The Tyrosine Phosphatase Activity of PTPN22 Is Involved in T Cell Development via the Regulation of TCR Expression
Abstract
:1. Introduction
2. Results
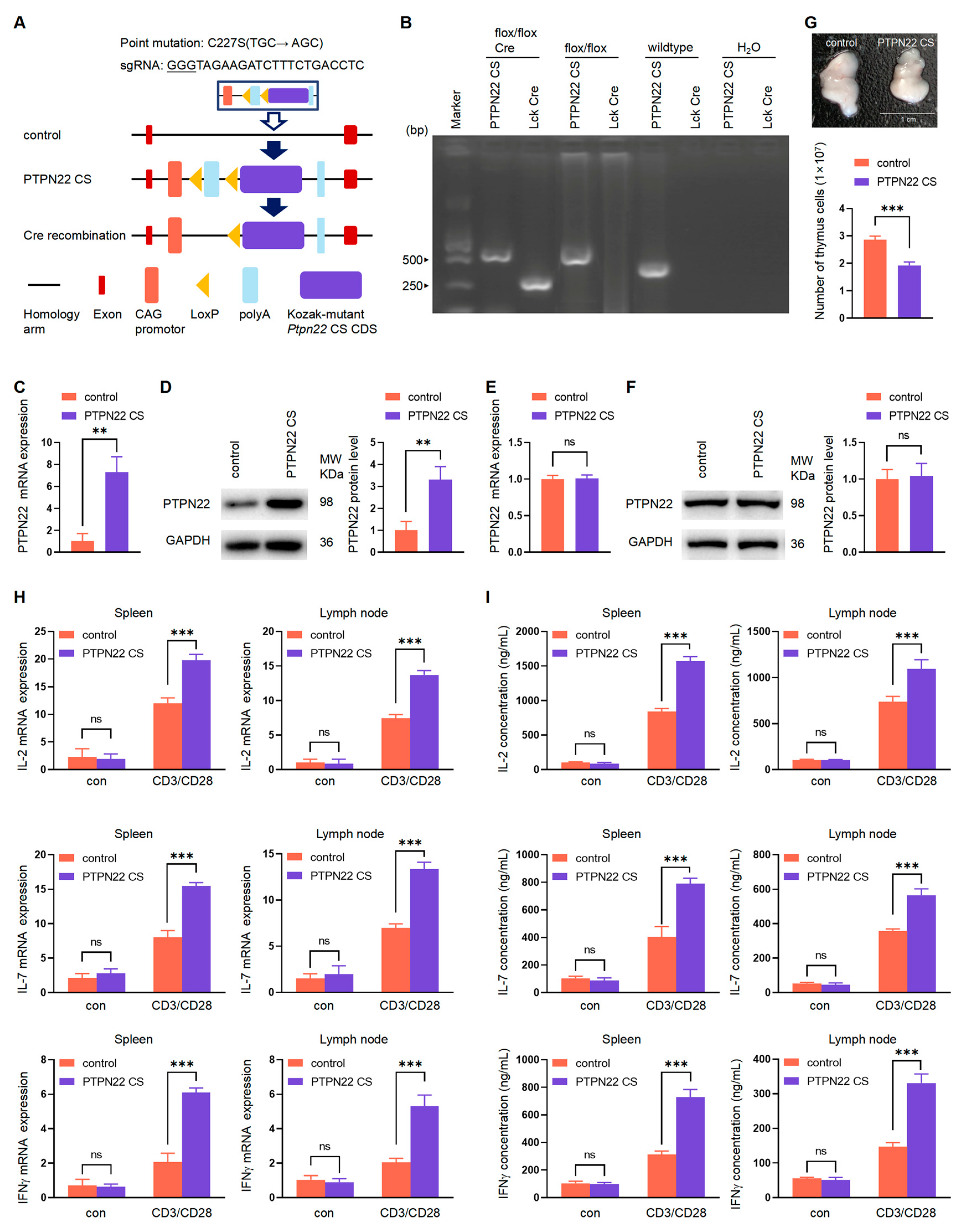
2.1. Production of the PTPN22 CS Transgenic Mice
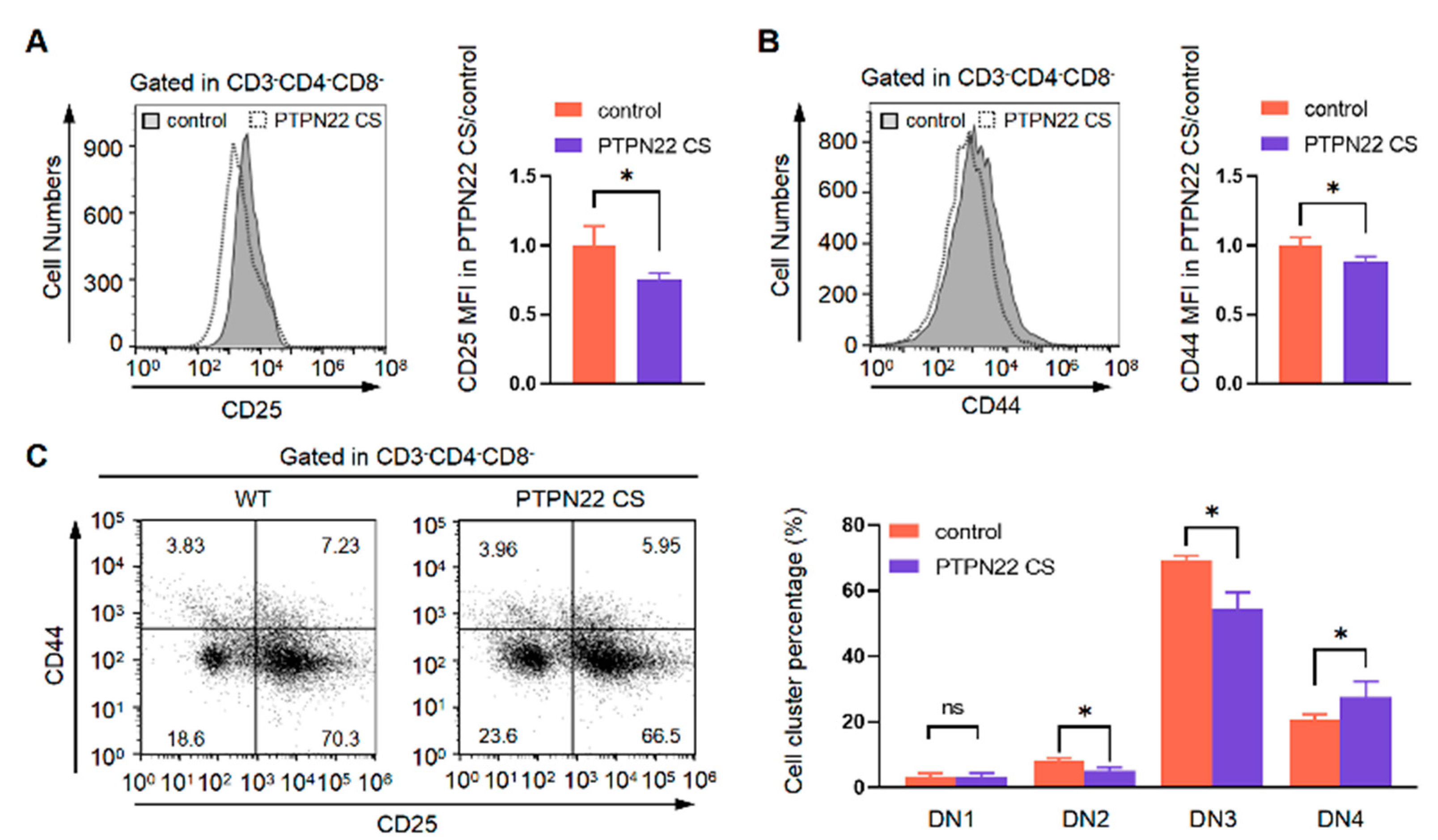
