A Network Comprised of miR-15b and miR-29a Is Involved in Vascular Endothelial Growth Factor Pathway Regulation in Thymus Adipose Tissue from Elderly Ischemic Cardiomyopathy Subjects
Abstract
1. Introduction
2. Results
2.1. Clinical and Biological Variables of Both Patient Groups
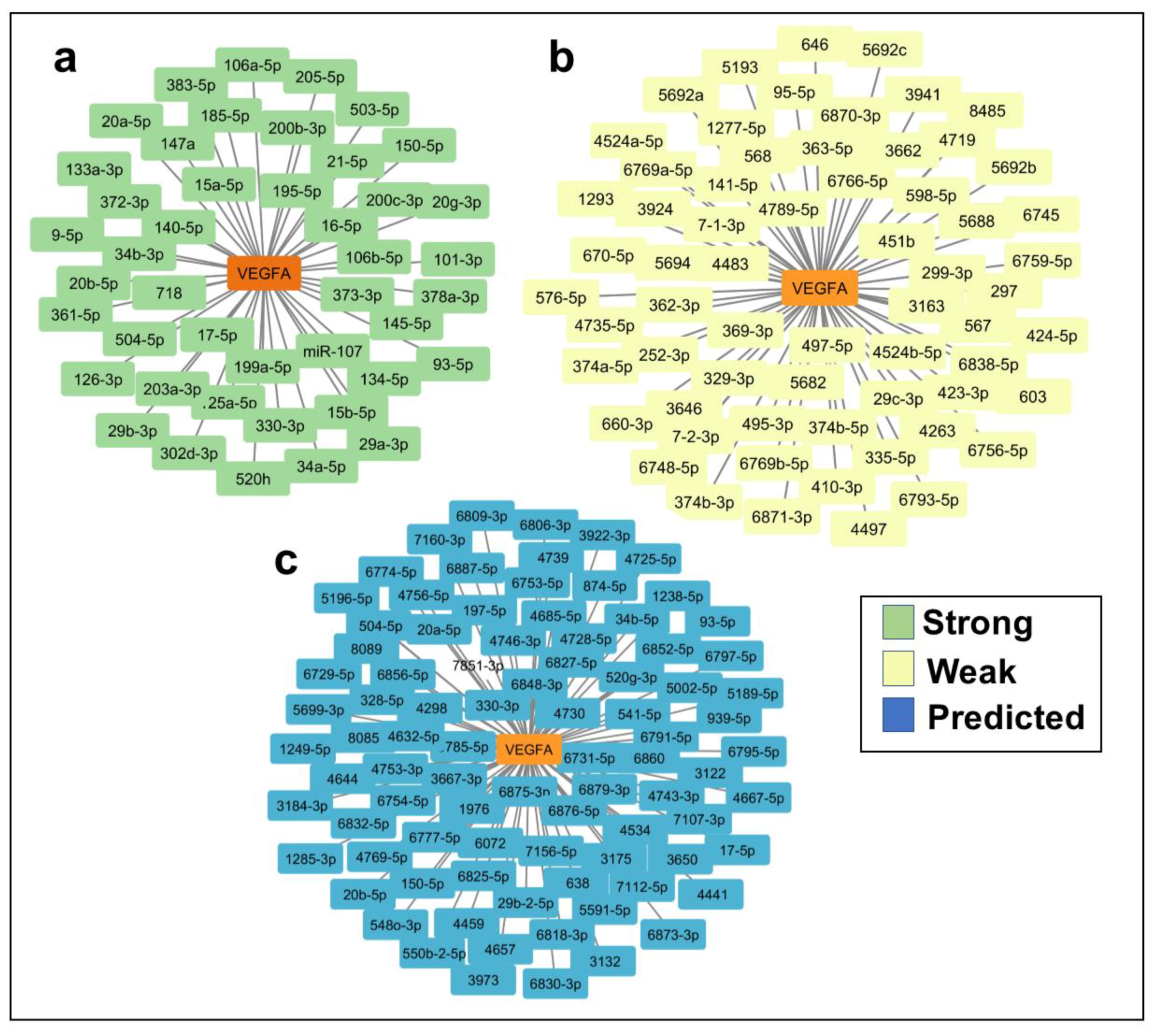
2.2. In Silico Identification of miRNAs Predicted to Regulate VEGFA and TargetScore Calculation of VEGFA miRNA Binding Sites
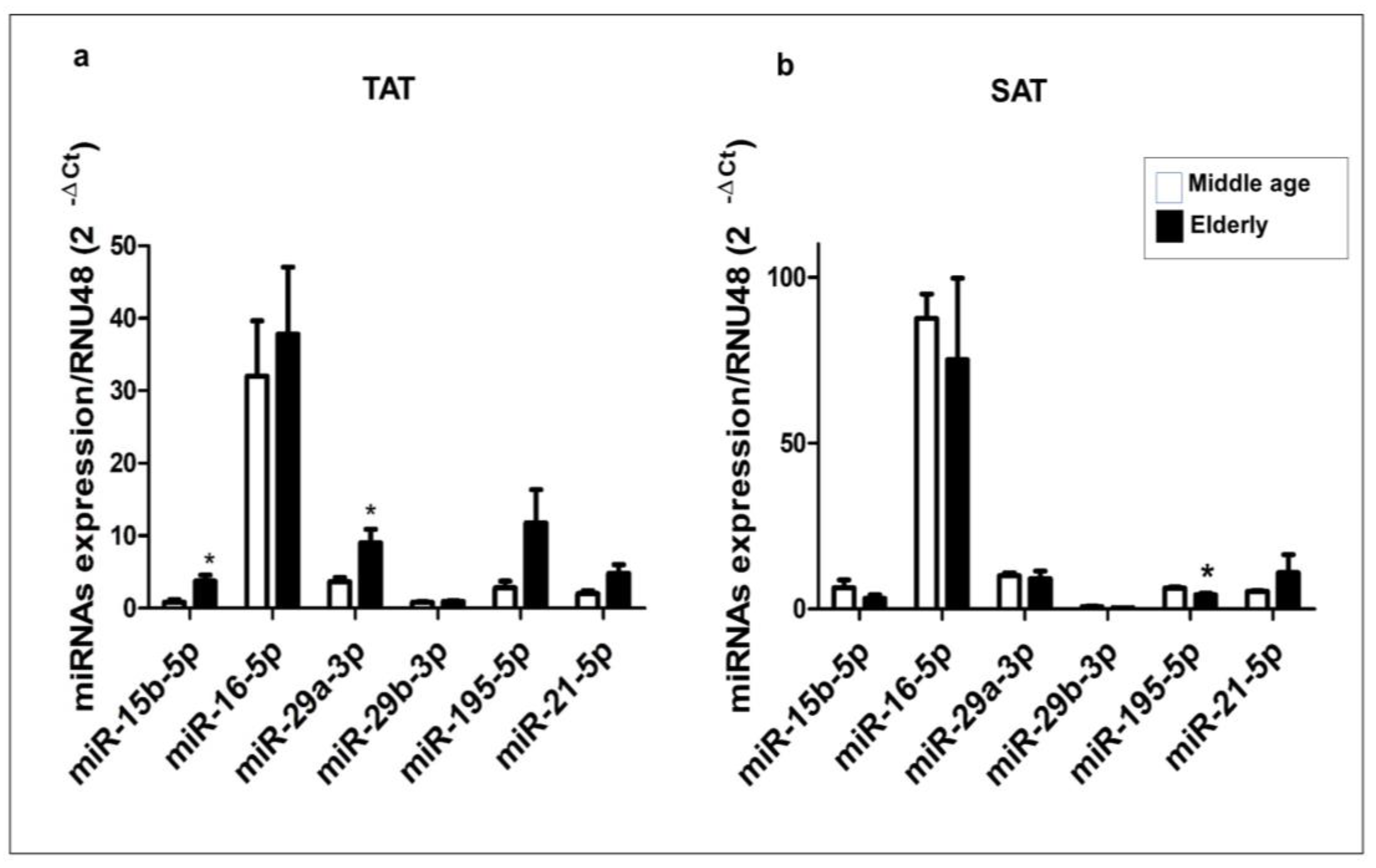
2.3. Expression Profile of miR-15b-5p, miR-16-5p, miR-29a-3p, miR-29b-3p, miR-195-5p, and miR-21-5p in Human TAT and SAT
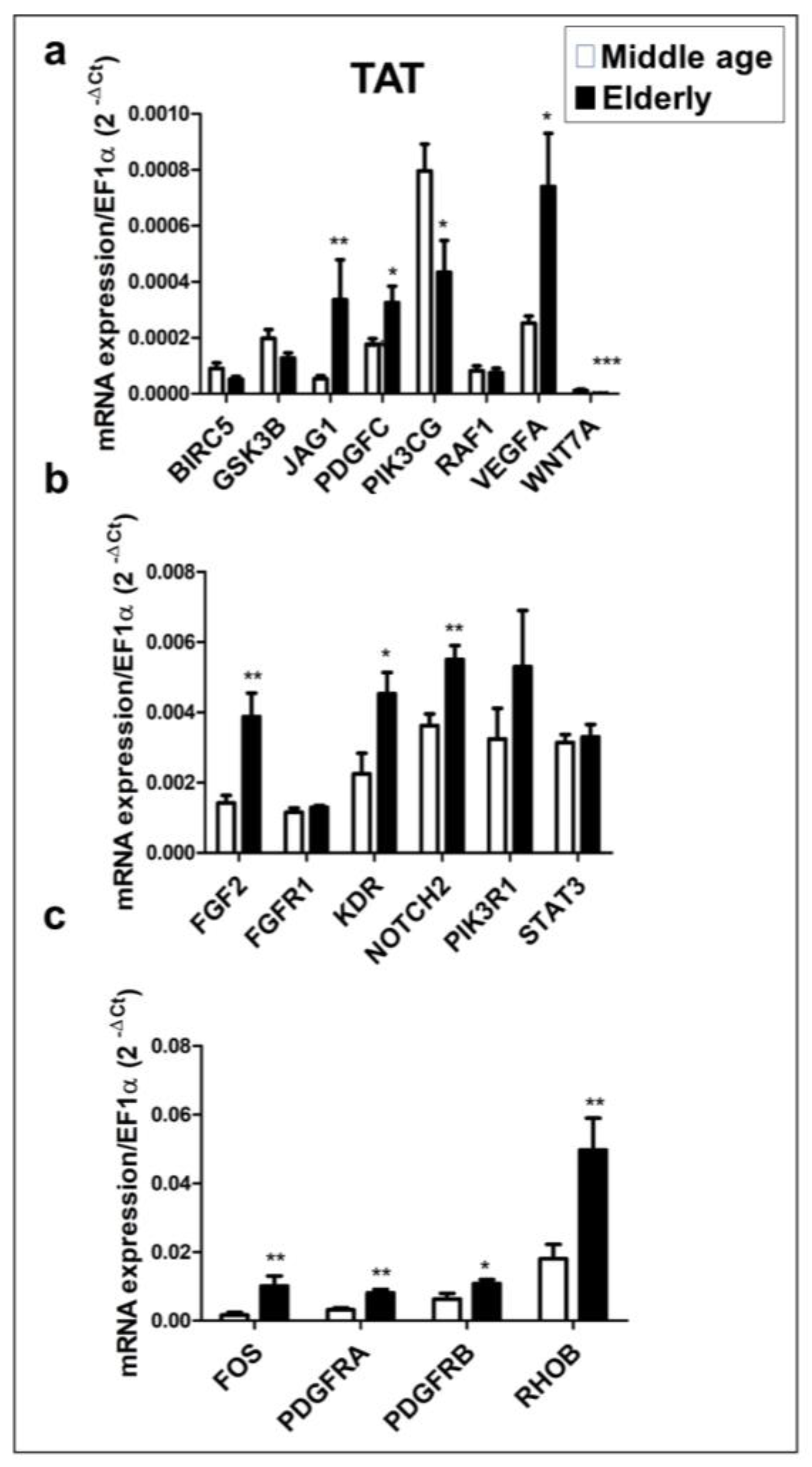
2.4. mRNA Expression Profile of Putative Target Genes in Human TAT
2.5. Interaction Network Model between VEGFA, the miRNAs, and Target Genes in Human TAT of Elderly Patients
3. Discussion
4. Materials and Methods
4.1. Ethical Issues
4.2. Participants and CABG Surgery
4.3. TAT and SAT Collection and Isolation of miRNA and mRNA
4.4. miRNA Extraction and RT-qPCR
4.5. mRNA Isolation and RT-qPCR
4.6. Statistical Analysis
5. Conclusions
6. Study Limitation
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Algoet, M.; Janssens, S.; Himmelreich, U.; Gsell, W.; Pusovnik, M.; Van den Eynde, J.; Oosterlinck, W. Myocardial ischemia-reperfusion injury and the influence of inflammation. Trends Cardiovasc. Med. 2022, 33, 357–366. [Google Scholar] [CrossRef] [PubMed]
- Damluji, A.A.; van Diepen, S.; Katz, J.N.; Menon, V.; Tamis-Holland, J.E.; Bakitas, M.; Cohen, M.G.; Balsam, L.B.; Chikwe, J.; American Heart Association Council on Clinical Cardiology; et al. Mechanical Complications of Acute Myocardial Infarction: A Scientific Statement From the American Heart Association. Circulation 2021, 144, e16–e35. [Google Scholar] [CrossRef] [PubMed]
