, 458, 143–148. [Google Scholar] [CrossRef]
- Wade, G.N.; Bartness, T.J. Effects of Photoperiod and Gonadectomy on Food Intake, Body Weight, and Body Composition in Siberian Hamsters. Am. J. Physiol.–Regul. Integr. Comp. Physiol. 1984, 15, R26–R30. [Google Scholar] [CrossRef]
- Togo, Y.; Otsuka, T.; Goto, M.; Furuse, M.; Yasuo, S. Photoperiod Regulates Dietary Preferences and Energy Metabolism in Young Developing Fischer 344 Rats but Not in Same-Age Wistar Rats. Am. J. Physiol.–Endocrinol. Metab. 2012, 303, 777–786. [Google Scholar] [CrossRef] [PubMed]
- Gibert-Ramos, A.; Ibars, M.; Salvadó, M.; Crescenti, A. Response to the Photoperiod in the White and Brown Adipose Tissues of Fischer 344 Rats Fed a Standard or Cafeteria Diet. J. Nutr. Biochem. 2019, 70, 82–90. [Google Scholar] [CrossRef]
- Larkin, L.M.; Moore, B.J.; Stern, J.S.; Horwitz, B.A. Effect of Photoperiod on Body Weight and Food Intake of Obese and Lean Zucker Rats. Life Sci. 1991, 49, 735–745. [Google Scholar] [CrossRef]
- Ibrahim, M.M. Subcutaneous and Visceral Adipose Tissue: Structural and Functional Differences. Obes. Rev. 2010, 11, 11–18. [Google Scholar] [CrossRef]
- Ali, A.T.; Hochfeld, W.E.; Myburgh, R.; Pepper, M.S. Adipocyte and Adipogenesis. Eur. J. Cell Biol. 2013, 92, 229–236. [Google Scholar] [CrossRef]
- Grigoraş, A.; Amalinei, C.; Balan, R.A.; Giuşcă, S.E.; Avădănei, E.R.; Lozneanu, L.; Căruntu, I.D. Adipocytes Spectrum–From Homeostasia to Obesity and Its Associated Pathology. Ann. Anat. 2018, 219, 102–120. [Google Scholar] [CrossRef] [PubMed]
- Cristancho, A.G.; Lazar, M.A. Forming Functional Fat: A Growing Understanding of Adipocyte Differentiation. Nat. Rev. Mol. Cell Biol. 2011, 12, 722–734. [Google Scholar] [CrossRef] [PubMed]
- Basoli, V.; Santaniello, S.; Cruciani, S.; Ginesu, G.C.; Cossu, M.L.; Delitala, A.P.; Serra, P.A.; Ventura, C.; Maioli, M. Melatonin and Vitamin D Interfere with the Adipogenic Fate of Adipose-Derived Stem Cells. Int. J. Mol. Sci. 2017, 18, 981. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Liu, M. Adipose Tissue in Control of Metabolism. J. Endocrinol. 2016, 231, R77–R99. [Google Scholar] [CrossRef]
- Dolfini, D.; Zambelli, F.; Pavesi, G.; Mantovani, R. A Perspective of Promoter Architecture from the CCAAT Box. Cell Cycle 2009, 8, 4127–4137. [Google Scholar] [CrossRef]
- Cho, Y.W.; Hong, S.H.; Jin, Q.; Wang, L.; Lee, J.E.; Gavrilova, O.; Ge, K. Histone Methylation Regulator PTIP Is Required for PPARγ and C/EBPα Expression and Adipogenesis. Cell Metab. 2009, 10, 27–39. [Google Scholar] [CrossRef]
- Moseti, D.; Regassa, A.; Kim, W.K. Molecular Regulation of Adipogenesis and Potential Anti-Adipogenic Bioactive Molecules. Int. J. Mol. Sci. 2016, 17, 124. [Google Scholar] [CrossRef]
