Using Fatty Acid-Binding Proteins as Potential Biomarkers to Discriminate between Parkinson’s Disease and Dementia with Lewy Bodies: Exploration of a Novel Technique
Abstract
:1. Introduction
2. Results
2.1. FABP3 as a Potential Biomarker for Lewy Body Diseases
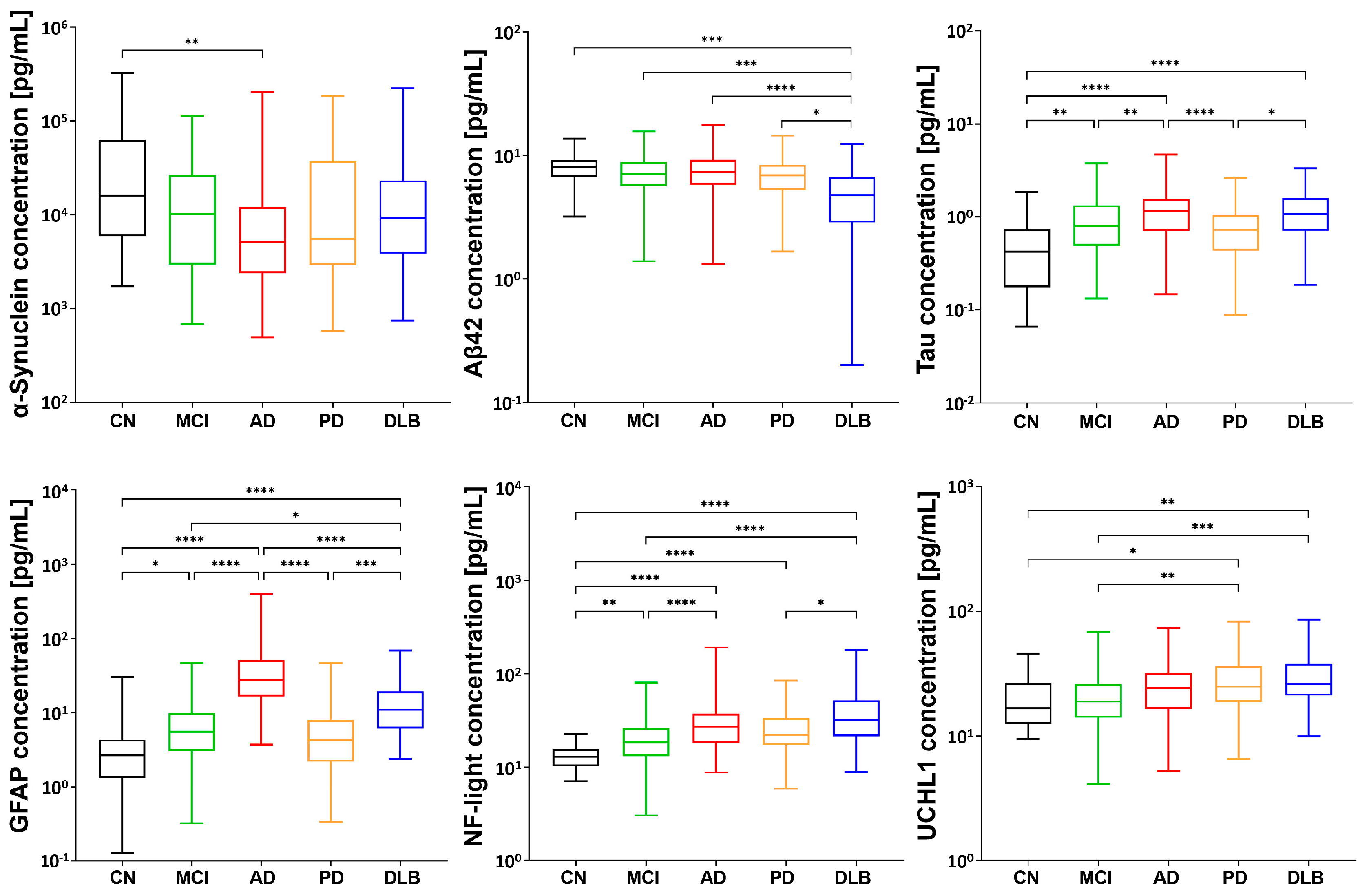
2.2. Distinctive Disease Profiling through Established Biomarkers
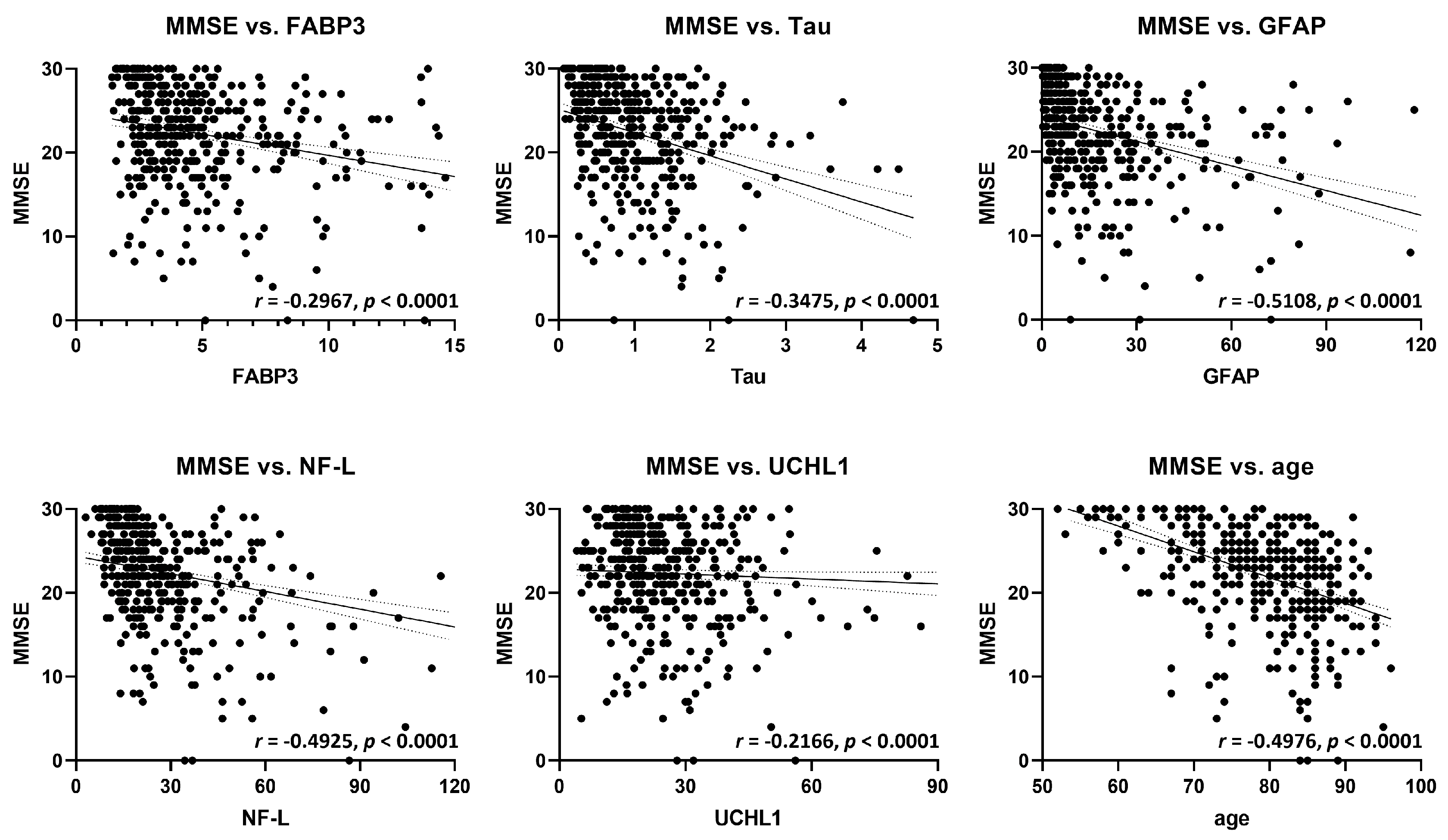
2.3. Correlation Analysis of FABP3 and Established Biomarkers with Cognitive Function
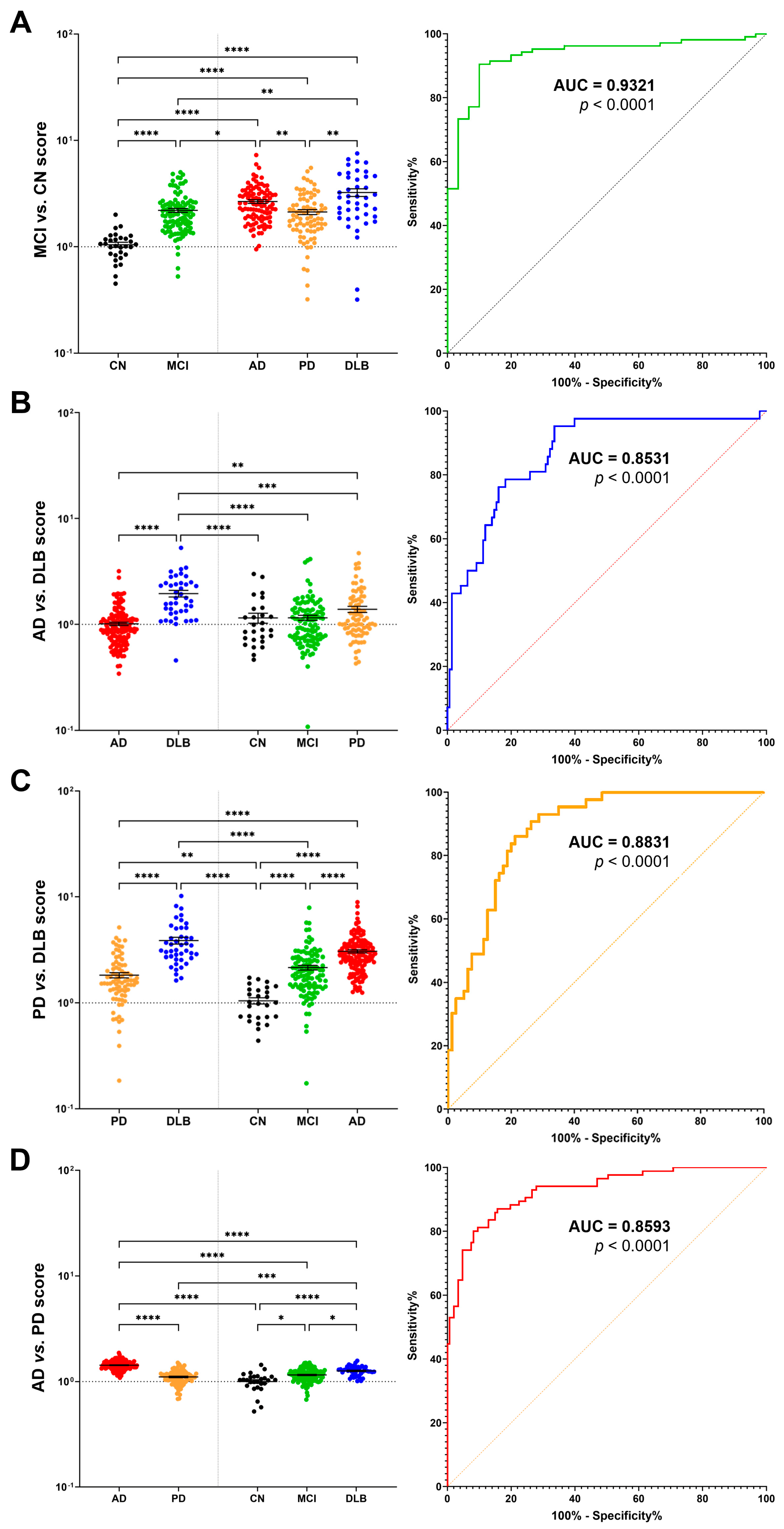
2.4. Multimarker Score for Accurate Disease Discrimination and Differential Diagnosis
3. Discussion
4. Materials and Methods
4.1. Study Population
4.2. Ethical Approval
4.3. Assays
4.4. Antibodies
4.5. Scoring Method
4.6. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shi, M.; Huber, B.R.; Zhang, J. Biomarkers for cognitive impairment in Parkinson disease. Brain Pathol. 2010, 20, 660–671. [Google Scholar] [CrossRef]
- Sezgin, M.; Bilgic, B.; Tinaz, S.; Emre, M. Parkinson’s Disease Dementia and Lewy Body Disease. Semin. Neurol. 2019, 39, 274–282. [Google Scholar] [CrossRef]
- Oizumi, H.; Yamasaki, K.; Suzuki, H.; Hasegawa, T.; Sugimura, Y.; Baba, T.; Fukunaga, K.; Takeda, A. Fatty Acid-Binding Protein 3 Expression in the Brain and Skin in Human Synucleinopathies. Front. Aging Neurosci. 2021, 13, 648982. [Google Scholar] [CrossRef] [PubMed]
- Kawahata, I.; Bousset, L.; Melki, R.; Fukunaga, K. Fatty Acid-Binding Protein 3 is Critical for alpha-Synuclein Uptake and MPP(+)-Induced Mitochondrial Dysfunction in Cultured Dopaminergic Neurons. Int. J. Mol. Sci. 2019, 20, 5358. [Google Scholar] [CrossRef] [PubMed]
