Lentinula edodes Cultured Extract and Rouxiella badensis subsp. acadiensis (Canan SV-53) Intake Alleviates Immune Deregulation and Inflammation by Modulating Signaling Pathways and Epigenetic Mechanisms
Abstract
:1. Introduction
2. Results
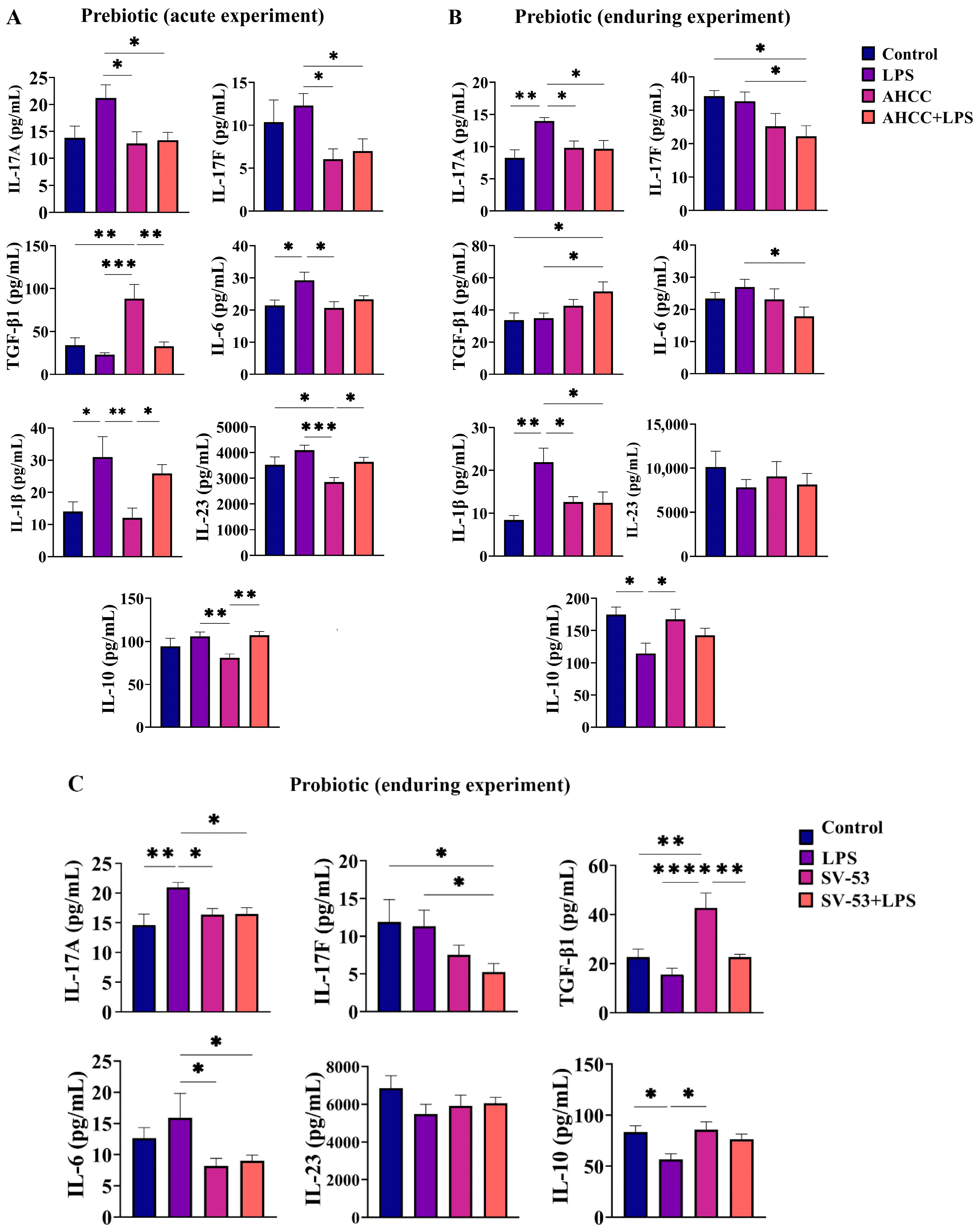
2.1. Effect of the Treatment on the Cytokine Concentrations in the Small Intestine
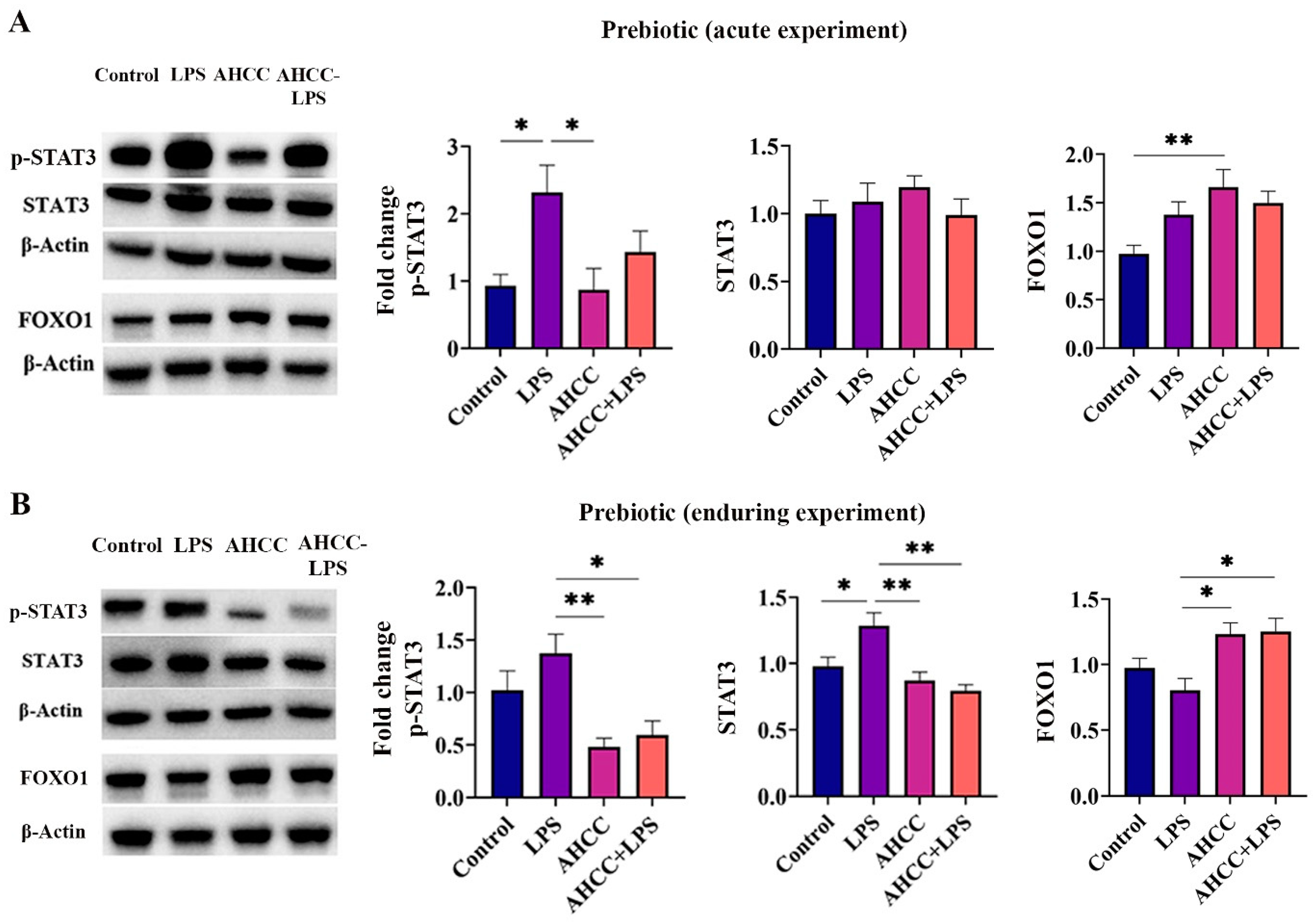
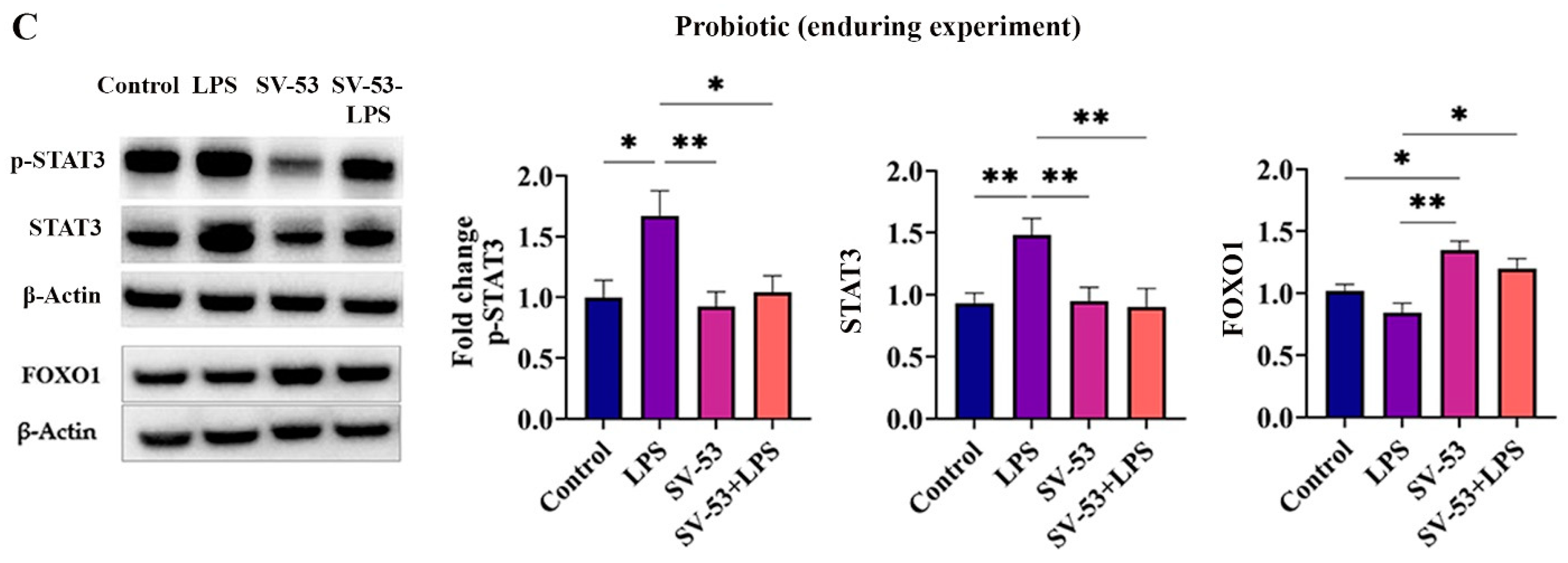
2.2. Effect of the Treatment on p-STAT3, STAT3, and FOXO1 Levels in the Small Intestine
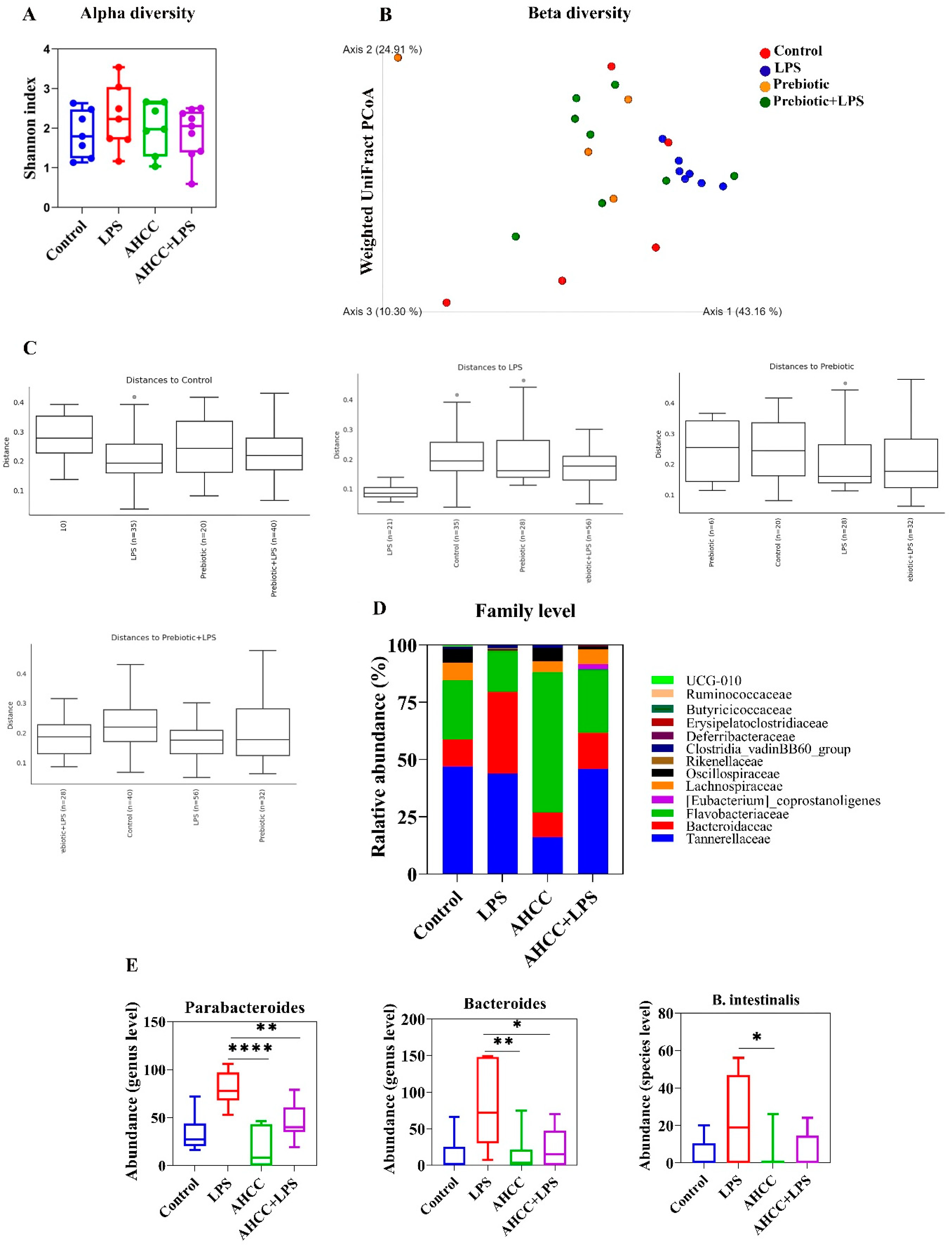
2.3. Effect of the Treatment on the Gut Microbiota of Mice in Pubertal Window
2.4. Effect of the Treatment on the miR-145 and miR-425 Expressions in the Small Intestine
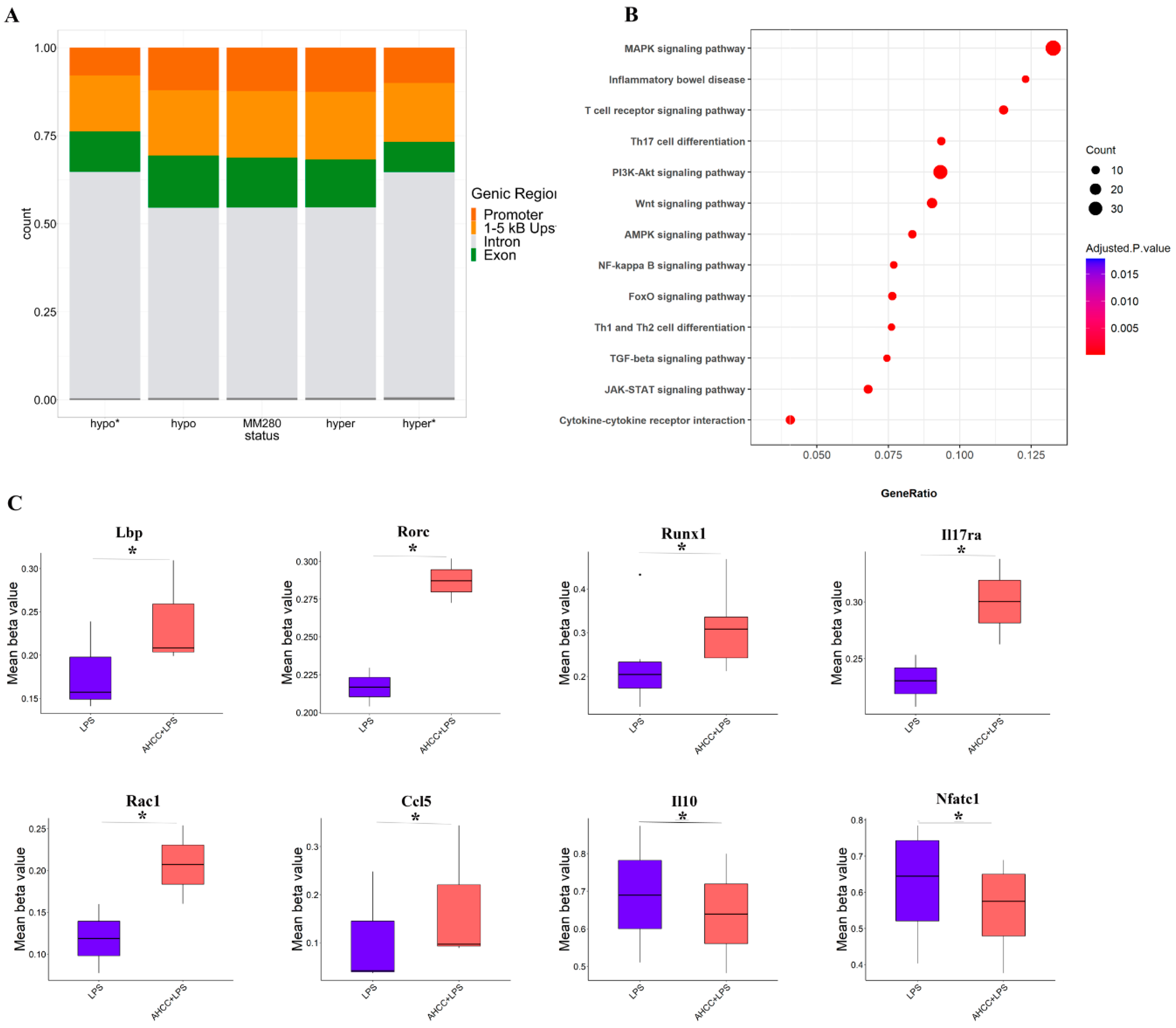
2.5. Effect of Treatment on DNA Methylation Status in Small Intestine of Mice
3. Discussion
4. Materials and Methods
4.1. Animals
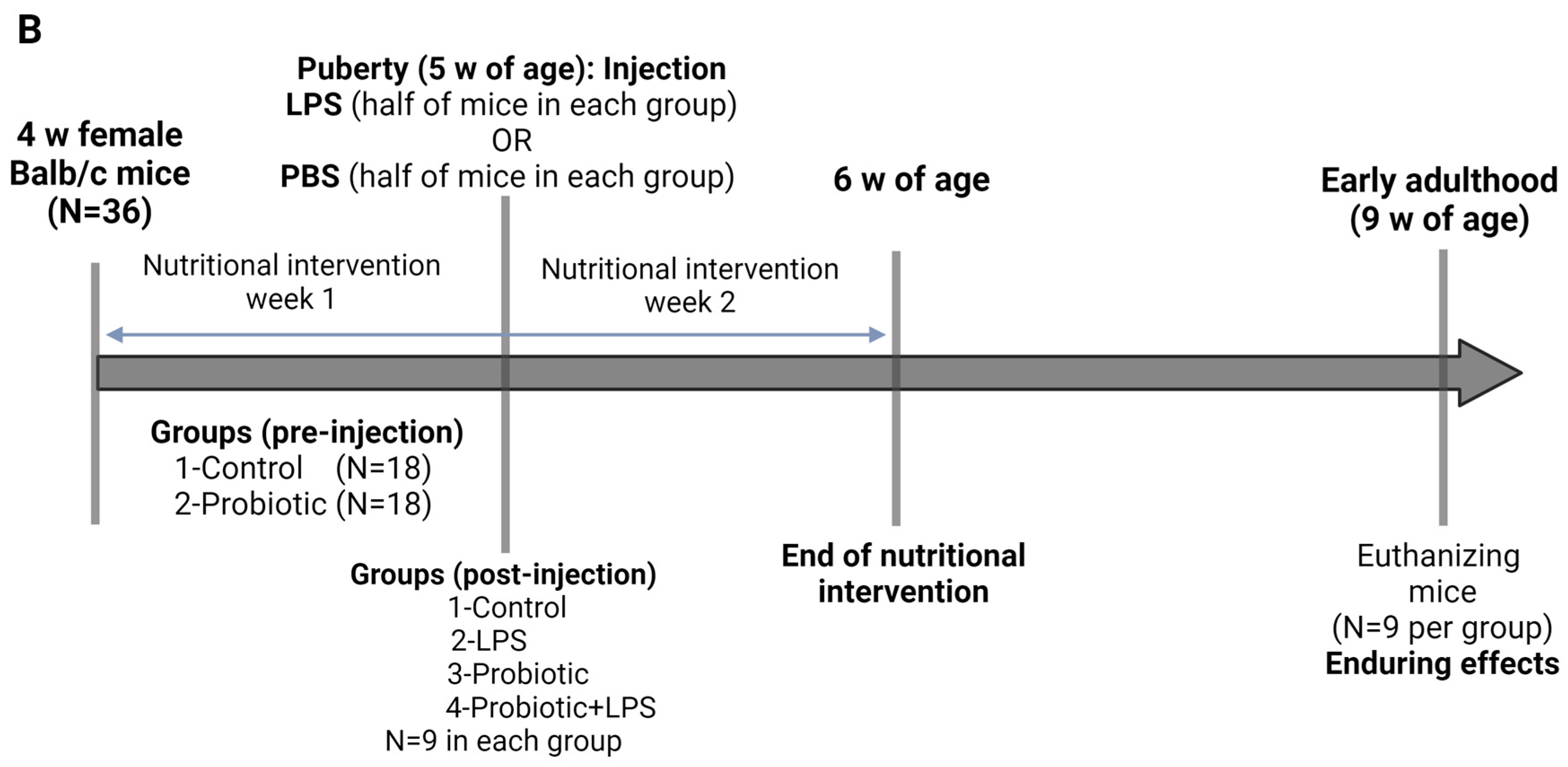
4.2. Study Design
4.2.1. Pubertal LPS-Prebiotic Model
4.2.2. Pubertal LPS-Probiotic Model
4.3. Determination of Cytokine Concentrations in the Small Intestine of the Mice by ELISA and Luminex Multiplex Assay
4.4. Determination of p-STAT, STAT3, and FOXO1 Levels in the Small Intestine of the Mice by Western Blotting
4.5. Determination of miRNAs Expression in the Small Intestine of the Mice by Real-Time Quantitative Reverse Transcription PCR (RT-qPCR)
4.6. Methylome-Wide Profiling and Data Analysis
4.7. Gut Microbiome Analysis
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shahbazi, R.; Yasavoli-Sharahi, H.; Alsadi, N.; Ismail, N.; Matar, C. Probiotics in Treatment of Viral Respiratory Infections and Neuroinflammatory Disorders. Molecules 2020, 25, 4891. [Google Scholar] [CrossRef]
- Wu, R.Y.; Jeffrey, M.P.; Johnson-Henry, K.C.; Green-Johnson, J.M.; Sherman, P.M. Impact of prebiotics, probiotics, and gut derived metabolites on host immunity. LymphoSign J. 2016, 4, 1–24. [Google Scholar] [CrossRef]
- Gagliardi, A.; Totino, V.; Cacciotti, F.; Iebba, V.; Neroni, B.; Bonfiglio, G.; Trancassini, M.; Passariello, C.; Pantanella, F.; Schippa, S. Rebuilding the Gut Microbiota Ecosystem. Int. J. Environ. Res. Public Health 2018, 15, 1679. [Google Scholar] [CrossRef] [PubMed]
- Murray, E.; Sharma, R.; Smith, K.B.; Mar, K.D.; Barve, R.; Lukasik, M.; Pirwani, A.F.; Malette-Guyon, E.; Lamba, S.; Thomas, B.J.; et al. Probiotic consumption during puberty mitigates LPS-induced immune responses and protects against stress-induced depression- and anxiety-like behaviors in adulthood in a sex-specific manner. Brain Behav. Immun. 2019, 81, 198–212. [Google Scholar] [CrossRef]
- Guo, S.; Al-Sadi, R.; Said, H.M.; Ma, T.Y. Lipopolysaccharide causes an increase in intestinal tight junction permeability in vitro and in vivo by inducing enterocyte membrane expression and localization of TLR-4 and CD14. Am. J. Pathol. 2013, 182, 375–387. [Google Scholar] [CrossRef] [PubMed]
- Yahfoufi, N.; Kadamani, A.K.; Aly, S.; Al Sharani, S.; Liang, J.; Butcher, J.; Stintzi, A.; Matar, C.; Ismail, N. Pubertal consumption of R. badensis subspecies acadiensis modulates LPS-induced immune responses and gut microbiome dysbiosis in a sex-specific manner. Brain Behav. Immun. 2023, 107, 62–75. [Google Scholar] [CrossRef]
- Chang, J.; Kim, B.M.; Chang, C.-H. Co-stimulation of TLR4 and Dectin-1 Induces the Production of Inflammatory Cytokines but not TGF-β for Th17 Cell Differentiation. Immune Netw. 2014, 14, 30–37. [Google Scholar] [CrossRef]
- Sutton, C.E.; Lalor, S.J.; Sweeney, C.M.; Brereton, C.F.; Lavelle, E.C.; Mills, K.H. Interleukin-1 and IL-23 induce innate IL-17 production from gammadelta T cells, amplifying Th17 responses and autoimmunity. Immunity 2009, 31, 331–341. [Google Scholar] [CrossRef]
