Association of Klotho with Coronary Artery Disease in Subjects with Type 2 Diabetes Mellitus and Preserved Kidney Function: A Case-Control Study
Abstract
:1. Introduction
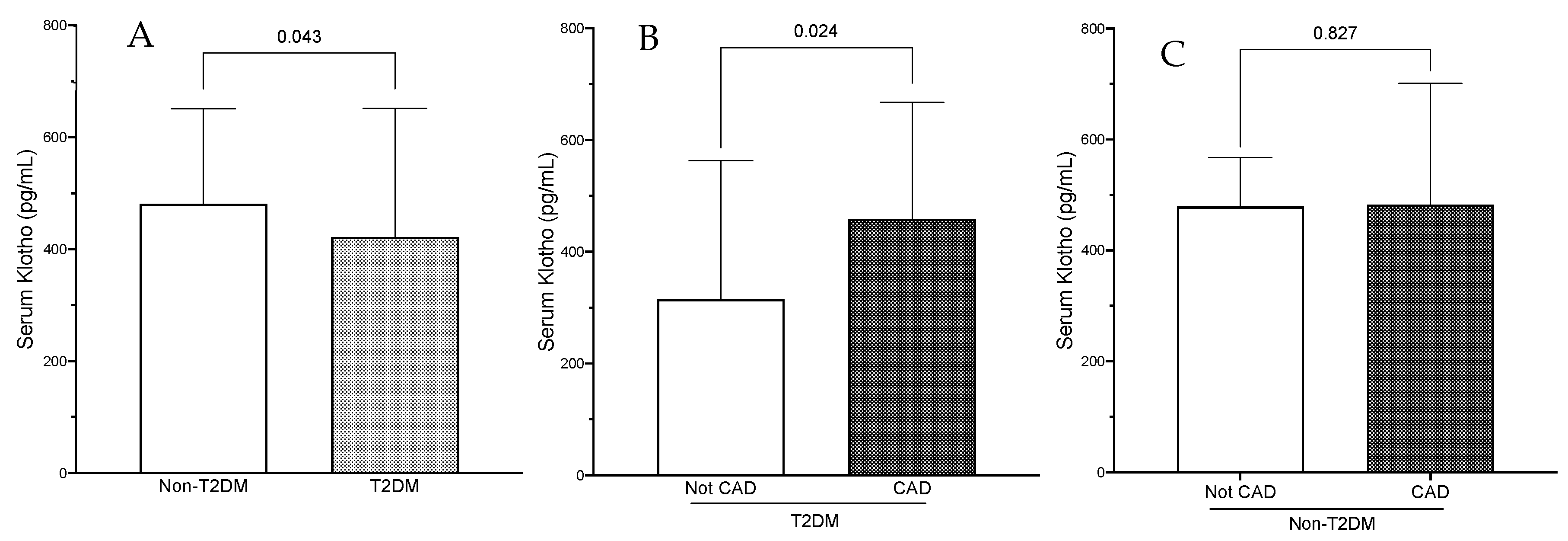
2. Results
3. Discussion
4. Materials and Methods
4.1. Study Design and Population
4.2. Coronary Angiography
4.3. Clinical and Biochemical Variables
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- IDF. Type 2 diabetes. In IDF Diabetes Atlas, 10th ed.; International Diabetes Federation: Brussels, Belgium, 2021; p. 14. [Google Scholar]
- Fowler, M.J. Microvascular and Macrovascular Complications of Diabetes. Clin. Diabetes 2008, 26, 77–82. [Google Scholar] [CrossRef]
- Grant, P.J.; Cosentino, F.; Marx, N. Diabetes and coronary artery disease: Not just a risk factor. Heart 2020, 106, 1357–1364. [Google Scholar] [CrossRef] [PubMed]
- Roberts, A.C.; Porter, K.E. Cellular and molecular mechanisms of endothelial dysfunction in diabetes. Diabetes Vasc. Dis. Res. 2013, 10, 472–482. [Google Scholar] [CrossRef] [PubMed]
- Jouven, X.; Lemaitre, R.N.; Rea, T.D.; Sotoodehnia, N.; Empana, J.P.; Siscovick, D.S. Diabetes, glucose level, and risk of sudden cardiac death. Eur. Heart J. 2005, 26, 2142–2147. [Google Scholar] [CrossRef]
- Hu, M.C.; Shiizaki, K.; Kuro-o, M.; Moe, O.W. Fibroblast growth factor 23 and Klotho: Physiology and pathophysiology of an endocrine network of mineral metabolism. Annu. Rev. Physiol. 2013, 75, 503–533. [Google Scholar] [CrossRef]
- Chen, C.D.; Tung, T.Y.; Liang, J.; Zeldich, E.; Tucker Zhou, T.B.; Turk, B.E.; Abraham, C.R. Identification of cleavage sites leading to the shed form of the anti-aging protein klotho. Biochemistry 2014, 53, 5579–5587. [Google Scholar] [CrossRef]
- Hu, M.C.; Kuro-o, M.; Moe, O.W. Klotho and chronic kidney disease. Contrib. Nephrol. 2013, 180, 47–63. [Google Scholar]
- Nie, F.; Wu, D.; Du, H.; Yang, X.; Yang, M.; Pang, X.; Xu, Y. Serum klotho protein levels and their correlations with the progression of type 2 diabetes mellitus. J. Diabetes Complicat. 2017, 31, 594–598. [Google Scholar] [CrossRef]
- Asai, O.; Nakatani, K.; Tanaka, T.; Sakan, H.; Imura, A.; Yoshimoto, S.; Samejima, K.; Yamaguchi, Y.; Matsui, M.; Akai, Y.; et al. Decreased renal α-Klotho expression in early diabetic nephropathy in humans and mice and its possible role in urinary calcium excretion. Kidney Int. 2012, 81, 539–547. [Google Scholar] [CrossRef]
- Lu, X.; Hu, M.C. Klotho/FGF23 Axis in chronic kidney disease and cardiovascular disease. Kidney Dis. 2017, 3, 15–23. [Google Scholar] [CrossRef]