- Fukui, N.; Yamamoto, H.; Miyabe, M.; Aoyama, Y.; Hongo, K.; Mizobata, T.; Kawahata, I.; Yabuki, Y.; Shinoda, Y.; Fukunaga, K.; et al. An alpha-synuclein decoy peptide prevents cytotoxic alpha-synuclein aggregation caused by fatty acid binding protein 3. J. Biol. Chem. 2021, 296, 100663. [Google Scholar] [CrossRef] [PubMed]
- Yabuki, Y.; Matsuo, K.; Kawahata, I.; Fukui, N.; Mizobata, T.; Kawata, Y.; Owada, Y.; Shioda, N.; Fukunaga, K. Fatty Acid Binding Protein 3 Enhances the Spreading and Toxicity of alpha-Synuclein in Mouse Brain. Int. J. Mol. Sci. 2020, 21, 2230. [Google Scholar] [CrossRef]
- Kawahata, I.; Sekimori, T.; Wang, H.; Wang, Y.; Sasaoka, T.; Bousset, L.; Melki, R.; Mizobata, T.; Kawata, Y.; Fukunaga, K. Dopamine D2 Long Receptors Are Critical for Caveolae-Mediated alpha-Synuclein Uptake in Cultured Dopaminergic Neurons. Biomedicines 2021, 9, 49. [Google Scholar] [CrossRef]
- Matsuo, K.; Kawahata, I.; Melki, R.; Bousset, L.; Owada, Y.; Fukunaga, K. Suppression of alpha-synuclein propagation after intrastriatal injection in FABP3 null mice. Brain Res. 2021, 1760, 147383. [Google Scholar] [CrossRef] [PubMed]
- Kawahata, I.; Fukunaga, K. Degradation of Tyrosine Hydroxylase by the Ubiquitin-Proteasome System in the Pathogenesis of Parkinson’s Disease and Dopa-Responsive Dystonia. Int. J. Mol. Sci. 2020, 21, 3779. [Google Scholar] [CrossRef]
- Haga, H.; Yamada, R.; Izumi, H.; Shinoda, Y.; Kawahata, I.; Miyachi, H.; Fukunaga, K. Novel fatty acid-binding protein 3 ligand inhibits dopaminergic neuronal death and improves motor and cognitive impairments in Parkinson’s disease model mice. Pharmacol. Biochem. Behav. 2020, 191, 172891. [Google Scholar] [CrossRef]
