Identification of Key TRIM Genes Involved in Response to Pseudomonas aeruginosa or Chlamydia spp. Infections in Human Cell Lines and in Mouse Organs
Abstract
:1. Introduction
2. Results
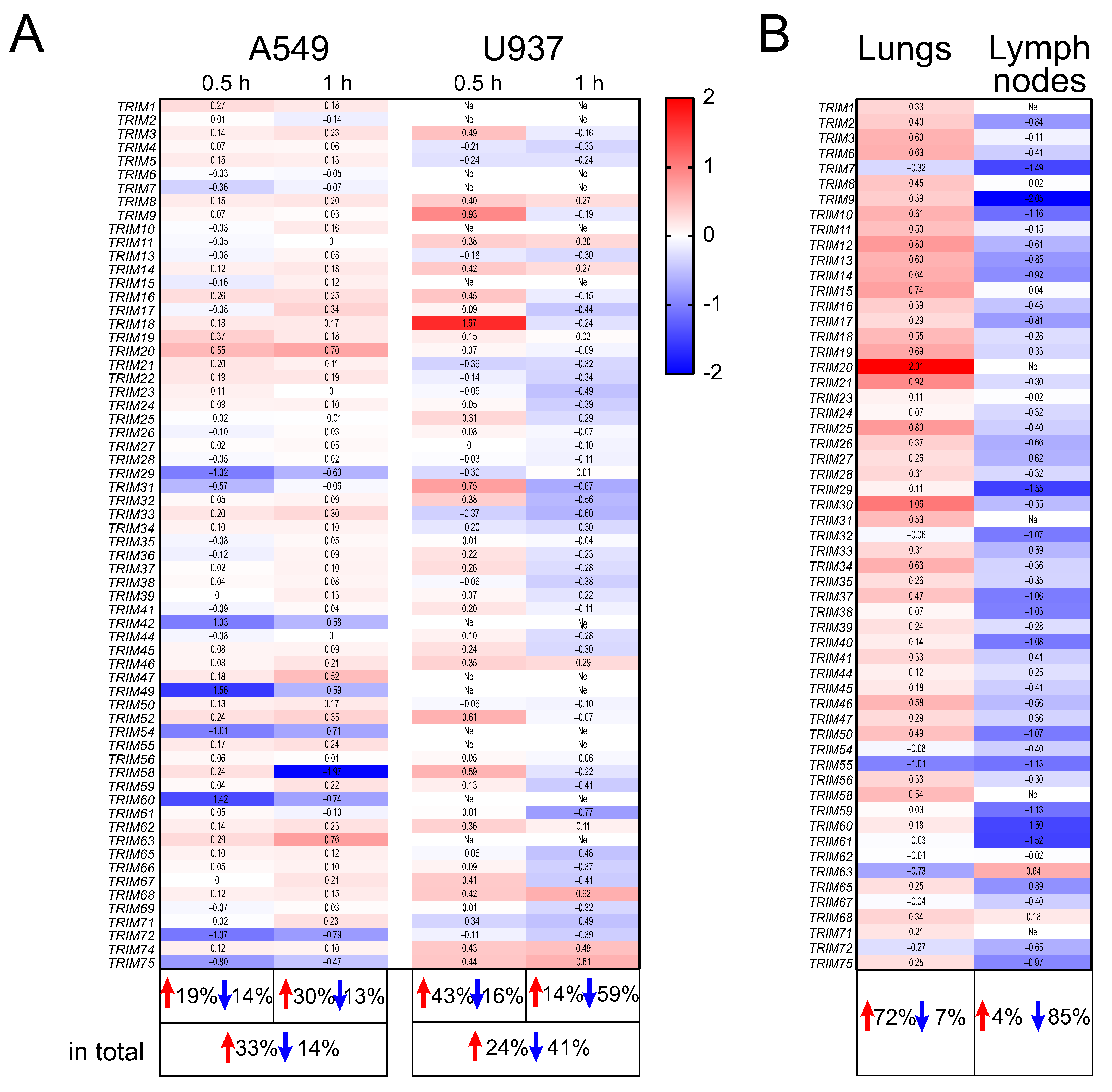
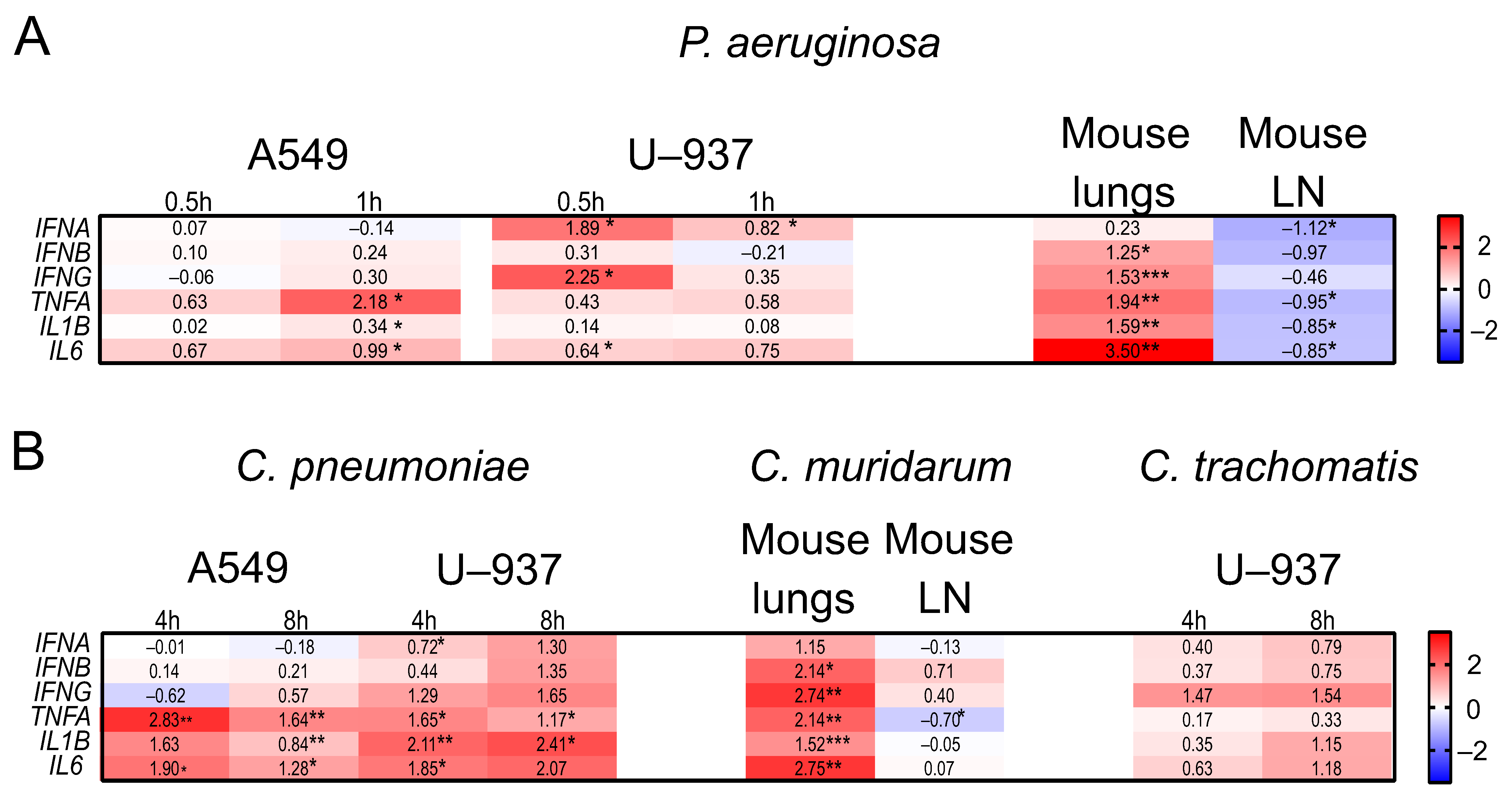
2.1. The Expression Profiles of TRIM Genes in Human A549 and U937 Cell Lines and in DBA/2 Mouse Organs after P. aeruginosa Infection
2.2. The Expression Profiles of TRIM Genes in Human A549, U937, and PC-3 Cell Lines and in DBA/2 Mouse Organs after Chlamydia spp. Infection
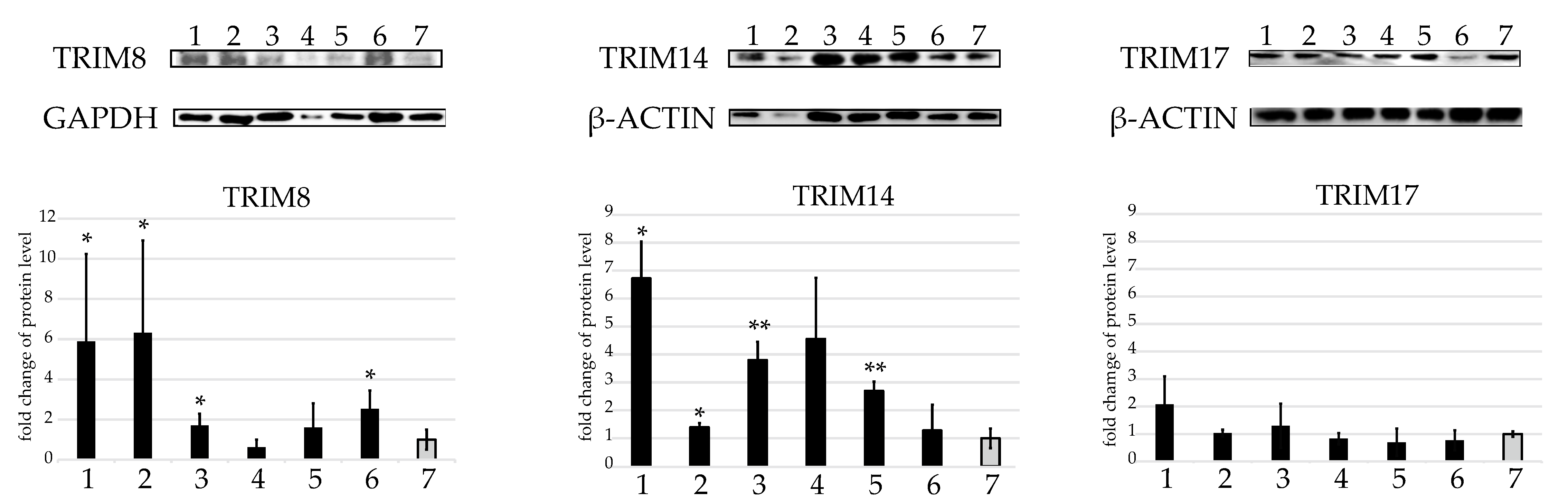
2.3. Western Blot Protein Assay of Several TRIMs of Interest in the U937 Cell Line
2.4. The Expression Profiles of IFNs and Inflammatory Genes in Human A549, U937, and PC-3 Cell Lines, and in DBA/2 Mouse Organs after P. aeruginosa and Chlamydia spp. Infections
3. Discussion
4. Materials and Methods
4.1. Cell Lines
4.2. Pathogens
4.3. Infection of the Cell Lines
4.4. Mouse Infection
4.5. Quantitative PCR
4.6. Western Blot
4.7. Bioinformatic Analysis
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shen, Z.; Wei, L.; Yu, Z.B.; Yao, Z.Y.; Cheng, J.; Wang, Y.T.; Song, X.T.; Li, M. The Roles of TRIMs in Antiviral Innate Immune Signaling. Front. Cell Infect. Microbiol. 2021, 15, 628275. [Google Scholar]
- Wang, L.; Ning, S. TRIMming Type I Interferon-Mediated Innate Immune Response in Antiviral and Antitumor Defense. Viruses 2021, 13, 279. [Google Scholar] [PubMed]
- Chen, Y.; Cao, S.; Sun, Y.; Li, C. Gene Expression Profiling of the TRIM Protein Family Reveals Potential Biomarkers for Indicating Tuberculosis Status. Microb. Pathog. 2018, 114, 385–392. [Google Scholar] [PubMed]
- Lou, J.; Wang, Y.; Zheng, X.; Qiu, W. TRIM22 regulates macrophage autophagy and enhances Mycobacterium tuberculosis clearance by targeting the nuclear factor–multiplicity κB/beclin 1 pathway. J. Cell Biochem. 2018, 119, 8971–8980. [Google Scholar] [PubMed]
- Wang, J.; Teng, J.L.L.; Zhao, D.; Ge, P.; Li, B.; Woo, P.C.Y.; Liu, C.H. The ubiquitin ligase TRIM27 functions as a host restriction factor antagonized by Mycobacterium tuberculosis PtpA during mycobacterial infection. Sci. Rep. 2016, 6, 34827. [Google Scholar]
