Function of Transcription Factors PoMYB12, PoMYB15, and PoMYB20 in Heat Stress and Growth of Pleurotus ostreatus
Abstract
1. Introduction
2. Results
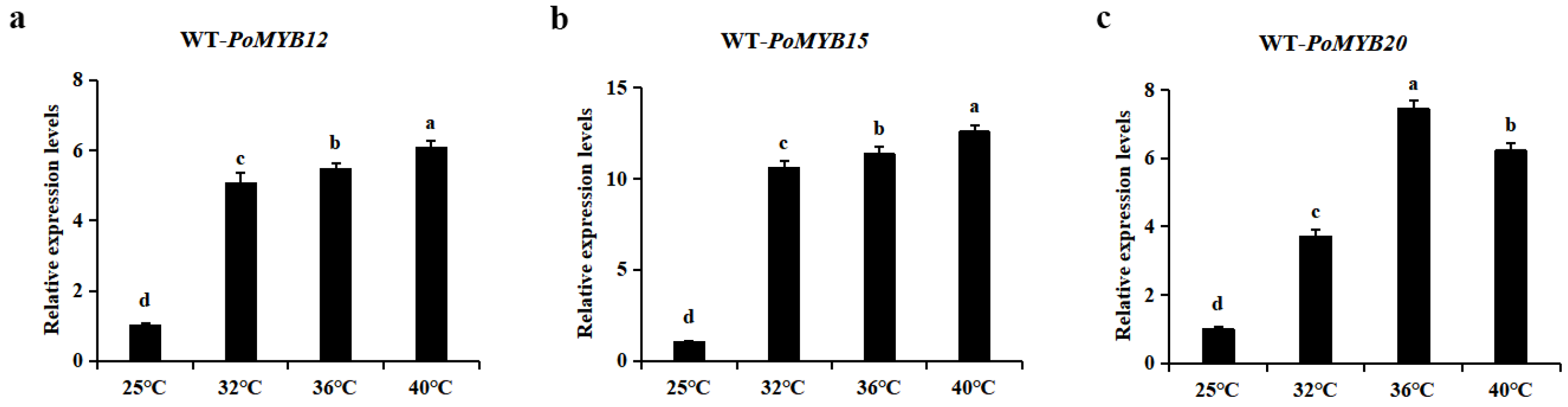
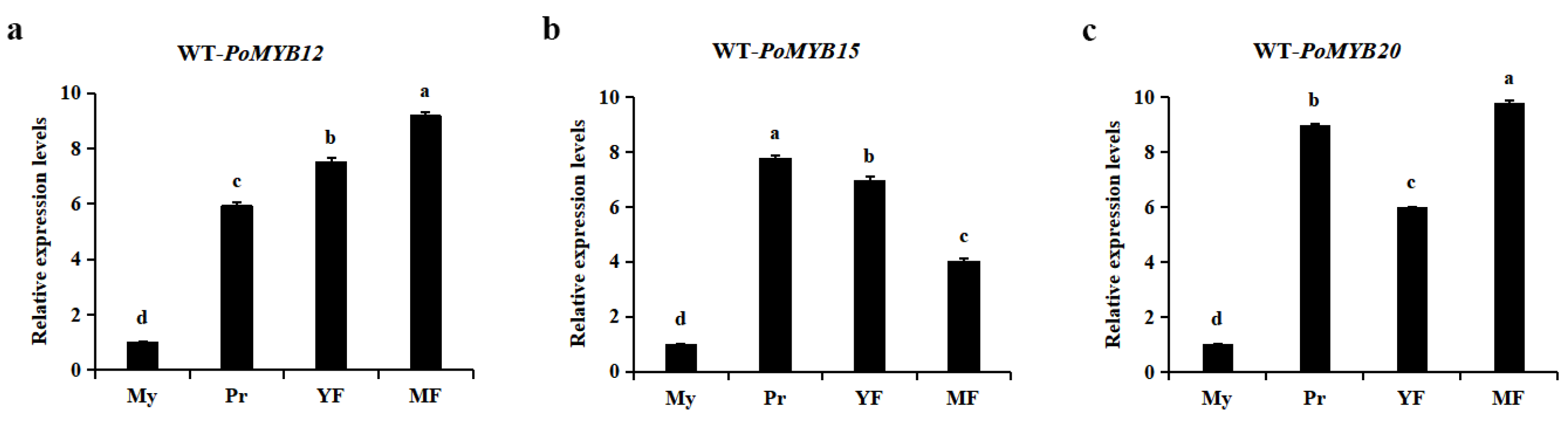
2.1. PoMYB12, PoMYB15, and PoMYB20 May Be Involved in the Response of P. ostreatus Mycelium to Heat Stress
2.2. PoMYB12, PoMYB15, and PoMYB20 Are Involved in the Response of P. ostreatus Mycelium to Heat Stress
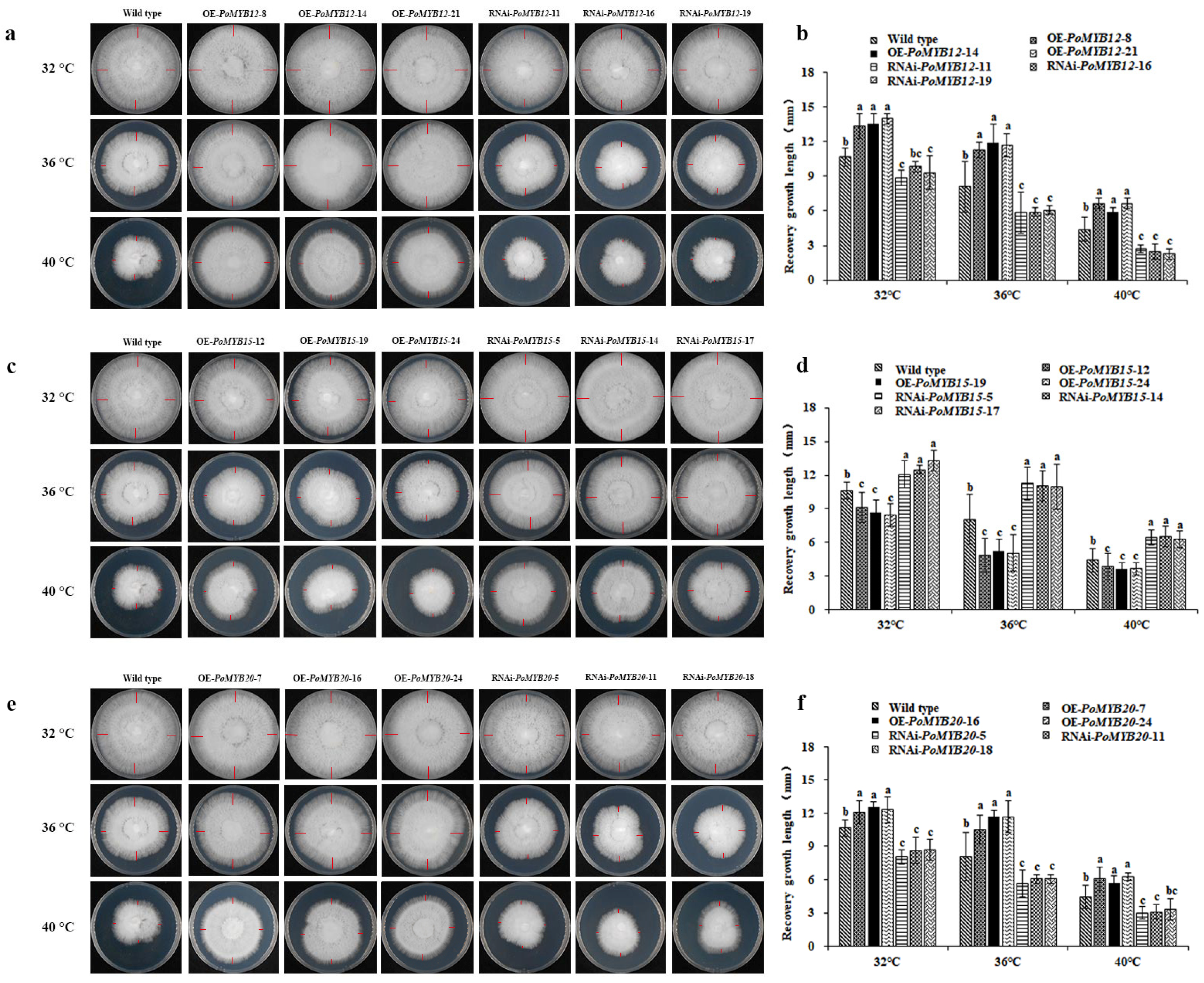
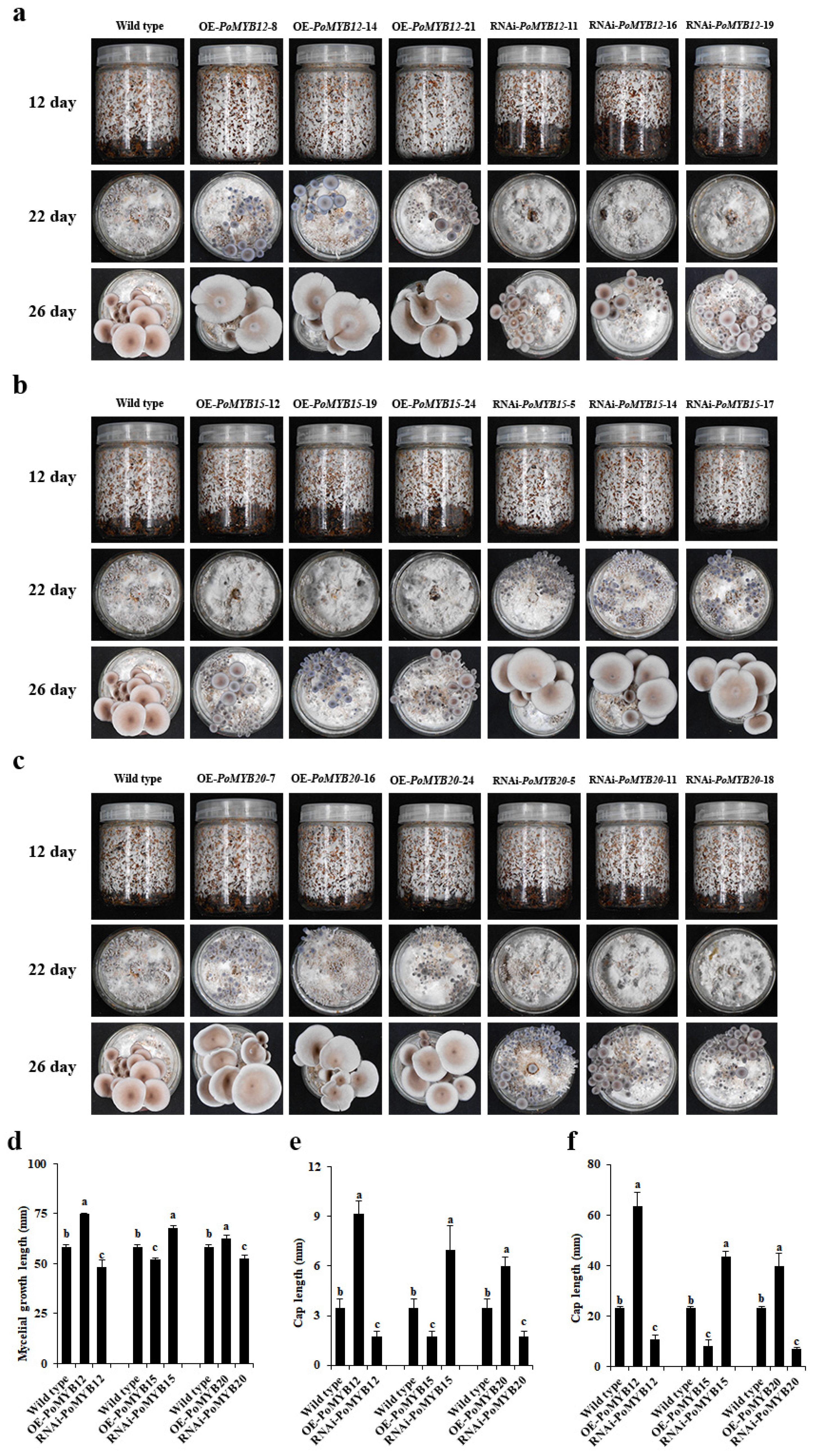
2.3. PoMYB12, PoMYB15, and PoMYB20 May Be Involved in the Growth and Development of P. ostreatus
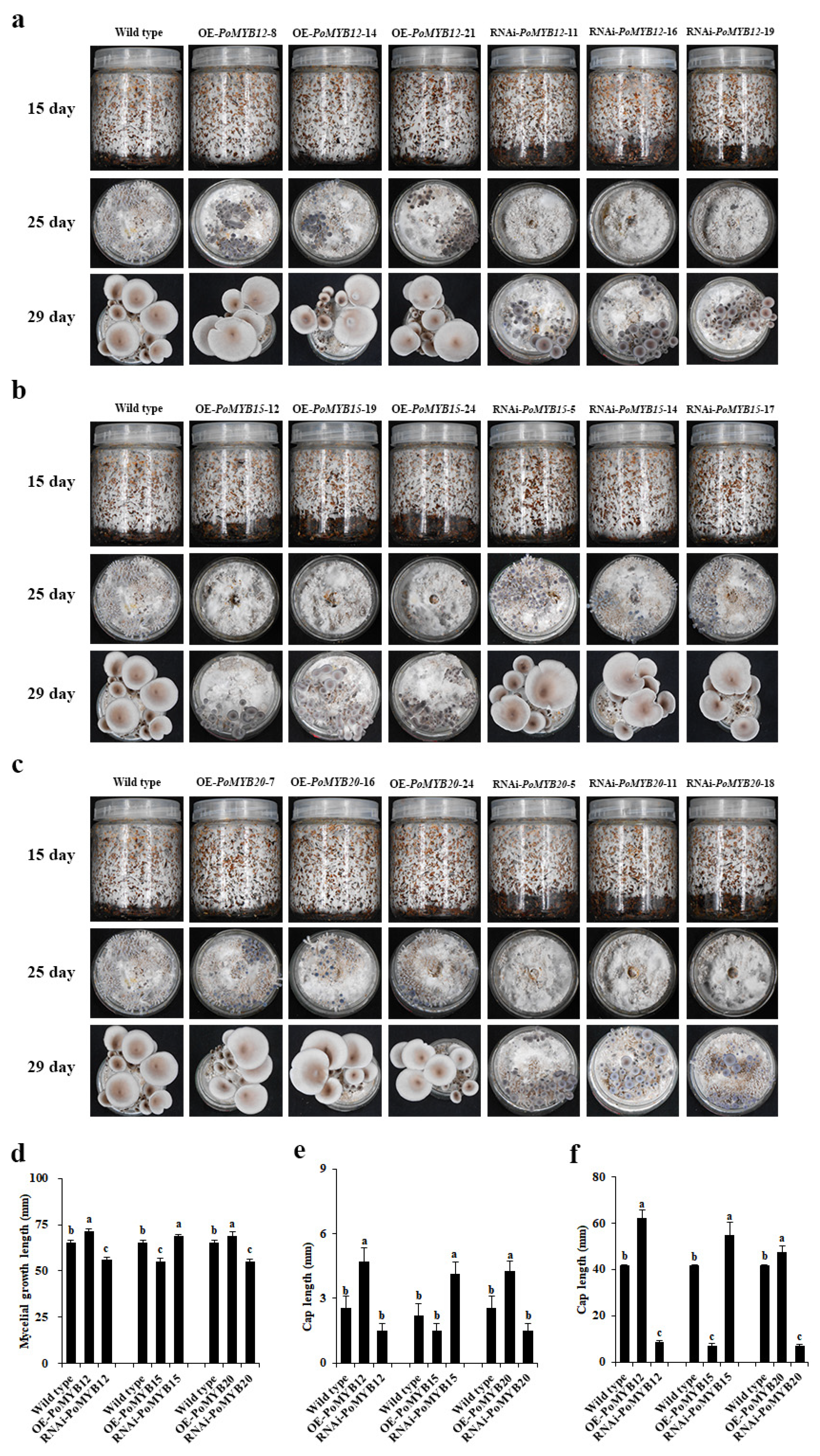
2.4. PoMYB12, PoMYB15, and PoMYB20 Are Involved in the Growth and Development of P. ostreatus
2.5. PoMYB12, PoMYB15, and PoMYB20 Are Involved in Growth and Development in Response to 36 °C Heat Stress of P. ostreatus
3. Discussion
4. Materials and Methods
4.1. Strains Tested
4.2. Construction of OE and RNAi Plasmids of PoMYB12, PoMYB15, PoMYB20
4.3. Recombinant Plasmids Transformed into Competent Cells of A. tumefaciens
4.4. A. tumefaciens-Mediated Recombinant Plasmid Transfer to the Mycelium of P. ostreatus
4.5. Detection of Gene Expression in WT Strain under Different Heat Stress Conditions
4.6. Detection of Heat Stress Resistance in PoMYB12, PoMYB15, and PoMYB20 Transformed Strains
4.7. Analysis of Gene Expression Levels and Effects of Transformed Strains on the Growth and Development of P. ostreatus
4.8. Growth and Development of Transformed Strains after 24 h of Response to 36 °C Heat Stress
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Feller, A.; Machemer, K.; Braun, E.L.; Grotewold, E. Evolutionary and comparative analysis of MYB and bHLH plant transcription factors. Plant J. 2011, 66, 94–116. [Google Scholar] [CrossRef]
