Inflammation and Digestive Cancer
Abstract
1. Introduction
2. The Mechanisms of the Carcinogenic Effects of Chronic Inflammation
2.1. Chronic Hepatitis
2.2. Inflammatory Bowel Disease
2.3. Chronic Gastritis and the Carcinogenic Effect of Helicobacter pylori
2.3.1. H. pylori Virulence and Direct Carcinogenic Effects
2.3.2. Inflammatory Host Factors
2.3.3. Accelerated Cell Death, Proliferation and Mutation Risk Caused by Inflammation
2.3.4. Lack of Acid Secretion
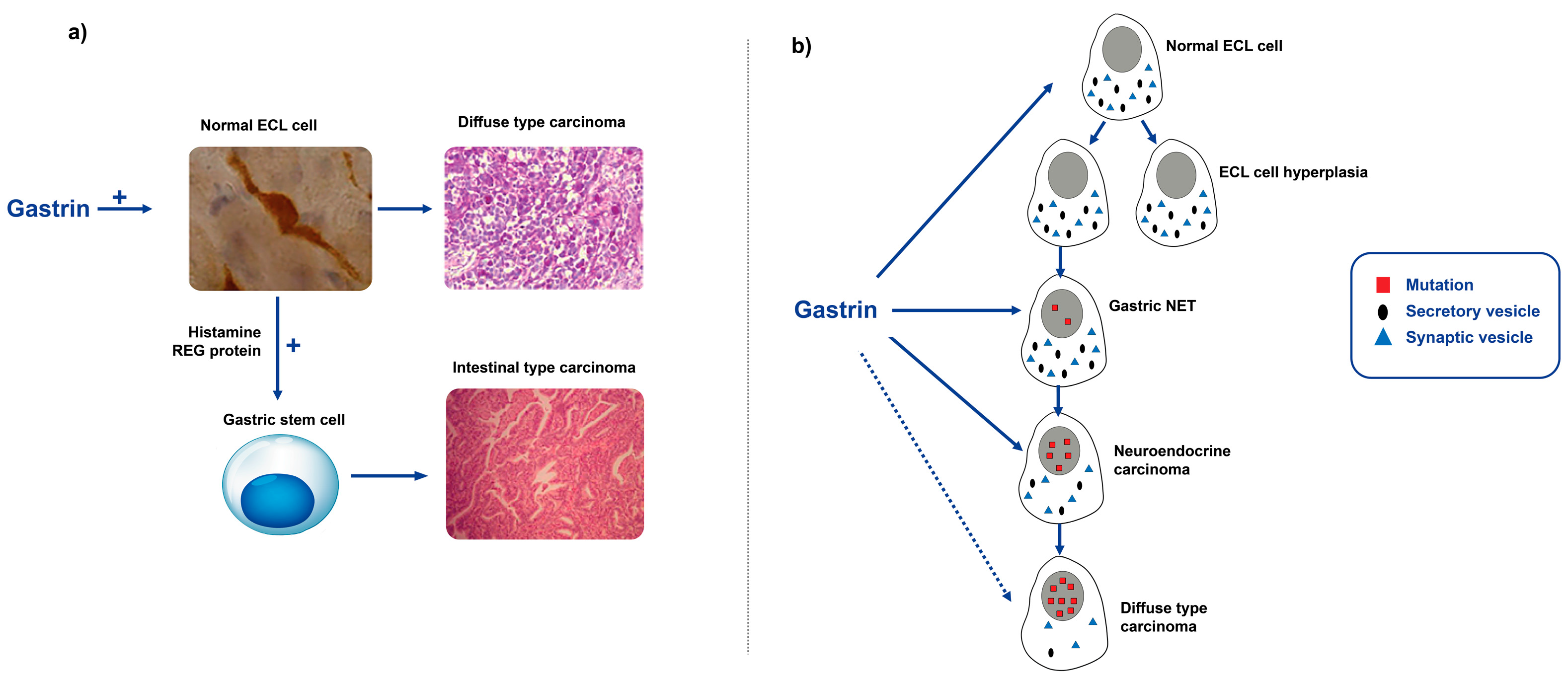
2.3.5. The Role of Gastrin in Gastric Carcinogenesis
3. Conclusions—Chronic Inflammation and Cancer
Author Contributions
Funding
Conflicts of Interest
References
- Méndez-López, L.F. Revisiting Epithelial Carcinogenesis. Int. J. Mol. Sci. 2022, 23, 7437. [Google Scholar] [CrossRef]
- Waldum, H.L.; Oberg, K.; Sordal, O.F.; Sandvik, A.K.; Gustafsson, B.I.; Mjones, P.; Fossmark, R. Not only stem cells, but also mature cells, particularly neuroendocrine cells, may develop into tumours: Time for a paradigm shift. Ther. Adv. Gastroenterol. 2018, 11, 1756284818775054. [Google Scholar] [CrossRef] [PubMed]
- Sell, S. On the stem cell origin of cancer. Am. J. Pathol. 2010, 176, 2584–2594. [Google Scholar] [CrossRef] [PubMed]
- Qvigstad, G.; Falkmer, S.; Westre, B.; Waldum, H.L. Clinical and histopathological tumour progression in ECL cell carcinoids (“ECLomas”). Apmis 1999, 107, 1085–1092. [Google Scholar] [CrossRef] [PubMed]
- Waldum, H.L.; Sandvik, A.K.; Brenna, E.; Fossmark, R.; Qvigstad, G.; Soga, J. Classification of tumours. J. Exp. Clin. Cancer Res. 2008, 27, 70. [Google Scholar] [CrossRef] [PubMed]
- Quante, M.; Wang, T.C. Inflammation and stem cells in gastrointestinal carcinogenesis. Physiology 2008, 23, 350–359. [Google Scholar] [CrossRef]
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 2018, 9, 7204–7218. [Google Scholar] [CrossRef]
- Lee, T.Y.; Hsu, Y.C.; Ho, H.J.; Lin, J.T.; Chen, Y.J.; Wu, C.Y. Daily aspirin associated with a reduced risk of hepatocellular carcinoma in patients with non-alcoholic fatty liver disease: A population-based cohort study. EClinicalMedicine 2023, 61, 102065. [Google Scholar] [CrossRef]
- Williams, C.S.; Mann, M.; DuBois, R.N. The role of cyclooxygenases in inflammation, cancer, and development. Oncogene 1999, 18, 7908–7916. [Google Scholar] [CrossRef]
- Li, B.; Cheung, K.S.; Wong, I.Y.; Leung, W.K.; Law, S. Nonaspirin nonsteroidal anti-inflammatory drugs and gastric cancer risk after Helicobacter pylori eradication: A territory-wide study. Cancer 2021, 127, 1805–1815. [Google Scholar] [CrossRef]
- Li, L.; Zhang, Y.; Qin, L. Effect of celecoxib plus standard chemotherapy on cancer prognosis: A systematic review and meta-analysis. Eur. J. Clin. Investig. 2023, 53, e13973. [Google Scholar] [CrossRef] [PubMed]
- Oh, T.K.; Song, I.A. Long-Term Glucocorticoid Use and Cancer Risk: A Population-Based Cohort Study in South Korea. Cancer Prev. Res. 2020, 13, 1017–1026. [Google Scholar] [CrossRef] [PubMed]
- Manna, R.; Rigante, D. The everchanging framework of autoinflammation. Intern. Emerg. Med. 2021, 16, 1759–1770. [Google Scholar] [CrossRef] [PubMed]
- Kanda, T.; Goto, T.; Hirotsu, Y.; Moriyama, M.; Omata, M. Molecular Mechanisms Driving Progression of Liver Cirrhosis towards Hepatocellular Carcinoma in Chronic Hepatitis B and C Infections: A Review. Int. J. Mol. Sci. 2019, 20, 1358. [Google Scholar] [CrossRef] [PubMed]
- Khanna, R.; Verma, S.K. Pediatric hepatocellular carcinoma. World J. Gastroenterol. 2018, 24, 3980–3999. [Google Scholar] [CrossRef]
- Kushwah, A.S.; Srivastava, K.; Banerjee, M. Differential expression of DNA repair genes and treatment outcome of chemoradiotherapy (CRT) in cervical cancer. Gene 2023, 868, 147389. [Google Scholar] [CrossRef]
- Wang, X.; Chen, Y.; Xu, M.; Cheng, K.; Duan, X.; Liao, W.; Wang, Y.; Lu, Y.; Duan, Z.; Wang, L. Virologic response maintenance and hepatocellular carcinoma in chronic hepatitis B patients treated with entecavir. Expert. Rev. Gastroenterol. Hepatol. 2021, 15, 1337–1344. [Google Scholar] [CrossRef]
