The Effects of Chronic Unpredictable Mild Stress and Semi-Pure Diets on the Brain, Gut and Adrenal Medulla in C57BL6 Mice
Abstract
1. Introduction
2. Results
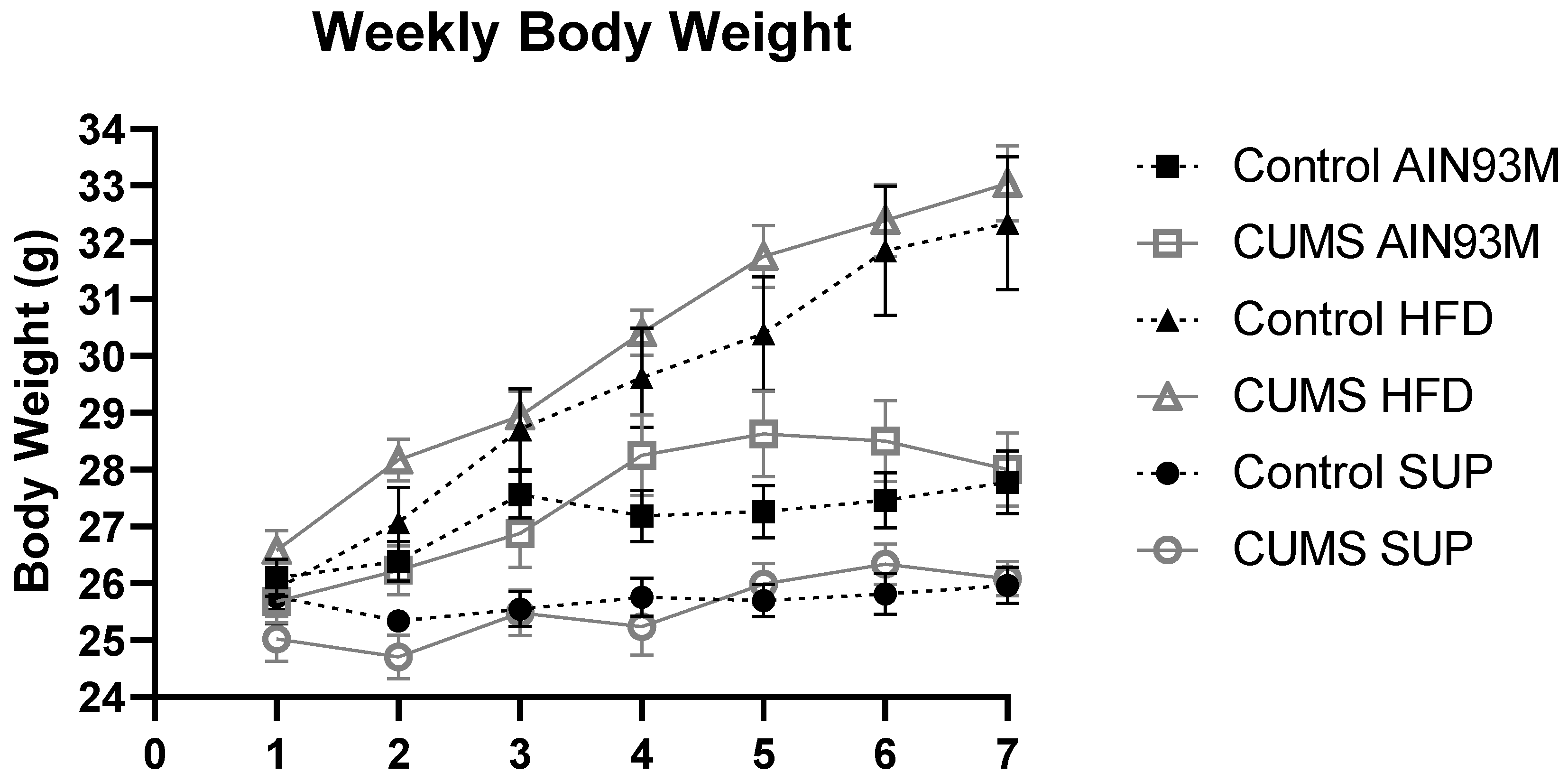
2.1. Body Weight
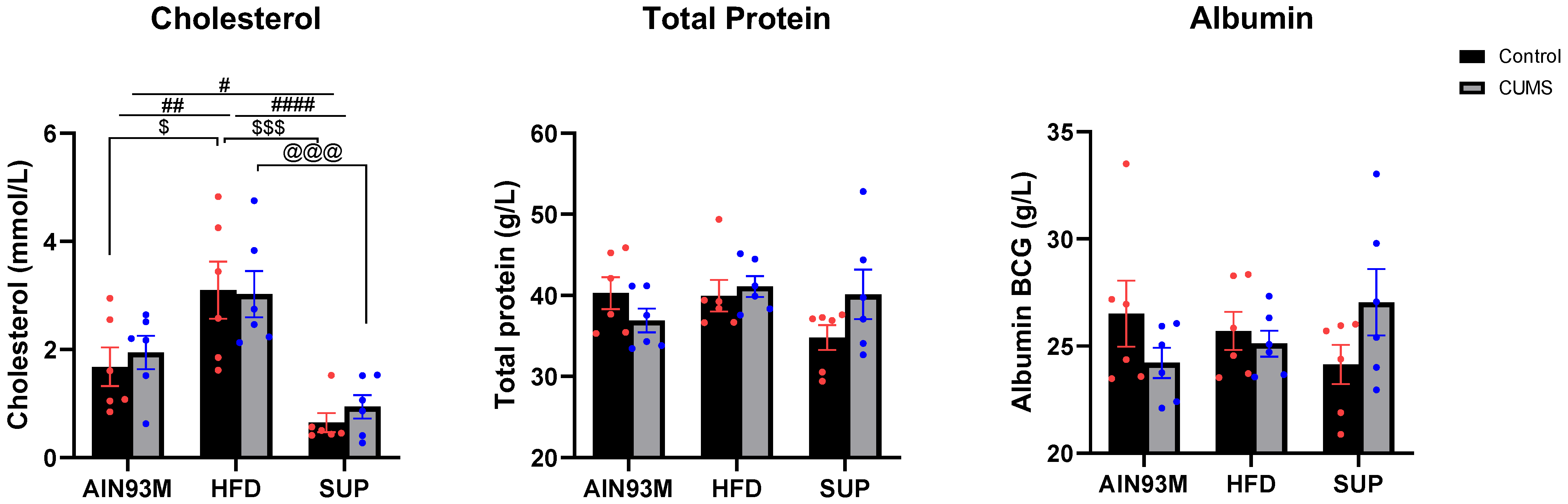
2.2. General Biochemistry
2.3. Behaviour
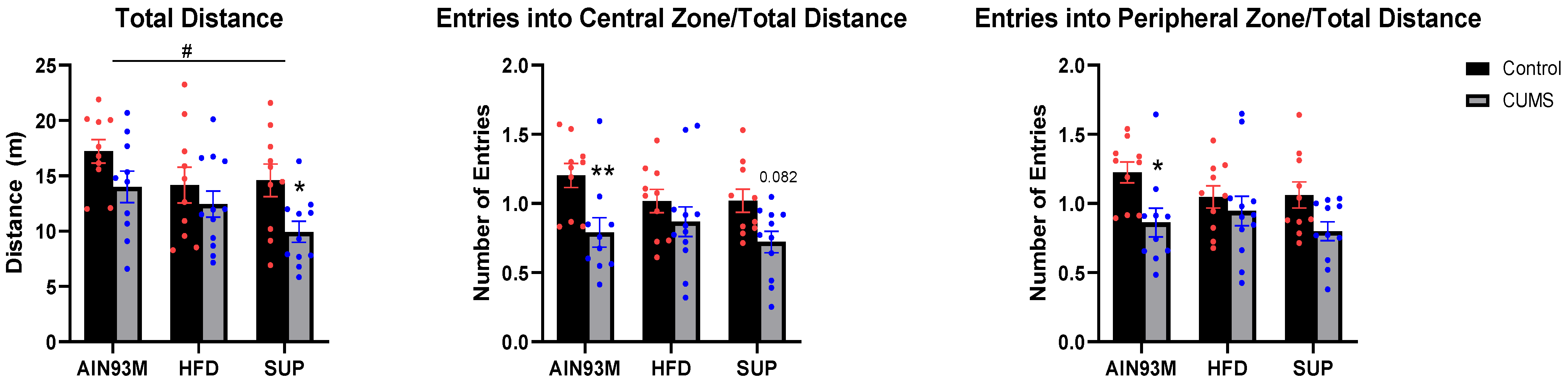
2.3.1. Open Field Test
2.3.2. Elevated Plus-Maze Test
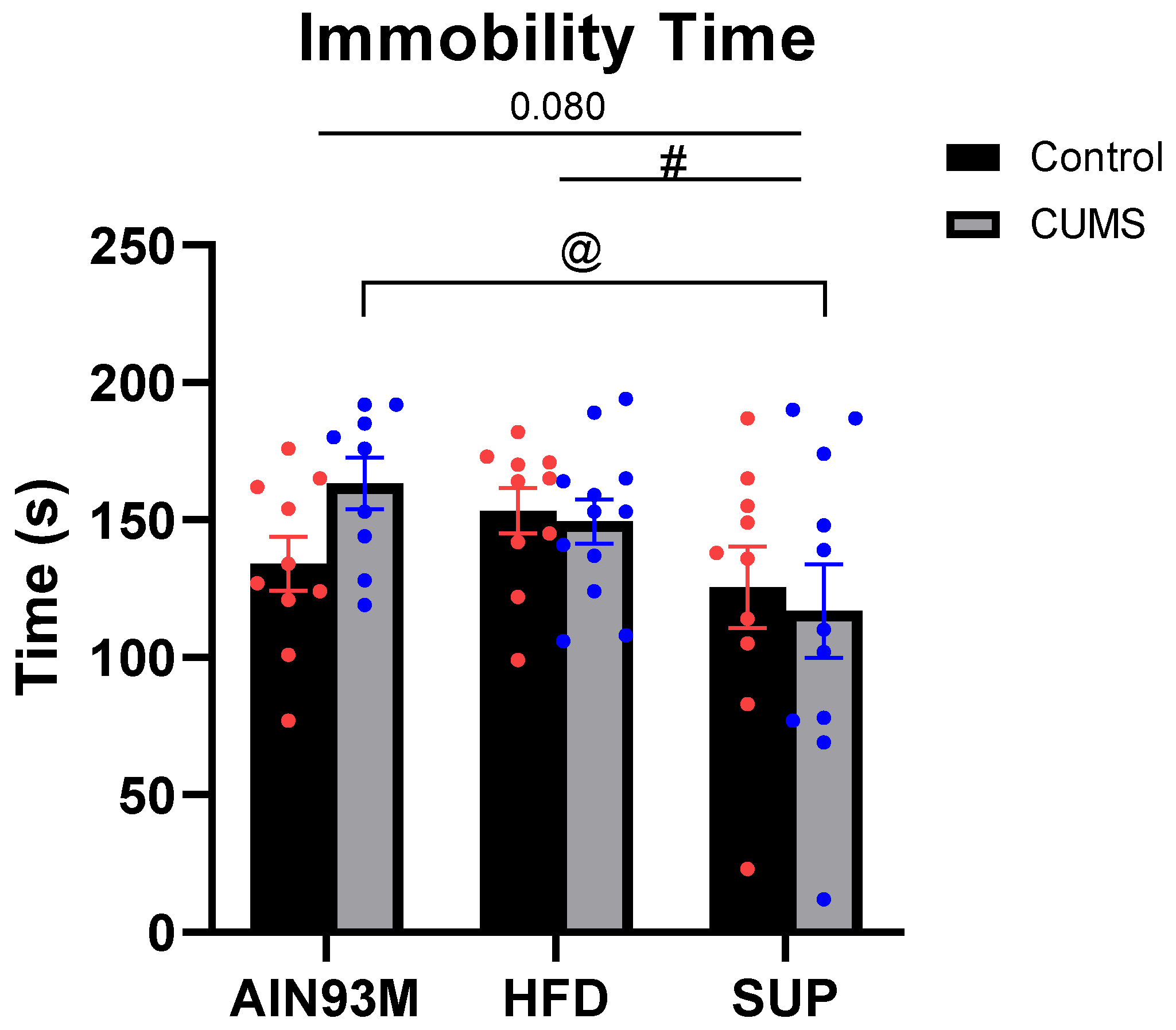
2.3.3. Tail Suspension Test
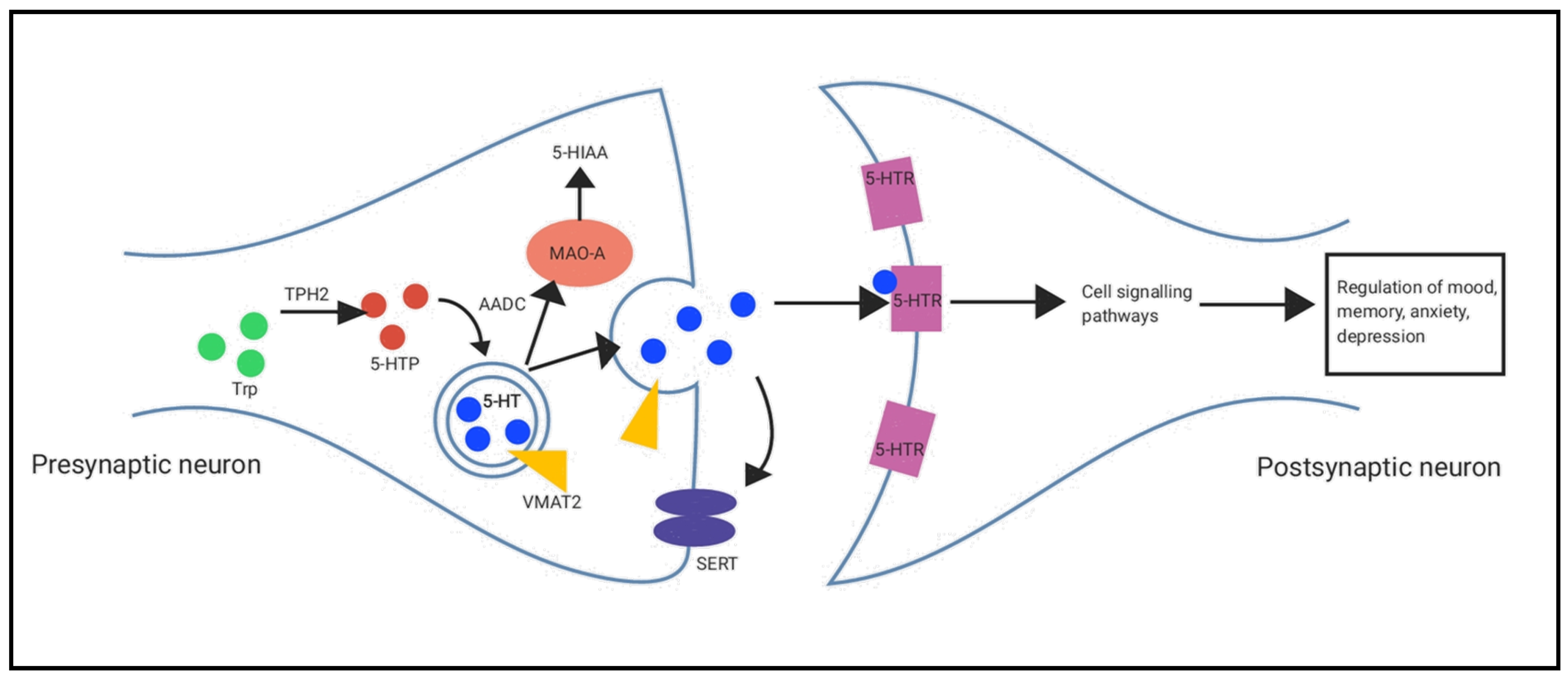
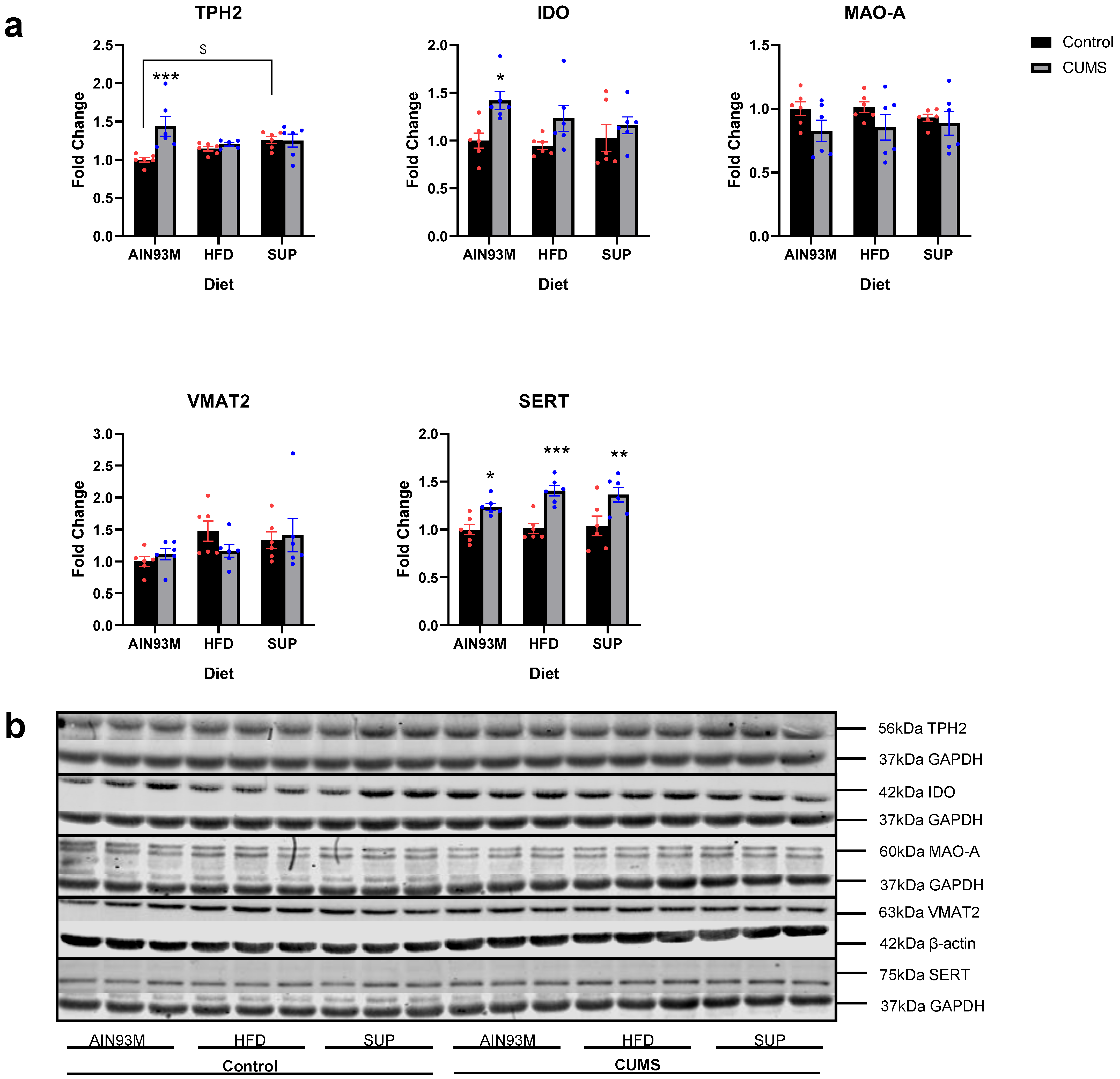
2.4. Serotonin Synthesis and Metabolism Pathways in the Hippocampus and Prefrontal Cortex
2.5. Mature Brain-Derived Neurotrophic Factor Levels in the Hippocampus and Prefrontal Cortex
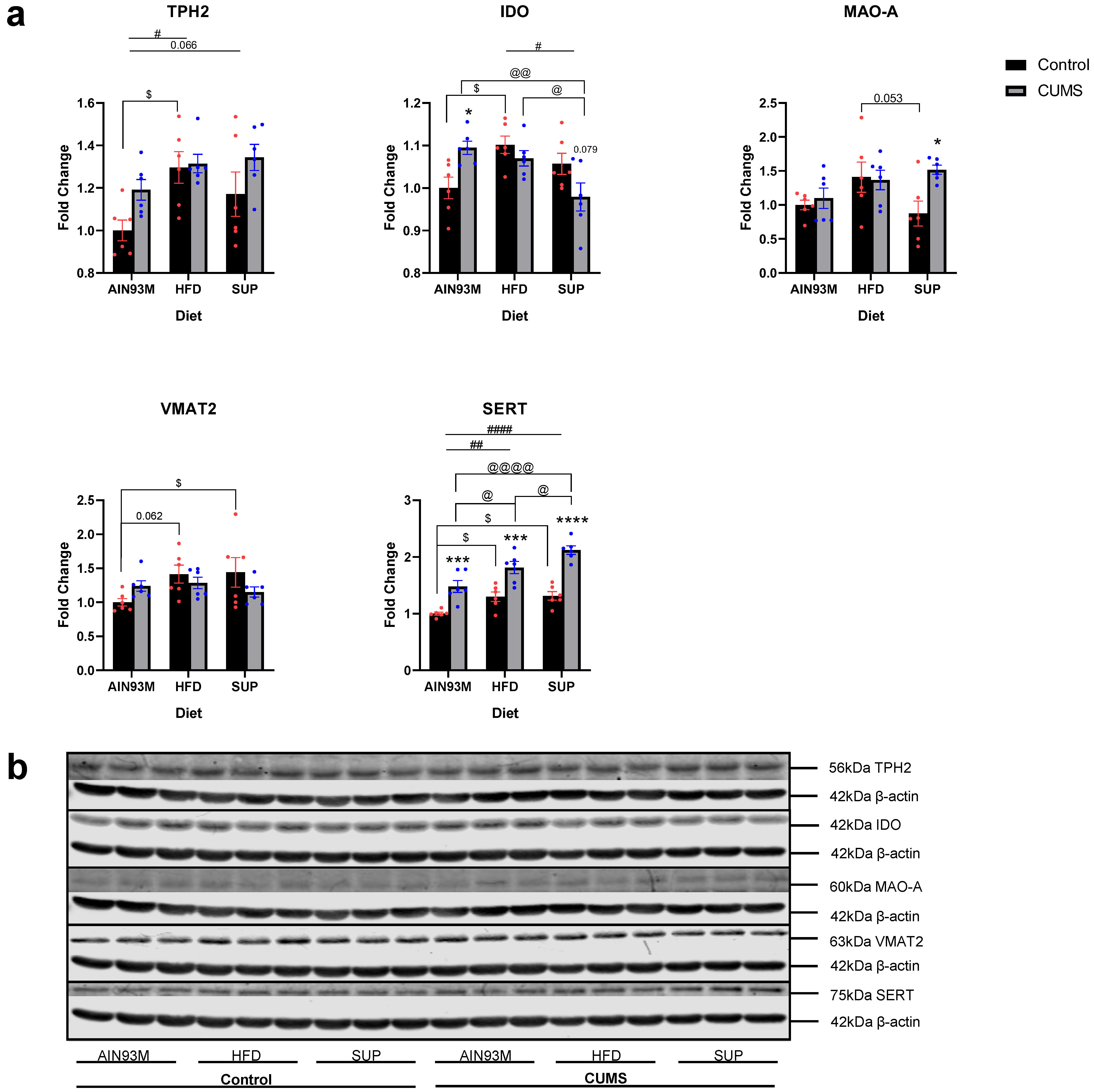
2.6. Serotonin Synthesis and Metabolism Pathways in the Colon
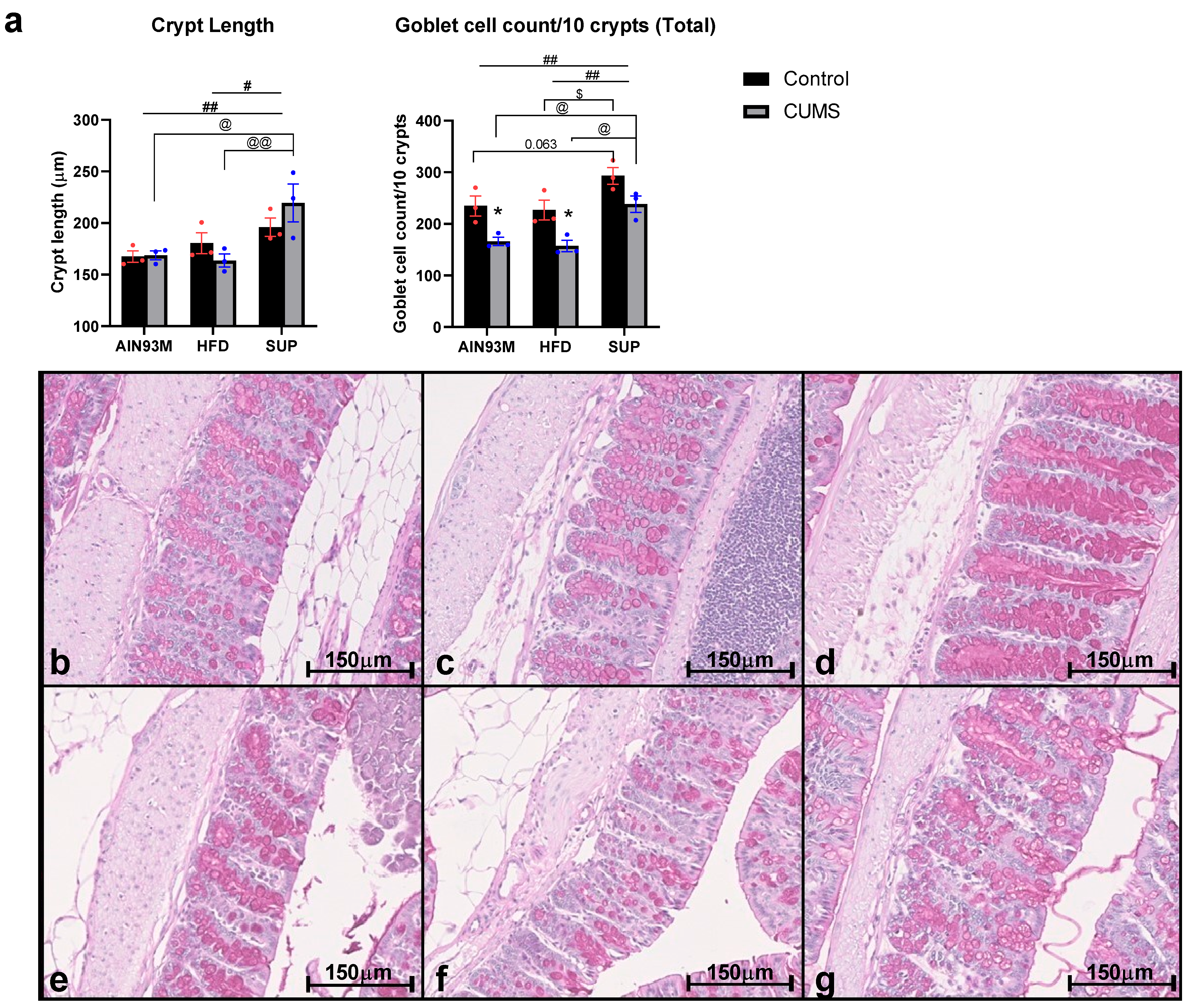
2.7. Colonic Histopathology
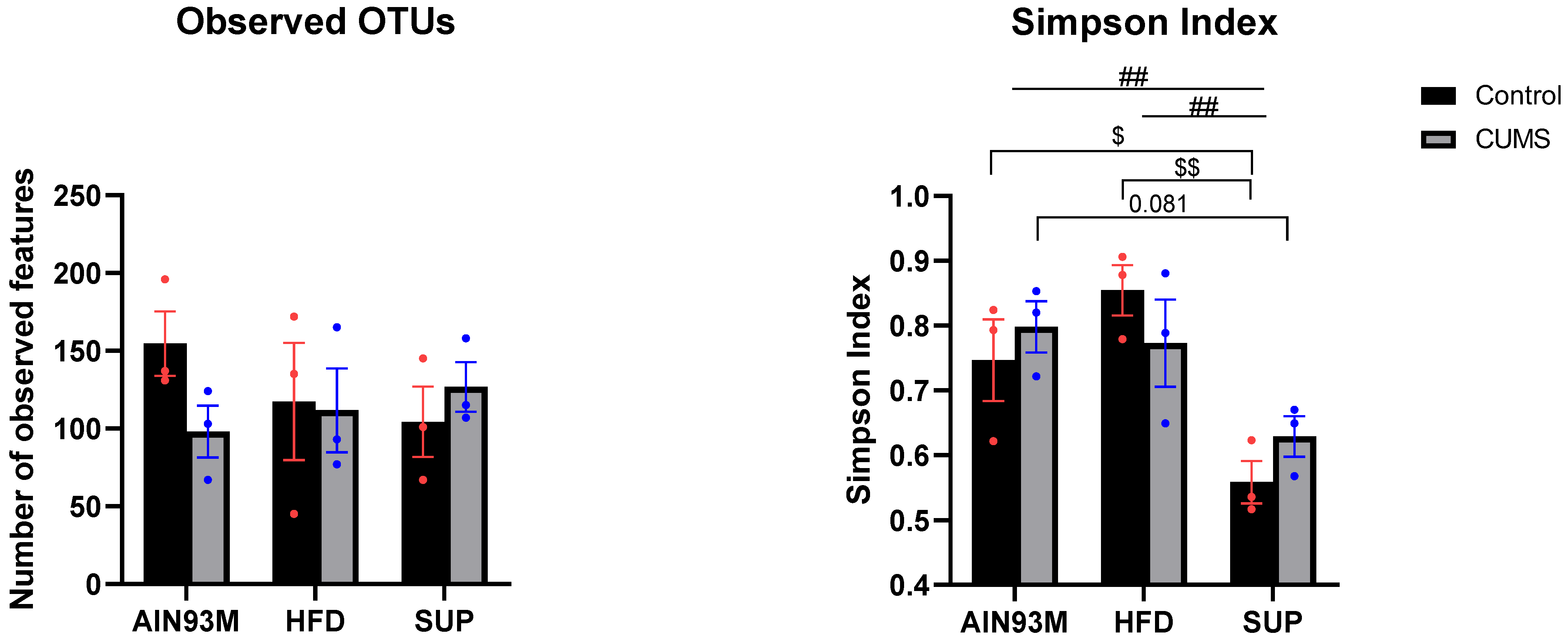
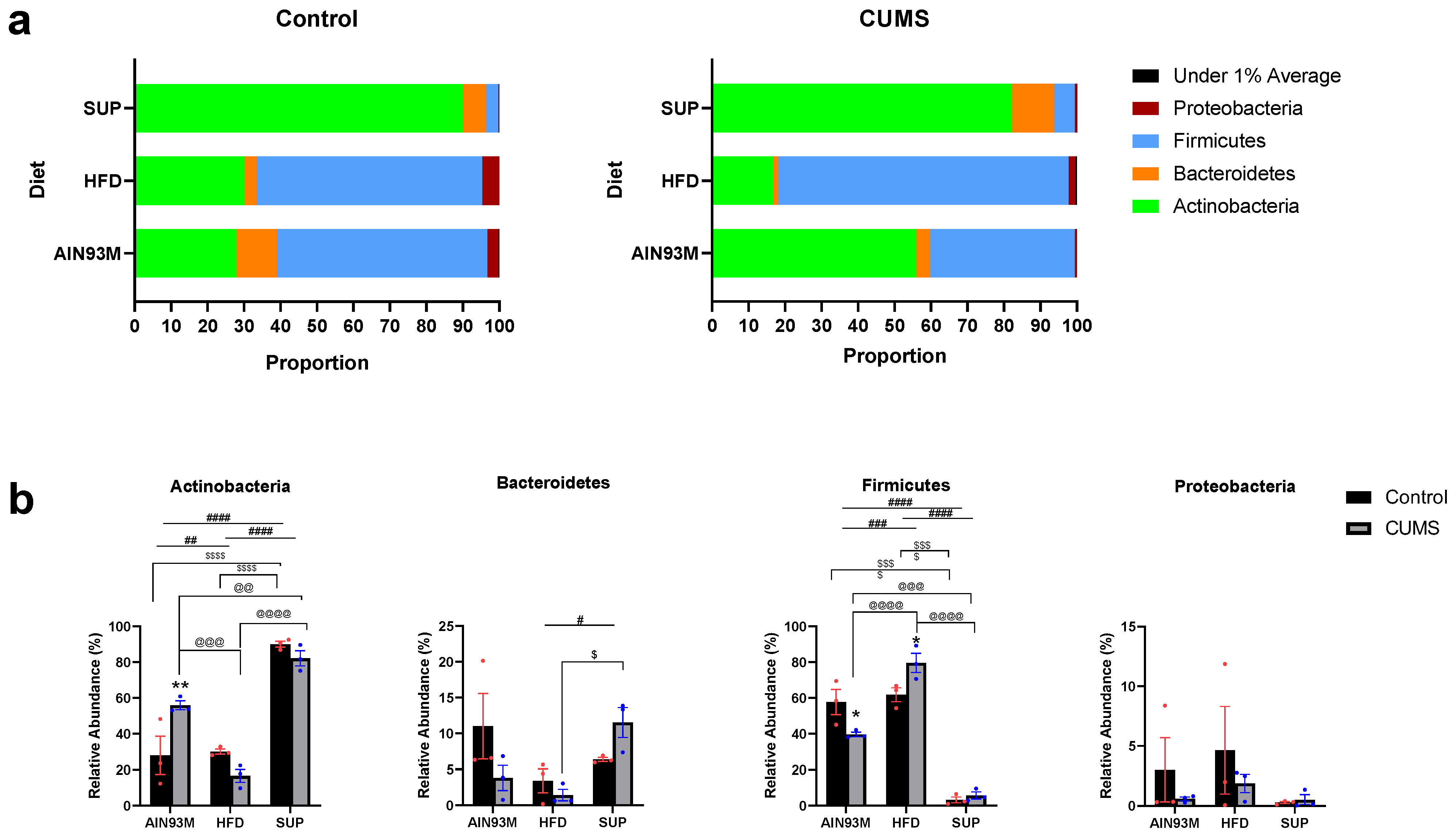
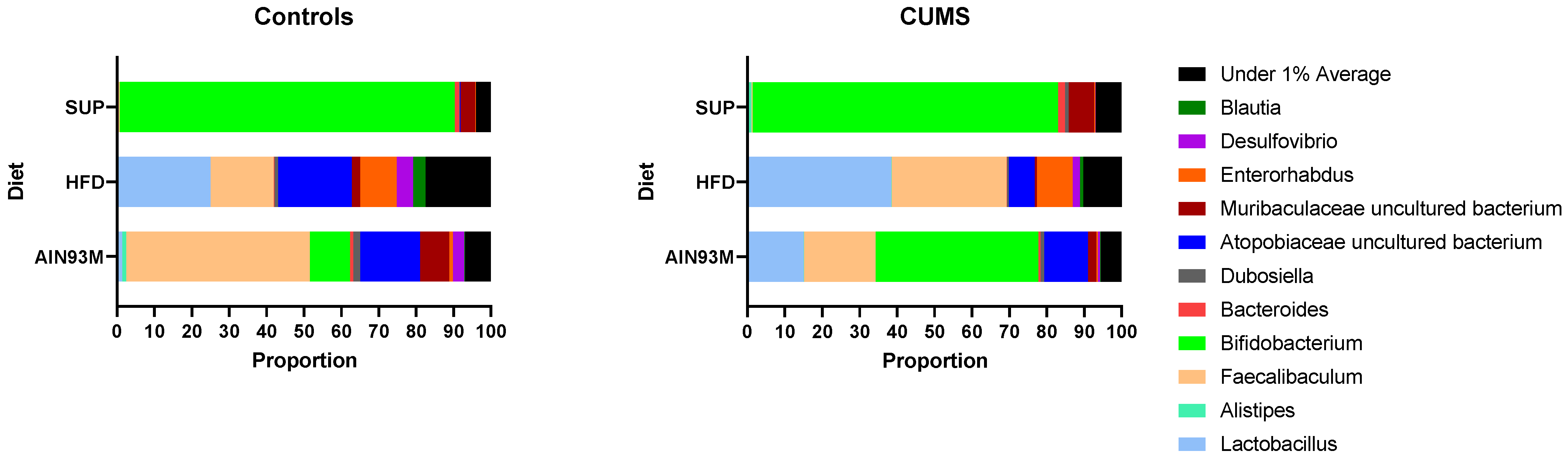
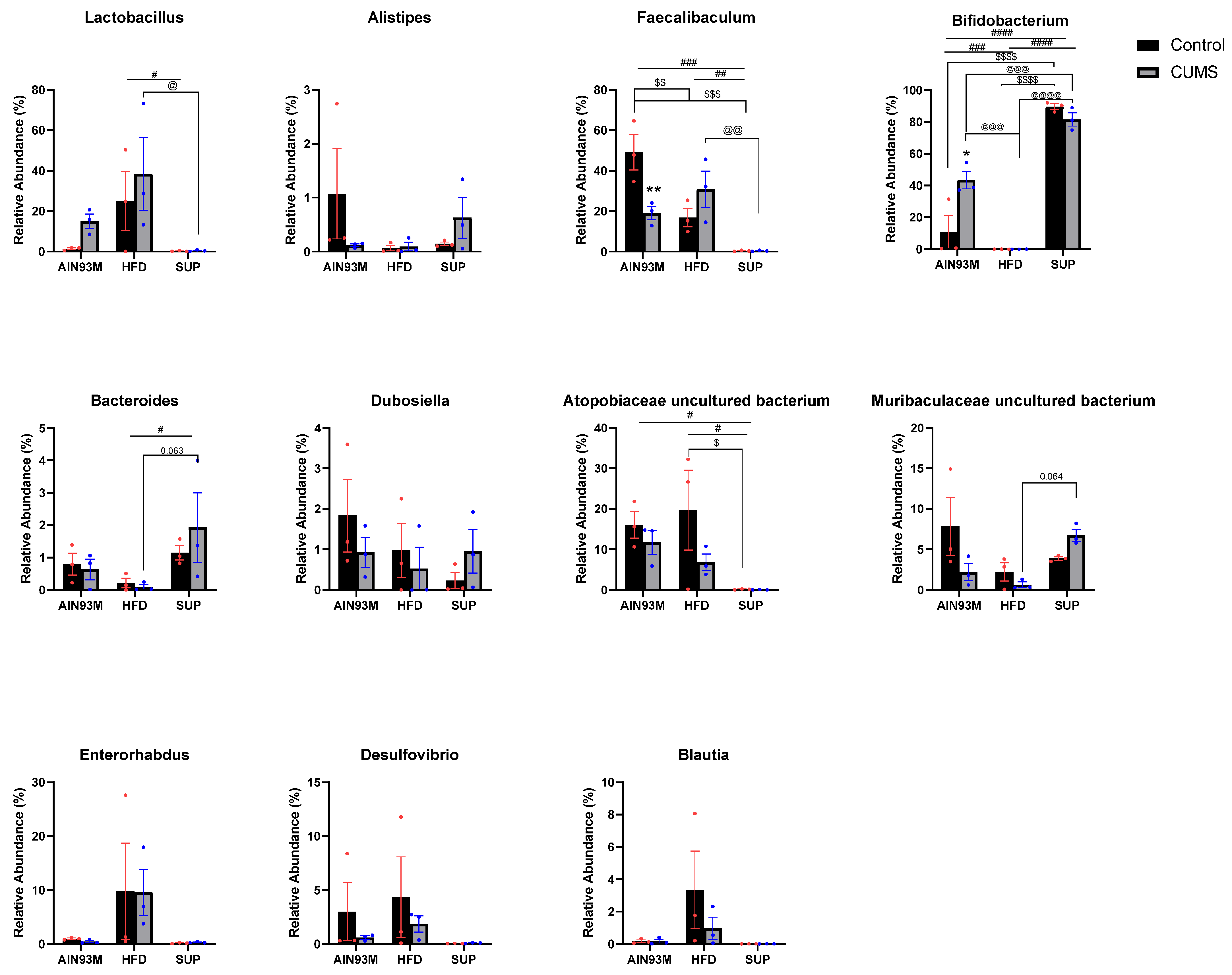
2.8. Gut Microbiota
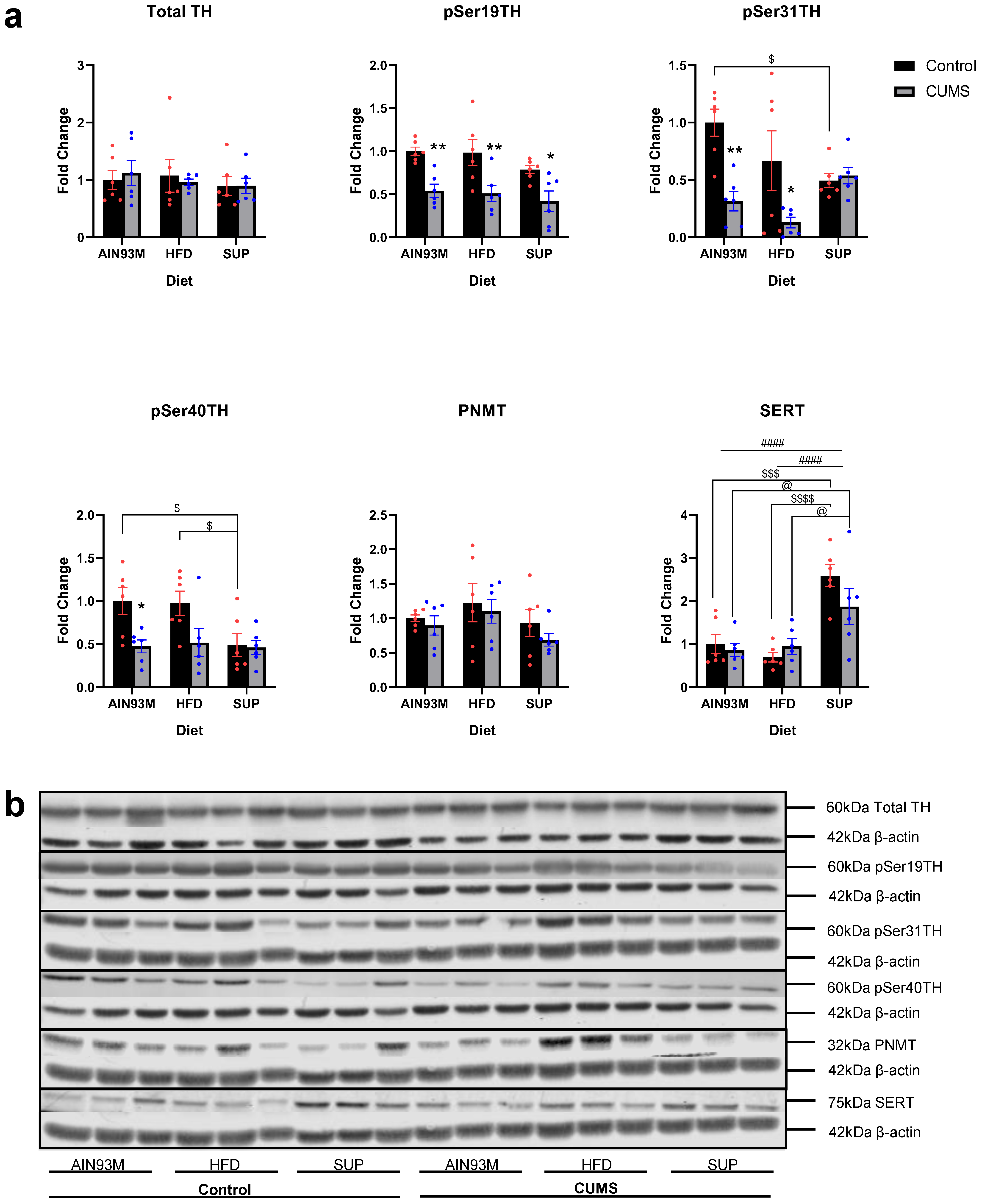
2.9. Adrenal Catecholamine Synthetic Enzymes and SERT
3. Discussion
3.1. General Biochemistry
3.2. Behaviour
3.3. Serotonin Synthesis and Metabolism Pathways in the Brain
3.4. Mature Brain-Derived Neurotrophic Factor Levels in the Hippocampus and Prefrontal Cortex
3.5. Colonic Serotonin Synthesis and Metabolism Pathways, Histopathology, and the Gut Microbiota
3.6. Adrenal Catecholamine Synthetic Enzymes and SERT
3.7. Clinical Implications
3.8. Study Limitations
4. Materials and Methods
4.1. Animals
4.2. Experimental Design
4.3. Behavioural Tests
4.3.1. Open Field Test
4.3.2. Elevated Plus-Maze Test
4.3.3. Tail Suspension Test
4.4. Blood Sample Collection and Analyses
4.5. Fresh Tissue Collection and Homogenisation
4.6. Western Blot
4.7. Histological Analyses
4.8. Faecal Sample Collection and 16S rRNA Sequencing
4.9. Microbiota Analyses
4.10. Statistical Analyses
5. Conclusions
- It may act as a “comfort food” since it prevented anxiety- and depressive-like behaviours following CUMS
- Reduced colonic SERT levels suggest reduced serotonin reuptake following CUMS, potentially exacerbating colonic inflammation.
- Decreased colonic goblet cell numbers following CUMS, similar to the AIN93M diet
- Lowered cholesterol levels below the reference range for C57Bl6 mice
- Increased anxiety-like behaviour is likely due to the inclusion of psyllium husk in the diet
- It had a protective effect on the mucus barrier of the colon by preventing a reduction in goblet cell number following CUMS
- These protective effects on the colon may have been driven by an increase in the species evenness of the gut microbiota and/or an increase in the abundance of Bifidobacteria
- Reduced the catecholamine synthetic capacity of tyrosine hydroxylase in the adrenal glands, possibly via upregulation of adrenal serotonin reuptake; however, the mechanisms governing this interaction require further study
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Primary Antibodies | |||
|---|---|---|---|
| Specificity | Species | Company | Dilution |
| IDO | Mouse | Santa Cruz (Cat# sc-137012, RRID:AB_2123436) | 1:5000 in 1% milk/1% BSA/TBST |
| Anti-monoamine oxidase-A (MAO-A) | Monoclonal Rabbit | ABclonal (Cat# A4105, RRID:AB_2863189) | 1:2000 in 5% BSA/TBST |
| Anti-mature brain-derived neurotrophic factor (mBDNF) | Polyclonal Rabbit | Osenses (Cat# 2728, RRID: Not available) | 1:4000 in 5% BSA/TBST |
| Phenylethanolamine N-methyltransferase (PNMT) | Polyclonal Rabbit | Abcam (Cat# Ab69579, RRID:AB_2050200) | 1:3000 in 5% BSA/TBST |
| Anti-phospho-Serine 19-tyrosine hydroxylase (pSer19TH) | Rabbit | Homemade † | 1:6000 TBST/Azide |
| Anti-phospho-Serine 31-tyrosine hydroxylase (pSer31TH) | Rabbit | Homemade † | 1:4000 in BSA/Azide |
