Abstract
Spinal muscular atrophy (SMA) linked to 5q is a recessive motor neuron disease characterized by progressive and diffuse weakness and muscular atrophy. SMA is the most common neurodegenerative disease in childhood with an incidence of approximately 1 in 6000–10,000 live births, being long considered a leading cause of hereditary mortality in infancy, worldwide. The classification of SMA is based on the natural history of the disease, with a wide clinical spectrum of onset and severity. We are currently in a new therapeutic era, that, thanks to the widespread use of the newly approved disease-modifying therapies and the possibility of an early administration, should lead to a deep change in the clinical scenario and, thus, in the history of SMA. With the aim to achieve a new view of SMA, in this review we consider different aspects of this neuromuscular disease: the historical perspective, the clinical features, the diagnostic process, the psychological outcome, innovation in treatments and therapies, the possibility of an early identification of affected infants in the pre-symptomatic phase through newborn screening programs.
1. Introduction
Spinal muscular atrophy (SMA) linked to 5q is an autosomal recessive neuromuscular disease characterized by degeneration of alpha motor neurons in the anterior horns of the spinal cord, resulting in progressive and symmetrical proximal muscle weakness [1,2,3,4,5]. In particular, the main clinical manifestation of SMA includes hypotonia, muscle weakness by denervation, followed by respiratory failure and atrophy of variable severity depending on the genotype [6]. SMA has long been considered the leading cause of hereditary death in infancy worldwide [5,6], being second for incidence only to cystic fibrosis. The widespread use of the newly approved disease-modifying therapies (DMTs) and the possibility of an early administration should radically change the history of SMA [7]. The incidence of SMA is estimated at 1 in 6000–10,000, with a carrier frequency of 1/40–1/60 [8,9]. Nowadays, we know that most cases of SMA have mutations in the motor neuron type 1 (SMN1) gene located at 5q13.12. This gene covers 20 kb and is located in the telomeric portion of DNA that is subject to reorganization and deletion. The centromeric duplicated element, known as the SMN2 gene, is highly homologous to SMN1 with more than 99% nucleotide identity. SMN1 and SMN2 differ in eight nucleotides, specifically, the c.840C > T nucleotide change in SMN2 is in a coding region and interrupts an exonic splicing enhancer in exon 7. Consequently, most SMN2 transcripts lack exon 7, making a non-functional protein. Only approximately 10 percent of the proteins produced by each SMN2 copy are estimated to be functional. SMN is an RNA-binding protein that contributes to many cellular processes and pathways and plays a critical role in snRNP complex assembly in the cytoplasm. Ninety-five percent of SMA patients have a homozygous deletion of exon 7 of SMN1 or gene conversion from SMN1 to SMN2 (5q-SMA patients) [10], while the remaining 5% are compound heterozygotes for a deletion of exon 7 in SMN1 and a point mutation. In order to obtain a new view and future perspectives of SMA, in this review we discuss different aspects of this severe neuromuscular disorder: the historical perspective, from the early description and manifestations to the molecular genetic characterization, the clinical features and its classification into five types, the diagnostic process, the psychological outcome, recent innovation in treatments and therapies, the possibility of an early identification of affected infants in the pre-symptomatic phase through newborn screening programs.
2. History of Spinal Muscular Atrophy
SMA, an inherited, progressive neuromuscular disease that can cause muscle atrophy, was first discovered in infants in the early 1890s by physicians Guido Werdnig and Johann Hoffman. Werdnig meticulously described the disease in two newborn siblings in 1891 and Hoffmann characterized what he used to term “infantile progressive muscular atrophy” and detailed the clinical presentation in seven patients from 1893 to 1900 [11]. In their papers, Werding and Hoffman were able to provide a rather complete picture of the clinical and pathologic aspects of infantile SMA [11]. Although the etymon of Werdnig–Hoffmann disease was later attributed to the severe childhood form of SMA, their cases were of moderate severity. The first descriptions of severe childhood SMA were reported by Sylvestre in 1899 and by Beevor in 1903 [12]. It was only in the 1950s that Wohlfart, Fez, and Eliasson described a milder form of SMA in which patients could maintain the ability to stay upright and to ambulate [12]. All cases described throughout the years showed an anterior horn cell degeneration and symmetrical, proximal predominant extremity weakness that also affects axial, intercostal, and bulbar musculature [12]. At the International Consortium on Spinal Muscular Atrophy, the different phenotypes described were classified into three types of SMA based on motor function and age of disease onset [13]. Later, a fourth category was added for adult-onset cases and a zero category for patients with pre-natal onset and death within the first few weeks of life. However, 25% of patients still elude a well-defined classification of the disease. The classification of SMA presented a mystery regarding the severity of the disease, namely how a genetic defect could cause a wide range of more or less severe phenotypes. That enigma was finally solved in 1995 in Melki’s laboratory, where it was discovered that 95% of SMA cases are caused by a homozygous deletion in exon 7 of the SMN1 gene, on chromosome 5q13.3 [14]. Figure 1 represents in a timeline the highlights in the history of SMA from the first reports and descriptions to the classification of different forms.

Figure 1.
SMA timeline. SMA was first reported by Werdnig in 1891 and then by Hoffmann in 1893 [10]. In 1899 and 1903, Sylvestre and Beevor recognized variability of muscle weakness severity [11]. In the 1950s, Wolhfart, Fez, and Eliasson described a milder form of SMA [11] and in 1995 Melki discovered that 95% of SMA cases are caused by a homozygous deletion in SMN1 [13].
In addition, in humans, there are two forms of the SMN gene: the telomeric form (SMN1) and the centromeric form (SMN2). The SMN1 gene produces the encoding SMN protein and the SMN2 gene is identical to SMN1 except for a nucleotic substitution at position 840 of a cytosine to a thymine that excludes exon 7 from transcription. In particular, the exclusion of exon 7 from the transcription process is not complete, and only a small portion of the total mRNA transcripts (10–15%) of the SMN2 gene contain exon 7 encoding a normal SMN protein. SMA patients have a non-functional SMN1 gene, and therefore the SMN2 gene plays a crucial role in the production of SMN protein. Therefore, SMA is a condition caused by a deficit in the SMN protein which causes selective motor neuron loss. Disease severity is explained by SMN2 copy variability but it is not the only phenotypic modifier [15,16]. Studies conducted in animal models have significantly contributed to the development of therapies for SMA. For example, Monani et al. conducted studies in mouse models. Mice lacked the SMN2 gene and consequently the deletion of the SMN gene was lethal. Monani et al. found that insertion of two copies of human SMN2 into mice caused the development of a severe SMA-like phenotype with motor neuron loss. In contrast, mice with eight copies of SMN2 were normal [17]. These models played a crucial role as they allowed molecular and biochemical studies and initiation of the development of therapies aimed at increasing SMN protein expression and preventing motor neuron loss [17].
3. Clinical Features of SMA
SMA is a monogenic, autosomal recessive neuromuscular disorder, characterized by muscle weakness and atrophy caused by degeneration of alpha motor neurons of the anterior horns of the spinal cord.
The weakness usually affects the lower limbs with diffuse areflexia. The onset of weakness ranges from birth to adulthood and is symmetric, proximal to distal, and progressive. Facial and ocular muscles are usually unaffected. SMA has been classified into five types based on severity of symptoms, age at onset, highest motor milestone achieved, and genotype (Table 1) [18]. Usually, the severity of clinical manifestations is inversely proportional to SMN2 copy number. SMN2 can only partially compensate for the loss of SMN1, resulting in a deficiency but not a complete depletion of SMN protein [19]. Literature data have shown that patients with SMA have normal or above-average cognitive abilities [20,21].

Table 1.
Classification of SMA into five types based on symptom severity, age at onset, highest motor milestone achieved, and genotype [2,19,21,22,23,24,25,26].
SMA type 0 (SMA 0) is congenital, and presents at birth as severe weakness, hypotonia, and respiratory distress. There may be a history of restricted movements in utero, joint contractures, areflexia, and atrial septal defect. Patients with SMA 0 have severe respiratory diseases and die within the first six months of life [16]. SMA 0 patients have a single copy of SMN2.
SMA type 1 (SMA 1) is the most common form of SMA (50–60% of cases) and manifests early in the first six months of life, with severe weakness and generalized hypotonia, poor motor abilities, areflexia, and feeding, swallowing, and breathing difficulties with premature death within 24 months [24]. The mean age at onset of symptoms is 2.5 months. Affected children may acquire head control and ability to roll, but then lose these abilities before the age of six months. They have a characteristic “bell-shaped” chest and abdominal breathing due to the weakness of the intercostal respiratory muscles with preservation of diaphragmatic muscles. There may also be fasciculation of the tongue [16]. SMA 1 patients typically have one to two copies of SMN2 [25].
SMA type 2 (SMA 2) patients usually manifest motor symptoms after the first 6 months of life. Children with SMA 2 may reach motor milestones slowly and, with supportive care only, they can achieve the ability to sit independently. Moreover, they may stand with support, but they are never able to walk without any help [22]. Common clinical manifestations include progressive proximal muscle weakness, lack of muscular tone, hand tremor, reduced or absent deep tendon reflexes, scoliosis, and progressive respiratory muscle weakness that results in a restrictive lung disease, potentially leading to death. These patients represent approximately 30% of cases and they usually have three copies of SMN2 [2].
SMA type 3 (SMA 3) symptoms begin after 18 months of age and are heterogeneous. Patients present a progressive proximal muscle weakness, and the legs are more severely affected than the arms [25]. SMA 3 can be subdivided into type 3a (clinical symptoms before the age of three) and type 3b (clinical symptoms after the age of three). The children generally reach the major milestones, including independent walking, but their motor performance levels vary widely. Some children are barely able to get up from a sitting position and walk a few steps without help, while others walk well and can climb stairs [27]. SMA 3 accounts for about 10% of the patient population and most of these have three or four copies of SMN2 [2].