- Jin, W.; Dong, C. IL-17 cytokines in immunity and inflammation. Emerg. Microbes Infect. 2013, 2, e60. [Google Scholar] [CrossRef]
- Geha, M.; Tsokos, M.G.; Bosse, R.E.; Sannikova, T.; Iwakura, Y.; Dalle Lucca, J.J.; De Waal Malefyt, R.; Tsokos, G.C. IL-17A Produced by Innate Lymphoid Cells Is Essential for Intestinal Ischemia-Reperfusion Injury. J. Immunol. 2017, 199, 2921–2929. [Google Scholar] [CrossRef]
- Cui, G. TH9, TH17, and TH22 Cell Subsets and Their Main Cytokine Products in the Pathogenesis of Colorectal Cancer. Front. Oncol. 2019, 9, 1002. [Google Scholar] [CrossRef] [PubMed]
- Littman, D.R.; Rudensky, A.Y. Th17 and regulatory T cells in mediating and restraining inflammation. Cell 2010, 140, 845–858. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.-H.; Ye, X.-Q.; Iwakura, Y. Interleukin-17 family members in health and disease. Int. Immunol. 2021, 33, 723–729. [Google Scholar] [CrossRef]
- Woś, I.; Tabarkiewicz, J. Effect of interleukin-6, -17, -21, -22, and -23 and STAT3 on signal transduction pathways and their inhibition in autoimmune arthritis. Immunol. Res. 2021, 69, 26–42. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.W.; Smith, A.J.; Yang, Y.; Selhorst, A.J.; Liu, Y.; Racke, M.K.; Lovett-Racke, A.E. IL-23R-activated STAT3/STAT4 is essential for Th1/Th17-mediated CNS autoimmunity. JCI Insight 2017, 2, e91663. [Google Scholar] [CrossRef]
- Mori, T.; Miyamoto, T.; Yoshida, H.; Asakawa, M.; Kawasumi, M.; Kobayashi, T.; Morioka, H.; Chiba, K.; Toyama, Y.; Yoshimura, A. IL-1β and TNFα-initiated IL-6-STAT3 pathway is critical in mediating inflammatory cytokines and RANKL expression in inflammatory arthritis. Int. Immunol. 2011, 23, 701–712. [Google Scholar] [CrossRef]
- Ichiyama, K.; Gonzalez-Martin, A.; Kim, B.S.; Jin, H.Y.; Jin, W.; Xu, W.; Sabouri-Ghomi, M.; Xu, S.; Zheng, P.; Xiao, C.; et al. The MicroRNA-183-96-182 Cluster Promotes T Helper 17 Cell Pathogenicity by Negatively Regulating Transcription Factor Foxo1 Expression. Immunity 2016, 44, 1284–1298. [Google Scholar] [CrossRef]
- Zenobia, C.; Hajishengallis, G. Basic biology and role of interleukin-17 in immunity and inflammation. Periodontol. 2000 2015, 69, 142–159. [Google Scholar] [CrossRef]
- Huang, J.; Xu, X.; Yang, J. miRNAs Alter T Helper 17 Cell Fate in the Pathogenesis of Autoimmune Diseases. Front. Immunol. 2021, 12, 593473. [Google Scholar] [CrossRef]
- Dupraz, L.; Magniez, A.; Rolhion, N.; Richard, M.L.; Da Costa, G.; Touch, S.; Mayeur, C.; Planchais, J.; Agus, A.; Danne, C.; et al. Gut microbiota-derived short-chain fatty acids regulate IL-17 production by mouse and human intestinal γδ T cells. Cell Rep. 2021, 36, 109332. [Google Scholar] [CrossRef]
- Ivanov, I.I.; Atarashi, K.; Manel, N.; Brodie, E.L.; Shima, T.; Karaoz, U.; Wei, D.; Goldfarb, K.C.; Santee, C.A.; Lynch, S.V.; et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell 2009, 139, 485–498. [Google Scholar] [CrossRef] [PubMed]
- Mukasa, R.; Balasubramani, A.; Lee, Y.K.; Whitley, S.K.; Weaver, B.T.; Shibata, Y.; Crawford, G.E.; Hatton, R.D.; Weaver, C.T. Epigenetic instability of cytokine and transcription factor gene loci underlies plasticity of the T helper 17 cell lineage. Immunity 2010, 32, 616–627. [Google Scholar] [CrossRef] [PubMed]
- Schmolka, N.; Serre, K.; Grosso, A.R.; Rei, M.; Pennington, D.J.; Gomes, A.Q.; Silva-Santos, B. Epigenetic and transcriptional signatures of stable versus plastic differentiation of proinflammatory γδ T cell subsets. Nat. Immunol. 2013, 14, 1093–1100. [Google Scholar] [CrossRef] [PubMed]