| Anti-phospho-Serine 40-tyrosine hydroxylase (pSer40TH) | Sheep | Homemade † | 1:2000 in TBST/Azide |
| Anti-serotonin transporter (SERT) | Polyclonal Rabbit | ABclonal (Cat# A14171, RRID:AB_2761030) | 1:1000 in TBST |
| Anti-tyrosine hydroxylase (TH) | Monoclonal Mouse | Sigma (Cat# T1299, RRID:AB_477560) | 1:7000 in 5% BSA/TBST |
| Anti-tryptophan hydroxylase 1 (TPH1) | Polyclonal Rabbit | ABclonal (Cat# A1569, RRID:AB_2763100) | 1:2000 in 5% BSA/TBST |
| Anti-tryptophan hydroxylase 2 (TPH2) | Polyclonal Rabbit | Sigma (Cat# SAB2102520, RRID:AB_10606926) | 1:1000 in 5% BSA/Azide |
| Anti-vesicular monoamine transporter 2 (VMAT2) | Monoclonal Mouse | Santa Cruz (Cat# sc-374079, RRID:AB_10917928) | 1:1000 in 5% BSA/Azide |
| Anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH) | Rabbit | ProteinTech (Cat# 10494-1-AP, RRID: AB_2263076) | 1:3000 in TBST |
| β-actin | Monoclonal Rabbit (High Dilution) | ABclonal (Cat# AC026, RRID:AB_2768234) | 1:2000 in TBST |
| β-actin | Monoclonal Mouse | Abcam (Cat# ab66338, RRID:AB_2289239) | 1:8000 in TBST |
| Secondary Antibodies | |||
| anti-rabbit IgG | Polyclonal Donkey | Li-Cor Biosciences (Cat# 926-32213, RRID:AB_621848) | 1:20,000 in TBST |
| anti-mouse IgG | Polyclonal Goat | Li-Cor Biosciences (Cat# 926-32210, RRID:AB_621842) | 1:20,000 in TBST |
| anti-goat IgG | Polyclonal Donkey | Li-Cor Biosciences (Cat# 926-32214, RRID:AB_621846) | 1:20,000 in TBST |
References
- Dunkley, P.R.; Dickson, P.W. Tyrosine hydroxylase phosphorylation in vivo. J. Neurochem. 2019, 149, 706–728. [Google Scholar] [CrossRef] [PubMed]
- Renna, M.E.; Fresco, D.M.; Mennin, D.S. Emotion regulation therapy and its potential role in the treatment of chronic stress-related pathology across disorders. Chronic Stress 2020, 4, 2470547020905787. [Google Scholar] [CrossRef] [PubMed]
- Santana, M.M.; Rosmaninho-Salgado, J.; Cortez, V.; Pereira, F.C.; Kaster, M.P.; Aveleira, C.A.; Ferreira, M.; Álvaro, A.R.; Cavadas, C. Impaired adrenal medullary function in a mouse model of depression induced by unpredictable chronic stress. Eur. Neuropsychopharmacol. 2015, 25, 1753–1766. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, P.J.; Cryan, J.F.; Dinan, T.G.; Clarke, G. Kynurenine pathway metabolism and the microbiota-gut-brain axis. Neuropharmacology 2017, 112, 399–412. [Google Scholar] [CrossRef]
- Correia, A.S.; Vale, N. Tryptophan metabolism in depression: A narrative review with a focus on serotonin and kynurenine pathways. Int. J. Mol. Sci. 2022, 23, 8493. [Google Scholar] [CrossRef]
- Perez-Caballero, L.; Torres-Sanchez, S.; Romero-López-Alberca, C.; González-Saiz, F.; Mico, J.A.; Berrocoso, E. Monoaminergic system and depression. Cell Tissue Res. 2019, 377, 107–113. [Google Scholar] [CrossRef]
- Belvederi Murri, M.; Pariante, C.; Mondelli, V.; Masotti, M.; Atti, A.R.; Mellacqua, Z.; Antonioli, M.; Ghio, L.; Menchetti, M.; Zanetidou, S.; et al. Hpa axis and aging in depression: Systematic review and meta-analysis. Psychoneuroendocrinology 2014, 41, 46–62. [Google Scholar] [CrossRef]
- Li, C.-C.; Jiang, N.; Gan, L.; Zhao, M.-J.; Chang, Q.; Liu, X.-M.; Pan, R.-L. Peripheral and cerebral abnormalities of the tryptophan metabolism in the depression-like rats induced by chronic unpredicted mild stress. Neurochem. Int. 2020, 138, 104771. [Google Scholar] [CrossRef]
- Lu, Q.; Mouri, A.; Yang, Y.; Kunisawa, K.; Teshigawara, T.; Hirakawa, M.; Mori, Y.; Yamamoto, Y.; Libo, Z.; Nabeshima, T. Chronic unpredictable mild stress-induced behavioral changes are coupled with dopaminergic hyperfunction and serotonergic hypofunction in mouse models of depression. Behav. Brain Res. 2019, 372, 112053. [Google Scholar] [CrossRef]