- Perelman, S.S.; Abrams, M.E.; Eitson, J.L.; Chen, D.; Jimenez, A.; Mettlen, M.; Schoggins, J.W.; Alto, N.M. Cell-Based Screen Identifies Human Interferon-Stimulated Regulators of Listeria monocytogenes Infection. PLoS Pathog. 2016, 12, e1006102. [Google Scholar]
- Hoffpauir, C.T.; Bell, S.L.; West, K.O.; Jing, T.; Wagner, A.R.; Torres-Odio, S.; Cox, J.S.; West, A.P.; Li, P.; Patrick, K.L.; et al. TRIM14 Is a Key Regulator of the Type I IFN Response during Mycobacterium tuberculosis Infection. J. Immunol. 2020, 205, 153–167. [Google Scholar]
- Hos, N.J.; Fischer, J.; Hos, D.; Hejazi, Z.; Calabrese, C.; Ganesan, R.; Murthy, A.M.V.; Rybniker, J.; Kumar, S.; Krönke, M.; et al. TRIM21 Is Targeted for Chaperone-Mediated Autophagy during Salmonella Typhimurium Infection. J. Immunol. 2020, 205, 2456–2467. [Google Scholar]
- Kamanova, J.; Sun, H.; Lara-Tejero, M.; Galán, J.E. The Salmonella Effector Protein SopA Modulates Innate Immune Responses by Targeting TRIM E3 Ligase Family Members. PLoS Pathog. 2016, 12, e1005552. [Google Scholar]
- OuYang, X.; Guo, J.; Jiang, H.; Zheng, Y.; Liu, P. TRIM32 Drives Pathogenesis in Streptococcal Toxic Shock-Like Syndrome and Streptococcus suis Meningitis by Regulating Innate Immune Responses. Infect. Immun. 2020, 88, e00957-19. [Google Scholar]
- Weiss, E.; Essaied, W.; Adrie, C.; Zahar, J.R.; Timsit, J.F. Treatment of severe hospital-acquired and ventilator-associated pneumonia: A systematic review of inclusion and judgment criteria used in randomized controlled trials. Crit Care 2017, 21, 162. [Google Scholar]
- Bastidas, R.J.; Elwell, C.A.; Engel, J.N.; Valdivia, R.H. Chlamydial intracellular survival strategies. Cold Spring Harb. Perspect. Med. 2013, 3, a010256. [Google Scholar] [PubMed]
- Morrison, R.P.; Caldwell, H.D. Immunity to murine chlamydial genital infection. Infect. Immun. 1978, 70, 2741–2751. [Google Scholar]
- Sellami, H.; Said-Sadier, N.; Znazen, A.; Gdoura, R.; Ojcius, D.M.; Hammami, A. Chlamydia trachomatis infection increases the expression of inflammatory tumorigenic cytokines and chemokines as well as components of the Toll-like receptor and NF-κB pathways in human prostate epithelial cells. Mol. Cell Probes 2014, 28, 147–154. [Google Scholar] [PubMed]
