Bacteria, Fungi, and Enzymes in Soil Treated with Sulcotrione and Terbuthylazine
Abstract
:1. Introduction
2. Results
2.1. Response of Soil Microorganisms to Herbicides
| Object | Org | Act | Fun |
|---|---|---|---|
| SulRD | 0.314 ± 0.100 ab | 0.164 ± 0.027 b | 0.026 ± 0.006 bc |
| Sul10RD | 0.074 ± 0.077 c | 0.026 ± 0.028 c | −0.175 ± 0.023 d |
| TezRD | 0.421 ± 0.122 a | 0.279 ± 0.077 ab | 0.254 ± 0.031 a |
| Tez10RD | 0.145 ± 0.074 bc | −0.128 ± 0.046 d | −0.105 ± 0.030 cd |
| SucRD | 0.342 ± 0.042 a | −0.135 ± 0.085 d | 0.087 ± 0.133 b |
| Suc10RD | 0.492 ± 0.012 a | 0.367 ± 0.007 a | −0.209 ± 0.023 d |
| Object | Org | Act | Fun |
|---|---|---|---|
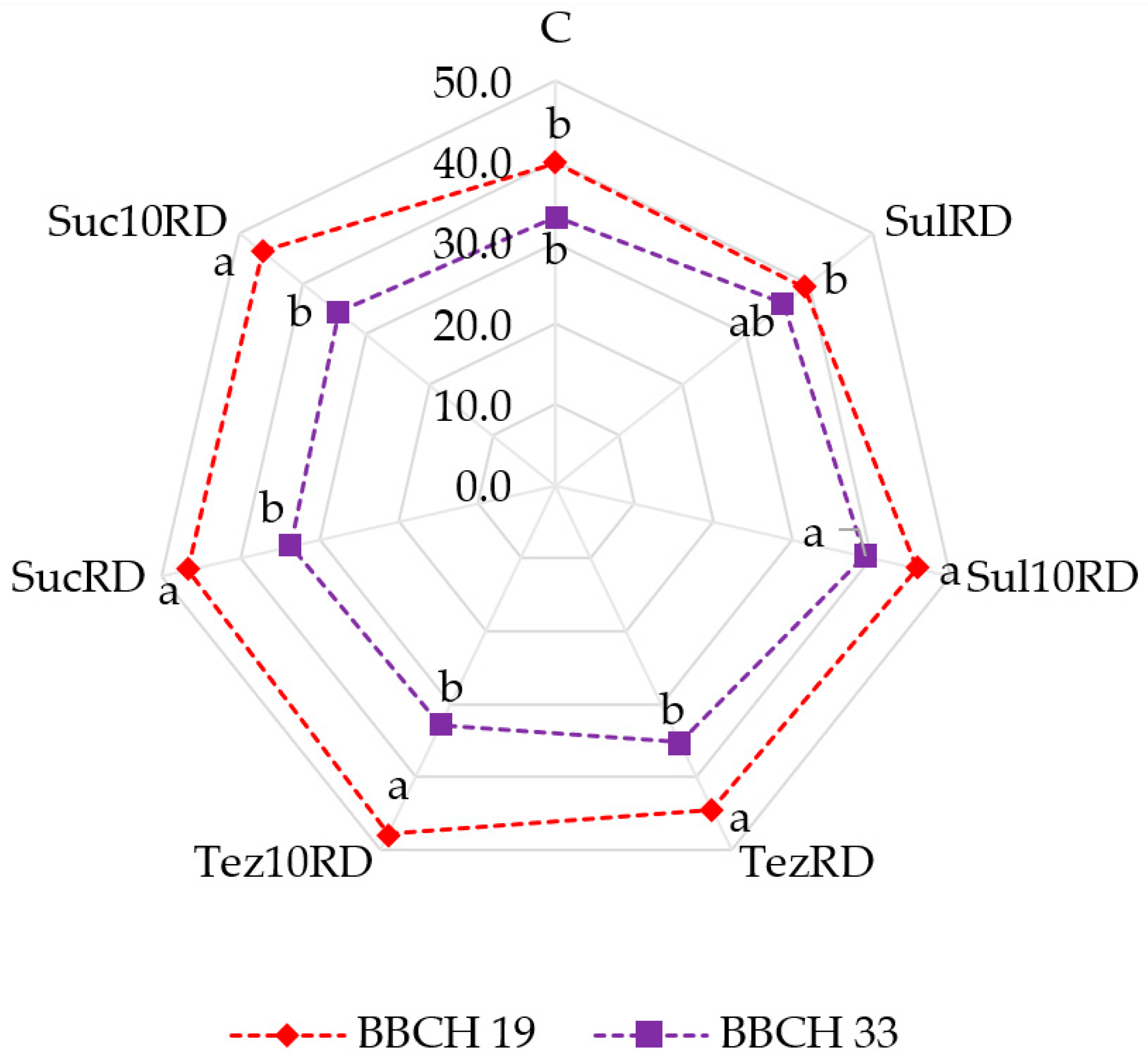
| C | 31.390 ± 1.005 a | 23.436 ± 0.324 a | 41.762 ± 0.127 a |
| SulRD | 29.567 ± 0.311 b | 24.065 ± 0.564 a | 38.886 ± 0.600 bc |
| Sul10RD | 28.528 ± 0.469 b | 20.441 ± 0.138 b | 37.105 ± 0.871 cd |
| TezRD | 31.448 ± 0.073 a | 23.173 ± 0.096 a | 35.877 ± 0.470 d |
| Tez10RD | 28.520 ± 0.361 b | 23.350 ± 1.043 a | 31.758 ± 1.552 e |
| SucRD | 29.090 ± 0.715 b | 23.962 ± 0.247 a | 39.801 ± 0.984 b |
| Suc10RD | 28.736 ± 0.297 b | 23.546 ± 0.127 a | 39.133 ± 0.908 bc |
| Object | Org | Act | Fun |
|---|---|---|---|
| C | 0.790 ± 0.019 c | 0.867 ± 0.003 bc | 0.538 ± 0.020 d |
| SulRD | 0.822 ± 0.0131 ab | 0.879 ± 0.001 b | 0.632 ± 0.005 cd |
| Sul10RD | 0.846 ± 0.003 a | 0.874 ± 0.003 b | 0.667 ± 0.009 bc |
| TezRD | 0.789 ± 0.010 c | 0.882 ± 0.005 ab | 0.705 ± 0.031 ab |
| Tez10RD | 0.848 ± 0.004 a | 0.889 ± 0.002 a | 0.763 ± 0.042 a |
| SucRD | 0.823 ± 0.013 ab | 0.870 ± 0.013 b | 0.585 ± 0.029 de |
| Suc10RD | 0.814 ± 0.001 bc | 0.852 ± 0.010 c | 0.580 ± 0.029 de |
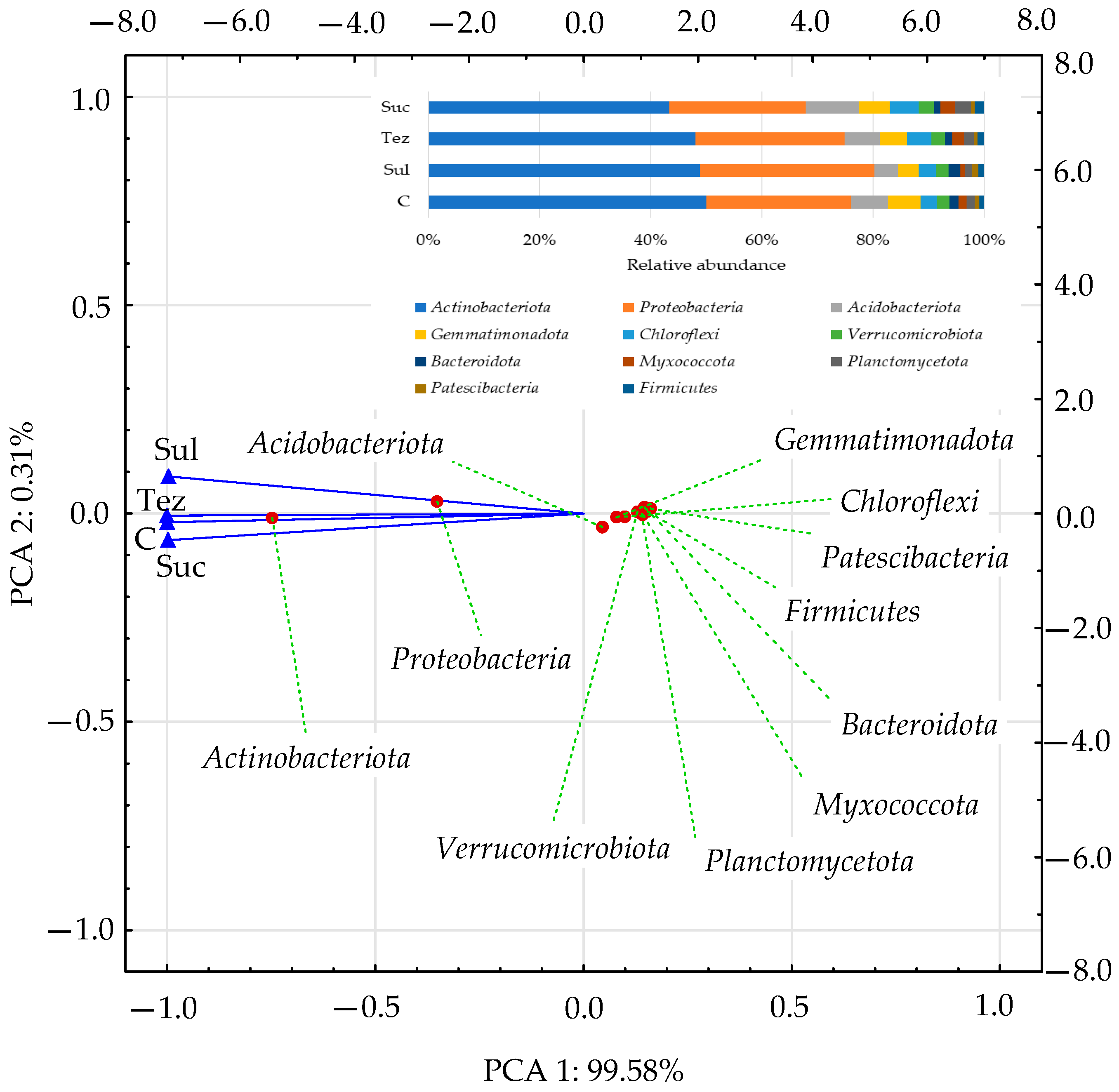
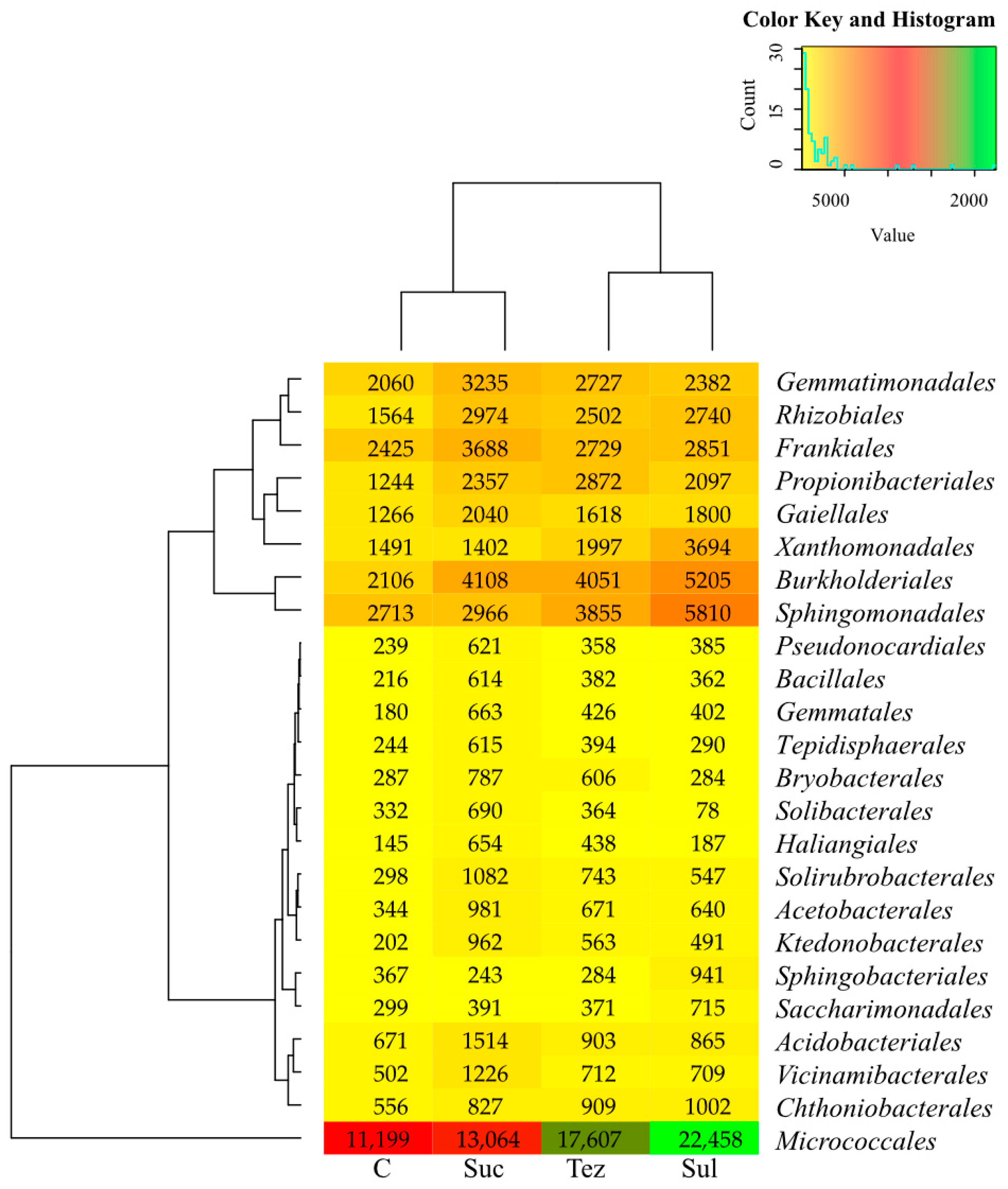
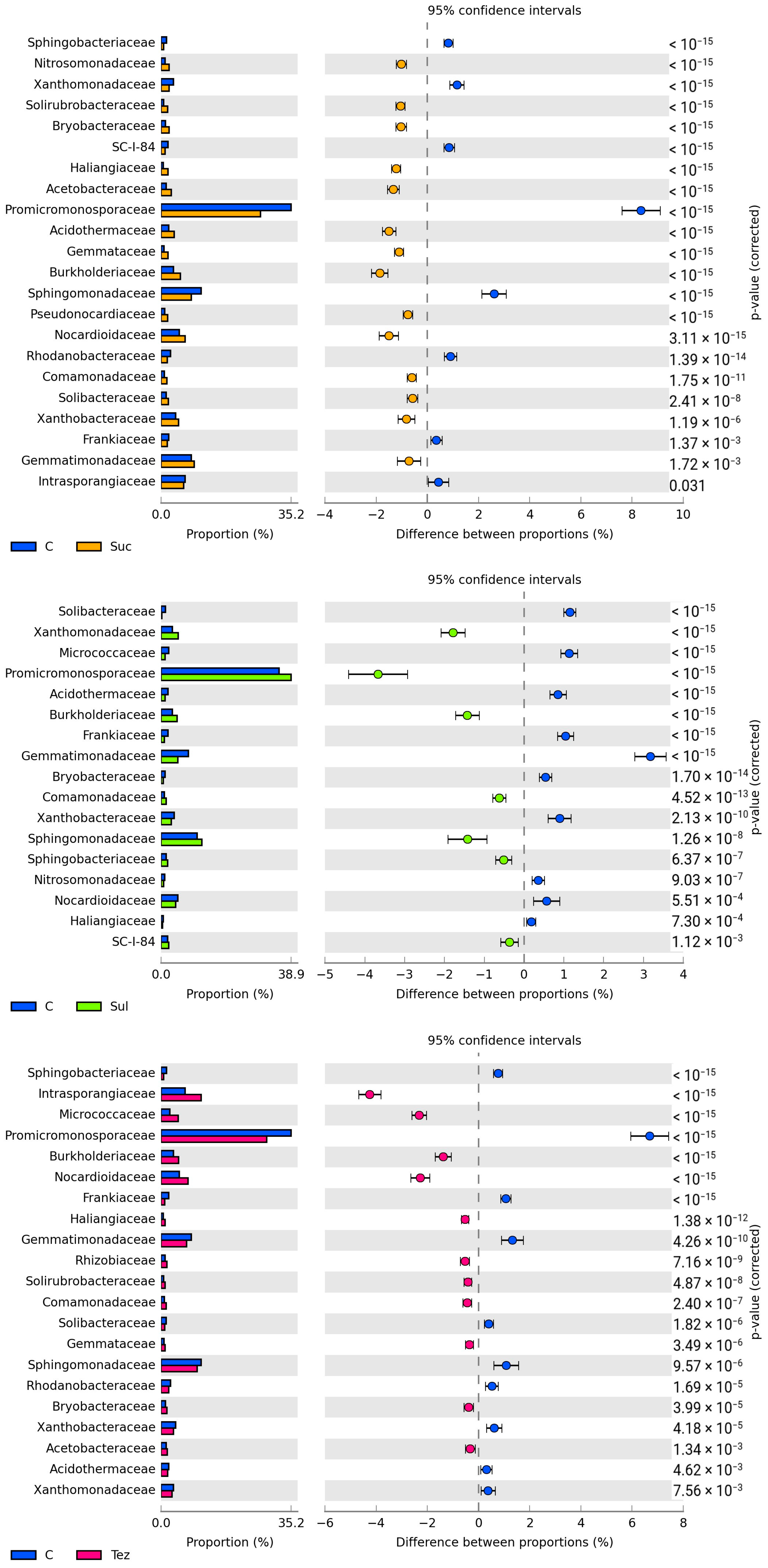

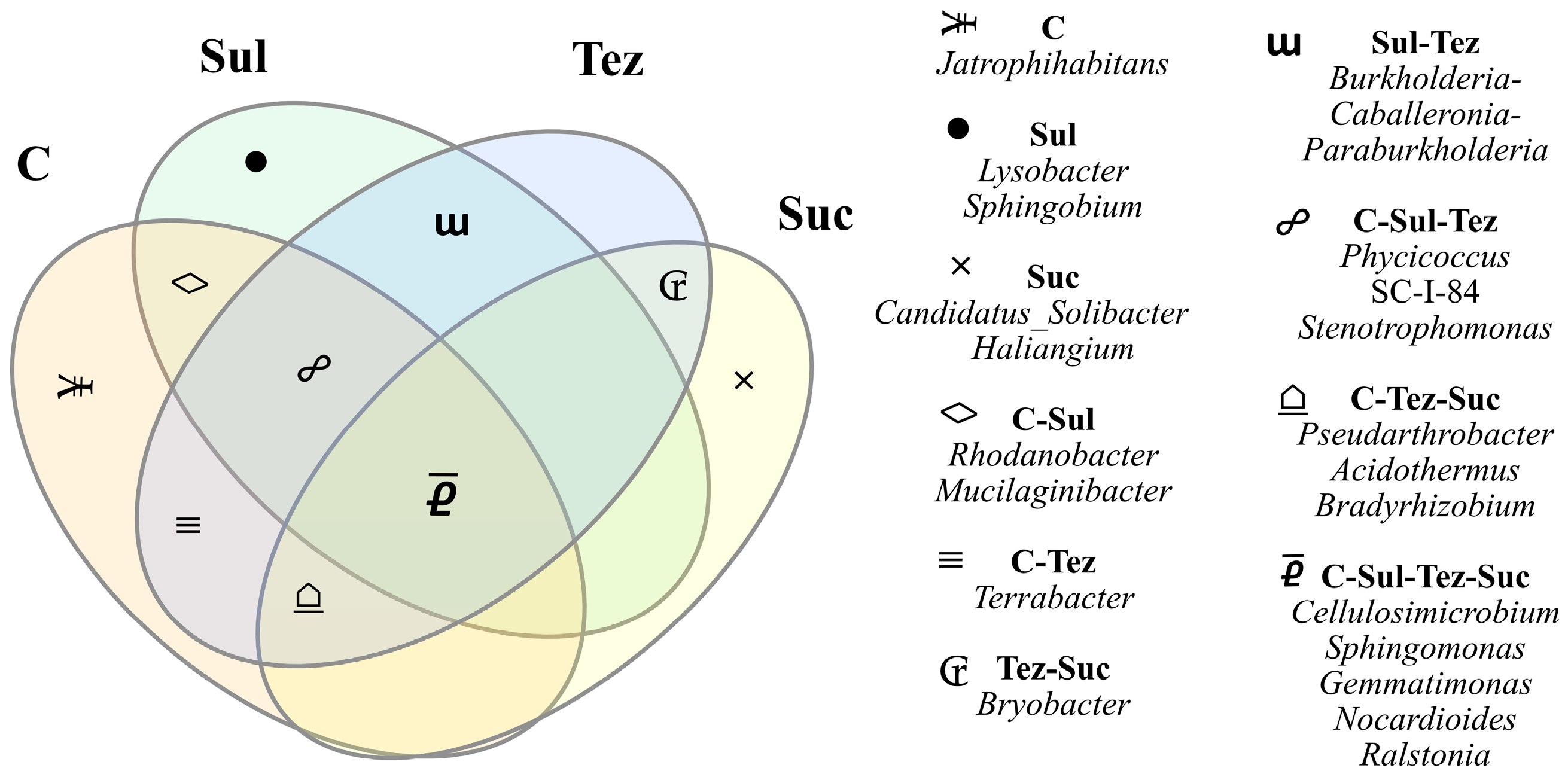
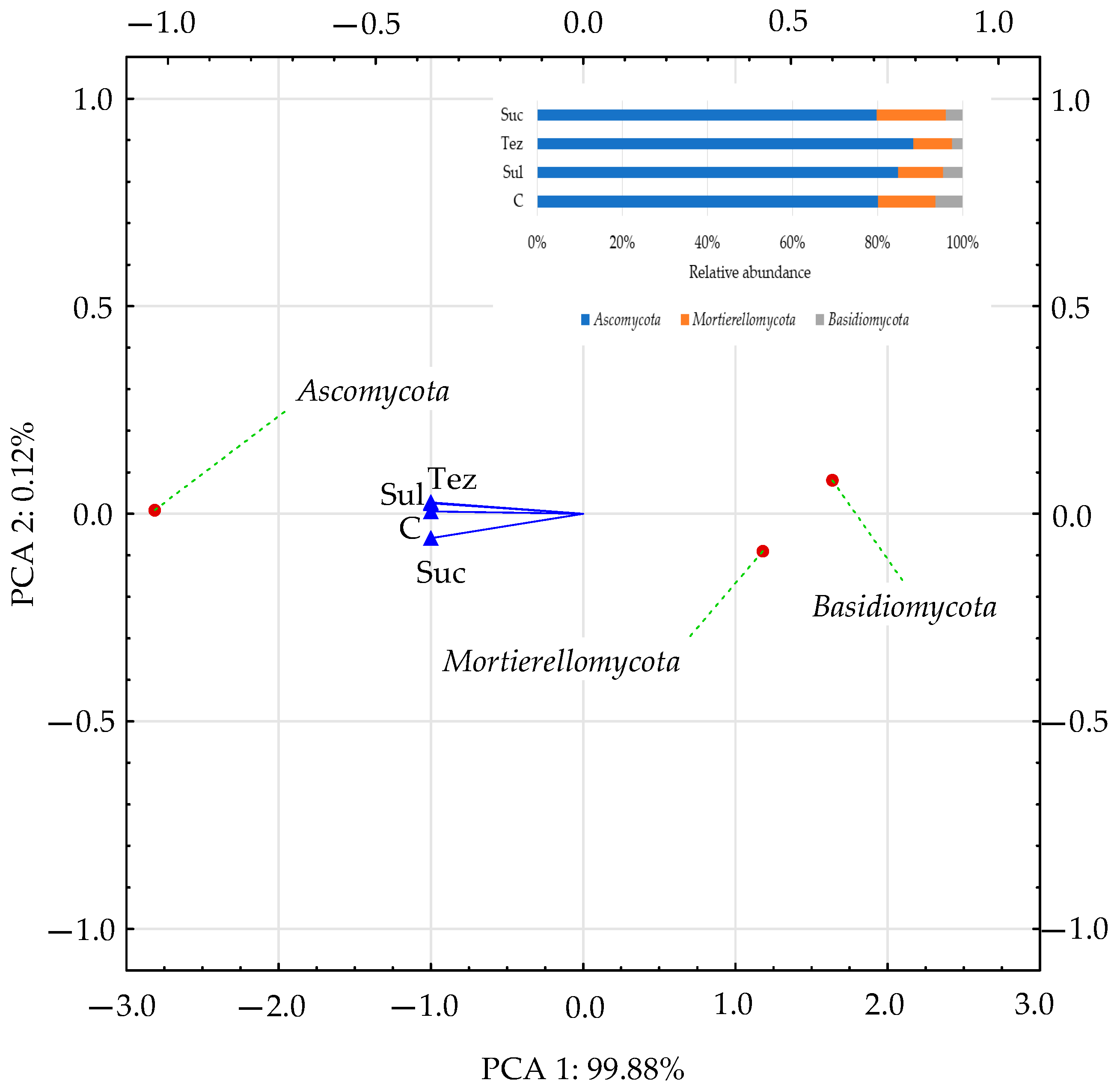
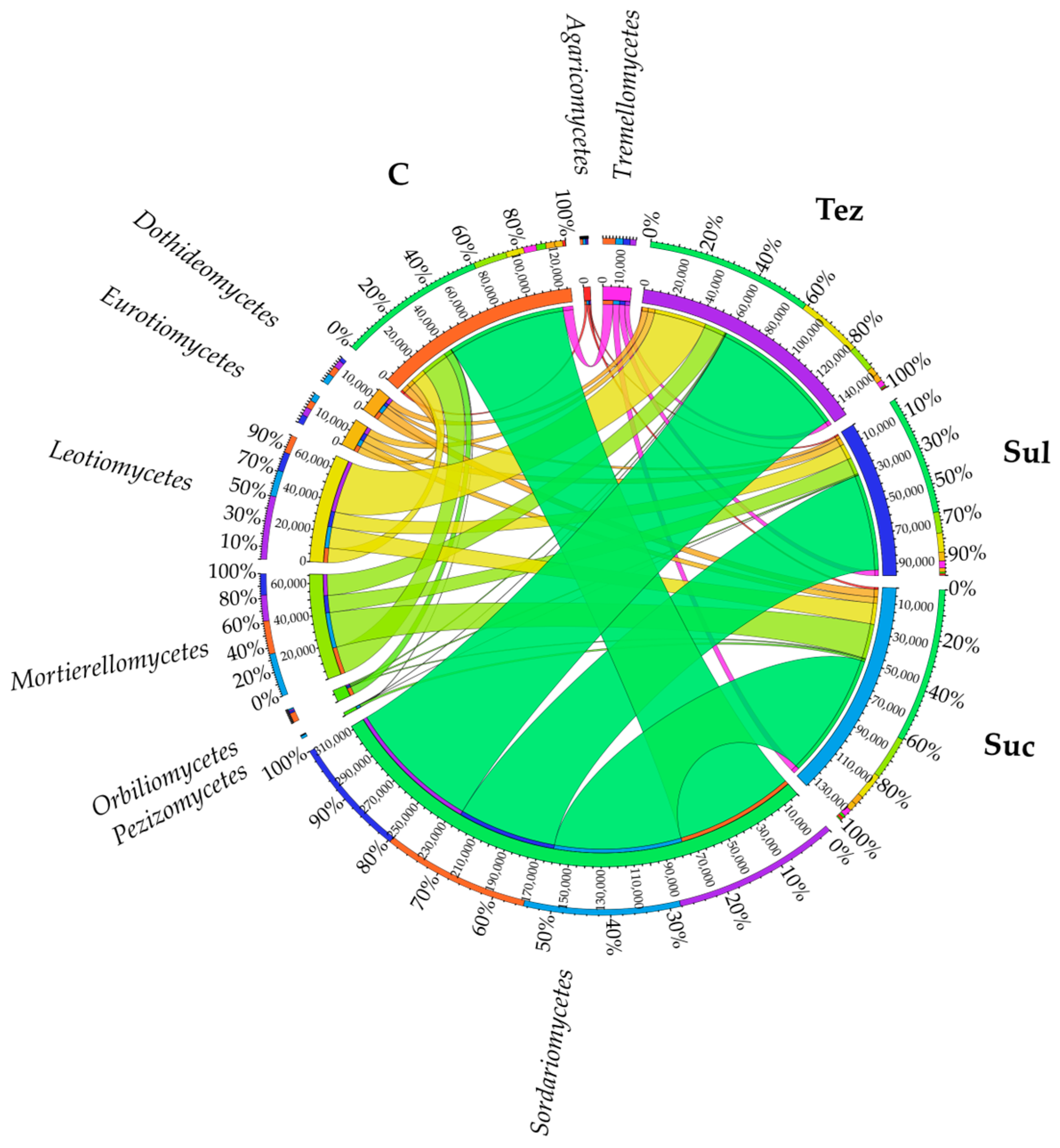
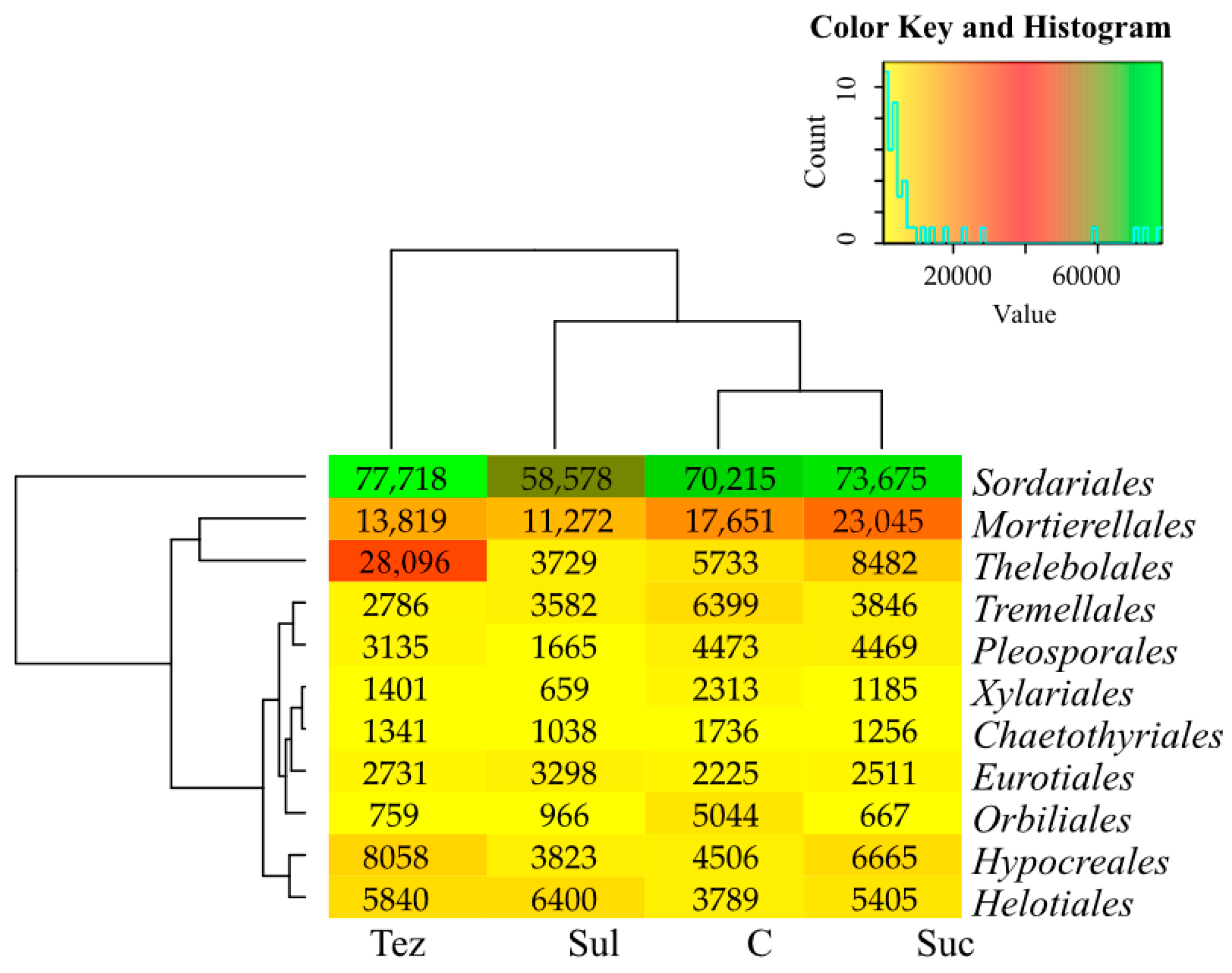
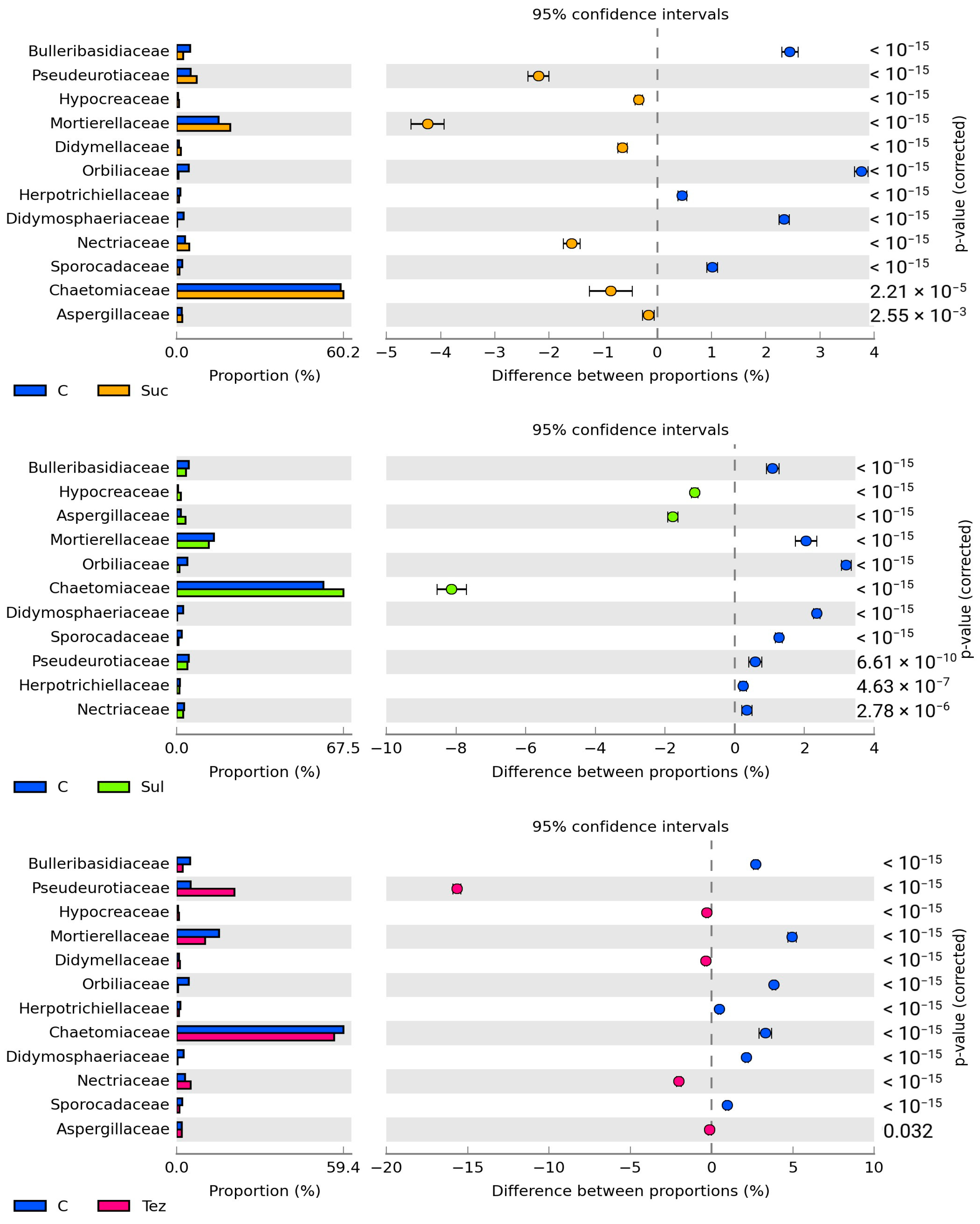
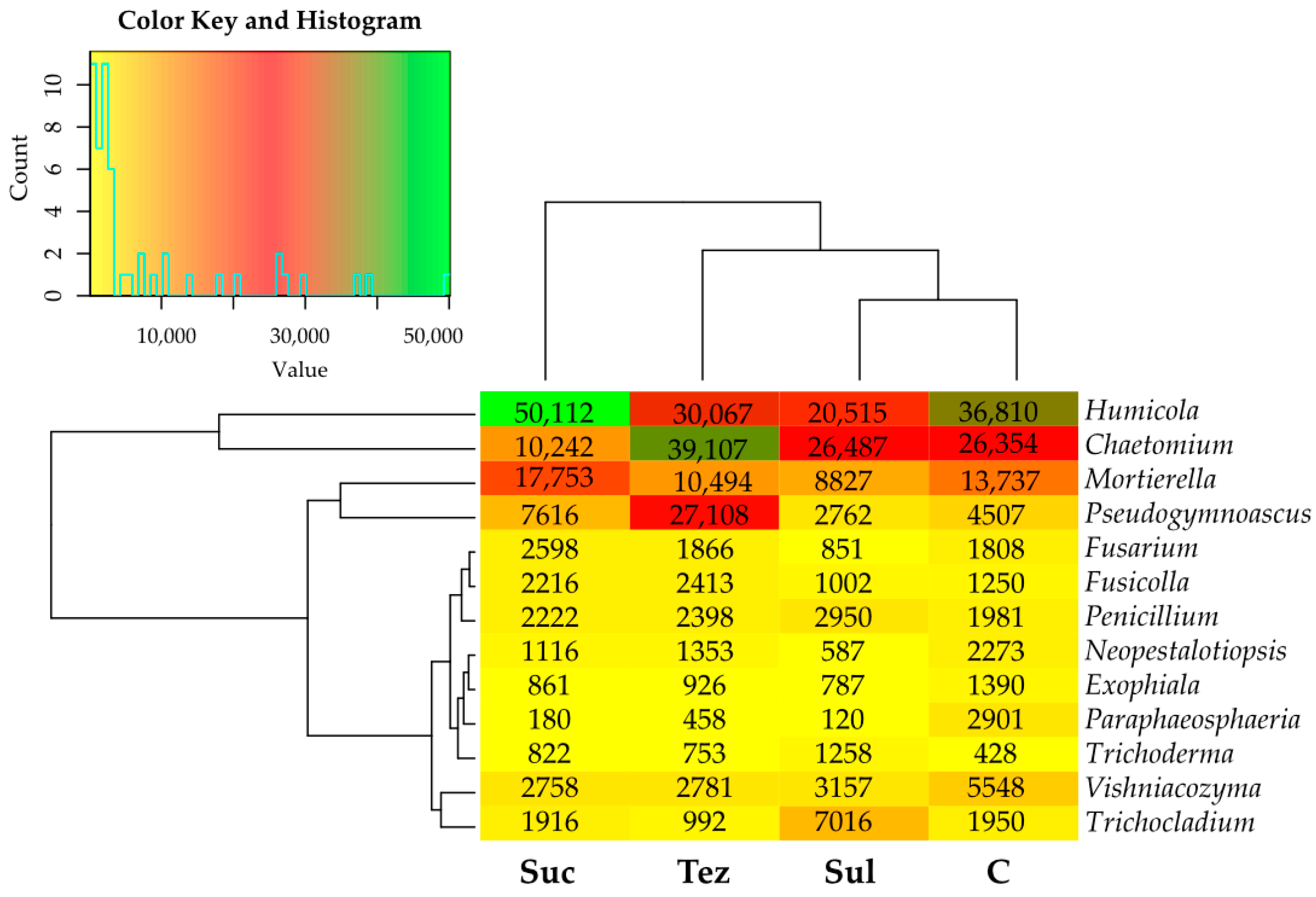
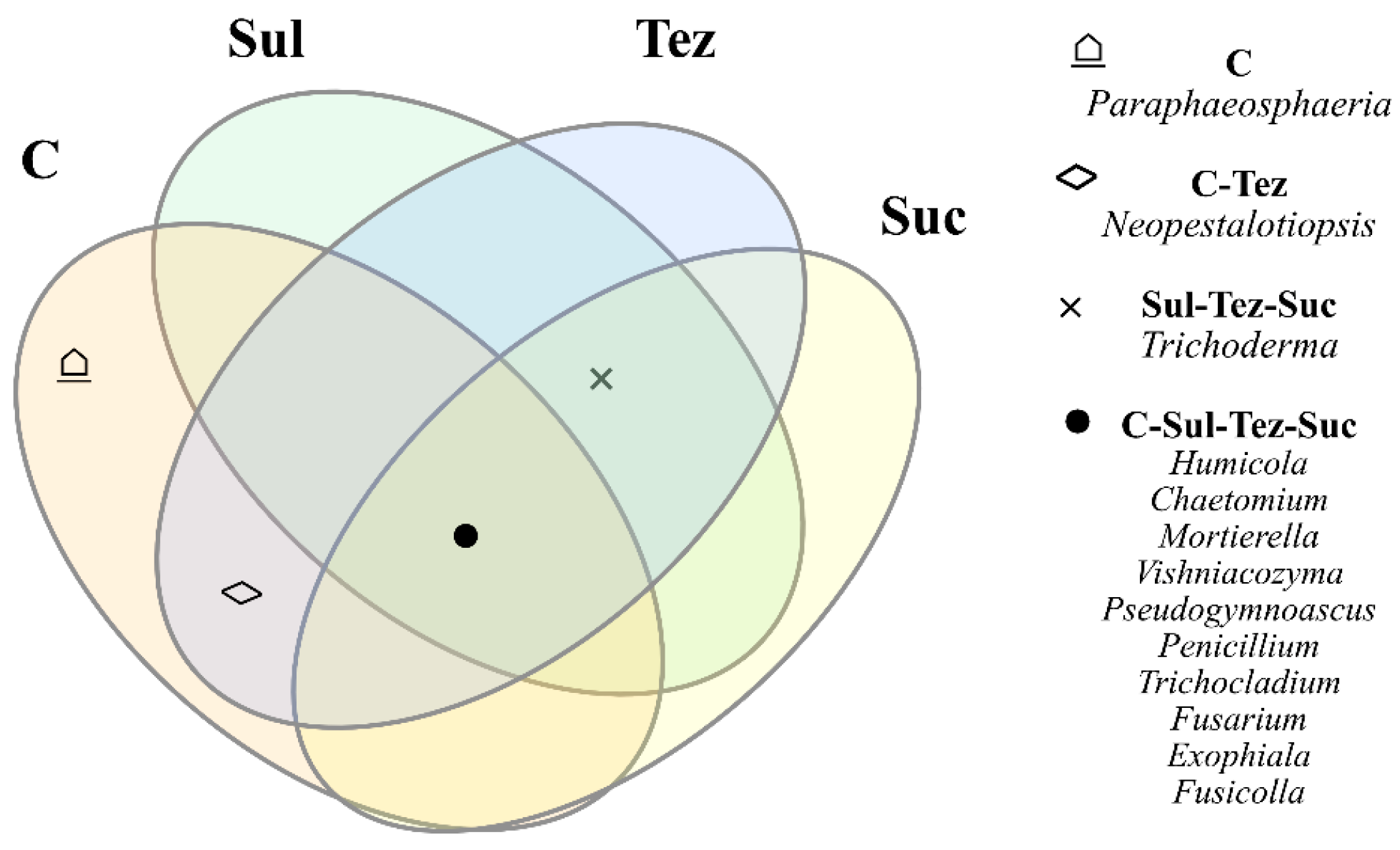
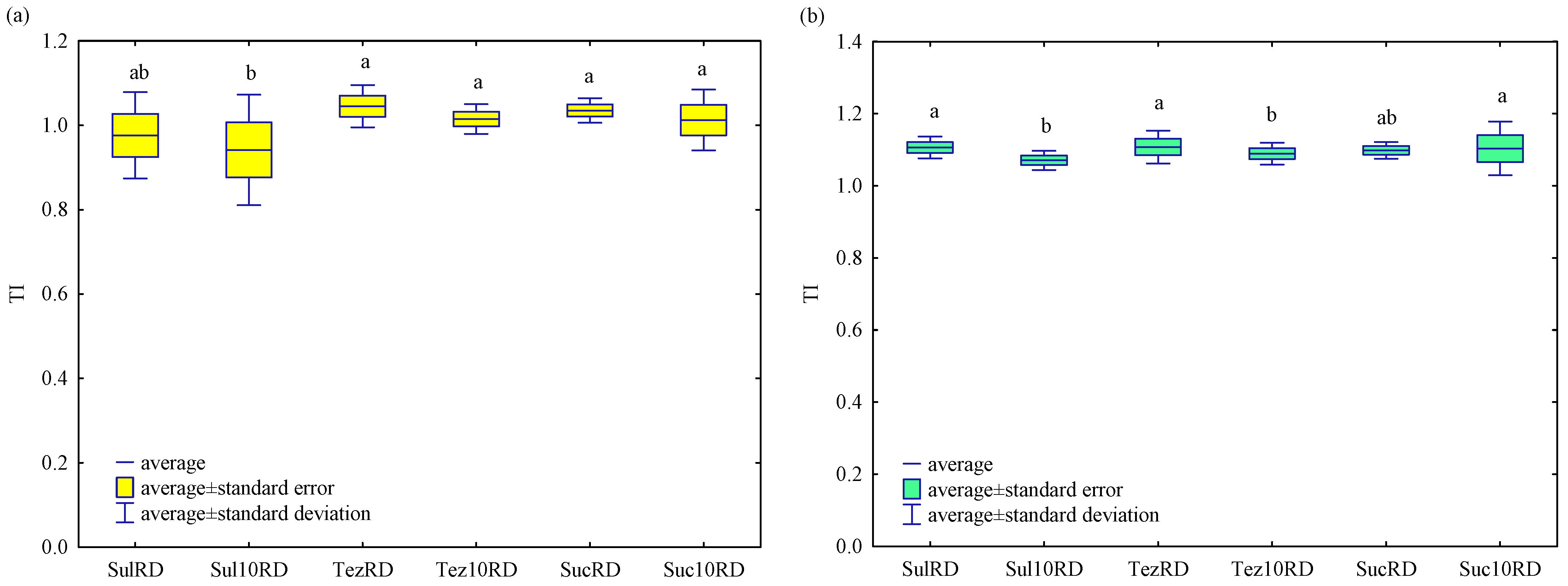
2.2. Response of Soil Enzymes to Herbicides
2.3. Response of Maize (Zea mays L.) to Herbicides
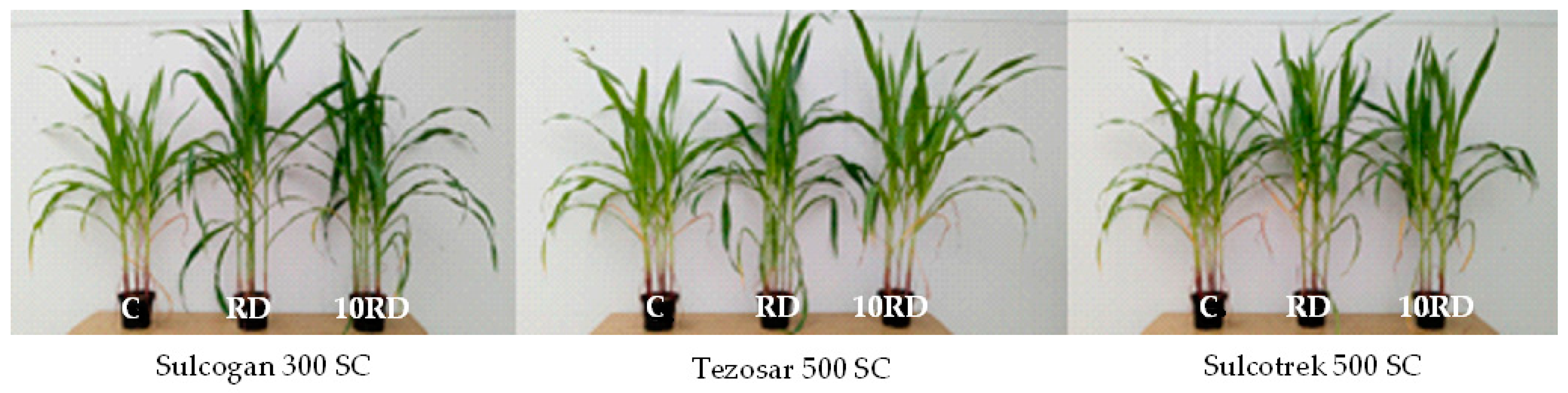
3. Discussion
3.1. Response of Soil Microorganisms to Herbicides
3.2. Response of Soil Enzymes to Herbicides
3.3. Response of Maize (Zea mays L.) to Herbicides
4. Materials and Methods
4.1. Experimental Design
- Soil without addition of herbicides (control);
- Soil with addition of herbicide Sulcogan 300 SC at a dose recommended by the manufacturer (0.150 mg kg−1 d.m. Soil per active substance—sulrd);
- Soil with addition of Sulcogan 300 SC herbicide at a dose 10-fold higher than recommended by the manufacturer (1.50 mg kg−1 d.m. Soil per active substance—Sul10RD);
- Soil with addition of herbicide Tezosar 500 SC at a dose recommended by the manufacturer (0.165 mg kg−1 d.m. Soil per active substance—tezrd);