- Rehman, J.; Traktuev, D.; Li, J.; Merfeld-Clauss, S.; Temm-Grove, C.J.; Bovenkerk, J.E.; Pell, C.L.; Johnstone, B.H.; Considine, R.V.; March, K.L. Secretion of angiogenic and antiapoptotic factors by human adipose stromal cells. Circulation 2004, 109, 1292–1298. [Google Scholar] [CrossRef] [PubMed]
- Kondo, K.; Shintani, S.; Shibata, R.; Murakami, H.; Murakami, R.; Imaizumi, M.; Kitagawa, Y.; Murohara, T. Implantation of adipose-derived regenerative cells enhances ischemia-induced angiogenesis. Arter. Thromb. Vasc. Biol. 2009, 29, 61–66. [Google Scholar] [CrossRef] [PubMed]
- El-Ftesi, S.B.; Chang, E.I.M.; Longaker, M.T.M.; Gurtner, G.C.M. Aging and diabetes impair the neovascular potential of adipose-derived stromal cells. Plast. Reconstr. Surg. 2009, 123, 475–485. [Google Scholar] [CrossRef]
- Efimenko, A.; Starostina, E.; Kalinina, N.; Stolzing, A. Angiogenic properties of aged adipose derived mesenchymal stem cells after hypoxic conditioning. J. Transl. Med. 2011, 9, 10. [Google Scholar] [CrossRef]
- Kvell, K.; Pongracz, J.E. Central Immune Senescence, Reversal Potentials. In Senescence; Nagata, T., Ed.; InTech: Rijeka, Croatia, 2012; Chapter 31. [Google Scholar]
- Aragüez, L.C.; Murri, M.; Olivera, W.O.; Salas, J.; Mayas, M.D.; Delgado-Lista, J.; Tinahones, F.; El Bekay, R. Thymus fat as an attractive source of angiogenic factors in elderly subjects with myocardial ischemia. AGE 2012, 35, 1263–1275. [Google Scholar] [CrossRef][Green Version]
- Salas, J.; Montiel, M.; Jiménez, E.; Valenzuela, M.; Valderrama, J.F.; Castillo, R.; González, S.; El Bekay, R. Angiogenic properties of adult human thymus fat. Cell Tissue Res. 2009, 338, 313–318. [Google Scholar] [CrossRef]
- Holmes, D.I.; Zachary, I. The vascular endothelial growth factor (VEGF) family: Angiogenic factors in health and disease. Genome Biol. 2005, 6, 209. [Google Scholar] [CrossRef][Green Version]