- Ross, S.E.; Erickson, R.L.; Gerin, I.; DeRose, P.M.; Bajnok, L.; Longo, K.A.; Misek, D.E.; Kuick, R.; Hanash, S.M.; Atkins, K.B.; et al. Microarray Analyses during Adipogenesis: Understanding the Effects of Wnt Signaling on Adipogenesis and the Roles of Liver X Receptor α in Adipocyte Metabolism. Mol. Cell Biol. 2002, 22, 5989–5999. [Google Scholar] [CrossRef]
- Kim, S.K.; Kong, C.S. Anti-Adipogenic Effect of Dioxinodehydroeckol via AMPK Activation in 3T3-L1 Adipocytes. Chem. Biol. Interact. 2010, 186, 24–29. [Google Scholar] [CrossRef]
- Kiehn, J.T.; Tsang, A.H.; Heyde, I.; Leinweber, B.; Kolbe, I.; Leliavski, A.; Oster, H. Circadian Rhythms in Adipose Tissue Physiology. Compr. Physiol. 2017, 7, 383–427. [Google Scholar] [CrossRef] [PubMed]
- Kuntz, M.J.; Harris, R.A. Pyruvate Dehydrogenase Kinase 4: Regulation by Thiazolidinediones and Implication in Glyceroneogenesis in Adipose Tissue. Encycl. Signal. Mol. 2018, 57, 1–9. [Google Scholar] [CrossRef]
- Schweiger, M.; Schreiber, R.; Haemmerle, G.; Lass, A.; Fledelius, C.; Jacobsen, P.; Tornqvist, H.; Zechner, R.; Zimmermann, R. Adipose Triglyceride Lipase and Hormone-Sensitive Lipase Are the Major Enzymes in Adipose Tissue Triacylglycerol Catabolism. J. Biol. Chem. 2006, 281, 40236–40241. [Google Scholar] [CrossRef] [PubMed]
- Richard, A.J.; White, U.; Elks, C.; Al, E. Adipose Tissue: Physiology to Metabolic Dysfunction Introduction: AN Historical Perspective on Adipose Tissue Biology; Feingold, K.R., Anawalt, B., Boyce, A., Eds.; Endotext [Internet]; MDText.com, Inc.: South Dartmouth, MA, USA, 2020; pp. 1–65. [Google Scholar]
- Wang, H.; Liu, L.; Lin, J.Z.; Aprahamian, T.R.; Farmer, S.R. Browning of White Adipose Tissue with Roscovitine Induces a Distinct Population of UCP1+Adipocytes. Cell Metab. 2016, 24, 835–847. [Google Scholar] [CrossRef]
- Lanthier, N.; Leclercq, I.A. Adipose Tissues as Endocrine Target Organs. Best Pract. Res. Clin. Gastroenterol. 2014, 28, 545–558. [Google Scholar] [CrossRef]
- Labbé, S.M.; Caron, A.; Chechi, K.; Laplante, M.; Lecomte, R.; Richard, D. Metabolic Activity of Brown, “Beige,” and White Adipose Tissues in Response to Chronic Adrenergic Stimulation in Male Mice. Am. J. Physiol.–Endocrinol. Metab. 2016, 311, E260–E268. [Google Scholar] [CrossRef]
- Moonen, M.P.B.; Nascimento, E.B.M.; van Marken Lichtenbelt, W.D. Human Brown Adipose Tissue: Underestimated Target in Metabolic Disease? BBA–Mol. Cell Biol. Lipids 2018, 1981, 30105–30107. [Google Scholar] [CrossRef]
- Villarroya, F.; Cereijo, R.; Gavaldà-Navarro, A.; Villarroya, J.; Giralt, M. Inflammation of Brown/Beige Adipose Tissues in Obesity and Metabolic Disease. J. Intern. Med. 2018, 284, 492–504. [Google Scholar] [CrossRef]
- Frigolet Vázquez-Vela, M.E.; Torres, N.; Tovar, A.R. White Adipose Tissue as Endocrine Organ and Its Role in Obesity. Arch. Med. Res. 2008, 39, 715–728. [Google Scholar] [CrossRef]