- Jiang, M.X.; Hong, X.; Liao, B.B.; Shi, S.Z.; Lai, X.F.; Zheng, H.Y.; Xie, L.; Wang, Y.; Wang, X.L.; Xin, H.B.; et al. Expression profiling of TRIM protein family in THP1-derived macrophages following TLR stimulation. Sci. Rep. 2017, 7, 42781. [Google Scholar]
- Carthagena, L.; Bergamaschi, A.; Luna, J.M.; David, A.; Uchil, P.D.; Margottin-Goguet, F.; Mothes, W.; Hazan, U.; Transy, C.; Pancino, G.; et al. Human TRIM gene expression in response to interferons. PLoS ONE 2009, 4, e4894. [Google Scholar]
- Ebenezer, D.L.; Fu, P.; Krishnan, Y.; Maienschein-Cline, M.; Hu, H.; Jung, S.; Madduri, R.; Arbieva, Z.; Harijith, A.; Natarajan, V. Genetic deletion of Sphk2 confers protection against Pseudomonas aeruginosa mediated differential expression of genes related to virulent infection and inflammation in mouse lung. BMC Genomics 2019, 20, 984. [Google Scholar]
- Virok, D.P.; Raffai, T.; Kókai, D.; Paróczai, D.; Bogdanov, A.; Veres, G.; Vécsei, L.; Poliska, S.; Tiszlavicz, L.; Somogyvári, F.; et al. Indoleamine 2,3-Dioxygenase Activity in Chlamydia muridarum and Chlamydia pneumoniae Infected Mouse Lung Tissues. Front. Cell Infect. Microbiol. 2019, 9, 192. [Google Scholar]
- Soderberg, K.A.; Payne, G.W.; Sato, A.; Medzhitov, R.; Segal, S.S.; Iwasaki, A. Innate control of adaptive immunity via remodeling of lymph node feed arteriole. Proc. Natl. Acad. Sci. USA 2005, 102, 16315–16320. [Google Scholar]
- Palm, N.W.; Medzhitov, R. Not so fast: Adaptive suppression of innate immunity. Nat. Med. 2007, 13, 1142–1144. [Google Scholar]
- Yang, L.; Xia, H. TRIM Proteins in Inflammation: From Expression to Emerging Regulatory Mechanisms. Inflammation 2021, 44, 811–820. [Google Scholar] [CrossRef]
- Yang, W.; Gu, Z.; Zhang, H.; Hu, H. To TRIM the Immunity: From Innate to Adaptive Immunity. Front. Immunol. 2020, 11, 02157. [Google Scholar] [CrossRef]
- McIsaac, S.M.; Stadnyk, A.W.; Lin, T.-J. Toll-like receptors in the host defense against Pseudomonas aeruginosa respiratory infection and cystic fibrosis. J. Leukoc. Biol. 2012, 92, 977–985. [Google Scholar] [CrossRef]