- Bhatti, G.K.; Khullar, N.; Sidhu, I.S.; Navik, U.S.; Reddy, A.P.; Reddy, P.H.; Bhatti, J.S. Emerging role of non-coding RNA in health and disease. Metab. Brain Dis. 2021, 36, 1119–1134. [Google Scholar] [CrossRef]
- Siebert, V.; Allencherril, J.; Ye, Y.; Wehrens, X.H.T.; Birnbaum, Y. The Role of Non-coding RNAs in Ischemic Myocardial Reperfusion Injury. Cardiovasc. Drugs Ther. 2019, 33, 489–498. [Google Scholar] [CrossRef] [PubMed]
- Enright, A.J.; John, B.; Gaul, U.; Tuschl, T.; Sander, C.; Marks, D.S. MicroRNA targets in Drosophila. Genome Biol. 2003, 5, R1. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, V.; Bell, G.W.; Nam, J.-W.; Bartel, D.P. Predicting effective microRNA target sites in mammalian mRNAs. eLife 2015, 4, e05005. [Google Scholar] [CrossRef] [PubMed]
- Lhamyani, S.; Gentile, A.-M.; Giráldez-Pérez, R.M.; Feijóo-Cuaresma, M.; Romero-Zerbo, S.Y.; Clemente-Postigo, M.; Zayed, H.; Oliva-Olivera, W.; Bermúdez-Silva, F.J.; Salas, J.; et al. miR-21 mimic blocks obesity in mice: A novel therapeutic option. Mol. Ther.-Nucleic Acids 2021, 26, 401–416. [Google Scholar] [CrossRef]
- Oliva-Olivera, W.; Coín-Aragüez, L.; Salas, J.; Lhamyani, S.; Gentile, A.-M.; García, E.S.; Hmadcha, A.; Zayed, H.; Vega-Rioja, A.; Tinahones, F.J.; et al. Myocardial Ischemic Subject’s Thymus Fat: A Novel Source of Multipotent Stromal Cells. PLoS ONE 2015, 10, e0144401. [Google Scholar] [CrossRef]
- Ding, M.-H.; Lozoya, E.G.; Rico, R.N.; Chew, S.A. The Role of Angiogenesis-Inducing microRNAs in Vascular Tissue Engineering. Tissue Eng. Part A 2020, 26, 1283–1302. [Google Scholar] [CrossRef]
- Che, P.; Liu, J.; Shan, Z.; Wu, R.; Yao, C.; Cui, J.; Zhu, X.; Wang, J.; Burnett, M.S.; Wang, S.; et al. miR-125a-5p impairs endothelial cell angiogenesis in aging mice via RTEF -1 downregulation. Aging Cell 2014, 13, 926–934. [Google Scholar] [CrossRef]