- Tan, J.T.M.; Nankivell, V.A.; Bilu, C.; Shemesh, T.; Nicholls, S.J.; Zimmet, P.; Kronfeld-Schor, N.; Brown, A.; Bursill, C.A. High-Energy Diet and Shorter Light Exposure Drives Markers of Adipocyte Dysfunction in Visceral and Subcutaneous Adipose Depots of Psammomys Obesus. Int. J. Mol. Sci. 2019, 20, 6291. [Google Scholar] [CrossRef]
- Liu, X.Y.; Yang, D.B.; Xu, Y.C.; Gronning, M.O.L.; Zhang, F.; Wang, D.H.; Speakman, J.R. Photoperiod Induced Obesity in the Brandt’s Vole (Lasiopodomys Brandtii): A Model of “Healthy Obesity”? DMM Dis. Model. Mech. 2016, 9, 1357–1366. [Google Scholar] [CrossRef] [PubMed]
- Kró, E.; Redman, P.; Thomson, P.J.; Williams, R.; Mayer, C.; Mercer, J.G.; Speakman, J.R. Effect of Photoperiod on Body Mass, Food Intake and Body Composition in the Field Vole, Microtus Agrestis. J. Exp. Biol. 2005, 208, 571–584. [Google Scholar] [CrossRef]
- Kooijman, S.; Van Den Berg, R.; Ramkisoensing, A.; Boon, M.R.; Kuipers, E.N.; Loef, M.; Zonneveld, T.C.M.; Lucassen, E.A.; Sips, H.C.M.; Chatzispyrou, I.A.; et al. Prolonged Daily Light Exposure Increases Body Fat Mass through Attenuation of Brown Adipose Tissue Activity. Proc. Natl. Acad. Sci. USA 2015, 112, 6748–6753. [Google Scholar] [CrossRef] [PubMed]
- Shoemaker, M.B.; Heideman, P. Reduced Body Mass, Food Intake, and Testis Size in Response to Short Photoperiod in Adult F344 Rats. BMC Physiol. 2002, 2, 11. [Google Scholar] [CrossRef]
- Ross, A.W.; Russell, L.; Helfer, G.; Thomson, L.M.; Dalby, M.J.; Morgan, P.J. Photoperiod Regulates Lean Mass Accretion, but Not Adiposity, in Growing F344 Rats Fed a High Fat Diet. PLoS One 2015, 10, 1–17. [Google Scholar] [CrossRef]
- Knopper, L.D.; Boily, P. The Energy Budget of Captive Siberian Hamsters, Phodopus Sungorus, Exposed to Photoperiod Changes: Mass Loss Is Caused by a Voluntary Decrease in Food Intake. Physiol. Biochem. Zool. 2000, 73, 517–522. [Google Scholar] [CrossRef]
- Ross, A.W.; Johnson, C.E.; Bell, L.M.; Reilly, L.; Duncan, J.S.; Barrett, P.; Heideman, P.D.; Morgan, P.J. Divergent Regulation of Hypothalamic Neuropeptide Y and Agouti-Related Protein by Photoperiod in F344 Rats with Differential Food Intake and Growth. J. Neuroendocrinol. 2009, 21, 610–619. [Google Scholar] [CrossRef]
- Mariné-Casadó, R.; Domenech-Coca, C.; del Bas, J.M.; Bladé, C.; Arola, L.; Caimari, A. The Exposure to Different Photoperiods Strongly Modulates the Glucose and Lipid Metabolisms of Normoweight Fischer 344 Rats. Front. Physiol. 2018, 9, 416. [Google Scholar] [CrossRef]
- Ibars, M.; Aragonès, G.; Ardid-Ruiz, A.; Gibert-Ramos, A.; Arola-Arnal, A.; Suárez, M.; Bladé, C. Seasonal Consumption of Polyphenol-Rich Fruits Affects the Hypothalamic Leptin Signaling System in a Photoperiod-Dependent Mode. Sci. Rep. 2018, 8, 13572. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, Z.; Vasilieva, N.; Khrushchova, A.; Wang, D. Effects of Short Photoperiod on Energy Intake, Thermogenesis, and Reproduction in Desert Hamsters (Phodopus Roborovskii). Integr. Zool. 2015, 10, 207–215. [Google Scholar] [CrossRef]