- Baldoni, E.; Genga, A.; Cominelli, E. Plant MYB transcription factors: Their role in drought response mechanisms. Int. J. Mol. Sci. 2015, 16, 15811–15851. [Google Scholar] [CrossRef] [PubMed]
- Li, J.L.; Han, G.L.; Sun, C.F.; Sui, N. Research advances of MYB transcription factors in plant stress resistance and breeding. Plant Signal Behav. 2019, 14, 1613131. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.J.; Ma, Z.T.; Chen, H.; Liu, M.Y. MYB gene family in potato (Solanum tuberosum L.): Genome-wide identification of hormone-responsive reveals their potential functions in growth and development. Int. J. Mol. Sci. 2019, 20, 4847. [Google Scholar] [CrossRef] [PubMed]
- Qu, X.X.; Zou, J.J.; Wang, J.X.; Yang, K.Z.; Wang, X.Q.; Le, J. A rice R2R3-type MYB transcription factor OsFLP positively regulates drought stress response via OsNAC. Int. J. Mol. Sci. 2022, 23, 5873. [Google Scholar] [CrossRef]
- Li, X.X.; Guo, C.; Li, Z.Y.; Wang, G.P.; Yang, J.S.; Chen, L.; Hu, Z.G.; Sun, J.H.; Gao, J.P.; Yang, A.G.; et al. Deciphering the roles of tobacco MYB transcription factors in environmental stress tolerance. Front. Plant Sci. 2022, 13, 998606. [Google Scholar] [CrossRef]
- Zhao, H.X.; Yao, P.F.; Zhao, J.L.; Wu, H.L.; Wang, S.; Chen, Y.; Hu, M.F.; Wang, T.; Li, C.L.; Wu, Q. A novel R2R3-MYB tran-scription factor FtMYB22 negatively regulates salt and drought stress through ABA-dependent pathway. Int. J. Mol. Sci. 2022, 23, 14549. [Google Scholar] [CrossRef]
- Tang, M.Q.; Liu, L.; Hu, X.; Zheng, H.Y.; Wang, Z.K.; Liu, Y.; Zhu, Q.; Cui, L.C.; Xie, S.Q. Genome-wide characterization of R2R3-MYB gene family in Santalum album and their expression analysis under cold stress. Front. Plant Sci. 2023, 14, 1142562. [Google Scholar] [CrossRef]
