MiR-148a-3p within HucMSC-Derived Extracellular Vesicles Suppresses Hsp90b1 to Prevent Fibroblast Collagen Synthesis and Secretion in Silica-Induced Pulmonary Fibrosis
Abstract
:1. Introduction
2. Results
2.1. Cultivation of hucMSCs and Identification of hucMSC-EVs
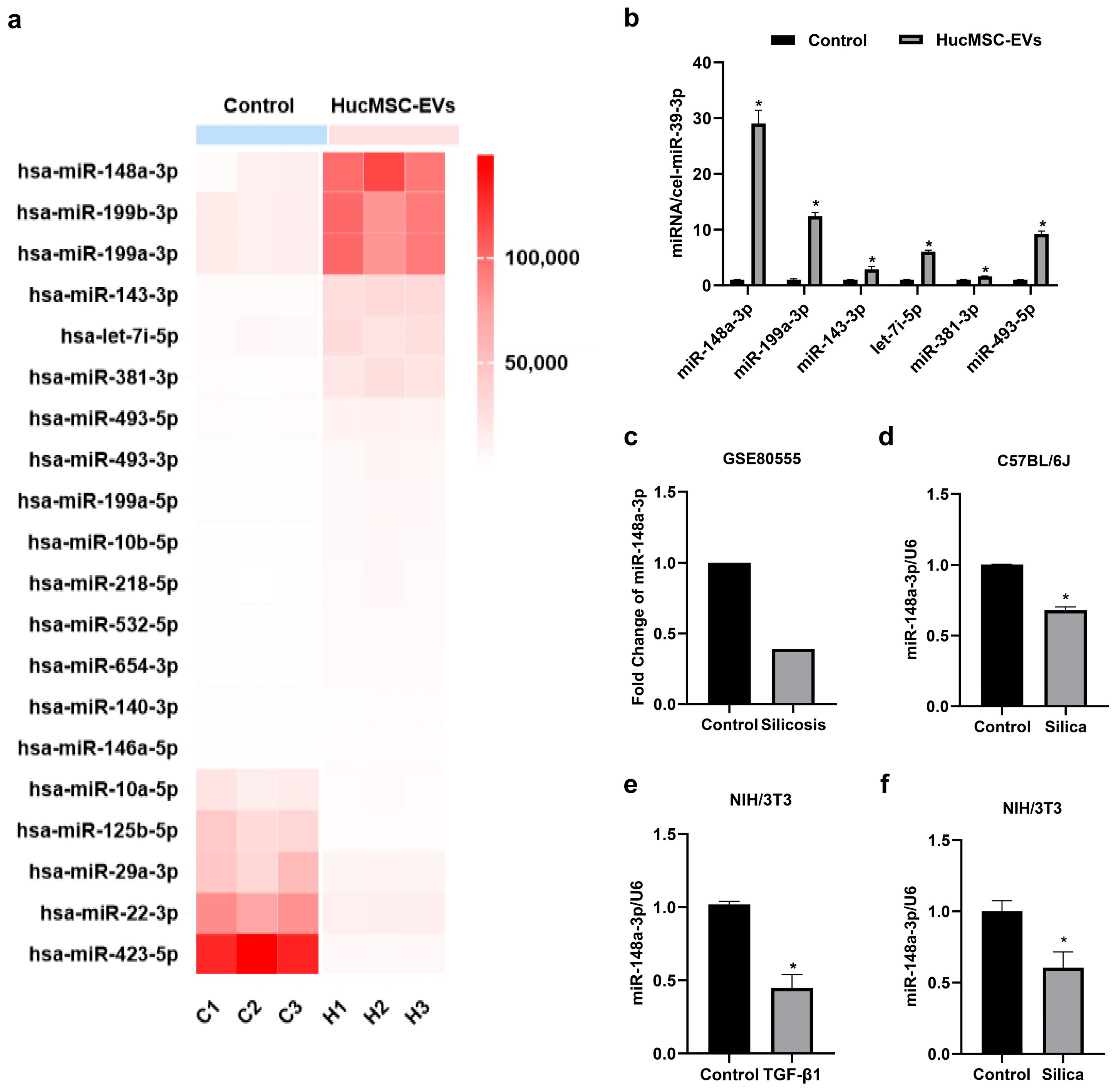
2.2. MiR-148a-3p Was Highly Expressed in hucMSC-EVs and Downregulated in Silica-Induced Pulmonary Fibrosis In Vitro and In Vivo
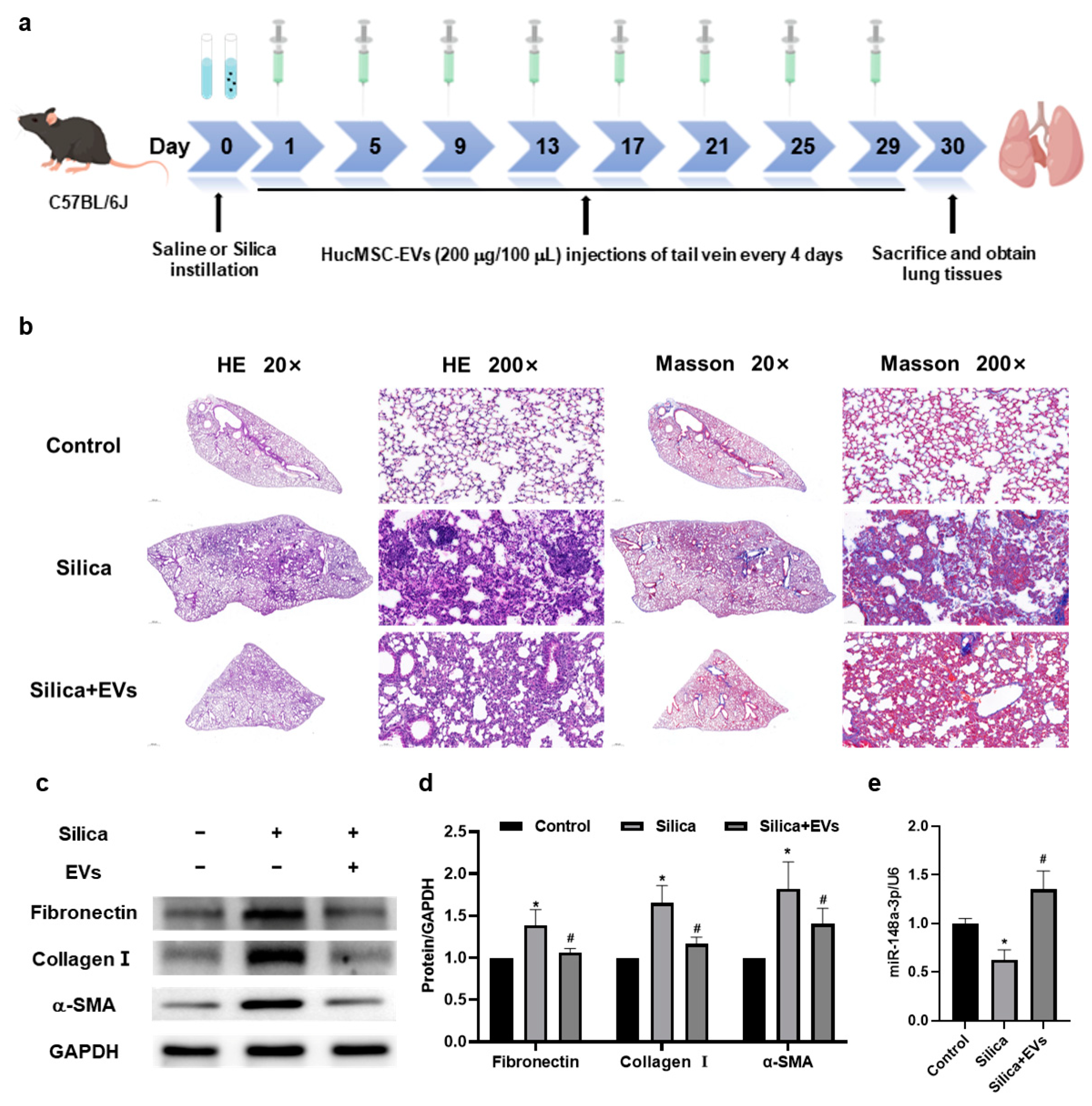
2.3. MiR-148a-3p Related to hucMSC-EVs’ Inhibition of Silica-Induced Pulmonary Fibrosis in Mice
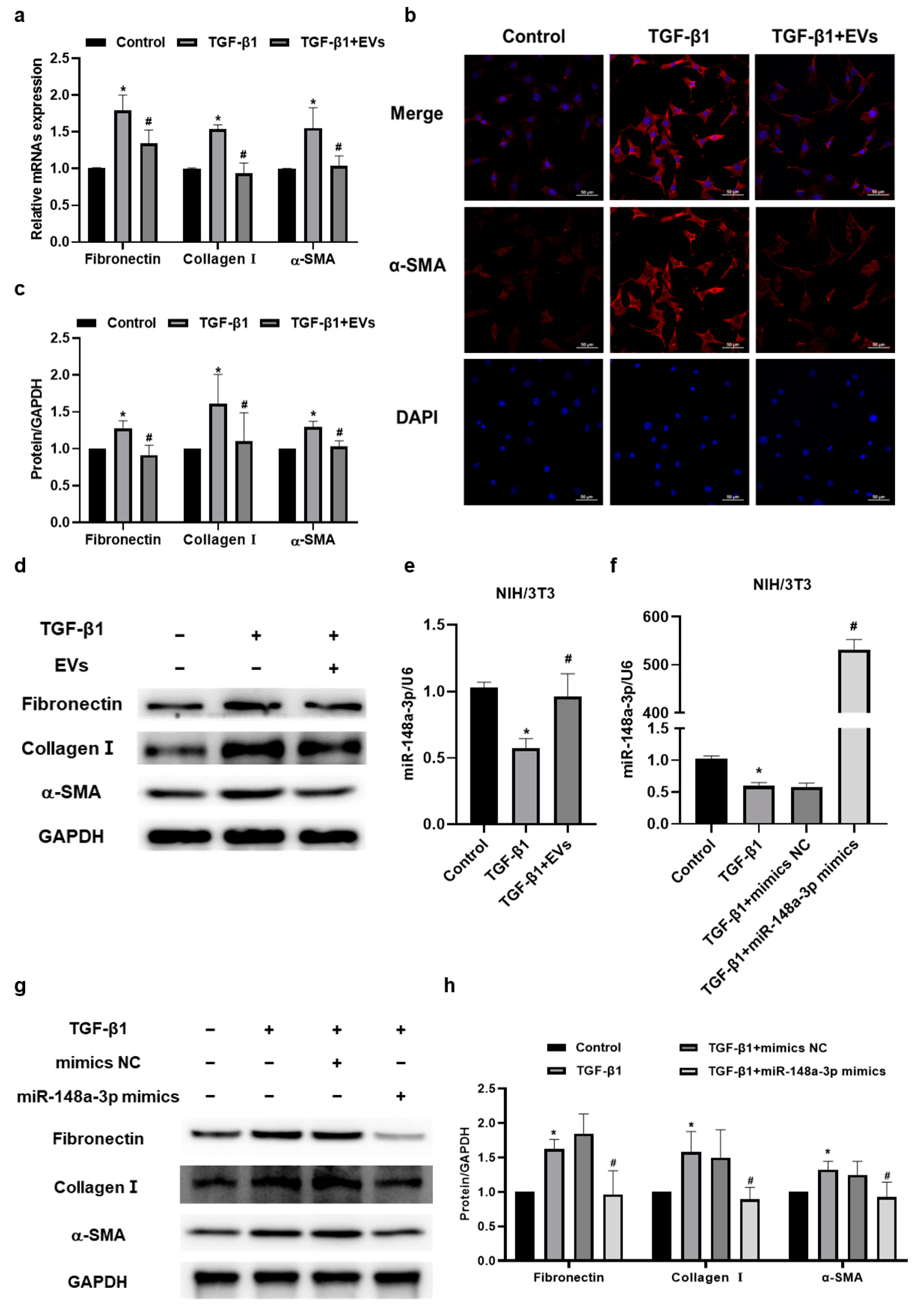
2.4. MiR-148a-3p in hucMSC-EVs Suppressed TGF-β1-Induced Collagen Synthesis and Secretion in NIH/3T3 Cells
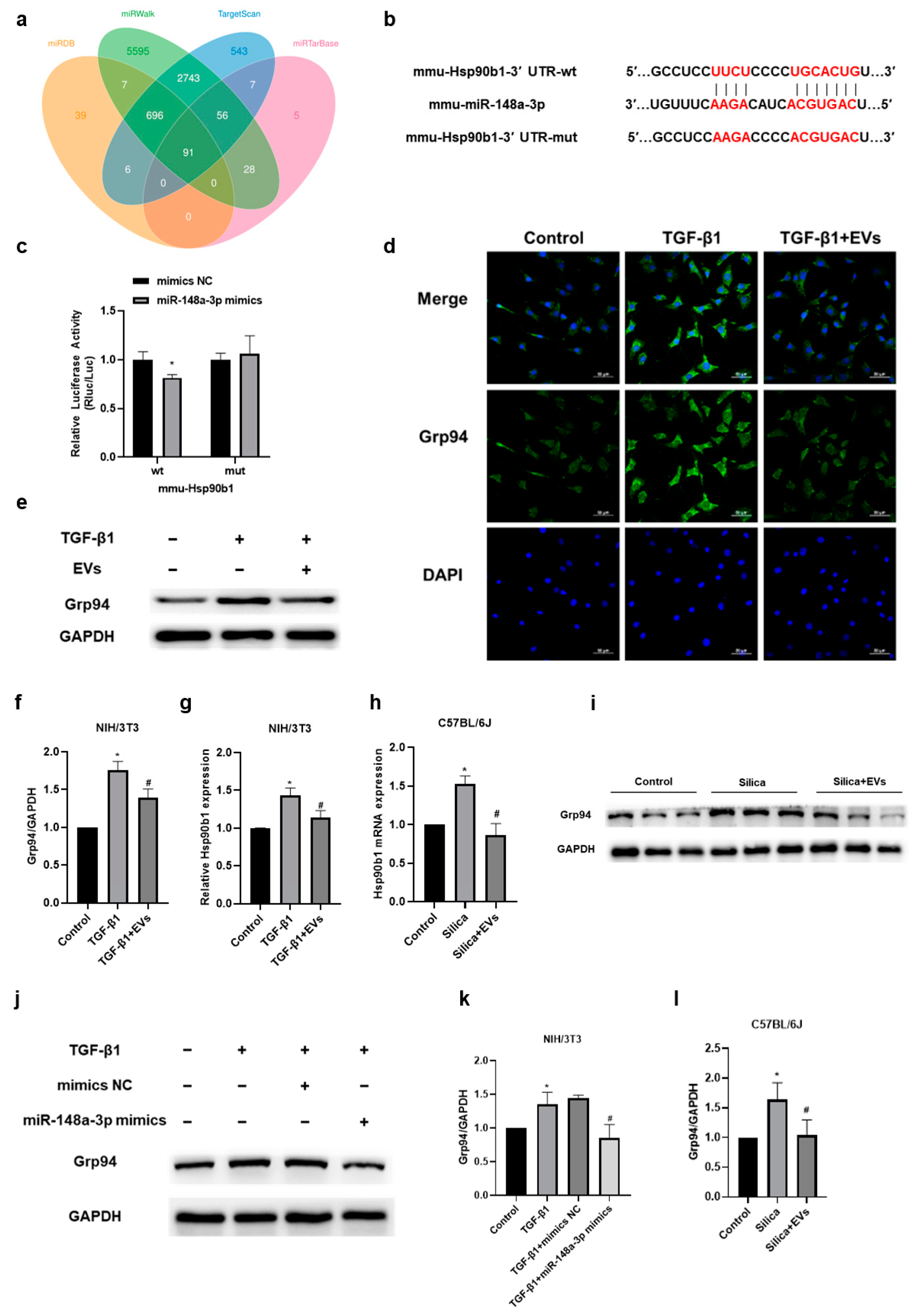
2.5. The Amelioratory Effect of hucMSC-EVs on Silica-Induced Pulmonary Fibrosis by Targeting Hsp90b1 via miR-148a-3p
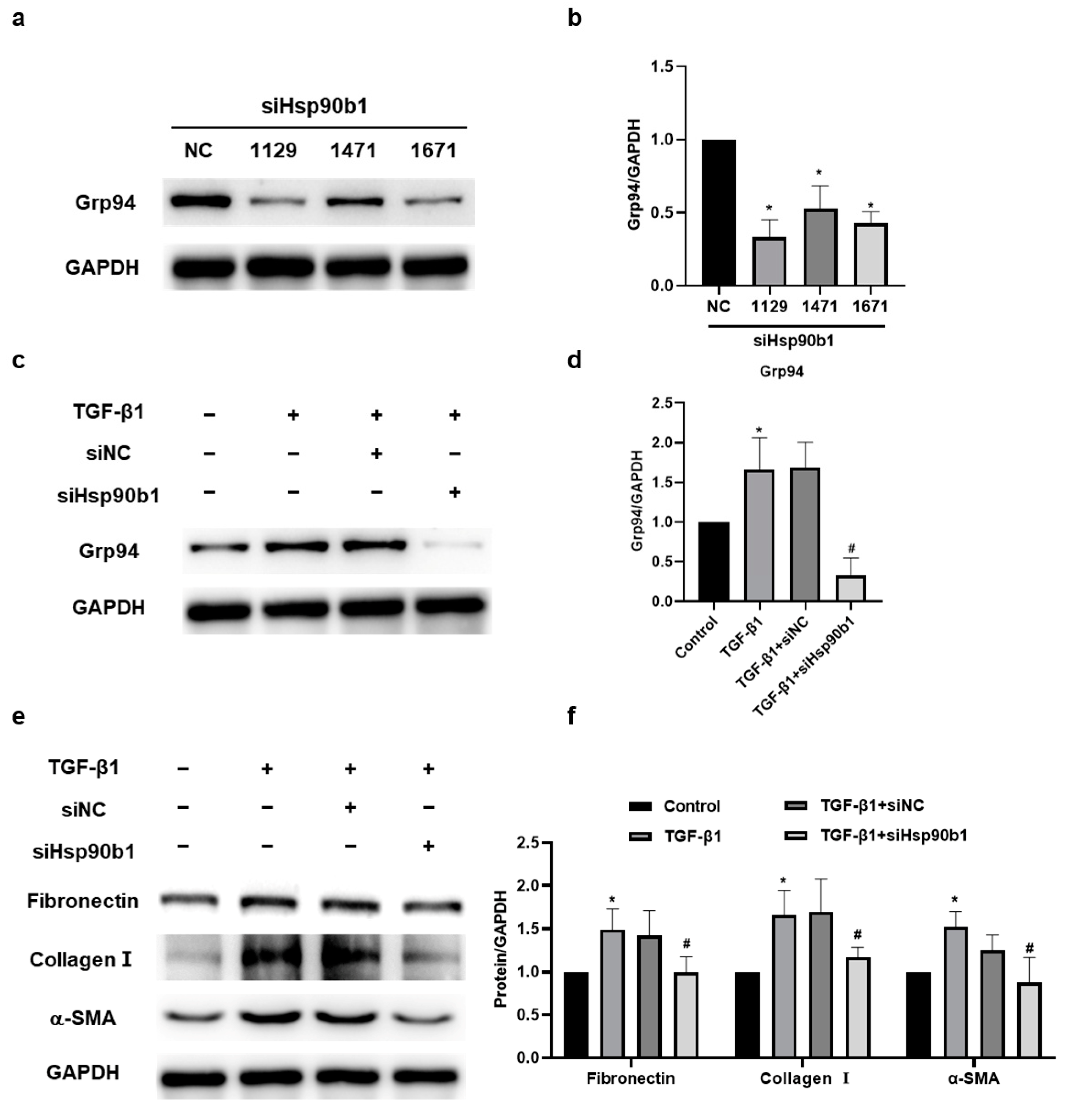
2.6. The Inhibition of Hsp90b1 Suppresses Fibrosis-Related Proteins in NIH/3T3 Cells
3. Discussion
4. Materials and Methods
4.1. HucMSCs Culture and hucMSC-EV Isolation and Identification
4.2. Animal Models
4.3. Histopathology
4.4. NIH/3T3 Cell Culture and Treatment
4.5. In Vitro Tracking
4.6. Western Blotting Analysis
4.7. RNA Isolation and Quantitative Real-Time PCR (qRT-PCR) Analysis
4.8. Immunofluorescence
4.9. Dual-Luciferase Reporter Gene Assay
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| α-SMA | alpha-smooth muscle actin |
| ECM | extracellular matrix |
| EVs | extracellular vesicles |
| FBS | fetal bovine serum |
| HE | hematoxylin and eosin |
| HRP | horse radish peroxidase |
| Hsp90 | heat shock protein 90 |
| Hsp90b1 | heat shock protein 90 beta family member 1 |
| HucMSCs | human umbilical cord mesenchymal stem cells |
| HucMSC-EVs | human umbilical cord mesenchymal stem cell-derived extracellular vesicles |
| MSCs | mesenchymal stem cells |
| MSC-EVs | mesenchymal stem cell-derived extracellular vesicles |
| MiRNA | microRNA |
| MRC-5-EVs | human embryonic lung fibroblast-derived extracellular vesicles |
| Mut | mutant |
| NIH/3T3 cell | murine fibroblast cell line |
| NTA | Nanoparticle Tracking Analysis |
| qRT-PCR | quantitative real-time polymerase chain reaction |
| SiRNAs | small interfering RNAs |