- Ghoreschi, K.; Laurence, A.; Yang, X.-P.; Tato, C.M.; McGeachy, M.J.; Konkel, J.E.; Ramos, H.L.; Wei, L.; Davidson, T.S.; Bouladoux, N.; et al. Generation of pathogenic TH17 cells in the absence of TGF-β signalling. Nature 2010, 467, 967–971. [Google Scholar] [CrossRef] [PubMed]
- Bi, K.; Zhang, X.; Chen, W.; Diao, H. MicroRNAs Regulate Intestinal Immunity and Gut Microbiota for Gastrointestinal Health: A Comprehensive Review. Genes 2020, 11, 1075. [Google Scholar] [CrossRef]
- Raisch, J.; Darfeuille-Michaud, A.; Nguyen, H.T.T. Role of microRNAs in the immune system, inflammation and cancer. World J. Gastroenterol. 2013, 19, 2985–2996. [Google Scholar] [CrossRef]
- Mikami, Y.; Philips, R.L.; Sciumè, G.; Petermann, F.; Meylan, F.; Nagashima, H.; Yao, C.; Davis, F.P.; Brooks, S.R.; Sun, H.W.; et al. MicroRNA-221 and -222 modulate intestinal inflammatory Th17 cell response as negative feedback regulators downstream of interleukin-23. Immunity 2021, 54, 514–525.e6. [Google Scholar] [CrossRef]
- Robichaud, S.; Shahbazi, R.; Matar, C. Role of probiotics in prevention of COVID-19 through modulation of gut–lung axis. In COVID-19 and Nutraceuticals, A Guidebook, 1st ed.; Prasad, C., Öztürk, G., Eds.; Bohr Publishers and New Century Health Publishers, LLC: New Delhi, India, 2021; pp. 33–60. [Google Scholar]
- Shahbazi, R.; Sharifzad, F.; Bagheri, R.; Alsadi, N.; Yasavoli-Sharahi, H.; Matar, C. Anti-Inflammatory and Immunomodulatory Properties of Fermented Plant Foods. Nutrients 2021, 13, 1516. [Google Scholar] [CrossRef]
- Mohammad, S.; Thiemermann, C. Role of Metabolic Endotoxemia in Systemic Inflammation and Potential Interventions. Front. Immunol. 2020, 11, 594150. [Google Scholar] [CrossRef]
- Pujari, R.; Banerjee, G. Impact of prebiotics on immune response: From the bench to the clinic. Immunol. Cell Biol. 2021, 99, 255–273. [Google Scholar] [CrossRef]
- Yuan, X.; Chen, R.; Zhang, Y.; Lin, X.; Yang, X. Gut microbiota: Effect of pubertal status. BMC Microbiol. 2020, 20, 334. [Google Scholar] [CrossRef]
- Yahfoufi, N.; Matar, C.; Ismail, N. Adolescence and Aging: Impact of Adolescence Inflammatory Stress and Microbiota Alterations on Brain Development, Aging, and Neurodegeneration. J. Gerontol. A Biol. Sci. Med. Sci. 2020, 75, 1251–1257. [Google Scholar] [CrossRef] [PubMed]
- Brenhouse, H.C.; Schwarz, J.M. Immunoadolescence: Neuroimmune development and adolescent behavior. Neurosci. Biobehav. Rev. 2016, 70, 288–299. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; van Mil, S.; Melanson, B.; Thomas, B.J.; Rooke, J.; Mallet, J.-F.; Matar, C.; Schwarz, J.M.; Ismail, N. Programming Effects of Pubertal Lipopolysaccharide Treatment in Male and Female CD-1 Mice. J. Immunol. 2019, 202, 2131–2140. [Google Scholar] [CrossRef]
- Yahfoufi, N.; Ah-Yen, E.G.; Chandrasegaram, R.; Aly, S.; Murack, M.; Kadamani, A.K.; Matar, C.; Ismail, N. Adolescent use of potential novel probiotic Rouxiella badensis subsp. acadiensis (Canan SV-53) mitigates pubertal LPS-Induced behavioral changes in adulthood in a sex-specific manner by modulating 5HT1A receptors expression in specific brain areas. Comp. Psychoneuroendocrinol. 2021, 7, 100063. [Google Scholar] [CrossRef]
- Yu, H.; Pardoll, D.; Jove, R. STATs in cancer inflammation and immunity: A leading role for STAT3. Nat. Rev. Cancer 2009, 9, 798–809. [Google Scholar] [CrossRef]
- Lainé, A.; Martin, B.; Luka, M.; Mir, L.; Auffray, C.; Lucas, B.; Bismuth, G.; Charvet, C. Foxo1 Is a T Cell-Intrinsic Inhibitor of the RORγt-Th17 Program. J. Immunol. 2015, 195, 1791–1803. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Wu, N.; Leong, M.C.; Zhang, W.; Ye, Z.; Li, R.; Huang, J.; Zhang, Z.; Li, L.; Yao, X.; et al. miR-145 improves metabolic inflammatory disease through multiple pathways. J. Mol. Cell Biol. 2019, 12, 152–162. [Google Scholar] [CrossRef] [PubMed]
