Effect of Linker Substituent Nature on Performance of Active Sites in UiO-66: Combined FT-IR and DFT Study
Abstract
:1. Introduction
2. Results and Discussion
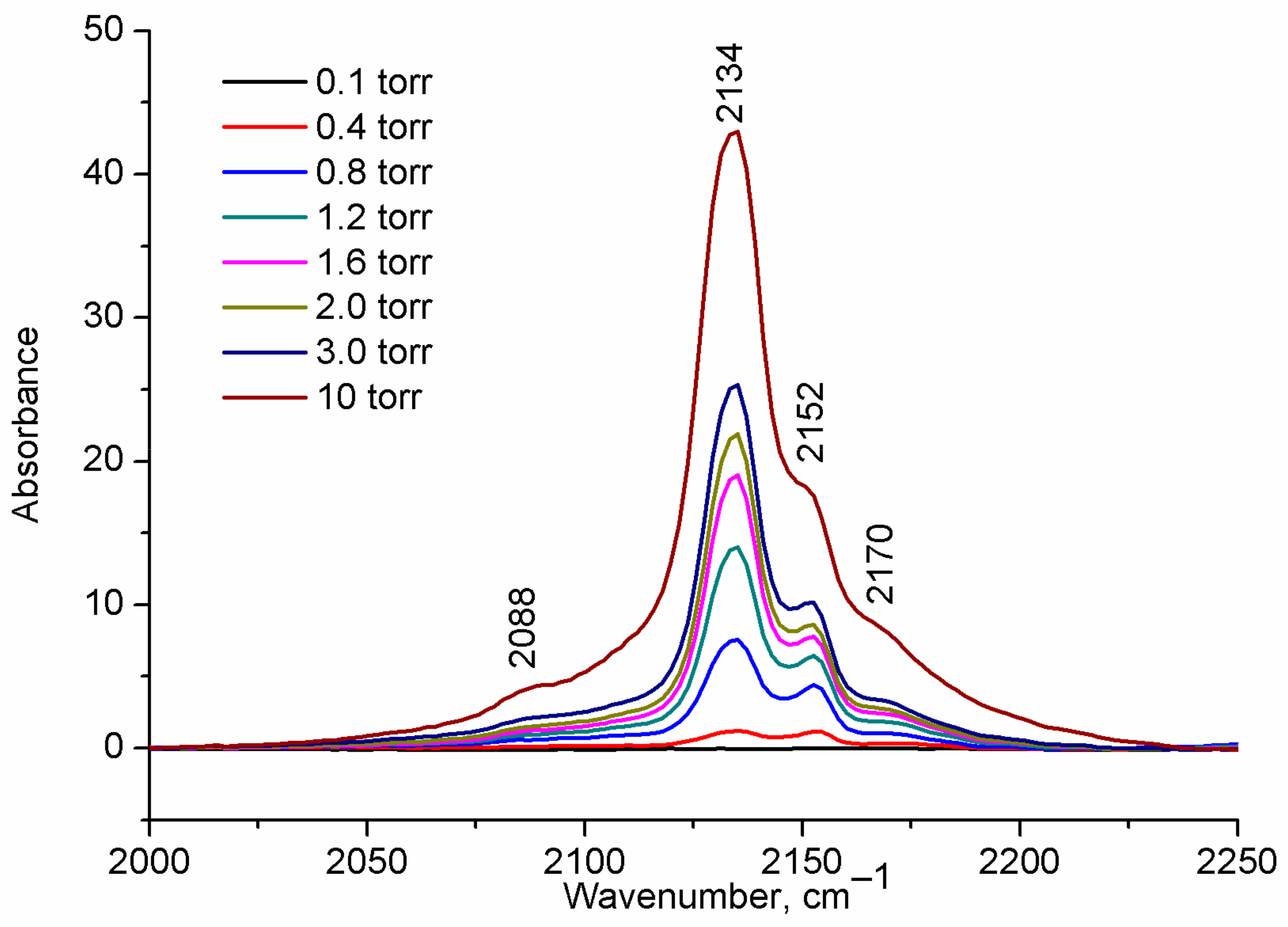
2.1. CO Adsorption on H-UiO-66
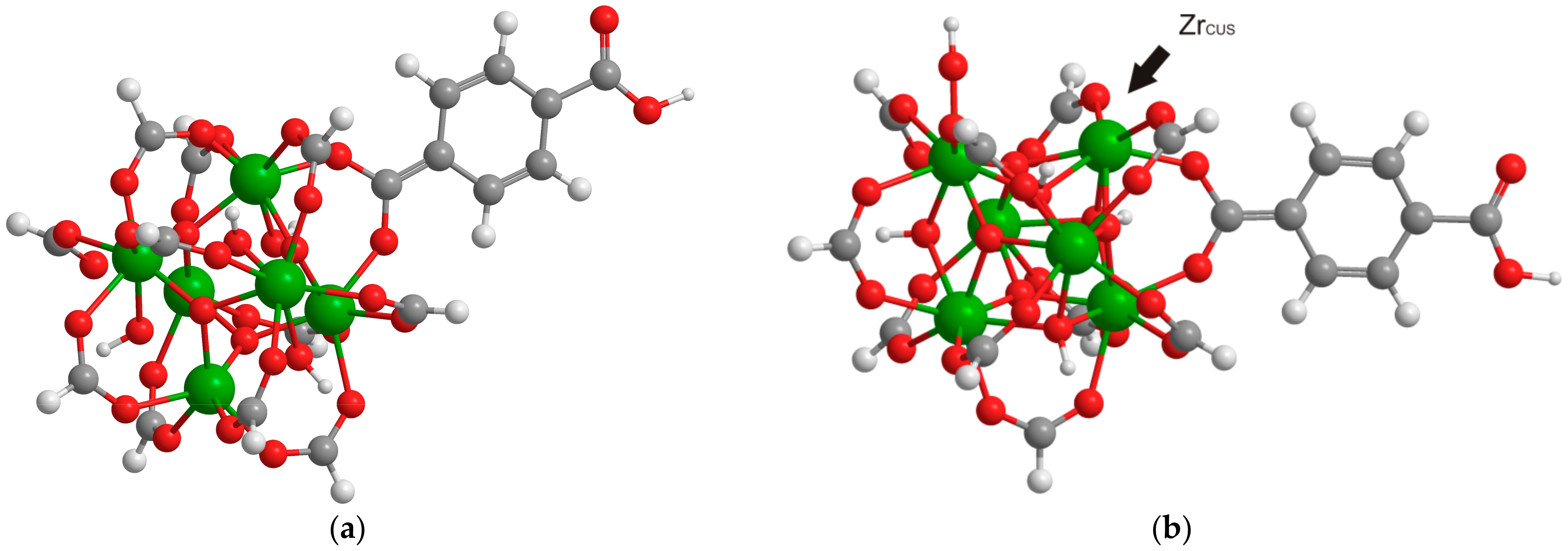
2.2. Effect of Substituent
3. Materials and Methods
4. Computational Details
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kirlikovali, K.O.; Hanna, S.L.; Son, F.A.; Farha, O.K. Back to the Basics: Developing Advanced Metal-Organic Frameworks Using Fundamental Chemistry Concepts. ACS Nanosci. Au 2023, 3, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Rowsell, J.L.; Yaghi, O.M. Metal–organic frameworks: A new class of porous materials. Microporous Mesoporous Mater. 2004, 73, 3–14. [Google Scholar] [CrossRef]
- Wang, C.; Liu, D.; Lin, W. Metal–organic frameworks as a tunable platform for designing functional molecular materials. J. Am. Chem. Soc. 2013, 135, 13222–13234. [Google Scholar] [CrossRef] [PubMed]
- Kumari, A.; Kaushal, S.; Pal Singh, P. Bimetallic metal-organic frameworks heterogeneous catalysts: Design, construction, and application. Mater. Today Energy 2021, 20, 100667. [Google Scholar] [CrossRef]
- Winarta, J.; Shan, B.; Mcintryre, S.M.; Ye, L.; Wang, C.; Liu, J.; Mu, B. A Decade of UiO-66 Research: A Historic Review of Dinamic Structure, Synthesis Mechanisms, and Characterization Techniques of an Archetypal Metal-Organic Framework. Cryst. Growth Des. 2020, 30, 1347–1362. [Google Scholar] [CrossRef]
- Cavka, J.H.; Jakobsen, S.; Olsbye, U.; Guillou, N.; Lamberti, C.; Bordiga, S.; Lillerud, K.P. A new zirconium inorganic building brick forming metal organic frameworks with exceptional stability. J. Am. Chem. Soc. 2008, 130, 13850–13851. [Google Scholar] [CrossRef]
- Valenzano, L.; Civalleri, B.; Chavan, S.; Bordiga, S.; Nilsen, M.H.; Jakobsen, S.; Lamberti, C. Disclosing the complex structure of UiO-66 metal organic framework: A synergic combination of experiment and theory. Chem. Mater. 2011, 23, 1700–1718. [Google Scholar] [CrossRef]