| TEM | Transmission Electron Microscope |
| TGF-β1 | transforming growth factor-β1 |
| Wt | wild-type |
| 3D | three-dimensional |
| 3′ UTR | 3′ untranslated region |
References
- Leung, C.C.; Yu, I.T.; Chen, W. Silicosis. Lancet 2012, 379, 2008–2018. [Google Scholar] [CrossRef] [PubMed]
- Hao, X.; Jin, Y.; Zhang, Y.; Li, S.; Cui, J.; He, H.; Guo, L.; Yang, F.; Liu, H. Inhibition of Oncogenic Src Ameliorates Silica-Induced Pulmonary Fibrosis via PI3K/AKT Pathway. Int. J. Mol. Sci. 2023, 24, 774. [Google Scholar] [CrossRef]
- Lian, X.; Chen, X.; Sun, J.; An, G.; Li, X.; Wang, Y.; Niu, P.; Zhu, Z.; Tian, L. MicroRNA-29b inhibits supernatants from silica-treated macrophages from inducing extracellular matrix synthesis in lung fibroblasts. Toxicol. Res. 2017, 6, 878–888. [Google Scholar] [CrossRef] [PubMed]
- Chanda, D.; Otoupalova, E.; Smith, S.R.; Volckaert, T.; De Langhe, S.P.; Thannickal, V.J. Developmental pathways in the pathogenesis of lung fibrosis. Mol. Asp. Med. 2019, 65, 56–69. [Google Scholar] [CrossRef] [PubMed]
- Pakshir, P.; Hinz, B. The big five in fibrosis: Macrophages, myofibroblasts, matrix, mechanics, and miscommunication. Matrix Biol. 2018, 68–69, 81–93. [Google Scholar] [CrossRef]
- Calabrese, F.; Lunardi, F.; Tauro, V.; Pezzuto, F.; Fortarezza, F.; Vedovelli, L.; Faccioli, E.; Balestro, E.; Schiavon, M.; Esposito, G.; et al. RNA Sequencing of Epithelial Cell/Fibroblastic Foci Sandwich in Idiopathic Pulmonary Fibrosis: New Insights on the Signaling Pathway. Int. J. Mol. Sci. 2022, 23, 3323. [Google Scholar] [CrossRef]
- Cheng, D.; Wang, Y.; Li, Z.; Xiong, H.; Sun, W.; Xi, S.; Zhou, S.; Liu, Y.; Ni, C. Liposomal UHRF1 siRNA shows lung fibrosis treatment potential through regulation of fibroblast activation. JCI Insight 2022, 7, e162831. [Google Scholar] [CrossRef]
- Richeldi, L.; Collard, H.R.; Jones, M.G. Idiopathic pulmonary fibrosis. Lancet 2017, 389, 1941–1952. [Google Scholar] [CrossRef]
- Kardia, E.; Zakaria, N.; Sarmiza Abdul Halim, N.S.; Widera, D.; Yahaya, B.H. The use of mesenchymal stromal cells in treatment of lung disorders. Regen. Med. 2017, 12, 203–216. [Google Scholar] [CrossRef]
- Geiger, S.; Hirsch, D.; Hermann, F.G. Cell therapy for lung disease. Eur. Respir. Rev. 2017, 26, 170044. [Google Scholar] [CrossRef]
