Mechanistic Insights into the Roles of the IL-17/IL-17R Families in Pancreatic Cancer
Abstract
:1. Introduction
2. The Overview of the IL-17/IL17R Families
2.1. The IL-17 Family Members
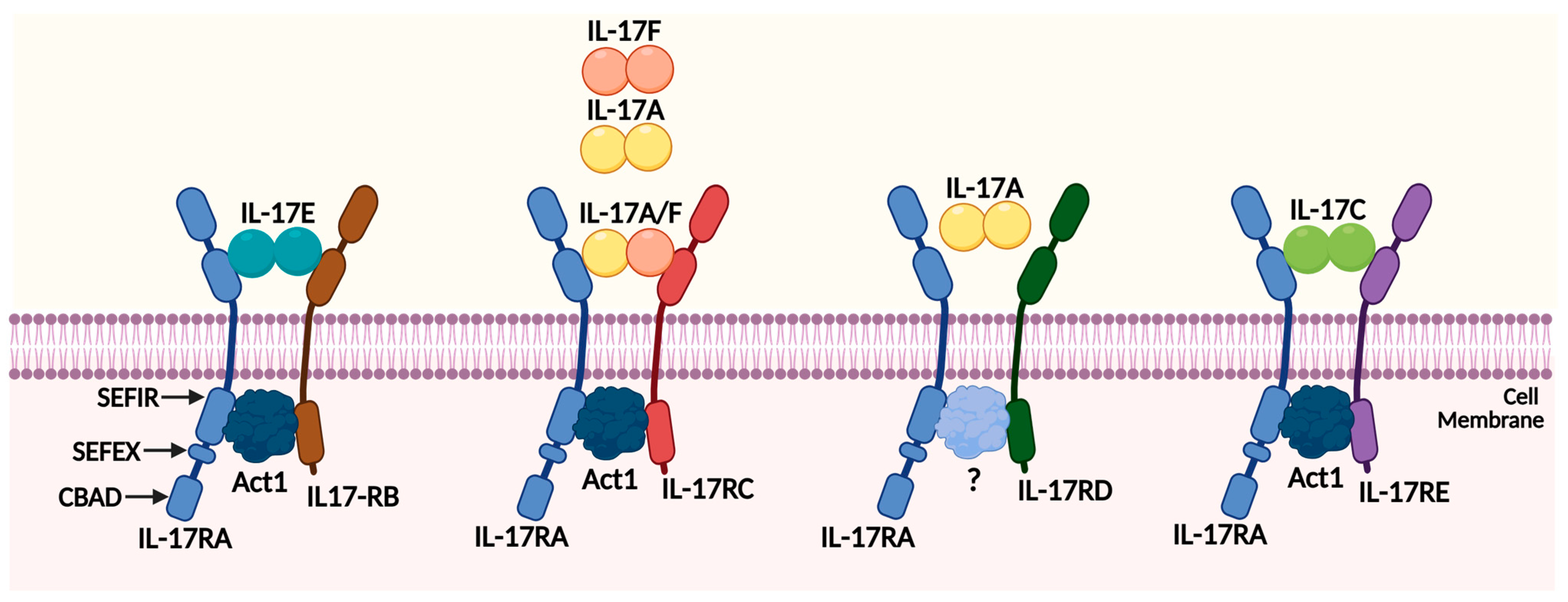
2.2. The IL-17 Receptor Family Members
2.3. The IL-17 Signaling Pathways
2.4. The Role of IL-17/IL17R Families in Inflammation and Tumor Immunity
3. Mechanistic Functions of the IL-17/IL17R Families in Pancreatic Cancer
3.1. The Role of IL-17/IL17R Families in the Pathogenesis of Pancreatic Cancer (PanIN and ADM Stages)
3.2. The Role of IL-17/IL17R Families in Pancreatic Cancer
3.3. The Role of IL-17/IL17R Families in Chemotherapy Resistance
4. Current Clinical Applications on Targeting IL-17/IL17R Family Member
5. Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, L.; Sanagapalli, S.; Stoita, A. Challenges in diagnosis of pancreatic cancer. World J. Gastroenterol. 2018, 24, 2047–2060. [Google Scholar] [CrossRef]
- Hu, C.; Li, M. In advanced pancreatic cancer: The value and significance of interventional therapy. J. Interv. Med. 2020, 3, 118–121. [Google Scholar] [CrossRef]
- Maomao, C.; He, L.; Dianqin, S.; Siyi, H.; Xinxin, Y.; Fan, Y.; Shaoli, Z.; Changfa, X.; Lin, L.; Ji, P.; et al. Current cancer burden in China: Epidemiology, etiology, and prevention. Cancer Biol. Med. 2022, 19, 1121–1138. [Google Scholar] [CrossRef]
- Chan, R.N.C.; Lee, T.T.L.; Chou, O.H.I.; So, J.; Chung, C.T.; Dee, E.C.; Ng, K.; Tang, P.; Roever, L.; Liu, T.; et al. Risk Factors of Pancreatic Cancer in Patients With Type 2 Diabetes Mellitus: The Hong Kong Diabetes Study. J. Endocr. Soc. 2022, 6, bvac138. [Google Scholar] [CrossRef]
- Maek, A.N.W.; Buranapraditkun, S.; Klaewsongkram, J.; Ruxrungtham, K. Increased interleukin-17 production both in helper T cell subset Th17 and CD4-negative T cells in human immunodeficiency virus infection. Viral Immunol. 2007, 20, 66–75. [Google Scholar] [CrossRef]
- Iwanaga, N.; Kolls, J.K. Updates on T helper type 17 immunity in respiratory disease. Immunology 2019, 156, 3–8. [Google Scholar] [CrossRef]
- Tsai, L.H.; Hsu, K.W.; Chiang, C.M.; Yang, H.J.; Liu, Y.H.; Yang, S.F.; Peng, P.H.; Cheng, W.C.; Wu, H.H. Targeting interleukin-17 receptor B enhances gemcitabine sensitivity through downregulation of mucins in pancreatic cancer. Sci. Rep. 2020, 10, 17817. [Google Scholar] [CrossRef]
- Marques, H.S.; de Brito, B.B.; da Silva, F.A.F.; Santos, M.L.C.; de Souza, J.C.B.; Correia, T.M.L.; Lopes, L.W.; Neres, N.S.M.; Dorea, R.; Dantas, A.C.S.; et al. Relationship between Th17 immune response and cancer. World J. Clin. Oncol. 2021, 12, 845–867. [Google Scholar] [CrossRef]
- Arif, S.; Moore, F.; Marks, K.; Bouckenooghe, T.; Dayan, C.M.; Planas, R.; Vives-Pi, M.; Powrie, J.; Tree, T.; Marchetti, P.; et al. Peripheral and islet interleukin-17 pathway activation characterizes human autoimmune diabetes and promotes cytokine-mediated beta-cell death. Diabetes 2011, 60, 2112–2119. [Google Scholar] [CrossRef]
- Hu, F.; Guo, F.; Zhu, Y.; Zhou, Q.; Li, T.; Xiang, H.; Shang, D. IL-17 in pancreatic disease: Pathogenesis and pharmacotherapy. Am. J. Cancer Res. 2020, 10, 3551–3564. [Google Scholar]
- Chandana, S.; Babiker, H.M.; Mahadevan, D. Therapeutic trends in pancreatic ductal adenocarcinoma (PDAC). Expert. Opin. Investig. Drugs 2019, 28, 161–177. [Google Scholar] [CrossRef]
- Sui, G.; Qiu, Y.; Yu, H.; Kong, Q.; Zhen, B. Interleukin-17 promotes the development of cisplatin resistance in colorectal cancer. Oncol. Lett. 2019, 17, 944–950. [Google Scholar] [CrossRef]
- Ge, Y.; Huang, M.; Yao, Y.M. Biology of Interleukin-17 and Its Pathophysiological Significance in Sepsis. Front. Immunol. 2020, 11, 1558. [Google Scholar] [CrossRef]