- Pandit, R.; Beerens, S.; Adan, R.A.H. Role of Leptin in Energy Expenditure: The Hypothalamic Perspective. Am. J. Physiol.–Regul. Integr. Comp. Physiol. 2017, 312, R938–R947. [Google Scholar] [CrossRef] [PubMed]
- Park, H.-K.; Ahima, R.S. Physiology of Leptin: Energy Homeostasis, Neuroendocrine Function and Metabolism. Metabolism 2015, 40, 1301–1315. [Google Scholar] [CrossRef] [PubMed]
- Fenselau, H.; Campbell, J.N.; Verstegen, A.M.J.; Madara, J.C.; Xu, J.; Shah, B.P.; Resch, J.M.; Yang, Z.; Mandelblat-Cerf, Y.; Livneh, Y.; et al. A Rapidly-Acting Glutamatergic ARC→PVH Satiety Circuit Postsynaptically Regulated by α-MSH. Nat Neurosci 2017, 20, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Vohra, M.S.; Benchoula, K.; Serpell, C.J.; Hwa, W.E. AgRP/NPY and POMC Neurons in the Arcuate Nucleus and Their Potential Role in Treatment of Obesity. Eur. J. Pharmacol. 2022, 915, 174611. [Google Scholar] [CrossRef] [PubMed]
- Vong, L.; Chianping, Y.; Yang, Z.; Choi, B.; Chua, S., Jr.; Lowell, B.B. Leptin Action on GABAergic Neurons Prevents Obesity and Reduces Inhibitory Tone to POMC Neurons. Neuron 2011, 23, 142–154. [Google Scholar] [CrossRef]
- Lau, J.; Farzi, A.; Qi, Y.; Heilbronn, R.; Mietzsch, M.; Shi, Y.C.; Herzog, H. CART Neurons in the Arcuate Nucleus and Lateral Hypothalamic Area Exert Differential Controls on Energy Homeostasis. Mol. Metab. 2018, 7, 102–118. [Google Scholar] [CrossRef]
- Caron, A.; Lemko, H.M.D.; Castorena, C.M.; Fujikawa, T.; Lee, S.; Lord, C.C.; Ahmed, N.; Lee, C.E.; Holland, W.L.; Liu, C.; et al. POMC Neurons Expressing Leptin Receptors Coordinate Metabolic Responses to Fasting via Suppression of Leptin Levels. elife 2018, 7, e33710. [Google Scholar] [CrossRef]
- Essner, R.A.; Smith, A.G.; Jamnik, A.A.; Ryba, A.R.; Trutner, Z.D.; Carter, M.E. AgRP Neurons Can Increase Food Intake during Conditions of Appetite Suppression and Inhibit Anorexigenic Parabrachial Neurons. J. Neurosci. 2017, 37, 8678–8687. [Google Scholar] [CrossRef]
- Morgan, P.J.; Ross, A.W.; Mercer, J.G.; Barrett, P. What Can We Learn from Seasonal Animals about the Regulation of Energy Balance? Prog. Brain Res. 2006, 153, 325–337. [Google Scholar] [CrossRef]
- Mercer, J.G.; Moar, K.M.; Logie, T.J.; Findlay, P.A.; Adam, C.L.; Morgan, P.J. Seasonally Inappropriate Body Weight Induced by Food Restriction: Effect on Hypothalamic Gene Expression in Male Siberian Hamsters. Endocrinology 2001, 142, 4173–4181. [Google Scholar] [CrossRef]