- Harrell, C.R.; Sadikot, R.; Pascual, J.; Fellabaum, C.; Jankovic, M.G.; Jovicic, N.; Djonov, V.; Arsenijevic, N.; Volarevic, V. Mesenchymal Stem Cell-Based Therapy of Inflammatory Lung Diseases: Current Understanding and Future Perspectives. Stem Cells Int. 2019, 2019, 4236973. [Google Scholar] [CrossRef] [PubMed]
- Konala, V.B.; Mamidi, M.K.; Bhonde, R.; Das, A.K.; Pochampally, R.; Pal, R. The current landscape of the mesenchymal stromal cell secretome: A new paradigm for cell-free regeneration. Cytotherapy 2016, 18, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Yang, J.; Fang, J.; Zhou, Y.; Candi, E.; Wang, J.; Hua, D.; Shao, C.; Shi, Y. The secretion profile of mesenchymal stem cells and potential applications in treating human diseases. Signal Transduct. Target Ther. 2022, 7, 92. [Google Scholar]
- Keshtkar, S.; Azarpira, N.; Ghahremani, M.H. Mesenchymal stem cell-derived extracellular vesicles: Novel frontiers in regenerative medicine. Stem Cell Res. Ther. 2018, 9, 63. [Google Scholar] [CrossRef]
- Vizoso, F.J.; Eiro, N.; Cid, S.; Schneider, J.; Perez-Fernandez, R. Mesenchymal Stem Cell Secretome: Toward Cell-Free Therapeutic Strategies in Regenerative Medicine. Int. J. Mol. Sci. 2017, 18, 1852. [Google Scholar] [CrossRef]
- Diener, C.; Keller, A.; Meese, E. Emerging concepts of miRNA therapeutics: From cells to clinic. Trends Genet. 2022, 38, 613–626. [Google Scholar] [CrossRef]
- Kilikevicius, A.; Meister, G.; Corey, D.R. Reexamining assumptions about miRNA-guided gene silencing. Nucleic Acids Res. 2022, 50, 617–634. [Google Scholar] [CrossRef]
- Wan, X.; Chen, S.; Fang, Y.; Zuo, W.; Cui, J.; Xie, S. Mesenchymal stem cell-derived extracellular vesicles suppress the fibroblast proliferation by downregulating FZD6 expression in fibroblasts via micrRNA-29b-3p in idiopathic pulmonary fibrosis. J. Cell Physiol. 2020, 235, 8613–8625. [Google Scholar] [CrossRef]
- Kadota, T.; Fujita, Y.; Araya, J.; Watanabe, N.; Fujimoto, S.; Kawamoto, H.; Minagawa, S.; Hara, H.; Ohtsuka, T.; Yamamoto, Y.; et al. Human bronchial epithelial cell-derived extracellular vesicle therapy for pulmonary fibrosis via inhibition of TGF-β-WNT crosstalk. J. Extracell. Vesicles 2021, 10, e12124. [Google Scholar] [CrossRef]