SMA type 4 (SMA 4) is the least common form of SMA (<5% of cases), and the life expectancy is normal [16]. SMA 4 was added to this classification to describe patients with onset in adulthood (>18 years) and mild course. This group includes patients who can walk in adulthood and have no problems with breathing and feeding [26]. SMN2 copies are usually between three and five [25].
4. SMA Diagnosis with Molecular Genetic Testing
Since 1995, when Lefebvre identified the SMA-determining gene [14], several diagnostic genetic tests have become commercially available to identify individuals with SMA. Many DNA tests are available to detect the absence of exon 7 of the SMN1 gene, used as a diagnostic test for SMA patients. According to a recent study [28], determining the exact copy number of the SMN2 gene is crucial to assess eligibility for treatment, so advanced techniques are needed to discriminate SMN1 from SMN2 [29]. Many techniques are used to detect copy number variations, but most cannot detect single-exon deletions or duplications. Current molecular methodologies for the detection and diagnosis of SMA by revealing SMN1 deletion and by SMN1 and SMN2 gene quantitation, respectively, include single-strand conformation polymorphism, restriction fragment length polymorphism, real-time polymerase chain reaction (RT-PCR), denaturing high-performance liquid chromatography, multiplex ligation probe amplification (MLPA), quantitative PCR (qPCR) and competitive PCR, high-resolution melting analysis, and liquid microbead assay [8]. More recently, digital PCR has shown a growing range of applications, being used not only to identify SMA individuals but also to determine SMN1 and SMN2 copy number [8]. As Mercuri et al. reported, the gold standard of SMA genetic testing is a quantitative assay of SMN1 and SMN2 using MLPA or quantitative polymerase chain reaction (qPCR) [30]. The qPCR is an assay that accurately quantifies SMN1 and SMN2 exon 7 copy number and SMN1 gene duplication. There are commercial kits that are engineered to detect certain gene conversions from SMN1 to SMN2 and SMN2 to SMN1 by checking the sequence identity of exon 7 and intron 7. These kits also can determine the presence of certain alleles associated with gene duplication [29] and enhanced splicing of SMN2 [31]. The disadvantages of multiplex qPCR include: (1) the use of genomic DNA (gDNA) isolated from whole blood collected in EDTA tubes or buccal swab with a concentration of 10–40 ng/µL, processed within 14 days after isolation; (2) the inability of detecting nonsense, frameshift, or missense mutations; (3) the distinction between samples with two copies of SMN1 on one chromosome and zero copies on the other (2 + 0 or healthy carriers) and samples with one genomic copy of SMN1 on each chromosome (1 + 1) can be carried out only by analyzing the genotype of gene duplication variants in certain populations [29]; (4) determining the gene copy number for all SMN exons, useful for rare cases of SMA with complicated gene structures, is not possible; (5) furthermore, the primers used in this technique have binding sites not containing polymorphic sites with minor allele frequencies (MAFs) greater than 0.005 (according to the Single Nucleotide Polymorphism Database (dbSNP) build 152). However, very rare polymorphisms located within the primer-binding sites may potentially impact the accurate quantification of the number of SMN1 and SMN2 copies [23]. Despite these disadvantages, the multiplex qPCR assay has the lowest cost and shortest duration (<4 h). Thus, results have demonstrated that the multiplex qPCR assay is rapid, accurate, and cost-effective and represents a high-throughput strategy. Considering the low cost, the high-throughput approach, and the high accuracy of the multiplex qPCR assay, it can be considered a routine tool for clinical diagnosis and for screening of SMA carriers. MLPA, on the other hand, is a multiplex PCR-based method using a single primer pair to amplify and quantify specific and multiple genomic loci (20 independent control loci for SMN1/2), in a single tube reaction from only 20 ng of a patient’s genomic DNA. MLPA technology allows the simultaneous detection of SMN1 and SMN2 copy numbers, thus facilitating diagnosis. The MLPA technique is more sensitive and allows a high degree of precision for the quantitative detection of three SMN1 copies or fewer. Commercial kits of probe sets are available and provide different SMA assays, fitting the genetic testing needs [32,33].
MLPA technology has several important limitations, including: (1) DNA sequence variants located in probe-binding sites of SMN1 alleles may interfere with probe hybridization and result in a false-positive carrier (one copy) or false-positive diagnostic (zero copies) result; (2) reactions are sensitive to contaminants but generate uninterpretable results; (3) MLPA cannot yet be used to investigate single cells, important for pre-implantation genetic diagnosis testing; (4) MLPA is not a suitable method to detect unknown point mutations; (5) MLPA probes are sensitive to small deletions, insertions, and mismatches; (6) MLPA requires a CE analyzer, which is a higher-cost option compared with slab gel electrophoresis for RFLP [23]. Advantages and disadvantages of multiplex qPCR and MLPA as molecular genetic tests for SMA diagnosis are summarized in Table 2.

Table 2.
Advantages and limitations of multiplex qPCR and MLPA as molecular genetic tests for SMA diagnosis [22,23,24,25].
5. Psychological Adjustment of Individuals with SMA
The psychological adjustment of individuals with SMA is challenging to discern due to limited quantitative research employing standardized instruments that focus on the subjective well-being of individuals with SMA. This situation arises from the difficulties inherent in conducting research within this specific context [34]. Nevertheless, previous research has explored various aspects of SMA individuals’ lives, including their quality of life, acceptance of the disease, self-esteem, emotions, and social roles across different age groups including adults, adolescents, and children. These studies investigated mostly the experience of individuals with SMA 3 and SMA 4, due to the difficulty of individuals with SMA 1 and SMA 2 in reaching adult age. In the last two cases, research analyzed the families’ experiences and psychological burden [35].
Regarding adult and adolescents, Wan et al. [36] conducted a qualitative research study on psychological well-being of individuals with SMA and the impact of the disease, identifying four recurring themes in their experiences: distress in response to changes in physical function, the influence of stigma on participants’ social expectations, resilience, and grit in the face of challenges posed by the illness. Another study showed that, despite the physical limitations imposed by their disease, participants generally reported satisfaction with their overall life [35]. Furthermore, it was noted that sociodemographic characteristics and clinical variables, such as motor function, were unrelated to psychological well-being, except in the case of females, who exhibited a higher susceptibility to negative emotions [34]. In terms of self-esteem, participants in this study displayed high self-esteem scores [34].
A recent study among adults highlighted that individuals with SMA perceived their health-related quality of life to be very similar to reference values of the general population except for the domain of physical functioning [37]. Conversely, a comprehensive American study suggested that perceived quality of life of respondents with SMA was lower in all domains compared to healthy subjects [38]. As a result, studies investigating the impact of illness severity on quality of life in individuals with SMA have reported contradictory findings. Other variables appear to play a significant role in psychological adjustment of individuals with SMA. It has been observed that disease acceptance is a correlate or predictor of subjective well-being among individuals with chronic physical disabilities [39,40]. A study among adults with various physical disabilities underscored that acceptance of the illness was positively associated with self-acceptance, well-being, positive relations, family satisfaction, and the presence of meaning, defined as the extent to which individuals grasp, make sense of, and find significance in their lives [41,42]. Indeed, Zhang et al. [43] noticed that the acceptance of one’s disease denotes the degree to which individuals integrate their lifestyle into the experience of dealing with the disability.
Regarding social relations, a study involving young individuals highlighted that social support from family, but not from friends, was significantly associated with better psychological adjustment [44]. The authors found significant associations between family support and age, as well as between friend support and motor functioning, in predicting functional ability [44]. Another study emphasized the importance of social relationships [36]. Wan and colleagues [36] reported that individuals with SMA often received informal support from family, friends, and peers to fill gaps in formal care. However, practical support provided by family and friends was seen as unsustainable over time. In general, social support from family and friends was reported as a facilitator for coping with the challenges of illness [45,46]. Furthermore, Fischer et al. [34] reported that adults with SMA were highly satisfied with their participation in social activities. Their research showed that societal participation explained 30–50% of the variance in psychological well-being [34]. Considering all these aspects, it can be argued that the acceptance of disability and social relationships can improve autonomy, perceived competence, and overall quality of life. Additionally, it could be hypothesized that these factors create a positive cycle, wherein individuals with a higher sense of well-being are more motivated to be active and to engage in social activities, and vice versa [34]. As Post et al. [47] suggested, well-being and mental health are closely related to an individual’s level of participation. Conversely, individuals with SMA typically rely on assistance from others, such as caregivers, to engage in various activities. This condition also prompts reflection on the caregivers who take care of individuals with SMA. A recent review on this topic highlighted that most studies have reported decreased quality of life, as well as moderate to high levels of burden and distress among caregivers of individuals with SMA. Another review conducted on parents of children with SMA 1 and 2 showed that parents feel sad, helpless, hopeless, and frustrated about their child’s future, due to the fact that they might lose their children at any time [35].
To alleviate the burden on families and facilitate the participation of individuals with SMA in social activities, healthcare policies should address the needs of families and provide support for caregiving, decision making, and activity organization [48], as has also been shown for other categories of chronic disease patients [49].
Regarding emotional states, some studies found an association between less severe physical limitations, such as ambulation, physical decline following long periods of stability, and increased emotional distress [37,50,51]. Subjective feelings of depression and anxiety were generally low in adults with SMA [52]. However, for school-aged individuals with SMA, there was a high prevalence of anxiety and depression [53]. In a research study involving children and adolescents aged 8–18, high levels of anxiety and depression were reported, and these were associated with factors such as respiratory system dysfunction, digestive system dysfunction, skeletal deformity, rehabilitation exercises, academic delay, specialized support from schools, household income levels, caregivers’ subjective anxiety, and caregivers’ expectations [53].