- Mucciolo, G.; Curcio, C.; Roux, C.; Li, W.Y.; Capello, M.; Curto, R.; Chiarle, R.; Giordano, D.; Satolli, M.A.; Lawlor, R.; et al. IL17A critically shapes the transcriptional program of fibroblasts in pancreatic cancer and switches on their protumorigenic functions. Proc. Natl. Acad. Sci. USA 2021, 118, e2020395118. [Google Scholar] [CrossRef]
- Roux, C.; Mucciolo, G.; Kopecka, J.; Novelli, F.; Riganti, C.; Cappello, P. IL17A Depletion Affects the Metabolism of Macrophages Treated with Gemcitabine. Antioxidants 2021, 10, 422. [Google Scholar] [CrossRef]
- Bastid, J.; Dejou, C.; Docquier, A.; Bonnefoy, N. The Emerging Role of the IL-17B/IL-17RB Pathway in Cancer. Front. Immunol. 2020, 11, 718. [Google Scholar] [CrossRef]
- Yang, Y.F.; Lee, Y.C.; Lo, S.; Chung, Y.N.; Hsieh, Y.C.; Chiu, W.C.; Yuan, S.F. A positive feedback loop of IL-17B-IL-17RB activates ERK/beta-catenin to promote lung cancer metastasis. Cancer Lett. 2018, 422, 44–55. [Google Scholar] [CrossRef]
- Wu, H.H.; Hwang-Verslues, W.W.; Lee, W.H.; Huang, C.K.; Wei, P.C.; Chen, C.L.; Shew, J.Y.; Lee, E.Y.; Jeng, Y.M.; Tien, Y.W.; et al. Targeting IL-17B-IL-17RB signaling with an anti-IL-17RB antibody blocks pancreatic cancer metastasis by silencing multiple chemokines. J. Exp. Med. 2015, 212, 333–349. [Google Scholar] [CrossRef]
- Huang, C.K.; Yang, C.Y.; Jeng, Y.M.; Chen, C.L.; Wu, H.H.; Chang, Y.C.; Ma, C.; Kuo, W.H.; Chang, K.J.; Shew, J.Y.; et al. Autocrine/paracrine mechanism of interleukin-17B receptor promotes breast tumorigenesis through NF-kappaB-mediated antiapoptotic pathway. Oncogene 2014, 33, 2968–2977. [Google Scholar] [CrossRef]
- Jamieson, K.C.; Wiehler, S.; Michi, A.N.; Proud, D. Rhinovirus Induces Basolateral Release of IL-17C in Highly Differentiated Airway Epithelial Cells. Front. Cell. Infect. Microbiol. 2020, 10, 103. [Google Scholar] [CrossRef] [PubMed]
- Vitiello, G.A.; Miller, G. Targeting the interleukin-17 immune axis for cancer immunotherapy. J. Exp. Med. 2020, 217, e20190456. [Google Scholar] [CrossRef] [PubMed]
- Jungnickel, C.; Schmidt, L.H.; Bittigkoffer, L.; Wolf, L.; Wolf, A.; Ritzmann, F.; Kamyschnikow, A.; Herr, C.; Menger, M.D.; Spieker, T.; et al. IL-17C mediates the recruitment of tumor-associated neutrophils and lung tumor growth. Oncogene 2017, 36, 4182–4190. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Sun, S.; Liu, D. IL-17D: A Less Studied Cytokine of IL-17 Family. Int. Arch. Allergy Immunol. 2020, 181, 618–623. [Google Scholar] [CrossRef] [PubMed]
- Saddawi-Konefka, R.; O’Sullivan, T.; Gross, E.T.; Washington, A., Jr.; Bui, J.D. Tumor-expressed IL-17D recruits NK cells to reject tumors. Oncoimmunology 2014, 3, e954853. [Google Scholar] [CrossRef]
- Benatar, T.; Cao, M.Y.; Lee, Y.; Lightfoot, J.; Feng, N.; Gu, X.; Lee, V.; Jin, H.; Wang, M.; Wright, J.A.; et al. IL-17E, a proinflammatory cytokine, has antitumor efficacy against several tumor types in vivo. Cancer Immunol. Immunother. 2010, 59, 805–817. [Google Scholar] [CrossRef]
- Fort, M.M.; Cheung, J.; Yen, D.; Li, J.; Zurawski, S.M.; Lo, S.; Menon, S.; Clifford, T.; Hunte, B.; Lesley, R.; et al. IL-25 induces IL-4, IL-5, and IL-13 and Th2-associated pathologies in vivo. Immunity 2001, 15, 985–995. [Google Scholar] [CrossRef]
- Mikkola, T.; Almahmoudi, R.; Salo, T.; Al-Samadi, A. Variable roles of interleukin-17F in different cancers. BMC Cancer 2022, 22, 54. [Google Scholar] [CrossRef]
- Ferreira, N.; Mesquita, I.; Baltazar, F.; Silvestre, R.; Granja, S. IL-17A and IL-17F orchestrate macrophages to promote lung cancer. Cell. Oncol. 2020, 43, 643–654. [Google Scholar] [CrossRef]
- Gaffen, S.L. Structure and signalling in the IL-17 receptor family. Nat. Rev. Immunol. 2009, 9, 556–567. [Google Scholar] [CrossRef]
- Ely, L.K.; Fischer, S.; Garcia, K.C. Structural basis of receptor sharing by interleukin 17 cytokines. Nat. Immunol. 2009, 10, 1245–1251. [Google Scholar] [CrossRef]