- MacLean, J.A.; King, M.L.; Okuda, H.; Hayashi, K. WNT7A Regulation by miR-15b in Ovarian Cancer. PLoS ONE 2016, 11, e0156109. [Google Scholar] [CrossRef]
- Kabekkodu, S.P.; Shukla, V.; Varghese, V.K.; Souza, J.D.; Chakrabarty, S.; Satyamoorthy, K. Clustered miRNAs and their role in biological functions and diseases. Biol. Rev. 2018, 93, 1955–1986. [Google Scholar] [CrossRef]
- Zhao, W.-J.; Zhang, H.-F.; Su, J.-Y. Downregulation of microRNA-195 promotes angiogenesis induced by cerebral infarction via targeting VEGFA. Mol. Med. Rep. 2017, 16, 5434–5440. [Google Scholar] [CrossRef]
- Yang, I.-P.; Tsai, H.-L.; Huang, C.-W.; Lu, C.-Y.; Miao, Z.-F.; Chang, S.-F.; Juo, S.-H.H.; Wang, J.-Y. High blood sugar levels significantly impact the prognosis of colorectal cancer patients through down-regulation of microRNA-16 by targeting Myb and VEGFR2. Oncotarget 2016, 7, 18837–18850. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Yin, M.; Wei, X.; Liu, J.; Wang, X.; Niu, C.; Kang, X.; Xu, J.; Zhou, Z.; Sun, S.; et al. Bach1 Represses Wnt/β-Catenin Signaling and Angiogenesis. Circ. Res. 2015, 117, 364–375. [Google Scholar] [CrossRef] [PubMed]
- Desjarlais, M.; Dussault, S.; Rivera, J.C.; Chemtob, S.; Rivard, A. MicroRNA Expression Profiling of Bone Marrow–Derived Proangiogenic Cells (PACs) in a Mouse Model of Hindlimb Ischemia: Modulation by Classical Cardiovascular Risk Factors. Front. Genet. 2020, 11, 947. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.N.; Jang, J.-P.; Han, J.M.; Jang, J.-H.; Ahn, J.S.; Jung, H.J. Antiangiogenic Potential of Microbial Metabolite Elaiophylin for Targeting Tumor Angiogenesis. Molecules 2018, 23, 563. [Google Scholar] [CrossRef]