- Mak, L.Y.; Huang, Q.; Wong, D.K.; Stamm, L.; Cheung, K.S.; Ko, K.L.; Yan, R.; Ouyang, L.; Fung, J.; Seto, W.K.; et al. Residual HBV DNA and pgRNA viraemia is associated with hepatocellular carcinoma in chronic hepatitis B patients on antiviral therapy. J. Gastroenterol. 2021, 56, 479–488. [Google Scholar] [CrossRef]
- Llovet, J.M.; Willoughby, C.E.; Singal, A.G.; Greten, T.F.; Heikenwälder, M.; El-Serag, H.B.; Finn, R.S.; Friedman, S.L. Nonalcoholic steatohepatitis-related hepatocellular carcinoma: Pathogenesis and treatment. Nat. Rev. Gastroenterol. Hepatol. 2023, 20, 487–503. [Google Scholar] [CrossRef]
- De, A.; Duseja, A. Natural History of Simple Steatosis or Nonalcoholic Fatty Liver. J. Clin. Exp. Hepatol. 2020, 10, 255–262. [Google Scholar] [CrossRef]
- Bengtsson, B.; Widman, L.; Wahlin, S.; Stål, P.; Björkström, N.K.; Hagström, H. The risk of hepatocellular carcinoma in cirrhosis differs by etiology, age and sex: A Swedish nationwide population-based cohort study. United Eur. Gastroenterol. J. 2022, 10, 465–476. [Google Scholar] [CrossRef] [PubMed]
- Carrat, F.; Fontaine, H.; Dorival, C.; Simony, M.; Diallo, A.; Hezode, C.; De Ledinghen, V.; Larrey, D.; Haour, G.; Bronowicki, J.P.; et al. Clinical outcomes in patients with chronic hepatitis C after direct-acting antiviral treatment: A prospective cohort study. Lancet 2019, 393, 1453–1464. [Google Scholar] [CrossRef] [PubMed]
- Ioannou, G.N. HCC surveillance after SVR in patients with F3/F4 fibrosis. J. Hepatol. 2021, 74, 458–465. [Google Scholar] [CrossRef]
- Polyak, S.J.; Crispe, I.N.; Baumert, T.F. Liver Abnormalities after Elimination of HCV Infection: Persistent Epigenetic and Immunological Perturbations Post-Cure. Pathogens 2021, 10, 44. [Google Scholar] [CrossRef] [PubMed]
- Brougham-Cook, A.; Jain, I.; Kukla, D.A.; Masood, F.; Kimmel, H.; Ryoo, H.; Khetani, S.R.; Underhill, G.H. High throughput interrogation of human liver stellate cells reveals microenvironmental regulation of phenotype. Acta Biomater. 2022, 138, 240–253. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zuo, L.; Lin, Z.; Yang, Z.; Chen, R.; Xu, Y. The relationship between aspirin consumption and hepatocellular carcinoma: A systematic review and meta-analysis. Eur. J. Med. Res. 2023, 28, 226. [Google Scholar] [CrossRef] [PubMed]
- Porter, R.J.; Arends, M.J.; Churchhouse, A.M.D.; Din, S. Inflammatory Bowel Disease-Associated Colorectal Cancer: Translational Risks from Mechanisms to Medicines. J. Crohn’s Colitis 2021, 15, 2131–2141. [Google Scholar] [CrossRef]
- Bargen, J.A.; Sauer, W.G.; Sloan, W.P.; Gage, R.P. The development of cancer in chronic ulcerative colitis. Gastroenterology 1954, 26, 32–37, passim. [Google Scholar] [CrossRef]
- Ekbom, A.; Helmick, C.; Zack, M.; Adami, H.O. Ulcerative colitis and colorectal cancer. A population-based study. N. Engl. J. Med. 1990, 323, 1228–1233. [Google Scholar] [CrossRef]
- Krugliak Cleveland, N.; Rubin, D.T.; Hart, J.; Weber, C.R.; Meckel, K.; Tran, A.L.; Aelvoet, A.S.; Pan, I.; Gonsalves, A.; Gaetano, J.N.; et al. Patients With Ulcerative Colitis and Primary Sclerosing Cholangitis Frequently Have Subclinical Inflammation in the Proximal Colon. Clin. Gastroenterol. Hepatol. 2018, 16, 68–74. [Google Scholar] [CrossRef]
- Fantini, M.C.; Guadagni, I. From inflammation to colitis-associated colorectal cancer in inflammatory bowel disease: Pathogenesis and impact of current therapies. Dig. Liver Dis. 2021, 53, 558–565. [Google Scholar] [CrossRef]
- Rubin, D.T.; Huo, D.; Kinnucan, J.A.; Sedrak, M.S.; McCullom, N.E.; Bunnag, A.P.; Raun-Royer, E.P.; Cohen, R.D.; Hanauer, S.B.; Hart, J.; et al. Inflammation is an independent risk factor for colonic neoplasia in patients with ulcerative colitis: A case-control study. Clin. Gastroenterol. Hepatol. 2013, 11, 1601–1608.e4. [Google Scholar] [CrossRef] [PubMed]
