Alteration of Blood Immune Biomarkers in MCI Patients with Different APOE Genotypes after Cognitive Training: A 1 Year Follow-Up Cohort Study
Abstract
1. Introduction
2. Results
2.1. General Characteristics of the Patient’s Cohort and the Assessment of Their Mental Status before and after the Cognitive Training
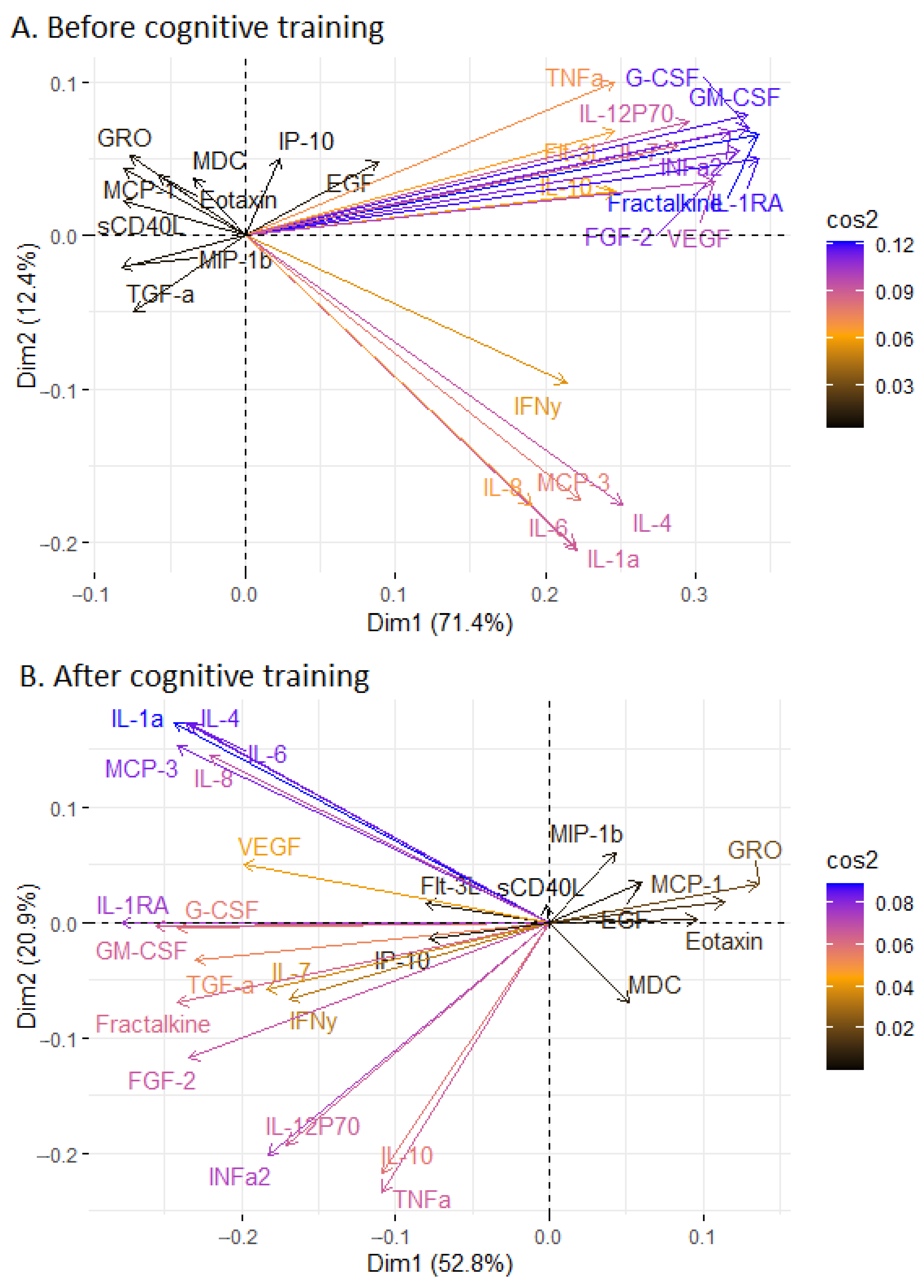
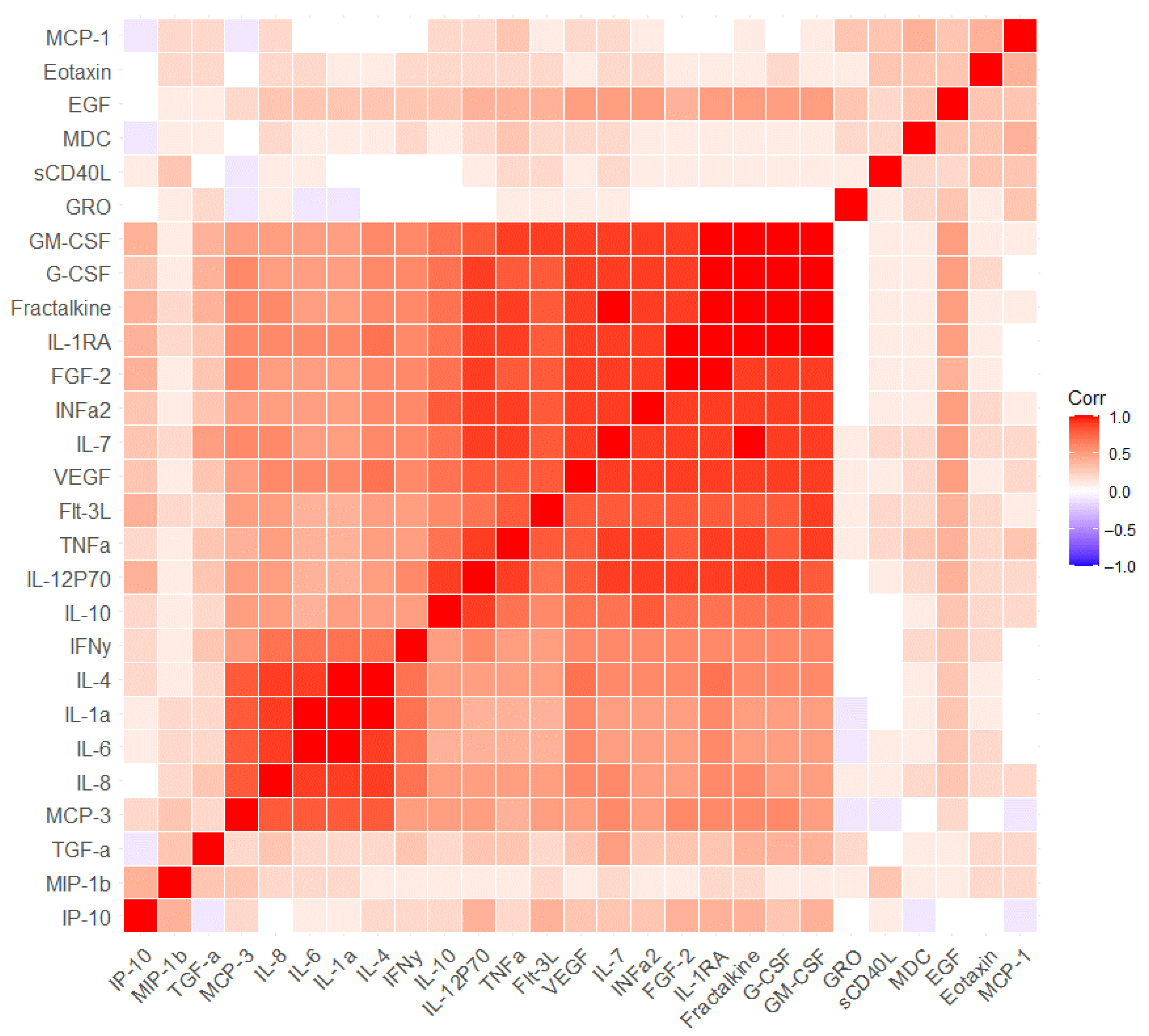
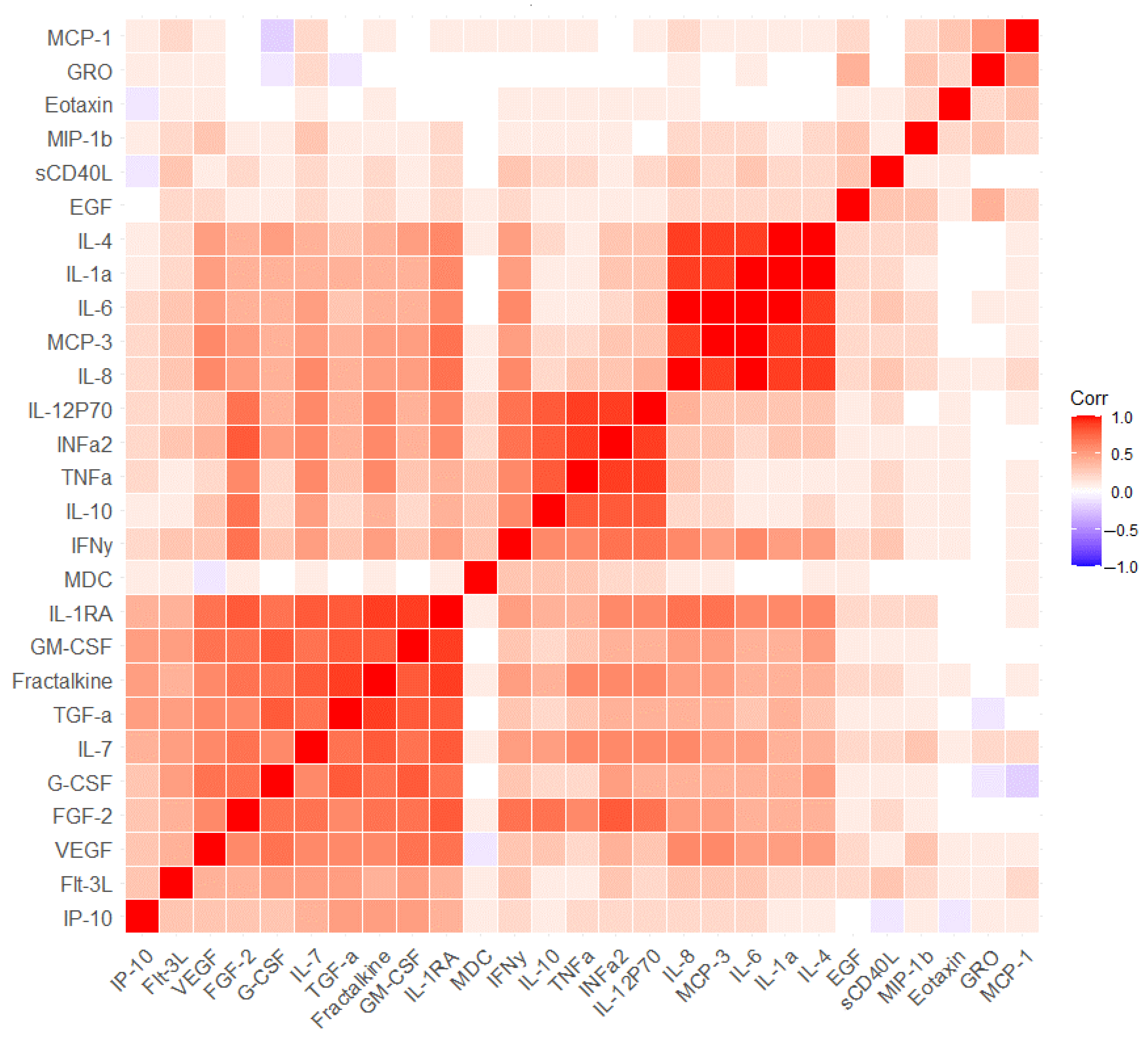
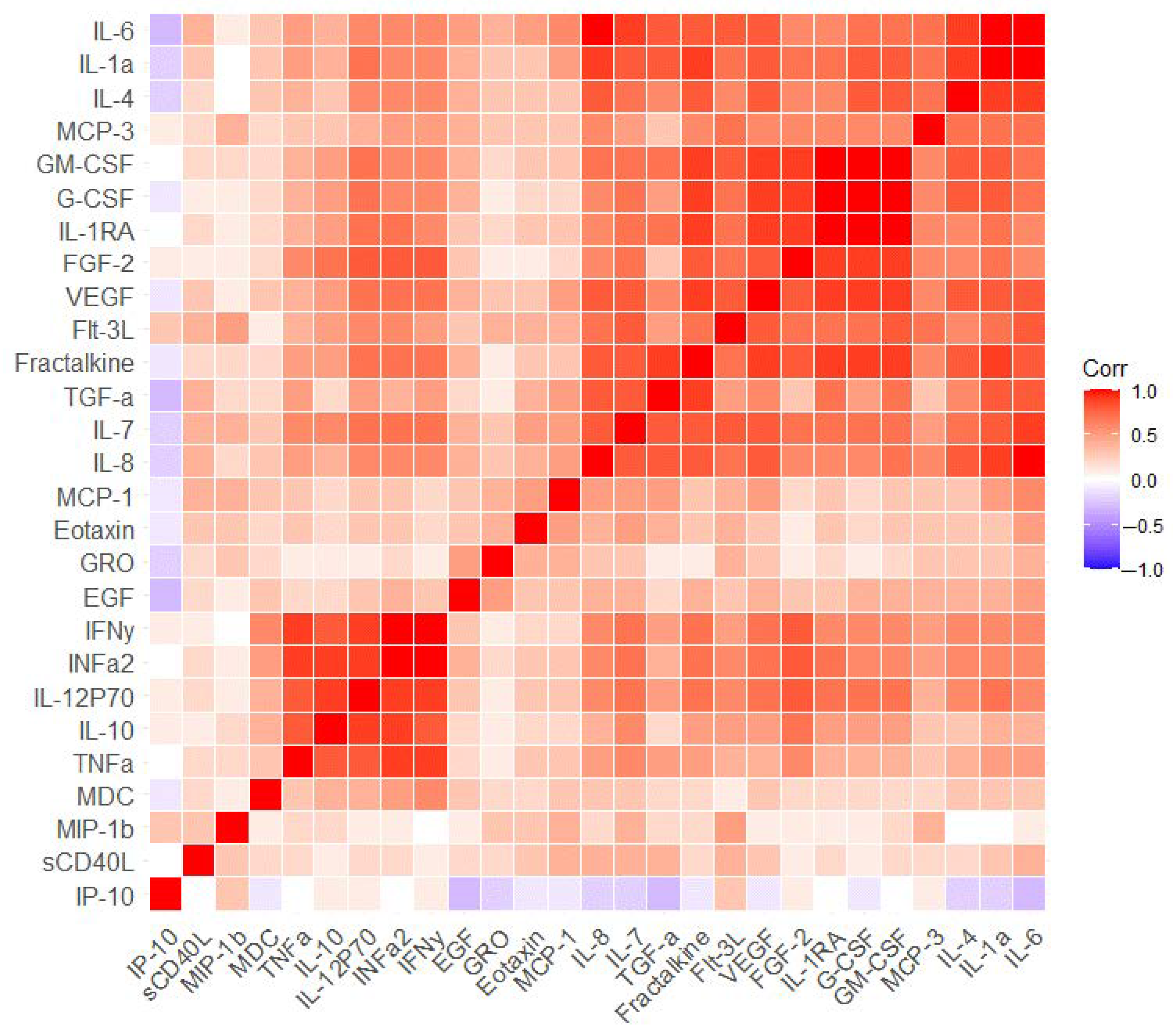
2.2. The Measurement of Serum Markers before and after the Cognitive Training
2.3. The Effect of APOE on Cognitive Scale Values and Associations with Serum Biomarkers
3. Discussion
4. Materials and Methods
4.1. Patients
4.2. Neurocognitive Training
- -
- CT-1 Correction of thinking and imagination (programming, regulation and control of complex activities);
- -
- CT-2 Correction of amnestic activity and memory disorders;
- -
- CT-3 Correction of attention and perception;
- -
- CT-4 Art therapy for patients with mild cognitive decline;
- -
- Cognitive warm-up—correction of praxis and gnosis.
