Upregulation of Orai Channels Contributes to Aging-Related Vascular Alterations in Rat Coronary Arteries
Abstract
:1. Introduction
2. Results
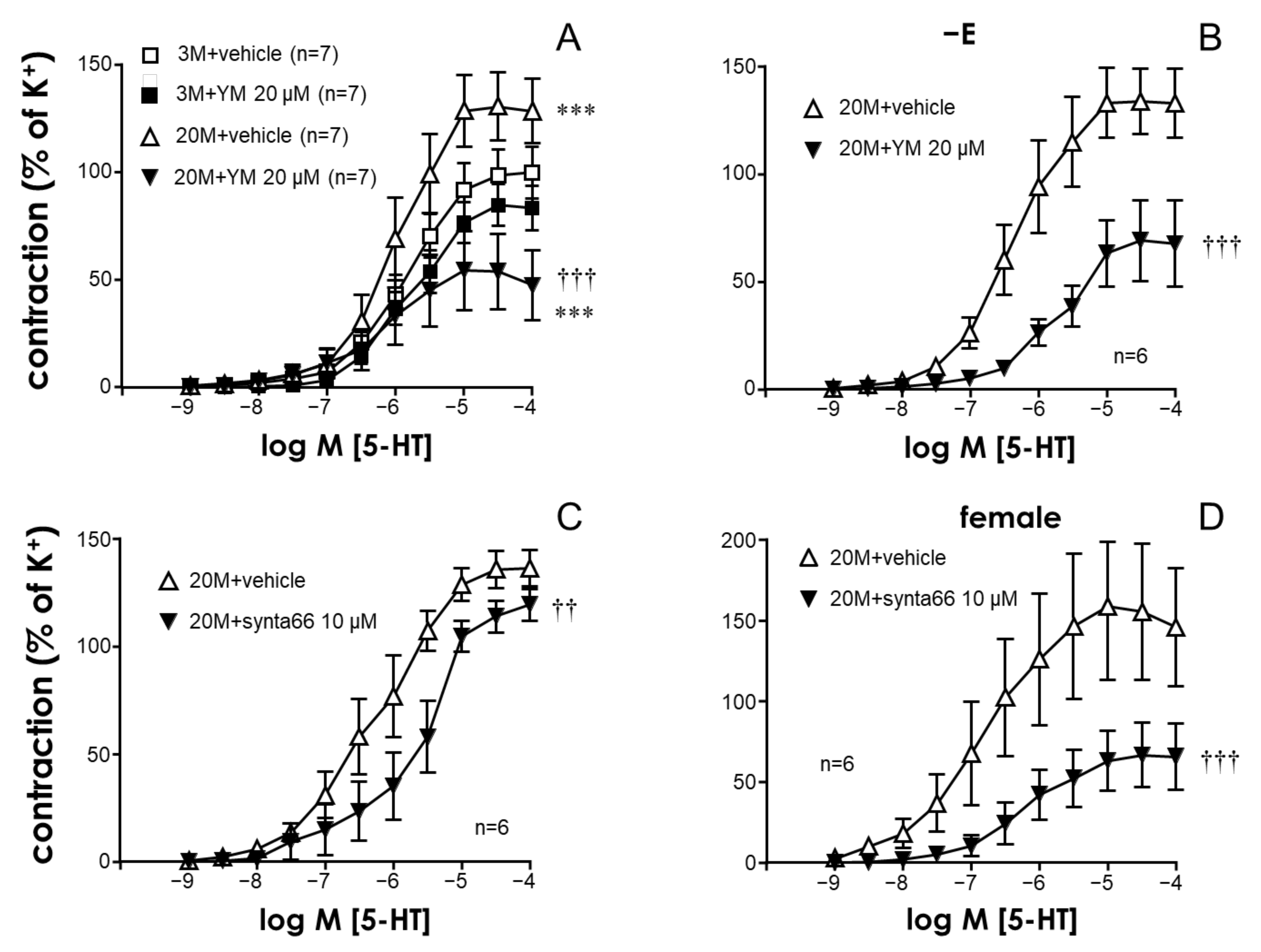
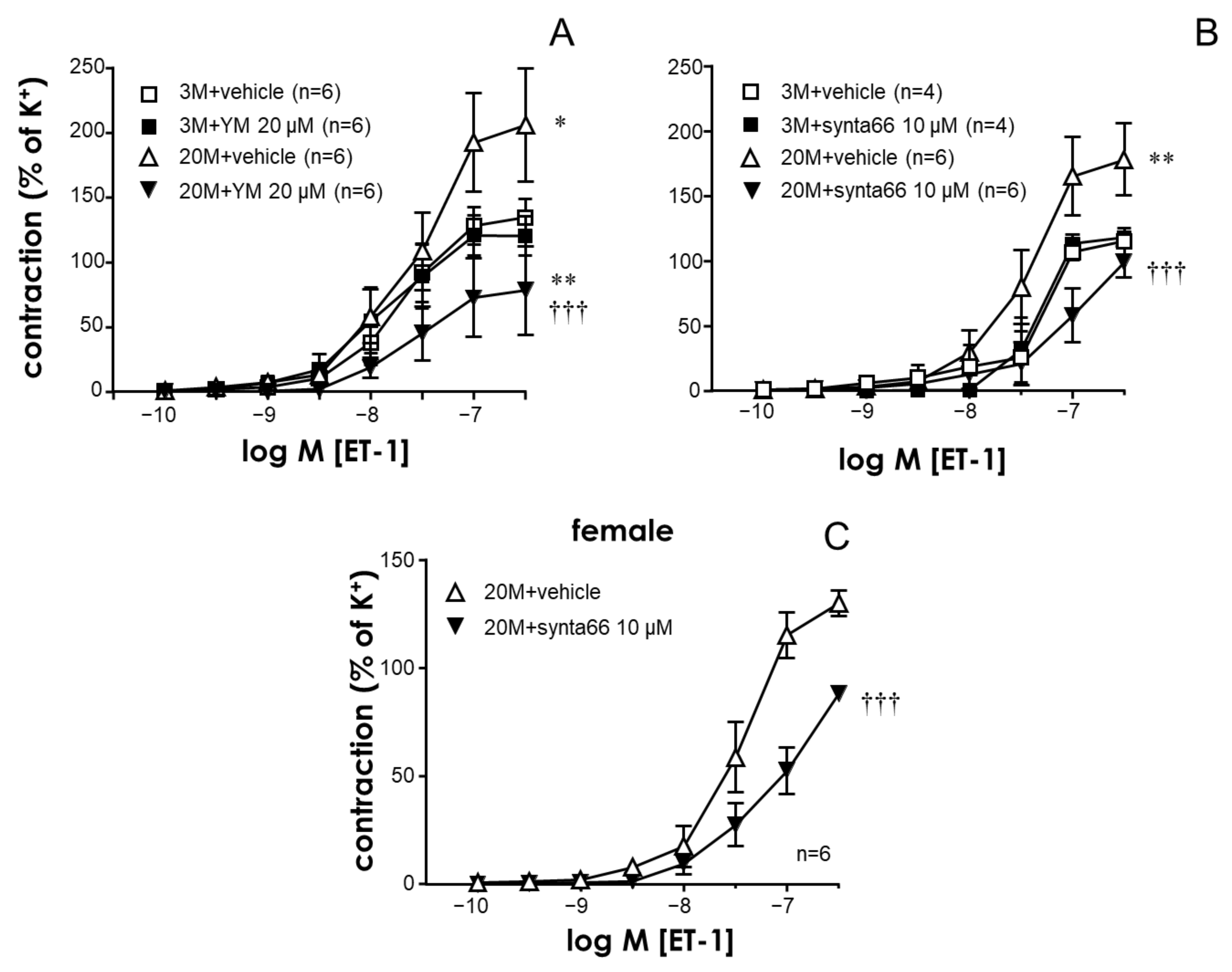
2.1. The STIM/Orai System Contributes to Aging-Induced Hypercontractility in Rat Coronary Arteries
2.2. STIM/Orai Inhibition Effects Are Lost under Smooth Muscle Depolarizing Conditions
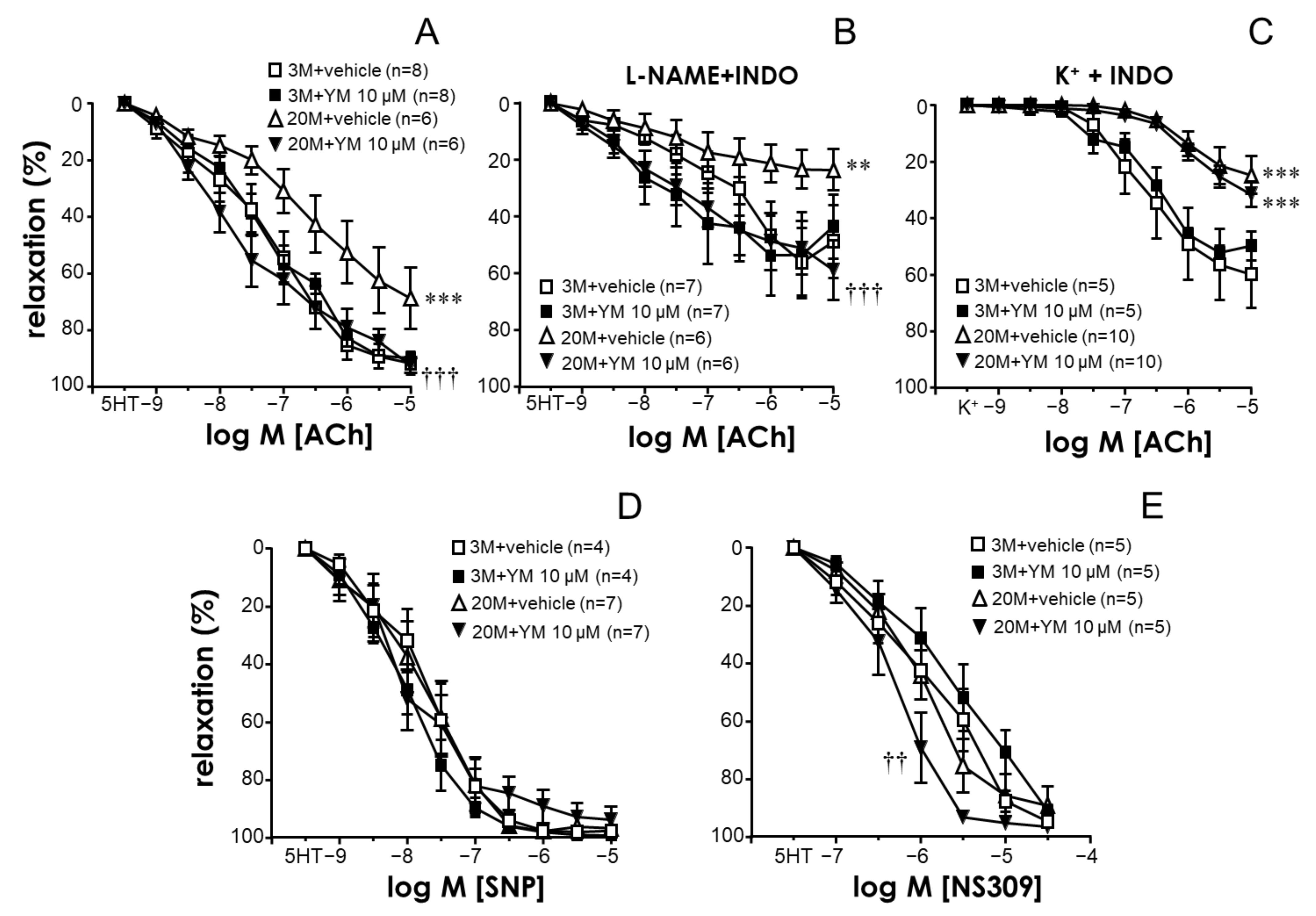
2.3. STIM/Orai Inhibition Improved Endothelium-Dependent Vasodilation in Coronary Arteries from Older Rats
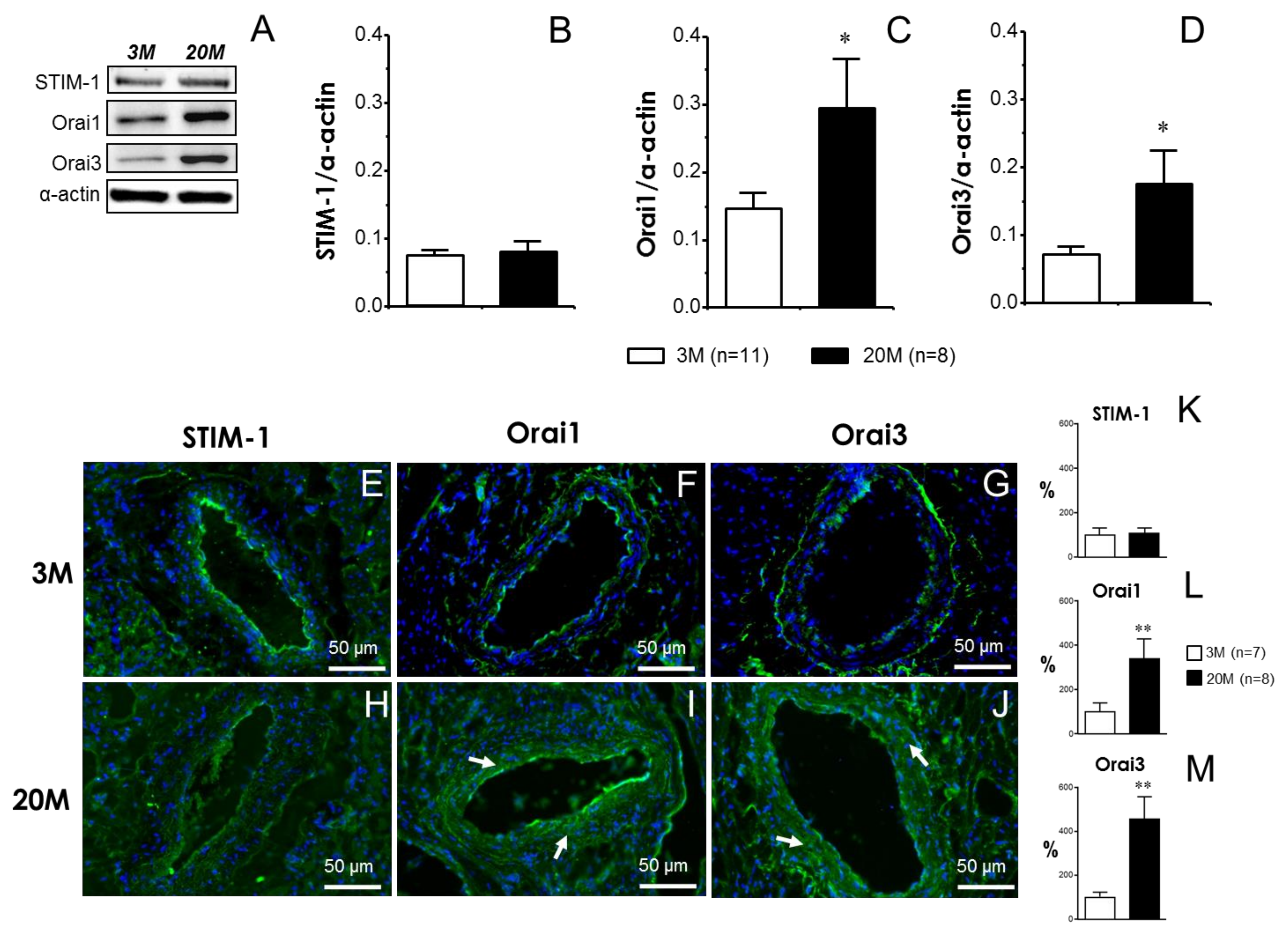
2.4. Functional Effects of STIM/Orai Inhibition Are Associated with the Increased Expression of Orai Channels in Coronary Arteries from Older Rats
3. Discussion
4. Materials and Methods
4.1. Experimental Animals
4.2. Functional Evaluation of Coronary Arteries
4.3. Western Blot Assays
4.4. Immunofluorescence Assays
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Benjamin, E.J.; Muntner, P.; Alonso, A.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Das, S.R.; et al. Heart Disease and Stroke Statistics-2019 Update: A Report From the American Heart Association. Circulation 2019, 139, e56–e528. [Google Scholar] [CrossRef] [PubMed]
- Ungvari, Z.; Tarantini, S.; Sorond, F.; Merkely, B.; Csiszar, A. Mechanisms of Vascular Aging, A Geroscience Perspective: JACC Focus Seminar. J. Am. Coll. Cardiol. 2020, 75, 931–941. [Google Scholar] [CrossRef] [PubMed]
- Amarasekera, A.T.; Chang, D.; Schwarz, P.; Tan, T.C. Vascular endothelial dysfunction may be an early predictor of physical frailty and sarcopenia: A meta-analysis of available data from observational studies. Exp. Gerontol. 2021, 148, 111260. [Google Scholar] [CrossRef] [PubMed]