- Gan, B.; Lim, C.; Chu, G.; Hua, S.; Ding, Z.; Collins, M.; Hu, J.; Jiang, S.; Fletcher-Sananikone, E.; Zhuang, L.; et al. FoxOs enforce a progression checkpoint to constrain mTORC1-activated renal tumorigenesis. Cancer Cell 2010, 18, 472–484. [Google Scholar] [CrossRef]
- Mallet, J.F.; Shahbazi, R.; Alsadi, N.; Matar, C. Polyphenol-Enriched Blueberry Preparation Controls Breast Cancer Stem Cells by Targeting FOXO1 and miR-145. Molecules 2021, 26, 4330. [Google Scholar] [CrossRef]
- Jiang, G.; Huang, C.; Li, J.; Huang, H.; Jin, H.; Zhu, J.; Wu, X.R.; Huang, C. Role of STAT3 and FOXO1 in the Divergent Therapeutic Responses of Non-metastatic and Metastatic Bladder Cancer Cells to miR-145. Mol. Cancer Ther. 2017, 16, 924–935. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; He, Q.; Guo, Z.; Xiong, F.; Li, Y.; Pan, Y.; Gao, C.; Li, L.; He, C. MicroRNA-425 facilitates pathogenic Th17 cell differentiation by targeting forkhead box O1 (Foxo1) and is associated with inflammatory bowel disease. Biochem. Biophys. Res. Commun. 2018, 496, 352–358. [Google Scholar] [CrossRef]
- Mallet, J.-F.; Shahbazi, R.; Alsadi, N.; Saleem, A.; Sobiesiak, A.; Arnason, J.T.; Matar, C. Role of a Mixture of Polyphenol Compounds Released after Blueberry Fermentation in Chemoprevention of Mammary Carcinoma: In Vivo Involvement of miR-145. Int. J. Mol. Sci. 2023, 24, 3677. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.-L.; Gao, N.; Xing, Y.; Zhang, H.-B.; Zhang, A.-L.; Liu, J.; He, J.-L.; Xu, Y.; Lin, W.-M.; Chen, Z.-M.; et al. Profiling the genome-wide DNA methylation pattern of porcine ovaries using reduced representation bisulfite sequencing. Sci. Rep. 2016, 6, 22138. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.; Rhee, J.-K. Genomic Effect of DNA Methylation on Gene Expression in Colorectal Cancer. Biology 2022, 11, 1388. [Google Scholar] [CrossRef]
- Herrera-Covarrubias, D.; Coria-Avila, G.; Hernandez, M.E.; Ismail, N. Stress during puberty facilitates precancerous prostate lesions in adult rats. Exp. Oncol. 2017, 39, 269–275. [Google Scholar] [CrossRef]
- Cho, I.; Yamanishi, S.; Cox, L.; Methe, B.A.; Zavadil, J.; Li, K.; Gao, Z.; Mahana, D.; Raju, K.; Teitler, I.; et al. Antibiotics in early life alter the murine colonic microbiome and adiposity. Nature 2012, 488, 621–626. [Google Scholar] [CrossRef]
- Leclercq, S.; Mian, F.M.; Stanisz, A.M.; Bindels, L.B.; Cambier, E.; Ben-Amram, H.; Koren, O.; Forsythe, P.; Bienenstock, J. Low-dose penicillin in early life induces long-term changes in murine gut microbiota, brain cytokines and behavior. Nat. Ccommun. 2017, 8, 15062. [Google Scholar] [CrossRef]
- Garcia-Montero, C.; Fraile-Martinez, O.; Gomez-Lahoz, A.M.; Pekarek, L.; Castellanos, A.J.; Noguerales-Fraguas, F.; Coca, S.; Guijarro, L.G.; Garcia-Honduvilla, N.; Asunsolo, A.; et al. Nutritional Components in Western Diet Versus Mediterranean Diet at the Gut Microbiota-Immune System Interplay. Implications for Health and Disease. Nutrients 2021, 13, 699. [Google Scholar] [CrossRef]
- Mallet, J.F.; Graham, E.; Ritz, B.W.; Homma, K.; Matar, C. Active Hexose Correlated Compound (AHCC) promotes an intestinal immune response in BALB/c mice and in primary intestinal epithelial cell culture involving toll-like receptors TLR-2 and TLR-4. Eur. J. Nutr. 2016, 55, 139–146. [Google Scholar] [CrossRef]
- Graham, E.A.; Mallet, J.F.; Jambi, M.; Nishioka, H.; Homma, K.; Matar, C. MicroRNA signature in the chemoprevention of functionally-enriched stem and progenitor pools (FESPP) by Active Hexose Correlated Compound (AHCC). Cancer Biol. Ther. 2017, 18, 765–774. [Google Scholar] [CrossRef]