6. SMA Treatments and Therapies
As SMA is a systemic disease, interdisciplinary management of respiratory, nutritional, gastroenterological, orthopedic, and psychosocial problems is required to care for patients with SMA. In 2007, general treatment recommendations were addressed and published in the first consensus statement pointing out the standards of care for SMA. However, the implementation of these standards of care can vary significantly depending on different factors such as cultural perspectives, socioeconomic aspects, and the availability of resources [54,55].
In the past decade, the introduction of new therapies has led to a significant change in the SMA treatment landscape. Today, multiple types of treatments are available for SMA, including SMN2 splicing modifiers and gene replacement therapy. Clinical studies have demonstrated the potential of both these therapy approaches to positively alter the course of SMA in humans [30,56].
6.1. FDA-Approved SMN-Based Therapies for SMA
In the absence of therapeutic intervention, the SMN2 gene produces mostly truncated SMN protein and only a small fraction of full-length SMN protein, due to poor inclusion of exon 7 in mature mRNA transcripts. However, FDA-approved therapies are aimed at increasing the inclusion of exon 7 in mature mRNA transcripts to enhance the production of full-length SMN protein [57].
FDA-approved therapies for SMA, including nusinersen, onasemnogene abeparvovec, and risdiplam, have improved motor function and lifespan for many patients. However, not all patients respond optimally, particularly those with one copy of SMN2 or those who receive post-symptomatic treatment. Furthermore, cost, availability, access, and the patient’s condition may limit the effectiveness of SMN-based therapy. Thus, SMN-independent strategies to improve motor function and quality of life are needed [58,59].
The first FDA-approved therapy for SMA was nusinersen (trade name Spinraza). The arrival of nusinersen in 2016 marked the first drug capable of altering the natural history of SMA in the United States, which followed in Europe the year after: the drug was approved in 2016 by the FDA and in 2017 by the EMA for all subtypes of 5q-SMA patients [60,61]. Nusinersen is an oligonucleotide based on antisense technology and an SMN2 splicing modifier able to alter the splicing process of SMN2 messenger RNA. This alteration leads to the production of a functional protein in the central nervous system (Figure 2a). However, it must be administered intrathecally, meaning it is directly injected into the fluid surrounding the central nervous system [60,61]. This invasive practice can only be performed in a hospital setting. Despite the inconvenience of the method of administration, nusinersen has been shown to increase survival without permanent respiratory support in SMA 1 and has increased motor function development in types 1–3 [62]. However, improvements in SMA 2 and 3 were less evident [63,64]. Studies have shown that, if administered before the onset of symptoms, nusinersen may result in near-normal motor development in children with SMA [65,66].

Figure 2.
FDA-approved SMN-based therapies for SMA and their mechanism of action [19,67]. (a) The different actions of SMN2 splicing modifiers such as nusinersen (ASO) and risdiplam (small molecules in comparison to the absence of therapeutic intervention). Both SMN2-targeted therapies promote the inclusion of exon 7 in mature mRNA transcripts. (b) The mechanism of SMN1-targeted therapy, by non-replicating self-complementing AAV-9, compared to the homozygous loss of functional SMN1 gene. The onasemnogene abeparvovec gene therapy carries full-length human SMN cDNA to guarantee the production of SMN functional protein.
Nusinersen has been associated with several adverse events, with the majority being pyrexia, upper respiratory tract infections, nasopharyngitis, vomiting, headache, and constipation. Serious adverse events were also reported, which were in line with the typical nature and frequency of events observed in the context of SMA or during lumbar puncture procedures [22,68]. In the past, a variety of side effects have been identified for different antisense oligonucleotides (ASOs). Thus, it is important to mention that, compared to other ASOs, nusinersen is safe and there is no evidence of clinically relevant problems [69]. However, nephrotoxicities as side effects on the kidney, including renal tubular degeneration, glomerulonephritis, and increased urinary protein levels, are a potential risk in nusinersen-treated patients [70]. Screening for SMA may lead to early detection and facilitate prompt treatment using nusinersen [71,72].
In March 2021, Italy received a new gene therapy drug called onasemnogene abeparvovec (Zolgensma). This drug was approved by the FDA in July 2019 and by the EMA in May 2020. It is intended for patients with symptomatic 5q-SMA type 1 SMA under six months of age and for pre-symptomatic 5q-SMA patients with 2–3 copies of SMN2. While it is reimbursable up to 21 kg in Europe, in Italy, it is only compensated up to 13.5 kg and up to two copies of SMN2. However, individuals with three copies of SMN2 who are symptomatic are still eligible [73,74].
Onasemnogene abeparvovec is a non-replicating self-complementing adeno-associated serotype 9 (AAV-9) viral vector that carries full-length human SMN cDNA controlled by the hybrid CMV enhancer/chicken β-actin promoter. The single intravenous administration over a 60 min period allows the vector to cross the blood–brain barrier into the central nervous system where it is endocytosed by cells, including motor neurons, and transduces host cells to transcribe its double-stranded DNA unit [57] (Figure 2b). Prior to and after infusion of onasemnogene abeparvovec, it is recommended to initiate an immunomodulatory regimen with corticosteroid administration to reduce the risk of side effects [75]. Serotype AAV9 targets cells efficiently and important improvements have been shown in motor function for SMA-affected individuals treated pre-symptomatically. Of interest, research studies in porcine and macaque models highlighted that post-symptomatic administration may have limited benefit because of inability to recover motor neurons [19,74]. Moreover, clinical trials demonstrated that affected individuals with three copies of SMN2 were able to reach motor abilities according to age and that the loss of previously gained motor skills are prevented by post-symptomatic treatment. However, less than half of SMA-affected patients treated post-symptomatically reach advanced motor skills such as walking or standing [76,77]. Furthermore, it has been shown that onasemnogene abeparvovec significantly improved airway function, crucial for SMA-affected individuals requiring permanent ventilation therapy [78]. However, the risk of neutralizing antibodies of maternal origin, serious liver complications, as well as the cost of the single-dose gene therapy are all potential drawbacks of this treatment [19].
Before infusing onasemnogen abeparvovec, it is recommended to test patients for the presence of anti-AAV9 antibodies. The half-life of IgG antibodies acquired passively is about 35 to 40 days. In general, in children, the level of antibodies may reflect maternal placental transfer, especially in infancy [79]. Therefore, a follow-up test for antibodies is strongly suggested, mainly in the case of positivity in the initial assay, within f two weeks to enable the decrease in moderately elevated titers to an acceptable level before treatment. It is important to specify that infants with an antibody titer above 1.5 cannot receive treatment, although only approximately 6% of infants present this titer [75,80]. As already described in clinical trials, the most common adverse reactions associated with onasemnogene abeparvovec were elevated aminotransferases and vomiting [81]. Moreover, warnings as indicated in the prescribing information are acute serious liver injury, elevated aminotransferases, elevated troponin-I, and thrombocytopenia. Following the rare occurrence of acute liver injury, serial monitoring of liver function, platelets, and troponin-I concentrations is recommended [81]. Following onasemnogene abeparvovec therapy, it is also important to mention the rare occurrence of severe adverse events, like thrombotic microangiopathy and atypical hemolytic uremia syndrome [81,82].
On 1 April, the European Commission gave the green light to a new drug, risdiplam (commercial name Evrysdi), which can modify diseases. It is the second splicing modifier approved by the FDA in July 2020 and designed for patients with SMA types 1, 2, or 3 with 5q-SMA and of two months of age and older. Being the only orally administered drug, families showed a lot of interest [83]. Unlike Spinraza, risdiplam is effective on the entire body, not only on the central nervous system [84] (Figure 2a). Research studies comparing Evrysdi with Spinraza showed that Evrysdi is indeed a valid alternative for SMA 1, as it can improve survival rates and motor function. However, it is important to note that, while Evrysdi can be used to treat patients as young as two months old, it is not necessarily the preferred choice of clinicians for patients diagnosed by a neonatal screening program and possibly treated before the age of two months [85,86]. Details of each disease-modifying therapy (DMT), as described above, are summarized in Table 3. Moreover, Figure 2 illustrates the mechanism of action of the FDA-approved SMN-based therapies for SMA.

Table 3.
FDA-approved SMN-based DMTs for SMA [4,18,20,46,47,48,49].
6.2. Neuroprotective Drugs
Neuroprotective strategies aimed at preventing dysfunction in motor neurons and associated circuits, but independently of survival motor neuron (SMN), have been attempted for SMA with limited success [19]. Although neuroprotective treatments have been used in other neurodegenerative diseases, such as Alzheimer’s and Parkinson’s disease, the complexity of the process of neurodegeneration suggests that targeting single-cell pathways may not be enough to halt or improve disease progression. Therefore, neuroprotective strategies would likely require SMN-targeted treatments to be effective. Combining neuroprotective strategies with SMN-dependent approaches could maximize therapeutic benefits [87].
6.3. Neuromuscular Junction Drugs
Targeting calcium homeostasis in developing motor nerve terminals can improve neuromuscular dysfunction and motor capacity. Neuromuscular activity drives muscle strength, endurance, and motor skills, which are crucial for daily activities such as wheelchair mobility, food preparation, hygiene, and computer use. Complementary treatments targeting neuromuscular junction function can improve motor skills and quality of life [19].
In conclusion, improving motor skills is essential for SMA patients to perform daily activities independently, enhancing their quality of life and reducing caregiver burden. It is worth emphasizing that the combination of neuroprotective strategies with SMN-dependent approaches and complementary treatments targeting neuromuscular junction function could maximize therapeutic benefits for SMA patients [19].
Most importantly, multidisciplinary management involving physiatrists, orthopedists, and physiotherapists is necessary to prescribe the aids to maintain the patient’s motor autonomy. Periodic and continuous monitoring by a nutritionist, pulmonologist, and neurologist are also important.