- Hall, J.N.; Bollini, P. Structure, characterization, and catalytic properties of open-metal sites in metal organic frameworks. React. Chem. Eng. 2019, 4, 207–222. [Google Scholar] [CrossRef]
- Vermoortele, F.; Vandichel, M.; Van de Voorde, B.; Ameloot, R.; Waroquier, M.; Van Speybroeck, V.; De Vos, D.E. Electronic Effects of Linker Substitution on Lewis Acid Catalysis Metal-Organic frameworks. Angew. Chem. Int. Ed. 2012, 51, 4887–4890. [Google Scholar] [CrossRef]
- Timofeeva, M.N.; Panchenko, V.N.; Won Jun, J.; Hasan, Z.; Matrosova, M.M.; Hwa Jhung, S. Effects of linker substitution on catalytic properties of porous zirconium terephthalate UiO-66 in acetalization of benzyldehyde with methanol. Appl. Catal. A 2014, 471, 91–97. [Google Scholar] [CrossRef]
- Blandez, J.F.; Santiago-Portillo, A.; Navalón, S.; Giménez-Marqués, M.; Álvaro, M.; Horcajada, P.; García, H. Influence of functionalization of terephthalate linker on the catalytic activity of UiO-66 for epoxide ring opening. J. Mol. Catal. A Chem. 2016, 425, 332–339. [Google Scholar] [CrossRef]
- Ji, P.; Drake, T.; Murakami, A.; Oliveres, P.; Skone, J.H.; Lin, W. Tuning Lewis Acidity of Metal-Organic Frameworks via Perfluorination of Bridging Ligands: Spectroscopic, Theoretical, and Catalytic Studies. J. Am. Chem. Soc. 2018, 140, 10553–10561. [Google Scholar] [CrossRef] [PubMed]
- Noh, J.; Kim, Y.; Park, H.; Lee, J.; Yoon, M.; Park, M.H.; Kim, Y.; Kim, M. Functional Group Effects on a Metal-Organic Framework Catalyst for CO2 Cycloaddition. J. Ind. Eng. Chem. 2018, 64, 478–483. [Google Scholar] [CrossRef]
- Torbina, V.; Ten, S.; Vodyankina, O. Effect of organic linker substituent on catalytic activity of UiO-66 metal-organic framework in selective oxidation of propylene glycol: Homolytic versus heterolytic activation of hydrogen peroxide. Mater. Today Chem. 2022, 24, 100776. [Google Scholar] [CrossRef]