- Jeon, Y.K.; Shin, M.J.; Saini, S.K.; Custodero, C.; Aggarwal, M.; Anton, S.D.; Leeuwenburgh, C.; Mankowski, R.T. Vascular dysfunction as a potential culprit of sarcopenia. Exp. Gerontol. 2021, 145, 111220. [Google Scholar] [CrossRef]
- Rodríguez-Mañas, L.; El-Assar, M.; Vallejo, S.; López-Dóriga, P.; Solís, J.; Petidier, R.; Montes, M.; Nevado, J.; Castro, M.; Gómez-Guerrero, C.; et al. Endothelial dysfunction in aged humans is related with oxidative stress and vascular inflammation. Aging Cell 2009, 8, 226–238. [Google Scholar] [CrossRef]
- Ma, L.; Wang, K.; Shang, J.; Cao, C.; Zhen, P.; Liu, X.; Wang, W.; Zhang, H.; Du, Y.; Liu, H. Anti-peroxynitrite treatment ameliorated vasorelaxation of resistance arteries in aging rats: Involvement with NO-sGC-cGKs pathway. PLoS ONE 2014, 9, e104788. [Google Scholar] [CrossRef]
- El Assar, M.; Fernández, A.; Sánchez-Ferrer, A.; Angulo, J.; Rodríguez-Mañas, L. Multivessel analysis of progressive vascular aging in the rat: Asynchronous vulnerability among vascular territories. Mech. Ageing Dev. 2018, 173, 39–49. [Google Scholar] [CrossRef]
- El Assar, M.; Angulo, J.; García-Rojo, E.; Sevilleja-Ortiz, A.; García-Gómez, B.; Fernández, A.; Sánchez-Ferrer, A.; La Fuente, J.M.; Romero-Otero, J.; Rodríguez-Mañas, L. Early manifestation of aging-related vascular dysfunction in human penile vasculature-A potential explanation for the role of erectile dysfunction as a harbinger of systemic vascular disease. Geroscience 2022, 44, 485–501. [Google Scholar] [CrossRef]
- Heusch, G. Coronary blood flow in heart failure: Cause, consequence and bystander. Basic. Res. Cardiol. 2022, 117, 1. [Google Scholar] [CrossRef]