- Wang, H.; Fukunaga, K.; Cheng, A.; Wang, Y.; Arimura, N.; Yoshino, H.; Sasaki, T.; Kawahata, I. Novel FABP3 ligand, HY-11-9, ameliorates neuropathological deficits in MPTP-induced Parkinsonism in mice. J. Pharmacol. Sci. 2023, 152, 30–38. [Google Scholar] [CrossRef]
- Guo, Q.; Kawahata, I.; Jia, W.; Wang, H.; Cheng, A.; Yabuki, Y.; Shioda, N.; Fukunaga, K. alpha-Synuclein decoy peptide protects mice against alpha-synuclein-induced memory loss. CNS Neurosci. Ther. 2023, 29, 1547–1560. [Google Scholar] [CrossRef]
- Guo, Q.; Kawahata, I.; Cheng, A.; Wang, H.; Jia, W.; Yoshino, H.; Fukunaga, K. Fatty acid-binding proteins 3 and 5 are involved in the initiation of mitochondrial damage in ischemic neurons. Redox Biol 2023, 59, 102547. [Google Scholar] [CrossRef]
- Wang, Y.; Shinoda, Y.; Cheng, A.; Kawahata, I.; Fukunaga, K. Epidermal Fatty Acid-Binding Protein 5 (FABP5) Involvement in Alpha-Synuclein-Induced Mitochondrial Injury under Oxidative Stress. Biomedicines 2021, 9, 110. [Google Scholar] [CrossRef]
- Guo, Q.; Kawahata, I.; Degawa, T.; Ikeda-Matsuo, Y.; Sun, M.; Han, F.; Fukunaga, K. Fatty Acid-Binding Proteins Aggravate Cerebral Ischemia-Reperfusion Injury in Mice. Biomedicines 2021, 9, 529. [Google Scholar] [CrossRef] [PubMed]
- Cheng, A.; Jia, W.; Kawahata, I.; Fukunaga, K. A novel fatty acid-binding protein 5 and 7 inhibitor ameliorates oligodendrocyte injury in multiple sclerosis mouse models. EBioMedicine 2021, 72, 103582. [Google Scholar] [CrossRef] [PubMed]
- Cheng, A.; Wang, Y.F.; Shinoda, Y.; Kawahata, I.; Yamamoto, T.; Jia, W.B.; Yamamoto, H.; Mizobata, T.; Kawata, Y.; Fukunaga, K. Fatty acid-binding protein 7 triggers alpha-synuclein oligomerization in glial cells and oligodendrocytes associated with oxidative stress. Acta Pharmacol. Sin. 2021, 43, 552–562. [Google Scholar] [CrossRef] [PubMed]
