Therapeutic Aspects of Prunus cerasus Extract in a Rabbit Model of Atherosclerosis-Associated Diastolic Dysfunction
Abstract
:1. Introduction
2. Results
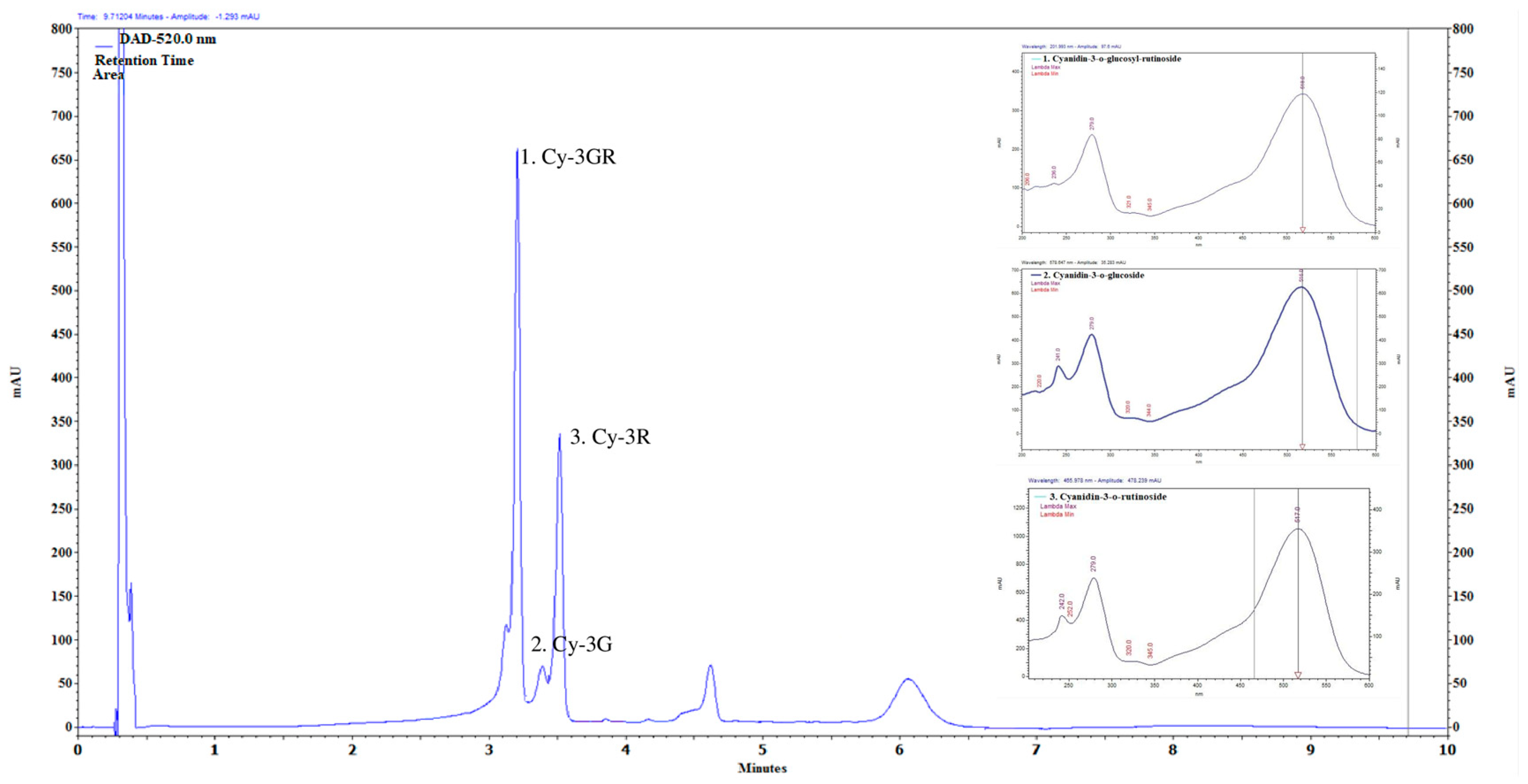
2.1. Main Compounds of Anthocyanin-Rich PCE
2.2. PCE Treatment Moderates Diet-Induced Dyslipidemia
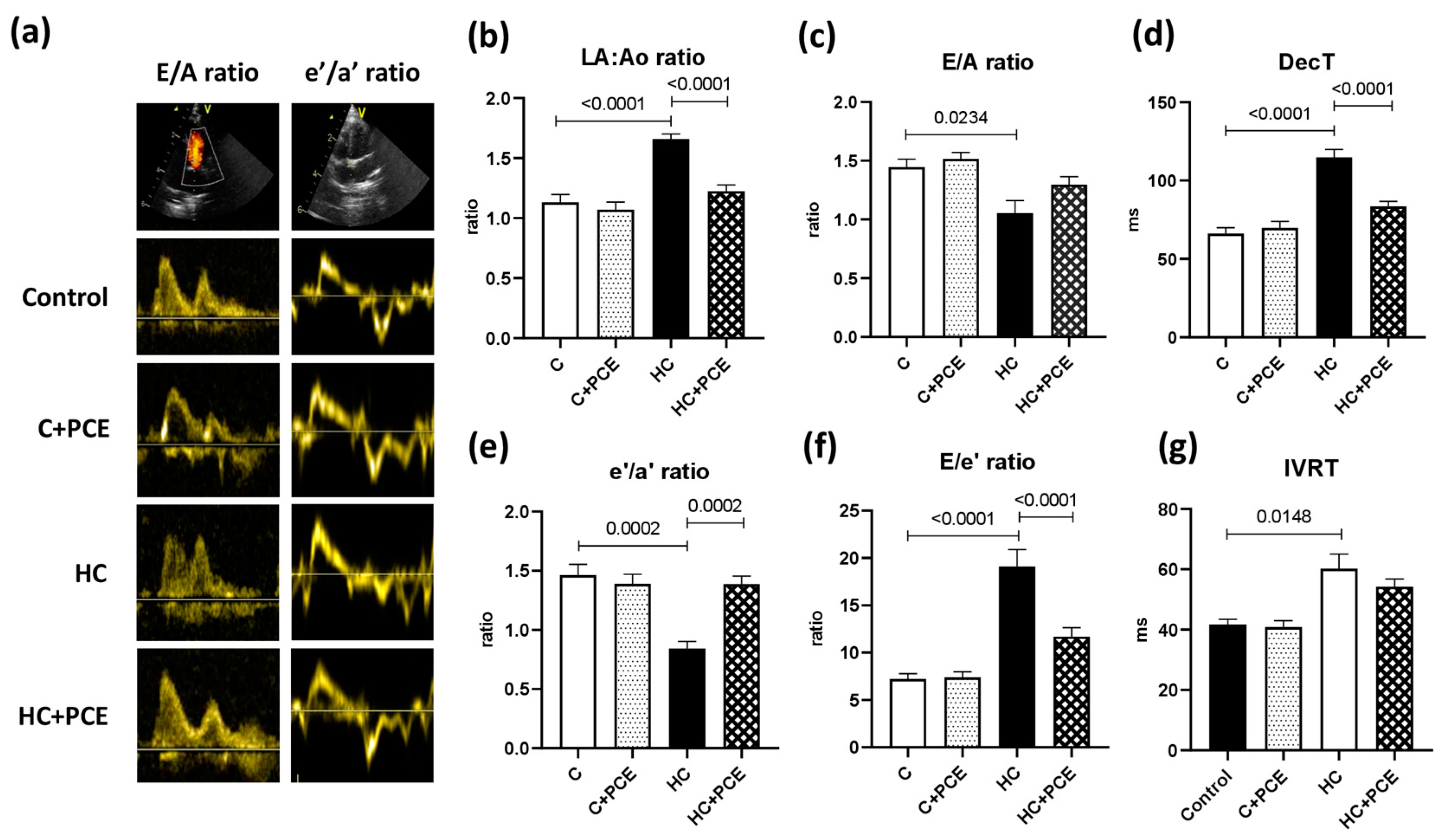
2.3. Atherosclerosis-Associated Cardiac Dysfunction Was Attenuated by Anthocyanin Administration
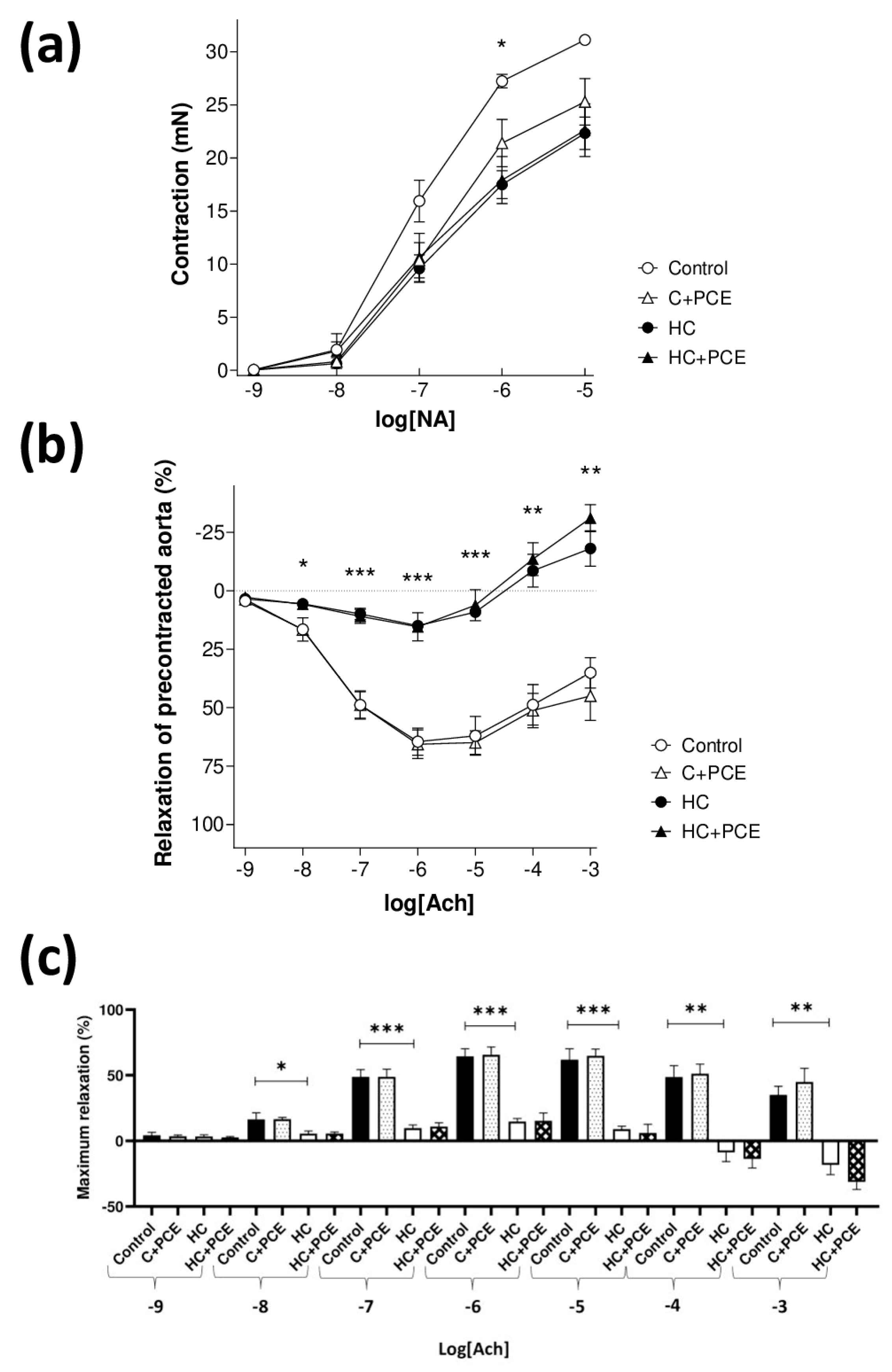
2.4. Anthocyanin-Rich PCE Did Not Improve the Endothelial Dysfunction Induced by Atherogenic Diet
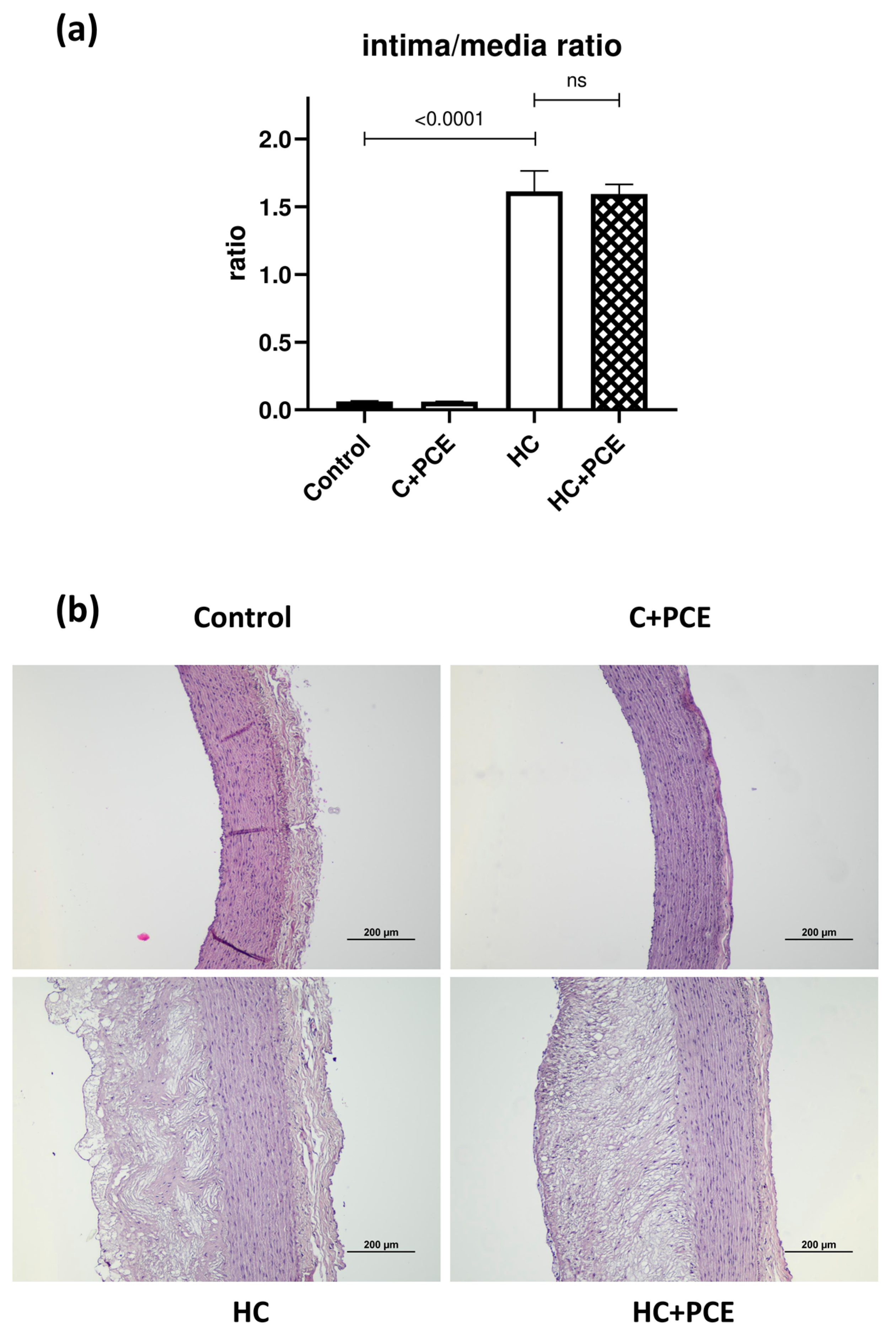
2.5. Already Existing Atherosclerotic Plaque Coverage Was Not Reduced by the PCE Treatment
2.6. PCE Administration Restored the Myocardial eNOS, PKG, SERCA2a, and HO-1 Levels Altered by the Cholesterol-Rich Diet
3. Discussion
3.1. Diastolic Dysfunction
3.2. Dyslipidemia and Lipid Profile
3.3. Endothelial Dysfunction and Atherosclerotic Plaque Coverage
3.4. Upregulation of the NO-cGMP-PKG Pathway Correlates with the Restoration of the Cardiac Function
3.5. Heme Oxygenase-1 Overexpression May Act as a Double-Edged Sword
4. Materials and Methods
4.1. Anthocyanins
4.2. Animals
4.3. Study Design
4.4. Serum Parameters
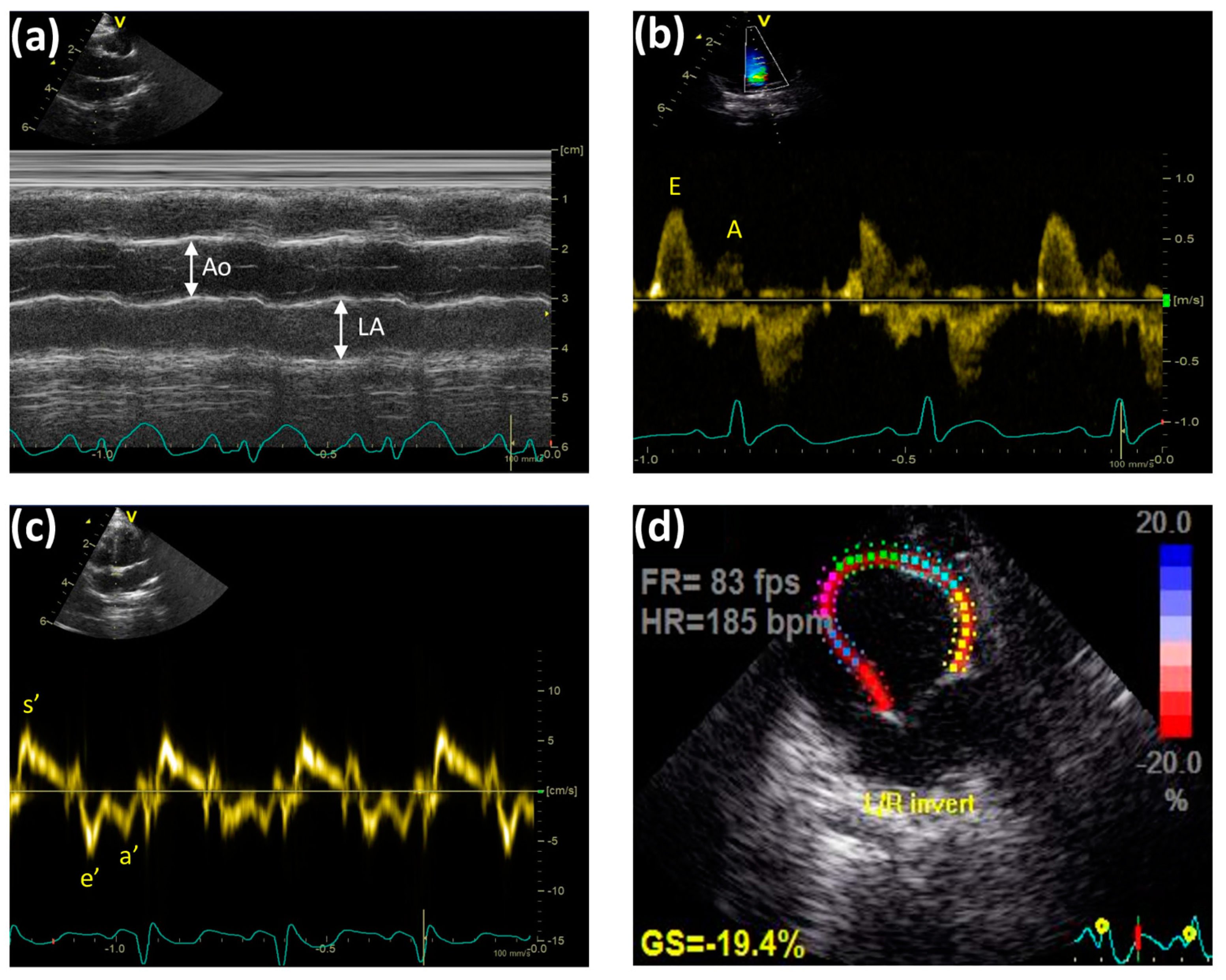
4.5. Transthoracic Echocardiographic Technique
4.6. Functional Vascular Assays
4.7. Western Blot
4.8. Histology
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Conrad, N.; Judge, A.; Tran, J.; Mohseni, H.; Hedgecott, D.; Crespillo, A.P.; Allison, M.; Hemingway, H.; Cleland, J.G.; McMurray, J.J.V.; et al. Temporal trends and patterns in heart failure incidence: A population-based study of 4 million individuals. Lancet 2018, 391, 572–580. [Google Scholar] [CrossRef] [PubMed]