- Kitagawa, M.; Sugiyama, H.; Morinaga, H.; Inoue, T.; Takiue, K.; Ogawa, A.; Yamanari, T.; Kikumoto, Y.; Uchida, H.A.; Kitamura, S.; et al. A decreased level of serum soluble Klotho is an independent biomarker associated with arterial stiffness in patients with chronic kidney disease. PLoS ONE 2013, 8, e56695. [Google Scholar] [CrossRef] [PubMed]
- Navarro-González, J.F.; Donate-Correa, J.; Muros de Fuentes, M.; Pérez-Hernández, H.; Martínez-Sanz, R.; Mora-Fernández, C. Reduced Klotho is associated with the presence and severity of coronary artery disease. Heart 2014, 100, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Donate-Correa, J.; Ferri, C.M.; Martín-Núñez, E.; Pérez-Delgado, N.; González-Luis, A.; Mora-Fernández, C.; Navarro-González, J.F. Klotho as a biomarker of subclinical atherosclerosis in patients with moderate to severe chronic kidney disease. Sci. Rep. 2021, 11, 15877. [Google Scholar] [CrossRef]
- Pan, H.C.; Chou, K.M.; Lee, C.C.; Yang, M.I.; Sun, C.Y. Circulating Klotho levels can predict long-term macrovascular outcomes in type 2 diabetic patients. Atherosclerosis 2018, 276, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Wang, Q.; Lv, C.; Qin, N.; Lei, S.; Yuan, Q.; Wang, G. The changes of serum sKlotho and NGAL levels and their correlation in type 2 diabetes mellitus patients with different stages of urinary albumin. Diabetes Res. Clin. Pract. 2014, 106, 343–350. [Google Scholar] [CrossRef]
- Van Ark, J.; Hammes, H.P.; Van Dijk, M.C.; Vervloet, M.G.; Wolffenbuttel, B.H.; Van Goor, H.; Hillebrands, J.L. Circulating alpha-klotho levels are not disturbed in patients with type 2 diabetes with and without macrovascular disease in the absence of nephropathy. Cardiovasc. Diabetol. 2013, 12, 116. [Google Scholar] [CrossRef] [PubMed]
- Keles, N.; Dogan, B.; Kalcik, M.; Caliskan, M.; Keles, N.N.; Aksu, F.; Bulut, M.; Kostek, O.; Isbilen, B.; Yilmaz, Y.; et al. Is serum Klotho protective against atherosclerosis in patients with type 1 diabetes mellitus? J. Diabetes Complicat. 2016, 30, 126–132. [Google Scholar] [CrossRef]
- Castelblanco, E.; Hernández, M.; Alonso, N.; Ribes-Betriu, A.; Real, J.; Granado-Casas, M.; Rossell, J.; Rojo-López, M.I.; Dusso, A.S.; Julve, J.; et al. Association of α-klotho with subclinical carotid atherosclerosis in subjects with type 1 diabetes mellitus. Cardiovasc. Diabetol. 2022, 21, 207. [Google Scholar] [CrossRef]
- Mao, Q.; Deng, M.; Zhao, J.; Zhou, D.; Chen, M.; Liu, Q.; Xu, S.; Zhao, X. Low serum Klotho reflects senile inflammation in middle-aged and elderly patients with coronary atherosclerosis. Cytokine 2023, 167, 156213. [Google Scholar] [CrossRef]
- Semba, R.D.; Cappola, A.R.; Sun, K.; Bandinelli, S.; Dalal, M.; Crasto, C.; Guralnik, J.M.; Ferrucci, L. Plasma klotho and cardiovascular disease in adults. J. Am. Geriatr. Soc. 2011, 59, 1596–1601. [Google Scholar] [CrossRef]