2.2. PTPN22 CS Promotes the Development of DN Cells
2.3. PTPN22 CS Promotes the Development of DP Cells
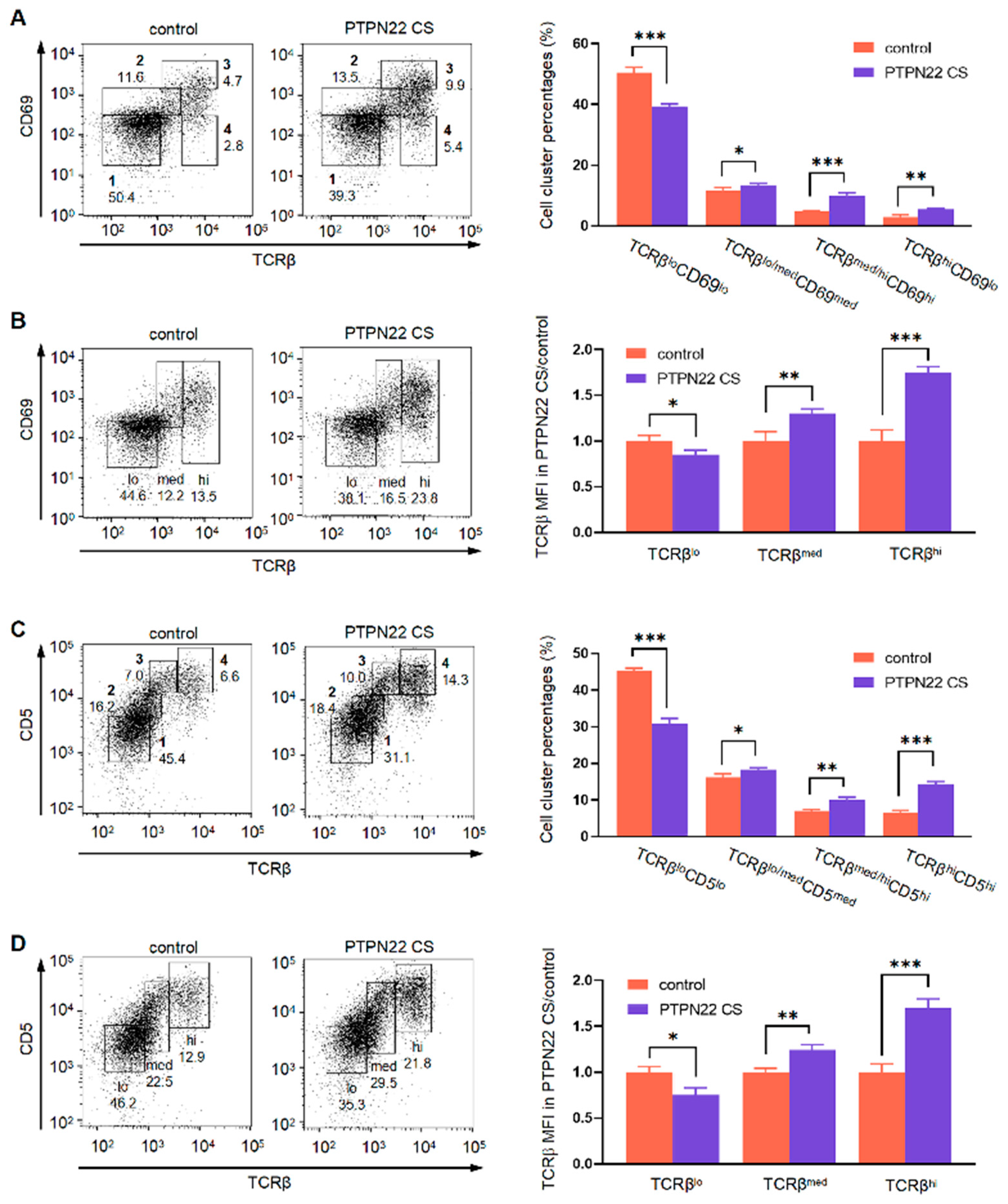
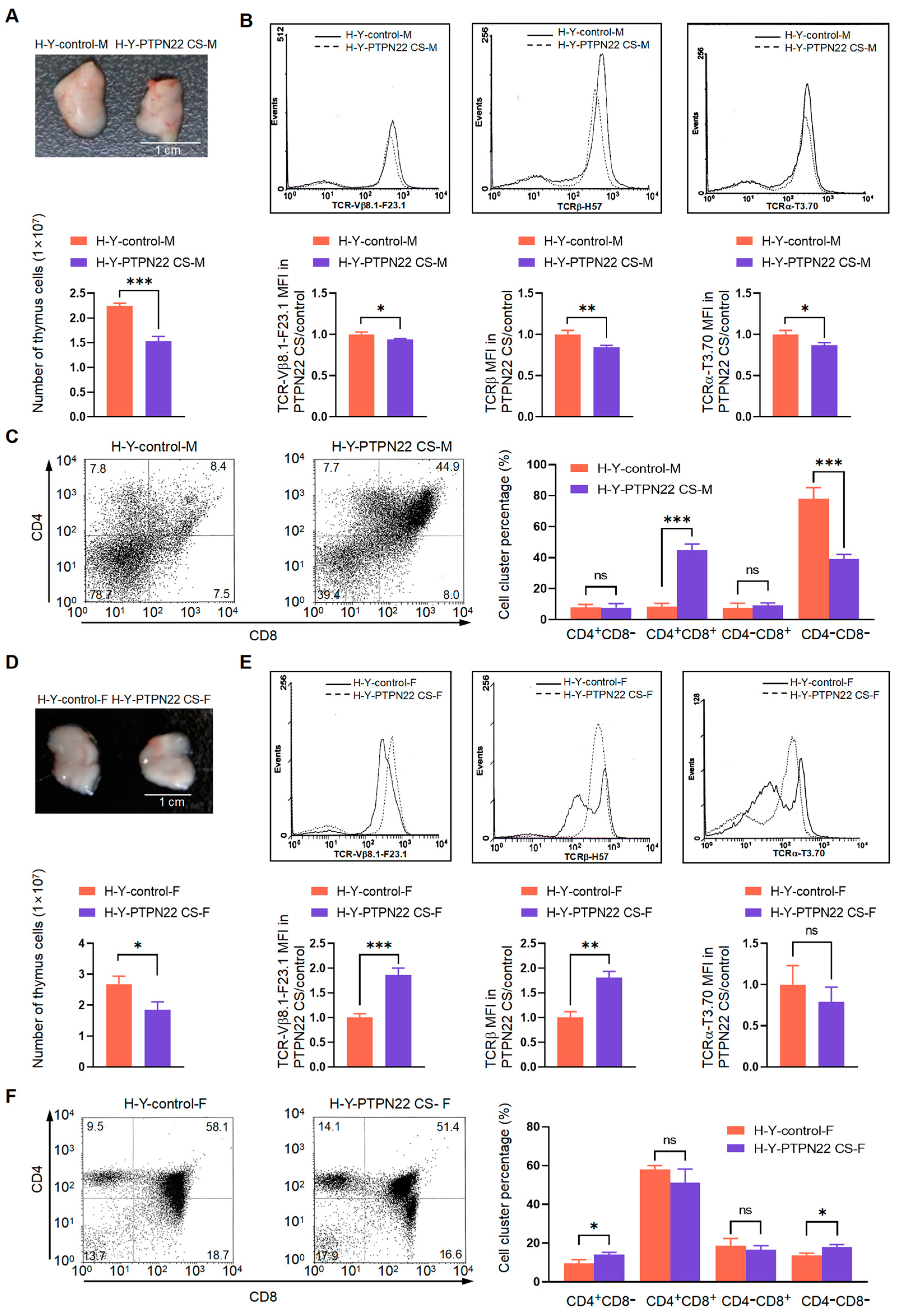
2.4. PTPN22 CS Promotes the Expression of TCRβmed/hi and CD3med/hi on T Cell Surface
2.5. PTPN22 CS Promotes the Positive and Negative Selection of T Cells