- Al-Kuhlani, M.; Lambert, G.; Pal, S.; de la Maza, L.; Ojcius, D.M. Immune response against Chlamydia trachomatis via toll-like receptors is negatively regulated by SIGIRR. PLoS ONE 2020, 15, e0230718. [Google Scholar] [CrossRef]
- Shimada, K.; Crother, T.R.; Arditi, M. Innate immune responses to Chlamydia pneumoniae infection: Role of TLRs, NLRs, and the inflammasome. Microbes Infect. 2012, 14, 1301–1307. [Google Scholar] [CrossRef] [PubMed]
- Ciesielska, A.; Matyjek, M.; Kwiatkowska, K. TLR4 and CD14 trafficking and its influence on LPS-induced pro-inflammatory signaling. Cell. Mol. Life Sci. 2021, 78, 1233–1261. [Google Scholar]
- Ozato, K.; Shin, D.M.; Chang, T.H.; Morse, H.C., 3rd. TRIM family proteins and their emerging roles in innate immunity. Nat. Rev. Immunol. 2008, 8, 849–860. [Google Scholar] [CrossRef] [PubMed]
- Rajsbaum, R.; Stoye, J.P.; O’Garra, A. Type I interferon-dependent and -independent expression of tripartite motif proteins in immune cells. Eur. J. Immunol. 2008, 38, 619–630. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.; Wang, Z.; Lin, H.; Lei, T.; Zhou, Z.; Huang, W.; Wu, X.; Zuo, L.; Wu, J.; Liu, Y.; et al. TRIM47 is a novel endothelial activation factor that aggravates lipopolysaccharide-induced acute lung injury in mice via K63-linked ubiquitination of TRAF2. Signal Transduct. Target. Ther. 2022, 7, 148. [Google Scholar] [CrossRef]
- Guo, L.; Dong, W.; Fu, X.; Lin, J.; Dong, Z.; Tan, X.; Zhang, T. Tripartite Motif 8 (TRIM8) Positively Regulates Pro-inflammatory Responses in Pseudomonas aeruginosa-Induced Keratitis Through Promoting K63-Linked Polyubiquitination of TAK1 Protein. Inflammation 2017, 40, 454–463. [Google Scholar] [CrossRef]
- Kimura, T.; Jia, J.; Kumar, S.; Choi, S.W.; Gu, Y.; Mudd, M.; Dupont, N.; Jiang, S.; Peters, R.; Farzam, F.; et al. Dedicated SNAREs and specialized TRIM cargo receptors mediate secretory autophagy. EMBO J. 2017, 36, 42–60. [Google Scholar] [CrossRef] [PubMed]
- Aral, K.; Berdeli, E.; Cooper, P.R.; Milward, M.R.; Kapila, Y.; Karadede Ünal, B.; Aral, C.A.; Berdeli, A. Differential expression of inflammasome regulatory transcripts in periodontal disease. J. Periodontol. 2020, 91, 606–616. [Google Scholar] [CrossRef]