- Zhu, Y.; Qu, J.; He, L.; Zhang, F.; Zhou, Z.; Yang, S.; Zhou, Y. Calcium in Vascular Smooth Muscle Cell Elasticity and Adhesion: Novel Insights Into the Mechanism of Action. Front. Physiol. 2019, 10, 852. [Google Scholar] [CrossRef]
- Collins, H.E.; Zhang, D.; Chatham, J.C. STIM and Orai Mediated Regulation of Calcium Signaling in Age-Related Diseases. Front. Aging 2022, 3, 876785. [Google Scholar] [CrossRef] [PubMed]
- Mancarella, S.; Potireddy, S.; Wang, Y.; Gao, H.; Gandhirajan, R.K.; Autieri, M.; Scalia, R.; Cheng, Z.; Wang, H.; Madesh, M.; et al. Targeted STIM deletion impairs calcium homeostasis, NFAT activation, and growth of smooth muscle. FASEB J. 2013, 27, 893–906. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, Y.; Zhou, Y.; Hendron, E.; Mancarella, S.; Andrake, M.D.; Rothberg, B.S.; Soboloff, J.; Gill, D.L. Distinct Orai-coupling domains in STIM1 and STIM2 define the Orai-activating site. Nat. Commun. 2014, 5, 3183. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, W.; Ruhle, B.; Motiani, R.K.; Schindl, R.; Muik, M.; Spinelli, A.M.; Bisaillon, J.M.; Shinde, A.V.; Fahrner, M.; et al. Store-independent Orai1/3 channels activated by intracrine leukotriene C4: Role in neointimal hyperplasia. Circ. Res. 2013, 112, 1013–1025. [Google Scholar] [CrossRef]
- Sevilleja-Ortiz, A.; El Assar, M.; García-Gómez, B.; La Fuente, J.M.; Alonso-Isa, M.; Romero-Otero, J.; Martínez-Salamanca, J.I.; Fernández, A.; Rodríguez-Mañas, L.; Angulo, J. STIM/Orai Inhibition as a Strategy for Alleviating Diabetic Erectile Dysfunction Through Modulation of Rat and Human Penile Tissue Contractility and in vivo Potentiation of Erectile Responses. J. Sex. Med. 2022, 19, 1733–1749. [Google Scholar] [CrossRef]