- Xing, H.; Zhang, X.; Xing, N.; Qu, H.; Zhang, K. Uncovering pharmacological mechanisms of zhi-zi-hou-po decoction in chronic unpredictable mild stress-induced rats through pharmacokinetics, monoamine neurotransmitter and neurogenesis. J. Ethnopharmacol. 2019, 243, 112079. [Google Scholar] [CrossRef]
- Zhao, S.; Yang, T.; Hou, X.; Zhang, H.; Zhao, Y.; Wang, H.; Sun, N.; Tan, H.; Zhang, J.; Fan, H. Chlorogenic acid ameliorates chronic stress-induced prefrontal cortex injury through activating the 5-ht/bdnf signaling pathway in rats. Food Biosci. 2022, 50, 102179. [Google Scholar] [CrossRef]
- Wang, Q.; Timberlake, M.A., II; Prall, K.; Dwivedi, Y. The recent progress in animal models of depression. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2017, 77, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Gasmi, A.; Aniqa, N.; Menzel, A.; Asma Gasmi, B.; Pivina, L.; Sàdaf, N.; Peana, M.; Chirumbolo, S.; Bjørklund, G. Neurotransmitters regulation and food intake: The role of dietary sources in neurotransmission. Molecules 2023, 28, 210. [Google Scholar] [CrossRef] [PubMed]
- Höglund, E.; Øverli, Ø.; Winberg, S. Tryptophan metabolic pathways and brain serotonergic activity: A comparative review. Front. Endocrinol. 2019, 10, 158. [Google Scholar] [CrossRef] [PubMed]
- Meng, C.; Feng, S.; Hao, Z.; Dong, C.; Liu, H. Antibiotics exposure attenuates chronic unpredictable mild stress-induced anxiety-like and depression-like behavior. Psychoneuroendocrinology 2022, 136, 105620. [Google Scholar] [CrossRef]
- Savitz, J. The kynurenine pathway: A finger in every pie. Mol. Psychiatry 2020, 25, 131–147. [Google Scholar] [CrossRef]
- Song, X.; Wang, W.; Ding, S.; Liu, X.; Wang, Y.; Ma, H. Puerarin ameliorates depression-like behaviors of chronic unpredictable mild stress mice by remodeling their gut microbiota. J. Affect. Disord. 2021, 290, 353–363. [Google Scholar] [CrossRef]
- Tan, J.; Li, X.; Zhu, Y.; Sullivan, M.A.; Deng, B.; Zhai, X.; Lu, Y. Antidepressant shugan jieyu capsule alters gut microbiota and intestinal microbiome function in rats with chronic unpredictable mild stress-induced depression. Front. Pharmacol. 2022, 13, 828595. [Google Scholar] [CrossRef]
- Zhang, W.; Qu, W.; Wang, H.; Yan, H. Antidepressants fluoxetine and amitriptyline induce alterations in intestinal microbiota and gut microbiome function in rats exposed to chronic unpredictable mild stress. Transl. Psychiatry 2021, 11, 131. [Google Scholar] [CrossRef]
- König, N.; Bimpisidis, Z.; Dumas, S.; Wallén-Mackenzie, Å. Selective knockout of the vesicular monoamine transporter 2 (vmat2) gene in calbindin2/calretinin-positive neurons results in profound changes in behavior and response to drugs of abuse. Front. Behav. Neurosci. 2020, 14, 578443. [Google Scholar] [CrossRef]
- Li, H.; Wang, P.; Huang, L.; Li, P.; Zhang, D. Effects of regulating gut microbiota on the serotonin metabolism in the chronic unpredictable mild stress rat model. Neurogastroenterol. Motil. 2019, 31, e13677. [Google Scholar] [CrossRef] [PubMed]
- Bashir, M.I.; Abdul Aziz, N.H.K.; Noor, D.A.M. Antidepressant-like effects of polygonum minus aqueous extract in chronic ultra-mild stress-induced depressive mice model. Behav. Sci. 2022, 12, 196. [Google Scholar] [CrossRef] [PubMed]
- Murawska-Ciałowicz, E.; Wiatr, M.; Ciałowicz, M.; Gomes de Assis, G.; Borowicz, W.; Rocha-Rodrigues, S.; Paprocka-Borowicz, M.; Marques, A. Bdnf impact on biological markers of depression-role of physical exercise and training. Int. J. Environ. Res. Public Health 2021, 18, 7553. [Google Scholar] [CrossRef]
- Popova, N.K.; Naumenko, V.S. Neuronal and behavioral plasticity: The role of serotonin and bdnf systems tandem. Expert Opin. Ther. Targets 2019, 23, 227–239. [Google Scholar] [CrossRef] [PubMed]
- Frankiensztajn, L.M.; Elliott, E.; Koren, O. The microbiota and the hypothalamus-pituitary-adrenocortical (hpa) axis, implications for anxiety and stress disorders. Curr. Opin. Neurobiol. 2020, 62, 76–82. [Google Scholar] [CrossRef]