- Dunlay, S.M.; Roger, V.L. Understanding the epidemic of heart failure: Past, present, and future. Curr. Heart Fail. Rep. 2014, 11, 404–415. [Google Scholar] [CrossRef]
- Roth, G.A.; Forouzanfar, M.H.; Moran, A.E.; Barber, R.; Nguyen, G.; Feigin, V.L.; Naghavi, M.; Mensah, G.A.; Murray, C.J. Demographic and epidemiologic drivers of global cardiovascular mortality. N. Engl. J. Med. 2015, 372, 1333–1341. [Google Scholar] [CrossRef] [PubMed]
- Deswal, A. Diastolic dysfunction and diastolic heart failure: Mechanisms and epidemiology. Curr. Cardiol. Rep. 2005, 7, 178–183. [Google Scholar] [CrossRef] [PubMed]
- Disease, G.B.D.; Injury, I.; Prevalence, C. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1789–1858. [Google Scholar] [CrossRef]
- Katz, A.M.; Zile, M.R. New molecular mechanism in diastolic heart failure. Circulation 2006, 113, 1922–1925. [Google Scholar] [CrossRef] [PubMed]
- van Heerebeek, L.; Hamdani, N.; Falcao-Pires, I.; Leite-Moreira, A.F.; Begieneman, M.P.; Bronzwaer, J.G.; van der Velden, J.; Stienen, G.J.; Laarman, G.J.; Somsen, A.; et al. Low myocardial protein kinase G activity in heart failure with preserved ejection fraction. Circulation 2012, 126, 830–839. [Google Scholar] [CrossRef]
- Forstermann, U.; Sessa, W.C. Nitric oxide synthases: Regulation and function. Eur. Heart J. 2012, 33, 829–837. [Google Scholar] [CrossRef]
- Cai, Z.; Wu, C.; Xu, Y.; Cai, J.; Zhao, M.; Zu, L. The NO-cGMP-PKG Axis in HFpEF: From Pathological Mechanisms to Potential Therapies. Aging Dis. 2023, 14, 46–62. [Google Scholar] [CrossRef]