- Korelitz, B.I.; Sultan, K.; Kothari, M.; Arapos, L.; Schneider, J.; Panagopoulos, G. Histological healing favors lower risk of colon carcinoma in extensive ulcerative colitis. World J. Gastroenterol. 2014, 20, 4980–4986. [Google Scholar] [CrossRef] [PubMed]
- Waldum, H.L.; Bjorvatn, B.; Burhol, P.G. Gastritis, peptic ulcer disease, inflammatory bowel disease, and stomach and colon cancers- are they all caused by viral infections? Med. Hypotheses 1981, 7, 1329–1338. [Google Scholar] [CrossRef]
- Tarris, G.; de Rougemont, A.; Charkaoui, M.; Michiels, C.; Martin, L.; Belliot, G. Enteric Viruses and Inflammatory Bowel Disease. Viruses 2021, 13, 104. [Google Scholar] [CrossRef] [PubMed]
- Gogokhia, L.; Round, J.L. Immune-bacteriophage interactions in inflammatory bowel diseases. Curr. Opin. Virol. 2021, 49, 30–35. [Google Scholar] [CrossRef]
- Everhov, Å.H.; Ludvigsson, J.F.; Järås, J.; Erichsen, R.; Pedersen, L.; Halfvarson, J.; Askling, J.; Ekbom, A.; Sørensen, H.T.; Olén, O. Colorectal Cancer in Childhood-onset Inflammatory Bowel Disease: A Scandinavian Register-based Cohort Study, 1969–2017. J. Pediatr. Gastroenterol. Nutr. 2022, 75, 480–484. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhu, W.; Zuo, L.; Shen, B. The Role of the Mesentery in Crohn’s Disease: The Contributions of Nerves, Vessels, Lymphatics, and Fat to the Pathogenesis and Disease Course. Inflamm. Bowel Dis. 2016, 22, 1483–1495. [Google Scholar] [CrossRef]
- Pochard, C.; Coquenlorge, S.; Freyssinet, M.; Naveilhan, P.; Bourreille, A.; Neunlist, M.; Rolli-Derkinderen, M. The multiple faces of inflammatory enteric glial cells: Is Crohn’s disease a gliopathy? Am. J. Physiol. Gastrointest. Liver Physiol. 2018, 315, G1–G11. [Google Scholar] [CrossRef]
- Everhov, Å.H.; Erichsen, R.; Järås, J.; Pedersen, L.; Halfvarson, J.; Askling, J.; Ekbom, A.; Ludvigsson, J.F.; Toft Sørensen, H.; Olén, O. Colorectal cancer in elderly-onset inflammatory bowel disease: A 1969-2017 Scandinavian register-based cohort study. Aliment. Pharmacol. Ther. 2022, 56, 1168–1182. [Google Scholar] [CrossRef]
- Yashiro, M. Ulcerative colitis-associated colorectal cancer. World J. Gastroenterol. 2014, 20, 16389–16397. [Google Scholar] [CrossRef]
- Ahn, C.; Negus, D.; Huang, W. Pyoderma gangrenosum: A review of pathogenesis and treatment. Expert. Rev. Clin. Immunol. 2018, 14, 225–233. [Google Scholar] [CrossRef]
- Maghfour, J.; Olson, J.; Conic, R.R.Z.; Mesinkovska, N.A. The Association between Alopecia and Inflammatory Bowel Disease: A Systematic Review and Meta-Analysis. Dermatology 2021, 237, 658–672. [Google Scholar] [CrossRef]
- Liu, Y.H.; Wu, Z.; Ding, J.Y.; Shi, Y.D. Microscopic Colitis Is Associated With a Reduced Risk of Colorectal Adenoma and Cancer: A Meta-Analysis. Inflamm. Bowel Dis. 2022, 28, 1584–1591. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.; Roland, J.T.; Barlow, B.J.; O’Neal, R.; Rich, A.E.; Nam, K.T.; Shi, C.; Goldenring, J.R. Cell lineage distribution atlas of the human stomach reveals heterogeneous gland populations in the gastric antrum. Gut 2014, 63, 1711–1720. [Google Scholar] [CrossRef] [PubMed]
- Guilford, P.; Hopkins, J.; Harraway, J.; McLeod, M.; McLeod, N.; Harawira, P.; Taite, H.; Scoular, R.; Miller, A.; Reeve, A.E. E-cadherin germline mutations in familial gastric cancer. Nature 1998, 392, 402–405. [Google Scholar] [CrossRef]
- Calvete, O.; Reyes, J.; Zuniga, S.; Paumard-Hernandez, B.; Fernandez, V.; Bujanda, L.; Rodriguez-Pinilla, M.S.; Palacios, J.; Heine-Suner, D.; Banka, S.; et al. Exome sequencing identifies ATP4A gene as responsible of an atypical familial type I gastric neuroendocrine tumour. Hum. Mol. Genet. 2015, 24, 2914–2922. [Google Scholar] [CrossRef] [PubMed]
- Hirabayashi, M.; Georges, D.; Clifford, G.M.; de Martel, C. Estimating the Global Burden of Epstein-Barr Virus-Associated Gastric Cancer: A Systematic Review and Meta-Analysis. Clin. Gastroenterol. Hepatol. 2023, 21, 922–930.e21. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, J.; Oliveira, C.; Malta, M.; Sousa, H. Epstein-Barr virus gene expression and latency pattern in gastric carcinomas: A systematic review. Future Oncol. 2017, 13, 567–579. [Google Scholar] [CrossRef]