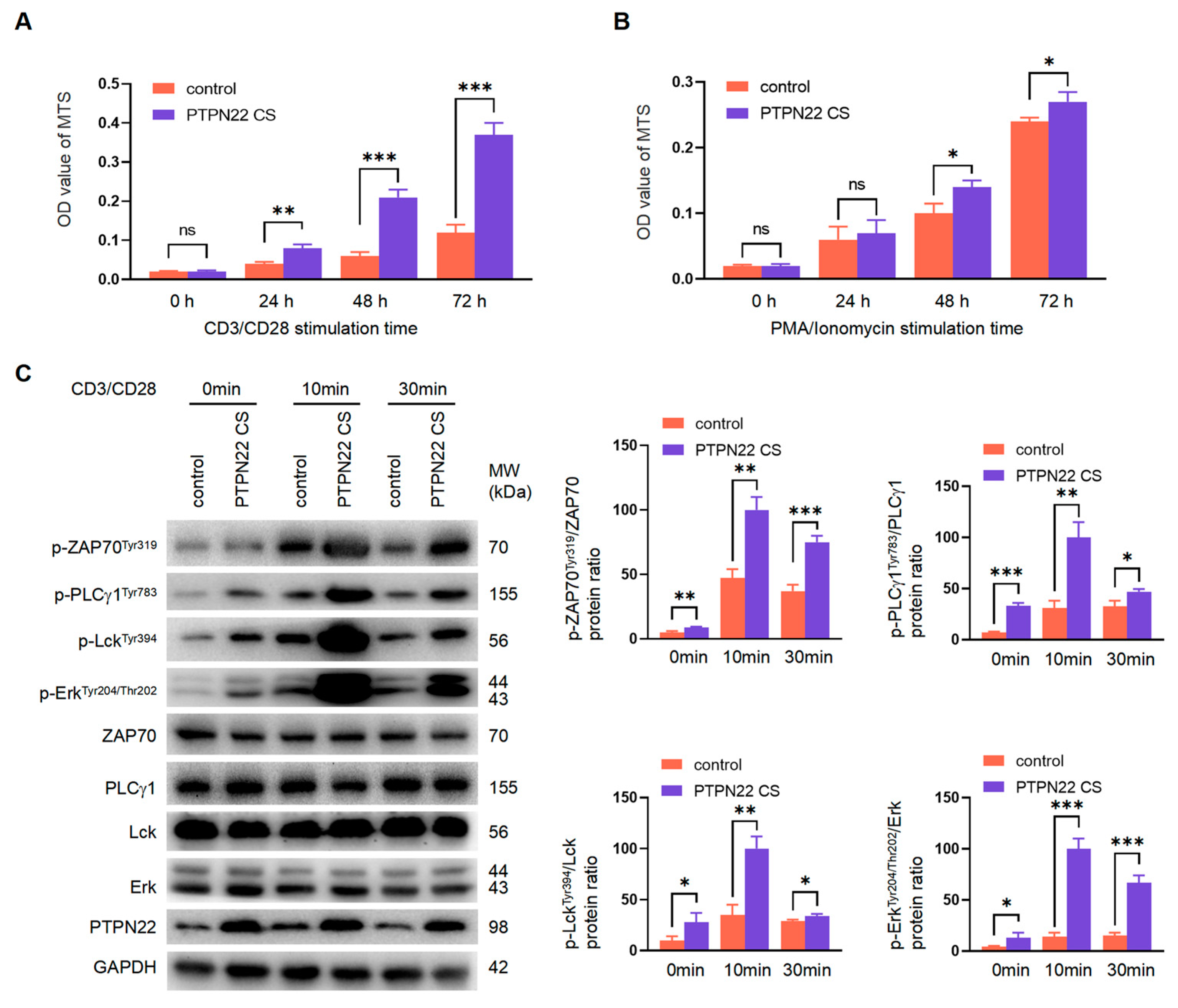
2.6. PTPN22 CS Regulates TCR Internalization and Recycling
2.7. PTPN22 CS Regulates T Cell Development Depending on the Upstream TCR Signaling Pathway
3. Discussion
4. Materials and Methods
4.1. Mice
4.2. Cell Culture