- Matar, C.Y.; Yahfoufi, N.; Mallet, J.F.; Ismail, N. Probiotic Compositions and Methods. PCT Patent No. PCT/CA2020/051385, 16 October 2021. [Google Scholar]
- Novotny-Nuñez, I.; Perdigón, G.; Matar, C.; Martínez Monteros, M.J.; Yahfoufi, N.; Cazorla, S.I.; Maldonado-Galdeano, C. Evaluation of Rouxiella badensis Subsp Acadiensis (Canan SV-53) as a Potential Probiotic Bacterium. Microorganisms 2023, 11, 1347. [Google Scholar] [CrossRef] [PubMed]
- Salvetti, E.; Tremblay, J.; Arbour, M.; Mallet, J.-F.; Masson, L.; Matar, C. Complete PacBio Single-Molecule Real-Time Sequence of a Novel Probiotic-Like Bacterium, Rouxiella badensis subsp. acadiensis, Isolated from the Biota of Wild Blueberries in the Acadian Forest. Microbiol. Resour. Announc. 2023, 12, e0134022. [Google Scholar] [CrossRef]
- Yahfoufi, N.; Alsadi, N.; Mallet, J.F.; Kulshreshtha, G.; Hincke, M.; Ismail, N.; Matar, C. Immunomodulation and Intestinal Morpho-Functional Aspects of a Novel Gram-Negative Bacterium Rouxiella badensis subsp. acadiensis. Front. Microbiol. 2021, 12, 569119. [Google Scholar] [CrossRef]
- Sylvia, K.E.; Demas, G.E. Acute intraperitoneal lipopolysaccharide influences the immune system in the absence of gut dysbiosis. Physiol. Rep. 2018, 6, e13639. [Google Scholar] [CrossRef]
- Esposito, P.; Ismail, N. Linking Puberty and the Gut Microbiome to the Pathogenesis of Neurodegenerative Disorders. Microorganisms 2022, 10, 2163. [Google Scholar] [CrossRef]
- Chen, L.; Zou, Y.; Peng, J.; Lu, F.; Yin, Y.; Li, F.; Yang, J. Lactobacillus acidophilus suppresses colitis-associated activation of the IL-23/Th17 axis. J. Immunol. Res. 2015, 2015, 909514. [Google Scholar] [CrossRef] [PubMed]
- Egwuagu, C.E. STAT3 in CD4+ T helper cell differentiation and inflammatory diseases. Cytokine 2009, 47, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Kim, J.; Min, H.; Seong, R.H. RORγt-driven T(H)17 Cell Differentiation Requires Epigenetic Control by the Swi/Snf Chromatin Remodeling Complex. iScience 2020, 23, 101106. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Ruan, G.; Cheng, Y.; Yi, A.; Chen, D.; Wei, Y. The role of Th17 cells in inflammatory bowel disease and the research progress. Front. Immunol. 2022, 13, 1055914. [Google Scholar] [CrossRef]
- Liu, X.; Lee, Y.S.; Yu, C.R.; Egwuagu, C.E. Loss of STAT3 in CD4+ T cells prevents development of experimental autoimmune diseases. J. Immunol. 2008, 180, 6070–6076. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Liao, X.; Agarwal, M.K.; Barnes, L.; Auron, P.E.; Stark, G.R. Unphosphorylated STAT3 accumulates in response to IL-6 and activates transcription by binding to NF-kappaB. Genes Dev. 2007, 21, 1396–1408. [Google Scholar] [CrossRef] [PubMed]
- Huber, S.; Gagliani, N.; Esplugues, E.; O’Connor, W., Jr.; Huber, F.J.; Chaudhry, A.; Kamanaka, M.; Kobayashi, Y.; Booth, C.J.; Rudensky, A.Y.; et al. Th17 cells express interleukin-10 receptor and are controlled by Foxp3− and Foxp3+ regulatory CD4+ T cells in an interleukin-10-dependent manner. Immunity 2011, 34, 554–565. [Google Scholar] [CrossRef]
- Hsu, P.; Santner-Nanan, B.; Hu, M.; Skarratt, K.; Lee, C.H.; Stormon, M.; Wong, M.; Fuller, S.J.; Nanan, R. IL-10 potentiates differentiation of human induced regulatory T cells via STAT3 and Foxo1. J. Immunol. 2015, 195, 3665–3674. [Google Scholar] [CrossRef] [PubMed]
- Chung, Y.; Chang, S.H.; Martinez, G.J.; Yang, X.O.; Nurieva, R.; Kang, H.S.; Ma, L.; Watowich, S.S.; Jetten, A.M.; Tian, Q.; et al. Critical regulation of early Th17 cell differentiation by interleukin-1 signaling. Immunity 2009, 30, 576–587. [Google Scholar] [CrossRef]