- Sevilleja-Ortiz, A.; El Assar, M.; García-Rojo, E.; García-Gómez, B.; Fernández, A.; Sánchez-Ferrer, A.; La Fuente, J.M.; Romero-Otero, J.; Rodríguez-Mañas, L.; Angulo, J. Ageing-induced hypercontractility is related to functional enhancement of STIM/Orai and upregulation of Orai 3 in rat and human penile tissue. Mech. Ageing Dev. 2021, 200, 111590. [Google Scholar] [CrossRef]
- El Assar, M.; García-Rojo, E.; Sevilleja-Ortiz, A.; Sánchez-Ferrer, A.; Fernández, A.; García-Gómez, B.; Romero-Otero, J.; Rodríguez-Mañas, L.; Angulo, J. Functional Role of STIM-1 and Orai1 in Human Microvascular Aging. Cells 2022, 11, 3675. [Google Scholar] [CrossRef] [PubMed]
- Angulo, J.; El Assar, M.; Sevilleja-Ortiz, A.; Fernández, A.; Sánchez-Ferrer, A.; Romero-Otero, J.; Martínez-Salamanca, J.I.; La Fuente, J.M.; Rodríguez-Mañas, L. Short-term pharmacological activation of Nrf2 ameliorates vascular dysfunction in aged rats and in pathological human vasculature. A potential target for therapeutic intervention. Redox Biol. 2019, 26, 101271. [Google Scholar] [CrossRef]
- Tschudi, M.R.; Lüscher, T.F. Age and hypertension differently affect coronary contractions to endothelin-1, serotonin, and angiotensins. Circulation 1995, 91, 2415–2422. [Google Scholar] [CrossRef]
- Simonsen, A.H.; Sheykhzade, M.; Berg Nyborg, N.C. Age- and endothelium-dependent changes in coronary artery reactivity to serotonin and calcium. Vasc. Pharmacol. 2004, 41, 43–49. [Google Scholar] [CrossRef]
- Ishikawa, J.; Ohga, K.; Yoshino, T.; Takezawa, R.; Ichikawa, A.; Kubota, H.; Yamada, T. A pyrazole derivative, YM-58483, potently inhibits store-operated sustained Ca2+ influx and IL-2 production in T lymphocytes. J. Immunol. 2003, 170, 4441–4449. [Google Scholar] [CrossRef]