4.3. Antibodies
4.4. Flow Cytometry
4.5. Proliferation Assays
4.6. RNA Isolation and Quantitative Real-Time PCR (RT-PCR)
4.7. Western Blotting
4.8. Enzyme-Linked Immunosorbent Assay
4.9. Internalization and Recycling Assays Using Flow Cytometry
4.10. Statistical Analysis
5. Conclusions
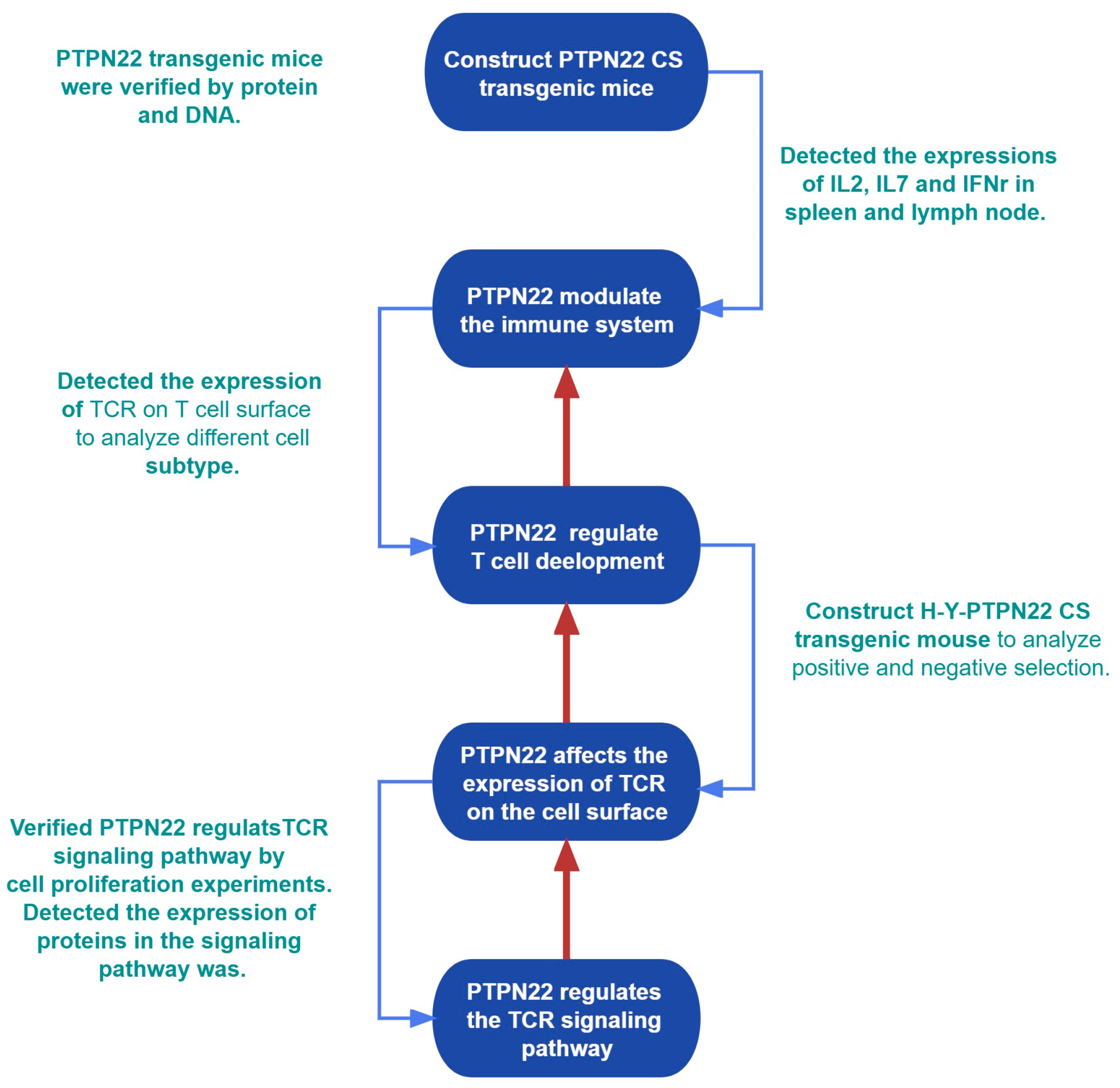
Study Scheme
- a)
- Constructed PTPN22 CS transgenic mice (CRISPR-Cas9 technology); verify the expression of protein and RNA of PTPN22 CS (Western blot, RT-PCR); detect the regulation of PTPN22 CS on thymus and peripheral immune organs (Flow cytometry; RT-PCR; ELISA).