- Zile, M.R.; Brutsaert, D.L. New concepts in diastolic dysfunction and diastolic heart failure: Part II: Causal mechanisms and treatment. Circulation 2002, 105, 1503–1508. [Google Scholar] [CrossRef]
- Marchio, P.; Guerra-Ojeda, S.; Vila, J.M.; Aldasoro, M.; Victor, V.M.; Mauricio, M.D. Targeting Early Atherosclerosis: A Focus on Oxidative Stress and Inflammation. Oxid. Med. Cell Longev. 2019, 2019, 8563845. [Google Scholar] [CrossRef] [PubMed]
- Franssen, C.; Chen, S.; Unger, A.; Korkmaz, H.I.; De Keulenaer, G.W.; Tschope, C.; Leite-Moreira, A.F.; Musters, R.; Niessen, H.W.; Linke, W.A.; et al. Myocardial Microvascular Inflammatory Endothelial Activation in Heart Failure With Preserved Ejection Fraction. JACC Heart Fail. 2016, 4, 312–324. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Liu, J.; Duan, H.; Li, R.; Peng, W.; Wu, C. Activation of Nrf2/HO-1 signaling: An important molecular mechanism of herbal medicine in the treatment of atherosclerosis via the protection of vascular endothelial cells from oxidative stress. J. Adv. Res. 2021, 34, 43–63. [Google Scholar] [CrossRef] [PubMed]
- Fiorelli, S.; Porro, B.; Cosentino, N.; Di Minno, A.; Manega, C.M.; Fabbiocchi, F.; Niccoli, G.; Fracassi, F.; Barbieri, S.; Marenzi, G.; et al. Activation of Nrf2/HO-1 Pathway and Human Atherosclerotic Plaque Vulnerability:an In Vitro and In Vivo Study. Cells 2019, 8, 356. [Google Scholar] [CrossRef] [PubMed]