4.3. Cognitive Impairment Scales
4.4. Serum Sample Collection
4.5. APOE Genotyping
4.6. Multiplex Assay for Cytokine and Chemokine Measurement
4.7. Statistical Processing
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AD | Alzheimer’s disease |
| Apo A1 | Apolipoprotein AI |
| APOE | Apolipoprotein |
| CCR3 | C-C chemokine receptor type 3 |
| CD14 | Cluster of differentiation 14 |
| CDT | Clock drawing test |
| CRP | C-reactive protein |
| CSF | Cerebrospinal fluid |
| EGF | Epidermal growth factor |
| FDF-2 | Fibroblast growth factor |
| FDR | False discovery rate |
| Flt-3L | Fms-related tyrosine kinase 3 ligand |
| G-CSF | Granulocyte colony-stimulating factor |
| GM-CSF | Granulocyte-macrophage colony-stimulating factor |
| GRO | Growth-regulated oncogene α |
| HADS | Hospital Anxiety and Depression Scale |
| HDL | High-density lipoprotein |
| IL-10 | Interleukin-10 |
| IL-12P70 | Interleukin-12P70 |
| IL-1a | Interleukin-1a |
| IL-1Ra | Interleukin-1Ra |
| IL-4 | Interleukin-4 |
| IL-6 | Interleukin-6 |
| IL-7 | Interleukin-7 |
| IL-8 | Interleukin-8 |
| INFa2 | Interferon α2 |
| INFγ | Interferon γ |
| Interleukin-1β | Interleukin-1β |
| IP-10 | Interferon γ-induced protein 10 kDa |
| LDL | Low-density lipoprotein |
| MCI | Mild cognitive impairment |
| MCP-1 | Monocyte Chemoattractant Protein 1 |
| MCP-3 | Monocyte chemotactic protein-3 |
| MDC | Macrophage-derived chemokine |
| MHIS | Modified Hachinski Ishemic Score |
| MIP-1β | Macrophage Inflammatory Protein 1β |
| MMSE | Mini-Mental State Examination |
| MoCA | Montreal Cognitive Assessment |
| MPO | Myeloperoxidase |
| NGAL | Neutrophil gelatinase-associated lipocalin |
| PCA | Principal component analysis |
| PET | Positron emission tomography |
| sCD40L | Soluble CD40 ligand |
| TGFa | Transforming Growth Factor Alpha |
| TNFα | Tumor necrosis factor α |
| VEGF | Vascular endothelial growth factor |
References
- Fernández-Calle, R.; Konings, S.C.; Frontiñán-Rubio, J.; García-Revilla, J.; Camprubí-Ferrer, L.; Svensson, M.; Martinson, I.; Boza-Serrano, A.; Venero, J.L.; Nielsen, H.M.; et al. APOE in the bullseye of neurodegenerative diseases: Impact of the APOE genotype in Alzheimer’s disease pathology and brain diseases. Mol. Neurodegener. 2022, 17, 62. [Google Scholar] [CrossRef] [PubMed]
- Livingston, G.; Huntley, J.; Sommerlad, A.; Ames, D.; Ballard, C.; Banerjee, S.; Brayne, C.; Burns, A.; Cohen-Mansfield, J.; Cooper, C.; et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet 2020, 396, 413–446. [Google Scholar] [CrossRef] [PubMed]
- Knopman, D.S.; Amieva, H.; Petersen, R.C.; Chételat, G.; Holtzman, D.M.; Hyman, B.T.; Nixon, R.A.; Jones, D.T. Alzheimer disease. Nat. Rev. Dis. Primers 2021, 7, 33. [Google Scholar] [CrossRef] [PubMed]
- Gale, S.A.; Acar, D.; Daffner, K.R. Dementia. Am. J. Med. 2018, 131, 1161–1169. [Google Scholar] [CrossRef] [PubMed]
- Pyenson, B.; Sawhney, T.G.; Steffens, C.; Rotter, D.; Peschin, S.; Scott, J.; Jenkins, E. The Real-World Medicare Costs of Alzheimer Disease: Considerations for Policy and Care. J. Manag. Care Spec. Pharm. 2019, 25, 800–809. [Google Scholar] [CrossRef]
- Luo, J.; Agboola, F.; Grant, E.; Morris, J.C.; Masters, C.L.; Albert, M.S.; Johnson, S.C.; McDade, E.M.; Fagan, A.M.; Benzinger, T.L.S.; et al. Accelerated longitudinal changes and ordering of Alzheimer disease biomarkers across the adult lifespan. Brain 2022, 145, 4459–4473. [Google Scholar] [CrossRef]
- Li, H.T.; Yuan, S.X.; Wu, J.S.; Gu, Y.; Sun, X. Predicting Conversion from MCI to AD Combining Multi-Modality Data and Based on Molecular Subtype. Brain Sci. 2021, 11, 674. [Google Scholar] [CrossRef]
- Arevalo-Rodriguez, I.; Smailagic, N.; Roqué-Figuls, M.; Ciapponi, A.; Sanchez-Perez, E.; Giannakou, A.; Pedraza, O.L.; Bonfill Cosp, X.; Cullum, S. Mini-Mental State Examination (MMSE) for the early detection of dementia in people with mild cognitive impairment (MCI). Cochrane Database Syst. Rev. 2021, 7, CD010783. [Google Scholar] [CrossRef] [PubMed]
- Misiak, B.; Leszek, J.; Kiejna, A. Metabolic syndrome, mild cognitive impairment and Alzheimer’s disease—The emerging role of systemic low-grade inflammation and adiposity. Brain Res. Bull. 2012, 89, 144–149. [Google Scholar] [CrossRef]
- Tien, Y.T.; Lee, W.J.; Liao, Y.C.; Wang, W.F.; Jhang, K.M.; Wang, S.J.; Fuh, J.L. Plasma Transthyretin as a Predictor of Amnestic Mild Cognitive Impairment Conversion to Dementia. Sci. Rep. 2019, 9, 18691. [Google Scholar] [CrossRef] [PubMed]
- Anderson, N.D. State of the science on mild cognitive impairment (MCI). CNS Spectr. 2019, 24, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Kormas, C.; Zalonis, I.; Evdokimidis, I.; Kapaki, E.; Potagas, C. The severity of executive dysfunction among different PD-MCI subtypes. Appl. Neuropsychol. Adult 2022, 29, 546–550. [Google Scholar] [CrossRef] [PubMed]
- Zubrikhina, M.O.; Abramova, O.V.; Yarkin, V.E.; Ushakov, V.L.; Ochneva, A.G.; Bernstein, A.V.; Burnaev, E.V.; Andreyuk, D.S.; Savilov, V.B.; Kurmishev, M.V.; et al. Machine learning approaches to mild cognitive impairment detection based on structural MRI data and morphometric features. Cogn. Syst. Res. 2023, 78, 87–95. [Google Scholar] [CrossRef]
- Campbell, N.L.; Unverzagt, F.; LaMantia, M.A.; Khan, B.A.; Boustani, M.A. Risk factors for the progression of mild cognitive impairment to dementia. Clin. Geriatr. Med. 2013, 29, 873–893. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Lutz, M.; Luo, S.; Alzheimer’s Disease Neuroimaging Initiative. Association Between Polygenic Risk Score and the Progression from Mild Cognitive Impairment to Alzheimer’s Disease. J. Alzheimer’s Dis. 2021, 84, 1323–1335. [Google Scholar] [CrossRef]
- Meghdadi, A.H.; Stevanović Karić, M.; McConnell, M.; Rupp, G.; Richard, C.; Hamilton, J.; Salat, D.; Berka, C. Resting state EEG biomarkers of cognitive decline associated with Alzheimer’s disease and mild cognitive impairment. PLoS ONE 2021, 16, e0244180. [Google Scholar] [CrossRef] [PubMed]
- Temmerman, J.; Engelborghs, S.; Bjerke, M.; D’haeseleer, M. Cerebrospinal fluid inflammatory biomarkers for disease progression in Alzheimer’s disease and multiple sclerosis: A systematic review. Front. Immunol. 2023, 14, 1162340. [Google Scholar] [CrossRef] [PubMed]
- Alzheimer’s Association. Changing the Trajectory of Alzheimer’s Disease: How a Treatment by 2025 Saves Lives and Dollars. Available online: www.alz.org (accessed on 15 December 2017).