- Whitley, S.K.; Balasubramani, A.; Zindl, C.L.; Sen, R.; Shibata, Y.; Crawford, G.E.; Weathington, N.M.; Hatton, R.D.; Weaver, C.T. IL-1R signaling promotes STAT3 and NF-κB factor recruitment to distal cis-regulatory elements that regulate Il17a/f transcription. J. Biol. Chem. 2018, 293, 15790–15800. [Google Scholar] [CrossRef]
- McAleer, J.P.; Liu, B.; Li, Z.; Ngoi, S.-M.; Dai, J.; Oft, M.; Vella, A.T. Potent intestinal Th17 priming through peripheral lipopolysaccharide-based immunization. J. Leukoc. Biol. 2010, 88, 21–31. [Google Scholar] [CrossRef]
- Chen, X.W.; Zhou, S.F. Inflammation, cytokines, the IL-17/IL-6/STAT3/NF-κB axis, and tumorigenesis. Drug Des. Devel. Ther. 2015, 9, 2941–2946. [Google Scholar] [CrossRef]
- Cabrera-Ortega, A.A.; Feinberg, D.; Liang, Y.; Rossa, C., Jr.; Graves, D.T. The Role of Forkhead Box 1 (FOXO1) in the Immune System: Dendritic Cells, T Cells, B Cells, and Hematopoietic Stem Cells. Crit. Rev. Immunol. 2017, 37, 1–13. [Google Scholar] [CrossRef]
- Graves, D.T.; Milovanova, T.N. Mucosal Immunity and the FOXO1 Transcription Factors. Front. Immunol. 2019, 10, 2530. [Google Scholar] [CrossRef]
- Shi, Y.; Zhong, L.; Li, Y.; Chen, Y.; Feng, S.; Wang, M.; Xia, Y.; Tang, S. Repulsive Guidance Molecule b Deficiency Induces Gut Microbiota Dysbiosis and Increases the Susceptibility to Intestinal Inflammation in Mice. Front. Microbiol. 2021, 12, 648915. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Wang, H.; Xu, S.; Zhuang, Y.; An, J.; Su, C.; Xia, Y.; Chen, J.; Xu, Z.Z.; Liu, Q.; et al. Alteration in gut microbiota is associated with dysregulation of cytokines and glucocorticoid therapy in systemic lupus erythematosus. Gut Microbes 2020, 11, 1758–1773. [Google Scholar] [CrossRef] [PubMed]
- Andrews, M.C.; Duong, C.P.M.; Gopalakrishnan, V.; Iebba, V.; Chen, W.-S.; Derosa, L.; Khan, M.A.W.; Cogdill, A.P.; White, M.G.; Wong, M.C.; et al. Gut microbiota signatures are associated with toxicity to combined CTLA-4 and PD-1 blockade. Nat. Med. 2021, 27, 1432–1441. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Gong, D.; Nguyen, D.N.; Zhang, X.; Hu, Q.; Lu, H.; Fredholm, M.; Sangild, P.T.; Gao, F. Early microbial colonization affects DNA methylation of genes related to intestinal immunity and metabolism in preterm pigs. DNA Res. 2018, 25, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Woo, V.; Alenghat, T. Epigenetic regulation by gut microbiota. Gut Microbes. 2022, 14, 2022407. [Google Scholar] [CrossRef]
- Fahmy, C.A.; Gamal-Eldeen, A.M.; El-Hussieny, E.A.; Raafat, B.M.; Mehanna, N.S.; Talaat, R.M.; Shaaban, M.T. Bifidobacterium longum suppresses murine colorectal cancer through the modulation of oncomirs and tumor suppressor mirnas. Nutr. Cancer 2019, 71, 688–700. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zheng, S.; Xin, N.; Dou, C.; Fu, L.; Zhang, X.; Chen, J.; Zhang, Y.; Geng, D.; Xiao, C.; et al. Identification of novel MicroRNA signatures linked to experimental autoimmune myasthenia gravis pathogenesis: Down-regulated miR-145 promotes pathogenetic Th17 cell response. J. Neuroimmune Pharmacol. 2013, 8, 1287–1302. [Google Scholar] [CrossRef]
- Li, R.; Shen, Q.; Wu, N.; He, M.; Liu, N.; Huang, J.; Lu, B.; Yao, Q.; Yang, Y.; Hu, R. MiR-145 improves macrophage-mediated inflammation through targeting Arf6. Endocrine 2018, 60, 73–82. [Google Scholar] [CrossRef]
- Xiao, S.; Zhu, H.; Luo, J.; Wu, Z.; Xie, M. miR-425-5p is associated with poor prognosis in patients with breast cancer and promotes cancer cell progression by targeting PTEN. Oncol. Rep. 2019, 42, 2550–2560. [Google Scholar] [CrossRef]
- Wu, Z.; Guo, J.; Zhang, Y.; Liu, J.; Ma, H.; Tang, Y. MiR-425-5p accelerated the proliferation, migration, and invasion of ovarian cancer cells via targeting AFF4. J. Ovarian Res. 2021, 14, 138. [Google Scholar] [CrossRef]