- Klingenspor, M.; Niggemann, H.; Heldmaier, G. Modulation of Leptin Sensitivity by Short Photoperiod Acclimation in the Djungarian Hamster, Phodopus Sungorus. J. Comp. Physiol.–B Biochem. Syst. Environ. Physiol. 2000, 170, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Reddy, A.B.; Cronin, A.S.; Ford, H.; Ebling, F.J.P. Seasonal Regulation of Food Intake and Body Weight in the Male Siberian Hamster: Studies of Hypothalamic Orexin (Hypocretin), Neuropeptide Y (NPY) and pro-Opiomelanocortin (POMC). Eur. J. Neurosci. 1999, 11, 3255–3264. [Google Scholar] [CrossRef] [PubMed]
- Tups, A.; Ellis, C.; Moar, K.M.; Logie, T.J.; Adam, C.L.; Mercer, J.G.; Klingenspor, M. Photoperiodic Regulation of Leptin Sensitivity in the Siberian Hamster, Phodopus Sungorus, Is Reflected in Arcuate Nucleus SOCS-3 (Suppressor of Cytokine Signaling) Gene Expression. Endocrinology 2004, 145, 1185–1193. [Google Scholar] [CrossRef]
- Pedroso, J.A.B.; Ramos-Lobo, A.M.; Donato, J. SOCS3 as a Future Target to Treat Metabolic Disorders. Hormones 2019, 18, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Ebling, F.J.P.; Arthurs, O.J.; Turney, B.W.; Cronin, A.S. Seasonal Neuroendocrine Rhythms in the Male Siberian Hamster Persist after Monosodium Glutamate-Induced Lesions of the Arcuate Nucleus in the Neonatal Period. J. Neuroendocrinol. 1998, 10, 701–712. [Google Scholar] [CrossRef] [PubMed]
- Helfer, G.; Ross, A.W.; Morgan, P.J. Neuromedin U Partly Mimics Thyroid-Stimulating Hormone and Triggers Wnt/β-Catenin Signalling in the Photoperiodic Response of F344 Rats. J. Neuroendocrinol. 2013, 25, 1264–1272. [Google Scholar] [CrossRef]
- Helfer, G.; Tups, A. Hypothalamic Wnt Signalling and Its Role in Energy Balance Regulation. J. Neuroendocrinol. 2016, 28, 1–9. [Google Scholar] [CrossRef]
- Helfer, G.; Ross, A.W.; Russell, L.; Thomson, L.M.; Shearer, K.D.; Goodman, T.H.; McCaffery, P.J.; Morgan, P.J. Photoperiod Regulates Vitamin A and Wnt/β-Catenin Signaling in F344 Rats. Endocrinology 2012, 153, 815–824. [Google Scholar] [CrossRef]
- Mariné-Casadó, R.; Domenech-Coca, C.; del Bas, J.M.; Bladé, C.; Arola, L.; Caimari, A. Intake of an Obesogenic Cafeteria Diet Affects Body Weight, Feeding Behavior, and Glucose and Lipid Metabolism in a Photoperiod-Dependent Manner in F344 Rats. Front. Physiol. 2018, 9, 1–16. [Google Scholar] [CrossRef]
- Gibert-Ramos, A.; Palacios-Jordan, H.; Salvadó, M.J.; Crescenti, A. Consumption of Out-of-Season Orange Modulates Fat Accumulation, Morphology and Gene Expression in the Adipose Tissue of Fischer 344 Rats. Eur. J. Nutr. 2020, 59, 621–631. [Google Scholar] [CrossRef]
- Demas, G.E.; Bowers, R.R.; Bartness, T.J.; Gettys, T.W. Photoperiodic Regulation of Gene Expression in Brown and White Adipose Tissue of Siberian Hamsters (Phodopus Sungorus). Am. J. Physiol.–Regul. Integr. Comp. Physiol. 2002, 282, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Ravussin, E.; Galgani, J.E. The Implication of Brown Adipose Tissue for Humans. Annu. Rev. Nutr. 2011, 31, 33–47. [Google Scholar] [CrossRef] [PubMed]