- Cheng, A.; Kawahata, I.; Wang, Y.; Jia, W.; Haoyang, W.; Sekimori, T.; Chen, Y.; Suzuki, H.; Takeda, A.; Stefanova, N.; et al. Epsin2, a novel target for multiple system atrophy therapy via alpha-synuclein/FABP7 propagation. Brain 2023, 146, 3172–3180. [Google Scholar] [CrossRef]
- Backstrom, D.C.; Eriksson Domellof, M.; Linder, J.; Olsson, B.; Ohrfelt, A.; Trupp, M.; Zetterberg, H.; Blennow, K.; Forsgren, L. Cerebrospinal Fluid Patterns and the Risk of Future Dementia in Early, Incident Parkinson Disease. JAMA Neurol. 2015, 72, 1175–1182. [Google Scholar] [CrossRef]
- Walker, L.; Stefanis, L.; Attems, J. Clinical and neuropathological differences between Parkinson’s disease, Parkinson’s disease dementia and dementia with Lewy bodies—current issues and future directions. J. Neurochem. 2019, 150, 467–474. [Google Scholar] [CrossRef]
- Jellinger, K.A.; Korczyn, A.D. Are dementia with Lewy bodies and Parkinson’s disease dementia the same disease? BMC Med. 2018, 16, 34. [Google Scholar] [CrossRef]
- Mollenhauer, B.; Cullen, V.; Kahn, I.; Krastins, B.; Outeiro, T.F.; Pepivani, I.; Ng, J.; Schulz-Schaeffer, W.; Kretzschmar, H.A.; McLean, P.J.; et al. Direct quantification of CSF alpha-synuclein by ELISA and first cross-sectional study in patients with neurodegeneration. Exp. Neurol. 2008, 213, 315–325. [Google Scholar] [CrossRef]
- Mollenhauer, B.; Steinacker, P.; Bahn, E.; Bibl, M.; Brechlin, P.; Schlossmacher, M.G.; Locascio, J.J.; Wiltfang, J.; Kretzschmar, H.A.; Poser, S.; et al. Serum heart-type fatty acid-binding protein and cerebrospinal fluid tau: Marker candidates for dementia with Lewy bodies. Neurodegener. Dis. 2007, 4, 366–375. [Google Scholar] [CrossRef] [PubMed]