- Brindley, R.L.; Bauer, M.B.; Blakely, R.D.; Currie, K.P. Serotonin and serotonin transporters in the adrenal medulla: A potential hub for modulation of the sympathetic stress response. ACS Chem. Neurosci. 2017, 8, 943–954. [Google Scholar] [CrossRef]
- Ong, L.; Guan, L.; Stutz, B.; Dickson, P.; Dunkley, P.; Bobrovskaya, L. The effects of footshock and immobilization stress on tyrosine hydroxylase phosphorylation in the rat locus coeruleus and adrenal gland. Neuroscience 2011, 192, 20–27. [Google Scholar] [CrossRef]
- Ong, L.K.; Guan, L.; Damanhuri, H.; Goodchild, A.K.; Bobrovskaya, L.; Dickson, P.W.; Dunkley, P.R. Neurobiological consequences of acute footshock stress: Effects on tyrosine hydroxylase phosphorylation and activation in the rat brain and adrenal medulla. J. Neurochem. 2014, 128, 547–560. [Google Scholar] [CrossRef]
- Funke, R.; Eichler, A.; Distler, J.; Golub, Y.; Kratz, O.; Moll, G.H. Stress system dysregulation in pediatric generalized anxiety disorder associated with comorbid depression. Stress Health 2017, 33, 518–529. [Google Scholar] [CrossRef]
- Marathe, S.V.; D’Almeida, P.L.; Virmani, G.; Bathini, P.; Alberi, L. Effects of monoamines and antidepressants on astrocyte physiology: Implications for monoamine hypothesis of depression. J. Exp. Neurosci. 2018, 12, 1179069518789149. [Google Scholar] [CrossRef]
- Taylor, A.M.; Holscher, H.D. A review of dietary and microbial connections to depression, anxiety, and stress. Nutr. Neurosci. 2020, 23, 237–250. [Google Scholar] [CrossRef] [PubMed]
- Arab, L.; Guo, R.; Elashoff, D. Lower depression scores among walnut consumers in nhanes. Nutrients 2019, 11, 275. [Google Scholar] [CrossRef] [PubMed]
- Leone, V.; Chang, E.B.; Devkota, S. Diet, microbes, and host genetics: The perfect storm in inflammatory bowel diseases. J. Gastroenterol. 2013, 48, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Martín-Peláez, S.; Camps-Bossacoma, M.; Massot-Cladera, M.; Rigo-Adrover, M.; Franch, À.; Pérez-Cano, F.J.; Castell, M. Effect of cocoa’s theobromine on intestinal microbiota of rats. Mol. Nutr. Food Res. 2017, 61, 1700238. [Google Scholar] [CrossRef]
- Pase, M.P.; Scholey, A.B.; Pipingas, A.; Kras, M.; Nolidin, K.; Gibbs, A.; Wesnes, K.; Stough, C. Cocoa polyphenols enhance positive mood states but not cognitive performance: A randomized, placebo-controlled trial. J. Psychopharmacol. 2013, 27, 451–458. [Google Scholar] [CrossRef]
- Paturi, G.; Mandimika, T.; Butts, C.A.; Zhu, S.; Roy, N.C.; McNabb, W.C.; Ansell, J. Influence of dietary blueberry and broccoli on cecal microbiota activity and colon morphology in mdr1a−/− mice, a model of inflammatory bowel diseases. Nutrition 2012, 28, 324–330. [Google Scholar] [CrossRef]
- Lee, J.; Purello, C.; Booth, S.L.; Bennett, B.; Wiley, C.D.; Korstanje, R. Chow diet in mouse aging studies: Nothing regular about it. GeroScience 2023, 45, 2079–2084. [Google Scholar] [CrossRef]
- Toyoda, A.; Shimonishi, H.; Sato, M.; Usuda, K.; Ohsawa, N.; Nagaoka, K. Effects of non-purified and semi-purified commercial diets on behaviors, plasma corticosterone levels, and cecum microbiome in c57bl/6j mice. Neurosci. Lett. 2018, 670, 36–40. [Google Scholar] [CrossRef]
- Jesch, E.D.; Carr, T.P. Food ingredients that inhibit cholesterol absorption. Prev. Nutr. Food Sci. 2017, 22, 67–80. [Google Scholar] [CrossRef]
- Deng, Z.; Meng, C.; Huang, H.; Song, S.; Fu, L.; Fu, Z. The different effects of psyllium husk and orlistat on weight control, the amelioration of hypercholesterolemia and non-alcohol fatty liver disease in obese mice induced by a high-fat diet. Food Funct. 2022, 13, 8829–8849. [Google Scholar] [CrossRef]