- Mao, Q.; Deng, M.; Zhao, J.; Zhou, D.; Tong, W.; Xu, S.; Zhao, X. Klotho ameliorates angiotension-II-induced endothelial senescence via restoration of autophagy by inhibiting Wnt3a/GSK-3β/mTOR signaling: A potential mechanism involved in prognostic performance of Klotho in coronary atherosclerotic disease. Mech. Ageing Dev. 2023, 211, 111789. [Google Scholar] [CrossRef] [PubMed]
- Kanbay, M.; Demiray, A.; Afsar, B.; Covic, A.; Tapoi, L.; Ureche, C.; Ortiz, A. Role of Klotho in the Development of Essential Hypertension. Hypertension 2021, 77, 740–750. [Google Scholar] [CrossRef] [PubMed]
- Fard, T.K.; Ahmadi, R.; Akbari, T.; Moradi, N.; Fadaei, R.; Fard, M.K.; Fallah, S. Klotho, FOXO1 and cytokines associations in patients with coronary artery disease. Cytokine 2021, 141, 155443. [Google Scholar] [CrossRef] [PubMed]
- Shimamura, Y.; Hamada, K.; Inoue, K.; Ogata, K.; Ishihara, M.; Kagawa, T.; Inoue, M.; Fujimoto, S.; Ikebe, M.; Yuasa, K.; et al. Serum levels of soluble secreted α-Klotho are decreased in the early stages of chronic kidney disease, making it a probable novel biomarker for early diagnosis. Clin. Exp. Nephrol. 2012, 16, 722–729. [Google Scholar] [CrossRef] [PubMed]
- Devaraj, S.; Syed, B.; Chien, A.; Jialal, I. Validation of an immunoassay for soluble Klotho protein: Decreased levels in diabetes and increased levels in chronic kidney disease. Am. J. Clin. Pathol. 2012, 137, 479–485. [Google Scholar] [CrossRef]
- Wang, Q.; Su, W.; Shen, Z.; Wang, R. Correlation between soluble alpha-klotho and renal function in patients with chronic kidney disease: A review and meta-analysis. Biomed. Res. Int. 2018, 2018, 9481475. [Google Scholar] [CrossRef]
- Liu, Q.F.; Ye, J.M.; Yu, L.X.; He, A.L.; Sun, Q.; He, D.W.; Li, S.S. Plasma s-Klotho is related to kidney function and predicts adverse renal outcomes in patients with advanced chronic kidney disease. J. Investig. Med. 2017, 66, 669–675. [Google Scholar] [CrossRef]
- Memmos, E.; Sarafidis, P.; Pateinakis, P.; Tsiantoulas, A.; Faitatzidou, D.; Giamalis, P.; Vasilikos, V.; Papagianni, A. Soluble Klotho is associated with mortality and cardiovascular events in hemodialysis. BMC Nephrol. 2019, 20, 217. [Google Scholar] [CrossRef]
- Lee, E.Y.; Kim, S.S.; Lee, J.S.; Kim, I.J.; Song, S.H. Soluble a-Klotho as a Novel Biomarker in the Early Stage of Nephropathy in Patients with Type 2 Diabetes. PLoS ONE 2014, 9, e102984. [Google Scholar] [CrossRef]
- Koga, S.; Ikeda, S.; Akashi, R.; Yonekura, T.; Kawano, H.; Maemura, K. Serum soluble Klotho is inversely related to coronary artery calcification assessed by intravascular ultrasound in patients with stable coronary artery disease. J. Cardiol. 2021, 77, 583–589. [Google Scholar] [CrossRef]