7. Newborn Screening for SMA
7.1. New Perspectives on SMA through Newborn Screening
SMA is a severe and progressive neuromuscular disease, the most common genetic cause of infant mortality, characterized by loss of motor neurons causing muscle weakness and atrophy [1,2,3,4,5]. Historically, diagnostic delay has always been a challenge for this rare and devastating disease, but, following improvement in supportive therapies and the availability of DMTs, a new scenario for SMA has been emerging, leading to a breakthrough in the natural history of affected children [88,89]. In fact, as detailed above, three DMTs have been approved by the U.S. Food and Drug Administration (FDA) since 2016: nusinersen in December 2016, onasemnogene abeparvovec in May 2019, and risdiplam in August 2020 [88]. Both pre-clinical trials in mouse models and clinical data demonstrated the importance of early and pre-symptomatic treatment in modulating the rapid and progressive degeneration associated with SMA [88,90,91,92]. Considering that the best outcomes have been described when treatments start as early as possible, hopefully before significant motor weakness or loss occur, time of diagnosis and treatment is crucial for SMA [8,90]. Of note, it has already been reported that motor neuron loss in SMA 1 patients is an irreversible process and begins in the peri-natal period, leading to a severe denervation in the first three months and loss of over 90% of motor units at 6 months [91]. With these recent advances in treatment aiming to increase the expression levels of SMN protein, the possibility to identify SMA-affected infants early in the pre-symptomatic phase through newborn screening (NBS) programs represents an imminent need and is essential for the achievement of maximal therapeutic benefit [2]. It is also important to consider that NBS for SMA gives the unprecedented opportunity to obtain the maximal benefit from these therapies without increasing the cost of treatment since most affected patients still need treatment once diagnosed [2]. As different authors already discussed, SMA can be recognized as “one of the golden candidates” for inclusion in NBS panels [89,93,94]. The idea to include SMA in NBS programs is strongly supported by the fulfilment of the Wilson and Jungner criteria and by experts that agree to establish NBS for SMA [95,96]. The ten principles for population-based screening decisions, outlined in a seminal WHO publication entitled “Principles and Practice of Screening for Disease” in 1968 [97], include considerations on the disease itself (prevalence, severity, natural history), the availability of an effective treatment and of a reliable screening test, and societal considerations (cost/effectiveness, false-positive results) [93]. Therefore, in recent years, thanks to national or regional pilot projects, several NBS programs have been implemented including a screening test for SMA in many countries. As detailed by Dangouloff et al. [98], SMA NBS was implemented in different countries. Australia [99,100], Belgium [101], Canada [93,94], Germany [102,103], Italy [89], Japan [104,105], Taiwan [106], and the United States [107] screen for SMA, in part of the country or in the whole country. In fact, the Advisory Committee on Heritable Disorders in Newborns and Children (ACHDNC) added NBS for SMA to the Recommended Uniform Screening Panel (RUSP) in July 2018 [98,103]. Of interest, as specified by Abiusi et al., the first Italian SMA NBS project showed the highest incidence reported so far (1:6059) [89].
7.2. Molecular Analysis as Newborn Blood Screening Test for SMA
As already discussed above, NBS for SMA started in many countries and regions as a pilot project. For this reason, ethical committee approval was required before implementing SMA NBS [98]. However, no biochemical marker has been identified to be used as a clinically meaningful biomarker in NBS [8,108] and the NBS assay is based on a very sensitive, accurate molecular testing with a high predictive power. The assay consists of a real-time PCR analysis able to detect the presence of the SMN1 gene from a dried blood spot (DBS) sample, more precisely by identifying homozygous deletions of exon 7 in the SMN1 gene in affected infants. As for routine pre-existing metabolic NBS workflow, DBS samples for SMA NBS are collected by pricking the newborn’s heel within 48–72 h of birth and by letting the drops of whole blood dry on a special filter paper card, better known as a Guthrie card. Having started as a pilot project and not being mandatory by law in many regions or countries, details on the SMA NBS pilot via informative sheets and consent forms are provided to all parents before sample collection. More precisely, local health personnel provide all the necessary information about SMA NBS to families. Then, neonatal DBS samples are sent to the NBS reference laboratory within 24–48 h from their collection. Most NBS laboratories perform testing on the day of DBS sample receipt or within 48 h of their arrival. The molecular screening test is usually performed on a 3.2 mm disk (approximately 3–3.2 μL whole blood) punched out from the DBS specimen, followed by DNA extraction.
The protocol designed for NBS is very quick and provides results in a very short time. It consists of two main steps consisting of DNA extraction and amplification analysis by real-time PCR. In particular, the SMA primary screening test allows the qualitative detection of exon 7 in the SMN1 gene.
A valid negative result for SMA is determined by amplification of the SMN1 gene (Figure 3a) while a valid positive result is determined by the absence of amplification of the SMN1 gene (Figure 3b).
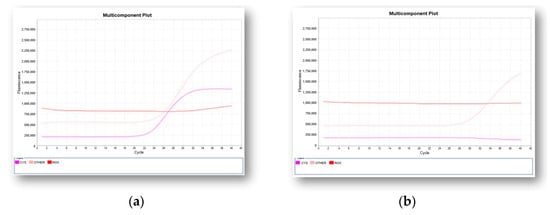
Figure 3.
Two representative real-time PCR multicomponent plots obtained by the qualitative detection of exon 7 in SMN1 gene. Internal control (pink curve), SMN1 gene (purple curve): (a) a negative result with the amplification of the SMN1 gene is shown; (b) a positive result as determined by the absence of amplification of SMN1 gene is reported.
In case of a negative SMA NBS result, no further action is needed, and no information is provided to families, as already agreed in NBS policy [89]. To confirm the positive result, the sample analysis must be briefly repeated. For a confirmed positive SMA NBS test, it is necessary to proceed with the recall of the newborn and confirmatory testing analysis. Most importantly, to provide information regarding SMA, on the significance of the positive test result, and to conduct a careful clinical evaluation of the infant, multidisciplinary counselling with medical geneticists, pediatric neurologists, and also a psychologist is essential for the family [89]. Figure 4 represents the SMA NBS workflow.
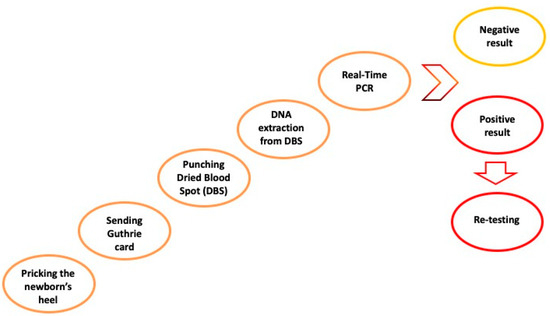
Figure 4.
SMA NBS workflow [6,53,57].
The molecular test for SMA NBS is inexpensive and, as has already occurred for other NBS analyses, it can be multiplex [2,109]. Bearing in mind the paramount need for a screening test for SMA, commercial real-time PCR assay kits have been developed by different companies. These include: Eonis™ SCID-SMA kit (PerkinElmer, Turku, Finland), SPOT-it™ TREC & SMN1 Screening Kit (ImmunoIVD, Nacka Strand, Sweden), NeoNat SCID-SMA REAL-TIME PCR KIT (Labsystems Diagnostics Oy, Vantaa, Finland), Targeted qPCR SMA and Targeted qPCR SMA FLEX (Zentech, Liège, Belgium), SALSA MC002 SMA Newborn Screen (MRC Holland, Amsterdam, the Netherlands), TaqMan™ SCID/SMA Assay (Applied Biosystems™, Thermo Fisher Scientific, Waltham, MA, USA). Some of the SMA assay kits are certified for use in diagnostic procedures (in vitro diagnostic, IVD), while some are for research use only (RUO). Anyway, most of the commercial kits available for SMA are all multiplex and intended for simultaneous screening of SMA and severe combined immunodeficiency (SCID) in newborns.
8. Discussion
In recent years, advances in treatment and care for SMA-affected patients significantly changed. As well known, SMA has historically been recognized as the leading cause of hereditary death in infancy, worldwide. However, thanks to three newly FDA-approved DMTs, implementation of NBS programs, and consequently the possibility of an early intervention, there is a reasonable opportunity to greatly and rapidly change the natural history of the disease [2,10]. In recent years, SMA NBS pilot projects not only pointed out a higher incidence of the disease than expected, but also demonstrated that the possibility to identify infants with SMA in the neonatal period leads to improved outcomes when SMA-specific DMTs are promptly provided. As Vill et al. assessed, pre-symptomatic therapy prevents the death of motor neurons, considering that in their study all pre-symptomatically treated affected individuals, even with two SMN2 copies, achieved normal motor development [103]. It should be recognized that determining the copy number of SMN2 in the diagnostic confirmation process is certainly a key step in defining the most suitable therapeutic option. At the same time, it is also a very delicate phase. Moreover, recommendations for the treatment of SMA patients soon after their identification by an NBS program are complicated by different barriers including administrative, institutional, and insurance-related ones. This aspect is very important when remembering that SMA infants identified by NBS tests should be potentially treated within 14 days of life [10]. In this review, the basic criteria for the inclusion of a rare disease in NBS programs have been reported, and the benefits of SMA NBS have also been discussed. As is well known, cost factors for SMA NBS represent a critical issue but, at the same time, a decisive factor [103], and only very preliminary health economic data or cost-effectiveness data for SMA NBS are available [98]. In this context, it is worth mentioning the Italian situation: in the last five years, many regions in Italy implemented NBS for SMA, particularly thanks to effective DMTs available and reimbursed by the National Health Service (SSN). For this reason, Ghetti G et al. started an evaluation study of the cost-effectiveness of SMA NBS in Italy, demonstrating that NBS, when followed by pre-symptomatic treatment for SMA, results in good value for money and is cost-effective for the Italian SSN [110].