- Soil with addition of Tezosar 500 SC herbicide at a dose 10-fold higher than recommended by the manufacturer (1.65 mg kg−1 d.m. Soil per active substance—Tez10RD);
- Soil with addition of herbicide Sulcotrek 500 SC at a dose recommended by the manufacturer (0.333 mg kg−1 d.m. Soil per active substance—sucrd);
- Soil with addition of Sulcotrek 500 SC herbicide at a dose 10-fold higher than recommended by the manufacturer (3.33 mg kg−1 d.m. soil per active substance—Suc10RD).
4.2. Soil
4.3. Herbicides
4.4. Conducting Microbiological Analyses of Soil
4.5. Conducting Enzymatic Analyses of Soil
4.6. Conducting Statistical and Bioinformatics Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ahmad, S.; Cui, D.; Zhong, G.; Liu, J. Microbial technologies employed for biodegradation of neonicotinoids in the agroecosystem. Front. Microbiol. 2021, 12, 759439. [Google Scholar] [CrossRef]
- Mishra, S.; Lin, Z.; Pang, S.; Zhang, W.; Bhatt, P.; Chen, S. Recent advanced technologies for the characterization of xenobiotic-degrading microorganisms and microbial communities. Front. Bioeng. Biotechnol. 2021, 9, 632059. [Google Scholar] [CrossRef] [PubMed]
- Roman, D.L.; Voiculescu, D.I.; Filip, M.; Ostafe, V.; Isvoran, A. Effects of triazole fungicides on soil microbiota and on the activities of enzymes found in soil: A review. Agriculture 2021, 11, 893. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, X.; Liu, W.; Ge, L. Plant and microorganism combined degradation of bensulfuron herbicide in eight different agricultural soils. Agronomy 2022, 12, 2989. [Google Scholar] [CrossRef]
- Machado, C.; Martins, I. Risk assessment of occupational pesticide exposure: Use of endpoints and surrogates. Regul. Toxicol. Pharmacol. 2018, 98, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Tonello, C.Z.; Correa, N.B.; Colla, L.M. Bioremediation of terbuthylazine contaminated soil: A bibliometric and systematic review. Conjecturas 2023, 23, 157–177. [Google Scholar] [CrossRef]
- Tahat, M.M.; Alananbeh, K.M.; Othman, Y.A.; Leskovar, D.I. Soil health and sustainable agriculture. Sustainability 2020, 12, 4859. [Google Scholar] [CrossRef]
- Rao, M.A.; Scelza, R.; Acevedo, F.; Diez, M.C.; Gianfreda, L. Enzymes as useful tools for environmental purposes. Chemosphere 2014, 107, 145–162. [Google Scholar] [CrossRef]
- Bhattacharyya, R.; Rabbi, S.M.F.; Zhang, Y.; Young, I.M.; Jones, A.R.; Dennis, P.G.; Menzies, N.W.; Kopittke, P.M.; Dalal, R.C. Soil organic carbon is significantly associated with the pore geometry, microbial diversity and enzyme activity of the macro-aggregates under different land uses. Sci. Total Environ. 2021, 778, 146286. [Google Scholar] [CrossRef]
- Yang, X.; Leng, Y.; Zhou, Z.; Shang, H.; Ni, K.; Ma, L.; Yi, X.; Cai, Y.; Ji, L.; Ruan, J.; et al. Ecological management model for the improvement of soil fertility through the regulation of rare microbial taxa in tea (Camellia sinensis L.) plantation soils. J. Environ. Manag. 2022, 308, 114595. [Google Scholar] [CrossRef]