- Han, Z.L.; Zhang, C.; Zhang, H.; Duan, Y.; Zou, Z.W.; Zhou, L.; Zhu, X.J.; Fang, W.P.; Ma, Y.C. CsMYB transcription factors participate in jasmonic acid signal transduction in response to cold stress in tea plant (Camellia sinensis). Plants 2022, 11, 2869. [Google Scholar] [CrossRef]
- Casaretto, J.A.; El-Kereamy, A.; Zeng, B.; Stiegelmeyer, S.M.; Chen, X.; Bi, Y.M.; Rothstein, S.J. Expression of OsMYB55 in maize activates stress-responsive genes and enhances heat and drought tolerance. BMC Genom. 2016, 17, 312. [Google Scholar] [CrossRef]
- Wu, Z.; Li, T.; Liu, X.Y.; Yuan, G.Z.; Hou, H.Z.; Teng, N.J. A novel R2R3-MYB transcription factor LlMYB305 from Lilium longiflorum plays a positive role in thermotolerance by activating heat-protective genes. Environ. Exp. Bot. 2021, 184, 104399. [Google Scholar] [CrossRef]
- Yang, B.C.; Song, Z.H.; Li, C.N.; Jiang, J.H.; Zhou, Y.Y.; Wang, R.P.; Wang, Q.; Ni, C.; Liang, Q.; Chen, H.D.; et al. RSM1, an Arabidopsis MYB protein, interacts with HY5/HYH to modulate seed germination and seedling development in response to abscisic acid and salinity. PLoS Genet. 2018, 14, e1007839. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.B.; Zhu, J.; Gao, J.F.; Wang, C.; Li, H.; Zhang, H.Q.; Zhang, S.; Wang, D.M.; Wang, Q.X.; Huang, H.; et al. Transcription factor AtMYB103 is required for anther development by regulating tapetum development, callose dissolution and exine formation in Arabidopsis. Plant J. 2007, 52, 528–538. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.C.; Hou, S.L.; Guo, X.F.; Jia, J.T.; Yang, W.G.; Liu, Z.J.; Chen, S.Y.; Li, X.X.; Qi, D.M.; Liu, G.; et al. A MYB-related transcription factor from sheepgrass, LcMYB2, promotes seed germination and root growth under drought stress. BMC Plant Biol. 2019, 19, 564. [Google Scholar] [CrossRef]