- Kramer, J.M.; Yi, L.; Shen, F.; Maitra, A.; Jiao, X.; Jin, T.; Gaffen, S.L. Evidence for ligand-independent multimerization of the IL-17 receptor. J. Immunol. 2006, 176, 711–715. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.H.; Dong, C. Signaling of interleukin-17 family cytokines in immunity and inflammation. Cell Signal. 2011, 23, 1069–1075. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.H.; Tsai, L.H.; Huang, C.K.; Hsu, P.H.; Chen, M.Y.; Chen, Y.I.; Hu, C.M.; Shen, C.N.; Lee, C.C.; Chang, M.C.; et al. Characterization of initial key steps of IL-17 receptor B oncogenic signaling for targeted therapy of pancreatic cancer. Sci. Transl. Med. 2021, 13, eabc2823. [Google Scholar] [CrossRef] [PubMed]
- Ho, A.W.; Gaffen, S.L. IL-17RC: A partner in IL-17 signaling and beyond. Semin. Immunopathol. 2010, 32, 33–42. [Google Scholar] [CrossRef]
- Girondel, C.; Meloche, S. Interleukin-17 Receptor D in Physiology, Inflammation and Cancer. Front. Oncol. 2021, 11, 656004. [Google Scholar] [CrossRef]
- Pande, S.; Yang, X.; Friesel, R. Interleukin-17 receptor D (Sef) is a multi-functional regulator of cell signaling. Cell. Commun. Signal. 2021, 19, 6. [Google Scholar] [CrossRef]
- Liao, R.; Sun, J.; Wu, H.; Yi, Y.; Wang, J.X.; He, H.W.; Cai, X.Y.; Zhou, J.; Cheng, Y.F.; Fan, J.; et al. High expression of IL-17 and IL-17RE associate with poor prognosis of hepatocellular carcinoma. J. Exp. Clin. Cancer Res. 2013, 32, 3. [Google Scholar] [CrossRef]
- Amatya, N.; Garg, A.V.; Gaffen, S.L. IL-17 Signaling: The Yin and the Yang. Trends Immunol. 2017, 38, 310–322. [Google Scholar] [CrossRef]
- McGeachy, M.J.; Cua, D.J.; Gaffen, S.L. The IL-17 Family of Cytokines in Health and Disease. Immunity 2019, 50, 892–906. [Google Scholar] [CrossRef]
- Chang, S.H.; Park, H.; Dong, C. Act1 adaptor protein is an immediate and essential signaling component of interleukin-17 receptor. J. Biol. Chem. 2006, 281, 35603–35607. [Google Scholar] [CrossRef]
- Zhao, J.; Chen, X.; Herjan, T.; Li, X. The role of interleukin-17 in tumor development and progression. J. Exp. Med. 2020, 217, e20190297. [Google Scholar] [CrossRef] [PubMed]
- Zenobia, C.; Hajishengallis, G. Basic biology and role of interleukin-17 in immunity and inflammation. Periodontol. 2000 2015, 69, 142–159. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-Carrozzi, V.; Sambandam, A.; Luis, E.; Lin, Z.; Jeet, S.; Lesch, J.; Hackney, J.; Kim, J.; Zhou, M.; Lai, J.; et al. IL-17C regulates the innate immune function of epithelial cells in an autocrine manner. Nat. Immunol. 2011, 12, 1159–1166. [Google Scholar] [CrossRef] [PubMed]
- Nies, J.F.; Panzer, U. IL-17C/IL-17RE: Emergence of a Unique Axis in T(H)17 Biology. Front. Immunol. 2020, 11, 341. [Google Scholar] [CrossRef]
- Yamaguchi, S.; Nambu, A.; Numata, T.; Yoshizaki, T.; Narushima, S.; Shimura, E.; Hiraishi, Y.; Arae, K.; Morita, H.; Matsumoto, K.; et al. The roles of IL-17C in T cell-dependent and -independent inflammatory diseases. Sci. Rep. 2018, 8, 15750. [Google Scholar] [CrossRef]
- Huang, J.; Lee, H.Y.; Zhao, X.; Han, J.; Su, Y.; Sun, Q.; Shao, J.; Ge, J.; Zhao, Y.; Bai, X.; et al. Interleukin-17D regulates group 3 innate lymphoid cell function through its receptor CD93. Immunity 2021, 54, 673–686 e674. [Google Scholar] [CrossRef]
- Borowczyk, J.; Buerger, C.; Tadjrischi, N.; Drukala, J.; Wolnicki, M.; Wnuk, D.; Modarressi, A.; Boehncke, W.H.; Brembilla, N.C. IL-17E (IL-25) and IL-17A Differentially Affect the Functions of Human Keratinocytes. J. Investig. Dermatol. 2020, 140, 1379–1389 e1372. [Google Scholar] [CrossRef]
- Yang, X.O.; Chang, S.H.; Park, H.; Nurieva, R.; Shah, B.; Acero, L.; Wang, Y.H.; Schluns, K.S.; Broaddus, R.R.; Zhu, Z.; et al. Regulation of inflammatory responses by IL-17F. J. Exp. Med. 2008, 205, 1063–1075. [Google Scholar] [CrossRef]