- Bargut, T.C.L.; Aguila, M.B.; Mandarim-de-Lacerda, C.A. Brown Adipose Tissue: Updates in Cellular and Molecular Biology. Tissue Cell 2016, 48, 452–460. [Google Scholar] [CrossRef] [PubMed]
- Stanford, K.I.; Middelbeek, R.J.; Townsend, K.L.; An, D.; Nygaard, E.B.; Hitchcox, K.M.; Markan, K.R.; Nakano, K.; Hirshman, M.F.; Tseng, Y.H.; et al. Brown Adipose Tissue Regulates Glucose Homeostasis and Insulin Sensitivity. J. Clin. Investig. 2013, 123, 215–223. [Google Scholar] [CrossRef]
- Suárez Carmona, W.; Sánchez Oliver, A.; González Jurado, J. Fisiopatología de La Obesidad: Perspectiva Actual. Rev. Chil. Nutr. 2017, 44, 226–233. [Google Scholar] [CrossRef]
- Labbé, S.M.; Caron, A.; Bakan, I.; Laplante, M.; Carpentier, A.C.; Lecomte, R.; Richard, D. In Vivo Measurement of Energy Substrate Contribution to Cold-Induced Brown Adipose Tissue Thermogenesis. FASEB J. 2015, 29, 2046–2058. [Google Scholar] [CrossRef]
- Carobbio, S.; Guénantin, A.; Vidal-puig, A.; Samuelson, I.; Bahri, M. Brown and Beige Fat: From Molecules to Physiology and Pathophysiology. BBA–Mol. Cell Biol. Lipids 2019, 1864, 37–50. [Google Scholar] [CrossRef]
- Villarroya, F.; Cereijo, R.; Villarroya, J.; Giralt, M. Brown Adipose Tissue as a Secretory Organ. Nat. Rev. Endocrinol. 2017, 13, 26–35. [Google Scholar] [CrossRef]
- Tan, D.X.; Manchester, L.C.; Fuentes-Broto, L.; Paredes, S.D.; Reiter, R.J. Significance and Application of Melatonin in the Regulation of Brown Adipose Tissue Metabolism: Relation to Human Obesity. Obes. Rev. 2011, 12, 167–188. [Google Scholar] [CrossRef]
- Halpern, B.; Mancini, M.C.; Bueno, C.; Barcelos, I.P.; De Melo, M.E.; Lima, M.S.; Carneiro, C.G.; Sapienza, M.T.; Buchpiguel, C.A.; Do Amaral, F.G.; et al. Melatonin Increases Brown Adipose Tissue Volume and Activity in Patients with Melatonin Deficiency: A Proof-of-Concept Study. Diabetes 2019, 68, 947–952. [Google Scholar] [CrossRef]
- Boon, P.; Visser, H.; Daan, S. Effect of Photoperiod on Body Mass, and Daily Energy Intake and Energy Expenditure in Young Rats. Physiol. Behav. 1997, 62, 913–919. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Xie, X.; Zhao, B.; Huang, L.; Shen, Q.; Ma, L.; Chen, Y.; Wu, T.; Fu, Z. Effects of Altered Photoperiod on Circadian Clock and Lipid Metabolism in Rats. Chronobiol. Int. 2017, 34, 1094–1104. [Google Scholar] [CrossRef] [PubMed]
- Powell, C.S.; Blaylock, M.L.; Wang, R.; Hunter, H.L.; Johanning, G.L.; Nagy, T.R. Effects of Energy Expenditure and Ucp1 on Photoperiod-Induced Weight Gain in Collared Lemmings. Obes. Res. 2002, 10, 541–550. [Google Scholar] [CrossRef]