- Huang, C.J.; Hu, G.J.; Li, F.F.; Li, Y.Q.; Wu, J.X.; Zhou, X.P. NbPHAN, a MYB transcription factor, regulates leaf development and affects drought tolerance in Nicotiana benthamiana. Physiol. Plant 2013, 149, 297–309. [Google Scholar]
- Kim, Y.; Kim, H.; Son, H.; Choi, G.J.; Kim, J.C.; Lee, Y.W. MYT3, a Myb-like transcription factor, affects fungal development and pathogenicity of Fusarium graminearum. PLoS ONE 2014, 9, e94359. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, P.J.; Li, H.H.; Wang, Y.L.; Long, L.K.; Li, K.; Zhang, X.L.; Pan, Y.Y.; Liu, G. A Myb transcription factor represses conidiation and cephalosporin C production in Acremonium chrysogenum. Fungal Genet. Biol. 2018, 118, 1–9. [Google Scholar] [CrossRef]
- Meng, L.; Lyu, X.M.; Shi, L.L.; Wang, Q.J.; Wang, L.; Zhu, M.J.; Mukhtar, L.; Xie, B.G.; Wang, W. The transcription factor FvHmg1 negatively regulates fruiting body development in Winter Mushroom Flammulina velutipes. Gene 2021, 785, 145618. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.J.; Hu, C.C.; Xie, B.G.; Zhang, L.; Yan, S.J.; Wei, W.; Tao, Y.X.; Li, S.J. A single transcription factor (PDD1) determines development and yield of winter mushroom (Flammulina velutipes). Appl. Environ. Microbiol. 2019, 85, e01735-19. [Google Scholar] [CrossRef]
- Liu, X.B.; Xia, E.H.; Li, M.; Cui, Y.Y.; Wang, P.M.; Zhang, J.X.; Xie, B.G.; Xu, J.P.; Yan, J.J.; Nagy, L.G.; et al. Transcriptome data reveal conserved patterns of fruiting body development and response to heat stress in the mushroom-forming fungus Flammulina filiformis. PLoS ONE 2020, 15, e0239890. [Google Scholar] [CrossRef]