- van Venrooij, N.A.; Pereira, R.C.; Tintut, Y.; Fishbein, M.C.; Tumber, N.; Demer, L.L.; Salusky, I.B.; Wesseling-Perry, K. FGF23 protein expression in coronary arteries is associated with impaired kidney function. Nephrol. Dial. Transpl. 2014, 29, 1525–1532. [Google Scholar] [CrossRef] [PubMed]
- Nattero-Chávez l Luque-Ramírez, M.; Moncayo, S.; Alonso-Díaz, S.; Fernández-Durán, E.; Redondo-López, S.; García-Ureña, M.; Escobar-Morreale, H.F. Circulating soluble klotho is not associated with an elevated ankle-brachial index as a surrogate marker of early arterial calcification in patients with type 1 diabetes mellitus and no evidence of renal dysfunction. Diabetes Metab. 2019, 45, 589–592. [Google Scholar] [CrossRef]
- Wang, Y.; Kuro-o, M.; Sun, Z. Klotho gene delivery suppresses Nox2 expression and attenuates oxidative stress in rat aortic smooth muscle cells via the cAMP-PKA pathway. Aging Cell 2012, 11, 410–417. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Banerjee, S.; Dey, N.; LeJeune, W.S.; Sarkar, P.S.; Brobey, R.; Rosenblatt, K.P.; Tilton, R.G.; Choudhary, S. Klotho depletion contributes to increased inflammation in kidney of the db/db mouse model of diabetes via RelA (Serine)536 phosphorylation. Diabetes 2011, 60, 1907–1916. [Google Scholar] [CrossRef] [PubMed]
- Buendía, P.; Ramírez, R.; Aljama, P.; Carracedo, J. Klotho Prevents Translocation of NFkB. Vitam. Horm. 2016, 101, 119–150. [Google Scholar] [PubMed]
- Cuarental, L.; Ribagorda, M.; Ceballos, M.I.; Pintor-Chocano, A.; Carriazo, S.M.; Dopazo, A.; Vazquez, E.; Suarez-Alvarez, B.; Cannata-Ortiz, P.; Sanz, A.B.; et al. The transcription factor Fosl1 preserves Klotho expression and protects from acute kidney injury. Kidney Int. 2023, 103, 686–701. [Google Scholar] [CrossRef]
- Yoon, H.E.; Lim, S.W.; Piao, S.G.; Song, J.H.; Kim, J.; Yang, C.W. Statin upregulates the expression of klotho, an anti-aging gene, in experimental cyclosporine nephropathy. Nephron Exp. Nephrol. 2012, 120, e123–e133. [Google Scholar] [CrossRef]
- Yoon, H.E.; Ghee, J.Y.; Piao, S.; Song, J.H.; Han, D.H.; Kim, S.; Ohashi, N.; Kobori, H.; Kuro-o, M.; Yang, C.W. Angiotensin II blockade upregulates the expression of Klotho, the anti-ageing gene, in an experimental model of chronic cyclosporine nephropathy. Nephrol. Dial. Transplant. 2011, 26, 800–813. [Google Scholar] [CrossRef]
- Chen, C.D.; Podvin, S.; Gillespie, E.; Leeman, S.E.; Abraham, C.R. Insulin stimulates the cleavage and release of the extracellular domain of Klotho by ADAM10 and ADAM17. Proc. Natl. Acad. Sci. USA 2007, 104, 19796–19801. [Google Scholar] [CrossRef]