- Aral, K.; Milward, M.R.; Cooper, P.R. Dysregulation of Inflammasomes in Human Dental Pulp Cells Exposed to Porphyromonas gingivalis and Fusobacterium nucleatum. J. Endod. 2020, 46, 1265–1272. [Google Scholar] [CrossRef]
- An, Y.; Ni, Y.; Xu, Z.; Shi, S.; He, J.; Liu, Y.; Deng, K.Y.; Fu, M.; Jiang, M.; Xin, H.B. TRIM59 expression is regulated by Sp1 and Nrf1 in LPS-activated macrophages through JNK signaling pathway. Cell Signal 2020, 67, 109522. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.; Zhu, Z.; Liu, S.; Hou, Y.; Tang, M.; Zhu, P.; Tian, Y.; Li, D.; Yan, D.; Zhu, X. TRIM59 Protects Mice From Sepsis by Regulating Inflammation and Phagocytosis in Macrophages. Front. Immunol. 2020, 11, 263. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Liu, X.; Zhang, J.; Qin, L.; Du, J.; Li, X.; Qian, S.; Chen, H.; Qian, P. TRIM67 Suppresses TNFalpha-Triggered NF-kB Activation by Competitively Binding Beta-TrCP to IkBa. Front. Immunol. 2022, 13, 793147. [Google Scholar] [CrossRef]
- Lu, M.; Zhu, X.; Yang, Z.; Zhang, W.; Sun, Z.; Ji, Q.; Chen, X.; Zhu, J.; Wang, C.; Nie, S. E3 ubiquitin ligase tripartite motif 7 positively regulates the TLR4-mediated immune response via its E3 ligase domain in macrophages. Mol. Immunol. 2019, 109, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Yi, W.; Wang, Z.; Ye, C.; Tian, S.; Li, X.; Zou, A.; Zhao, X.; Yuan, Y.; Wang, X.; et al. TRIM21 negatively regulated Corynebacterium pseudotuberculosis-induced inflammation and is critical for the survival of C. pseudotuberculosis infected C57BL6 mice. Vet. Microbiol. 2021, 261, 109209. [Google Scholar] [CrossRef] [PubMed]
- Nagre, N.; Cong, X.; Terrazas, C.; Pepper, I.; Schreiber, J.M.; Fu, H.; Sill, J.M.; Christman, J.W.; Satoskar, A.R.; Zhao, X. Inhibition of Macrophage Complement Receptor CRIg by TRIM72 Polarizes Innate Immunity of the Lung. Am. J. Respir. Cell Mol. Biol. 2018, 58, 756–766. [Google Scholar] [CrossRef]
- Sheremet, A.B.; Zigangirova, N.A.; Zayakin, E.S.; Luyksaar, S.I.; Kapotina, L.N.; Nesterenko, L.N.; Kobets, N.V.; Gintsburg, A.L. Small molecule inhibitor of type three secretion system belonging to a class 2, 4-disubstituted-4H-[1, 3, 4]-thiadiazine-5-ones improves survival and decreases bacterial loads in an airway Pseudomonas aeruginosa infection in mice. BioMed. Res. Int. 2018, 2018, 5810767. [Google Scholar] [CrossRef]