- Verma, A.H.; Richardson, J.P.; Zhou, C.; Coleman, B.M.; Moyes, D.L.; Ho, J.; Huppler, A.R.; Ramani, K.; McGeachy, M.J.; Mufazalov, I.A.; et al. Oral epithelial cells orchestrate innate type 17 responses to Candida albicans through the virulence factor candidalysin. Sci. Immunol. 2017, 2, eaam8834. [Google Scholar] [CrossRef]
- Teunissen, M.B.; Koomen, C.W.; de Waal Malefyt, R.; Wierenga, E.A.; Bos, J.D. Interleukin-17 and interferon-gamma synergize in the enhancement of proinflammatory cytokine production by human keratinocytes. J. Investig. Dermatol. 1998, 111, 645–649. [Google Scholar] [CrossRef]
- Fabre, T.; Kared, H.; Friedman, S.L.; Shoukry, N.H. IL-17A enhances the expression of profibrotic genes through upregulation of the TGF-beta receptor on hepatic stellate cells in a JNK-dependent manner. J. Immunol. 2014, 193, 3925–3933. [Google Scholar] [CrossRef] [PubMed]
- Hall, S.L.; Baker, T.; Lajoie, S.; Richgels, P.K.; Yang, Y.; McAlees, J.W.; van Lier, A.; Wills-Karp, M.; Sivaprasad, U.; Acciani, T.H.; et al. IL-17A enhances IL-13 activity by enhancing IL-13-induced signal transducer and activator of transcription 6 activation. J. Allergy Clin. Immunol. 2017, 139, 462–471 e414. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Sun, H.; Wu, S.; Tan, H.; Sun, Y.; Liu, X.; Si, S.; Xu, L.; Huang, J.; Zhou, W.; et al. IL-17A promotes CXCR2-dependent angiogenesis in a mouse model of liver cancer. Mol. Med. Rep. 2019, 20, 1065–1074. [Google Scholar] [CrossRef] [PubMed]
- Swaidani, S.; Liu, C.; Zhao, J.; Bulek, K.; Li, X. TRAF Regulation of IL-17 Cytokine Signaling. Front. Immunol. 2019, 10, 1293. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yi, T.; Kortylewski, M.; Pardoll, D.M.; Zeng, D.; Yu, H. IL-17 can promote tumor growth through an IL-6-Stat3 signaling pathway. J. Exp. Med. 2009, 206, 1457–1464. [Google Scholar] [CrossRef]
- Liao, Y.; Zhao, J.; Bulek, K.; Tang, F.; Chen, X.; Cai, G.; Jia, S.; Fox, P.L.; Huang, E.; Pizarro, T.T.; et al. Inflammation mobilizes copper metabolism to promote colon tumorigenesis via an IL-17-STEAP4-XIAP axis. Nat. Commun. 2020, 11, 900. [Google Scholar] [CrossRef]
- Garo, L.P.; Ajay, A.K.; Fujiwara, M.; Gabriely, G.; Raheja, R.; Kuhn, C.; Kenyon, B.; Skillin, N.; Kadowaki-Saga, R.; Saxena, S.; et al. MicroRNA-146a limits tumorigenic inflammation in colorectal cancer. Nat. Commun. 2021, 12, 2419. [Google Scholar] [CrossRef]
- Guo, N.; Shen, G.; Zhang, Y.; Moustafa, A.A.; Ge, D.; You, Z. Interleukin-17 Promotes Migration and Invasion of Human Cancer Cells Through Upregulation of MTA1 Expression. Front. Oncol. 2019, 9, 546. [Google Scholar] [CrossRef]
- Bie, Q.; Sun, C.; Gong, A.; Li, C.; Su, Z.; Zheng, D.; Ji, X.; Wu, Y.; Guo, Q.; Wang, S.; et al. Non-tumor tissue derived interleukin-17B activates IL-17RB/AKT/beta-catenin pathway to enhance the stemness of gastric cancer. Sci. Rep. 2016, 6, 25447. [Google Scholar] [CrossRef]
- Mochizuki, K.; He, S.; Zhang, Y. Notch and inflammatory T-cell response: New developments and challenges. Immunotherapy 2011, 3, 1353–1366. [Google Scholar] [CrossRef]
- Chen, J.; Ye, X.; Pitmon, E.; Lu, M.; Wan, J.; Jellison, E.R.; Adler, A.J.; Vella, A.T.; Wang, K. IL-17 inhibits CXCL9/10-mediated recruitment of CD8(+) cytotoxic T cells and regulatory T cells to colorectal tumors. J. Immunother. Cancer 2019, 7, 324. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Bechara, R.; Zhao, J.; McGeachy, M.J.; Gaffen, S.L. IL-17 receptor-based signaling and implications for disease. Nat. Immunol. 2019, 20, 1594–1602. [Google Scholar] [CrossRef] [PubMed]
- Carr, R.M.; Fernandez-Zapico, M.E. Pancreatic cancer microenvironment, to target or not to target? EMBO Mol. Med. 2016, 8, 80–82. [Google Scholar] [CrossRef] [PubMed]