- Reis, J.F.; Monteiro, V.V.; de Souza Gomes, R.; do Carmo, M.M.; da Costa, G.V.; Ribera, P.C.; Monteiro, M.C. Action mechanism and cardiovascular effect of anthocyanins: A systematic review of animal and human studies. J. Transl. Med. 2016, 14, 315. [Google Scholar] [CrossRef]
- Krga, I.; Milenkovic, D. Anthocyanins: From Sources and Bioavailability to Cardiovascular-Health Benefits and Molecular Mechanisms of Action. J. Agric. Food Chem. 2019, 67, 1771–1783. [Google Scholar] [CrossRef]
- Kalt, W.; Cassidy, A.; Howard, L.R.; Krikorian, R.; Stull, A.J.; Tremblay, F.; Zamora-Ros, R. Recent Research on the Health Benefits of Blueberries and Their Anthocyanins. Adv. Nutr. 2020, 11, 224–236. [Google Scholar] [CrossRef]
- Aboonabi, A.; Singh, I.; Rose’ Meyer, R. Cytoprotective effects of berry anthocyanins against induced oxidative stress and inflammation in primary human diabetic aortic endothelial cells. Chem. Biol. Interact. 2020, 317, 108940. [Google Scholar] [CrossRef]
- Wang, L.S.; Stoner, G.D. Anthocyanins and their role in cancer prevention. Cancer Lett. 2008, 269, 281–290. [Google Scholar] [CrossRef]
- Li, D.; Zhang, Y.; Liu, Y.; Sun, R.; Xia, M. Purified anthocyanin supplementation reduces dyslipidemia, enhances antioxidant capacity, and prevents insulin resistance in diabetic patients. J. Nutr. 2015, 145, 742–748. [Google Scholar] [CrossRef]
- Pojer, E.; Mattivi, F.; Johnson, D.; Stockley, C.S. The Case for Anthocyanin Consumption to Promote Human Health: A Review. Compr. Rev. Food Sci. Food Saf. 2013, 12, 483–508. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Yu, Z.; Tang, Q.; Song, H.; Gao, Z.; Chen, W.; Zheng, X. Honeysuckle anthocyanin supplementation prevents diet-induced obesity in C57BL/6 mice. Food Funct. 