- Lan, X.; Du, H.; Peng, W.; Liu, Y.; Fang, Z.; Song, T. Functional diversity of the soil culturable microbial community in eucalyptus plantations of different ages in Guangxi, South China. Forests 2019, 10, 1083. [Google Scholar] [CrossRef]
- Li, M.; Wei, Y.; Yin, Y.; Ding, H.; Zhu, W.; Zhou, Y. The effect of intercropping Mulberry (Morus alba L.) with Peanut (Arachis hypogaea L.), on the soil rhizosphere microbial community. Forests 2022, 13, 1757. [Google Scholar] [CrossRef]
- He, X.; Su, Y.; Liang, Y.; Chen, X.; Zhu, H.; Wang, K. Land reclamation and short-term cultivation change soil microbial communities and bacterial metabolic profiles. J. Sci. Food Agric. 2012, 92, 1103–1111. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Chen, S.B.; Chen, L.; Wang, D. Responses of soil microbial communities and their network interactions to saline-alkaline stress in Cd-contaminated soils. Environ. Pollut. 2019, 252, 1609–1621. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yang, J.; Xie, X.; Chen, X.; Pu, L.; Zhang, X. Soil microbial succession with soil development since costal reclamation. Catena 2020, 187, 104393. [Google Scholar] [CrossRef]
- Li, Y.; Zeng, C.; Long, M. Variation of soil nutrients and bacterial community diversity of different land utilization types in Yangtze River Basin, Chongqing Municipality. PeerJ 2020, 8, e9386. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Pokharel, P.; Liu, G.; Chen, J. Reclamation of desert land to different land-use types changes soil bacterial community composition in a desert-oasis ecotone. Land Degrad. Dev. 2021, 32, 1389–1399. [Google Scholar] [CrossRef]
- Morgante, V.; Flores, C.; Fadic, X.; Gonzalez, M.; Hernandez, M.; Cereceda-Balic, F.; Seeger, M. Influence of microorganisms and leaching on simazine attenuation in agricultural soil. J. Environ. Manag. 2012, 95, S300–S305. [Google Scholar] [CrossRef]
- Song, J.L.; Gu, J.G.; Zhai, Y.; Wu, W.; Wang, H.S.; Ruan, Z.Y.; Shi, Y.H.; Yan, Y.C. Biodegradation of nicosulfuron by a Talaromyces flavus LZM1. Bioresour. Technol. 2013, 140, 243–248. [Google Scholar] [CrossRef]
- Kumar, S.; Kaushik, G.; Dar, M.A.; Nimesh, S.; López-Chuken, U.J.; Villarreal-Chiu, J.F. Microbial degradation of organophosphate pesticides: A review. Pedosphere 2018, 28, 190–208. [Google Scholar] [CrossRef]
- Asim, N.; Hassan, M.; Shafique, F.; Ali, M.; Nayab, H.; Shafi, N.; Khawaja, S.; Manzoor, S. Characterizations of novel pesticide-degrading bacterial strains from industrial wastes found in the industrial cities of Pakistan and their biodegradation potential. PeerJ 2021, 9, e12211. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, P.; Gangola, S.; Bhandari, G.; Zhang, W.; Maithani, D.; Mishra, S.; Chen, S. New insights into the degradation of synthetic pollutants in contaminated environments. Chemosphere 2021, 30, 128827. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Hernández, M.L.; Sánchez-Salinas, E.; Dantán-González, E.; Castrejón-Godínez, M.L. Pesticide biodegradation: Mechanisms, genetics and strategies to enhance the process. Biodegrad. Life Sci. 2013, 10, 251–287. [Google Scholar] [CrossRef]