- O’Toole, P.W.; Shiels, P.G. The role of the microbiota in sedentary lifestyle disorders and ageing: Lessons from the animal kingdom. J. Intern. Med. 2020, 287, 271–282. [Google Scholar] [CrossRef]
- Karlsson, F.H.; Fåk, F.; Nookaew, I.; Tremaroli, V.; Fagerberg, B.; Petranovic, D.; Bäckhed, F.; Nielsen, J. Symptomatic atherosclerosis is associated with an altered gut metagenome. Nat. Commun. 2012, 3, 1245. [Google Scholar] [CrossRef] [PubMed]
- Boulangé, C.L.; Neves, A.L.; Chilloux, J.; Nicholson, J.K.; Dumas, M.E. Impact of the gut microbiota on inflammation, obesity, and metabolic disease. Genome Med. 2016, 8, 42. [Google Scholar] [PubMed]
| Non-T2DM | T2DM | p | All Subjects | |
|---|---|---|---|---|
| Characteristics | ||||
| N | 200 | 133 | 333 | |
| Age (years) | 64.6 ± 11.3 | 66.3 ± 10.2 | 0.27 | 65.3 ± 10.9 |
| Sex (% male) | 135 (67.5) | 85 (63.9) | 0.49 | 220 (66.1) |
| BMI (kg/m2) | 25.3 ± 2.8 | 27.1 ± 2.1 | 0.09 | 26.1 ± 3.2 |
| SBP (mm Hg) | 123 ± 15.1 | 128 ± 11.5 | 0.12 | 127 ± 12.3 |
| DBP (mm Hg) | 74.5 ± 8.6 | 75.8 ± 9.7 | 0.13 | 74.9 ± 9.1 |
| Comorbidities (%) | ||||
| Significant CAD | 167 (83.5) | 103 (77.4) | 0.17 | 270 (81.1) |
| Obesity | 28 (14) | 14 (10.5) | 0.35 | 42 (12.6) |
| Hypertension | 61 (30.5) | 37 (27.8) | 0.59 | 98 (29.4) |
| Former smokers | 40 (20) | 28 (21.1) | 0.46 | 68 (20.4) |
| Current smokers | 63 (31.5) | 21 (23.3) | 0.04 | 94 (28.2) |
| Dyslipidemia | 88 (44) | 54 (40.6) | 0.54 | 142 (42.6) |
| Laboratory data | ||||
| T-cholesterol (mg/dL) | 172.6 (145–201.5) | 168 (140–190) | 0.14 | 171 (143–198) |
| HDL-C (mg/dL) | 40.5 (33–48) | 37 (31–43.5) | 0.05 | 38 (32–46) |
| LDL-C (mg/dL) | 103 (80–127) | 94 (74.5–112) | <0.01 | 100.4 (76–121.8) |
| TG (mg/dL) | 125 (92.3–162.8) | 142 (114.5–197.5) | <0.001 | 135 (102–172) |
| TyG index | 4.68 (4.53–4.79) | 5.04 (4.9–5.25) | <0.001 | 4.8 (4.61–5.02) |
| FG (mg/dL) | 90 (86–95) | 158 (138–200) | <0.001 | 97 (89–147.5) |
| Hb1ac (%) | 5.37 ± 0.37 | 7.57 ± 0.42 | <0.001 | 6.28 ± 1.46 |
| eGFR (mL/min/1.73 m2) | 101.1 ± 9.2 | 97.5 ± 8.3 | 0.11 | 99.5 ± 3.3 |
| Creatinine (mg/dL) | 0.89 (0.75–1.05) | 0.82 (0.7–1.02) | 0.04 | 0.86 (0.74–1.04) |
| ACR (mg/g) | 5.82 (3.54–15.9) | 8.38 (4.08–16.96) | 0.08 | 6.83 (3.68–16.04) |
| Uric acid (mg/dL) | 5.54 (4.58–6.69) | 5.36 (4.46–6.21) | 0.29 | 5.43 (5.5–6.6) |
| Calcium (mg/dL) | 9.32 ± 0.31 | 9.33 ± 0.38 | 0.56 | 9.32 ± 0.34 |
| Phosphate (mg/dL) | 3.54 ± 0.53 | 3.55 ± 0.67 | 0.71 | 3.54 ± 0.71 |
| NLR (/mL) | 2.3 (1.69–3.24) | 1.78 (2.32–3.61) | 0.28 | 2.31 (1.74–3.33) |
| MLR (/mL) | 0.33 (0.26–0.49) | 0.32 (0.24–0.44) | 0.06 | 0.33 (0.24–0.46) |