- Tokuda, T.; Salem, S.A.; Allsop, D.; Mizuno, T.; Nakagawa, M.; Qureshi, M.M.; Locascio, J.J.; Schlossmacher, M.G.; El-Agnaf, O.M. Decreased alpha-synuclein in cerebrospinal fluid of aged individuals and subjects with Parkinson’s disease. Biochem. Biophys. Res. Commun. 2006, 349, 162–166. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.X.; Mok, S.S.; Laughton, K.M.; McLean, C.A.; Cappai, R.; Masters, C.L.; Culvenor, J.G.; Horne, M.K. Plasma alpha-synuclein is decreased in subjects with Parkinson’s disease. Exp. Neurol. 2007, 204, 583–588. [Google Scholar] [CrossRef]
- Bougea, A.; Stefanis, L.; Paraskevas, G.P.; Emmanouilidou, E.; Vekrelis, K.; Kapaki, E. Plasma alpha-synuclein levels in patients with Parkinson’s disease: A systematic review and meta-analysis. Neurol. Sci. 2019, 40, 929–938. [Google Scholar] [CrossRef] [PubMed]
- Calabresi, P.; Mechelli, A.; Natale, G.; Volpicelli-Daley, L.; Di Lazzaro, G.; Ghiglieri, V. Alpha-synuclein in Parkinson’s disease and other synucleinopathies: From overt neurodegeneration back to early synaptic dysfunction. Cell Death Dis. 2023, 14, 176. [Google Scholar] [CrossRef]
- Lin, C.H.; Yang, S.Y.; Horng, H.E.; Yang, C.C.; Chieh, J.J.; Chen, H.H.; Liu, B.H.; Chiu, M.J. Plasma alpha-synuclein predicts cognitive decline in Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 2017, 88, 818–824. [Google Scholar] [CrossRef]
- Wood, H. Parkinson disease: Plasma alpha-synuclein—A potential marker of cognitive impairment in Parkinson disease. Nat. Rev. Neurol. 2017, 13, 450. [Google Scholar]