- Cakmak, H.; Gokmen, E.; Bozkurt, G.; Kocaturk, T.; Ergin, K. Effects of sunitinib and bevacizumab on VEGF and miRNA levels on corneal neovascularization. Cutan. Ocul. Toxicol. 2017, 37, 191–195. [Google Scholar] [CrossRef]
- Cignarelli, A.; Perrini, S.; Nigro, P.; Ficarella, R.; Barbaro, M.; Peschechera, A.; Porro, S.; Natalicchio, A.; Laviola, L.; Puglisi, F.; et al. Long-acting insulin analog detemir displays reduced effects on adipocyte differentiation of human subcutaneous and visceral adipose stem cells. Nutr. Metab. Cardiovasc. Dis. 2015, 26, 333–344. [Google Scholar] [CrossRef]
- Gaebler, N.; Haggenmüller, B.; Kapapa, M.; Serra, A.; Tews, D.; Funcke, J.-B.; Brandt, S.; Ioannidis, V.; Schön, M.; Möller, P.; et al. Age- and BMI-Associated Expression of Angiogenic Factors in White Adipose Tissue of Children. Int. J. Mol. Sci. 2019, 20, 5204. [Google Scholar] [CrossRef]
- Gentile, A.; Lhamyani, S.; Coín-Aragüez, L.; Clemente-Postigo, M.; Olivera, W.O.; Romero-Zerbo, S.; García-Serrano, S.; García-Escobar, E.; Zayed, H.; Doblado, E.; et al. miR-20b, miR-296, and Let-7f Expression in Human Adipose Tissue is Related to Obesity and Type 2 Diabetes. Obesity 2018, 27, 245–254. [Google Scholar] [CrossRef]
- Hindy, G.; Mollet, I.G.; Rukh, G.; Ericson, U.; Orho-Melander, M. Several type 2 diabetes-associated variants in genes annotated to WNT signaling interact with dietary fiber in relation to incidence of type 2 diabetes. Genes Nutr. 2016, 11, 1–9. [Google Scholar] [CrossRef]
- Boucher, J.M.; Ryzhova, L.; Harrington, A.; Davis-Knowlton, J.; Turner, J.E.; Cooper, E.; Maridas, D.; Ryzhov, S.; Rosen, C.J.; Vary, C.P.; et al. Pathological Conversion of Mouse Perivascular Adipose Tissue by Notch Activation. Arter. Thromb. Vasc. Biol. 2020, 40, 2227–2243. [Google Scholar] [CrossRef]