- Ripa, K.T.; Mårdh, P.A. Cultivation of Chlamydia trachomatis in cycloheximide-treated mccoy cells. J. Clin. Microbiol. 1977, 6, 328–331. [Google Scholar] [CrossRef] [PubMed]
- Miyashita, N.; Matsumoto, A. Establishment of a particle-counting method for purified elementary bodies of chlamydiae and evaluation of sensitivities of the IDEIA Chlamydia kit and DNA probe by using the purified elementary bodies. J. Clin. Microbiol. 1992, 30, 2911–2916. [Google Scholar] [CrossRef]
- Campbell, L.A.; Kuo, C.C. Cultivation and laboratory maintenance of Chlamydia pneumoniae. Curr. Protoc. Microbiol. 2009, 12, 11B.1.14. [Google Scholar]
- Jiang, X.; Shen, C.; Yu, H.; Karunakaran, K.P.; Brunham, R.C. Differences in innate immune responses correlate with differences in murine susceptibility to Chlamydia muridarum pulmonary infection. Immunology 2010, 129, 556–566. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)). Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Smith, P.K.; Krohn, R.I.; Hermanson, G.T.; Mallia, A.K.; Gartner, F.H.; Provenzano, M.D.; Fujimoto, E.K.; Goeke, N.M.; Olson, B.J.; Klenk, D.C. Measurement of protein using bicinchoninic acid. Anal. Biochem. 1985, 150, 76–85. [Google Scholar] [CrossRef]
- Kim, D.; Paggi, J.M.; Park, C.; Bennett, C.; Salzberg, S.L. Graph-based genome alignment and genotyping with HISAT2 and HISAT-genotype. Nat. Biotechnol. 2019, 37, 907–915. [Google Scholar] [CrossRef]
- Danecek, P.; Bonfield, J.K.; Liddle, J.; Marshall, J.; Ohan, V.; Pollard, M.O.; Whitwham, A.; Keane, T.; McCarthy, S.A.; Davies, R.M.; et al. Twelve years of SAMtools and BCFtools. GigaScience 2021, 10, giab008. [Google Scholar] [CrossRef]
- Putri, G.H.; Anders, S.; Pyl, P.T.; Pimanda, J.E.; Zanini, F. Analysing high-throughput sequencing data in Python with HTSeq 2.0. Bioinformatics 2022, 38, 2943–2945. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023; Available online: https://www.R-project.org/ (accessed on 21 April 2021).