- Senanarong, V.; Wachirutmangur, L.; Rattanabunnakit, C.; Srivanitchapoom, P.; Udomphanthurak, S. Plasma alpha synuclein (a-syn) as a potential biomarker of diseases with synucleinopathy. Alzheimer’s Dement. 2020, 16, e044409. [Google Scholar] [CrossRef]
- Okuzumi, A.; Hatano, T.; Matsumoto, G.; Nojiri, S.; Ueno, S.I.; Imamichi-Tatano, Y.; Kimura, H.; Kakuta, S.; Kondo, A.; Fukuhara, T.; et al. Propagative alpha-synuclein seeds as serum biomarkers for synucleinopathies. Nat. Med. 2023, 29, 1448–1455. [Google Scholar] [CrossRef]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef]
- Hughes, C.P.; Berg, L.; Danziger, W.L.; Coben, L.A.; Martin, R.L. A new clinical scale for the staging of dementia. Br. J. Psychiatry 1982, 140, 566–572. [Google Scholar] [CrossRef] [PubMed]
- Goetz, C.G.; Tilley, B.C.; Shaftman, S.R.; Stebbins, G.T.; Fahn, S.; Martinez-Martin, P.; Poewe, W.; Sampaio, C.; Stern, M.B.; Dodel, R.; et al. Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): Scale presentation and clinimetric testing results. Mov. Disord. 2008, 23, 2129–2170. [Google Scholar] [CrossRef] [PubMed]
- Pagani, F.; Bonora, R.; Bonetti, G.; Panteghini, M. Evaluation of a sandwich enzyme-linked immunosorbent assay for the measurement of serum heart fatty acid-binding protein. Ann. Clin. Biochem. 2002, 39 Pt 4, 404–405. [Google Scholar] [CrossRef] [PubMed]
- Bland, M. An Introduction to Medical Statistics; Oxford University Press: Oxford, UK, 2015. [Google Scholar]
- Tønnesen, S.; Kaufmann, T.; Doan, N.T.; Alnaes, D.; Cordova-Palomera, A.; Meer, D.V.; Rokicki, J.; Moberget, T.; Gurholt, T.P.; Haukvik, U.K.; et al. White matter aberrations and age-related trajectories in patients with schizophrenia and bipolar disorder revealed by diffusion tensor imaging. Sci. Rep. 2018, 8, 14129. [Google Scholar] [CrossRef]