- Chen, Z.; Yu, H.; Shi, X.; Warren, C.R.; Lotta, L.A.; Friesen, M.; Meissner, T.B.; Langenberg, C.; Wabitsch, M.; Wareham, N.; et al. Functional Screening of Candidate Causal Genes for Insulin Resistance in Human Preadipocytes and Adipocytes. Circ. Res. 2019, 126, 330–346. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Pande, S.; Koza, R.A.; Friesel, R. Sprouty1 regulates gonadal white adipose tissue growth through a PDGFRα/β-Akt pathway. Adipocyte 2021, 10, 574–586. [Google Scholar] [CrossRef] [PubMed]
- Kakudo, N.; Kushida, S.; Suzuki, K.; Matsumoto, N.; Kusumoto, K. The effect of C3 transferase on human adipose-derived stem cells. Hum. Cell 2011, 24, 165–169. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Shi, Y.; Wu, M.; Zhang, F.; Xu, M.; He, Z.; Tang, M. JAG1 enhances angiogenesis in triple-negative breast cancer through promoting the secretion of exosomal lncRNA MALAT1. Genes Dis. 2023, 10, 2167–2178. [Google Scholar] [CrossRef]
- Hodges, N.A.; Suarez-Martinez, A.D.; Murfee, W.L. Understanding angiogenesis during aging: Opportunities for discoveries and new models. J. Appl. Physiol. 2018, 125, 1843–1850. [Google Scholar] [CrossRef]
- Roshandel, E.; Noorazar, L.; Farhadihosseinabadi, B.; Mehdizadeh, M.; Kazemi, M.H.; Parkhideh, S. PI3 kinase signaling pathway in hematopoietic cancers: A glance in miRNA’s role. J. Clin. Lab. Anal. 2021, 35, e23725. [Google Scholar] [CrossRef]
- Semba, S.; Itoh, N.; Ito, M.; Youssef, E.M.; Harada, M.; Moriya, T.; Kimura, W.; Yamakawa, M. Down-regulation of PIK3CG, a catalytic subunit of phosphatidylinositol 3-OH kinase, by CpG hypermethylation in human colorectal carcinoma. Clin. Cancer Res. 2002, 8, 3824–3831. [Google Scholar]