- Noh, J.H.; Shin, J.Y.; Lee, J.H.; Park, Y.S.; Lee, I.S.; Kim, G.H.; Na, H.K.; Ahn, J.Y.; Jung, K.W.; Kim, D.H.; et al. Clinical Significance of Epstein-Barr Virus and Helicobacter pylori Infection in Gastric Carcinoma. Gut Liver 2023, 17, 69–77. [Google Scholar] [CrossRef]
- Waldum, H.L.; Kleveland, P.M.; Sordal, O.F. Helicobacter pylori and gastric acid: An intimate and reciprocal relationship. Ther. Adv. Gastroenterol. 2016, 9, 836–844. [Google Scholar] [CrossRef]
- Lauren, P. The two histological main types of gastric carcinoma: Diffuse and so-called intestinal-type carcinoma. An attemt at a histo-clinical classification. Acta Pathol. Microbiol. Scand. 1965, 64, 31–49. [Google Scholar] [CrossRef] [PubMed]
- Bertuccio, P.; Chatenoud, L.; Levi, F.; Praud, D.; Ferlay, J.; Negri, E.; Malvezzi, M.; La Vecchia, C. Recent patterns in gastric cancer: A global overview. Int. J. Cancer 2009, 125, 666–673. [Google Scholar] [CrossRef]
- Marshall, B.J.; Warren, J.R. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet 1984, 1, 1311–1315. [Google Scholar] [CrossRef] [PubMed]
- Parsonnet, J.; Friedman, G.D.; Vandersteen, D.P.; Chang, Y.; Vogelman, J.H.; Orentreich, N.; Sibley, R.K. Helicobacter pylori infection and the risk of gastric carcinoma. N. Engl. J. Med. 1991, 325, 1127–1131. [Google Scholar] [CrossRef] [PubMed]
- Uemura, N.; Okamoto, S.; Yamamoto, S.; Matsumura, N.; Yamaguchi, S.; Yamakido, M.; Taniyama, K.; Sasaki, N.; Schlemper, R.J. Helicobacter pylori infection and the development of gastric cancer. N. Engl. J. Med. 2001, 345, 784–789. [Google Scholar] [CrossRef] [PubMed]
- Hansson, L.E.; Nyren, O.; Hsing, A.W.; Bergstrom, R.; Josefsson, S.; Chow, W.H.; Fraumeni, J.F., Jr.; Adami, H.O. The risk of stomach cancer in patients with gastric or duodenal ulcer disease. N. Engl. J. Med. 1996, 335, 242–249. [Google Scholar] [CrossRef]
- Zamcheck, N.; Grable, E.; Ley, A.; Norman, L. Occurrence of gastric cancer among patients with pernicious anemia at the Boston City Hospital. N. Engl. J. Med. 1955, 252, 1103–1110. [Google Scholar] [CrossRef]
- Sjöblom, S.M.; Sipponen, P.; Miettinen, M.; Karonen, S.L.; Jrvinen, H.J. Gastroscopic screening for gastric carcinoids and carcinoma in pernicious anemia. Endoscopy 1988, 20, 52–56. [Google Scholar] [CrossRef]
- Rugge, M.; Bricca, L.; Guzzinati, S.; Sacchi, D.; Pizzi, M.; Savarino, E.; Farinati, F.; Zorzi, M.; Fassan, M.; Dei Tos, A.P.; et al. Autoimmune gastritis: Long-term natural history in naïve Helicobacter pylori-negative patients. Gut 2022, 72, 30–38. [Google Scholar] [CrossRef]
- Waldum, H.L. Conclusion that autoimmune gastritis does not predispose to gastric cancer is unproven. Gut 2023. [Google Scholar] [CrossRef] [PubMed]
- Usui, Y.; Taniyama, Y.; Endo, M.; Koyanagi, Y.N.; Kasugai, Y.; Oze, I.; Ito, H.; Imoto, I.; Tanaka, T.; Tajika, M.; et al. Helicobacter pylori, Homologous-Recombination Genes, and Gastric Cancer. N. Engl. J. Med. 2023, 388, 1181–1190. [Google Scholar] [CrossRef] [PubMed]
- Müller, A.; He, J. A Double Whammy on Gastric Cancer Risk. N. Engl. J. Med. 2023, 388, 1225–1229. [Google Scholar] [CrossRef] [PubMed]
- Waldum, H.L.; Burhol, P.G. Serum group I pepsinogens. Scand. J. Gastroenterol. 1981, 16, 449–451. [Google Scholar] [CrossRef]
- Wang, F.; Meng, W.; Wang, B.; Qiao, L. Helicobacter pylori-induced gastric inflammation and gastric cancer. Cancer Lett. 2014, 345, 196–202. [Google Scholar] [CrossRef]
- Take, S.; Mizuno, M.; Ishiki, K.; Kusumoto, C.; Imada, T.; Hamada, F.; Yoshida, T.; Yokota, K.; Mitsuhashi, T.; Okada, H. Risk of gastric cancer in the second decade of follow-up after Helicobacter pylori eradication. J. Gastroenterol. 2020, 55, 281–288. [Google Scholar] [CrossRef]
- Huang, J.Q.; Sridhar, S.; Chen, Y.; Hunt, R.H. Meta-analysis of the relationship between Helicobacter pylori seropositivity and gastric cancer. Gastroenterology 1998, 114, 1169–1179. [Google Scholar] [CrossRef]