- Gandhirajan, R.K.; Meng, S.; Chandramoorthy, H.C.; Mallilankaraman, K.; Mancarella, S.; Gao, H.; Razmpour, R.; Yang, X.-F.; Houser, S.R.; Chen, J.; et al. Blockade of NOX2 and STIM1 signaling limits lipopolysaccharide-induced vascular inflammation. J. Clin. Investig. 2013, 123, 887–902. [Google Scholar] [CrossRef]
- Bomfim, G.H.S.; Mendez-Lopez, I.; Arranz-Tagarro, J.A.; Carbonel, A.A.F.; Roman-Campos, D.; Padín, J.F.; Garcia, A.G.; Jurkiewicz, A.; Jurkiewicz, N.H. Functional Upregulation of STIM-1/Orai-1-Mediated Store-Operated Ca2+ Contributing to the Hypertension Development Elicited by Chronic EtOH Consumption. Curr. Vasc. Pharmacol. 2017, 15, 265–281. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Xin, P.; Yoast, R.E.; Emrich, S.M.; Johnson, M.T.; Pathak, T.; Benson, J.C.; Azimi, I.; Gill, D.L.; Monteith, G.R.; et al. Distinct pharmacological profiles of ORAI1, ORAI2, and ORAI3 channels. Cell Calcium 2020, 91, 102281. [Google Scholar] [CrossRef] [PubMed]
- Shawer, H.; Norman, K.; Cheng, C.W.; Foster, R.; Beech, D.J.; Bailey, M.A. ORAI1 Ca2+ Channel as a Therapeutic Target in Pathological Vascular Remodelling. Front. Cell Dev. Biol. 2021, 9, 653812. [Google Scholar] [CrossRef] [PubMed]
- Waldherr, L.; Tiffner, A.; Mishra, D.; Sallinger, M.; Schober, R.; Frischauf, I.; Schmidt, T.; Handl, V.; Sagmeister, P.; Köckinger, M.; et al. Blockage of Store-Operated Ca2+ Influx by Synta66 is Mediated by Direct Inhibition of the Ca2+ Selective Orai1 Pore. Cancers 2020, 12, 2876. [Google Scholar] [CrossRef]