- Bass, C.; Field, L. Gene amplification and insecticide resistance. Pest Manag. Sci. 2011, 67, 886–890. [Google Scholar] [CrossRef]
- Książek-Trela, P.; Szpyrka, E. The effect of natural and biological pesticides on the degradation of synthetic pesticides. Plant Prot. Sci. 2022, 58, 273–291. [Google Scholar] [CrossRef]
- Saafan, A.E.; Azmy, A.F.; Amin, M.A.; Ahmed, S.H.; Essam, T.M. Isolation and characterization of two malathion degrading Pseudomonas sp. in Egypt. Afr. J. Biotechnol. 2016, 15, 1661–1672. [Google Scholar] [CrossRef]
- Mollea, C.; Francesca, B.; Bernardo, R. Fungal biodegradation of naphthalene: Microcosms studies. Chemosphere 2005, 60, 636–643. [Google Scholar] [CrossRef]
- Novotny, C.; Svobodova, K.; Erbanova, P.; Cajthaml, T.; Kasinath, A.; Lang, E. Lignionolytic fungi in bioremediation extracellular enzyme production and degradation rate. Soil Biol. Biochem. 2004, 36, 1545–1551. [Google Scholar] [CrossRef]
- Diez, M.C. Biological aspects involved in the degradation of organic pollutants. J. Soil Sci. Plant Nutr. 2010, 10, 244–267. [Google Scholar] [CrossRef]
- Streletskii, R.; Astaykina, A.; Krasnov, G.; Gorbatov, V. Changes in bacterial and fungal community of soil under treatment of pesticides. Agronomy 2022, 12, 124. [Google Scholar] [CrossRef]
- Pinheiro, M.; Garnier, P.; Beguet, J.; Martin Laurent, F.; Vieublé Gonod, L. The millimetre scale distribution of 2,4-D and its degraders drives the fate of 2,4-D at the soil core scale. Soil Biol. Biochem. 2015, 88, 90–100. [Google Scholar] [CrossRef]
- Tecon, R.; Or, D. Biophysical processes supporting the diversity of microbial life in soil. FEMS Microbiol. Rev. 2017, 41, 599–623. [Google Scholar] [CrossRef] [PubMed]
- Schuster, S.; Kreft, J.U.; Brenner, N.; Wessely, F.; Theißen, G.; Ruppin, E.; Schroeter, A. Cooperation and cheating in microbial exoenzyme production—Theoretical analysis for biotechnological applications. Biotechnol. J. 2010, 5, 751–758. [Google Scholar] [CrossRef] [PubMed]
- Pileggi, M.; Pileggi, S.A.; Sadowsky, M.J. Herbicide bioremediation: From strains to bacterial communities. Heliyon 2020, 6, e05767. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.Z.; Gong, Z.Q.; Zhang, Y.L.; Li, P.J. Growth, cadmium uptake and accumulation of maize Zea mays L. under the effects of arbuscular mycorrhizal fungi. Ecotoxicology 2014, 23, 1979–1986. [Google Scholar] [CrossRef]
- Sariwati, A.; Purnomo, A.S. The effect of Pseudomonas aeruginosa addition on 1, 1, 1-trichloro-2, 2-bis (4-chlorophenyl)ethane (DDT) biodegradation by brown rot fungus Fomitopsis pinicola. Indones. J. Chem. 2018, 18, 75–81. [Google Scholar] [CrossRef]
- Dong, W.; Chen, Q.; Hou, Y.; Li, S.; Zhuang, K.; Huang, F.; Zhou, J.; Li, Z.; Wang, J.; Fu, L.; et al. Metabolic pathway involved in 2-methyl-6-ethylaniline degradation by Sphingobium sp. strain MEA3-1 and cloning of the novel flavin-dependent monooxygenase system meaBA. Appl. Environ. Microbiol. 2015, 81, 8254–8264. [Google Scholar] [CrossRef]