- Agarwal, M.; Khan, S. Plasma Lipids as Biomarkers for Alzheimer’s Disease: A Systematic Review. Cureus 2020, 12, e12008. [Google Scholar] [CrossRef]
- Hughes, T.M.; Rosano, C.; Evans, R.W.; Kuller, L.H. Brain cholesterol metabolism, oxysterols, and dementia. J. Alzheimer’s Dis. 2013, 33, 891–911. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Bui, H.; Vemuri, P.; Graff-Radford, J.; Jack, C.R., Jr.; Petersen, R.C.; Mielke, M.M. Lipidomic Network of Mild Cognitive Impairment from the Mayo Clinic Study of Aging. J. Alzheimer’s Dis. 2021, 81, 533–543. [Google Scholar] [CrossRef] [PubMed]
- Grimm, M.O.W.; Michaelson, D.M.; Hartmann, T. Omega-3 fatty acids, lipids, and apoE lipidation in Alzheimer’s disease: A rationale for multi-nutrient dementia prevention. J. Lipid Res. 2017, 58, 2083–2101. [Google Scholar] [CrossRef]
- Jeong, W.; Lee, H.; Cho, S.; Seo, J. ApoE4-Induced Cholesterol Dysregulation and Its Brain Cell Type-Specific Implications in the Pathogenesis of Alzheimer’s Disease. Mol. Cells 2019, 42, 739–746. [Google Scholar] [CrossRef]
- Rao, R.V.; Subramaniam, K.G.; Gregory, J.; Bredesen, A.L.; Coward, C.; Okada, S.; Kelly, L.; Bredesen, D.E. Rationale for a Multi-Factorial Approach for the Reversal of Cognitive Decline in Alzheimer’s Disease and MCI: A Review. Int. J. Mol. Sci. 2023, 24, 1659. [Google Scholar] [CrossRef]
- Li, R.; Wang, T.J.; Lyu, P.Y.; Liu, Y.; Chen, W.H.; Fan, M.Y.; Xu, J. Effects of Plasma Lipids and Statins on Cognitive Function. Chin. Med. J. 2018, 131, 471–476. [Google Scholar] [CrossRef]
- Solomon, A.; Kåreholt, I.; Ngandu, T.; Wolozin, B.; Macdonald, S.W.; Winblad, B.; Nissinen, A.; Tuomilehto, J.; Soininen, H.; Kivipelto, M. Serum total cholesterol, statins and cognition in non-demented elderly. Neurobiol. Aging 2009, 30, 1006–1009. [Google Scholar] [CrossRef]
- Panza, F.; Frisardi, V.; Seripa, D.; Imbimbo, B.P.; Sancarlo, D.; D’Onofrio, G.; Addante, F.; Paris, F.; Pilotto, A.; Solfrizzi, V. Metabolic syndrome, mild cognitive impairment, and dementia. Curr. Alzheimer Res. 2011, 8, 492–509. [Google Scholar] [CrossRef]
- Carlsson, C.M. Type 2 diabetes mellitus, dyslipidemia, and Alzheimer’s disease. J. Alzheimer’s Dis. 2010, 20, 711–722. [Google Scholar] [CrossRef]
- Duro, M.V.; Ebright, B.; Yassine, H.N. Lipids and brain inflammation in APOE4-associated dementia. Curr. Opin. Lipidol. 2022, 33, 16–24. [Google Scholar] [CrossRef]
- Fang, Y.; Doyle, M.F.; Chen, J.; Alosco, M.L.; Mez, J.; Satizabal, C.L.; Qiu, W.Q.; Murabito, J.M.; Lunetta, K.L. Association between inflammatory biomarkers and cognitive aging. PLoS ONE 2022, 17, e0274350. [Google Scholar] [CrossRef]
- Dionisio-Santos, D.A.; Olschowka, J.A.; O’Banion, M.K. Exploiting microglial and peripheral immune cell crosstalk to treat Alzheimer’s disease. J. Neuroinflamm. 2019, 16, 74. [Google Scholar] [CrossRef]
- Yasuno, F.; Kazui, H.; Kajimoto, K.; Ihara, M.; Morita, N.; Taguchi, A.; Yamamoto, A.; Matsuoka, K.; Takahashi, M.; Nakagawara, J.; et al. Mutual effect of cerebral amyloid β and peripheral lymphocytes in cognitively normal older individuals. Int. J. Geriatr. Psychiatry 2017, 32, e93–e99. [Google Scholar] [CrossRef] [PubMed]
- Bradburn, S.; Sarginson, J.; Murgatroyd, C.A. Association of Peripheral Interleukin-6 with Global Cognitive Decline in Non-demented Adults: A Meta-Analysis of Prospective Studies. Front. Aging Neurosci. 2018, 9, 438. [Google Scholar] [CrossRef] [PubMed]
- Beydoun, M.A.; Weiss, J.; Obhi, H.K.; Beydoun, H.A.; Dore, G.A.; Liang, H.; Evans, M.K.; Zonderman, A.B. Cytokines are associated with longitudinal changes in cognitive performance among urban adults. Brain Behav. Immun. 2019, 80, 474–487. [Google Scholar] [CrossRef] [PubMed]
- Buchhave, P.; Zetterberg, H.; Blennow, K.; Minthon, L.; Janciauskiene, S.; Hansson, O. Soluble TNF receptors are associated with Aβ metabolism and conversion to dementia in subjects with mild cognitive impairment. Neurobiol. Aging 2010, 31, 1877–1884. [Google Scholar] [CrossRef] [PubMed]
- Bawa, K.K.; Krance, S.H.; Herrmann, N.; Cogo-Moreira, H.; Ouk, M.; Yu, D.; Wu, C.Y.; Black, S.E.; Lanctôt, K.L.; Swardfager, W.; et al. A peripheral neutrophil-related inflammatory factor predicts a decline in executive function in mild Alzheimer’s disease. J. Neuroinflammation 2020, 17, 84. [Google Scholar] [CrossRef]
- Oberlin, L.E.; Erickson, K.I.; Mackey, R.; Klunk, W.E.; Aizenstein, H.; Lopresti, B.J.; Kullerm, L.H.; Lopez, O.L.; Snitz, B.E. Peripheral inflammatory biomarkers predict the deposition and progression of amyloid-β in cognitively unimpaired older adults. Brain Behav. Immun. 2021, 95, 178–189. [Google Scholar] [CrossRef] [PubMed]
- Morgan, A.R.; Touchard, S.; Leckey, C.; O’Hagan, C.; Nevado-Holgado, A.J.; NIMA Consortium; Barkhof, F.; Bertram, L.; Blin, O.; Bos, I.; et al. Inflammatory biomarkers in Alzheimer’s disease plasma. Alzheimer’s Dement. 2019, 15, 776–787. [Google Scholar] [CrossRef]
- Bettcher, B.M.; Tansey, M.G.; Dorothée, G.; Heneka, M.T. Peripheral and central immune system crosstalk in Alzheimer disease—A research prospectus. Nat. Rev. Neurol. 2021, 17, 689–701. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Wang, L.; Ge, C.; Liu, X.; Chen, M.; Zhang, C. Effect of Process-Based Multi-Task Cognitive Training Program on Executive Function in Older Adults with Mild Cognitive Impairment: Study Rationale and Protocol Design for a Randomized Controlled Trial. Front. Psychiatry 2020, 11, 655. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Zeng, H.; Pan, L.; Wang, X.; Liu, M. Acupressure and Cognitive Training Can Improve Cognitive Functions of Older Adults with Mild Cognitive Impairment: A Randomized Controlled Trial. Front. Psychol. 2021, 12, 726083. [Google Scholar] [CrossRef]
- da Silva, T.B.L.; Bratkauskas, J.S.; Barbosa, M.E.C.; da Silva, G.A.; Zumkeller, M.G.; de Moraes, L.C.; Lessa, P.P.; Cardoso, N.P.; Ordonez, T.N.; Brucki, S.M.D. Long-term studies in cognitive training for older adults: A systematic review. Dement. Neuropsychol. 2022, 16, 135–152. [Google Scholar] [CrossRef]
- Peng, Z.; Jiang, H.; Wang, X.; Huang, K.; Zuo, Y.; Wu, X.; Abdullah, A.S.; Yang, L. The Efficacy of Cognitive Training for Elderly Chinese Individuals with Mild Cognitive Impairment. BioMed Res. Int. 2019, 2019, 4347281. [Google Scholar] [CrossRef]
