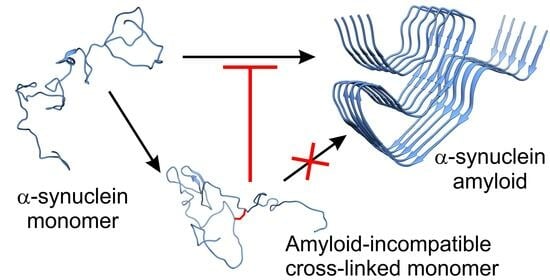
Cross-Linked α-Synuclein as Inhibitor of Amyloid Formation
Abstract
1. Introduction
2. Results
2.1. Analysis of α-Synuclein Structures for Potential Cross-Linking Residue Pairs
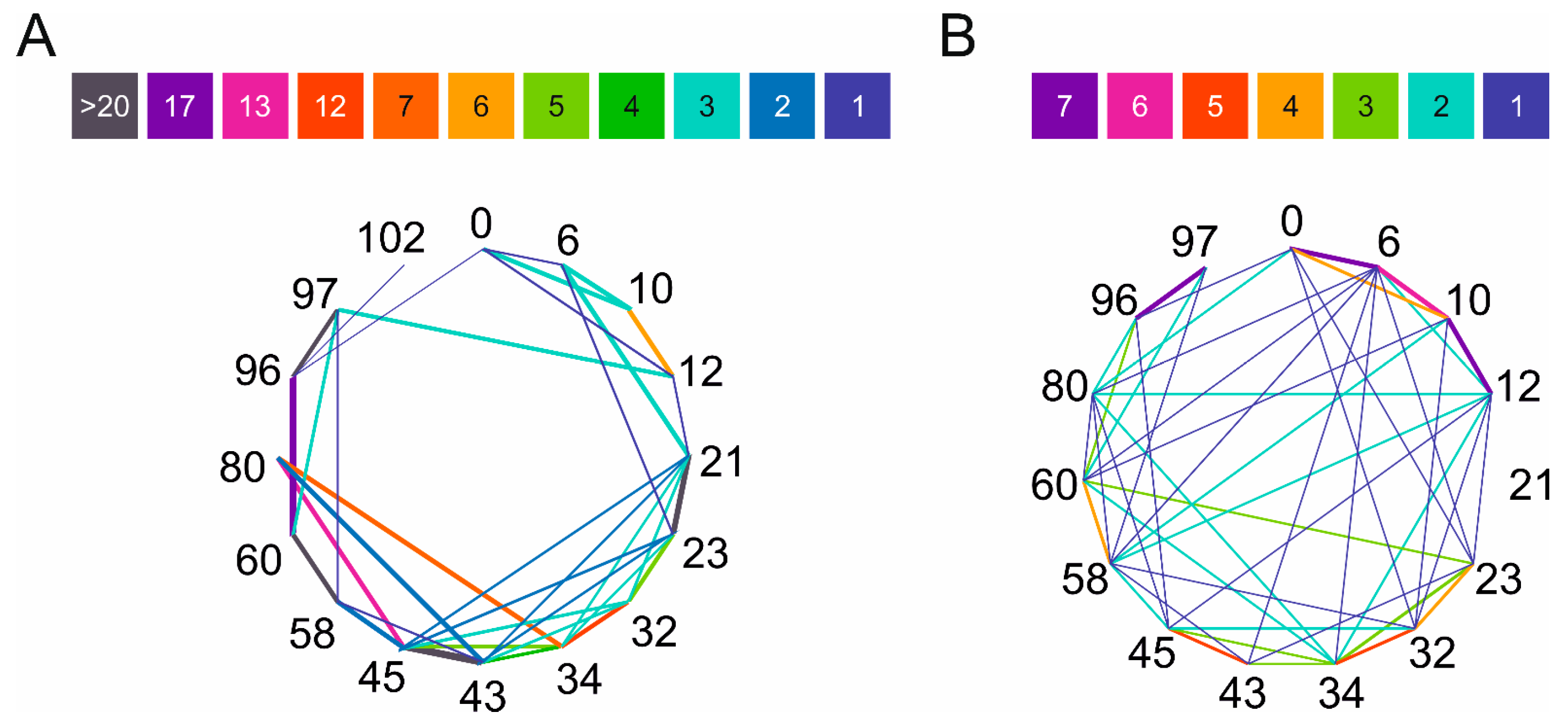
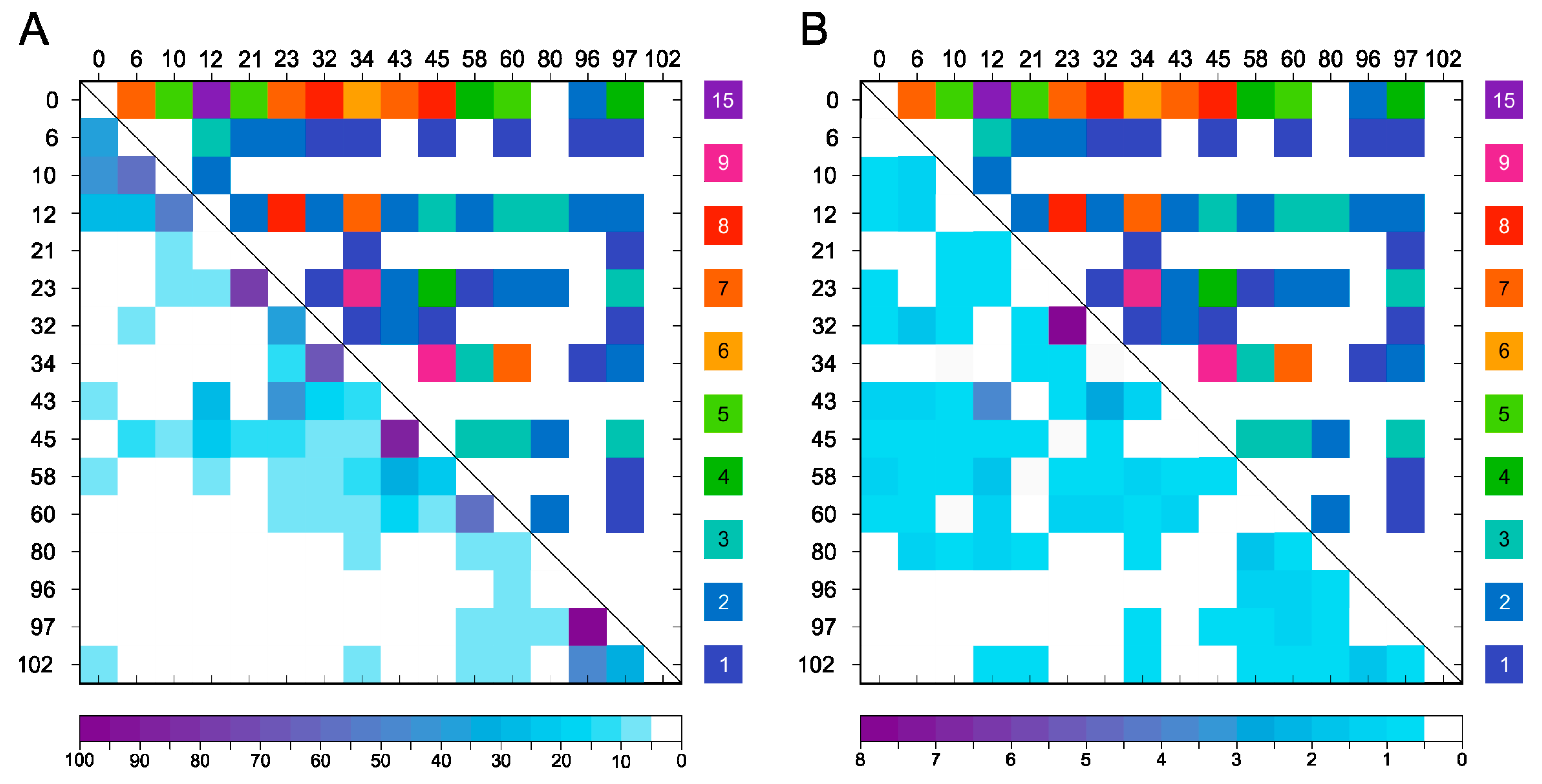
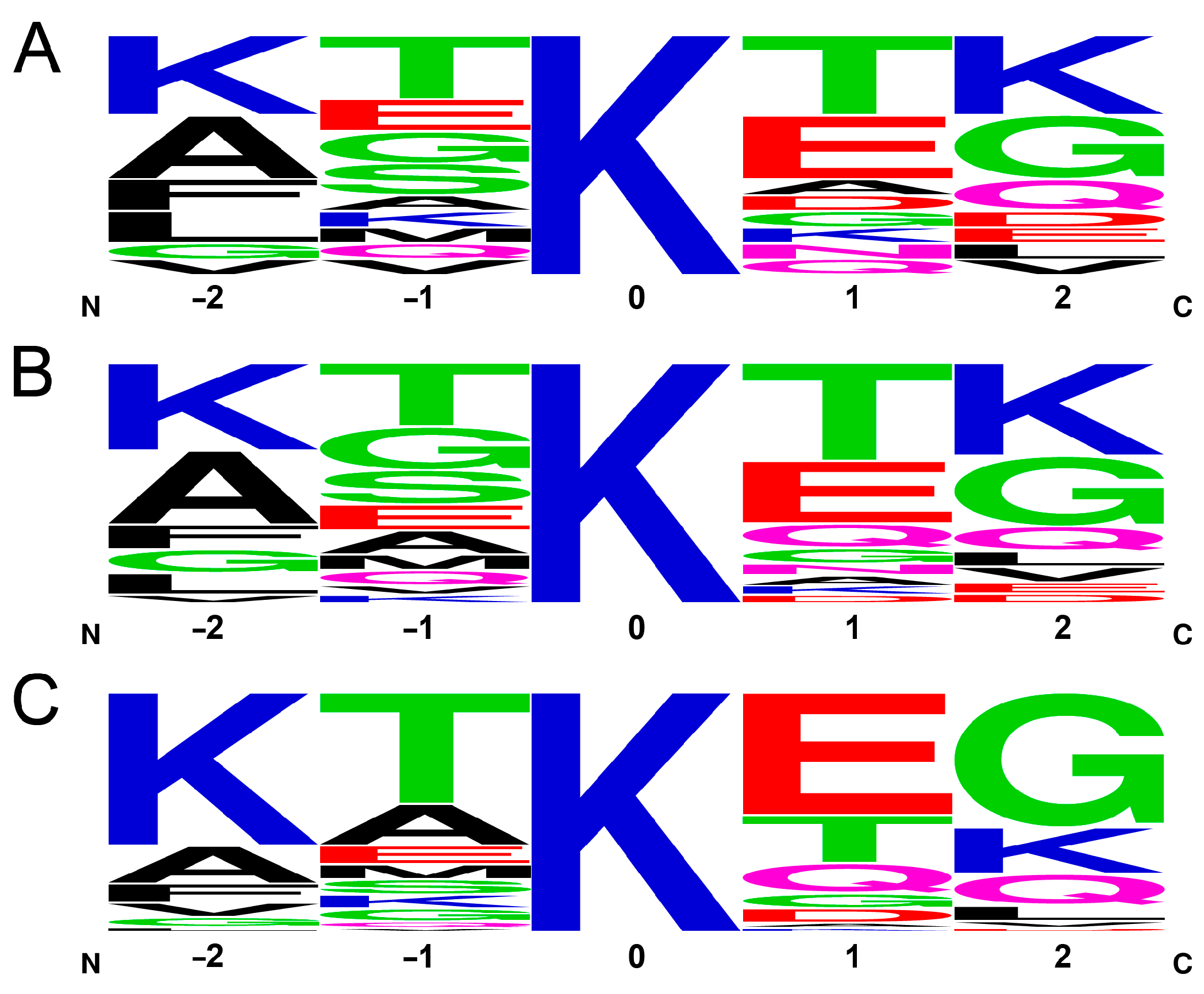
2.2. Chemical Cross-Linking and Identification by Mass Spectrometry
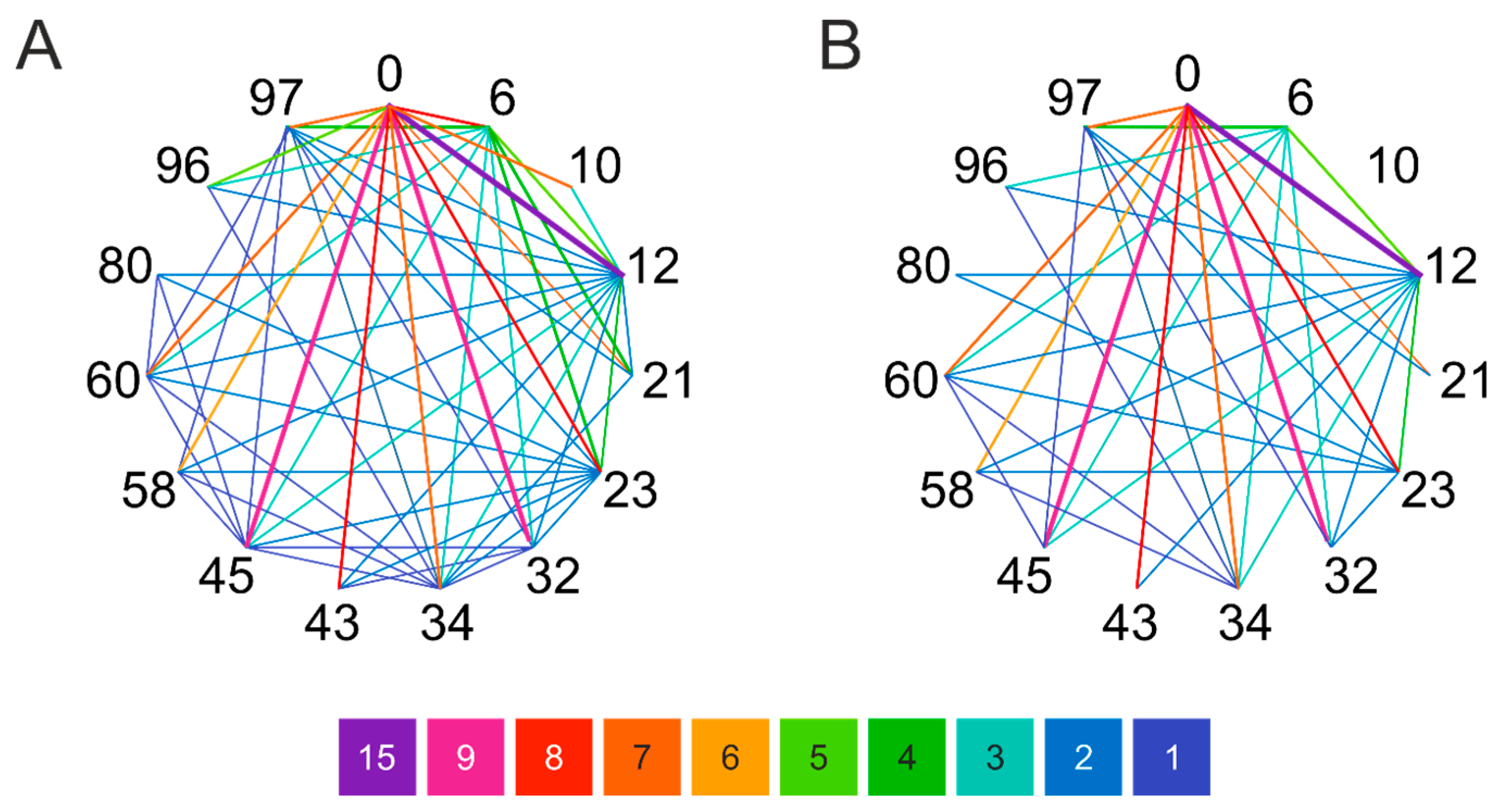
2.3. Comparison of Experimental Cross-Linking Results with Distance Analysis on Amyloid Structures
2.4. Distance Analysis of Amino Groups in MD Simulation of Monomeric α-Synuclein
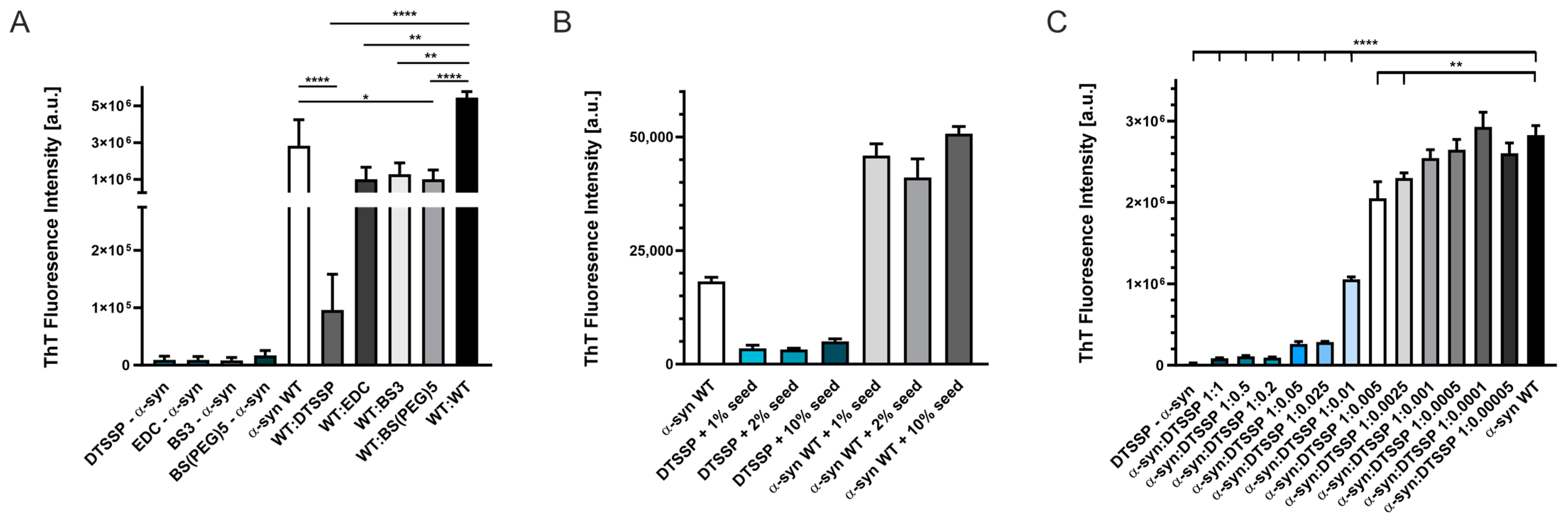
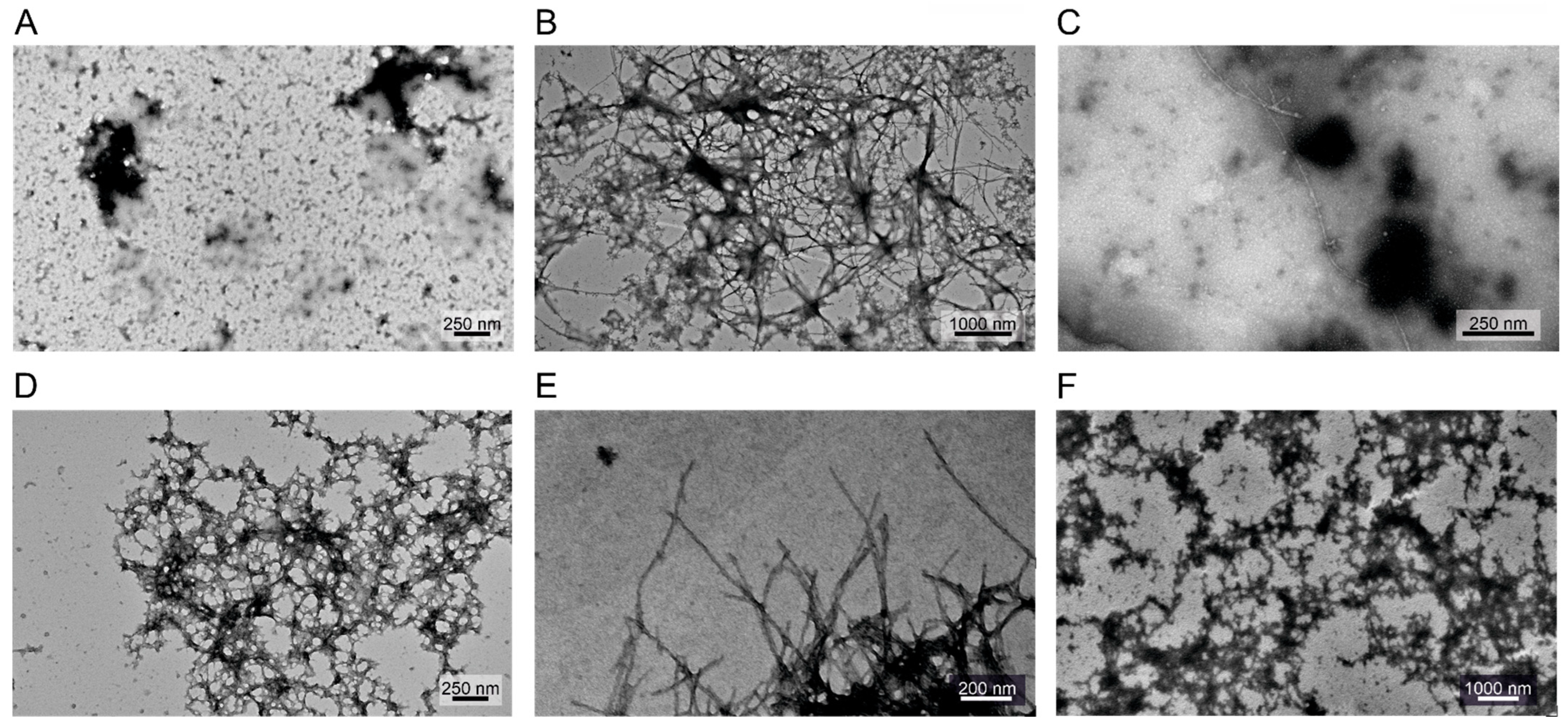
2.5. Effect of the Introduced Cross-Links on the Fibrillation and Aggregate Morphology of α-Syn
3. Discussion
4. Materials and Methods
4.1. Expression and Purification of α-Synuclein
4.2. Cross-Linking Reactions of α-Synuclein by Chosen Agents and Optimization
4.3. Protein Measurement with LC–MS and Data Analysis
4.4. Sample Preparation for LC–MS/MS Analysis, Measurement, and Data Evaluation
4.5. MD Simulations and Trajectory Analysis for Potential Cross-Linking Pairs
4.6. α-Synuclein Aggregation and Thioflavin T Fluorescence Assay
4.7. Transmission Electron Microscopy (TEM)
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Souza, J.M.; Giasson, B.I.; Chen, Q.; Lee, V.M.-Y.; Ischiropoulos, H. Dityrosine Cross-Linking Promotes Formation of Stable α-Synuclein Polymers. J. Biol. Chem. 2000, 275, 18344–18349. [Google Scholar] [CrossRef] [PubMed]
- Bitan, G.; Teplow, D.B. Rapid Photochemical Cross-Linking—A New Tool for Studies of Metastable, Amyloidogenic Protein Assemblies. Acc. Chem. Res. 2004, 37, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Konno, T.; Morii, T.; Hirata, A.; Sato, S.-I.; Oiki, S.; Ikura, K. Covalent Blocking of Fibril Formation and Aggregation of Intracellular Amyloidgenic Proteins by Transglutaminase-Catalyzed Intramolecular Cross-Linking. Biochemistry 2005, 44, 2072–2079. [Google Scholar] [CrossRef] [PubMed]