- Ma, J.; Liu, J.; Wang, Z.; Gu, X.; Fan, Y.; Zhang, W.; Xu, L.; Zhang, J.; Cai, D. NF-kappaB-dependent MicroRNA-425 upregulation promotes gastric cancer cell growth by targeting PTEN upon IL-1β induction. Mol. Cancer 2014, 13, 40. [Google Scholar] [CrossRef]
- Park, B.S.; Lee, J.-O. Recognition of lipopolysaccharide pattern by TLR4 complexes. Exp. Mol. Med. 2013, 45, e66. [Google Scholar] [CrossRef]
- Ma, S.; Patel, S.A.; Abe, Y.; Chen, N.; Patel, P.R.; Cho, B.S.; Abbasi, N.; Zeng, S.; Schnabl, B.; Chang, J.T.; et al. RORγt phosphorylation protects against T cell-mediated inflammation. Cell Rep. 2022, 38, 110520. [Google Scholar] [CrossRef] [PubMed]
- Hall, J.A.; Pokrovskii, M.; Kroehling, L.; Kim, B.R.; Kim, S.Y.; Wu, L.; Lee, J.Y.; Littman, D.R. Transcription factor RORα enforces stability of the Th17 cell effector program by binding to a Rorc cis-regulatory element. Immunity 2022, 55, 2027–2043.e9. [Google Scholar] [CrossRef] [PubMed]
- Chi, X.; Jin, W.; Zhao, X.; Xie, T.; Shao, J.; Bai, X.; Jiang, Y.; Wang, X.; Dong, C. RORγt expression in mature Th17 cells safeguards their lineage specification by inhibiting conversion to Th2 cells. Sci. Adv. 2022, 8, eabn7774. [Google Scholar] [CrossRef] [PubMed]
- Zhong, X.; Wu, Y.; Liu, Y.; Zhu, F.; Li, X.; Li, D.; Li, Z.; Zeng, L.; Qiao, J.; Chen, X.; et al. Increased RUNX1 expression in patients with immune thrombocytopenia. Hum. Immunol. 2016, 77, 687–691. [Google Scholar] [CrossRef]
- Zhang, F.; Meng, G.; Strober, W. Interactions among the transcription factors Runx1, RORγt and Foxp3 regulate the differentiation of interleukin 17–producing T cells. Nat. Immunol. 2008, 9, 1297–1306. [Google Scholar] [CrossRef]
- Rex, D.A.B.; Dagamajalu, S.; Gouda, M.M.; Suchitha, G.P.; Chanderasekaran, J.; Raju, R.; Prasad, T.S.K.; Bhandary, Y.P. A comprehensive network map of IL-17A signaling pathway. J. Cell Commun. Signal 2023, 17, 209–215. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, X.; Wang, J.; Wang, X.; Zhang, Y. Immunogenic Cell Death Associated Molecular Patterns and the Dual Role of IL17RA in Interstitial Cystitis/Bladder Pain Syndrome. Biomolecules 2023, 13, 421. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, D.; Wang, L.; Wang, S.; Roden, A.C.; Zhao, H.; Li, X.; Prakash, Y.S.; Matteson, E.L.; Tschumperlin, D.J.; et al. Profibrotic effect of IL-17A and elevated IL-17RA in idiopathic pulmonary fibrosis and rheumatoid arthritis-associated lung disease support a direct role for IL-17A/IL-17RA in human fibrotic interstitial lung disease. Am. J. Physiol. 2019, 316, L487–L497. [Google Scholar] [CrossRef]
- Sanlioglu, S.; Williams, C.M.; Samavati, L.; Butler, N.S.; Wang, G.; McCray, P.B., Jr.; Ritchie, T.C.; Hunninghake, G.W.; Zandi, E.; Engelhardt, J.F. Lipopolysaccharide induces Rac1-dependent reactive oxygen species formation and coordinates tumor necrosis factor-alpha secretion through IKK regulation of NF-kappa B. J. Biol. Chem. 2001, 276, 30188–30198. [Google Scholar] [CrossRef] [PubMed]
- Kurdi, A.T.; Bassil, R.; Olah, M.; Wu, C.; Xiao, S.; Taga, M.; Frangieh, M.; Buttrick, T.; Orent, W.; Bradshaw, E.M.; et al. Tiam1/Rac1 complex controls Il17a transcription and autoimmunity. Nat. Commun. 2016, 7, 13048. [Google Scholar] [CrossRef] [PubMed]