- Weir, E.K.; Cabrera, J.A.; Mahapatra, S.; Peterson, D.A.; Hong, Z. The role of ion channels in hypoxic pulmonary vasoconstriction. Adv. Exp. Med. Biol. 2010, 661, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Csiszar, A.; Ungvari, Z.; Edwards, J.G.; Kaminski, P.; Wolin, M.S.; Koller, A.; Kaley, G. Aging-induced phenotypic changes and oxidative stress impair coronary arteriolar function. Circ. Res. 2002, 90, 1159–1166. [Google Scholar] [CrossRef]
- Hotta, K.; Chen, B.; Behnke, B.J.; Ghosh, P.; Stabley, J.N.; Bramy, J.A.; Sepulveda, J.L.; Delp, M.D.; Muller-Delp, J.M. Exercise training reverses age-induced diastolic dysfunction and restores coronary microvascular function. J. Physiol. 2017, 595, 3703–3719. [Google Scholar] [CrossRef]
- Christophersen, P.; Wulff, H. Pharmacological gating modulation of small- and intermediate-conductance Ca(2+)-activated K(+) channels (KCa2.x and KCa3.1). Channels 2015, 9, 336–343. [Google Scholar] [CrossRef]
- Edwards, G.; Félétou, M.; Weston, A.H. Endothelium-derived hyperpolarising factors and associated pathways: A synopsis. Pflugers Arch. 2010, 459, 863–879. [Google Scholar] [CrossRef] [PubMed]
- Garland, C.J.; Dora, K.A. EDH: Endothelium-dependent hyperpolarization and microvascular signalling. Acta Physiol. 2017, 219, 152–161. [Google Scholar] [CrossRef] [PubMed]
- Guéguinou, M.; ChantÔme, A.; Fromont, G.; Bougnoux, P.; Vandier, C.; Potier-Cartereau, M. KCa and Ca2+ Channels: The Complex Thought. Biochim. Biophys. Acta 2014, 1843, 2322–2333. [Google Scholar] [CrossRef] [PubMed]
- Climent, B.; Moreno, L.; Martínez, P.; Contreras, C.; Sánchez, A.; Pérez-Vizcaíno, F.; García-Sacristán, A.; Rivera, L.; Prieto, D. Upregulation of SK3 and IK1 channels contributes to the enhanced endothelial calcium signaling and the preserved coronary relaxation in obese Zucker rats. PLoS ONE 2014, 9, e109432. [Google Scholar] [CrossRef]