- Kuwabara, T.; Ishikawa, F.; Kondo, M.; Kakiuchi, T. The Role of IL-17 and Related Cytokines in Inflammatory Autoimmune Diseases. Mediators Inflamm. 2017, 2017, 3908061. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Liu, S.; Parajuli, K.R.; Zhang, W.; Zhang, K.; Mo, Z.; Liu, J.; Chen, Z.; Yang, S.; Wang, A.R.; et al. Interleukin-17 promotes prostate cancer via MMP7-induced epithelial-to-mesenchymal transition. Oncogene 2017, 36, 687–699. [Google Scholar] [CrossRef]
- Ha, H.; Debnath, B.; Neamati, N. Role of the CXCL8-CXCR1/2 Axis in Cancer and Inflammatory Diseases. Theranostics 2017, 7, 1543–1588. [Google Scholar] [CrossRef] [PubMed]
- He, D.; Li, H.; Yusuf, N.; Elmets, C.A.; Athar, M.; Katiyar, S.K.; Xu, H. IL-17 mediated inflammation promotes tumor growth and progression in the skin. PLoS ONE 2012, 7, e32126. [Google Scholar] [CrossRef]
- Yamaguchi, Y.; Fujio, K.; Shoda, H.; Okamoto, A.; Tsuno, N.H.; Takahashi, K.; Yamamoto, K. IL-17B and IL-17C are associated with TNF-alpha production and contribute to the exacerbation of inflammatory arthritis. J. Immunol. 2007, 179, 7128–7136. [Google Scholar] [CrossRef]
- Chang, S.H. T helper 17 (Th17) cells and interleukin-17 (IL-17) in cancer. Arch. Pharm. Res. 2019, 42, 549–559. [Google Scholar] [CrossRef]
- Kotsiliti, E. IL-17A-producing CD8(+) T cells in pancreatic cancer. Nat. Rev. Gastroenterol. Hepatol. 2023, 20, 202. [Google Scholar] [CrossRef]
- Kuen, D.S.; Kim, B.S.; Chung, Y. IL-17-Producing Cells in Tumor Immunity: Friends or Foes? Immune Netw. 2020, 20, e6. [Google Scholar] [CrossRef] [PubMed]
- Najafi, S.; Mirshafiey, A. The role of T helper 17 and regulatory T cells in tumor microenvironment. Immunopharmacol. Immunotoxicol. 2019, 41, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Yang, W.; Tang, B.; Wang, X.; Zhang, Q.; Li, W.; Li, L. The protective and pathogenic role of Th17 cell plasticity and function in the tumor microenvironment. Front. Immunol. 2023, 14, 1192303. [Google Scholar] [CrossRef]
- Qian, X.; Gu, L.; Ning, H.; Zhang, Y.; Hsueh, E.C.; Fu, M.; Hu, X.; Wei, L.; Hoft, D.F.; Liu, J. Increased Th17 cells in the tumor microenvironment is mediated by IL-23 via tumor-secreted prostaglandin E2. J. Immunol. 2013, 190, 5894–5902. [Google Scholar] [CrossRef] [PubMed]
- Coffelt, S.B.; Kersten, K.; Doornebal, C.W.; Weiden, J.; Vrijland, K.; Hau, C.S.; Verstegen, N.J.M.; Ciampricotti, M.; Hawinkels, L.; Jonkers, J.; et al. IL-17-producing gammadelta T cells and neutrophils conspire to promote breast cancer metastasis. Nature 2015, 522, 345–348. [Google Scholar] [CrossRef]
- Jarocki, M.; Karska, J.; Kowalski, S.; Kielb, P.; Nowak, L.; Krajewski, W.; Saczko, J.; Kulbacka, J.; Szydelko, T.; Malkiewicz, B. Interleukin 17 and Its Involvement in Renal Cell Carcinoma. J. Clin. Med. 2022, 11, 4973. [Google Scholar] [CrossRef]
- Punt, S.; Fleuren, G.J.; Kritikou, E.; Lubberts, E.; Trimbos, J.B.; Jordanova, E.S.; Gorter, A. Angels and demons: Th17 cells represent a beneficial response, while neutrophil IL-17 is associated with poor prognosis in squamous cervical cancer. Oncoimmunology 2015, 4, e984539. [Google Scholar] [CrossRef]
- Wang, X.; Yang, L.; Huang, F.; Zhang, Q.; Liu, S.; Ma, L.; You, Z. Inflammatory cytokines IL-17 and TNF-alpha up-regulate PD-L1 expression in human prostate and colon cancer cells. Immunol. Lett. 2017, 184, 7–14. [Google Scholar] [CrossRef]
- He, S.; Fei, M.; Wu, Y.; Zheng, D.; Wan, D.; Wang, L.; Li, D. Distribution and clinical significance of Th17 cells in the tumor microenvironment and peripheral blood of pancreatic cancer patients. Int. J. Mol. Sci. 2011, 12, 7424–7437. [Google Scholar] [CrossRef]
- Gnerlich, J.L.; Mitchem, J.B.; Weir, J.S.; Sankpal, N.V.; Kashiwagi, H.; Belt, B.A.; Porembka, M.R.; Herndon, J.M.; Eberlein, T.J.; Goedegebuure, P.; et al. Induction of Th17 cells in the tumor microenvironment improves survival in a murine model of pancreatic cancer. J. Immunol. 2010, 185, 4063–4071. [Google Scholar] [CrossRef]