| hs-CRP (mg/L) | 3.2 (1.92–6.23) | 3.3 (2–6.7) | 0.74 | 3.2 (2–6.6) |
| TNFα (pg/mL) | 1.99 (1.35–2.55) | 2.09 (1.52–2.99) | 0.12 | 2.06 (1.43–2.77) |
| IL6 (pg/mL) | 6.12 (3.49–10.35) | 6.36 (3.3–12.14) | 0.52 | 6.3 (3.3–10.76) |
| Klotho (pg/mL) | 480.8 (332.2–651) | 421.9 (252–651.4) | 0.04 | 466.3 (290.5–651.4) |
| iFGF23 (pg/mL) | 583.3 (471.7–790.1) | 570.7 (427.3–706.1) | 0.11 | 577 (443.1–766.1) |
| Medication | ||||
| Statin (%) | 104 (52) | 84 (63.2) | 0.04 | 188 (56.5) |
| ACEI/ARB (%) | 80 (40) | 61 (45.9) | 0.29 | 141 (42.3) |
| Non-T2DM | T2DM | p | All Subjects | |
|---|---|---|---|---|
| SSI | 30.13 (16.1–44.94) | 33.5 (16.13–54.13) | 0.39 | 32.25 (16.1–51.13) |
| Obstruction (%) | ||||
| LCA | 55.12 ± 29.1 | 54.2 ± 29.21 | 0.61 | 54.75 ± 29.1 |
| RCA | 25.86 ± 34.66 | 33.16 ± 37.4 | 0.02 | 28.78 ± 35.91 |
| LAD | 33.43 ± 38.92 | 34.59 ± 40.05 | 0.33 | 33.89 ± 39.32 |
| CA | 14.54 ± 30.82 | 14.77 ± 31.1 | 0.94 | 14.63 ± 30.89 |
| No CAD | CAD | p | |
|---|---|---|---|
| Characteristics | |||
| N | 30 | 103 | |
| Age (years) | 63 (54.8–73) | 68 (61–75) | 0.16 |
| Sex (% male) | 20 (66.7) | 65 (63.1) | 0.83 |
| BMI (kg/m2) | 26.8 ± 2.2 | 27.3 ± 2.9 | 0.13 |
| SBP (mm Hg) | 129 ± 10.1 | 132 ± 11.1 | 0.04 |
| DBP (mm Hg) | 75.3 ± 7.7 | 75.9 ± 9.1 | 0.47 |
| Comorbidities | |||
| Obesity | 4 (13.3) | 10 (9.7) | 0.52 |
| Hypertension (%) | 6 (20) | 31 (30.1) | 0.36 |
| Former smoker (%) | 7 (23.3) | 21 (20.4) | 0.12 |
| Current smokers (%) | 7 (23.3) | 19 (18.4) | 0.21 |
| Dyslipidemia (%) | 10 (33) | 44 (42.7) | 0.41 |
| Laboratory data | |||
| T-cholesterol (mg/dL) | 160.5 (134.5–188.5) | 168 (142–193) | 0.47 |
| HDL-C (mg/dL) | 34 (27.3–42.8) | 38 (31–44) | 0.26 |
| LDL-C (mg/dL) | 90.5 (70.3–118.5) | 95 (75–110) | 0.84 |
| TG (mg/dL) | 142.5 (114.5–200) | 142 (114–197) | 0.97 |
| TyG index | 5.08 (4.9–5.26) | 5.03 (4.9–5.25) | 0.76 |
| FG (mg/dL) | 133.8 (67.2–222) | 162 (142–196) | 0.28 |
| Hb1ac (%) | 7.42 ± 0.2 | 7.58 ± 0.21 | 0.11 |
| eGFR (mL/min/1.73 m2) | 99.8 ± 10.3 | 96.9 ± 9.3 | 0.16 |
| Creatinine (mg/dL) | 0.84 (0.71–0.99) | 0.82 (0.7–1.1) | 0.64 |
| ACR (mg/g) | 5.76 (3.22–13.84) | 9 (4.3–19) | 0.08 |
| Uric acid (mg/dL) | 5.36 (4.35–6.89) | 5.36 (4.45–6.11) | 0.99 |
| Calcium (mg/dL) | 9.3 ± 0.34 | 9.36 ± 0.28 | 0.85 |
| Phosphate (mg/dL) | 3.55 ± 0.71 | 3.55 ± 0.42 | 0.98 |
| NLR (/mL) | 2.58 (1.62–3.89) | 2.2 (1.8–3.52) | 0.69 |
| MLR (/mL) | 0.33 (0.23–0.46) | 0.32 (0.24–0.43) | 0.86 |
| hs-CRP (mg/L) | 2.75 (1.25–5.9) | 3.9 (2.1–6.8) | 0.03 |
| TNFα (pg/mL) | 1.87 (1.34–2.72) | 2.22 (1.57–3.1) | 0.04 |
| IL6 (pg/mL) | 5.03 (2.48–8.97) | 7.5 (3.51–12–25) | 0.03 |
| Klotho (pg/mL) | 314.5 (6.15–562.81) | 458.97 (275.2–667.2) | 0.02 |