- Oliva-Olivera, W.; Coín-Aragüez, L.; Lhamyani, S.; Salas, J.; Gentile, A.; Romero-Zerbo, S.; Zayed, H.; Valderrama, J.; Tinahones, F.J.; El Bekay, R. Differences in the neovascular potential of thymus versus subcutaneous adipose-derived stem cells from patients with myocardial ischaemia. J. Tissue Eng. Regen. Med. 2017, 12, e1772–e1784. [Google Scholar] [CrossRef]
- Staels, W.; Heremans, Y.; Heimberg, H.; De Leu, N. VEGF-A and blood vessels: A beta cell perspective. Diabetologia 2019, 62, 1961–1968. [Google Scholar] [CrossRef]
- Akl, M.R.; Nagpal, P.; Ayoub, N.M.; Tai, B.; Prabhu, S.A.; Capac, C.M.; Gliksman, M.; Goy, A.; Suh, K.S. Molecular and clinical significance of fibroblast growth factor 2 (FGF2/bFGF) in malignancies of solid and hematological cancers for personalized therapies. Oncotarget 2016, 7, 44735–44762. [Google Scholar] [CrossRef]
- Nakamura, Y. Multiple Therapeutic Applications of RBM-007, an Anti-FGF2 Aptamer. Cells 2021, 10, 1617. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, H.; Chen, Y.; Fu, J.; Lei, Y.; Sun, J.; Tang, B. Platelet-derived growth factor D promotes the angiogenic capacity of endothelial progenitor cells. Mol. Med. Rep. 2018, 19, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Laschke, M.W.; Menger, M.D. Microvascular Fragments in Microcirculation Research and Regenerative Medicine. Tissue Eng. Part B Rev. 2022, 28, 1109–1120. [Google Scholar] [CrossRef] [PubMed]


| Variables | Middle-Aged (n = 8) | Elderly (n = 10) | p Value |
|---|---|---|---|
| Age (years) | 56.63 ± 1.72 | 74 ± 1.09 | 0.0001 |
| Sex (M/W) | 5/3 | 6/4 | NS |
| BMI (kg/m2) | 28.75 ± 1.74 | 29.40 ± 1.44 | NS |
| Glucose (mg/dL) | 104.12 ± 7.61 | 101.50 ± 5.33 | NS |
| Triglycerides (mg/dL) | 110.12 ± 10.36 | 116.80 ± 12.53 | NS |