- b)
- PTPN22 CS regulates T cell development. This process is defined by the expression of TCR on the surface of T cells (Flow cytometry).
- c)
- Study the causes of changes in TCR expression on the T cell surface (flow cytometry, Western blot).
- d)
- Study the signal pathway on which PTPN22 regulates the internalization and recycling of TCR (MTS, Western blot).
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Godfrey, D.I.; Kennedy, J.; Suda, T.; Zlotnik, A. Pillars article: A developmental pathway involving four phenotypically and functionally distinct subsets of CD3-CD4-CD8- triple-negative adult mouse thymocytes defined by CD44 and CD25 expression. J. Immunol. 1993, 189, 4203–4211. [Google Scholar]
- Hwang, J.R.; Byeon, Y.; Kim, D.; Park, S.G. Recent insights of T cell receptor-mediated signaling pathways for T cell activation and development. Exp. Mol. Med. 2020, 52, 750–761. [Google Scholar] [CrossRef] [PubMed]
- Falk, I.; Nerz, G.; Haidl, I.; Krotkova, A.; Eichmann, K. Immature thymocytes that fail to express TCRbeta and/or TCRgamma delta proteins die by apoptotic cell death in the CD44(-)CD25(-) (DN4) subset. Eur. J. Immunol. 2001, 31, 3308–3317. [Google Scholar] [CrossRef] [PubMed]
- Bluestone, J.A.; Pardoll, D.; Sharrow, S.O.; Fowlkes, B.J. BJF Characterization of murine thymocytes with CD3-associated T-cell receptor structures. Nature 1987, 326, 82–84. [Google Scholar] [CrossRef]
- Wang, H.; Holst, J.; Woo, S.R.; Guy, C.; Bettini, M.; Wang, Y.; Shafer, A.; Naramura, M.; Mingueneau, M.; Dragone, L.L.; et al. Tonic ubiquitylation controls T-cell receptor:CD3 complex expression during T-cell development. EMBO J. 2010, 29, 1285–1298. [Google Scholar] [CrossRef] [PubMed]
- Palmer, E.; Naeher, D. Affinity threshold for thymic selection through a T-cell receptor-co-receptor zipper. Nat. Rev. Immunol. 2009, 9, 207–213. [Google Scholar] [CrossRef]
- Fu, G.; Casas, J.; Rigaud, S.; Rybakin, V.; Lambolez, F.; Brzostek, J.; Hoerter, J.A.; Paster, W.; Acuto, O.; Cheroutre, H.; et al. Themis sets the signal threshold for positive and negative selection in T-cell development. Nature 2013, 504, 441–445. [Google Scholar] [CrossRef]
- Palacios, E.H.; Weiss, A. Function of the Src-family kinases, Lck and Fyn, in T-cell development and activation. Oncogene 2004, 23, 7990–8000. [Google Scholar] [CrossRef]
- Salmond, R.J.; Filby, A.; Qureshi, I.; Caserta, S.; Zamoyska, R. T-cell receptor proximal signaling via the Src-family kinases, Lck and Fyn, influences T-cell activation, differentiation, and tolerance. Immunol. Rev. 2009, 228, 9–22. [Google Scholar] [CrossRef]
- Daniels, M.A.; Teixeiro, E.; Gill, J.; Hausmann, B.; Roubaty, D.; Holmberg, K.; Werlen, G.; Holländer, G.A.; Gascoigne, N.R.; Palmer, E. Thymic selection threshold defined by compartmentalization of Ras/MAPK signalling. Nature 2006, 444, 724–729. [Google Scholar] [CrossRef]
- Lo, W.L.; Shah, N.H.; Rubin, S.A.; Zhang, W.; Horkova, V.; Fallahee, I.R.; Stepanek, O.; Zon, L.I.; Kuriyan, J.; Weiss, A. Slow phosphorylation of a tyrosine residue in LAT optimizes T cell ligand discrimination. Nat. Immunol. 2019, 20, 1481–1493. [Google Scholar] [CrossRef]
- Alcover, A.; Alarcon, B.; Di Bartolo, V. Cell Biology of T Cell Receptor Expression and Regulation. Annu. Rev. Immunol. 2018, 36, 103–125. [Google Scholar] [CrossRef]
- Alcover, A.; Alarcon, B. Internalization and intracellular fate of TCR-CD3 complexes. Crit. Rev. Immunol. 2000, 20, 325–346. [Google Scholar] [CrossRef] [PubMed]