2013, 4, 1654–1661. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Ling, W.; Guo, H.; Song, F.; Ye, Q.; Zou, T.; Li, D.; Zhang, Y.; Li, G.; Xiao, Y.; et al. Anti-inflammatory effect of purified dietary anthocyanin in adults with hypercholesterolemia: A randomized controlled trial. Nutr. Metab. Cardiovasc. Dis. 2013, 23, 843–849. [Google Scholar] [CrossRef]
- Xu, L.; Tian, Z.; Chen, H.; Zhao, Y.; Yang, Y. Anthocyanins, Anthocyanin-Rich Berries, and Cardiovascular Risks: Systematic Review and Meta-Analysis of 44 Randomized Controlled Trials and 15 Prospective Cohort Studies. Front. Nutr. 2021, 8, 747884. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Xia, M.; Yang, Y.; Liu, F.; Li, Z.; Hao, Y.; Mi, M.; Jin, T.; Ling, W. Purified anthocyanin supplementation improves endothelial function via NO-cGMP activation in hypercholesterolemic individuals. Clin. Chem. 2011, 57, 1524–1533. [Google Scholar] [CrossRef]
- Parzonko, A.; Oswit, A.; Bazylko, A.; Naruszewicz, M. Anthocyans-rich Aronia melanocarpa extract possesses ability to protect endothelial progenitor cells against angiotensin II induced dysfunction. Phytomedicine 2015, 22, 1238–1246. [Google Scholar] [CrossRef]
- Getz, G.S.; Reardon, C.A. Animal models of atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 1104–1115. [Google Scholar] [CrossRef]
- Yin, W.; Carballo-Jane, E.; McLaren, D.G.; Mendoza, V.H.; Gagen, K.; Geoghagen, N.S.; McNamara, L.A.; Gorski, J.N.; Eiermann, G.J.; Petrov, A.; et al. Plasma lipid profiling across species for the identification of optimal animal models of human dyslipidemia. J. Lipid Res. 2012, 53, 51–65. [Google Scholar] [CrossRef]