- Group", H.a.C.C. Gastric cancer and Helicobacter pylori: A combined analysis of 12 case control studies nested within prospective cohorts. Gut 2001, 49, 347–353. [Google Scholar] [CrossRef]
- Wotherspoon, A.C.; Ortiz-Hidalgo, C.; Falzon, M.R.; Isaacson, P.G. Helicobacter pylori-associated gastritis and primary B-cell gastric lymphoma. Lancet 1991, 338, 1175–1176. [Google Scholar] [CrossRef]
- Kay, J.; Thadhani, E.; Samson, L.; Engelward, B. Inflammation-induced DNA damage, mutations and cancer. DNA Repair 2019, 83, 102673. [Google Scholar] [CrossRef]
- Zavros, Y.; Merchant, J.L. The immune microenvironment in gastric adenocarcinoma. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 451–467. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.Q.; Zheng, G.F.; Sumanac, K.; Irvine, E.J.; Hunt, R.H. Meta-analysis of the relationship between cagA seropositivity and gastric cancer. Gastroenterology 2003, 125, 1636–1644. [Google Scholar] [CrossRef] [PubMed]
- Parsonnet, J.; Friedman, G.D.; Orentreich, N.; Vogelman, H. Risk for gastric cancer in people with CagA positive or CagA negative Helicobacter pylori infection. Gut 1997, 40, 297–301. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, S.; Crabtree, J.E.; Forman, D.; Kurosawa, M. Association between infections with CagA-positive or -negative strains of Helicobacter pylori and risk for gastric cancer in young adults. Research Group on Prevention of Gastric Carcinoma Among Young Adults. Am. J. Gastroenterol. 1999, 94, 3455–3459. [Google Scholar] [CrossRef]
- Freire de Melo, F.; Marques, H.S.; Rocha Pinheiro, S.L.; Lemos, F.F.B.; Silva Luz, M.; Nayara Teixeira, K.; Souza, C.L.; Oliveira, M.V. Influence of Helicobacter pylori oncoprotein CagA in gastric cancer: A critical-reflective analysis. World J. Clin. Oncol. 2022, 13, 866–879. [Google Scholar] [CrossRef]
- Sicinschi, L.A.; Correa, P.; Peek, R.M.; Camargo, M.C.; Piazuelo, M.B.; Romero-Gallo, J.; Hobbs, S.S.; Krishna, U.; Delgado, A.; Mera, R.; et al. CagA C-terminal variations in Helicobacter pylori strains from Colombian patients with gastric precancerous lesions. Clin. Microbiol. Infect. 2010, 16, 369–378. [Google Scholar] [CrossRef]
- Kobayashi, D.; Uchida, K.; Furukawa, A.; Ito, T.; Maruta, L.M.; Seidler, H.B.K.; Felipe-Silva, A.; Sekine, M.; Ando, N.; Toyama, Y.; et al. Immunohistochemical Differentiation between Western and East Asian Types of CagA-Positive Helicobacter pylori in Gastric Biopsy Samples. Can. J. Gastroenterol. Hepatol. 2022, 2022, 1371089. [Google Scholar] [CrossRef]
- Yamaoka, Y.; Kita, M.; Kodama, T.; Sawai, N.; Kashima, K.; Imanishi, J. Induction of various cytokines and development of severe mucosal inflammation by cagA gene positive Helicobacter pylori strains. Gut 1997, 41, 442–451. [Google Scholar] [CrossRef]
- Kuipers, E.J.; Pérez-Pérez, G.I.; Meuwissen, S.G.; Blaser, M.J. Helicobacter pylori and atrophic gastritis: Importance of the cagA status. J. Natl. Cancer Inst. 1995, 87, 1777–1780. [Google Scholar] [CrossRef]
- Ishikura, N.; Usui, Y.; Ito, H.; Kasugai, Y.; Oze, I.; Kato, S.; Yatabe, Y.; Nakamura, S.; Matsuo, K. Helicobacter pylori (HP) infection alone, but not HP-induced atrophic gastritis, increases the risk of gastric lymphoma: A case-control study in Japan. Ann. Hematol. 2019, 98, 1981–1987. [Google Scholar] [CrossRef]
- Keikha, M.; Sahebkar, A.; Yamaoka, Y.; Karbalaei, M. Helicobacter pylori cagA status and gastric mucosa-associated lymphoid tissue lymphoma: A systematic review and meta-analysis. J. Health Popul. Nutr. 2022, 41, 2. [Google Scholar] [CrossRef] [PubMed]
- Sjoblom, S.M.; Sipponen, P.; Karonen, S.L.; Jarvinen, H.J. Mucosal argyrophil endocrine cells in pernicious anaemia and upper gastrointestinal carcinoid tumours. J. Clin. Pathol. 1989, 42, 371–377. [Google Scholar] [CrossRef]