- Onnis, A.; Baldari, C.T. Orchestration of Immunological Synapse Assembly by Vesicular Trafficking. Front. Cell Dev. Biol. 2019, 7, 110. [Google Scholar] [CrossRef] [PubMed]
- Cloutier, J.F.; Veillette, A. Association of inhibitory tyrosine protein kinase p50csk with protein tyrosine phosphatase PEP in T cells and other hemopoietic cells. EMBO J. 1996, 15, 4909–4918. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.; Dadi, H.; Shaoul, E.; Sharfe, N.; Roifman, C.M. Cloning and Characterization of a Lymphoid-Specific, Inducible Human Protein Tyrosine Phosphatase, Lyp. Blood 1999, 93, 2013–2024. [Google Scholar] [CrossRef]
- Cloutier, J.F.; Veillette, A. Cooperative inhibition of T-cell antigen receptor signaling by a complex between a kinase and a phosphatase. J. Exp. Med. 1999, 189, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Katrekar, A.; Honigberg, L.A.; Smith, A.M.; Conn, M.T.; Tang, J.; Jeffery, D.; Mortara, K.; Sampang, J.; Williams, S.R.; et al. Identification of substrates of human protein-tyrosine phosphatase PTPN22. J. Biol. Chem. 2006, 281, 11002–11010. [Google Scholar] [CrossRef]
- Zhang, X.; Bai, B.; Wang, T.; Zhao, J.; Zhang, N.; Zhao, Y.; Wang, X.; Yu, Y.; Wang, B. PTPN22 interacts with EB1 to regulate T-cell receptor signaling. FASEB J. 2020, 34, 8959–8974. [Google Scholar] [CrossRef]
- Tizaoui, K.; Kim, S.H.; Jeong, G.H.; Kronbichler, A.; Lee, K.S.; Lee, K.H.; Shin, J.I. Association of PTPN22 1858C/T Polymorphism with Autoimmune Diseases, A Systematic Review and Bayesian Approach. J. Clin. Med. 2019, 8, 347. [Google Scholar] [CrossRef]
- Crabtree, J.N.; He, W.; Guan, W.; Flage, M.; Miller, M.S.; Peterson, E.J. Autoimmune Variant PTPN22 C1858T Is Associated With Impaired Responses to Influenza Vaccination. J. Infect. Dis. 2016, 214, 248–257. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zahir, N.; Jiang, Q.; Miliotis, H.; Heyraud, S.; Meng, X.; Dong, B.; Xie, G.; Qiu, F.; Hao, Z.; et al. The autoimmune disease-associated PTPN22 variant promotes calpain-mediated Lyp/Pep degradation associated with lymphocyte and dendritic cell hyperresponsiveness. Nat. Genet. 2011, 43, 902–907. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, K.; Martin, F.; Huang, G.; Tumas, D.; Diehl, L.; Chan, A.C. PEST domain-enriched tyrosine phosphatase (PEP) regulation of effector/memory T cells. Science 2004, 303, 685–689. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.I.; Martin, D.R.; Fung-Leung, W.P.; Teh, H.S.; Miller, R.G. Peripheral deletion of mature CD8+ antigen-specific T cells after in vivo exposure to male antigen. J. Immunol. 1992, 148, 3740–3745. [Google Scholar] [CrossRef] [PubMed]
- Van Ewijk, W.K.P.; Von Boehmer, H. Immunohistology of T cell differentiation in the thymus of H-Y-specific T cell receptor alpha/beta transgenic mice. Eur. J. Immunol. 1990, 20, 129–137. [Google Scholar] [CrossRef]
- Han, S.J.; Lee, J.H.; Kim, C.G.; Hong, S.H. Identification of p115 as a PLCgamma1-binding protein and the role of Src homology domains of PLCgamma1 in the vesicular transport. Biochem. Biophys. Res. Commun. 2003, 300, 649–655. [Google Scholar] [CrossRef]
- Wang, B.; Lemay, S.; Tsai, S.; Veillette, A. SH2 domain-mediated interaction of inhibitory protein tyrosine kinase Csk with protein tyrosine, phosphatase-HSCF. Mol. Cell. Biol. 2001, 21, 1077–1088. [Google Scholar] [CrossRef]
- Veillette, A.; Rhee, I.; Souza, C.M.; Davidson, D. PEST family phosphatases in immunity, autoimmunity, and autoinflammatory disorders. Immunol. Rev. 2009, 228, 312–324. [Google Scholar] [CrossRef]