- Xu, C.; Hou, L.; Zhao, J.; Wang, Y.; Jiang, F.; Jiang, Q.; Zhu, Z.; Tian, L. Exosomal let-7i-5p from three-dimensional cultured human umbilical cord mesenchymal stem cells inhibits fibroblast activation in silicosis through targeting TGFBR1. Ecotoxicol. Environ. Saf. 2022, 233, 113302. [Google Scholar] [CrossRef]
- Zhao, J.; Jiang, Q.; Xu, C.; Jia, Q.; Wang, H.; Xue, W.; Wang, Y.; Zhu, Z.; Tian, L. MiR-26a-5p from HucMSC-derived extracellular vesicles inhibits epithelial mesenchymal transition by targeting Adam17 in silica-induced lung fibrosis. Ecotoxicol. Environ. Saf. 2023, 257, 114950. [Google Scholar] [CrossRef]
- Tian, S.; Zhou, X.; Zhang, M.; Cui, L.; Li, B.; Liu, Y.; Su, R.; Sun, K.; Hu, Y.; Yang, F.; et al. Mesenchymal stem cell-derived exosomes protect against liver fibrosis via delivering miR-148a to target KLF6/STAT3 pathway in macrophages. Stem Cell Res. Ther. 2022, 13, 330. [Google Scholar] [CrossRef]
- Xiong, J.; Ni, J.; Chen, C.; Wang, K. miR-148a-3p regulates alcoholic liver fibrosis through targeting ERBB3. Int. J. Mol. Med. 2020, 46, 1003–1012. [Google Scholar] [CrossRef]
- Woo, S.J.; Kim, Y.; Jung, H.; Lee, J.J.; Hong, J.Y. MicroRNA 148a Suppresses Tuberculous Fibrosis by Targeting NOX4 and POLDIP2. Int. J. Mol. Sci. 2022, 23, 2999. [Google Scholar] [CrossRef]
- Chen, B.; Piel, W.H.; Gui, L.; Bruford, E.; Monteiro, A. The HSP90 family of genes in the human genome: Insights into their divergence and evolution. Genomics 2005, 86, 627–637. [Google Scholar] [CrossRef]
- Cabaud-Gibouin, V.; Durand, M.; Quéré, R.; Girodon, F.; Garrido, C.; Jego, G. Heat-Shock Proteins in Leukemia and Lymphoma: Multitargets for Innovative Therapeutic Approaches. Cancers 2023, 15, 984. [Google Scholar]
- Husain, H.; Waseem, M.; Ahmad, R. Proteomic and molecular evidences of Il1rl2, Ric8a, Krt18 and Hsp90b1 modulation during experimental hepatic fibrosis and pomegranate supplementation. Int. J. Biol. Macromol. 2021, 185, 696–707. [Google Scholar] [CrossRef]
- San-Miguel, B.; Crespo, I.; Sánchez, D.I.; González-Fernández, B.; Ortiz de Urbina, J.J.; Tuñón, M.J.; González-Gallego, J. Melatonin inhibits autophagy and endoplasmic reticulum stress in mice with carbon tetrachloride-induced fibrosis. J. Pineal. Res. 2015, 59, 151–162. [Google Scholar] [CrossRef]