- Antonodimitrakis, P.; Tsolakis, A.; Welin, S.; Kozlovacki, G.; Oberg, K.; Granberg, D. Gastric carcinoid in a patient infected with Helicobacter pylori: A new entity? World J. Gastroenterol. 2011, 17, 3066–3068. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Iwafuchi, M.; Ueki, J.; Yoshimura, A.; Mochizuki, T.; Motoyama, H.; Sugimura, K.; Honma, T.; Narisawa, R.; Ichida, T.; et al. Gastric carcinoid tumors without autoimmune gastritis in Japan: A relationship with Helicobacter pylori infection. Dig. Dis. Sci. 2002, 47, 579–585. [Google Scholar] [CrossRef] [PubMed]
- Waldum, H.; Fossmark, R. Gastritis, Gastric Polyps and Gastric Cancer. Int. J. Mol. Sci. 2021, 22, 6548. [Google Scholar] [CrossRef] [PubMed]
- El-Omar, E.M.; Carrington, M.; Chow, W.H.; McColl, K.E.; Bream, J.H.; Young, H.A.; Herrera, J.; Lissowska, J.; Yuan, C.C.; Rothman, N.; et al. Interleukin-1 polymorphisms associated with increased risk of gastric cancer. Nature 2000, 404, 398–402. [Google Scholar] [CrossRef]
- Troost, E.; Hold, G.L.; Smith, M.G.; Chow, W.H.; Rabkin, C.S.; McColl, K.E.; El-Omar, E.M. The role of interleukin-1beta and other potential genetic markers as indicators of gastric cancer risk. Can. J. Gastroenterol. 2003, 17 (Suppl. B), 397060. [Google Scholar] [CrossRef]
- Macarthur, M.; Hold, G.L.; El-Omar, E.M. Inflammation and Cancer II. Role of chronic inflammation and cytokine gene polymorphisms in the pathogenesis of gastrointestinal malignancy. Am. J. Physiol. Gastrointest. Liver Physiol. 2004, 286, G515–G520. [Google Scholar] [CrossRef]
- McLean, M.H.; El-Omar, E.M. Genetic aspects of inflammation. Curr. Opin. Pharmacol. 2009, 9, 370–374. [Google Scholar] [CrossRef]
- Ye, B.D.; Kim, S.G.; Park, J.H.; Kim, J.S.; Jung, H.C.; Song, I.S. The interleukin-8-251 A allele is associated with increased risk of noncardia gastric adenocarcinoma in Helicobacter pylori-infected Koreans. J. Clin. Gastroenterol. 2009, 43, 233–239. [Google Scholar] [CrossRef]
- Pretre, V.; Papadopoulos, D.; Regard, J.; Pelletier, M.; Woo, J. Interleukin-1 (IL-1) and the inflammasome in cancer. Cytokine 2022, 153, 155850. [Google Scholar] [CrossRef] [PubMed]
- Preston-Martin, S.; Pike, M.C.; Ross, R.K.; Jones, P.A.; Henderson, B.E. Increased cell division as a cause of human cancer. Cancer Res. 1990, 50, 7415–7421. [Google Scholar] [PubMed]
- Peek, R.M., Jr.; Moss, S.F.; Tham, K.T.; Pérez-Pérez, G.I.; Wang, S.; Miller, G.G.; Atherton, J.C.; Holt, P.R.; Blaser, M.J. Helicobacter pylori cagA+ strains and dissociation of gastric epithelial cell proliferation from apoptosis. J. Natl. Cancer Inst. 1997, 89, 863–868. [Google Scholar] [CrossRef]
- Tomasetti, C.; Vogelstein, B. Cancer etiology. Variation in cancer risk among tissues can be explained by the number of stem cell divisions. Science 2015, 347, 78–81. [Google Scholar] [CrossRef] [PubMed]
- Martinsen, T.C.; Bergh, K.; Waldum, H.L. Gastric juice: A barrier against infectious diseases. Basic Clin. Pharmacol. Toxicol. 2005, 96, 94–102. [Google Scholar] [CrossRef]
- Berstad, A. A modified hemoglobin substrate method for the estimation of pepsin in gastric juice. Scand. J. Gastroenterol. 1970, 5, 343–348. [Google Scholar] [CrossRef]
- Wilder-Smith, C.H.; Spirig, C.; Krech, T.; Merki, H.S. Bactericidal factors in gastric juice. Eur. J. Gastroenterol. Hepatol. 1992, 4, 885–891. [Google Scholar]
- Blair, A.J., 3rd; Richardson, C.T.; Walsh, J.H.; Feldman, M. Variable contribution of gastrin to gastric acid secretion after a meal in humans. Gastroenterology 1987, 92, 944–949. [Google Scholar] [CrossRef]
- Saffouri, B.; Weir, G.C.; Bitar, K.N.; Makhlouf, G.M. Gastrin and somatostatin secretion by perfused rat stomach: Functional linkage of antral peptides. Am. J. Physiol. 1980, 238, G495–G501. [Google Scholar] [CrossRef]
- Hansson, L.R.; Engstrand, L.; Nyrén, O.; Lindgren, A. Prevalence of Helicobacter pylori infection in subtypes of gastric cancer. Gastroenterology 1995, 109, 885–888. [Google Scholar] [CrossRef]
- Fossmark, R.; Sagatun, L.; Nordrum, I.S.; Sandvik, A.K.; Waldum, H.L. Hypergastrinemia is associated with adenocarcinomas in the gastric corpus and shorter patient survival. Apmis 2015, 123, 509–514. [Google Scholar] [CrossRef] [PubMed]