- Sherman, D.S.; Mauser, J.; Nuno, M.; Sherzai, D. The Efficacy of Cognitive Intervention in Mild Cognitive Impairment (MCI): A Meta-Analysis of Outcomes on Neuropsychological Measures. Neuropsychol. Rev. 2017, 27, 440–484. [Google Scholar] [CrossRef]
- Maki, Y.; Hattori, H. Rehabilitative Support for Persons with Dementia and Their Families to Acquire Self-Management Attitude and Improve Social Cognition and Sense of Cognitive Empathy. Geriatrics 2019, 4, 26. [Google Scholar] [CrossRef]
- Shimada, H.; Doi, T.; Lee, S.; Makizako, H. Reversible predictors of reversion from mild cognitive impairment to normal cognition: A 4-year longitudinal study. Alzheimer’s Res. Ther. 2019, 11, 24. [Google Scholar] [CrossRef]
- Jeong, M.K.; Park, K.W.; Ryu, J.K.; Kim, G.M.; Jung, H.H.; Park, H. Multi-Component Intervention Program on Habitual Physical Activity Parameters and Cognitive Function in Patients with Mild Cognitive Impairment: A Randomized Controlled Trial. Int. J. Environ. Res. Public Health 2021, 18, 6240. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Jackson, N.; Larson, S.; Ward, K.T. Teaching Geriatrics and Transitions of Care to Internal Medicine Resident Physicians. Geriatrics 2020, 5, 72. [Google Scholar] [CrossRef] [PubMed]
- Guazzarini, A.G.; Casanova, G.; Buchholz, F.; Kozori, M.; Lavolpe, S.; Lichtwarck, B.; Margioti, E.; Mendes, A.; Montandon, M.L.; Murasecco, I.; et al. The Special Care Unit for People with Behavioral and Psychological Symptoms of Dementia (SCU-B) in the Context of the Project “RECage-Respectful Caring for Agitated Elderly”: A Qualitative Study. Int. J. Environ. Res. Public Health 2022, 19, 16913. [Google Scholar] [CrossRef]
- Zorkina, Y.; Syunyakov, T.; Abramova, O.; Andryushchenko, A.; Andreuyk, D.; Abbazova, E.; Goncharov, D.; Rakova, A.; Andriushchenko, N.; Gryadunov, D.; et al. Positive Effect of Cognitive Training in Older Adults with Different APOE Genotypes and COVID-19 History: A 1-Year Follow-Up Cohort Study. Diagnostics 2022, 12, 2312. [Google Scholar] [CrossRef]
- Giau, V.V.; Bagyinszky, E.; An, S.S.A. Potential Fluid Biomarkers for the Diagnosis of Mild Cognitive Impairment. Int. J. Mol. Sci. 2019, 20, 4149. [Google Scholar] [CrossRef]
- Greenberg, B.D.; Pettigrew, C.; Soldan, A.; Wang, J.; Wang, M.C.; Darrow, J.A.; Albert, M.S.; Moghekar, A. CSF Alzheimer Disease Biomarkers: Time-Varying Relationships with MCI Symptom Onset and Associations with Age, Sex, and ApoE4. Neurology 2022, 99, e1640–e1650. [Google Scholar] [CrossRef] [PubMed]
- Varesi, A.; Carrara, A.; Pires, V.G.; Floris, V.; Pierella, E.; Savioli, G.; Prasad, S.; Esposito, C.; Ricevuti, G.; Chirumbolo, S.; et al. Blood-Based Biomarkers for Alzheimer’s Disease Diagnosis and Progression: An Overview. Cells 2022, 11, 1367. [Google Scholar] [CrossRef] [PubMed]
- Van Hulle, C.; Jonaitis, E.M.; Betthauser, T.J.; Batrla, R.; Wild, N.; Kollmorgen, G.; Andreasson, U.; Okonkwo, O.; Bendlin, B.B.; Asthana, S.; et al. An examination of a novel multipanel of CSF biomarkers in the Alzheimer’s disease clinical and pathological continuum. Alzheimer’s Dement. 2021, 17, 431–445. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, J.T. The importance of longitudinal cohort studies in understanding risk and protective factors for dementia. Int. Psychogeriatr. 2014, 26, 541–542. [Google Scholar] [CrossRef]
- Zorkina, Y.; Abramova, O.; Ushakova, V.; Andreyuk, D.; Andriushchenko, N.; Pavlov, K.; Savilov, V.; Soloveva, K.; Kurmishev, M.; Syunyakov, T.; et al. Inflammatory biomarkers and lipid metabolism parameters in women with mild cognitive impairment and dementia. Women Health 2023, 63, 285–295. [Google Scholar] [CrossRef]
- Burigina, L.A.; Gavrilova, S.I.; Kostyuk, G.P.; Pak, M.V.; Kurmishev, M.V.; Savilov, V.B.; Starodubcev, S.V.; Urchenko, I.E. Psychosocial therapy and neurocognitive rehabilitation of elderly patients with cognitive disorders. In Structural and Functional Model of Rehabilitation Program “Memory Clinic”; Kostyuk, G.P., Ed.; Scientific Research Institute of Health Organization and Medical Management of the Department of Health of the City of Moscow: Moscow, Russia, 2019. (In Russian) [Google Scholar]
- Kostyuk, G.P.; Kourmyshev, M.V.; Savilov, V.B.; Efremova, D.N.; Pak, M.V.; Burigina, L.A. Recovery of cognitive function in elderly persons in a special medico-rehabilitation unit ‘the Memory clinic’. Soc. Clin. Psychiatry. 2017, 27, 25–31. [Google Scholar]
- Zaldua, S.; Damen, F.C.; Pisharody, R.; Thomas, R.; Fan, K.D.; Ekkurthi, G.K.; Scheinman, S.B.; Alahmadi, S.; Marottoli, F.M.; Alford, S.; et al. Epidermal growth factor treatment of female mice that express APOE4 at an age of advanced pathology mitigates behavioral and cerebrovascular dysfunction. Heliyon 2020, 6, e03919. [Google Scholar] [CrossRef]
- Hu, Q.; Xu, S.; Ye, C.; Jia, J.; Zhou, L.; Hu, G. Novel Pituitary Actions of Epidermal Growth Factor: Receptor Specificity and Signal Transduction for UTS1, EGR1, and MMP13 Regulation by EGF. Int. J. Mol. Sci. 2019, 20, 5172. [Google Scholar] [CrossRef]
- Lim, N.S.; Swanson, C.R.; Cherng, H.R.; Unger, T.L.; Xie, S.X.; Weintraub, D.; Marek, K.; Stern, M.B.; Siderowf, A.; PARS Investigators; et al. Plasma EGF and cognitive decline in Parkinson’s disease and Alzheimer’s disease. Ann. Clin. Transl. Neurol. 2016, 3, 346–355. [Google Scholar] [CrossRef]
- Klyucherev, T.O.; Olszewski, P.; Shalimova, A.A.; Chubarev, V.N.; Tarasov, V.V.; Attwood, M.M.; Syvänen, S.; Schiöth, H.B. Advances in the development of new biomarkers for Alzheimer’s disease. Transl. Neurodegener. 2022, 11, 25. [Google Scholar] [CrossRef]
- Marksteiner, J.; Kemmler, G.; Weiss, E.M.; Knaus, G.; Ullrich, C.; Mechtcheriakov, S.; Oberbauer, H.; Auffinger, S.; Hinterhölzl, J.; Hinterhuber, H.; et al. Five out of 16 plasma signaling proteins are enhanced in plasma of patients with mild cognitive impairment and Alzheimer’s disease. Neurobiol. Aging 2011, 32, 539–540. [Google Scholar] [CrossRef] [PubMed]
- Björkqvist, M.; Ohlsson, M.; Minthon, L.; Hansson, O. Evaluation of a previously suggested plasma biomarker panel to identify Alzheimer’s disease. PLoS ONE 2012, 7, e29868. [Google Scholar] [CrossRef] [PubMed]
- Mansour, H.M.; Fawzy, H.M.; El-Khatib, A.S.; Khattab, M.M. Repurposed anti-cancer epidermal growth factor receptor inhibitors: Mechanisms of neuroprotective effects in Alzheimer’s disease. Neural Regen. Res. 2022, 17, 1913–1918. [Google Scholar] [CrossRef] [PubMed]