- Otto, G.P.; Rathkolb, B.; Oestereicher, M.A.; Lengger, C.J.; Moerth, C.; Micklich, K.; Fuchs, H.; Gailus-Durner, V.; Wolf, E.; Hrabě de Angelis, M. Clinical chemistry reference intervals for c57bl/6j, c57bl/6n, and c3heb/fej mice (mus musculus). J. Am. Assoc. Lab. Anim. Sci. JAALAS 2016, 55, 375–386. [Google Scholar] [PubMed]
- Hu, C.; Luo, Y.; Wang, H.; Kuang, S.; Liang, G.; Yang, Y.; Mai, S.; Yang, J. Re-evaluation of the interrelationships among the behavioral tests in rats exposed to chronic unpredictable mild stress. PLoS ONE 2017, 12, e0185129. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Shi, R.; Wang, J.; Wang, J.F.; Li, X.M. Unpredictable chronic mild stress not chronic restraint stress induces depressive behaviours in mice. Neuroreport 2014, 25, 1151–1155. [Google Scholar] [CrossRef] [PubMed]
- Mineur, Y.S.; Belzung, C.; Crusio, W.E. Effects of unpredictable chronic mild stress on anxiety and depression-like behavior in mice. Behav. Brain Res. 2006, 175, 43–50. [Google Scholar] [CrossRef]
- Barnard, D.E.; Lewis, S.M.; Teter, B.B.; Thigpen, J.E. Open-and closed-formula laboratory animal diets and their importance to research. J. Am. Assoc. Lab. Anim. Sci. 2009, 48, 709–713. [Google Scholar]
- Takahashi, E.; Ono, E. Effects of semi-purified diet on depressive behaviors in aged mice. Biochem. Biophys. Rep. 2021, 28, 101152. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, X.; Luo, S.; Chu, C.; Wu, D.; Liu, R.; Wang, L.; Wang, J.; Liu, X. Extract of sesame cake and sesamol alleviate chronic unpredictable mild stress-induced depressive-like behaviors and memory deficits. J. Funct. Foods 2018, 42, 237–247. [Google Scholar] [CrossRef]
- Yang, J.L.; Liu, D.X.; Jiang, H.; Pan, F.; Ho, C.S.H.; Ho, R.C.M. The effects of a high-fat diet combined with chronic unpredictable mild stress on depression-like behavior and leptin/leprb in male rats. Sci. Rep. 2016, 6, 35239. [Google Scholar] [CrossRef]
- Noronha, S.S.R.; Lima, P.M.; Campos, G.S.V.; Chírico, M.T.T.; Abreu, A.R.; Figueiredo, A.B.; Silva, F.C.S.; Chianca, D.A.; Lowry, C.A.; De Menezes, R.C.A. Association of a high-fat diet with neuroinflammation, anxiety-like defensive behavioral responses, and altered thermoregulatory responses in male rats. Brain Behav. Immun. 2019, 80, 500–511. [Google Scholar] [CrossRef]
- Moreira Júnior, R.E.; de Carvalho, L.M.; dos Reis, D.C.; Cassali, G.D.; Faria, A.M.C.; Maioli, T.U.; Brunialti-Godard, A.L. Diet-induced obesity leads to alterations in behavior and gut microbiota composition in mice. J. Nutr. Biochem. 2021, 92, 108622. [Google Scholar] [CrossRef]
- Lyte, M.; Chapel, A.; Lyte, J.M.; Ai, Y.; Proctor, A.; Jane, J.-L.; Phillips, G.J. Resistant starch alters the microbiota-gut brain axis: Implications for dietary modulation of behavior. PLoS ONE 2016, 11, e0146406. [Google Scholar] [CrossRef] [PubMed]
- Suneson, K.; Asp, M.; Träskman-Bendz, L.; Westrin, Å.; Ambrus, L.; Lindqvist, D. Low total cholesterol and low-density lipoprotein associated with aggression and hostility in recent suicide attempters. Psychiatry Res. 2019, 273, 430–434. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.-X.; Tao, C.; Gao, X.-R.; Wang, L.-L.; Jiang, F.-H.; Wang, C.; Fang, K.; Chen, X.-X.; Chen, Z.; Ge, J.-F. Bdnf-related imbalance of copine 6 and synaptic plasticity markers couples with depression-like behavior and immune activation in cums rats. Front. Neurosci. 2018, 12, 731. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Qin, X.; Yu, Z.; Chen, Y.; Tang, L.; Shan, W. Effects of high-fat diet on the formation of depressive-like behavior in mice. Food Funct. 2021, 12, 6416–6431. [Google Scholar] [CrossRef] [PubMed]
- Gapski, A.; Gomes, T.M.; Bredun, M.A.; Ferreira-Lima, N.E.; Ludka, F.K.; Bordignon-Luiz, M.T.; Burin, V.M. Digestion behavior and antidepressant-like effect promoted by acute administration of blueberry extract on mice. Food Res. Int. 2019, 125, 108618. [Google Scholar] [CrossRef]