- Pogwizd, S.M.; Bers, D.M. Rabbit models of heart disease. Drug Discov. Today Dis. Models 2008, 5, 185–193. [Google Scholar] [CrossRef]
- Nagueh, S.F.; Smiseth, O.A.; Appleton, C.P.; Byrd, B.F., 3rd; Dokainish, H.; Edvardsen, T.; Flachskampf, F.A.; Gillebert, T.C.; Klein, A.L.; Lancellotti, P.; et al. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2016, 29, 277–314. [Google Scholar] [CrossRef]
- Sharifov, O.F.; Schiros, C.G.; Aban, I.; Denney, T.S.; Gupta, H. Diagnostic Accuracy of Tissue Doppler Index E/e’ for Evaluating Left Ventricular Filling Pressure and Diastolic Dysfunction/Heart Failure With Preserved Ejection Fraction: A Systematic Review and Meta-Analysis. J. Am. Heart Assoc. 2016, 5, e002530. [Google Scholar] [CrossRef] [PubMed]
- Douglas, P.S. The left atrium: A biomarker of chronic diastolic dysfunction and cardiovascular disease risk. J. Am. Coll. Cardiol. 2003, 42, 1206–1207. [Google Scholar] [CrossRef] [PubMed]
- Bruch, C.; Schmermund, A.; Dagres, N.; Katz, M.; Bartel, T.; Erbel, R. Tei-Index in coronary artery disease--validation in patients with overall cardiac and isolated diastolic dysfunction. Z. Kardiol. 2002, 91, 472–480. [Google Scholar] [CrossRef]
- Stange, E.; Agostini, B.; Paenberg, J. Changes in rabbit lipoprotein properties by dietary cholesterol, and saturated and polyunsaturated fats. Atherosclerosis 1975, 22, 125–148. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Xia, M.; Ma, J.; Hao, Y.; Liu, J.; Mou, H.; Cao, L.; Ling, W. Anthocyanin supplementation improves serum LDL- and HDL-cholesterol concentrations associated with the inhibition of cholesteryl ester transfer protein in dyslipidemic subjects. Am. J. Clin. Nutr. 2009, 90, 485–492. [Google Scholar] [CrossRef] [PubMed]