- Li, S.; Zhang, J.; Feng, G.; Jiang, L.; Chen, Z.; Xin, W.; Zhang, X. The Emerging Role of Extracellular Vesicles from Mesenchymal Stem Cells and Macrophages in Pulmonary Fibrosis: Insights into miRNA Delivery. Pharmaceuticals 2022, 15, 1276. [Google Scholar] [CrossRef]
- Sun, W.; Li, Y.; Ma, D.; Liu, Y.; Xu, Q.; Cheng, D.; Li, G.; Ni, C. ALKBH5 promotes lung fibroblast activation and silica-induced pulmonary fibrosis through miR-320a-3p and FOXM1. Cell. Mol. Biol. Lett. 2022, 27, 26. [Google Scholar] [CrossRef]
- Yuan, J.; Li, P.; Pan, H.; Xu, Q.; Xu, T.; Li, Y.; Wei, D.; Mo, Y.; Zhang, Q.; Chen, J.; et al. miR-770-5p inhibits the activation of pulmonary fibroblasts and silica-induced pulmonary fibrosis through targeting TGFBR1. Ecotoxicol. Environ. Saf. 2021, 220, 112372. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Staron, M.; Hong, F.; Wu, B.X.; Sun, S.; Morales, C.; Crosson, C.E.; Tomlinson, S.; Kim, I.; Wu, D.; et al. Essential roles of grp94 in gut homeostasis via chaperoning canonical Wnt pathway. Proc. Natl. Acad. Sci. USA 2013, 110, 6877–6882. [Google Scholar] [CrossRef] [PubMed]
- Zhang, E.; Geng, X.; Shan, S.; Li, P.; Li, S.; Li, W.; Yu, M.; Peng, C.; Wang, S.; Shao, H.; et al. Exosomes derived from bone marrow mesenchymal stem cells reverse epithelial-mesenchymal transition potentially via attenuating Wnt/β-catenin signaling to alleviate silica-induced pulmonary fibrosis. Toxicol. Mech. Methods 2021, 31, 655–666. [Google Scholar] [CrossRef]
- Bandeira, E.; Oliveira, H.; Silva, J.D.; Menna-Barreto, R.F.S.; Takyia, C.M.; Suk, J.S.; Witwer, K.W.; Paulaitis, M.E.; Hanes, J.; Rocco, P.R.M.; et al. Therapeutic effects of adipose-tissue-derived mesenchymal stromal cells and their extracellular vesicles in experimental silicosis. Respir. Res. 2018, 19, 104. [Google Scholar] [CrossRef] [PubMed]
- Caplan, A.I. Mesenchymal Stem Cells: Time to Change the Name! Stem Cells Transl. Med. 2017, 6, 1445–1451. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.Y.; Zhou, T.Y.; Zhang, Z.D.; Liu, H.Y.; Zheng, Z.Y.; Xie, H.Q. Current therapeutic strategies for respiratory diseases using mesenchymal stem cells. MedComm 2021, 2, 351–380. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Li, Q.; Lian, X.; Zhu, Z.; Chen, X.; Pei, W.; Li, S.; Abbas, A.; Wang, Y.; Tian, L. MicroRNA-29b Mediates Lung Mesenchymal-Epithelial Transition and Prevents Lung Fibrosis in the Silicosis Model. Mol. Ther. Nucleic Acids 2019, 14, 20–31. [Google Scholar] [CrossRef] [PubMed]