- Son, H.; Jung, S.; Shin, J.H.; Kang, M.J.; Kim, H.J. Anti-stress and anti-depressive effects of spinach extracts on a chronic stress-induced depression mouse model through lowering blood corticosterone and increasing brain glutamate and glutamine levels. J. Clin. Med. 2018, 7, 406. [Google Scholar] [CrossRef]
- Lim, D.W.; Han, T.; Jung, J.; Song, Y.; Um, M.Y.; Yoon, M.; Kim, Y.T.; Cho, S.; Kim, I.-H.; Han, D.; et al. Chlorogenic acid from hawthorn berry (crataegus pinnatifida fruit) prevents stress hormone-induced depressive behavior, through monoamine oxidase b-reactive oxygen species signaling in hippocampal astrocytes of mice. Mol. Nutr. Food Res. 2018, 62, 1800029. [Google Scholar] [CrossRef]
- Sakakibara, H.; Shimoi, K. Anti-stress effects of polyphenols: Animal models and human trials. Food Funct. 2020, 11, 5702–5717. [Google Scholar] [CrossRef]
- Pourhamzeh, M.; Moravej, F.G.; Arabi, M.; Shahriari, E.; Mehrabi, S.; Ward, R.; Ahadi, R.; Joghataei, M.T. The roles of serotonin in neuropsychiatric disorders. Cell. Mol. Neurobiol. 2022, 42, 1671–1692. [Google Scholar] [CrossRef]
- Li, H.; Wang, P.; Zhou, Y.; Zhao, F.; Gao, X.; Wu, C.; Wu, T.; Jiang, L.; Zhang, D. Correlation between intestinal microbiotal imbalance and 5-ht metabolism, immune inflammation in chronic unpredictable mild stress male rats. Genes Brain Behav. 2022, 21, e12806. [Google Scholar] [CrossRef]
- Donner, N.C.; Kubala, K.H.; Hassell, J.E., Jr.; Lieb, M.W.; Nguyen, K.T.; Heinze, J.D.; Drugan, R.C.; Maier, S.F.; Lowry, C.A. Two models of inescapable stress increase tph2 mrna expression in the anxiety-related dorsomedial part of the dorsal raphe nucleus. Neurobiol. Stress 2018, 8, 68–81. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Garcia, A.L.; Meng, Q.; Canetta, S.; Gardier, A.M.; Guiard, B.P.; Kellendonk, C.; Dranovsky, A.; Leonardo, E.D. Serotonin signaling through prefrontal cortex 5-ht1a receptors during adolescence can determine baseline mood-related behaviors. Cell Rep. 2017, 18, 1144–1156. [Google Scholar] [CrossRef] [PubMed]
- Schwarcz, R.; Stone, T.W. The kynurenine pathway and the brain: Challenges, controversies and promises. Neuropharmacology 2017, 112, 237–247. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Gao, T.; Zeng, T.; Huang, P.; Wong, N.-K.; Dong, Z.; Li, Y.; Deng, G.; Wu, Z.; Lv, Z. Blockade of indoleamine 2, 3-dioxygenase 1 ameliorates hippocampal neurogenesis and bold-fmri signals in chronic stress precipitated depression. Aging (Albany NY) 2021, 13, 5875. [Google Scholar] [CrossRef] [PubMed]
- Sears, B.; Perry, M. The role of fatty acids in insulin resistance. Lipids Health Dis. 2015, 14, 121. [Google Scholar] [CrossRef]
- Bruta, K.; Vanshika; Bhasin, K.; Bhawana. The role of serotonin and diet in the prevalence of irritable bowel syndrome: A systematic review. Transl. Med. Commun. 2021, 6, 1. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, Q.; Jia, M.; Fu, S.; Pan, J.; Chu, C.; Liu, X.; Liu, X.; Liu, Z. (+)-sesamin attenuates chronic unpredictable mild stress-induced depressive-like behaviors and memory deficits via suppression of neuroinflammation. J. Nutr. Biochem. 2019, 64, 61–71. [Google Scholar] [CrossRef]
- Ghaffari-Nasab, A.; Badalzadeh, R.; Mohaddes, G.; Javani, G.; Ebrahimi-kalan, A.; Alipour, M.R. Young plasma induces antidepressant-like effects in aged rats subjected to chronic mild stress by suppressing indoleamine 2,3-dioxygenase enzyme and kynurenine pathway in the prefrontal cortex. Neurochem. Res. 2022, 47, 358–371. [Google Scholar] [CrossRef]
- Galland, L. Diet and inflammation. Nutr. Clin. Pract. 2010, 25, 634–640. [Google Scholar] [CrossRef]
- Smidowicz, A.; Regula, J. Effect of nutritional status and dietary patterns on human serum c-reactive protein and interleukin-6 concentrations. Adv. Nutr. 2015, 6, 738–747. [Google Scholar] [CrossRef]