- Bartness, T.J.; Wade, G.N. Photoperiodic Control of Body Weight and Energy Metabolism in Syrian Hamsters (Mesocricetus Auratus): Role of Pineal Gland, Melatonin, Gonads, and Diet. Endocrinology 1984, 114, 492–498. [Google Scholar] [CrossRef] [PubMed]
- Wen, X.; Zhang, B.; Wu, B.; Xiao, H.; Li, Z.; Li, R.; Xu, X.; Li, T. Signaling Pathways in Obesity: Mechanisms and Therapeutic Interventions. Signal Transduct. Target. Ther. 2022, 7, 298. [Google Scholar] [CrossRef] [PubMed]
- Sinitskaya, N.; Schuster-Klein, C.; Guardiola-Lemaitre, B.; Gourmelen, S.; Pévet, P.; Challet, E. Short Day-Length Increases Sucrose Consumption and Adiposity in Rats Fed a High-Fat Diet. Psychoneuroendocrinology 2008, 33, 1269–1278. [Google Scholar] [CrossRef]
- Pan, A.; Schernhammer, E.S.; Sun, Q.; Hu, F.B. Rotating Night Shift Work and Risk of Type 2 Diabetes: Two Prospective Cohort Studies in Women. PLoS Med. 2011, 8, e1001141. [Google Scholar] [CrossRef]
- Fonken, L.K.; Aubrecht, T.G.; Meléndez-Fernández, O.H.; Weil, Z.M.; Nelson, R.J. Dim Light at Night Disrupts Molecular Circadian Rhythms and Affects Metabolism. J. Biol. Rhythms 2013, 28, 262–271. [Google Scholar] [CrossRef]
- Opperhuizen, A.L.; Stenvers, D.J.; Jansen, R.D.; Foppen, E.; Fliers, E.; Kalsbeek, A. Light at Night Acutely Impairs Glucose Tolerance in a Time-, Intensity- and Wavelength-Dependent Manner in Rats. Diabetologia 2017, 60, 1333–1343. [Google Scholar] [CrossRef]
- Park, Y.M.M.; White, A.J.; Jackson, C.L.; Weinberg, C.R.; Sandler, D.P. Association of Exposure to Artificial Light at Night While Sleeping with Risk of Obesity in Women. JAMA Intern. Med. 2019, 179, 1061–1071. [Google Scholar] [CrossRef]
- Wehr, T.A.; Giesen, H.A.; Moul, D.E.; Turner, E.H.; Schwartz, P.J. Suppression of Men’s Responses to Seasonal Changes in Day Length by Modern Artificial Lighting. Am. J. Physiol.–Regul. Integr. Comp. Physiol. 1995, 269, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Arreaza-Gil, V.; Escobar-Martínez, I.; Suárez, M.; Bravo, F.I.; Muguerza, B.; Arola-Arnal, A.; Torres-Fuentes, C. Gut Seasons: Photoperiod Effects on Fecal Microbiota in Healthy and Cafeteria-Induced Obese Fisher 344 Rats. Nutrients 2022, 14, 722. [Google Scholar] [CrossRef] [PubMed]


| Species | M/F | Photoperiods (Light/Dark Hours) | Body Weight | Adiposity | WAT Metabolism | Reference |
|---|---|---|---|---|---|---|
| F344 rats | M | 18/6–6/18 | = | ↓ SP | SP ↓ Hypertrophy ↑ Hyperplasia ↓ Adipogenesis ↓ Lipogenesis ↓ Browning | [18] |
| Siberian hamsters | M | 16/8–8/16 | ↓ SP | ↓ SP | SP ↑ Lipolysis | [9] |
| Field voles | M | 16/8–8/16 | ↓ SP | ↓ SP | SP = Lipogenesis | [43] |
| Brandt voles | M | 16/8–8/16 | ↓ SP | ↓ SP | SP ↓ Lipogenesis ↓ Hypertrophy LP ↑ Browning | [42] |
| Psammomys obesus rats | M | 12/12–5/19 | = | = | SP ↓ Adipogenesis ↓ Browning | [41] |