- Huang, B.; Baek, S.H. Trim13 Potentiates Toll-Like Receptor 2-Mediated Nuclear Factor κB Activation via K29-Linked Polyubiquitination of Tumor Necrosis Factor Receptor-Associated Factor 6. Mol. Pharmacol. 2017, 91, 307–316. [Google Scholar] [CrossRef] [PubMed]


| P. aeruginosa | Chlamydia spp. | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| A549/PA | Mouse Lung/ PA | U937/PA | Mouse LN/PA | A549/CP | PC-3/CT | Mouse Lung/CM | U937/CP | U937/CT | Mouse LN/CM | |
| A549/PA | 16 1, 17, 18, 19, 20, 21, 47, 52 2, 63 | 16, 17, 18, 19, 20, 21 | no | no | 16, 18, 19, 20, 21, 47, 63 | 16, 17, 47 | 19, 20, 21 | no | no | no |
| Mouse lung/PA | 16, 17, 18, 19, 20, 21 | 1, 6, 8, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 25, 26, 27, 30, 34, 56, 58, 68 | 8, 14, 68 | no | 1, 8, 13, 14, 15, 16, 18, 19, 20, 21, 25, 27, 56, 68 | 16, 17, 27 | 6, 8, 11, 14, 15, 19, 20, 21, 26, 30, 34, 56, 68 | 8, 14, 68 | 8, 14, 26, 68 | no |
| U937/PA | no | 8, 14, 68 | 8, 14, 68, 74 | no | 8, 14, 68, 74 | no | 8, 14, 68 | 8, 14, 68 | 8, 14, 68, 74 | no |
| Mouse LN/PA | no | no | no | no | no | no | no | no | no | no |
| A549/CP | 16, 18, 19, 20, 21, 47, 63 | 1, 8, 13, 14, 15, 16, 18, 19, 20, 21, 25, 27, 56, 68 | 8, 14, 68, 74 | no | 1, 4, 5, 8, 9, 13, 14, 15, 16, 18, 19, 20, 21, 22, 23, 25, 27, 31, 35, 36, 39, 47, 50, 55, 56, 61, 63, 65, 68, 69, 71, 74 | 16, 27, 47 | 8, 14, 15, 19, 20, 21, 56, 68 | 8, 14, 68 | 8, 14, 36, 68, 74 | no |
| PC-3/CT | no | 16, 17, 27 | no | no | 16, 27, 47 | 3, 16, 17, 27, 33, 37, 47 | no | no | no | no |
| Mouse lung/CM | 19, 20, 21 | 6, 8, 11, 14, 15, 19, 20, 21, 26, 30, 34, 56, 68 | 8, 14, 68 | no | 8, 14, 15, 19, 20, 21, 56, 68 | no | 6, 8, 11, 14, 15, 19, 20, 21, 26, 30, 34, 56, 68 | 8, 14, 68 | 8, 14, 26, 68 | no |
| U937/CP | no | 8, 14, 68 | 8, 14, 68 | no | 8, 14, 68 | no | 8, 14, 68 | 8, 14, 68 | 8, 14, 68 | no |
| U937/CT | no | 8, 14, 26, 68 | 8, 14, 68, 74 | no | 8, 14, 36, 68, 74 | no | 8, 14, 26, 68 | 8, 14, 68 | 8, 14, 26, 36, 68, 74 | no |
| Mouse LN/CM | no | no | no | no | no | no | no | no | no | no |
| P. aeruginosa | Chlamydia spp. | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| A549/PA | Mouse Lung/PA | U937/PA | Mouse LN/PA | A549/CP | PC-3/CT | Mouse Lung/CM | U937/CP | U937/CT | Mouse LN/CM | |
| A549/PA | 7 1 | 7 | no | 7 | no | no | 7 | no | no | 7 |
| Mouse lung/PA | 7 | 7, 63 | no | 7 | no | no | 7, 63 | no | no | 7 |
| U937/PA | no | no | 58, 59, 61 2, 65, 66, 71 | 59, 65 | no | no | 65 | 58 | 65 | 65 |