- Wilhelmus, M.M.M.; van Dam, A.-M.; Drukarch, B. Tissue Transglutaminase: A Novel Pharmacological Target in Preventing Toxic Protein Aggregation in Neurodegenerative Diseases. Eur. J. Pharmacol. 2008, 585, 464–472. [Google Scholar] [CrossRef]
- Maina, M.B.; Burra, G.; Al-Hilaly, Y.K.; Mengham, K.; Fennell, K.; Serpell, L.C. Metal- and UV-Catalyzed Oxidation Results in Trapped Amyloid-β Intermediates Revealing That Self-Assembly Is Required for Aβ-Induced Cytotoxicity. iScience 2020, 23, 101537. [Google Scholar] [CrossRef]
- Fraenkel-Conrat, H.; Olcott, H.S. The Reaction of Formaldehyde with Proteins. V. Cross-Linking between Amino and Primary Amide or Guanidyl Groups. J. Am. Chem. Soc. 1948, 70, 2673–2684. [Google Scholar] [CrossRef]
- Sutherland, B.W.; Toews, J.; Kast, J. Utility of Formaldehyde Cross-Linking and Mass Spectrometry in the Study of Protein-Protein Interactions. J. Mass Spectrom. 2008, 43, 699–715. [Google Scholar] [CrossRef]
- Segers-Nolten, I.M.J.; Wilhelmus, M.M.M.; Veldhuis, G.; van Rooijen, B.D.; Drukarch, B.; Subramaniam, V. Tissue Transglutaminase Modulates α-Synuclein Oligomerization. Protein Sci. 2008, 17, 1395–1402. [Google Scholar] [CrossRef]
- Trnka, M.J.; Baker, P.R.; Robinson, P.J.J.; Burlingame, A.L.; Chalkley, R.J. Matching Cross-Linked Peptide Spectra: Only as Good as the Worse Identification. Mol. Cell. Proteom. 2014, 13, 420–434. [Google Scholar] [CrossRef]
- Chiti, F.; Dobson, C.M. Protein Misfolding, Functional Amyloid, and Human Disease. Annu. Rev. Biochem. 2006, 75, 333–366. [Google Scholar] [CrossRef]