- Hou, L.D.; Wang, L.N.; Wu, X.L.; Gao, W.; Zhang, J.X.; Huang, C.Y. Expression patterns of two pal genes of Pleurotus ostreatus across developmental stages and under heat stress. BMC Microbiol. 2019, 19, 231. [Google Scholar] [CrossRef] [PubMed]
- Hou, L.D.; Zhao, M.R.; Huang, C.Y.; He, Q.; Zhang, L.G.; Zhang, J.X. Alternative oxidase gene induced by nitric oxide is involved in the regulation of ROS and enhances the resistance of Pleurotus ostreatus to heat stress. Microb. Cell Fact. 2021, 20, 137. [Google Scholar] [CrossRef] [PubMed]
- Ling, Y.Y.; Ling, Z.L.; Zhao, R.L. Construction of a heat-resistant strain of Lentinus edodes by fungal Hsp20 protein overexpression and genetic transformation. Front. Microbiol. 2022, 13, 1009885. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.J.; An, Q.; Dai, Y.C. Physiological and biochemical characteristics of the Pleurotus ostreatus CCEF89. Mycosystema 2015, 34, 621–631. [Google Scholar]
- Liu, X.R.; Ye, L.Y.; Zhang, L.J.; Xie, B.G.; Wu, X.P. Mating type analyses of cultivated Pleurotus pulmonarius in China. Mycosystema 2021, 40, 3109–3117. [Google Scholar]
- Wang, L.N.; Gao, W.; Wu, X.; Zhao, M.R.; Qu, J.B.; Huang, C.Y.; Zhang, J.X. Genome-wide characterization and expression analyses of Pleurotus ostreatus MYB transcription factors during developmental stages and under heat stress based on de novo sequenced genome. Int. J. Mol. Sci. 2018, 19, 2052. [Google Scholar] [CrossRef]
- Hou, L.D.; Liu, Z.Q.; Yan, K.X.; Xu, L.J.; Chang, M.C.; Meng, J.L. Mnsod1 promotes the development of Pleurotus ostreatus and enhances the tolerance of mycelia to heat stress. Microb. Cell Fact. 2022, 21, 155. [Google Scholar] [CrossRef]