- Hochstrasser, T.; Ehrlich, D.; Marksteiner, J.; Sperner-Unterweger, B.; Humpel, C. Matrix metalloproteinase-2 and epidermal growth factor are decreased in platelets of Alzheimer patients. Curr. Alzheimer Res. 2012, 9, 982–989. [Google Scholar] [CrossRef] [PubMed]
- Thomas, R.; Zuchowska, P.; Morris, A.W.; Marottoli, F.M.; Sunny, S.; Deaton, R.; Gann, P.H.; Tai, L.M. Epidermal growth factor prevents APOE4 and amyloid-beta-induced cognitive and cerebrovascular deficits in female mice. Acta Neuropathol. Commun. 2016, 4, 111. [Google Scholar] [CrossRef]
- Zhu, C.; Xu, B.; Sun, X.; Zhu, Q.; Sui, Y. Targeting CCR3 to Reduce Amyloid-β Production, Tau Hyperphosphorylation, and Synaptic Loss in a Mouse Model of Alzheimer’s Disease. Mol. Neurobiol. 2017, 54, 7964–7978. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, A.L.; Gama, C.S.; Rocha, N.P.; Teixeira, M.M. Revisiting the Role of Eotaxin-1/CCL11 in Psychiatric Disorders. Front. Psychiatry 2018, 9, 241. [Google Scholar] [CrossRef]
- Rege, S.V.; Teichert, A.; Masumi, J.; Dhande, O.S.; Harish, R.; Higgins, B.W.; Lopez, Y.; Akrapongpisak, L.; Hackbart, H.; Caryotakis, S.; et al. CCR3 plays a role in murine age-related cognitive changes and T-cell infiltration into the brain. Commun. Biol. 2023, 6, 292. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.H.; Long, L.; Tang, Y.C.; Zhang, J.T.; Hut, H.T.; Tang, F.R. CCR3, CCR2A and macrophage inflammatory protein (MIP)-1a, monocyte chemotactic protein-1 (MCP-1) in the mouse hippocampus during and after pilocarpine-induced status epilepticus (PISE). Neuropathol. Appl. Neurobiol. 2009, 35, 496–514. [Google Scholar] [CrossRef]
- Grewal, R.; Haghighi, M.; Huang, S.; Smith, A.G.; Cao, C.; Lin, X.; Lee, D.C.; Teten, N.; Hill, A.M.; Selenica, M.B. Identifying biomarkers of dementia prevalent among amnestic mild cognitively impaired ethnic female patients. Alzheimer’s Res. Ther. 2016, 8, 43. [Google Scholar] [CrossRef][Green Version]
- Lalli, M.A.; Bettcher, B.M.; Arcila, M.L.; Garcia, G.; Guzman, C.; Madrigal, L.; Ramirez, L.; Acosta-Uribe, J.; Baena, A.; Wojta, K.J.; et al. Whole-genome sequencing suggests a chemokine gene cluster that modifies age at onset in familial Alzheimer’s disease. Mol. Psychiatry 2015, 20, 1294–1300. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Sun, Y.; Xie, X.; Zhao, Y. Blood and CSF chemokines in Alzheimer’s disease and mild cognitive impairment: A systematic review and meta-analysis. Alzheimer’s Res. Ther. 2023, 15, 107. [Google Scholar] [CrossRef]
- Yang, J.; Kong, C.; Jia, L.; Li, T.; Quan, M.; Li, Y.; Lyu, D.; Li, F.; Jin, H.; Li, Y.; et al. Association of accelerated long-term forgetting and senescence-related blood-borne factors in asymptomatic individuals from families with autosomal dominant Alzheimer’s disease. Alzheimer’s Res. Ther. 2021, 13, 107. [Google Scholar] [CrossRef] [PubMed]
- Villeda, S.A.; Luo, J.; Mosher, K.I.; Zou, B.; Britschgi, M.; Bieri, G.; Stan, T.M.; Fainberg, N.; Ding, Z.; Eggel, A.; et al. The ageing systemic milieu negatively regulates neurogenesis and cognitive function. Nature 2011, 477, 90–94. [Google Scholar] [CrossRef]
- Sanchez-Sanchez, J.L.; Giudici, K.V.; Guyonnet, S.; Delrieu, J.; Li, Y.; Bateman, R.J.; Parini, A.; Vellas, B.; de Souto Barreto, P.; MAPT/DSA Group. Plasma MCP-1 and changes on cognitive function in community-dwelling older adults. Alzheimer’s Res. Ther. 2022, 14, 5. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Anshita, D.; Ravichandiran, V. MCP-1: Function, regulation, and involvement in disease. Int. Immunopharmacol. 2021, 101 Pt B, 107598. [Google Scholar] [CrossRef]
- Bettcher, B.M.; Fitch, R.; Wynn, M.J.; Lalli, M.A.; Elofson, J.; Jastrzab, L.; Mitic, L.; Miller, Z.A.; Rabinovici, G.D.; Miller, B.L.; et al. MCP-1 and eotaxin-1 selectively and negatively associate with memory in MCI and Alzheimer’s disease dementia phenotypes. Alzheimer’s Dement. 2016, 3, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Flores-Aguilar, L.; Iulita, M.F.; Orciani, C.; Tanna, N.; Yang, J.; Bennett, D.A.; Cuello, A.C. Cognitive and brain cytokine profile of non-demented individuals with cerebral amyloid-beta deposition. J. Neuroinflamm. 2021, 18, 147. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.J.; Liao, Y.C.; Wang, Y.F.; Lin, I.F.; Wang, S.J.; Fuh, J.L. Plasma MCP-1 and Cognitive Decline in Patients with Alzheimer’s Disease and Mild Cognitive Impairment: A Two-year Follow-up Study. Sci. Rep. 2018, 8, 1280. [Google Scholar] [CrossRef] [PubMed]
- Ausó, E.; Gómez-Vicente, V.; Esquiva, G. Biomarkers for Alzheimer’s Disease Early Diagnosis. J. Pers. Med. 2020, 10, 114. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Shen, Y.Y.; Zhang, X.P.; Gui, L.; Cai, M.; Peng, G.P.; Pan, X.D.; Zhang, J.; Gan, D.; Li, B.; et al. Diagnostic potential of urinary monocyte chemoattractant protein-1 for Alzheimer’s disease and amnestic mild cognitive impairment. Eur. J. Neurol. 2020, 27, 1429–1435. [Google Scholar] [CrossRef] [PubMed]
- Teoh, N.S.N.; Gyanwali, B.; Lai, M.K.P.; Chai, Y.L.; Chong, J.R.; Chong, E.J.Y.; Chen, C.; Tan, C.S.; Hilal, S. Association of Interleukin-6 and Interleukin-8 with Cognitive Decline in an Asian Memory Clinic Population. J. Alzheimer’s Dis. 2023, 92, 445–455. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.M.; Song, J.; Kim, S.; Han, C.; Park, M.H.; Koh, Y.; Jo, S.A.; Kim, Y.Y. Identification of peripheral inflammatory markers between normal control and Alzheimer’s disease. BMC Neurol. 2011, 11, 51. [Google Scholar] [CrossRef]
- Vaz, M.; Domingues, C.; Trindade, D.; Barra, C.; Oliveira, J.M.; Rosa, I.M.; da Cruz e Silva, O.A.B.; Henriques, A.G. IL-8 and MCP-1 Impact on Tau Phosphorylation and Phosphatase Activity. Curr. Alzheimer Res. 2020, 17, 985–1000. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.; Oakley, A.E.; Monteiro, M.; Tuomela, K.; Allan, L.M.; Mukaetova-Ladinska, E.B.; O’Brien, J.T.; Kalaria, R.N. Multiplex analyte assays to characterize different dementias: Brain inflammatory cytokines in poststroke and other dementias. Neurobiol. Aging 2016, 38, 56–67. [Google Scholar] [CrossRef]