Importantly, as rightly argued by Abiusi et al., the spread of SMA NBS will point out the need to define accurate and univocal guidelines as well as standard analytical procedures for SMA molecular testing, both for primary screening tests and diagnostic confirmation analysis [89]. It is noteworthy that in the coming years the identification of SMA patients will be fulfilled by molecular testing rather than by a clinical picture, leading to the impellent demand for rewriting the natural history of this severe genetic neuromuscular disease as well as the standards of care [89].
Following this line, even the psychological support in the NBS context will change, and it should be addressed to parents. In most cases, parents tend to be dissatisfied with the quality and depth of the information received and prefer in-person visits, finding them clearer, more welcoming, and reassuring [111]. In particular, they need a caring, empathic, and safe setting of communication [111]. It will also be important for health professionals to improve communication resources to mitigate the impact of positive screening results and to offer psychosocial interventions to support families in the future [112].
In conclusion, the new scenario for SMA, following implementation of NBS programs and the availability of recently approved DMTs, which together ensure an early intervention, reveals an urgent need: the identification of clinically meaningful biomarkers, as a follow-up tool, essential to measure and evaluate the disease across time. Therefore, many efforts are being made to define SMA biomarkers of importance to highlight the underlying mechanisms of the disease but also to detect the disease progression, allowing for more appropriate and personalized timing and dosing of therapy for affected patients [108].
Author Contributions
Writing—original draft preparation, I.A. and R.F.; clinical investigation, visualization, R.G. and A.D.; psychological investigation, data curation, L.L. and A.B.; molecular methodology, E.A., S.N., M.R. and C.P.; supervision, V.D.L. and L.S.; writing—review and editing, conceptualization, C.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Scarciolla, O.; Stuppia, L.; De Angelis, M.V.; Murru, S.; Palka, C.; Giuliani, R.; Pace, M.; Di Muzio, A.; Torrente, I.; Morella, A.; et al. Spinal muscular atrophy genotyping by gene dosage using multiple ligation-dependent probe amplification. Neurogenetics 2006, 7, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Glascock, J.; Sampson, J.; Haidet-Phillips, A.; Connolly, A.; Darras, B.; Day, J.; Finkel, R.; Howell, R.R.; Klinger, K.; Kuntz, N.; et al. Treatment Algorithm for Infants Diagnosed with Spinal Muscular Atrophy through Newborn Screening. J. Neuromuscul. Dis. 2018, 5, 145–158. [Google Scholar] [CrossRef] [PubMed]
- Butchbach, M.E.R. Genomic Variability in the Survival Motor Neuron Genes (SMN1 and SMN2): Implications for Spinal Muscular Atrophy Phenotype and Therapeutics Development. Int. J. Mol. Sci. 2021, 22, 7896. [Google Scholar] [CrossRef]
- Kolb, S.J.; Kissel, J.T. Spinal muscular atrophy: A timely review. Arch. Neurol. 2011, 68, 979–984. [Google Scholar] [CrossRef] [PubMed]
- Kolb, S.J.; Kissel, J.T. Spinal Muscular Atrophy. Neurol. Clin. 2015, 33, 831–846. [Google Scholar] [CrossRef]
- Brzustowicz, L.M.; Lehner, T.; Castilla, L.H.; Penchaszadeh, G.K.; Wilhelmsen, K.C.; Daniels, R.; Davies, K.E.; Leppert, M.; Ziter, F.; Wood, D. Genetic mapping of chronic childhood-onset spinal muscular atrophy to chromosome 5q11.2-13.3. Nature 1990, 344, 540–541. [Google Scholar] [CrossRef]
- Groen, E.J.N.; Talbot, K.; Gillingwater, T.H. Advances in therapy for spinal muscular atrophy: Promises and challenges. Nat. Rev. Neurol. 2018, 14, 214–224. [Google Scholar] [CrossRef]
- Phan, H.C.; Taylor, J.L.; Hannon, H.; Howell, R. Newborn screening for spinal muscular atrophy: Anticipating an imminent need. Semin. Perinatol. 2015, 39, 217–229. [Google Scholar] [CrossRef]
- Ogino, S.; Wilson, R.B. Genetic testing and risk assessment for spinal muscular atrophy (SMA). Hum. Genet. 2002, 111, 477–500. [Google Scholar] [CrossRef]
- Butterfield, R.J. Spinal Muscular Atrophy Treatments, Newborn Screening, and the Creation of a Neurogenetics Urgency. Semin. Pediatr. Neurol. 2021, 38, 100899. [Google Scholar] [CrossRef]
- Iannaccone, S.T. Spinal muscular atrophy. Semin. Neurol. 1998, 18, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Nadeau, A.; D’Anjou, G.; Debray, G.; Robitaille, Y.; Simard, L.R.; Vanasse, M. A newborn with spinal muscular atrophy type 0 presenting with a clinicopathological picture suggestive of myotubular myopathy. J. Child. Neurol. 2007, 22, 1301–1304. [Google Scholar] [CrossRef] [PubMed]
- Munsat, T.L.; Davies, K.E. International SMA consortium meeting. (26–28 June 1992, Bonn, Germany). Neuromuscul. Disord. 1992, 2, 423–428. [Google Scholar] [CrossRef] [PubMed]
- Lefebvre, S.; Bürglen, L.; Reboullet, S.; Clermont, O.; Burlet, P.; Viollet, L.; Benichou, B.; Cruaud, C.; Millasseau, P.; Zeviani, M.; et al. Identification and characterization of a spinal muscular atrophy-determining gene. Cell 1995, 80, 155–165. [Google Scholar] [CrossRef]
- Mailman, M.D.; Heinz, J.W.; Papp, A.C.; Snyder, P.J.; Sedra, M.S.; Wirth, B.; Burghes, A.H.M.; Prior, T.W. Molecular analysis of spinal muscular atrophy and modification of the phenotype by SMN2. Genet. Med. 2002, 4, 20–26. [Google Scholar] [CrossRef]
- Prior, T.W.; Krainer, A.R.; Hua, Y.; Swoboda, K.J.; Snyder, P.C.; Bridgeman, S.J.; Burghes, A.H.M.; Kissel, J.T. A positive modifier of spinal muscular atrophy in the SMN2 gene. Am. J. Hum. Genet. 2009, 85, 408–413. [Google Scholar] [CrossRef]
- Monani, U.R.; Sendtner, M.; Coovert, D.D.; Parsons, D.W.; Andreassi, C.; Le, T.T.; Jablonka, S.; Schrank, B.; Rossoll, W.; Prior, T.W.; et al. The human centromeric survival motor neuron gene (SMN2) rescues embryonic lethality in Smn(-/-) mice and results in a mouse with spinal muscular atrophy. Hum. Mol. Genet. 2000, 9, 333–339. [Google Scholar] [CrossRef]
- Rouzier, C.; Chaussenot, A.; Paquis-Flucklinger, V. Molecular diagnosis and genetic counseling for spinal muscular atrophy (SMA). Arch. Pediatr. 2020, 27, 7S9–7S14. [Google Scholar] [CrossRef]
- Ojala, K.S.; Reedich, E.J.; DiDonato, C.J.; Meriney, S.D. In Search of a Cure: The Development of Therapeutics to Alter the Progression of Spinal Muscular Atrophy. Brain Sci. 2021, 11. [Google Scholar] [CrossRef]
- Prior, T.W.; Leach, M.E.; Finanger, E. Spinal Muscular Atrophy. In GeneReviews; University of Washington: Seattle, WA, USA, 1993. [Google Scholar]
- Polido, G.J.; Miranda, M.M.V.D.; Carvas, N.; Mendonça, R.D.H.; Caromano, F.A.; Reed, U.C.; Zanoteli, E.; Voos, M.C. Cognitive performance of children with spinal muscular atrophy: A systematic review. Dement. Neuropsychol. 2019, 13, 436–443. [Google Scholar] [CrossRef]
- Darras, B.T.; Farrar, M.A.; Mercuri, E.; Finkel, R.S.; Foster, R.; Hughes, S.G.; Bhan, I.; Farwell, W.; Gheuens, S. An Integrated Safety Analysis of Infants and Children with Symptomatic Spinal Muscular Atrophy (SMA) Treated with Nusinersen in Seven Clinical Trials. CNS Drugs 2019, 33, 919–932. [Google Scholar] [CrossRef] [PubMed]
- Prior, T.W.; Nagan, N.; Sugarman, E.A.; Batish, S.D.; Braastad, C. Technical standards and guidelines for spinal muscular atrophy testing. Genet. Med. 2011, 13, 686–694. [Google Scholar] [CrossRef] [PubMed]
- Babić, M.; Banović, M.; Berečić, I.; Banić, T.; Babić Leko, M.; Ulamec, M.; Junaković, A.; Kopić, J.; Sertić, J.; Barišić, N.; et al. Molecular Biomarkers for the Diagnosis, Prognosis, and Pharmacodynamics of Spinal Muscular Atrophy. J. Clin. Med. 2023, 12. [Google Scholar] [CrossRef]