- Climent, B.; Sánchez, A.; Moreno, L.; Pérez-Vizcaíno, F.; García-Sacristán, A.; Rivera, L.; Prieto, D. Underlying mechanisms preserving coronary basal tone and NO-mediated relaxation in obesity: Involvement of β1 subunit-mediated upregulation of BKCa channels. Atherosclerosis 2017, 263, 227–236. [Google Scholar] [CrossRef]
- Kassan, M.; Zhang, W.; Aissa, K.A.; Stolwijk, J.; Trebak, M.; Matrougui, K. Differential role for stromal interacting molecule 1 in the regulation of vascular function. Pflugers Arch. 2015, 467, 1195–1202. [Google Scholar] [CrossRef]
- Premer, C.; Kanelidis, A.J.; Hare, J.M.; Schulman, I.H. Rethinking Endothelial Dysfunction as a Crucial Target in Fighting Heart Failure. Mayo Clin. Proc. Innov. Qual. Outcomes 2019, 3, 1–13. [Google Scholar] [CrossRef]
- Alem, M.M. Endothelial Dysfunction in Chronic Heart Failure: Assessment, Findings, Significance, and Potential Therapeutic Targets. Int. J. Mol. Sci. 2019, 20, 3198. [Google Scholar] [CrossRef]
- Oneglia, A.; Nelson, M.D.; Merz, C.N.B. Sex Differences in Cardiovascular Aging and Heart Failure. Curr. Heart Fail. Rep. 2020, 17, 409–423. [Google Scholar] [CrossRef]
- Shah, S.J.; Lam, C.S.P.; Svedlund, S.; Saraste, A.; Hage, C.; Tan, R.-S.; Beussink-Nelson, L.; Faxén, U.L.; Fermer, M.L.; A Broberg, M.; et al. Prevalence and correlates of coronary microvascular dysfunction in heart failure with preserved ejection fraction: PROMIS-HFpEF. Eur. Heart J. 2018, 39, 3439–3450. [Google Scholar] [CrossRef]