- Huang, P.; Gao, T.; Dong, Z.; Zhou, C.; Lai, Y.; Pan, T.; Liu, Y.; Zhao, X.; Sun, X.; Hua, H.; et al. Neural circuitry among connecting the hippocampus, prefrontal cortex and basolateral amygdala in a mouse depression model: Associations correlations between bdnf levels and bold–fmri signals. Brain Res. Bull. 2018, 142, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Ho, C.S.; McIntyre, R.S.; Wang, W.; Ho, R.C. Agomelatine-induced modulation of brain-derived neurotrophic factor (bdnf) in the rat hippocampus. Life Sci. 2018, 210, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Diniz, D.M.; Bosch, K.; Calabrese, F.; Brivio, P.; Riva, M.A.; Grandjean, J.; Homberg, J.R. Bdnf overexpression in the prelimbic cortex does not reduce anxiety-and depression-like behavior in serotonin knockout rats. bioRxiv 2020. [Google Scholar] [CrossRef]
- Koopman, N.; Katsavelis, D.; Ten Hove, A.S.; Brul, S.; de Jonge, W.J.; Seppen, J. The multifaceted role of serotonin in intestinal homeostasis. Int. J. Mol. Sci. 2021, 22, 9487. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Li, Y.; Tang, W.; Sun, Q.; Chen, L.; Wang, X.; Liu, Q.; Yu, S.; Yu, S.; Liu, C. Chronic unpredictable mild stress in rats induces colonic inflammation. Front. Physiol. 2019, 10, 1228. [Google Scholar] [CrossRef] [PubMed]
- Gustafsson, J.K.; Johansson, M.E. The role of goblet cells and mucus in intestinal homeostasis. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 785–803. [Google Scholar] [CrossRef]
- Fang, J.; Wang, H.; Zhou, Y.; Zhang, H.; Zhou, H.; Zhang, X. Slimy partners: The mucus barrier and gut microbiome in ulcerative colitis. Exp. Mol. Med. 2021, 53, 772–787. [Google Scholar] [CrossRef]
- Herselman, M.F.; Bailey, S.; Bobrovskaya, L. The Effects of Stress and Diet on the “Brain-Gut” and “Gut-Brain” Pathways in Animal Models of Stress and Depression. Int. J. Mol. Sci. 2022, 23, 2013. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, L.; Shi, B.; Huang, F.; Gao, Y.; Miao, Z.; Ma, K.; Zhan, Z.; Zou, W.; Liu, M. Comparison of the chronic unpredictable mild stress and the maternal separation in mice postpartum depression modeling. Biochem. Biophys. Res. Commun. 2022, 632, 24–31. [Google Scholar] [CrossRef]
- Walter, J.; Armet, A.M.; Finlay, B.B.; Shanahan, F. Establishing or exaggerating causality for the gut microbiome: Lessons from human microbiota-associated rodents. Cell 2020, 180, 221–232. [Google Scholar] [CrossRef]
- Ali-Sisto, T.; Tolmunen, T.; Toffol, E.; Viinamäki, H.; Mäntyselkä, P.; Valkonen-Korhonen, M.; Honkalampi, K.; Ruusunen, A.; Velagapudi, V.; Lehto, S.M. Purine metabolism is dysregulated in patients with major depressive disorder. Psychoneuroendocrinology 2016, 70, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Tian, P.; Zhu, H.; Zou, R.; Zhao, J.; Zhang, H.; Wang, G.; Chen, W. Lactobacillus paracasei ccfm1229 and lactobacillus rhamnosus ccfm1228 alleviated depression- and anxiety-related symptoms of chronic stress-induced depression in mice by regulating xanthine oxidase activity in the brain. Nutrients 2022, 14, 1294. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhong, Z.; Wang, B.; Wang, Y. Xiaoyao san ameliorates high-fat diet-induced anxiety and depression via regulating gut microbiota in mice. Biomed. Pharmacother. 2022, 156, 113902. [Google Scholar] [CrossRef] [PubMed]
- Kumer, S.C.; Vrana, K.E. Intricate regulation of tyrosine hydroxylase activity and gene expression. J. Neurochem. 1996, 67, 443–462. [Google Scholar] [CrossRef] [PubMed]
- Toska, K.; Kleppe, R.; Armstrong, C.G.; Morrice, N.A.; Cohen, P.; Haavik, J. Regulation of tyrosine hydroxylase by stress-activated protein kinases. J. Neurochem. 2002, 83, 775–783. [Google Scholar] [CrossRef] [PubMed]