- Ding, D.C.; Chang, Y.H.; Shyu, W.C.; Lin, S.Z. Human umbilical cord mesenchymal stem cells: A new era for stem cell therapy. Cell Transpl. 2015, 24, 339–347. [Google Scholar] [CrossRef]
- Hoffmann, A.; Floerkemeier, T.; Melzer, C.; Hass, R. Comparison of in vitro-cultivation of human mesenchymal stroma/stem cells derived from bone marrow and umbilical cord. J. Tissue Eng. Regen. Med. 2017, 11, 2565–2581. [Google Scholar] [CrossRef]
- Yin, S.; Ji, C.; Wu, P.; Jin, C.; Qian, H. Human umbilical cord mesenchymal stem cells and exosomes: Bioactive ways of tissue injury repair. Am. J. Transl. Res. 2019, 11, 1230–1240. [Google Scholar]
- Zhang, K.; Na, T.; Wang, L.; Gao, Q.; Yin, W.; Wang, J.; Yuan, B.Z. Human diploid MRC-5 cells exhibit several critical properties of human umbilical cord-derived mesenchymal stem cells. Vaccine 2014, 32, 6820–6827. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Zhao, W.; Yang, A.; Xu, A.; Wang, H.; Cong, M.; Liu, T.; Wang, P.; You, H. Integrated analysis of microRNA and gene expression profiles reveals a functional regulatory module associated with liver fibrosis. Gene 2017, 636, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Huang, F.; Zhang, R.; Luo, H. LncRNA Neat1 expedites the progression of liver fibrosis in mice through targeting miR-148a-3p and miR-22-3p to upregulate Cyth3. Cell Cycle 2021, 20, 490–507. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Luo, Z.; Pan, Y.; Zheng, W.; Li, W.; Zhang, Z.; Xiong, P.; Xu, D.; Du, M.; Wang, B.; et al. H19/miR-148a/USP4 axis facilitates liver fibrosis by enhancing TGF-β signaling in both hepatic stellate cells and hepatocytes. J. Cell. Physiol. 2019, 234, 9698–9710. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.Q.; Tian, H.T.; Liu, H.; Xie, R. The role of macrophage-derived TGF-β1 on SiO(2)-induced pulmonary fibrosis: A review. Toxicol. Ind. Health 2021, 37, 240–250. [Google Scholar] [CrossRef]
- Rocha-Parise, M.; Santos, L.M.; Damoiseaux, J.G.; Bagatin, E.; Lido, A.V.; Torello, C.O.; Cohen Tervaert, J.W.; Queiroz, M.L. Lymphocyte activation in silica-exposed workers. Int. J. Hyg. Environ. Health 2014, 217, 586–591. [Google Scholar] [CrossRef]