- McGuigan, J.E.; Trudeau, W.L. Serum gastrin concentrations in pernicious anemia. N. Engl. J. Med. 1970, 282, 358–361. [Google Scholar] [CrossRef] [PubMed]
- Comfort, M.W. Gastric acidity before and after development of gastric cancer: Its etiologic, diagnostic and prognostic significance. Ann. Intern. Med. 1951, 34, 1331–1348. [Google Scholar] [CrossRef] [PubMed]
- Grossman, M.I.; Kirsner, J.B.; Gillespie, I.E. Basal and histalog-stimulated gastric secretion in control subjects and in patients with peptic ulcer or gastric cancer. Gastroenterology 1963, 45, 14–26. [Google Scholar] [CrossRef]
- Morson, B.C. Intestinal metaplasia of the gastric mucosa. Br. J. Cancer 1955, 9, 365–376. [Google Scholar] [CrossRef]
- Siurala, M.; Seppala, K. Atrophic gastritis as a possible precursor of gastric carcinoma and pernicious anemia. Results of follow-up examinations. Acta Med. Scand. 1960, 166, 455–474. [Google Scholar] [CrossRef]
- Azzopardi, J.G.; Pollock, D.J. Argentaffin and argyrophil cells in gastric carcinoma. J. Pathol. Bacteriol. 1963, 86, 443–451. [Google Scholar] [CrossRef]
- Wilander, E. Achylia, pernicious anaemia, ECL cells and gastric carcinoids. Virchows Arch. A Pathol. Anat. Histol. 1980, 387, 371–373. [Google Scholar] [CrossRef]
- Havu, N. Enterochromaffin-like cell carcinoids of gastric mucosa in rats after life-long inhibition of gastric secretion. Digestion 1986, 35 (Suppl. S1), 42–55. [Google Scholar] [CrossRef]
- Poynter, D.; Pick, C.R.; Harcourt, R.A.; Selway, S.A.; Ainge, G.; Harman, I.W.; Spurling, N.W.; Fluck, P.A.; Cook, J.L. Association of long lasting unsurmountable histamine H2 blockade and gastric carcinoid tumours in the rat. Gut 1985, 26, 1284–1295. [Google Scholar] [CrossRef]
- Hakanson, R.; Sundler, F. Proposed mechanism of induction of gastric carcinoids: The gastrin hypothesis. Eur. J. Clin. Investig. 1990, 20 (Suppl. S1), S65–S71. [Google Scholar] [CrossRef] [PubMed]
- Waldum, H. Are diffuse gastric carcinomas neuroendocrine tumours ECL-omas? (Reply). Eur. J. Gastroenterol. Hepatol. 1991, 3, 863–865. [Google Scholar]
- Waldum, H.L.; Aase, S.; Kvetnoi, I.; Brenna, E.; Sandvik, A.K.; Syversen, U.; Johnsen, G.; Vatten, L.; Polak, J.M. Neuroendocrine differentiation in human gastric carcinoma. Cancer 1998, 83, 435–444. [Google Scholar] [CrossRef]
- Qvigstad, G.; Sandvik, A.K.; Brenna, E.; Aase, S.; Waldum, H.L. Detection of chromogranin A in human gastric adenocarcinomas using a sensitive immunohistochemical technique. Histochem. J. 2000, 32, 551–556. [Google Scholar] [CrossRef] [PubMed]
- Qvigstad, G.; Qvigstad, T.; Westre, B.; Sandvik, A.K.; Brenna, E.; Waldum, H.L. Neuroendocrine differentiation in gastric adenocarcinomas associated with severe hypergastrinemia and/or pernicious anemia. Apmis 2002, 110, 132–139. [Google Scholar] [CrossRef]
- Bakkelund, K.; Fossmark, R.; Nordrum, I.; Waldum, H. Signet ring cells in gastric carcinomas are derived from neuroendocrine cells. J. Histochem. Cytochem. 2006, 54, 615–621. [Google Scholar] [CrossRef]
- Sørdal, Ø.; Qvigstad, G.; Nordrum, I.S.; Sandvik, A.K.; Gustafsson, B.I.; Waldum, H. The PAS positive material in gastric cancer cells of signet ring type is not mucin. Exp. Mol. Pathol. 2014, 96, 274–278. [Google Scholar] [CrossRef]
- Schott, M.; Sagert, C.; Willenberg, H.S.; Schinner, S.; Ramp, U.; Varro, A.; Raffel, A.; Eisenberger, C.; Zacharowski, K.; Perren, A.; et al. Carcinogenic hypergastrinemia: Signet-ring cell carcinoma in a patient with multiple endocrine neoplasia type 1 with Zollinger-Ellison’s syndrome. J. Clin. Endocrinol. Metab. 2007, 92, 3378–3382. [Google Scholar] [CrossRef]
- Mjones, P.; Nordrum, I.S.; Sordal, O.; Sagatun, L.; Fossmark, R.; Sandvik, A.; Waldum, H.L. Expression of the Cholecystokinin-B Receptor in Neoplastic Gastric Cells. Horm. Cancer 2018, 9, 40–54. [Google Scholar] [CrossRef]
- Waldum, H.; Mjønes, P. Towards Understanding of Gastric Cancer Based upon Physiological Role of Gastrin and ECL Cells. Cancers 2020, 12, 3477. [Google Scholar] [CrossRef]