- Yin, Z.; Liu, Y.; Zhou, S.; Yang, Z.; Yang, W. Constructing zirconium based metal-organic frameworks based electrically-driven self-cleaning membrane for removal of tetracycline: Effect of ligand substitution. Chem. Eng. J. 2022, 450, 138100. [Google Scholar] [CrossRef]
- Biswas, S.; Zhang, J.; Li, Z.; Liu, Y.Y.; Grzywa, M.; Sun, L.; Volkmer, D.; Van Der Voort, P. Enhanced selectivity of CO 2 over CH 4 in sulphonate-, carboxylate-and iodo-functionalized UiO-66 frameworks. Dalton Trans. 2013, 42, 4730–4737. [Google Scholar] [CrossRef] [PubMed]
- Cmarik, G.E.; Kim, M.; Cohen, S.M.; Walton, K.S. Tuning the adsorption properties of UiO-66 via ligand functionalization. Langmuir 2012, 28, 15606–15613. [Google Scholar] [CrossRef]
- Biswas, S.; Van Der Voort, P. A general strategy for the synthesis of functionalised UiO-66 frameworks: Characterisation, stability and CO2 adsorption properties. Eur. J. Inorg. Chem. 2013, 2013, 2154–2160. [Google Scholar] [CrossRef]
- Yuan, N.; Gong, X.; Sun, W.; Yu, C. Advanced applications of Zr-based MOFs in the removal of water pollutants. Chemosphere 2021, 267, 128863. [Google Scholar] [CrossRef]
- Treger, M.; Hannebauer, A.; Schaate, A.; Budde, J.L.; Behrens, P.; Schneider, A.M. Tuning the optical properties of the metal–organic framework UiO-66 via ligand functionalization. Phys. Chem. Chem. Phys. 2023, 25, 6333–6341. [Google Scholar] [CrossRef]
- Rosen, A.S.; Fung, V.; Huck, P.; O’Donnell, C.T.; Horton, M.K.; Truhlar, D.G.; Snurr, R.Q. High-throughput predictions of metal–organic framework electronic properties: Theoretical challenges, graph neural networks, and data exploration. NPJ Comput. Mater. 2022, 8, 112. [Google Scholar] [CrossRef]
- Panchenko, V.N.; Jhung, S.H.; Timofeeva, M.V. Spectroscopic methods as instruments for the prediction of catalytic behavior of metal-organic frameworks. Russ. Chem. Bull. Int. Ed. 2015, 64, 1772–1783. [Google Scholar] [CrossRef]