- Park, S.J.; Hahn, H.J.; Oh, S.R.; Lee, H.J. Theophylline Attenuates BLM-Induced Pulmonary Fibrosis by Inhibiting Th17 Differentiation. Int. J. Mol. Sci. 2023, 24, 1019. [Google Scholar] [CrossRef]
- Lei, X.; He, N.; Zhu, L.; Zhou, M.; Zhang, K.; Wang, C.; Huang, H.; Chen, S.; Li, Y.; Liu, Q.; et al. Mesenchymal Stem Cell-Derived Extracellular Vesicles Attenuate Radiation-Induced Lung Injury via miRNA-214-3p. Antioxid. Redox Signal 2021, 35, 849–862. [Google Scholar] [CrossRef]
- Qu, Y.; Zhang, Q.; Cai, X.; Li, F.; Ma, Z.; Xu, M.; Lu, L. Exosomes derived from miR-181-5p-modified adipose-derived mesenchymal stem cells prevent liver fibrosis via autophagy activation. J. Cell Mol. Med. 2017, 21, 2491–2502. [Google Scholar] [CrossRef]
- Luo, Q.; Guo, D.; Liu, G.; Chen, G.; Hang, M.; Jin, M. Exosomes from MiR-126-Overexpressing Adscs Are Therapeutic in Relieving Acute Myocardial Ischaemic Injury. Cell Physiol. Biochem. 2017, 44, 2105–2116. [Google Scholar] [CrossRef]
- Xiao, K.; He, W.; Guan, W.; Hou, F.; Yan, P.; Xu, J.; Zhou, T.; Liu, Y.; Xie, L. Mesenchymal stem cells reverse EMT process through blocking the activation of NF-κB and Hedgehog pathways in LPS-induced acute lung injury. Cell Death Dis. 2020, 11, 863. [Google Scholar] [CrossRef] [PubMed]
- Tkach, M.; Théry, C. Communication by Extracellular Vesicles: Where We Are and Where We Need to Go. Cell 2016, 164, 1226–1232. [Google Scholar] [CrossRef] [PubMed]
- Crivelli, B.; Chlapanidas, T.; Perteghella, S.; Lucarelli, E.; Pascucci, L.; Brini, A.T.; Ferrero, I.; Marazzi, M.; Pessina, A.; Torre, M.L. Mesenchymal stem/stromal cell extracellular vesicles: From active principle to next generation drug delivery system. J. Control. Release 2017, 262, 104–117. [Google Scholar] [CrossRef] [PubMed]
- Sreedhar, A.S.; Kalmár, E.; Csermely, P.; Shen, Y.F. Hsp90 isoforms: Functions, expression and clinical importance. FEBS Lett. 2004, 562, 11–15. [Google Scholar] [CrossRef]
- Korfei, M.; Schmitt, S.; Ruppert, C.; Henneke, I.; Markart, P.; Loeh, B.; Mahavadi, P.; Wygrecka, M.; Klepetko, W.; Fink, L.; et al. Comparative proteomic analysis of lung tissue from patients with idiopathic pulmonary fibrosis (IPF) and lung transplant donor lungs. J. Proteome Res. 2011, 10, 2185–2205. [Google Scholar] [CrossRef]