- Eisenberg, D.; Jucker, M. The Amyloid State of Proteins in Human Diseases. Cell 2012, 148, 1188–1203. [Google Scholar] [CrossRef]
- Knowles, T.P.J.; Vendruscolo, M.; Dobson, C.M. The Amyloid State and Its Association with Protein Misfolding Diseases. Nat. Rev. Mol. Cell Biol. 2014, 15, 384–396. [Google Scholar] [CrossRef] [PubMed]
- Walsh, D.M.; Selkoe, D.J. A Critical Appraisal of the Pathogenic Protein Spread Hypothesis of Neurodegeneration. Nat. Rev. Neurosci. 2016, 17, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Alam, P.; Bousset, L.; Melki, R.; Otzen, D.E. A-Synuclein Oligomers and Fibrils: A Spectrum of Species, a Spectrum of Toxicities. J. Neurochem. 2019, 150, 522–534. [Google Scholar] [CrossRef] [PubMed]
- Fändrich, M. Oligomeric Intermediates in Amyloid Formation: Structure Determination and Mechanisms of Toxicity. J. Mol. Biol. 2012, 421, 427–440. [Google Scholar] [CrossRef] [PubMed]
- Paudel, H.K.; Li, W. Heparin-Induced Conformational Change in Microtubule-Associated Protein Tau as Detected by Chemical Cross-Linking and Phosphopeptide Mapping. J. Biol. Chem. 1999, 274, 8029–8038. [Google Scholar] [CrossRef] [PubMed]
- Steigenberger, B.; Albanese, P.; Heck, A.J.R.; Scheltema, R.A. To Cleave or Not to Cleave in XL-MS? J. Am. Soc. Mass Spectrom. 2020, 31, 196–206. [Google Scholar] [CrossRef]
- Ding, Y.-H.; Fan, S.-B.; Li, S.; Feng, B.-Y.; Gao, N.; Ye, K.; He, S.-M.; Dong, M.-Q. Increasing the Depth of Mass-Spectrometry-Based Structural Analysis of Protein Complexes through the Use of Multiple Cross-Linkers. Anal. Chem. 2016, 88, 4461–4469. [Google Scholar] [CrossRef]
- Iacobucci, C.; Piotrowski, C.; Aebersold, R.; Amaral, B.C.; Andrews, P.; Bernfur, K.; Borchers, C.; Brodie, N.I.; Bruce, J.E.; Cao, Y.; et al. First Community-Wide, Comparative Cross-Linking Mass Spectrometry Study. Anal. Chem. 2019, 91, 6953–6961. [Google Scholar] [CrossRef]