- Fiala, G.J.; Schaffer, A.M.; Merches, K.; Morath, A.; Swann, J.; Herr, L.A.; Hils, M.; Esser, C.; Minguet, S.; Schamel, W.W. Proximal Lck Promoter-Driven Cre Function Is Limited in Neonatal and Ineffective in Adult gammadelta T Cell Development. J. Immunol. 2019, 203, 569–579. [Google Scholar] [CrossRef]
- Allen, J.M.; Forbush, K.A.; Perlmutter, R.M. Functional dissection of the lck proximal promoter. Mol. Cell. Biol. 1992, 12, 2758–2768. [Google Scholar]
- Takahama, Y. Journey through the thymus, stromal guides for T-cell development and selection. Nat. Rev. Immunol. 2006, 6, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J. T Helper Cell Differentiation, Heterogeneity, and Plasticity. Cold Spring Harb. Perspect. Biol. 2018, 10, a030338. [Google Scholar] [CrossRef] [PubMed]
- Shao, F.; Zheng, P.; Yu, D.; Zhou, Z.; Jia, L. Follicular helper T cells in type 1 diabetes. FASEB J. 2020, 34, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Subrahmanyam, P.B.; Maecker, H.T. Mass Cytometry Analysis of T-Helper Cells. Methods Mol. Biol. 2021, 2285, 49–63. [Google Scholar]
- von Boehmer, H.; Teh, H.S.; Kisielow, P. The thymus selects the useful, neglects the useless and destroys the harmful. Immunol. Today 1989, 10, 57–61. [Google Scholar] [CrossRef]
- Irla, M. Instructive Cues of Thymic T Cell Selection. Annu. Rev. Immunol. 2022, 40, 95–119. [Google Scholar] [CrossRef]
- Evnouchidou, I.; Caillens, V.; Koumantou, D.; Saveanu, L. The role of endocytic trafficking in antigen T cell receptor activation. Biomed. J. 2022, 45, 310–320. [Google Scholar] [CrossRef]
- Han, S.J.; Lee, J.H.; Hong, S.H.; Dai Park, S.; Kim, C.G.; Song, M.D.; Park, T.K.; Kim, C.G. AP180 binds to the C-terminal SH2 domain of phospholipase C-gamma1 and inhibits its enzymatic activity. Biochem. Biophys. Res. Commun. 2002, 290, 35–41. [Google Scholar] [CrossRef]
- Rouquette-Jazdanian, A.K.; Sommers, C.L.; Kortum, R.L.; Morrison, D.K.; Samelson, L.E. LAT-independent Erk activation via Bam32-PLC-gamma1-Pak1 complexes, GTPase-independent Pak1 activation. Mol. Cell 2012, 48, 298–312. [Google Scholar] [CrossRef]
- Chen, X.; Li, X.M.; Gu, W.; Wang, D.; Chen, Y.; Guo, X.J. LAT alleviates Th2/Treg imbalance in an OVA-induced allergic asthma mouse model through LAT-PLC-γ1 interaction. Int. Immunopharmacol. 2017, 44, 9–15. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bai, B.; Li, T.; Zhao, J.; Zhao, Y.; Zhang, X.; Wang, T.; Zhang, N.; Wang, X.; Ba, X.; Xu, J.; et al. The Tyrosine Phosphatase Activity of PTPN22 Is Involved in T Cell Development via the Regulation of TCR Expression. Int. J. Mol. Sci. 2023, 24, 14505. https://doi.org/10.3390/ijms241914505
Bai B, Li T, Zhao J, Zhao Y, Zhang X, Wang T, Zhang N, Wang X, Ba X, Xu J, et al. The Tyrosine Phosphatase Activity of PTPN22 Is Involved in T Cell Development via the Regulation of TCR Expression. International Journal of Molecular Sciences. 2023; 24(19):14505. https://doi.org/10.3390/ijms241914505
Chicago/Turabian StyleBai, Bin, Tong Li, Jiahui Zhao, Yanjiao Zhao, Xiaonan Zhang, Tao Wang, Na Zhang, Xipeng Wang, Xinlei Ba, Jialin Xu, and et al. 2023. "The Tyrosine Phosphatase Activity of PTPN22 Is Involved in T Cell Development via the Regulation of TCR Expression" International Journal of Molecular Sciences 24, no. 19: 14505. https://doi.org/10.3390/ijms241914505
APA StyleBai, B., Li, T., Zhao, J., Zhao, Y., Zhang, X., Wang, T., Zhang, N., Wang, X., Ba, X., Xu, J., Yu, Y., & Wang, B. (2023). The Tyrosine Phosphatase Activity of PTPN22 Is Involved in T Cell Development via the Regulation of TCR Expression. International Journal of Molecular Sciences, 24(19), 14505. https://doi.org/10.3390/ijms241914505