| C57BL/6 mice | M | 12/12–16/8 | ↑ LP | ↑ LP | LP ↓ Glucose uptake | [44] |
| Species | M/F | Photoperiods (Light/Dark Hours) | Carbohydrates | Proteins | Fat | Reference |
|---|---|---|---|---|---|---|
| F344 rats | M | 16/8–8/16 | ↑ LP | ↑ LP | ND | [17] |
| F344 rats | M | 16/8–8/16 | Not measured | ↑ LP | Not measured | [46] |
| F344 rats | M | 18/6–6/18 (Control: 12/12) | ↓ LP and SP | Not measured | ND | [49,50] |
| Species | M/F | Photoperiods (Light/Dark Hours) and Diet | Adiposity | Food Intake | Energy Expenditure | Reference |
|---|---|---|---|---|---|---|
| F344 rats | M | 18/6–6/18 STD or CAF | LP-CAF ↓ Lean/fat mass ratio | SP ↓ Cumulative food intake LP-CAF ↑ Preference for fat-rich foods SP-CAF and LP-CAF ↓ CH Consumption and ↑ Npy ↑ Ghsr | SP-CAF ↑ RQ → ↑ CH oxidation ↓ fat oxidation rates SP-CAF and LP-CAF ↓ EE | [50,70] |
| F344 rats | M | 18/6–6/18 STD or CAF | SP-STD and SP-CAF ↑ Number of smaller adipocytes SP-CAF ↑ Acacα, C/ebpα and FASN | No data | SP-CAF ↑ BAT (%) ↑ lean mass (%) | [18] |
| F344 rats | M | 16/8–8/16 STD or CAF | SP-CAF and LP-CAF Doubled adiposity ↑ TG, NEFAs | SP-HFD and LP-HFD ↓ Food and protein intake and ↑ Leptin SP-HFD ↓↓ AgRP and Pomc | LP-STD and LP-CAF ↑ Lean mass | [46] |
| Psammomys obesus rats | M | 12/12–5/19 LED or HED | SP-LED and SP-HED ↑ Larger adipocytes SP-HED Larger adipocytes and ↑ Pparα ↓↓ Pparγ, C/ebpα and Adipoq SP-HED and LP-HED ↓ Pparγ (visceral WAT) | No data | SP-HED ↓↓ Pgc-1α (scWAT) → ↓ browning ↑ Ucp1 (visceral WAT) | [41] |
| Zucker rats | M | 14/10–10/14 Genetically obese or lean | LP-Lean ↑ Visceral WAT LP-Obese ↑↑ total fat | SP-Obese and LP-Obese ↑↑ Food consumption | LP-Obese ↑ BAT SP-Obese ↓ lean mass gain SP-Lean and SP-Obese ↑↑ mitochondrial content | [19] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Navarro-Masip, È.; Caron, A.; Mulero, M.; Arola, L.; Aragonès, G. Photoperiodic Remodeling of Adiposity and Energy Metabolism in Non-Human Mammals. Int. J. Mol. Sci. 2023, 24, 1008. https://doi.org/10.3390/ijms24021008
Navarro-Masip È, Caron A, Mulero M, Arola L, Aragonès G. Photoperiodic Remodeling of Adiposity and Energy Metabolism in Non-Human Mammals. International Journal of Molecular Sciences. 2023; 24(2):1008. https://doi.org/10.3390/ijms24021008
Chicago/Turabian StyleNavarro-Masip, Èlia, Alexandre Caron, Miquel Mulero, Lluís Arola, and Gerard Aragonès. 2023. "Photoperiodic Remodeling of Adiposity and Energy Metabolism in Non-Human Mammals" International Journal of Molecular Sciences 24, no. 2: 1008. https://doi.org/10.3390/ijms24021008
APA StyleNavarro-Masip, È., Caron, A., Mulero, M., Arola, L., & Aragonès, G. (2023). Photoperiodic Remodeling of Adiposity and Energy Metabolism in Non-Human Mammals. International Journal of Molecular Sciences, 24(2), 1008. https://doi.org/10.3390/ijms24021008