- Hadjiivanov, K.I.; Vayssilov, G.N. Characterization of Oxide Surfaces and Zeolites by Carbon Monoxide as an IR Probe Molecule. Adv. Catal. 2002, 47, 307–511. [Google Scholar] [CrossRef]
- Eischens, R.; Pliskin, W.; Francis, S. Infrared Spectra of Chemisorbed Carbon Monoxide. J. Chem. Phys. 1954, 22, 1786–1787. [Google Scholar] [CrossRef]
- Wiersum, A.D.; Soubeyrand-Lenoir, E.; Yang, Q.; Moulin, B.; Guillerm, V.; Yahia, M.B.; Bourrelly, S.; Vimont, A.; Miller, S.; Vagner, C.; et al. An Evaluation of UiO-66 for Gas-Based Applications. Chem. Asian J. 2011, 6, 3270–3280. [Google Scholar] [CrossRef]
- Driscoll, D.M.; Troya, D.; Usov, P.M.; Maynes, A.J.; Morris, A.J.; Morris, J.R. Characterization of Undercoordinated Zr Defect Sites in UiO-66 with Vibrational Spectroscopy of Adsorbes CO. J. Phys. Chem. C 2018, 122, 14582–14589. [Google Scholar] [CrossRef]
- Driscoll, D.M.; Troya, D.; Usov, P.M.; Maynes, A.J.; Morris, A.J.; Morris, J.R. Geometry and energetic of CO adsorption on hydroxylated UiO-66. Phys. Chem. Chem. Phys. 2019, 21, 5078–5085. [Google Scholar] [CrossRef]
- Cirujano, F.G.; Llabrés, I.; Xamena, F.X. Tuning the Catalytic Properties of UiO-66 Metal-Organic Frameworks: From Lewis to Defect-induced Brønsted Acidity. J. Phys. Chem. Lett. 2020, 11, 4879–4890. [Google Scholar] [CrossRef]
- Chakarova, K.; Strauss, I.; Mihaylov, M.; Drenchev, N.; Hadjiivanov, K. Evolution of acid and basic sites in UiO-66 and UiO-66-NH2 frameworks: FTIR study by probe molecules. Microporous Mesoporous Mater. 2019, 281, 110–122. [Google Scholar] [CrossRef]
- Mina-Camilde, N.; Manzanares, I.C.; Caballero, J.F. Molecular Constants of Carbon Monoxide at v = 0, 1, 2, and 3: A Vibrational Spectroscopy Experiment in Physical Chemistry. J. Chem. Educ. 1996, 73, 804. [Google Scholar] [CrossRef]
- Taddei, M. When defects turn into virtues: The curious case of zirconium-based metal-organic frameworks. Coord. Chem. Rev. 2017, 343, 1–24. [Google Scholar] [CrossRef]