- Lv, J.H.; Xu, Y.; Dan, X.M.; Yang, Y.C.; Mao, C.L.; Ma, X.X.; Zhu, J.; Sun, M.; Jin, Y.R.; Huang, L.K. Genomic survey of MYB gene family in six pearl millet (Pennisetum glaucum) varieties and their response to abiotic stresses. Genetica 2023, 151, 251–265. [Google Scholar] [CrossRef]
- Jin, X.; Ackah, M.; Acheampong, A.; Zhang, Q.N.; Wang, L.; Lin, Q.; Qiu, W.G. Genome-wide identification of candidate genes associated with heat stress in mulberry (Morus alba L.). Curr. Issues Mol. Biol. 2023, 45, 4151–4167. [Google Scholar] [CrossRef]
- Zhang, C.Y.; Liu, H.C.; Zhang, X.S.; Guo, Q.X.; Bian, S.M.; Wang, J.Y.; Zhai, L.L. VcMYB4a, an R2R3-MYB transcription factor from Vaccinium corymbosum, negatively regulates salt, drought, and temperature stress. Gene 2020, 757, 144935. [Google Scholar] [CrossRef]
- Qiu, F.X.; Zheng, Y.J.; Lin, Y.; Woldegiorgis, S.T.; Xu, S.C.; Feng, C.Q.; Huang, G.P.; Shen, H.L.; Xu, Y.Y.; Kabore, M.A.F.; et al. Integrated ATAC-Seq and RNA-Seq data analysis to reveal OsbZIP14 function in rice in response to heat stress. Int. J. Mol. Sci. 2023, 24, 5619. [Google Scholar] [CrossRef] [PubMed]
- Valsecchi, I.; Sarikaya-Bayram, Ö.; Hoi, J.W.S.; Muszkieta, L.; Gibbons, J.; Prevost, M.C.; Mallet, A.; Krijnse-Locker, J.; Ibrahim-Granet, O.; Mouyna, I.; et al. MybA, a transcription factor involved in conidiation and conidial viability of the human pathogen Aspergillus fumigatus. Mol. Microbiol. 2017, 105, 880–900. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Son, H.; Lee, J.; Min, K.; Choi, G.J.; Kim, J.C.; Lee, Y.W. A putative transcription factor MYT1 is required for female fertility in the Ascomycete Gibberella zeae. PLoS ONE 2011, 6, e25586. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lin, Y.; Son, H.; Min, K.; Lee, J.; Choi, G.J.; Kim, J.C.; Lee, Y.W. A putative transcription factor MYT2 regulates perithecium size in the Ascomycete Gibberella zeae. PLoS ONE 2012, 7, e37859. [Google Scholar] [CrossRef][Green Version]