- King, G.J.; Jones, A.; Kobe, B.; Huber, T.; Mouradov, D.; Hume, D.A.; Ross, I.L. Identification of Disulfide-Containing Chemical Cross-Links in Proteins Using MALDI-TOF/TOF-Mass Spectrometry. Anal. Chem. 2008, 80, 5036–5043. [Google Scholar] [CrossRef]
- Grazia Spillantini, M.; Anthony Crowther, R.; Jakes, R.; Cairns, N.J.; Lantos, P.L.; Goedert, M. Filamentous α-Synuclein Inclusions Link Multiple System Atrophy with Parkinson’s Disease and Dementia with Lewy Bodies. Neurosci. Lett. 1998, 251, 205–208. [Google Scholar] [CrossRef] [PubMed]
- Spillantini, M.G.; Crowther, R.A.; Jakes, R.; Hasegawa, M.; Goedert, M. α-Synuclein in Filamentous Inclusions of Lewy Bodies from Parkinson’s Disease and Dementia with Lewy Bodies. Proc. Natl. Acad. Sci. USA 1998, 95, 6469–6473. [Google Scholar] [CrossRef] [PubMed]
- Brodie, N.I.; Petrotchenko, E.V.; Borchers, C.H. The Novel Isotopically Coded Short-Range Photo-Reactive Crosslinker 2,4,6-Triazido-1,3,5-Triazine (TATA) for Studying Protein Structures. J. Proteom. 2016, 149, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zaer, S.; Drori, P.; Zamel, J.; Joron, K.; Kalisman, N.; Lerner, E.; Dokholyan, N.V. The Structural Heterogeneity of α-Synuclein Is Governed by Several Distinct Subpopulations with Interconversion Times Slower than Milliseconds. Structure 2021, 29, 1048–1064.e6. [Google Scholar] [CrossRef] [PubMed]
- Koder Hamid, M.; Månsson, L.K.; Meklesh, V.; Persson, P.; Skepö, M. Molecular Dynamics Simulations of the Adsorption of an Intrinsically Disordered Protein: Force Field and Water Model Evaluation in Comparison with Experiments. Front. Mol. Biosci. 2022, 9, 958175. [Google Scholar] [CrossRef] [PubMed]
- Bendor, J.T.; Logan, T.P.; Edwards, R.H. The Function of α-Synuclein. Neuron 2013, 79, 1044–1066. [Google Scholar] [CrossRef] [PubMed]