- Dunkley, P.R.; Bobrovskaya, L.; Graham, M.E.; Von Nagy-Felsobuki, E.I.; Dickson, P.W. Tyrosine hydroxylase phosphorylation: Regulation and consequences. J. Neurochem. 2004, 91, 1025–1043. [Google Scholar] [CrossRef] [PubMed]
- Yuenyongchaiwat, K.; Baker, I.S.; Sheffield, D. Symptoms of anxiety and depression are related to cardiovascular responses to active, but not passive, coping tasks. Braz. J. Psychiatry 2016, 39, 110–117. [Google Scholar] [CrossRef][Green Version]
- Bialkowska, A.B.; Ghaleb, A.M.; Nandan, M.O.; Yang, V.W. Improved swiss-rolling technique for intestinal tissue preparation for immunohistochemical and immunofluorescent analyses. JoVE J. Vis. Exp. 2016, 113, e54161. [Google Scholar]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F. Reproducible, interactive, scalable and extensible microbiome data science using qiime 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. Dada2: High-resolution sample inference from illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The silva ribosomal rna gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2012, 41, D590–D596. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, P.; Parfrey, L.W.; Yarza, P.; Gerken, J.; Pruesse, E.; Quast, C.; Schweer, T.; Peplies, J.; Ludwig, W.; Glöckner, F.O. The silva and “all-species living tree project (ltp)” taxonomic frameworks. Nucleic Acids Res. 2014, 42, D643–D648. [Google Scholar] [CrossRef] [PubMed]
- Gordon, S.L.; Bobrovskaya, L.; Dunkley, P.R.; Dickson, P.W. Differential regulation of human tyrosine hydroxylase isoforms 1 and 2 in situ: Isoform 2 is not phosphorylated at ser35. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2009, 1793, 1860–1867. [Google Scholar] [CrossRef] [PubMed]



| Comparison 1 | Comparison 2 | Sample Size | Permutations | Pseudo-F | p-Value | q-Value |
|---|---|---|---|---|---|---|
| Control AIN93M | Control HFD | 6 | 999 | 2.151254 | 0.078 | 0.138462 |
| Control SUP | 6 | 999 | 21.727793 | 0.114 | 0.138462 | |
| Control HFD | Control SUP | 6 | 999 | 8.108095 | 0.089 | 0.138462 |
| CUMS AIN93M | Control AIN93M | 6 | 999 | 6.558550 | 0.110 | 0.138462 |
| Control HFD | 6 | 999 | 3.450790 | 0.120 | 0.138462 | |
| Control SUP | 6 | 999 | 16.032741 | 0.094 | 0.138462 | |
| CUMS HFD | 6 | 999 | 5.742530 | 0.095 | 0.138462 | |
| CUMS SUP | 6 | 999 | 7.407984 | 0.078 | 0.138462 | |
| CUMS HFD | Control AIN93M | 6 | 999 | 2.498870 | 0.103 | 0.138462 |
| Control HFD | 6 | 999 | 1.021247 | 0.471 | 0.504643 | |
| Control SUP | 6 | 999 | 12.977976 | 0.118 | 0.138462 | |
| CUMS SUP | 6 | 999 | 9.760858 | 0.088 | 0.138462 | |
| CUMS SUP | Control AIN93M | 6 | 999 | 13.238861 | 0.099 | 0.138462 |
| Control HFD | 6 | 999 | 6.395057 | 0.108 | 0.138462 | |
| Control SUP | 6 | 999 | 0.700730 | 0.672 | 0.672000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Herselman, M.F.; Bobrovskaya, L. The Effects of Chronic Unpredictable Mild Stress and Semi-Pure Diets on the Brain, Gut and Adrenal Medulla in C57BL6 Mice. Int. J. Mol. Sci. 2023, 24, 14618. https://doi.org/10.3390/ijms241914618
Herselman MF, Bobrovskaya L. The Effects of Chronic Unpredictable Mild Stress and Semi-Pure Diets on the Brain, Gut and Adrenal Medulla in C57BL6 Mice. International Journal of Molecular Sciences. 2023; 24(19):14618. https://doi.org/10.3390/ijms241914618
Chicago/Turabian StyleHerselman, Mauritz Frederick, and Larisa Bobrovskaya. 2023. "The Effects of Chronic Unpredictable Mild Stress and Semi-Pure Diets on the Brain, Gut and Adrenal Medulla in C57BL6 Mice" International Journal of Molecular Sciences 24, no. 19: 14618. https://doi.org/10.3390/ijms241914618
APA StyleHerselman, M. F., & Bobrovskaya, L. (2023). The Effects of Chronic Unpredictable Mild Stress and Semi-Pure Diets on the Brain, Gut and Adrenal Medulla in C57BL6 Mice. International Journal of Molecular Sciences, 24(19), 14618. https://doi.org/10.3390/ijms241914618