- Shearer, G.C.; Chavan, S.; Ethiraj, J.; Vitillo, J.G.; Svelle, S.; Olsbye, U.; Lamberti, C.; Bordiga, S.; Lillerud, K.P. Tuned to perfection: Ironing out the defects in metal–organic framework UiO-66. Chem. Mater. 2014, 26, 4068–4071. [Google Scholar] [CrossRef]
- Han, Y.; Liu, M.; Li, K.; Zuo, Y.; Wei, Y.; Xu, S.; Zhang, G.; Song, C.; Zhang, Z.; Guo, X. Facile synthesis of morphology and size-controlled zirconium metal–organic framework UiO-66: The role of hydrofluoric acid in crystallization. CrystEngComm 2015, 17, 6434–6440. [Google Scholar] [CrossRef]
- Katz, M.J.; Brown, Z.J.; Colón, Y.J.; Siu, P.W.; Scheidt, K.A.; Snurr, R.Q.; Hupp, J.T.; Farha, O.K. A facile synthesis of UiO-66, UiO-67 and their derivatives. Chem. Commun. 2013, 49, 9449–9451. [Google Scholar] [CrossRef]
- Hammet, L.P. Some Relations between Reaction Rates and Equilibrium Constants. Chem. Rev. 1935, 17, 125–136. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09., Revision C.01.01; Gaussian, Inc.: Wallingford, CT, USA, 2010. [Google Scholar]
- Øien, S.; Wragg, D.; Reinsch, H.; Svelle, S.; Bordiga, S.; Lamberti, C.; Lillerud, K.P. Detailed Structure Analysis of Atomic Positions and Defects in Zirconium Metal–Organic Frameworks. Cryst. Growth Des. 2014, 14, 5370–5372. [Google Scholar] [CrossRef]
- Chai, J.-D.; Head-Gordon, M. Long-range corrected hybrid density functionals with damped atom–atom dispersion corrections. Phys. Chem. Chem. Phys. 2008, 10, 6615–6620. [Google Scholar] [CrossRef]
- Wadt, W.R.; Hay, P.J. Ab initio effective core potentials for molecular calculations—Potentials for main group elements Na to Bi. J. Chem. Phys. 1985, 82, 284–298. [Google Scholar] [CrossRef]
- Francl, M.M.; Pietro, W.J.; Hehre, W.J.; Binkley, J.S.; DeFrees, D.J.; Pople, J.A.; Gordon, M.S. Self-Consistent Molecular Orbital Methods. 23. A polarization-type basis set for 2nd-row elements. J. Chem. Phys. 1982, 77, 3654–3665. [Google Scholar] [CrossRef]

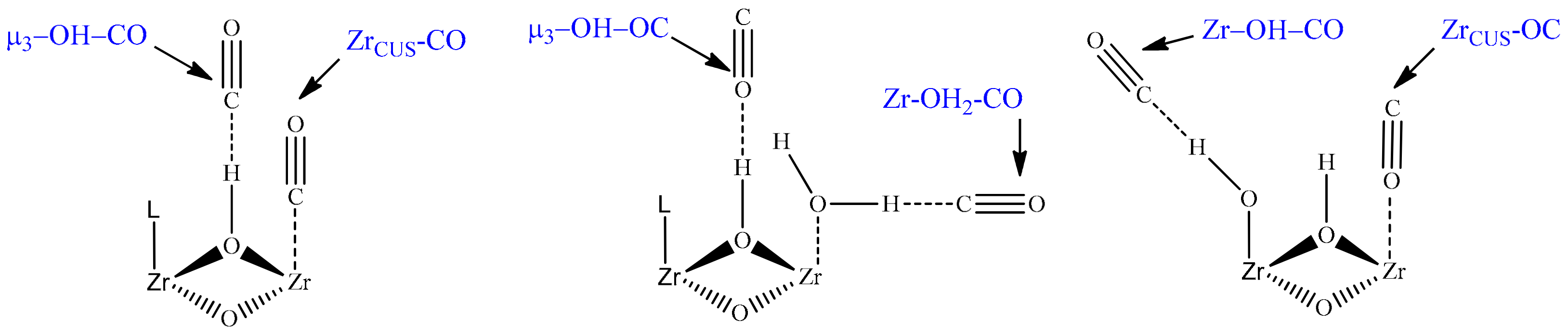
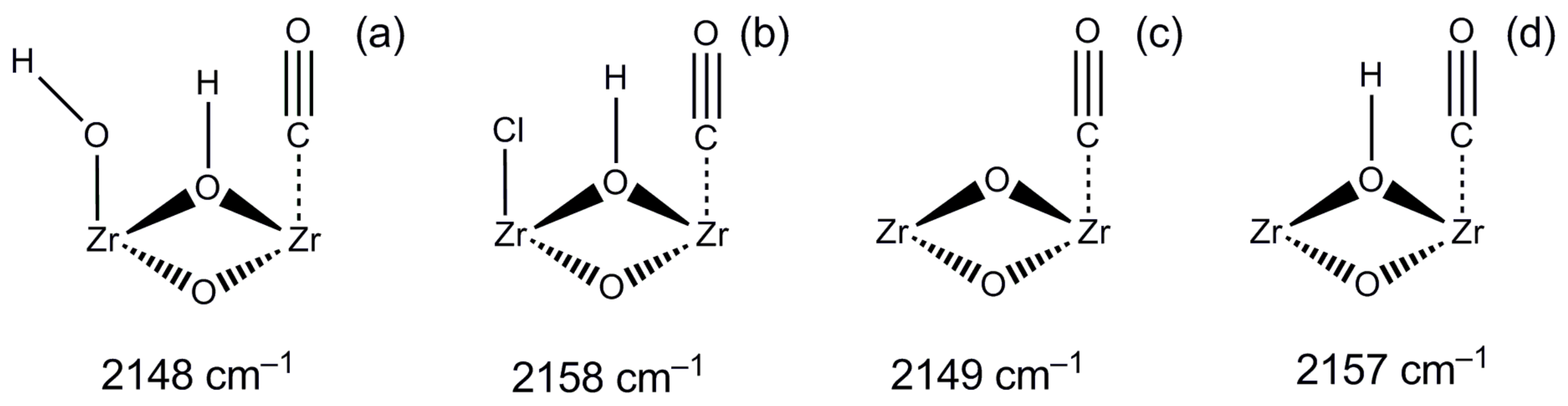


| Centers of CO Adsorption (See Figure 3) | Reference | |||||