- Amaral, M.; Kokh, D.B.; Bomke, J.; Wegener, A.; Buchstaller, H.P.; Eggenweiler, H.M.; Matias, P.; Sirrenberg, C.; Wade, R.C.; Frech, M. Protein Conformational Flexibility Modulates Kinetics and Thermodynamics of Drug Binding. Nat. Commun. 2017, 8, 2276. [Google Scholar] [CrossRef]
- Kumar, S.; Kumar, R.; Kumari, M.; Kumari, R.; Saha, S.; Bhavesh, N.S.; Maiti, T.K. Ellagic Acid Inhibits α-Synuclein Aggregation at Multiple Stages and Reduces Its Cytotoxicity. ACS Chem. Neurosci. 2021, 12, 1919–1930. [Google Scholar] [CrossRef]
- Nguyen, P.H.; Ramamoorthy, A.; Sahoo, B.R.; Zheng, J.; Faller, P.; Straub, J.E.; Dominguez, L.; Shea, J.-E.; Dokholyan, N.V.; De Simone, A.; et al. Amyloid Oligomers: A Joint Experimental/Computational Perspective on Alzheimer’s Disease, Parkinson’s Disease, Type II Diabetes, and Amyotrophic Lateral Sclerosis. Chem. Rev. 2021, 121, 2545–2647. [Google Scholar] [CrossRef]
- Robustelli, P.; Ibanez-de-Opakua, A.; Campbell-Bezat, C.; Giordanetto, F.; Becker, S.; Zweckstetter, M.; Pan, A.C.; Shaw, D.E. Molecular Basis of Small-Molecule Binding to α-Synuclein. J. Am. Chem. Soc. 2022, 144, 2501–2510. [Google Scholar] [CrossRef]
- Saurabh, S.; Nadendla, K.; Purohit, S.S.; Sivakumar, P.M.; Cetinel, S. Fuzzy Drug Targets: Disordered Proteins in the Drug-Discovery Realm. ACS Omega 2023, 8, 9729–9747. [Google Scholar] [CrossRef]
- Dettmer, U.; Newman, A.J.; Luth, E.S.; Bartels, T.; Selkoe, D. In Vivo Cross-Linking Reveals Principally Oligomeric Forms of α-Synuclein and β-Synuclein in Neurons and Non-Neural Cells. J. Biol. Chem. 2013, 288, 6371–6385. [Google Scholar] [CrossRef] [PubMed]
- Abeyawardhane, D.L.; Curry, A.M.; Forney, A.K.; Roberts, J.W.; Lucas, H.R. Biometals as Conformational Modulators of α-Synuclein Photochemical Crosslinking. J. Biol. Inorg. Chem. 2019, 24, 1261–1268. [Google Scholar] [CrossRef] [PubMed]