- Liu, Z.Q.; Deng, B.; Yuan, H.; Zhang, B.F.; Liu, J.Y.; Meng, J.L.; Chang, M.C. Transcription factor FfMYB15 regulates the expression of cellulase gene FfCEL6B during mycelial growth of Flammulina filiformis. Microb. Cell Fact. 2022, 21, 216. [Google Scholar] [CrossRef]
- Wang, L.N.; Huang, Q.H.; Zhang, L.L.; Wang, Q.F.; Liang, L.; Liao, B. Genome-wide characterization and comparative analysis of MYB transcription factors in Ganoderma species. G3 Genes Genomes Genet. 2020, 10, 2653–2660. [Google Scholar] [CrossRef]
- Zhang, G.; Yan, P.; Leng, D.; Shang, L.; Zhang, C.; Wu, Z.; Wang, Z. Functional roles of LaeA-like genes in fungal growth, cellulase activity, and secondary metabolism in Pleurotus ostreatus. J. Fungi 2022, 8, 902. [Google Scholar] [CrossRef] [PubMed]
- Hou, L.; Huang, C.; Wu, X.; Zhang, J.; Zhao, M. Nitric oxide negatively regulates the rapid formation of Pleurotus ostreatus primordia by inhibiting the mitochondrial aco gene. J. Fungi 2022, 8, 1055. [Google Scholar] [CrossRef]
- Qi, Y.; Chen, H.; Zhang, M.; Wen, Q.; Qiu, L.; Shen, J. Identification and expression analysis of Pofst3 suggests a role during Pleurotus ostreatus primordia formation. Fungal Biol. 2019, 123, 200–208. [Google Scholar] [CrossRef]
- Qi, Y.; Zhang, R.; Zhang, M.; Wen, Q.; Shen, J. Effects of exogenous ascorbic acid on the mycelia growth and primordia formation of Pleurotus ostreatus. J. Basic Microbiol. 2021, 61, 736–744. [Google Scholar] [CrossRef]