- Shapir, N.; Rosendahl, C.; Johnson, G.; Andreina, M.; Sadowsky, M.J.; Wackett, L.P. Substrate specificity and colorimetric assay for recombinant TrzN derived from Arthrobacter aurescens TC1. Appl. Environ. Microbiol. 2007, 71, 2214–2220. [Google Scholar] [CrossRef]
- Petric, I.; Bru, D.; Udikovic-Kolic, N.; Hrsak, D.; Philippot, L.; Martin-Laurent, F. Evidence for shifts in the structure and abundance of the microbial community in a long-term PCB-contaminated soil under bioremediation. J. Hazard. Mater. 2011, 195, 254–260. [Google Scholar] [CrossRef]
- Merlin, C.; Devers, M.; Béguet, J.; Boggio, B.; Rouard, N.; Martin-Laurent, F. Evaluation of the ecotoxicological impact of the organochlorine chlordecone on soil microbial community structure, abundance, and function. Environ. Sci. Pollut. Res. 2015, 23, 4185–4198. [Google Scholar] [CrossRef]
- Romdhane, S.; Devers-Lamrani, M.; Barthelmebs, L.; Calvayrac, C.; Bertrand, C.; Cooper, J.-F.; Dayan, F.E.; Martin-Laurent, F. Ecotoxicological impact of the bioherbicide leptospermone on the microbial community of two arable soils. Front. Microbiol. 2016, 7, 775. [Google Scholar] [CrossRef] [PubMed]
- Condette, J.C.; Khorsi-Cauet, H.; Morlière, P.; Zabijak, L.; Reygner, J.; Bach, V.; Gay-Quéheillard, J. Increased gut permeability and bacterial translocation after chronic chlorpyrifos exposure in rats. PLoS ONE 2014, 9, e102217. [Google Scholar] [CrossRef] [PubMed]
- Henn, C.; Monteiro, D.A.; Boscolo, M.; Da Silva, R.; Gomes, E. Biodegradation of atrazine and ligninolytic enzyme production by basidiomycete strains. BMC Microbiol. 2020, 20, 266. [Google Scholar] [CrossRef]
- Floch, C.; Chevremont, A.C.; Joanico, K.; Capowiez, Y.; Criquet, S. Indicators of pesticide contamination: Soil enzyme compared to functional diversity of bacterial communities via Biolog® Ecoplates. Eur. J. Soil Biol. 2011, 47, 256–263. [Google Scholar] [CrossRef]
- Huang, Y.; Xiao, L.; Li, F.; Xiao, M.; Lin, D.; Long, X.; Wu, Z. Microbial degradation of pesticide residues and an emphasis on the degradation of cypermethrin and 3-phenoxy benzoic acid: A review. Molecules 2018, 23, 2313. [Google Scholar] [CrossRef] [PubMed]
- Emurotu, M.O.; Anyanwu, C.U. Effect of atrazine and butachlor on some soil enzymes activities at different concentrations. Pelagia Res. Libr. 2016, 2, 9–15. [Google Scholar]
- Khan, I.A.; Hassan, G.; Malik, N.; Khan, R.; Khan, H.; Khan, S.A. Effect of herbicides on yield and yield components of hybrid maize (Zea mays). Planta Daninha 2016, 34, 729–736. [Google Scholar] [CrossRef]
- Grzanka, M.; Sobiech, Ł.; Idziak, R.; Skrzypczak, G. Effect of the time of herbicide application and the properties of the spray solution on the efficacy of weed control in maize (Zea mays L.) cultivation. Agriculture 2022, 12, 353. [Google Scholar] [CrossRef]
- Yu, Q.Q.; Lu, F.F.; Ma, L.Y.; Yang, H.; Song, N.H. Residues of reduced herbicides terbuthylazine, ametryn, and atrazine and toxicology to maize and the environment through salicylic acid. ACS Omega 2021, 6, 27396–27404. [Google Scholar] [CrossRef]