- Schmid, A.W.; Chiappe, D.; Pignat, V.; Grimminger, V.; Hang, I.; Moniatte, M.; Lashuel, H.A. Dissecting the Mechanisms of Tissue Transglutaminase-Induced Cross-Linking of α-Synuclein. J. Biol. Chem. 2009, 284, 13128–13142. [Google Scholar] [CrossRef]
- Nemes, Z.; Petrovski, G.; Aerts, M.; Sergeant, K.; Devreese, B.; Fésüs, L. Transglutaminase-Mediated Intramolecular Cross-Linking of Membrane-Bound α-Synuclein Promotes Amyloid Formation in Lewy Bodies. J. Biol. Chem. 2009, 284, 27252–27264. [Google Scholar] [CrossRef] [PubMed]
- Sahin, C.; Østerlund, E.C.; Österlund, N.; Costeira-Paulo, J.; Pedersen, J.N.; Christiansen, G.; Nielsen, J.; Grønnemose, A.L.; Amstrup, S.K.; Tiwari, M.K.; et al. Structural Basis for Dityrosine-Mediated Inhibition of α-Synuclein Fibrillization. J. Am. Chem. Soc. 2022, 144, 11949–11954. [Google Scholar] [CrossRef]
- Zöller, J.; Hong, S.; Eisinger, M.L.; Anderson, M.; Radloff, M.; Desch, K.; Gennis, R.; Langer, J.D. Ligand Binding and Conformational Dynamics of the E. Coli Nicotinamide Nucleotide Transhydrogenase Revealed by Hydrogen/Deuterium Exchange Mass Spectrometry. Comput. Struct. Biotechnol. J. 2022, 20, 5430–5439. [Google Scholar] [CrossRef]
- Gaucher, S.P.; Hadi, M.Z.; Young, M.M. Influence of Crosslinker Identity and Position on Gas-Phase Dissociation of Lys-Lys Crosslinked Peptides. J. Am. Soc. Mass Spectrom. 2006, 17, 395–405. [Google Scholar] [CrossRef][Green Version]
- Brodie, N.I.; Makepeace, K.A.T.; Petrotchenko, E.V.; Borchers, C.H. Isotopically-Coded Short-Range Hetero-Bifunctional Photo-Reactive Crosslinkers for Studying Protein Structure. J. Proteom. 2015, 118, 12–20. [Google Scholar] [CrossRef]