- Burr, P.; Reddivari, A.K.R. Spinal Muscle Atrophy. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- D’Amico, A.; Mercuri, E.; Tiziano, F.D.; Bertini, E. Spinal muscular atrophy. Orphanet J. Rare Dis. 2011, 6, 71. [Google Scholar] [CrossRef] [PubMed]
- Bartels, B.; Montes, J.; van der Pol, W.L.; de Groot, J.F. Physical exercise training for type 3 spinal muscular atrophy. Cochrane Database Syst. Rev. 2019, 3, CD012120. [Google Scholar] [CrossRef] [PubMed]
- Strunk, A.; Abbes, A.; Stuitje, A.; Hettinga, C.; Sepers, E.; Snetselaar, R.; Schouten, J.; Asselman, F.-L.; Cuppen, I.; Lemmink, H.; et al. Validation of a Fast, Robust, Inexpensive, Two-Tiered Neonatal Screening Test algorithm on Dried Blood Spots for Spinal Muscular Atrophy. Int. J. Neonatal Screen. 2019, 5, 21. [Google Scholar] [CrossRef] [PubMed]
- Luo, M.; Liu, L.; Peter, I.; Zhu, J.; Scott, S.A.; Zhao, G.; Eversley, C.; Kornreich, R.; Desnick, R.J.; Edelmann, L. An Ashkenazi Jewish SMN1 haplotype specific to duplication alleles improves pan-ethnic carrier screening for spinal muscular atrophy. Genet. Med. 2014, 16, 149–156. [Google Scholar] [CrossRef]
- Mercuri, E.; Finkel, R.S.; Muntoni, F.; Wirth, B.; Montes, J.; Main, M.; Mazzone, E.S.; Vitale, M.; Snyder, B.; Quijano-Roy, S.; et al. Diagnosis and management of spinal muscular atrophy: Part 1: Recommendations for diagnosis, rehabilitation, orthopedic and nutritional care. Neuromuscul. Disord. 2018, 28, 103–115. [Google Scholar] [CrossRef]
- Vezain, M.; Saugier-Veber, P.; Goina, E.; Touraine, R.; Manel, V.; Toutain, A.; Fehrenbach, S.; Frébourg, T.; Pagani, F.; Tosi, M.; et al. A rare SMN2 variant in a previously unrecognized composite splicing regulatory element induces exon 7 inclusion and reduces the clinical severity of spinal muscular atrophy. Hum. Mutat. 2010, 31, E1110–E1125. [Google Scholar] [CrossRef]
- Arkblad, E.L.; Darin, N.; Berg, K.; Kimber, E.; Brandberg, G.; Lindberg, C.; Holmberg, E.; Tulinius, M.; Nordling, M. Multiplex ligation-dependent probe amplification improves diagnostics in spinal muscular atrophy. Neuromuscul. Disord. 2006, 16, 830–838. [Google Scholar] [CrossRef]
- Passon, N.; Dubsky de Wittenau, G.; Jurman, I.; Radovic, S.; Bregant, E.; Molinis, C.; Damante, G.; Lonigro, I.R. Quick MLPA test for quantification of SMN1 and SMN2 copy numbers. Mol. Cell. Probes 2010, 24, 310–314. [Google Scholar] [CrossRef] [PubMed]
- Fischer, M.J.; Asselman, F.-L.; Kruitwagen-van Reenen, E.T.; Verhoef, M.; Wadman, R.I.; Visser-Meily, J.M.A.; van der Pol, W.L.; Schröder, C.D. Psychological well-being in adults with spinal muscular atrophy: The contribution of participation and psychological needs. Disabil. Rehabil. 2020, 42, 2262–2270. [Google Scholar] [CrossRef] [PubMed]
- Sari, D.M.; Wijaya, L.C.G.; Sitorus, W.D.R.; Dewi, M.M. Psychological burden in spinal muscular atrophy patients and their families: A systematic review. Egypt. J. Neurol. Psychiatry Neurosurg. 2022, 58, 140. [Google Scholar] [CrossRef]
- Wan, H.W.Y.; Carey, K.A.; D’Silva, A.; Kasparian, N.A.; Farrar, M.A. “Getting ready for the adult world”: How adults with spinal muscular atrophy perceive and experience healthcare, transition and well-being. Orphanet J. Rare Dis. 2019, 14, 74. [Google Scholar] [CrossRef] [PubMed]
- Kruitwagen-Van Reenen, E.T.; Wadman, R.I.; Visser-Meily, J.M.; van den Berg, L.H.; Schröder, C.; van der Pol, W.L. Correlates of health related quality of life in adult patients with spinal muscular atrophy. Muscle Nerve 2016, 54, 850–855. [Google Scholar] [CrossRef]
- Iannaccone, S.T.; Hynan, L.S.; Morton, A.; Buchanan, R.; Limbers, C.A.; Varni, J.W. The PedsQLTM in pediatric patients with Spinal Muscular Atrophy: Feasibility, reliability, and validity of the Pediatric Quality of Life InventoryTM Generic Core Scales and Neuromuscular Module. Neuromuscul. Disord. 2009, 19, 805–812. [Google Scholar] [CrossRef]
- Ditchman, N.; Sung, C.; Easton, A.B.; Johnson, K.S.; Batchos, E. Symptom severity and life satisfaction in brain injury: The mediating role of disability acceptance and social self-efficacy. NeuroRehabilitation 2017, 40, 531–543. [Google Scholar] [CrossRef]
- Park, E.-Y. Rasch Analysis of the Disability Acceptance Scale for Individuals With Cerebral Palsy. Front. Neurol. 2019, 10. [Google Scholar] [CrossRef]
- Steger, M.F.; Oishi, S.; Kashdan, T.B. Meaning in Life across the Life Span: Levels and Correlates of Meaning in Life from Emerging Adulthood to Older Adulthood. J. Posit. Psychol. 2009, 4, 43–52. [Google Scholar] [CrossRef]
- Szcześniak, M.; Świątek, A.H.; Cieślak, M.; Świdurska, D. Disease Acceptance and Eudemonic Well-Being Among Adults With Physical Disabilities: The Mediator Effect of Meaning in Life. Front. Psychol. 2020, 11, 525560. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, Y.; Deng, X.; Deng, C.; Pan, Y.; Hu, A. The Correlation Between Quality of Life and Acceptability of Disability in Patients With Facial Burn Scars. Front. Bioeng. Biotechnol. 2019, 7. [Google Scholar] [CrossRef] [PubMed]
- Wilson, S.; Washington, L.A.; Engel, J.M.; Ciol, M.A.; Jensen, M.P. Perceived social support, psychological adjustment, and functional ability in youths with physical disabilities. Rehabil. Psychol. 2006, 51, 322–330. [Google Scholar] [CrossRef]
- Lamb, C.; Peden, A. Understanding the Experience of Living with Spinal Muscular Atrophy. J. Neurosci. Nurs. 2008, 40, 250–256. [Google Scholar] [CrossRef]
- Ho, H.-M.; Tseng, Y.-H.; Hsin, Y.-M.; Chou, F.-H.; Lin, W.-T. Living with illness and self-transcendence: The lived experience of patients with spinal muscular atrophy. J. Adv. Nurs. 2016, 72, 2695–2705. [Google Scholar] [CrossRef]
- Post, M.W.M.; van der Zee, C.H.; Hennink, J.; Schafrat, C.G.; Visser-Meily, J.M.A.; van Berlekom, S.B. Validity of the utrecht scale for evaluation of rehabilitation-participation. Disabil. Rehabil. 2012, 34, 478–485. [Google Scholar] [CrossRef] [PubMed]
- Brandt, M.; Johannsen, L.; Inhestern, L.; Bergelt, C. Parents as informal caregivers of children and adolescents with spinal muscular atrophy: A systematic review of quantitative and qualitative data on the psychosocial situation, caregiver burden, and family needs. Orphanet J. Rare Dis. 2022, 17, 274. [Google Scholar] [CrossRef] [PubMed]
- Bramanti, S.M.; Trumello, C.; Lombardi, L.; Babore, A. COVID-19 and chronic disease patients: Perceived stress, worry, and emotional regulation strategies. Rehabil. Psychol. 2021, 66, 380–385. [Google Scholar] [CrossRef] [PubMed]
- Kruitwagen-van Reenen, E.T.; van der Pol, L.; Schröder, C.; Wadman, R.I.; van den Berg, L.H.; Visser-Meily, J.M.A.; Post, M.W.M. Social participation of adult patients with spinal muscular atrophy: Frequency, restrictions, satisfaction, and correlates. Muscle Nerve 2018, 58, 805–811. [Google Scholar] [CrossRef]
- Boardman, F.K.; Young, P.J.; Griffiths, F.E. Impairment Experiences, Identity and Attitudes Towards Genetic Screening: The Views of People with Spinal Muscular Atrophy. J. Genet. Couns. 2018, 27, 69–84. [Google Scholar] [CrossRef]
- Günther, R.; Wurster, C.D.; Cordts, I.; Koch, J.C.; Kamm, C.; Petzold, D.; Aust, E.; Deschauer, M.; Lingor, P.; Ludolph, A.C.; et al. Patient-Reported Prevalence of Non-motor Symptoms Is Low in Adult Patients Suffering From 5q Spinal Muscular Atrophy. Front. Neurol. 2019, 10. [Google Scholar] [CrossRef]
- Yao, M.; Xia, Y.; Feng, Y.; Ma, Y.; Hong, Y.; Zhang, Y.; Chen, J.; Yuan, C.; Mao, S. Anxiety and depression in school-age patients with spinal muscular atrophy: A cross-sectional study. Orphanet J. Rare Dis. 2021, 16, 385. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.H.; Finkel, R.S.; Bertini, E.S.; Schroth, M.; Simonds, A.; Wong, B.; Aloysius, A.; Morrison, L.; Main, M.; Crawford, T.O.; et al. Consensus statement for standard of care in spinal muscular atrophy. J. Child. Neurol. 2007, 22, 1027–1049. [Google Scholar] [CrossRef] [PubMed]