- Idziak, R.; Woznica, Z. Efficacy of reduced rates of soil-applied dimethenamid-P and pendimethalin mixture followed by postemergence herbicides in maize. Agriculture 2020, 10, 163. [Google Scholar] [CrossRef]
- Idziak, R.; Waligóra, H.; Szuba, V. The influence of agronomical and chemical weed control on weeds of corn. J. Plant Prot. Res. 2022, 62, 215–222. [Google Scholar] [CrossRef]
- Sobiech, Ł.; Grzanka, M.; Skrzypczak, G.; Idziak, R.; Włodarczak, S.; Ochowiak, M. Effect of adjuvants and pH adjuster on the efficacy of sulcotrione herbicide. Agronomy 2020, 10, 530. [Google Scholar] [CrossRef]
- Thiour-Mauprivez, C.; Devers-Lamrani, M.; Bru, D.; Béguet, J.; Spor, A.; Mounier, A.; Alletto, L.; Calvayrac, C.; Barthelmebs, L.; Martin-Laurent, F. Assessing the effects of β-triketone herbicides on the soil bacterial and hppd communities: A lab-to-field experiment. Front. Microbiol. 2021, 11, 610298. [Google Scholar] [CrossRef] [PubMed]
- Mendes, K.F.; Collegari, S.A.; Pimpinato, R.F.; Tornisielo, V.L. Glucose mineralization in soils of contrasting textures under application of s-metolachlor, terbuthylazine, and mesotrione, alone and in a mixture. Bragantia 2017, 77, 152–159. [Google Scholar] [CrossRef]
- Alptekin, H.; Ozkan, A.; Gurbuz, R.; Kulak, M. Management of weeds in maize by sequential or individual applications of pre- and post-emergence herbicides. Agriculture 2023, 13, 421. [Google Scholar] [CrossRef]
- Zhang, S.; Qiu, C.B.; Zhou, Y.; Jin, Z.P.; Yang, H. Bioaccumulation and degradation of pesticide fluroxypyr are associated with toxic tolerance in green alga Chlamydomonas reinhardtii. Ecotoxicology 2011, 20, 337–347. [Google Scholar] [CrossRef]
- Huang, W.; Lu, Y.; Chen, L.; Sun, D.; An, Y. Impact of pesticide/fertilizer mixtures on the rhizosphere microbial community of field-grown sugarcane. 3 Biotech 2021, 11, 210. [Google Scholar] [CrossRef]
- Sangiorgio, D.; Spinelli, F.; Vandelle, E. The unseen effect of pesticides: The impact on phytobiota structure and functions. Front. Agron. 2022, 4, 936032. [Google Scholar] [CrossRef]
- Kwak, M.-J.; Kong, H.G.; Choi, K.; Kwon, S.-K.; Song, J.Y.; Lee, J.; Lee, P.A.; Choi, S.Y.; Seo, M.; Lee, H.J.; et al. Rhizosphere microbiome structure alters to enable wilt resistance in tomato. Nat. Biotechnol. 2018, 36, 1100–1109. [Google Scholar] [CrossRef]
- Park, Y.S.; Ryu, C.M. Understanding plant social networking system: Avoiding deleterious microbiota but calling beneficials. Int. J. Mol. Sci. 2021, 22, 3319. [Google Scholar] [CrossRef]
- Dicke, M. Plant phenotypic plasticity in the phytobiome: A volatile issue. Curr. Opin. Plant Biol. 2016, 32, 17–23. [Google Scholar] [CrossRef] [PubMed]
- IUSS Working Group WRB. World Reference Base for Soil Resources, Update 2015. International Soil Classification System for Naming Soils and Creating Legends for Soil Maps; World Soil Resources Reports No. 106; FAO: Rome, Italy, 2015. [Google Scholar]
- Wyszkowska, J.; Borowik, A.; Zaborowska, M.; Kucharski, J. Sensitivity of Zea mays and soil microorganisms to the toxic effect of chromium (VI). Int. J. Mol. Sci. 2023, 24, 178. [Google Scholar] [CrossRef] [PubMed]