| Mouse LN/PA | 7 | 7 | 59, 65 | 7, 12, 13, 14, 17, 21, 26, 27, 28, 32, 37, 38, 41, 46, 47, 56, 59, 65, 72 | no | no | 7, 32, 65 | no | 65 | 7, 26, 27, 28, 32, 38, 41, 46, 47, 56, 65, 72 |
| A549/CP | no | no | no | no | 67 | no | 67 | no | no | 67 |
| PC-3/CT | no | no | no | no | no | no | no | no | no | no |
| Mouse lung/CM | 7 | 7, 63 | 65 | 7, 32, 65 | 67 | no | 1, 7, 32, 55, 63, 65, 67 | no | 65 | 7, 32, 65, 67 |
| U937/CP | no | no | 58 | no | no | no | no | 58 | no | no |
| U937/CT | no | no | 65 | 65 | no | no | 65 | no | 65 | 65 |
| Mouse LN/CM | 7 | 7 | 65 | 7, 26, 27, 28, 32, 38, 41, 46, 47, 56, 65, 72 | 67 | no | 7, 32, 65, 67 | no | 65 | 3, 7, 8, 18, 23, 24, 26, 27, 28, 32, 33, 35, 38, 39, 41, 45, 46, 47, 56, 65, 67, 72 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stepanenko, E.; Bondareva, N.; Sheremet, A.; Fedina, E.; Tikhomirov, A.; Gerasimova, T.; Poberezhniy, D.; Makarova, I.; Tarantul, V.; Zigangirova, N.; et al. Identification of Key TRIM Genes Involved in Response to Pseudomonas aeruginosa or Chlamydia spp. Infections in Human Cell Lines and in Mouse Organs. Int. J. Mol. Sci. 2023, 24, 13290. https://doi.org/10.3390/ijms241713290
Stepanenko E, Bondareva N, Sheremet A, Fedina E, Tikhomirov A, Gerasimova T, Poberezhniy D, Makarova I, Tarantul V, Zigangirova N, et al. Identification of Key TRIM Genes Involved in Response to Pseudomonas aeruginosa or Chlamydia spp. Infections in Human Cell Lines and in Mouse Organs. International Journal of Molecular Sciences. 2023; 24(17):13290. https://doi.org/10.3390/ijms241713290
Chicago/Turabian StyleStepanenko, Ekaterina, Natalia Bondareva, Anna Sheremet, Elena Fedina, Alexei Tikhomirov, Tatiana Gerasimova, Daniil Poberezhniy, Irina Makarova, Vyacheslav Tarantul, Nailya Zigangirova, and et al. 2023. "Identification of Key TRIM Genes Involved in Response to Pseudomonas aeruginosa or Chlamydia spp. Infections in Human Cell Lines and in Mouse Organs" International Journal of Molecular Sciences 24, no. 17: 13290. https://doi.org/10.3390/ijms241713290
APA StyleStepanenko, E., Bondareva, N., Sheremet, A., Fedina, E., Tikhomirov, A., Gerasimova, T., Poberezhniy, D., Makarova, I., Tarantul, V., Zigangirova, N., & Nenasheva, V. (2023). Identification of Key TRIM Genes Involved in Response to Pseudomonas aeruginosa or Chlamydia spp. Infections in Human Cell Lines and in Mouse Organs. International Journal of Molecular Sciences, 24(17), 13290. https://doi.org/10.3390/ijms241713290