- Steckel, M.L. Intelligence and Birth Order in Family. J. Soc. Psychol. 1930, 1, 329–344. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kawahata, I.; Sekimori, T.; Oizumi, H.; Takeda, A.; Fukunaga, K. Using Fatty Acid-Binding Proteins as Potential Biomarkers to Discriminate between Parkinson’s Disease and Dementia with Lewy Bodies: Exploration of a Novel Technique. Int. J. Mol. Sci. 2023, 24, 13267. https://doi.org/10.3390/ijms241713267
Kawahata I, Sekimori T, Oizumi H, Takeda A, Fukunaga K. Using Fatty Acid-Binding Proteins as Potential Biomarkers to Discriminate between Parkinson’s Disease and Dementia with Lewy Bodies: Exploration of a Novel Technique. International Journal of Molecular Sciences. 2023; 24(17):13267. https://doi.org/10.3390/ijms241713267
Chicago/Turabian StyleKawahata, Ichiro, Tomoki Sekimori, Hideki Oizumi, Atsushi Takeda, and Kohji Fukunaga. 2023. "Using Fatty Acid-Binding Proteins as Potential Biomarkers to Discriminate between Parkinson’s Disease and Dementia with Lewy Bodies: Exploration of a Novel Technique" International Journal of Molecular Sciences 24, no. 17: 13267. https://doi.org/10.3390/ijms241713267
APA StyleKawahata, I., Sekimori, T., Oizumi, H., Takeda, A., & Fukunaga, K. (2023). Using Fatty Acid-Binding Proteins as Potential Biomarkers to Discriminate between Parkinson’s Disease and Dementia with Lewy Bodies: Exploration of a Novel Technique. International Journal of Molecular Sciences, 24(17), 13267. https://doi.org/10.3390/ijms241713267