- Iulita, M.F.; Ganesh, A.; Pentz, R.; Flores Aguilar, L.; Gubert, P.; Ducatenzeiler, A.; Christie, S.; Wilcock, G.K.; Cuello, A.C. Identification and Preliminary Validation of a Plasma Profile Associated with Cognitive Decline in Dementia and At-Risk Individuals: A Retrospective Cohort Analysis. J. Alzheimer’s Dis. 2019, 67, 327–341. [Google Scholar] [CrossRef]
- Aksnes, M.; Aass, H.C.D.; Tiiman, A.; Terenius, L.; Bogdanović, N.; Vukojević, V.; Knapskog, A.B. Serum Amyloidogenic Nanoplaques and Cytokines in Alzheimer’s Disease: Pilot Study in a Small Naturalistic Memory Clinic Cohort. J. Alzheimer’s Dis. 2022, 86, 1459–1470. [Google Scholar] [CrossRef]
- Parhizkar, S.; Holtzman, D.M. APOE mediated neuroinflammation and neurodegeneration in Alzheimer’s disease. Semin. Immunol. 2022, 59, 101594. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, D.S.; Sagare, A.; Pachicano, M.; Sweeney, M.D.; Toga, A.; Zlokovic, B.; Chui, H.; Joe, E.; Schneider, L.; Morris, J.C.; et al. Early neuroinflammation is associated with lower amyloid and tau levels in cognitively normal older adults. Brain Behav. Immun. 2021, 94, 299–307. [Google Scholar] [CrossRef]
- Capogna, E.; Watne, L.O.; Sørensen, Ø.; Guichelaar, C.J.; Idland, A.V.; Halaas, N.B.; Blennow, K.; Zetterberg, H.; Walhovd, K.B.; Fjell, A.M.; et al. Associations of neuroinflammatory IL-6 and IL-8 with brain atrophy, memory decline, and core AD biomarkers—In cognitively unimpaired older adults. Brain Behav. Immun. 2023, 113, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Kloske, C.M.; Barnum, C.J.; Batista, A.F.; Bradshaw, E.M.; Brickman, A.M.; Bu, G.; Dennison, J.; Gearon, M.D.; Goate, A.M.; Haass, C.; et al. APOE and immunity: Research highlights. Alzheimer’s Dement. J. Alzheimer’s Assoc. 2023, 19, 2677–2696. [Google Scholar] [CrossRef]
- Li, Z.; Zhu, Y.; Kang, Y.; Qin, S.; Chai, J. Neuroinflammation as the Underlying Mechanism of Postoperative Cognitive Dysfunction and Therapeutic Strategies. Front. Cell. Neurosci. 2022, 16, 843069. [Google Scholar] [CrossRef] [PubMed]
- Bradburn, S.; Murgatroyd, C.; Ray, N. Neuroinflammation in mild cognitive impairment and Alzheimer’s disease: A meta-analysis. Ageing Res. Rev. 2019, 50, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Jagust, W. Imaging the evolution and pathophysiology of Alzheimer disease. Nat. Rev. Neurosci. 2018, 19, 687–700. [Google Scholar] [CrossRef]
- Akiyama, H.; Barger, S.; Barnum, S.; Bradt, B.; Bauer, J.; Cole, G.M.; Cooper, N.R.; Eikelenboom, P.; Emmerling, M.; Fiebich, B.L.; et al. Inflammation and Alzheimer’s disease. Neurobiol. Aging 2000, 21, 383–421. [Google Scholar] [CrossRef] [PubMed]
- Zverova, M.; Kitzlerova, E.; Fisar, Z.; Jirak, R.; Hroudova, J.; Benakova, H.; Lelkova, P.; Martasek, P.; Raboch, J. Interplay between the APOE Genotype and Possible Plasma Biomarkers in Alzheimer’s Disease. Curr. Alzheimer Res. 2018, 15, 938–950. [Google Scholar] [CrossRef] [PubMed]
- Khan, T.A.; Shah, T.; Prieto, D.; Zhang, W.; Price, J.; Fowkes, G.R.; Cooper, J.; Talmud, P.J.; Humphries, S.E.; Sundstrom, J.; et al. Apolipoprotein E genotype, cardiovascular biomarkers and risk of stroke: Systematic review and meta-analysis of 14,015 stroke cases and pooled analysis of primary biomarker data from up to 60,883 individuals. Int. J. Epidemiol. 2013, 42, 475–492. [Google Scholar] [CrossRef] [PubMed]
- Kritharides, L.; Nordestgaard, B.G.; Tybjærg-Hansen, A.; Kamstrup, P.R.; Afzal, S. Effect of APOE ε Genotype on Lipoprotein(a) and the Associated Risk of Myocardial Infarction and Aortic Valve Stenosis. J. Clin. Endocrinol. Metab. 2017, 102, 3390–3399. [Google Scholar] [CrossRef]
- Wu, S.; Hsu, L.A.; Teng, M.S.; Lin, J.F.; Chou, H.H.; Lee, M.C.; Wu, Y.M.; Su, C.W.; Ko, Y.L. Interactive effects of C-reactive protein levels on the association between APOE variants and triglyceride levels in a Taiwanese population. Lipids Health Dis. 2016, 15, 94. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Supasitthumrong, T.; Tunvirachaisakul, C.; Aniwattanapong, D.; Tangwongchai, S.; Chuchuen, P.; Tawankanjanachot, I.; Snabboon, T.; Hemrungrojn, S.; Carvalho, A.F.; Maes, M. Peripheral Blood Biomarkers Coupled with the Apolipoprotein E4 Genotype Are Strongly Associated with Semantic and Episodic Memory Impairments in Elderly Subjects with Amnestic Mild Cognitive Impairment and Alzheimer’s Disease. J. Alzheimer’s Dis. 2019, 71, 797–811. [Google Scholar] [CrossRef] [PubMed]
- Lima, T.A.; Adler, A.L.; Minett, T.; Matthews, F.E.; Brayne, C.; Marioni, R.E.; Medical Research Council Cognitive Function and Ageing Study. C-reactive protein, APOE genotype and longitudinal cognitive change in an older population. Age Ageing 2014, 43, 289–292. [Google Scholar] [CrossRef] [PubMed]
- Vasunilashorn, S.; Glei, D.A.; Lan, C.Y.; Brookmeyer, R.; Weinstein, M.; Goldman, N. Apolipoprotein E is associated with blood lipids and inflammation in Taiwanese older adults. Atherosclerosis 2011, 219, 349–354. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Civeira-Marín, M.; Cenarro, A.; Marco-Benedí, V.; Bea, A.M.; Mateo-Gallego, R.; Moreno-Franco, B.; Ordovás, J.M.; Laclaustra, M.; Civeira, F.; Lamiquiz-Moneo, I. APOE Genotypes Modulate Inflammation Independently of Their Effect on Lipid Metabolism. Int. J. Mol. Sci. 2022, 23, 12947. [Google Scholar] [CrossRef]
- Elahi, F.M.; Casaletto, K.B.; La Joie, R.; Walters, S.M.; Harvey, D.; Wolf, A.; Edwards, L.; Rivera-Contreras, W.; Karydas, A.; Cobigo, Y.; et al. Plasma biomarkers of astrocytic and neuronal dysfunction in early- and late-onset Alzheimer’s disease. Alzheimer’s Dement. 2020, 16, 681–695. [Google Scholar] [CrossRef]
- Rocha, N.P.; Scalzo, P.L.; Barbosa, I.G.; Souza, M.S.; Morato, I.B.; Vieira, E.L.; Christo, P.P.; Teixeira, A.L.; Reis, H.J. Cognitive Status Correlates with CXCL10/IP-10 Levels in Parkinson’s Disease. Park. Dis. 2014, 2014, 903796. [Google Scholar] [CrossRef]
- Turner, J.A.; Padgett, C.; McDonald, S.; Ahuja, K.D.K.; Francis, H.M.; Lim, C.K.; Honan, C.A. Innate immunity impacts social-cognitive functioning in people with multiple sclerosis and healthy individuals: Implications for IL-1ra and urinary immune markers. Brain Behav. Immun. Health 2021, 14, 100254. [Google Scholar] [CrossRef]
- Galimberti, D.; Schoonenboom, N.; Scheltens, P.; Fenoglio, C.; Bouwman, F.; Venturelli, E.; Guidi, I.; Blankenstein, M.A.; Bresolin, N.; Scarpini, E. Intrathecal chemokine synthesis in mild cognitive impairment and Alzheimer disease. Arch. Neurol. 2006, 63, 538–543. [Google Scholar] [CrossRef]