- Ulmer, T.S.; Bax, A.; Cole, N.B.; Nussbaum, R.L. Structure and Dynamics of Micelle-Bound Human α-Synuclein. J. Biol. Chem. 2005, 280, 9595–9603. [Google Scholar] [CrossRef]
- Kang, L.; Moriarty, G.M.; Woods, L.A.; Ashcroft, A.E.; Radford, S.E.; Baum, J. N-Terminal Acetylation of α-Synuclein Induces Increased Transient Helical Propensity and Decreased Aggregation Rates in the Intrinsically Disordered Monomer. Protein Sci. 2012, 21, 911–917. [Google Scholar] [CrossRef]
- Theillet, F.-X.; Binolfi, A.; Bekei, B.; Martorana, A.; Rose, H.M.; Stuiver, M.; Verzini, S.; Lorenz, D.; van Rossum, M.; Goldfarb, D.; et al. Structural Disorder of Monomeric α-Synuclein Persists in Mammalian Cells. Nature 2016, 530, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Pál-Gábor, H.; Gombos, L.; Micsonai, A.; Kovács, E.; Petrik, E.; Kovács, J.; Gráf, L.; Fidy, J.; Naiki, H.; Goto, Y.; et al. Mechanism of Lysophosphatidic Acid-Induced Amyloid Fibril Formation of β2-Microglobulin in Vitro under Physiological Conditions. Biochemistry 2009, 48, 5689–5699. [Google Scholar] [CrossRef] [PubMed]
- Kuipers, B.J.H.; Gruppen, H. Prediction of Molar Extinction Coefficients of Proteins and Peptides Using UV Absorption of the Constituent Amino Acids at 214 Nm to Enable Quantitative Reverse Phase High-Performance Liquid Chromatography-Mass Spectrometry Analysis. J. Agric. Food Chem. 2007, 55, 5445–5451. [Google Scholar] [CrossRef] [PubMed]
- Anthis, N.J.; Clore, G.M. Sequence-Specific Determination of Protein and Peptide Concentrations by Absorbance at 205 Nm. Protein Sci. 2013, 22, 851–858. [Google Scholar] [CrossRef]
- Perez-Riverol, Y.; Bai, J.; Bandla, C.; García-Seisdedos, D.; Hewapathirana, S.; Kamatchinathan, S.; Kundu, D.J.; Prakash, A.; Frericks-Zipper, A.; Eisenacher, M.; et al. The PRIDE Database Resources in 2022: A Hub for Mass Spectrometry-Based Proteomics Evidences. Nucleic Acids Res. 2022, 50, D543–D552. [Google Scholar] [CrossRef]