| Cholesterol (mg/dL) | 129.00 ± 14.60 | 156.00 ± 10.06 | NS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gentile, A.M.; Lhamyani, S.; Mengual Mesa, M.; Pavón-Morón, F.J.; Pearson, J.R.; Salas, J.; Clemente-Postigo, M.; Pérez Costillas, L.; Fuster, G.O.; El Bekay Rizky, R. A Network Comprised of miR-15b and miR-29a Is Involved in Vascular Endothelial Growth Factor Pathway Regulation in Thymus Adipose Tissue from Elderly Ischemic Cardiomyopathy Subjects. Int. J. Mol. Sci. 2023, 24, 14456. https://doi.org/10.3390/ijms241914456
Gentile AM, Lhamyani S, Mengual Mesa M, Pavón-Morón FJ, Pearson JR, Salas J, Clemente-Postigo M, Pérez Costillas L, Fuster GO, El Bekay Rizky R. A Network Comprised of miR-15b and miR-29a Is Involved in Vascular Endothelial Growth Factor Pathway Regulation in Thymus Adipose Tissue from Elderly Ischemic Cardiomyopathy Subjects. International Journal of Molecular Sciences. 2023; 24(19):14456. https://doi.org/10.3390/ijms241914456
Chicago/Turabian StyleGentile, Adriana Mariel, Said Lhamyani, María Mengual Mesa, Francisco Javier Pavón-Morón, John R. Pearson, Julián Salas, Mercedes Clemente-Postigo, Lucía Pérez Costillas, Gabriel Olveira Fuster, and Rajaa El Bekay Rizky. 2023. "A Network Comprised of miR-15b and miR-29a Is Involved in Vascular Endothelial Growth Factor Pathway Regulation in Thymus Adipose Tissue from Elderly Ischemic Cardiomyopathy Subjects" International Journal of Molecular Sciences 24, no. 19: 14456. https://doi.org/10.3390/ijms241914456
APA StyleGentile, A. M., Lhamyani, S., Mengual Mesa, M., Pavón-Morón, F. J., Pearson, J. R., Salas, J., Clemente-Postigo, M., Pérez Costillas, L., Fuster, G. O., & El Bekay Rizky, R. (2023). A Network Comprised of miR-15b and miR-29a Is Involved in Vascular Endothelial Growth Factor Pathway Regulation in Thymus Adipose Tissue from Elderly Ischemic Cardiomyopathy Subjects. International Journal of Molecular Sciences, 24(19), 14456. https://doi.org/10.3390/ijms241914456