- Pinto, T.; Machado, L.; Bulgacov, T.M.; Rodrigues-Júnior, A.L.; Costa, M.; Ximenes, R.; Sougey, E.B. Is the Montreal Cognitive Assessment (MoCA) screening superior to the Mini-Mental State Examination (MMSE) in the detection of mild cognitive impairment (MCI) and Alzheimer’s Disease (AD) in the elderly? Int. Psychogeriatr. 2019, 31, 491–504. [Google Scholar] [CrossRef]
- Zhuang, L.; Yang, Y.; Gao, J. Cognitive assessment tools for mild cognitive impairment screening. J. Neurol. 2021, 268, 1615–1622. [Google Scholar] [CrossRef]
- Creavin, S.T.; Wisniewski, S.; Noel-Storr, A.H.; Trevelyan, C.M.; Hampton, T.; Rayment, D.; Thom, V.M.; Nash, K.J.; Elhamoui, H.; Milligan, R.; et al. Mini-Mental State Examination (MMSE) for the detection of dementia in clinically unevaluated people aged 65 and over in community and primary care populations. Cochrane Database Syst. Rev. 2016, 13, CD011145. [Google Scholar] [CrossRef]
- Julian, L.J. Measures of anxiety: State-Trait Anxiety Inventory (STAI), Beck Anxiety Inventory (BAI), and Hospital Anxiety and Depression Scale-Anxiety (HADS-A). Arthritis Care Res. 2011, 63, S467–S472. [Google Scholar] [CrossRef] [PubMed]
- Bjelland, I.; Dahl, A.A.; Haug, T.T.; Neckelmann, D. The validity of the Hospital Anxiety and Depression Scale. An updated literature review. J. Psychosom. Res. 2002, 52, 69–77. [Google Scholar] [CrossRef] [PubMed]

| Variable/Statistic | Categories | Value |
|---|---|---|
| Age, Mean (SE) | 72.4 (0.64) | |
| Sex, n (%) | Female | 121 (89.0) |
| Male | 15 (11.0) | |
| Education and work | ||
| Vocational school, n (%) | No | 66 (49.6) |
| Yes | 67 (50.4) | |
| Not Magister, n (%) | No | 126 (94.7) |
| Yes | 7 (5.3) | |
| Magister, n (%) | No | 46 (34.1) |
| Yes | 89 (65.9) | |
| Years of University, n (%) | 0 | 41 (30.6) |
| 1–3 | 5 (3.6) | |
| 5 | 59 (44.0) | |
| 6 | 24 (17.9) | |
| >7 | 5 (3.6) | |
| Total years of school + vocational school, n (%) | 8 | 1 (0.7) |
| 10 | 78 (57.8) | |
| 11 | 7 (5.2) | |
| 12 | 18 (13.3) | |
| 13 | 20 (14.8) | |
| 14 | 11 (8.1) | |
| Years of university, mean (SE) | 3.66 (0.22) | |
| Total years of school + vocational school, mean (SE) | 11.07 (0.12) | |
| Type of work, n (%) | Intellectual | 116 (86.6) |
| Technical | 18 (13.4) | |
| Family | ||
| Family, n (%) | Yes | 95 (70.4) |
| No | 40 (29.6) | |
| Number of children, n (%) | 0 | 17 (12.7) |
| 1 | 54 (40.3) | |
| 2 | 58 (43.3) | |
| 3 | 5 (3.7) | |
| Scales | Baseline Examination | 1 Year | F | df | p | ||
|---|---|---|---|---|---|---|---|
| Mean | Std. Error of Mean | Mean | Std. Error of Mean | ||||
| MoCA 1 | 23.72 | 0.28 | 24.32 | 0.33 | 5.26 | 1 | 0.02 |
| MMSE 2 | 26.80 | 0.16 | 28.29 | 0.17 | 68.3 | 1 | <0.001 |
| HADS 3 | 12.85 | 0.50 | 12.41 | 0.49 | 0.65 | 1 | 0.42 |
| Immune Parameters | First Visit | Second Visit | F | p | FDR | ||
|---|---|---|---|---|---|---|---|
| Mean | Std. Error of Mean | Mean | Std. Error of Mean | ||||
| EGF | 77.9 | 4.7 | 106.2 | 6.9 | 17 | 0.00007 | 0.002 |
| FGF 2 | 115.7 | 16.3 | 124 | 10.3 | 0.4 | 0.53 | 0.75 |
| Eotaxin | 135.3 | 7 | 161.6 | 10.4 | 7.17 | 0.008 | 0.04 |
| TGFα | 5.2 | 0.4 | 10.7 | 3.1 | 1.13 | 0.29 | 0.56 |
| G-CSF | 109.1 | 33.3 | 84.1 | 11.8 | 0.55 | 0.46 | 0.78 |
| Fractalkine | 240.7 | 92.3 | 248.6 | 49.8 | 0.01 | 0.91 | 0.98 |
| INFα2 | 63.6 | 11.3 | 62.5 | 12.6 | 0.001 | 0.98 | 0.98 |
| IFNγ | 12.3 | 4.2 | 13.2 | 4.1 | 0.07 | 0.79 | 0.93 |
| GRO | 1409.1 | 77 | 1735.9 | 87.4 | 13.42 | 0.0004 | 0.004 |
| IL-10 | 4.5 | 0.6 | 3.8 | 0.6 | 0.06 | 0.81 | 0.91 |
| MCP-3 | 57.4 | 6.3 | 47.9 | 5.8 | 0.11 | 0.74 | 0.91 |
| MDC | 682 | 36.6 | 799.2 | 59 | 5.93 | 0.016 | 0.07 |
| IL-12P70 | 7.5 | 2.3 | 7.6 | 2.2 | 0.003 | 0.96 | 1.00 |
| sCD40L | 2931.6 | 159.7 | 3095.4 | 79.5 | 1 | 0.32 | 0.58 |
| IL-1RA | 32.9 | 12.5 | 22.3 | 3.3 | 0.45 | 0.5 | 0.79 |
| IL-1a | 81.6 | 20.4 | 73.8 | 15.7 | 1.22 | 0.27 | 0.56 |
| IL-4 | 369 | 78.6 | 322.8 | 69 | 0.34 | 0.56 | 0.76 |
| IL-6 | 25 | 5.9 | 31.9 | 6.3 | 3.42 | 0.07 | 0.21 |
| IL-7 | 15.6 | 1.7 | 17 | 1.2 | 1.49 | 0.23 | 0.52 |
| IL-8 | 15.8 | 1.8 | 19.9 | 1.9 | 8.16 | 0.005 | 0.03 |
| IP-10 | 269.1 | 20.1 | 230 | 15 | 3.5 | 0.06 | 0.20 |
| MCP-1 | 543.8 | 27.9 | 640.8 | 22.8 | 13.46 | 0.0003 | 0.004 |
| MIP-1 β | 42.1 | 2.2 | 43.5 | 2.8 | 0.42 | 0.52 | 0.78 |
| TNFa | 16.9 | 1.7 | 21.3 | 3.2 | 2.84 | 0.09 | 0.24 |
| VEGF | 101.5 | 17.3 | 128.6 | 11.1 | 3.85 | 0.053 | 0.20 |
| Flt-3L | 43.0 | 5.6 | 42.1 | 2.9 | 1.85 | 0.18 | 0.45 |
| GM-CSF | 19.6 | 10.2 | 14.4 | 2.7 | 0.23 | 0.63 | 0.81 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abramova, O.; Zorkina, Y.; Ushakova, V.; Gryadunov, D.; Ikonnikova, A.; Fedoseeva, E.; Emelyanova, M.; Ochneva, A.; Morozova, I.; Pavlov, K.; et al. Alteration of Blood Immune Biomarkers in MCI Patients with Different APOE Genotypes after Cognitive Training: A 1 Year Follow-Up Cohort Study. Int. J. Mol. Sci. 2023, 24, 13395. https://doi.org/10.3390/ijms241713395
Abramova O, Zorkina Y, Ushakova V, Gryadunov D, Ikonnikova A, Fedoseeva E, Emelyanova M, Ochneva A, Morozova I, Pavlov K, et al. Alteration of Blood Immune Biomarkers in MCI Patients with Different APOE Genotypes after Cognitive Training: A 1 Year Follow-Up Cohort Study. International Journal of Molecular Sciences. 2023; 24(17):13395. https://doi.org/10.3390/ijms241713395
Chicago/Turabian StyleAbramova, Olga, Yana Zorkina, Valeriya Ushakova, Dmitry Gryadunov, Anna Ikonnikova, Elena Fedoseeva, Marina Emelyanova, Aleksandra Ochneva, Irina Morozova, Konstantin Pavlov, and et al. 2023. "Alteration of Blood Immune Biomarkers in MCI Patients with Different APOE Genotypes after Cognitive Training: A 1 Year Follow-Up Cohort Study" International Journal of Molecular Sciences 24, no. 17: 13395. https://doi.org/10.3390/ijms241713395
APA StyleAbramova, O., Zorkina, Y., Ushakova, V., Gryadunov, D., Ikonnikova, A., Fedoseeva, E., Emelyanova, M., Ochneva, A., Morozova, I., Pavlov, K., Syunyakov, T., Andryushchenko, A., Savilov, V., Kurmishev, M., Andreuyk, D., Shport, S., Gurina, O., Chekhonin, V., Kostyuk, G., & Morozova, A. (2023). Alteration of Blood Immune Biomarkers in MCI Patients with Different APOE Genotypes after Cognitive Training: A 1 Year Follow-Up Cohort Study. International Journal of Molecular Sciences, 24(17), 13395. https://doi.org/10.3390/ijms241713395