- Storz, P. Acinar cell plasticity and development of pancreatic ductal adenocarcinoma. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 296–304. [Google Scholar] [CrossRef] [PubMed]
- Habbe, N.; Shi, G.; Meguid, R.A.; Fendrich, V.; Esni, F.; Chen, H.; Feldmann, G.; Stoffers, D.A.; Konieczny, S.F.; Leach, S.D.; et al. Spontaneous induction of murine pancreatic intraepithelial neoplasia (mPanIN) by acinar cell targeting of oncogenic Kras in adult mice. Proc. Natl. Acad. Sci. USA 2008, 105, 18913–18918. [Google Scholar] [CrossRef] [PubMed]
- McAllister, F.; Leach, S.D. Targeting IL-17 for pancreatic cancer prevention. Oncotarget 2014, 5, 9530–9531. [Google Scholar] [CrossRef]
- McAllister, F.; Bailey, J.M.; Alsina, J.; Nirschl, C.J.; Sharma, R.; Fan, H.; Rattigan, Y.; Roeser, J.C.; Lankapalli, R.H.; Zhang, H.; et al. Oncogenic Kras activates a hematopoietic-to-epithelial IL-17 signaling axis in preinvasive pancreatic neoplasia. Cancer Cell. 2014, 25, 621–637. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chen, H.; Jiang, R.; Hong, X.; Peng, J.; Chen, W.; Jiang, J.; Li, J.; Huang, D.; Dai, H.; et al. Interleukin-17 activates and synergizes with the notch signaling pathway in the progression of pancreatic ductal adenocarcinoma. Cancer Lett. 2021, 508, 1–12. [Google Scholar] [CrossRef]
- Zhang, Y.; Zoltan, M.; Riquelme, E.; Xu, H.; Sahin, I.; Castro-Pando, S.; Montiel, M.F.; Chang, K.; Jiang, Z.; Ling, J.; et al. Immune Cell Production of Interleukin 17 Induces Stem Cell Features of Pancreatic Intraepithelial Neoplasia Cells. Gastroenterology 2018, 155, 210–223.e3. [Google Scholar] [CrossRef]
- Loncle, C.; Bonjoch, L.; Folch-Puy, E.; Lopez-Millan, M.B.; Lac, S.; Molejon, M.I.; Chuluyan, E.; Cordelier, P.; Dubus, P.; Lomberk, G.; et al. IL17 Functions through the Novel REG3beta-JAK2-STAT3 Inflammatory Pathway to Promote the Transition from Chronic Pancreatitis to Pancreatic Cancer. Cancer Res. 2015, 75, 4852–4862. [Google Scholar] [CrossRef]
- Chellappa, S.; Hugenschmidt, H.; Hagness, M.; Line, P.D.; Labori, K.J.; Wiedswang, G.; Tasken, K.; Aandahl, E.M. Regulatory T cells that co-express RORgammat and FOXP3 are pro-inflammatory and immunosuppressive and expand in human pancreatic cancer. Oncoimmunology 2016, 5, e1102828. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, H.; Huang, S.; Chu, Q. KRAS Mutations in Solid Tumors: Characteristics, Current Therapeutic Strategy, and Potential Treatment Exploration. J. Clin. Med. 2023, 12, 709. [Google Scholar] [CrossRef]
- Zhang, Y.; Chandra, V.; Riquelme Sanchez, E.; Dutta, P.; Quesada, P.R.; Rakoski, A.; Zoltan, M.; Arora, N.; Baydogan, S.; Horne, W.; et al. Interleukin-17-induced neutrophil extracellular traps mediate resistance to checkpoint blockade in pancreatic cancer. J. Exp. Med. 2020, 217, e20190354. [Google Scholar] [CrossRef]
- Karabulut, S.; Afsar, C.U.; Karabulut, M.; Alis, H.; Kilic, L.; Cikot, M.; Yasasever, C.T.; Aykan, N.F. Evaluation of Serum Interleukin-17 (IL-17) Levels as a Diagnostic Marker in Pancreatic Adenocarcinoma. J. Gastrointest. Cancer 2016, 47, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.A.; Singh, N.; Gunjan, D.; Gopi, S.; Dash, N.R.; Gupta, S.; Saraya, A. Increased circulating Th17 cell populations in patients with pancreatic ductal adenocarcinoma. Immunogenetics 2023. [Google Scholar] [CrossRef] [PubMed]
- Picard, F.S.R.; Lutz, V.; Brichkina, A.; Neuhaus, F.; Ruckenbrod, T.; Hupfer, A.; Raifer, H.; Klein, M.; Bopp, T.; Pfefferle, P.I.; et al. IL-17A-producing CD8(+) T cells promote PDAC via induction of inflammatory cancer-associated fibroblasts. Gut 2023, 72, 1510–1522. [Google Scholar] [CrossRef]