- Kinoshita, Y.; Ishihara, S.; Kadowaki, Y.; Fukui, H.; Chiba, T. Reg protein is a unique growth factor of gastric mucosal cells. J. Gastroenterol. 2004, 39, 507–513. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.T.; Zeng, L.; Yang, J.; Zeng, C.; Chen, Y. Analysis of the Incidence and Survival of Gastric Cancer Based on the Lauren Classification: A Large Population-Based Study Using SEER. Front. Oncol. 2020, 10, 1212. [Google Scholar] [CrossRef] [PubMed]
- Waldum, H.L.; Sordal, O.F.; Mjones, P.G. The Enterochromaffin-like [ECL] Cell-Central in Gastric Physiology and Pathology. Int. J. Mol. Sci. 2019, 20, 2444. [Google Scholar] [CrossRef]
- Waldum, H.L.; Fossmark, R. Types of Gastric Carcinomas. Int. J. Mol. Sci. 2018, 19, 4109. [Google Scholar] [CrossRef] [PubMed]
- Waldum, H.L.; Hauso, O.; Sordal, O.F.; Fossmark, R. Gastrin May Mediate the Carcinogenic Effect of Helicobacter pylori Infection of the Stomach. Dig. Dis. Sci. 2015, 60, 1522–1527. [Google Scholar] [CrossRef] [PubMed]
- Fossmark, R.; Calvete, O.; Mjones, P.; Benitez, J.; Waldum, H.L. ECL-cell carcinoids and carcinoma in patients homozygous for an inactivating mutation in the gastric H(+) K(+) ATPase alpha subunit. Apmis 2016, 124, 561–566. [Google Scholar] [CrossRef]
- Solcia, E.; Capella, C.; Fiocca, R.; Rindi, G.; Rosai, J. Gastric argyrophil carcinoidosis in patients with Zollinger-Ellison syndrome due to type 1 multiple endocrine neoplasia. A newly recognized association. Am. J. Surg. Pathol. 1990, 14, 503–513. [Google Scholar] [CrossRef]
- Feurle, G.E. Argyrophil cell hyperplasia and a carcinoid tumour in the stomach of a patient with sporadic Zollinger-Ellison syndrome. Gut 1994, 35, 275–277. [Google Scholar] [CrossRef]
- Cadiot, G.; Vissuzaine, C.; Potet, F.; Mignon, M. Fundic argyrophil carcinoid tumor in a patient with sporadic-type Zollinger-Ellison syndrome. Dig. Dis. Sci. 1995, 40, 1275–1278. [Google Scholar] [CrossRef]
- Richards, M.L.; Gauger, P.; Thompson, N.W.; Giordano, T.J. Regression of type II gastric carcinoids in multiple endocrine neoplasia type 1 patients with Zollinger-Ellison syndrome after surgical excision of all gastrinomas. World J. Surg. 2004, 28, 652–658. [Google Scholar] [CrossRef]
- Waldum, H.L.; Brenna, E.; Sandvik, A.K. Long-term safety of proton pump inhibitors: Risks of gastric neoplasia and infections. Expert. Opin. Drug Saf. 2002, 1, 29–38. [Google Scholar] [PubMed]
- Fossmark, R.; Qvigstad, G.; Waldum, H.L. Gastric cancer: Animal studies on the risk of hypoacidity and hypergastrinemia. World J. Gastroenterol. 2008, 14, 1646–1651. [Google Scholar] [CrossRef] [PubMed]
- Waldum, H.L.; Brenna, E.; Sandvik, A.K. Relationship of ECL cells and gastric neoplasia. Yale J. Biol. Med. 1998, 71, 325–335. [Google Scholar] [PubMed]

| Organ | Pathogenesis | Disease/Agent | Mechanism of Carcinogenesis | ||
|---|---|---|---|---|---|
| Causative Agent Is Carcinogenic | Increased Proliferation | Secondary Hormonal Changes | |||
| Liver | HBV | ++ | + | ||
| HCV | ? | ++ | |||
| Steatohepatitis | ++ | ||||
| Colon | Ulcerative colitis | ? | + | ||
| Crohn’s disease | ? | + | |||
| Stomach | Oxyntic atrophy | Helicobacter pylori gastritis | + | ++ | |
| Autoimmune gastritis | + | ++ | |||
| Epstein-Barr virus | + | + | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Waldum, H.; Fossmark, R. Inflammation and Digestive Cancer. Int. J. Mol. Sci. 2023, 24, 13503. https://doi.org/10.3390/ijms241713503
Waldum H, Fossmark R. Inflammation and Digestive Cancer. International Journal of Molecular Sciences. 2023; 24(17):13503. https://doi.org/10.3390/ijms241713503
Chicago/Turabian StyleWaldum, Helge, and Reidar Fossmark. 2023. "Inflammation and Digestive Cancer" International Journal of Molecular Sciences 24, no. 17: 13503. https://doi.org/10.3390/ijms241713503
APA StyleWaldum, H., & Fossmark, R. (2023). Inflammation and Digestive Cancer. International Journal of Molecular Sciences, 24(17), 13503. https://doi.org/10.3390/ijms241713503