- Bairey Merz, C.N.; Pepine, C.J.; Walsh, M.N.; Fleg, J.L. Ischemia and No Obstructive Coronary Artery Disease (INOCA): Developing Evidence-Based Therapies and Research Agenda for the Next Decade. Circulation 2017, 135, 1075–1092. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Bruns, A.-F.; Hou, B.; Rode, B.; Webster, P.J.; Bailey, M.A.; Appleby, H.L.; Moss, N.K.; Ritchie, J.E.; Yuldasheva, N.Y.; et al. Orai3 Surface Accumulation and Calcium Entry Evoked by Vascular Endothelial Growth Factor. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 1987–1994. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Angulo, J.; Fernández, A.; Sevilleja-Ortiz, A.; Sánchez-Ferrer, A.; Rodríguez-Mañas, L.; El Assar, M. Upregulation of Orai Channels Contributes to Aging-Related Vascular Alterations in Rat Coronary Arteries. Int. J. Mol. Sci. 2023, 24, 13402. https://doi.org/10.3390/ijms241713402
Angulo J, Fernández A, Sevilleja-Ortiz A, Sánchez-Ferrer A, Rodríguez-Mañas L, El Assar M. Upregulation of Orai Channels Contributes to Aging-Related Vascular Alterations in Rat Coronary Arteries. International Journal of Molecular Sciences. 2023; 24(17):13402. https://doi.org/10.3390/ijms241713402
Chicago/Turabian StyleAngulo, Javier, Argentina Fernández, Alejandro Sevilleja-Ortiz, Alberto Sánchez-Ferrer, Leocadio Rodríguez-Mañas, and Mariam El Assar. 2023. "Upregulation of Orai Channels Contributes to Aging-Related Vascular Alterations in Rat Coronary Arteries" International Journal of Molecular Sciences 24, no. 17: 13402. https://doi.org/10.3390/ijms241713402
APA StyleAngulo, J., Fernández, A., Sevilleja-Ortiz, A., Sánchez-Ferrer, A., Rodríguez-Mañas, L., & El Assar, M. (2023). Upregulation of Orai Channels Contributes to Aging-Related Vascular Alterations in Rat Coronary Arteries. International Journal of Molecular Sciences, 24(17), 13402. https://doi.org/10.3390/ijms241713402