- Ding, Y.; Liang, S.; Lei, J.H.; Chen, L.G.; Kothe, E.; Ma, A. Agrobacterium tumefaciens mediated fused egfp-hph gene expression under the control of gpd promoter in Pleurotus ostreatus. Microbiol. Res. 2011, 166, 314–322. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.N. Characterization and Function Analysis of Catalase Genes in Pleurotus ostreatus. Ph.D. Thesis, Chinese Academy of Agricultural Sciences, Beijing, China, 2019. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Guadarrama-Mendoza, P.C.; Valencia del Toro, G.; Ramírez-Carrillo, R.; Robles-Martínez, F.; Yáñez-Fernández, J.; Garín-Aguilar, M.E.; Hernández, G.G.; Bravo-Vila, G. Morphology and mycelial growth rate of Pleurotus spp. strains from the Mexican mixtec region. Braz. J. Microbiol. 2014, 45, 861–872. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, H.; Liu, Z.; Guo, L.; Hou, L.; Meng, J.; Chang, M. Function of Transcription Factors PoMYB12, PoMYB15, and PoMYB20 in Heat Stress and Growth of Pleurotus ostreatus. Int. J. Mol. Sci. 2023, 24, 13559. https://doi.org/10.3390/ijms241713559
Yuan H, Liu Z, Guo L, Hou L, Meng J, Chang M. Function of Transcription Factors PoMYB12, PoMYB15, and PoMYB20 in Heat Stress and Growth of Pleurotus ostreatus. International Journal of Molecular Sciences. 2023; 24(17):13559. https://doi.org/10.3390/ijms241713559
Chicago/Turabian StyleYuan, Hui, Zongqi Liu, Lifeng Guo, Ludan Hou, Junlong Meng, and Mingchang Chang. 2023. "Function of Transcription Factors PoMYB12, PoMYB15, and PoMYB20 in Heat Stress and Growth of Pleurotus ostreatus" International Journal of Molecular Sciences 24, no. 17: 13559. https://doi.org/10.3390/ijms241713559
APA StyleYuan, H., Liu, Z., Guo, L., Hou, L., Meng, J., & Chang, M. (2023). Function of Transcription Factors PoMYB12, PoMYB15, and PoMYB20 in Heat Stress and Growth of Pleurotus ostreatus. International Journal of Molecular Sciences, 24(17), 13559. https://doi.org/10.3390/ijms241713559