- Bladen, C.L.; Thompson, R.; Jackson, J.M.; Garland, C.; Wegel, C.; Ambrosini, A.; Pisano, P.; Walter, M.C.; Schreiber, O.; Lusakowska, A.; et al. Mapping the differences in care for 5,000 spinal muscular atrophy patients, a survey of 24 national registries in North America, Australasia and Europe. J. Neurol. 2014, 261, 152–163. [Google Scholar] [CrossRef] [PubMed]
- Finkel, R.S.; Chiriboga, C.A.; Vajsar, J.; Day, J.W.; Montes, J.; De Vivo, D.C.; Yamashita, M.; Rigo, F.; Hung, G.; Schneider, E.; et al. Treatment of infantile-onset spinal muscular atrophy with nusinersen: A phase 2, open-label, dose-escalation study. Lancet 2016, 388, 3017–3026. [Google Scholar] [CrossRef]
- Day, J.W.; Howell, K.; Place, A.; Long, K.; Rossello, J.; Kertesz, N.; Nomikos, G. Advances and limitations for the treatment of spinal muscular atrophy. BMC Pediatr. 2022, 22, 632. [Google Scholar] [CrossRef]
- Ramos-Platt, L.; Elman, L.; Shieh, P.B. Experience and Perspectives in the US on the Evolving Treatment Landscape in Spinal Muscular Atrophy. Int. J. Gen. Med. 2022, 15, 7341–7353. [Google Scholar] [CrossRef]
- Nishio, H.; Niba, E.T.E.; Saito, T.; Okamoto, K.; Takeshima, Y.; Awano, H. Spinal Muscular Atrophy: The Past, Present, and Future of Diagnosis and Treatment. Int. J. Mol. Sci. 2023, 24, 11939. [Google Scholar] [CrossRef]
- Ottesen, E.W. ISS-N1 makes the First FDA-approved Drug for Spinal Muscular Atrophy. Transl. Neurosci. 2017, 8, 1–6. [Google Scholar] [CrossRef]
- Qiu, J.; Wu, L.; Qu, R.; Jiang, T.; Bai, J.; Sheng, L.; Feng, P.; Sun, J. History of development of the life-saving drug “Nusinersen” in spinal muscular atrophy. Front. Cell. Neurosci. 2022, 16, 942976. [Google Scholar] [CrossRef]
- Vázquez-Costa, J.F.; Povedano, M.; Nascimiento-Osorio, A.E.; Moreno Escribano, A.; Kapetanovic Garcia, S.; Dominguez, R.; Exposito, J.M.; González, L.; Marco, C.; Medina Castillo, J.; et al. Nusinersen in adult patients with 5q spinal muscular atrophy: A multicenter observational cohorts’ study. Eur. J. Neurol. 2022, 29, 3337–3346. [Google Scholar] [CrossRef]
- Park, J.-M.; Min, Y.-S.; Park, D.; Park, J.-S. Effect of Nusinersen in a late onset spinal muscular atrophy patient for 14 months: A case report. Medicine 2021, 100, e24236. [Google Scholar] [CrossRef] [PubMed]
- Belančić, A.; Strbad, T.; Kučan Štiglić, M.; Vitezić, D. Effectiveness of Nusinersen in Type 1, 2 and 3 Spinal Muscular Atrophy: Croatian Real-World Data. J. Clin. Med. 2023, 12, 2839. [Google Scholar] [CrossRef] [PubMed]
- Dunaway Young, S.; Montes, J.; Glanzman, A.M.; Gee, R.; Day, J.W.; Finkel, R.S.; Darras, B.T.; De Vivo, D.C.; Gambino, G.; Foster, R.; et al. Nusinersen Treatment of Children with Later-Onset Spinal Muscular Atrophy and Scoliosis Is Associated with Improvements or Stabilization of Motor Function. J. Clin. Med. 2023, 12, 4901. [Google Scholar] [CrossRef]
- Crawford, T.O.; Swoboda, K.J.; De Vivo, D.C.; Bertini, E.; Hwu, W.-L.; Finkel, R.S.; Kirschner, J.; Kuntz, N.L.; Nazario, A.N.; Parsons, J.A.; et al. Continued benefit of nusinersen initiated in the presymptomatic stage of spinal muscular atrophy: 5-year update of the NURTURE study. Muscle Nerve 2023, 68, 157–170. [Google Scholar] [CrossRef] [PubMed]
- Schorling, D.C.; Pechmann, A.; Kirschner, J. Advances in Treatment of Spinal Muscular Atrophy—New Phenotypes, New Challenges, New Implications for Care. J. Neuromuscul. Dis. 2020, 7, 1–13. [Google Scholar] [CrossRef]
- Zhong, Z.-J.; Zheng, P.-M.; Dou, H.-H.; Wang, J.-G. Adverse events in the treatment of spinal muscular atrophy in children and adolescents with nusinersen: A systematic review and meta-analysis. Front. Pediatr. 2023, 11, 1152318. [Google Scholar] [CrossRef] [PubMed]
- Stolte, B.; Nonnemacher, M.; Kizina, K.; Bolz, S.; Totzeck, A.; Thimm, A.; Wagner, B.; Deuschl, C.; Kleinschnitz, C.; Hagenacker, T. Nusinersen treatment in adult patients with spinal muscular atrophy: A safety analysis of laboratory parameters. J. Neurol. 2021, 268, 4667–4679. [Google Scholar] [CrossRef]
- Wu, H.; Wahane, A.; Alhamadani, F.; Zhang, K.; Parikh, R.; Lee, S.; McCabe, E.M.; Rasmussen, T.P.; Bahal, R.; Zhong, X.-B.; et al. Nephrotoxicity of marketed antisense oligonucleotide drugs. Curr. Opin. Toxicol. 2022, 32, 100373. [Google Scholar] [CrossRef]
- Albrechtsen, S.S.; Born, A.P.; Boesen, M.S. Nusinersen treatment of spinal muscular atrophy—A systematic review. Dan. Med. J. 2020, 67, A02200100. [Google Scholar]
- Gidaro, T.; Servais, L. Nusinersen treatment of spinal muscular atrophy: Current knowledge and existing gaps. Dev. Med. Child. Neurol. 2019, 61, 19–24. [Google Scholar] [CrossRef]
- Mahajan, R. Onasemnogene Abeparvovec for Spinal Muscular Atrophy: The Costlier Drug Ever. Int. J. Appl. Basic Med. Res. 2019, 9, 127–128. [Google Scholar] [CrossRef] [PubMed]
- Kotulska, K.; Fattal-Valevski, A.; Haberlova, J. Recombinant Adeno-Associated Virus Serotype 9 Gene Therapy in Spinal Muscular Atrophy. Front. Neurol. 2021, 12, 726468. [Google Scholar] [CrossRef] [PubMed]
- Kichula, E.A.; Proud, C.M.; Farrar, M.A.; Kwon, J.M.; Saito, K.; Desguerre, I.; McMillan, H.J. Expert recommendations and clinical considerations in the use of onasemnogene abeparvovec gene therapy for spinal muscular atrophy. Muscle Nerve 2021, 64, 413–427. [Google Scholar] [CrossRef] [PubMed]
- Strauss, K.A.; Farrar, M.A.; Muntoni, F.; Saito, K.; Mendell, J.R.; Servais, L.; McMillan, H.J.; Finkel, R.S.; Swoboda, K.J.; Kwon, J.M.; et al. Onasemnogene abeparvovec for presymptomatic infants with three copies of SMN2 at risk for spinal muscular atrophy: The Phase III SPR1NT trial. Nat. Med. 2022, 28, 1390–1397. [Google Scholar] [CrossRef]
- Jędrzejowska, M. Advances in Newborn Screening and Presymptomatic Diagnosis of Spinal Muscular Atrophy. Degener. Neurol. Neuromuscul. Dis. 2020, 10, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Panagiotou, P.; Kanaka-Gantenbein, C.; Kaditis, A.G. Changes in Ventilatory Support Requirements of Spinal Muscular Atrophy (SMA) Patients Post Gene-Based Therapies. Children 2022, 9, 1207. [Google Scholar] [CrossRef] [PubMed]
- Mendell, J.R.; Connolly, A.M.; Lehman, K.J.; Griffin, D.A.; Khan, S.Z.; Dharia, S.D.; Quintana-Gallardo, L.; Rodino-Klapac, L.R. Testing preexisting antibodies prior to AAV gene transfer therapy: Rationale, lessons and future considerations. Mol. Ther. Methods Clin. Dev. 2022, 25, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Gorovits, B.; Azadeh, M.; Buchlis, G.; Harrison, T.; Havert, M.; Jawa, V.; Long, B.; McNally, J.; Milton, M.; Nelson, R.; et al. Evaluation of the Humoral Response to Adeno-Associated Virus-Based Gene Therapy Modalities Using Total Antibody Assays. AAPS J. 2021, 23, 108. [Google Scholar] [CrossRef]
- Chand, D.H.; Zaidman, C.; Arya, K.; Millner, R.; Farrar, M.A.; Mackie, F.E.; Goedeker, N.L.; Dharnidharka, V.R.; Dandamudi, R.; Reyna, S.P. Thrombotic Microangiopathy Following Onasemnogene Abeparvovec for Spinal Muscular Atrophy: A Case Series. J. Pediatr. 2021, 231, 265–268. [Google Scholar] [CrossRef]
- Guillou, J.; de Pellegars, A.; Porcheret, F.; Frémeaux-Bacchi, V.; Allain-Launay, E.; Debord, C.; Denis, M.; Péréon, Y.; Barnérias, C.; Desguerre, I.; et al. Fatal thrombotic microangiopathy case following adeno-associated viral SMN gene therapy. Blood Adv. 2022, 6, 4266–4270. [Google Scholar] [CrossRef]
- Dhillon, S. Risdiplam: First Approval. Drugs 2020, 80, 1853–1858. [Google Scholar] [CrossRef] [PubMed]