- Bellaye, P.S.; Shimbori, C.; Yanagihara, T.; Carlson, D.A.; Hughes, P.; Upagupta, C.; Sato, S.; Wheildon, N.; Haystead, T.; Ask, K.; et al. Synergistic role of HSP90α and HSP90β to promote myofibroblast persistence in lung fibrosis. Eur. Respir. J. 2018, 51, 1700386. [Google Scholar] [CrossRef]
- Sibinska, Z.; Tian, X.; Korfei, M.; Kojonazarov, B.; Kolb, J.S.; Klepetko, W.; Kosanovic, D.; Wygrecka, M.; Ghofrani, H.A.; Weissmann, N.; et al. Amplified canonical transforming growth factor-β signalling via heat shock protein 90 in pulmonary fibrosis. Eur. Respir. J. 2017, 49, 1501941. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, Q.; Zhao, J.; Jia, Q.; Wang, H.; Xue, W.; Ning, F.; Wang, J.; Wang, Y.; Zhu, Z.; Tian, L. MiR-148a-3p within HucMSC-Derived Extracellular Vesicles Suppresses Hsp90b1 to Prevent Fibroblast Collagen Synthesis and Secretion in Silica-Induced Pulmonary Fibrosis. Int. J. Mol. Sci. 2023, 24, 14477. https://doi.org/10.3390/ijms241914477
Jiang Q, Zhao J, Jia Q, Wang H, Xue W, Ning F, Wang J, Wang Y, Zhu Z, Tian L. MiR-148a-3p within HucMSC-Derived Extracellular Vesicles Suppresses Hsp90b1 to Prevent Fibroblast Collagen Synthesis and Secretion in Silica-Induced Pulmonary Fibrosis. International Journal of Molecular Sciences. 2023; 24(19):14477. https://doi.org/10.3390/ijms241914477
Chicago/Turabian StyleJiang, Qiyue, Jing Zhao, Qiyue Jia, Hongwei Wang, Wenming Xue, Fuao Ning, Jiaxin Wang, Yan Wang, Zhonghui Zhu, and Lin Tian. 2023. "MiR-148a-3p within HucMSC-Derived Extracellular Vesicles Suppresses Hsp90b1 to Prevent Fibroblast Collagen Synthesis and Secretion in Silica-Induced Pulmonary Fibrosis" International Journal of Molecular Sciences 24, no. 19: 14477. https://doi.org/10.3390/ijms241914477
APA StyleJiang, Q., Zhao, J., Jia, Q., Wang, H., Xue, W., Ning, F., Wang, J., Wang, Y., Zhu, Z., & Tian, L. (2023). MiR-148a-3p within HucMSC-Derived Extracellular Vesicles Suppresses Hsp90b1 to Prevent Fibroblast Collagen Synthesis and Secretion in Silica-Induced Pulmonary Fibrosis. International Journal of Molecular Sciences, 24(19), 14477. https://doi.org/10.3390/ijms241914477