- Kucharski, J.; Tomkiel, M.; Baćmaga, M.; Borowik, A.; Wyszkowska, J. Enzyme activity and microorganisms diversity in soil contaminated with the herbicide Boreal 58 WG. J. Environ. Sci. Health B 2016, 51, 446–454. [Google Scholar] [CrossRef] [PubMed]
- Borowik, A.; Wyszkowska, J.; Kucharski, J. Impact of various grass species on soil bacteriobiome. Diversity 2020, 12, 212. [Google Scholar] [CrossRef]
- Sarathchandra, S.U.; Burch, G.; Cox, N.R. Growth patterns of bacterial communities in the rhizoplane and rhizosphere of with clover (Trifolium repens L.) and perennial ryegrass (Lolium perenne L.) in long-term pasture. Appl. Soil Ecol. 1997, 6, 293–299. [Google Scholar] [CrossRef]
- De Leij, F.A.A.M.; Whipps, J.M.; Lynch, J.M. The use of colony development for the characterization of bacterial communities in soil and on roots. Microb. Ecol. 1993, 27, 81–97. [Google Scholar] [CrossRef]
- Ferris, M.J.; Muyzer, G.; Ward, D.M. Denaturing gradient gel electrophoresis profiles of 16S rRNA-defined populations inhabiting a hot spring microbial mat community. Appl. Environ. Microbiol. 1996, 62, 340–346. [Google Scholar] [CrossRef]
- Öhlinger, R. Dehydrogenase activity with the substrate TTC. In Methods in Soil Biology; Schinner, F., Ohlinger, R., Kandler, E., Margesin, R., Eds.; Springer: Berlin/Heidelberg, Germany, 1996; pp. 241–243. [Google Scholar]
- Johnson, J.L.; Temple, K.L. Some variables affecting the measurement of catalase activity in soil. Soil Sci. Soc. Am. J. 1964, 28, 207–209. [Google Scholar] [CrossRef]
- Alef, K.; Nannipieri, P. (Eds.) Methods in Applied Soil Microbiology and Biochemistry; Academic London: London, UK, 1998; pp. 316–365. [Google Scholar]
- Baćmaga, M.; Wyszkowska, J.; Kucharski, J.; Borowik, A.; Kaczyński, P. Possibilities of restoring homeostasis of soil exposed to terbuthylazine by its supplementation with HumiAgra preparation. Appl. Soil Ecol. 2022, 178, 104582. [Google Scholar] [CrossRef]
- Wyszkowska, J.; Borowik, A.; Zaborowska, M.; Kucharski, J. The usability of sorbents in restoring enzymatic activity in soils polluted with petroleum-derived products. Materials 2023, 16, 3738. [Google Scholar] [CrossRef]
- TIBCO Software Inc. Statistica (Data Analysis Software System), Version 13. 2017. Available online: http://statistica.io (accessed on 15 May 2023).
- Borowik, A.; Wyszkowska, J.; Wyszkowski, M. Resistance of aerobic microorganisms and soil enzyme response to soil contamination with Ekodiesel Ultra Fuel. Environ. Sci. Pollut. Res. 2017, 24, 24346–24363. [Google Scholar] [CrossRef] [PubMed]
- Boros-Lajszner, E.; Wyszkowska, J.; Kucharski, J. Application of white mustard and oats in the phytostabilisation of soil contaminated with cadmium with the addition of cellulose and urea. J. Soils Sediments 2020, 20, 931–942. [Google Scholar] [CrossRef]
- Parks, D.H.; Tyson, G.W.; Hugenholtz, P.; Beiko, R.G. STAMP: Statistical analysis of taxonomic and functional profiles. Bioinformatics 2014, 30, 3123–3124. [Google Scholar] [CrossRef] [PubMed]