- Kakazu, J.; Walker, N.L.; Babin, K.C.; Trettin, K.A.; Lee, C.; Sutker, P.B.; Kaye, A.M.; Kaye, A.D. Risdiplam for the Use of Spinal Muscular Atrophy. Orthop. Rev. 2021, 13, 25579. [Google Scholar] [CrossRef] [PubMed]
- Menduti, G.; Rasà, D.M.; Stanga, S.; Boido, M. Drug Screening and Drug Repositioning as Promising Therapeutic Approaches for Spinal Muscular Atrophy Treatment. Front. Pharmacol. 2020, 11, 592234. [Google Scholar] [CrossRef] [PubMed]
- Ribero, V.A.; Daigl, M.; Martí, Y.; Gorni, K.; Evans, R.; Scott, D.A.; Mahajan, A.; Abrams, K.R.; Hawkins, N. How does risdiplam compare with other treatments for Types 1-3 spinal muscular atrophy: A systematic literature review and indirect treatment comparison. J. Comp. Eff. Res. 2022, 11, 347–370. [Google Scholar] [CrossRef]
- Bowerman, M.; Becker, C.G.; Yáñez-Muñoz, R.J.; Ning, K.; Wood, M.J.A.; Gillingwater, T.H.; Talbot, K.; UK SMA Research Consortium. Therapeutic strategies for spinal muscular atrophy: SMN and beyond. Dis. Model. Mech. 2017, 10, 943–954. [Google Scholar] [CrossRef]
- Deng, S.; Lee, B.H.; Ciafaloni, E. Parent Perceptions in Choosing Treatment for Infants With Spinal Muscular Atrophy Diagnosed Through Newborn Screening. J. Child. Neurol. 2022, 37, 43–49. [Google Scholar] [CrossRef]
- Abiusi, E.; Vaisfeld, A.; Fiori, S.; Novelli, A.; Spartano, S.; Faggiano, M.V.; Giovanniello, T.; Angeloni, A.; Vento, G.; Santoloci, R.; et al. Experience of a 2-year spinal muscular atrophy NBS pilot study in Italy: Towards specific guidelines and standard operating procedures for the molecular diagnosis. J. Med. Genet. 2023, 60, 697–705. [Google Scholar] [CrossRef]
- Farrar, M.A.; Park, S.B.; Vucic, S.; Carey, K.A.; Turner, B.J.; Gillingwater, T.H.; Swoboda, K.J.; Kiernan, M.C. Emerging therapies and challenges in spinal muscular atrophy. Ann. Neurol. 2017, 81, 355–368. [Google Scholar] [CrossRef]
- Swoboda, K.J.; Prior, T.W.; Scott, C.B.; McNaught, T.P.; Wride, M.C.; Reyna, S.P.; Bromberg, M.B. Natural history of denervation in SMA: Relation to age, SMN2 copy number, and function. Ann. Neurol. 2005, 57, 704–712. [Google Scholar] [CrossRef]
- De Vivo, D.C.; Bertini, E.; Swoboda, K.J.; Hwu, W.-L.; Crawford, T.O.; Finkel, R.S.; Kirschner, J.; Kuntz, N.L.; Parsons, J.A.; Ryan, M.M.; et al. Nusinersen initiated in infants during the presymptomatic stage of spinal muscular atrophy: Interim efficacy and safety results from the Phase 2 NURTURE study. Neuromuscul. Disord. 2019, 29, 842–856. [Google Scholar] [CrossRef]
- McMillan, H.J.; Kernohan, K.D.; Yeh, E.; Amburgey, K.; Boyd, J.; Campbell, C.; Dowling, J.J.; Gonorazky, H.; Marcadier, J.; Tarnopolsky, M.A.; et al. Newborn Screening for Spinal Muscular Atrophy: Ontario Testing and Follow-up Recommendations. Can. J. Neurol. Sci. 2021, 48, 504–511. [Google Scholar] [CrossRef] [PubMed]
- Kernohan, K.D.; McMillan, H.J.; Yeh, E.; Lacaria, M.; Kowalski, M.; Campbell, C.; Dowling, J.J.; Gonorazky, H.; Marcadier, J.; Tarnopolsky, M.A.; et al. Ontario Newborn Screening for Spinal Muscular Atrophy: The First Year. Can. J. Neurol. Sci. 2022, 49, 821–823. [Google Scholar] [CrossRef] [PubMed]
- Andermann, A.; Blancquaert, I.; Beauchamp, S.; Déry, V. Revisiting Wilson and Jungner in the genomic age: A review of screening criteria over the past 40 years. Bull. World Health Organ. 2008, 86, 317–319. [Google Scholar] [CrossRef]
- Prior, T.W.; Nagan, N. Spinal Muscular Atrophy: Overview of Molecular Diagnostic Approaches. Curr. Protoc. Hum. Genet. 2016, 88, 9.27.1–9.27.13. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.M.; Jungner, Y.G. Principles and practice of mass screening for disease. Bol. Oficina Sanit. Panam. 1968, 65, 281–393. [Google Scholar]
- Dangouloff, T.; Vrščaj, E.; Servais, L.; Osredkar, D.; SMA NBS World Study Group. Newborn screening programs for spinal muscular atrophy worldwide: Where we stand and where to go. Neuromuscul. Disord. 2021, 31, 574–582. [Google Scholar] [CrossRef]
- Kariyawasam, D.S.T.; Russell, J.S.; Wiley, V.; Alexander, I.E.; Farrar, M.A. The implementation of newborn screening for spinal muscular atrophy: The Australian experience. Genet. Med. 2020, 22, 557–565. [Google Scholar] [CrossRef]
- D’Silva, A.M.; Kariyawasam, D.S.T.; Best, S.; Wiley, V.; Farrar, M.A.; NSW SMA NBS Study Group. Integrating newborn screening for spinal muscular atrophy into health care systems: An Australian pilot programme. Dev. Med. Child. Neurol. 2022, 64, 625–632. [Google Scholar] [CrossRef]
- Boemer, F.; Caberg, J.-H.; Beckers, P.; Dideberg, V.; di Fiore, S.; Bours, V.; Marie, S.; Dewulf, J.; Marcelis, L.; Deconinck, N.; et al. Three years pilot of spinal muscular atrophy newborn screening turned into official program in Southern Belgium. Sci. Rep. 2021, 11, 19922. [Google Scholar] [CrossRef]
- Vill, K.; Kölbel, H.; Schwartz, O.; Blaschek, A.; Olgemöller, B.; Harms, E.; Burggraf, S.; Röschinger, W.; Durner, J.; Gläser, D.; et al. One Year of Newborn Screening for SMA—Results of a German Pilot Project. J. Neuromuscul. Dis. 2019, 6, 503–515. [Google Scholar] [CrossRef]
- Vill, K.; Schwartz, O.; Blaschek, A.; Gläser, D.; Nennstiel, U.; Wirth, B.; Burggraf, S.; Röschinger, W.; Becker, M.; Czibere, L.; et al. Newborn screening for spinal muscular atrophy in Germany: Clinical results after 2 years. Orphanet J. Rare Dis. 2021, 16, 153. [Google Scholar] [CrossRef]
- Shinohara, M.; Niba, E.T.E.; Wijaya, Y.O.S.; Takayama, I.; Mitsuishi, C.; Kumasaka, S.; Kondo, Y.; Takatera, A.; Hokuto, I.; Morioka, I.; et al. A Novel System for Spinal Muscular Atrophy Screening in Newborns: Japanese Pilot Study. Int. J. Neonatal Screen. 2019, 5, 41. [Google Scholar] [CrossRef] [PubMed]
- Sawada, T.; Kido, J.; Sugawara, K.; Yoshida, S.; Ozasa, S.; Nomura, K.; Okada, K.; Fujiyama, N.; Nakamura, K. Newborn screening for spinal muscular atrophy in Japan: One year of experience. Mol. Genet. Metab. Rep. 2022, 32, 100908. [Google Scholar] [CrossRef] [PubMed]
- Chien, Y.-H.; Chiang, S.-C.; Weng, W.-C.; Lee, N.-C.; Lin, C.-J.; Hsieh, W.-S.; Lee, W.-T.; Jong, Y.-J.; Ko, T.-M.; Hwu, W.-L. Presymptomatic Diagnosis of Spinal Muscular Atrophy Through Newborn Screening. J. Pediatr. 2017, 190, 124–129.e1. [Google Scholar] [CrossRef] [PubMed]
- Hale, K.; Ojodu, J.; Singh, S. Landscape of Spinal Muscular Atrophy Newborn Screening in the United States: 2018-2021. Int. J. Neonatal Screen. 2021, 7, 33. [Google Scholar] [CrossRef]
- Pino, M.G.; Rich, K.A.; Kolb, S.J. Update on Biomarkers in Spinal Muscular Atrophy. Biomark. Insights 2021, 16, 11772719211035644. [Google Scholar] [CrossRef]
- Taylor, J.L.; Lee, F.K.; Yazdanpanah, G.K.; Staropoli, J.F.; Liu, M.; Carulli, J.P.; Sun, C.; Dobrowolski, S.F.; Hannon, W.H.; Vogt, R.F. Newborn blood spot screening test using multiplexed real-time PCR to simultaneously screen for spinal muscular atrophy and severe combined immunodeficiency. Clin. Chem. 2015, 61, 412–419. [Google Scholar] [CrossRef]
- Ghetti, G.; Mennini, F.; Marcellusi, A.; Bischof, M.; Pistillo, G.; Pane, M. PCR145 Cost-Effectiveness Analysis of Newborn Screening for Spinal Muscular Atrophy (SMA) in Italy. Value Heal. 2022, 25, S419. [Google Scholar] [CrossRef]
- Bani, M.; Russo, S.; Raggi, E.; Gasperini, S.; Motta, S.; Menni, F.; Furlan, F.; Cefalo, G.; Paci, S.; Banderali, G.; et al. Parents’ experience of the communication process of positivity at newborn screening for metabolic diseases: A qualitative study. Child. Care. Health Dev. 2023. [Google Scholar] [CrossRef]
- Buchbinder, M.; Timmermans, S. Newborn Screening for Metabolic Disorders. Clin. Pediatr. 2012, 51, 739–744. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).