| iFGF23 (pg/mL) | 571.2 (444.5–675.4) | 570.21 (421.3–707.3) | 0.91 |
| Medication | |||
| Statin (%) | 21 (70) | 63 (61.2) | 0.38 |
| ACEI/ARB (%) | 11 (36.7) | 50 (48.5) | 0.25 |
| Non-T2DM | T2DM | |||
|---|---|---|---|---|
| r | p | r | p | |
| SSI | −0.3 | 0.675 | 0.233 | 0.007 |
| Obs LCA (%) | −0.074 | 0.297 | 0.212 | 0.014 |
| Obs RCA (%) | 0.028 | 0.696 | 0.182 | 0.036 |
| Obs LAD (%) | −0.063 | 0.379 | 0.152 | 0.082 |
| Obs CA (%) | 0.114 | 0.108 | 0.029 | 0.739 |
| Stenosis Severity Index | Adjusted R2 | ß | SE | t | p |
|---|---|---|---|---|---|
| Non-T2DM subjects | 0.045 | <0.05 | |||
| TNFα (pg/mL) | 0.130 | 0.228 | 1.837 | 0.048 | |
| ACR (mg/g) | 0.129 | 0.178 | 1.849 | 0.046 | |
| T2DM subjects | 0.153 | <0.01 | |||
| Dyslipidemia | 0.206 | 3.852 | 2.469 | 0.015 | |
| Klotho (pg/mL) | 0.184 | 0.007 | 2.201 | 0.030 | |
| ACR (mg/g) | 0.228 | 0.225 | 2.735 | 0.007 |
| Unadjusted | Model 1 | Model 2 | Model 3 | |||||
|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | |
| Klotho (pg/mL) | 1.003 (1.002–1.003) | <0.001 | 1.001 (1.0–1.003) | 0.037 | 1.001 (1.0–1.003) | 0.039 | 1.001 (1.0–1.003) | 0.041 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Donate-Correa, J.; Martín-Núñez, E.; Mora-Fernández, C.; González-Luis, A.; Martín-Olivera, A.; Navarro-González, J.F. Association of Klotho with Coronary Artery Disease in Subjects with Type 2 Diabetes Mellitus and Preserved Kidney Function: A Case-Control Study. Int. J. Mol. Sci. 2023, 24, 13456. https://doi.org/10.3390/ijms241713456
Donate-Correa J, Martín-Núñez E, Mora-Fernández C, González-Luis A, Martín-Olivera A, Navarro-González JF. Association of Klotho with Coronary Artery Disease in Subjects with Type 2 Diabetes Mellitus and Preserved Kidney Function: A Case-Control Study. International Journal of Molecular Sciences. 2023; 24(17):13456. https://doi.org/10.3390/ijms241713456
Chicago/Turabian StyleDonate-Correa, Javier, Ernesto Martín-Núñez, Carmen Mora-Fernández, Ainhoa González-Luis, Alberto Martín-Olivera, and Juan F. Navarro-González. 2023. "Association of Klotho with Coronary Artery Disease in Subjects with Type 2 Diabetes Mellitus and Preserved Kidney Function: A Case-Control Study" International Journal of Molecular Sciences 24, no. 17: 13456. https://doi.org/10.3390/ijms241713456
APA StyleDonate-Correa, J., Martín-Núñez, E., Mora-Fernández, C., González-Luis, A., Martín-Olivera, A., & Navarro-González, J. F. (2023). Association of Klotho with Coronary Artery Disease in Subjects with Type 2 Diabetes Mellitus and Preserved Kidney Function: A Case-Control Study. International Journal of Molecular Sciences, 24(17), 13456. https://doi.org/10.3390/ijms241713456