- Li, J.; Wu, X.; Schiffmann, L.; MacVicar, T.; Zhou, C.; Wang, Z.; Li, D.; Camacho, O.V.; Heuchel, R.; Odenthal, M.; et al. IL-17B/RB Activation in Pancreatic Stellate Cells Promotes Pancreatic Cancer Metabolism and Growth. Cancers 2021, 13, 5338. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Cheng, H.; Luo, G.; Lu, Y.; Jin, K.; Guo, M.; Ni, Q.; Yu, X. Circulating regulatory T cell subsets predict overall survival of patients with unresectable pancreatic cancer. Int. J. Oncol. 2017, 51, 686–694. [Google Scholar] [CrossRef]
- Song, Y.; Ji, B.; Jiang, C.X.; Chen, Z.M.; Yao, N.H.; Mukaida, N.; Huang, H. IL17RB expression might predict prognosis and benefit from gemcitabine in patients with resectable pancreatic cancer. Pathol. Res. Pract. 2019, 215, 152650. [Google Scholar] [CrossRef]
- Amrutkar, M.; Gladhaug, I.P. Pancreatic Cancer Chemoresistance to Gemcitabine. Cancers 2017, 9, 157. [Google Scholar] [CrossRef]
- Senthebane, D.A.; Rowe, A.; Thomford, N.E.; Shipanga, H.; Munro, D.; Mazeedi, M.; Almazyadi, H.A.M.; Kallmeyer, K.; Dandara, C.; Pepper, M.S.; et al. The Role of Tumor Microenvironment in Chemoresistance: To Survive, Keep Your Enemies Closer. Int. J. Mol. Sci. 2017, 18, 1586. [Google Scholar] [CrossRef]
- Cochaud, S.; Giustiniani, J.; Thomas, C.; Laprevotte, E.; Garbar, C.; Savoye, A.M.; Cure, H.; Mascaux, C.; Alberici, G.; Bonnefoy, N.; et al. IL-17A is produced by breast cancer TILs and promotes chemoresistance and proliferation through ERK1/2. Sci. Rep. 2013, 3, 3456. [Google Scholar] [CrossRef]
- Skrypek, N.; Duchene, B.; Hebbar, M.; Leteurtre, E.; van Seuningen, I.; Jonckheere, N. The MUC4 mucin mediates gemcitabine resistance of human pancreatic cancer cells via the Concentrative Nucleoside Transporter family. Oncogene 2013, 32, 1714–1723. [Google Scholar] [CrossRef]
- Beck, K.M.; Koo, J. Brodalumab for the treatment of plaque psoriasis: Up-to-date. Expert. Opin. Biol. Ther. 2019, 19, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Lechner, M.G.; Cheng, M.I.; Patel, A.Y.; Hoang, A.T.; Yakobian, N.; Astourian, M.; Pioso, M.S.; Rodriguez, E.D.; McCarthy, E.C.; Hugo, W.; et al. Inhibition of IL-17A Protects against Thyroid Immune-Related Adverse Events while Preserving Checkpoint Inhibitor Antitumor Efficacy. J. Immunol. 2022, 209, 696–709. [Google Scholar] [CrossRef] [PubMed]
- Corraliza-Gorjon, I.; Somovilla-Crespo, B.; Santamaria, S.; Garcia-Sanz, J.A.; Kremer, L. New Strategies Using Antibody Combinations to Increase Cancer Treatment Effectiveness. Front. Immunol. 2017, 8, 1804. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, S.; Girault, A.; Ohresser, M.; Lereclus, E.; Paintaud, G.; Lecomte, T.; Raoul, W. Monoclonal Antibodies Targeting the IL-17/IL-17RA Axis: An Opportunity to Improve the Efficiency of Anti-VEGF Therapy in Fighting Metastatic Colorectal Cancer? Clin. Colorectal Cancer 2018, 17, e109–e113. [Google Scholar] [CrossRef]
- Innocenti, F.; Owzar, K.; Cox, N.L.; Evans, P.; Kubo, M.; Zembutsu, H.; Jiang, C.; Hollis, D.; Mushiroda, T.; Li, L.; et al. A genome-wide association study of overall survival in pancreatic cancer patients treated with gemcitabine in CALGB 80303. Clin. Cancer Res. 2012, 18, 577–584. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Liu, R.; Wang, B.; Lian, J.; Yao, Y.; Sun, H.; Zhang, C.; Fang, L.; Guan, X.; Shi, J.; et al. Blocking IL-17A enhances tumor response to anti-PD-1 immunotherapy in microsatellite stable colorectal cancer. J. Immunother. Cancer 2021, 9, e001895. [Google Scholar] [CrossRef]
- Han, X.; Ye, J.; Huang, R.; Li, Y.; Liu, J.; Meng, T.; Song, D. Pan-cancer analysis reveals interleukin-17 family members as biomarkers in the prediction for immune checkpoint inhibitor curative effect. Front. Immunol. 2022, 13, 900273. [Google Scholar] [CrossRef]

| Cytokine Ligands | Receptors | Relative Cell Types | Sources | Downstream Functions |