- Krzywiński, M.; Schein, J.; Birol, I.; Connors, J.; Gascoyne, R.; Horsman, D.; Jones, S.J.; Marra, M.A. Circos: An information aesthetic for comparative genomics. Genome Res. 2009, 19, 1639–1645. [Google Scholar] [CrossRef] [PubMed]
- RStudio Team. R Studio: Integrated Development; RStudio, Inc.: Boston, MA, USA, 2019; Available online: http://www.rstudio.com/ (accessed on 24 July 2023).
- Warnes, G.R.; Bolker, B.; Bonebakker, L.; Gentleman, R.; Huber, W.; Liaw, A.; Lumley, T.; Maechler, M.; Magnusson, M.; Moeller, S.; et al. Gplots: Various R Programming Tools for Plotting Data. R Package Version 2.17.0. 2020. Available online: https://CRAN.R-Project.org/package=gplots (accessed on 24 July 2023).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019; Available online: https://www.gbif.org/tool/81287/r-a-language-and-environment-for-statistical-computing (accessed on 24 July 2023).
- Heberle, H.; Meirelles, G.V.; da Silva, F.R.; Telles, G.P.; Minghim, R. InteractiVenn: A web-based tool for the analysis of sets through Venn diagrams. BMC Bioinform. 2015, 16, 169. [Google Scholar] [CrossRef] [PubMed]

| Object | Deh | Cat | Pal | Pac | Glu | Aryl | Ure |
|---|---|---|---|---|---|---|---|
| SulRD | −0.142 ± 0.012 b | 0.003 ± 0.008 a | −0.044 ± 0.059 c | 0.078 ± 0.006 e | −0.015 ± 0.011 a | 0.379 ± 0.000 b | −0.233 ± 0.016 a |
| Sul10RD | −0.155 ± 0.010 b | −0.049 ± 0.025 b | −0.220 ± 0.008 d | 0.221 ± 0.004 bc | 0.011 ± 0.001 a | 0.486 ± 0.000 a | −0.340 ± 0.000 c |
| TezRD | −0.107 ± 0.005 ab | 0.001 ± 0.000 a | 0.160 ± 0.050 b | 0.298 ± 0.008 a | 0.004 ± 0.020 a | 0.197 ± 0.034 c | −0.339 ± 0.000 c |
| Tez10RD | −0.081 ± 0.024 a | 0.037 ± 0.000 a | 0.447 ± 0.046 a | 0.205 ± 0.005 c | −0.070 ± 0.001 b | 0.151 ± 0.034 cd | −0.362 ± 0.016 c |
| SucRD | −0.112 ± 0.002 ab | 0.026 ± 0.000 a | 0.548 ± 0.008 a | 0.155 ± 0.012 d | 0.019 ± 0.032 a | 0.203 ± 0.034 c | −0.278 ± 0.016 b |
| Suc10RD | −0.079 ± 0.027 a | −0.051 ± 0.016 b | −0.073 ± 0.021 c | 0.244 ± 0.005 b | 0.009 ± 0.001 a | 0.104 ± 0.017 d | −0.510 ± 0.016 d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baćmaga, M.; Wyszkowska, J.; Borowik, A.; Kucharski, J. Bacteria, Fungi, and Enzymes in Soil Treated with Sulcotrione and Terbuthylazine. Int. J. Mol. Sci. 2023, 24, 14469. https://doi.org/10.3390/ijms241914469
Baćmaga M, Wyszkowska J, Borowik A, Kucharski J. Bacteria, Fungi, and Enzymes in Soil Treated with Sulcotrione and Terbuthylazine. International Journal of Molecular Sciences. 2023; 24(19):14469. https://doi.org/10.3390/ijms241914469
Chicago/Turabian StyleBaćmaga, Małgorzata, Jadwiga Wyszkowska, Agata Borowik, and Jan Kucharski. 2023. "Bacteria, Fungi, and Enzymes in Soil Treated with Sulcotrione and Terbuthylazine" International Journal of Molecular Sciences 24, no. 19: 14469. https://doi.org/10.3390/ijms241914469
APA StyleBaćmaga, M., Wyszkowska, J., Borowik, A., & Kucharski, J. (2023). Bacteria, Fungi, and Enzymes in Soil Treated with Sulcotrione and Terbuthylazine. International Journal of Molecular Sciences, 24(19), 14469. https://doi.org/10.3390/ijms241914469