- Pronk, S.; Páll, S.; Schulz, R.; Larsson, P.; Bjelkmar, P.; Apostolov, R.; Shirts, M.R.; Smith, J.C.; Kasson, P.M.; van der Spoel, D.; et al. GROMACS 4.5: A High-Throughput and Highly Parallel Open Source Molecular Simulation Toolkit. Bioinformatics 2013, 29, 845–854. [Google Scholar] [CrossRef]
- Aliev, A.E.; Kulke, M.; Khaneja, H.S.; Chudasama, V.; Sheppard, T.D.; Lanigan, R.M. Motional Timescale Predictions by Molecular Dynamics Simulations: Case Study Using Proline and Hydroxyproline Sidechain Dynamics. Proteins 2014, 82, 195–215. [Google Scholar] [CrossRef]
- Jorgensen, W.L.; Chandrasekhar, J.; Madura, J.D.; Impey, R.W.; Klein, M.L. Comparison of Simple Potential Functions for Simulating Liquid Water. J. Chem. Phys. 1983, 79, 926–935. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Murvai, N.; Gellen, G.; Micsonai, A.; Schlosser, G.; Kardos, J. Cross-Linked α-Synuclein as Inhibitor of Amyloid Formation. Int. J. Mol. Sci. 2023, 24, 13403. https://doi.org/10.3390/ijms241713403
Murvai N, Gellen G, Micsonai A, Schlosser G, Kardos J. Cross-Linked α-Synuclein as Inhibitor of Amyloid Formation. International Journal of Molecular Sciences. 2023; 24(17):13403. https://doi.org/10.3390/ijms241713403
Chicago/Turabian StyleMurvai, Nikoletta, Gabriella Gellen, András Micsonai, Gitta Schlosser, and József Kardos. 2023. "Cross-Linked α-Synuclein as Inhibitor of Amyloid Formation" International Journal of Molecular Sciences 24, no. 17: 13403. https://doi.org/10.3390/ijms241713403
APA StyleMurvai, N., Gellen, G., Micsonai, A., Schlosser, G., & Kardos, J. (2023). Cross-Linked α-Synuclein as Inhibitor of Amyloid Formation. International Journal of Molecular Sciences, 24(17), 13403. https://doi.org/10.3390/ijms241713403