|---|---|---|---|---|
| IL-17A | IL-17RA and IL-17RC IL-17RA and IL-17RD | Th17 cells, αβ T cells, γδ T cells, iNKT cells, and LTi-like cells | Skin, gut, lung, pancreas | Proinflammatory; Promote cancer progression [13]; Regulate immune cells; T cell activation to neutrophil mobilization and activation [42] |
| IL-17B | IL-17RB | Neutrophils, chondrocytes, neurons, naïve, memory, and germinal center B cells | Stomach, pancreas, intestine, rheumatoid synovial tissues | Control immune cell trafficking to inflamed tissues; Promote cancer cell survival, proliferation and migration [16]; |
| IL-17C | IL-17RA and IL-17RE | Epithelial cells | In the mucosa of the mouth, skin, airway epithelium thymus and spleen | Regulate the innate immune function of epithelial cells [43]; Induce innate immune functions in bacterial, fungal, and brain infections [44]; Host defense against pathogens [45] |
| IL-17D | Unknown | T cells, smooth muscle cells, epithelial cells, mast cells | Skeletal muscle, brain, adipose tissue, heart, lung and pancreas [23] | Against dextran sulfate sodium (DSS)-induced colitis [46] |
| IL-17E | IL-17RA and IL-17RB | Mast cells, Epithelial cells, Th2 cells, NKT cells, Mast cells | Brain, kidney, lung, prostate, testis, spinal cord, adrenal gland | Anti-inflammatory and proinflammatory; Promote both proliferation and differentiation of keratinocytes [47] |
| IL-17F | IL-17RA and IL-17RC | Th17 cells, neutrophil, NK cells, γδ T cells, Mast cells | Skin, Joint | Antitumor effects; Neutrophil recruitments; Regulate the expression of inflammatory chemokines and cytokines [48] |
| IL-17 Subtype and Receptors | Mechanism Models | Biological Effects | Reference |
|---|---|---|---|
| IL-17A | Il-17A expression increased | Patients with PC in stable and remission stages | [95] |
| Il-17A expression decreased | Patients with unresectable advanced PC | ||
| IL-17 exerts effects on IL-17RA of PanIN epithelial cells | Accelerating the progression and PanIN | [84] | |
| IL-17A recruits neutrophils | Immunosuppressive microenvironment in PDAC | [90] | |
| Il-17 A-producing CD8+ T cells | Promoting PDAC progression | [70,93] | |
| IL-17 A activates the gp130-JAK2-STAT3 pathway | Promoting acinar-ductal metaplasia and PanIN development | [87] | |
| IL-17B/IL-17RB | Activating the ERK1/2 signaling pathway | Promoting the invasion and metastasis of PC | [18] |
| IL-17RB expression increased | Predicting the prognosis of patients with resectable PC | [96] | |
| Anti-IL-17RB monoclonal antibody | Inhibiting of tumor metastasis | [16] | |
| IL-17E | Activating the NF-κB signaling pathway | Exerting an antitumor effect in combination with drugs | [25] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Z.; Qiao, S.; Yang, L.; Sun, M.; Li, B.; Lu, A.; Li, F. Mechanistic Insights into the Roles of the IL-17/IL-17R Families in Pancreatic Cancer. Int. J. Mol. Sci. 2023, 24, 13539. https://doi.org/10.3390/ijms241713539
Chen Z, Qiao S, Yang L, Sun M, Li B, Lu A, Li F. Mechanistic Insights into the Roles of the IL-17/IL-17R Families in Pancreatic Cancer. International Journal of Molecular Sciences. 2023; 24(17):13539. https://doi.org/10.3390/ijms241713539
Chicago/Turabian StyleChen, Zheng, Shuangying Qiao, Liu Yang, Meiheng Sun, Boyue Li, Aiping Lu, and Fangfei Li. 2023. "Mechanistic Insights into the Roles of the IL-17/IL-17R Families in Pancreatic Cancer" International Journal of Molecular Sciences 24, no. 17: 13539. https://doi.org/10.3390/ijms241713539
APA StyleChen, Z., Qiao, S., Yang, L., Sun, M., Li, B., Lu, A., & Li, F. (2023). Mechanistic Insights into the Roles of the IL-17/IL-17R Families in Pancreatic Cancer. International Journal of Molecular Sciences, 24(17), 13539. https://doi.org/10.3390/ijms241713539