- Bandow, K.; Kusuyama, J.; Shamoto, M.; Kakimoto, K.; Ohnishi, T.; Matsuguchi, T. LPS-induced chemokine expression in both MyD88-dependent and -independent manners is regulated by Cot/Tpl2-ERK axis in macrophages. FEBS Lett. 2012, 586, 1540–1546. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Awasthi, A.; Yosef, N.; Quintana, F.J.; Xiao, S.; Peters, A.; Wu, C.; Kleinewietfeld, M.; Kunder, S.; Hafler, D.A.; et al. Induction and molecular signature of pathogenic TH17 cells. Nat. Immunol. 2012, 13, 991–999. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Li, Q.; Chuang, P.Y.; Lu, G.; Liu, R.; Yang, J.; Peng, L.; Dai, Y.; Zheng, Z.; Qi, C.-F.; et al. Regulation of Pathogenic Th17 Cell Differentiation by IL-10 in the Development of Glomerulonephritis. Am. J. Pathol. 2013, 183, 402–412. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Gurung, P.; Malireddi, R.K.S.; Vogel, P.; Kanneganti, T.-D.; Geiger, T.L. IL-10 engages macrophages to shift Th17 cytokine dependency and pathogenicity during T-cell-mediated colitis. Nat. Commun. 2015, 6, 6131. [Google Scholar] [CrossRef]
- Reppert, S.; Zinser, E.; Holzinger, C.; Sandrock, L.; Koch, S.; Finotto, S. NFATc1 deficiency in T cells protects mice from experimental autoimmune encephalomyelitis. Eur. J. Immunol. 2015, 45, 1426–1440. [Google Scholar] [CrossRef]
- Nelson, J.F.; Karelus, K.; Felicio, L.S.; Johnson, T.E. Genetic influences on the timing of puberty in mice. Biol. Reprod. 1990, 42, 649–655. [Google Scholar] [CrossRef]
- Zhou, W.; Triche, T.J., Jr.; Laird, P.W.; Shen, H. SeSAMe: Reducing artifactual detection of DNA methylation by Infinium BeadChips in genomic deletions. Nucleic Acids Res. 2018, 46, e123. [Google Scholar] [CrossRef]
- Ritchie, M.E.; Phipson, B.; Wu, D.; Hu, Y.; Law, C.W.; Shi, W.; Smyth, G.K. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015, 43, e47. [Google Scholar] [CrossRef]
- Peters, T.J.; Buckley, M.J.; Statham, A.L.; Pidsley, R.; Samaras, K.; V Lord, R.; Clark, S.J.; Molloy, P.L. De novo identification of differentially methylated regions in the human genome. Epigenetic Chromatin 2015, 8, 6. [Google Scholar] [CrossRef] [PubMed]
- Amplicon PCR; PCR Clean-Up; Index PCR. 16s Metagenomic Sequencing Library Preparation; Illumina: San Diego, CA, USA, 2013. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shahbazi, R.; Yasavoli-Sharahi, H.; Alsadi, N.; Sharifzad, F.; Fang, S.; Cuenin, C.; Cahais, V.; Chung, F.F.-L.; Herceg, Z.; Matar, C. Lentinula edodes Cultured Extract and Rouxiella badensis subsp. acadiensis (Canan SV-53) Intake Alleviates Immune Deregulation and Inflammation by Modulating Signaling Pathways and Epigenetic Mechanisms. Int. J. Mol. Sci. 2023, 24, 14610. https://doi.org/10.3390/ijms241914610
Shahbazi R, Yasavoli-Sharahi H, Alsadi N, Sharifzad F, Fang S, Cuenin C, Cahais V, Chung FF-L, Herceg Z, Matar C. Lentinula edodes Cultured Extract and Rouxiella badensis subsp. acadiensis (Canan SV-53) Intake Alleviates Immune Deregulation and Inflammation by Modulating Signaling Pathways and Epigenetic Mechanisms. International Journal of Molecular Sciences. 2023; 24(19):14610. https://doi.org/10.3390/ijms241914610
Chicago/Turabian StyleShahbazi, Roghayeh, Hamed Yasavoli-Sharahi, Nawal Alsadi, Farzaneh Sharifzad, Sandra Fang, Cyrille Cuenin, Vincent Cahais, Felicia Fei-Lei Chung, Zdenko Herceg, and Chantal Matar. 2023. "Lentinula edodes Cultured Extract and Rouxiella badensis subsp. acadiensis (Canan SV-53) Intake Alleviates Immune Deregulation and Inflammation by Modulating Signaling Pathways and Epigenetic Mechanisms" International Journal of Molecular Sciences 24, no. 19: 14610. https://doi.org/10.3390/ijms241914610
APA StyleShahbazi, R., Yasavoli-Sharahi, H., Alsadi, N., Sharifzad, F., Fang, S., Cuenin, C., Cahais, V., Chung, F. F.-L., Herceg, Z., & Matar, C. (2023). Lentinula edodes Cultured Extract and Rouxiella badensis subsp. acadiensis (Canan SV-53) Intake Alleviates Immune Deregulation and Inflammation by Modulating Signaling Pathways and Epigenetic Mechanisms. International Journal of Molecular Sciences, 24(19), 14610. https://doi.org/10.3390/ijms241914610