|---|---|---|---|---|---|---|
| ZrCUS-CO | μ3-OH-CO | μ3-OH–OC | Zr-OH2-CO | ZrCUS-OC | Physisorbed | |
| 2172 | 2155 | - | - | - | 2136 | [25] |
| 2180 (2194) | 2154 (2170) | - | - | - | 2148 | [26] |
| - | 2152 (2179) | 2124 (2121) | - | - | [27] | |
| 2180 | 2153 | 2126 | - | - | 2136 and 2132 | [29] |
| 2178 | 2153–2154 | - | 2142 | - | 2137–2138 | [28] |
| 2170 (2157) | 2152 (2140) | 2100 (2087) | - (2124) | 2088 (2066) | 2134 | This work |
| Sample | CO Adsorption Sites (See Figure 3) | ||
|---|---|---|---|
| ZrCUS-CO | μ3-OH-CO | ZrCUS-OC | |
| NH2-UiO-66 | 2168 | 2153 | 2086 |
| (2156) | (2140) | (2064) | |
| H-UiO-66 | 2170 | 2152 | 2088 |
| (2157) | (2141) | (2066) | |
| NO2-UiO-66 | 2172 | 2153 | 2090 |
| (2159) | (2140) | (2068) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torbina, V.V.; Salaev, M.A.; Paukshtis, E.A.; Liotta, L.F.; Vodyankina, O.V. Effect of Linker Substituent Nature on Performance of Active Sites in UiO-66: Combined FT-IR and DFT Study. Int. J. Mol. Sci. 2023, 24, 14893. https://doi.org/10.3390/ijms241914893
Torbina VV, Salaev MA, Paukshtis EA, Liotta LF, Vodyankina OV. Effect of Linker Substituent Nature on Performance of Active Sites in UiO-66: Combined FT-IR and DFT Study. International Journal of Molecular Sciences. 2023; 24(19):14893. https://doi.org/10.3390/ijms241914893
Chicago/Turabian StyleTorbina, Viktoriia V., Mikhail A. Salaev, Evgeniy A. Paukshtis, Leonarda F. Liotta, and Olga V. Vodyankina. 2023. "Effect of Linker Substituent Nature on Performance of Active Sites in UiO-66: Combined FT-IR and DFT Study" International Journal of Molecular Sciences 24, no. 19: 14893. https://doi.org/10.3390/ijms241914893
APA StyleTorbina, V. V., Salaev, M. A., Paukshtis, E. A., Liotta, L. F., & Vodyankina, O. V. (2023). Effect of Linker Substituent Nature on Performance of Active Sites in UiO-66: Combined FT-IR and DFT Study. International Journal of Molecular Sciences, 24(19), 14893. https://doi.org/10.3390/ijms241914893