- Vogel, R.A.; Corretti, M.C.; Gellman, J. Cholesterol, cholesterol lowering, and endothelial function. Prog. Cardiovasc. Dis. 1998, 41, 117–136. [Google Scholar] [CrossRef]
- Ludmer, P.L.; Selwyn, A.P.; Shook, T.L.; Wayne, R.R.; Mudge, G.H.; Alexander, R.W.; Ganz, P. Paradoxical vasoconstriction induced by acetylcholine in atherosclerotic coronary arteries. N. Engl. J. Med. 1986, 315, 1046–1051. [Google Scholar] [CrossRef]
- Davignon, J.; Ganz, P. Role of endothelial dysfunction in atherosclerosis. Circulation 2004, 109, III-27–III-32. [Google Scholar] [CrossRef]
- Chen, J.Y.; Ye, Z.X.; Wang, X.F.; Chang, J.; Yang, M.W.; Zhong, H.H.; Hong, F.F.; Yang, S.L. Nitric oxide bioavailability dysfunction involves in atherosclerosis. Biomed. Pharmacother. 2018, 97, 423–428. [Google Scholar] [CrossRef]
- Paulus, W.J.; Tschope, C. A novel paradigm for heart failure with preserved ejection fraction: Comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J. Am. Coll. Cardiol. 2013, 62, 263–271. [Google Scholar] [CrossRef]
- Huang, Y.; Walker, K.E.; Hanley, F.; Narula, J.; Houser, S.R.; Tulenko, T.N. Cardiac systolic and diastolic dysfunction after a cholesterol-rich diet. Circulation 2004, 109, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.W.; Ikeda, K.; Yamori, Y. Upregulation of endothelial nitric oxide synthase by cyanidin-3-glucoside, a typical anthocyanin pigment. Hypertension 2004, 44, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Sozanski, T.; Kucharska, A.Z.; Wisniewski, J.; Fleszar, M.G.; Rapak, A.; Gomulkiewicz, A.; Dziegiel, P.; Magdalan, J.; Nowak, B.; Szumny, D.; et al. The iridoid loganic acid and anthocyanins from the cornelian cherry (Cornus mas L.) fruit increase the plasma l-arginine/ADMA ratio and decrease levels of ADMA in rabbits fed a high-cholesterol diet. Phytomedicine 2019, 52, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Quintieri, A.M.; Baldino, N.; Filice, E.; Seta, L.; Vitetti, A.; Tota, B.; De Cindio, B.; Cerra, M.C.; Angelone, T. Malvidin, a red wine polyphenol, modulates mammalian myocardial and coronary performance and protects the heart against ischemia/reperfusion injury. J. Nutr. Biochem. 2013, 24, 1221–1231. [Google Scholar] [CrossRef] [PubMed]
- Otterbein, L.E.; Foresti, R.; Motterlini, R. Heme Oxygenase-1 and Carbon Monoxide in the Heart: The Balancing Act Between Danger Signaling and Pro-Survival. Circ. Res. 2016, 118, 1940–1959. [Google Scholar] [CrossRef] [PubMed]
- Chiang, S.K.; Chen, S.E.; Chang, L.C. The Role of HO-1 and Its Crosstalk with Oxidative Stress in Cancer Cell Survival. Cells 2021, 10, 2401. [Google Scholar] [CrossRef]
- Csonka, C.; Sarkozy, M.; Pipicz, M.; Dux, L.; Csont, T. Modulation of Hypercholesterolemia-Induced Oxidative/Nitrative Stress in the Heart. Oxid. Med. Cell Longev. 2016, 2016, 3863726. [Google Scholar] [CrossRef]
- Kishimoto, Y.; Kondo, K.; Momiyama, Y. The Protective Role of Heme Oxygenase-1 in Atherosclerotic Diseases. Int. J. Mol. Sci. 2019, 20, 3628. [Google Scholar] [CrossRef]
- Barbagallo, I.; Galvano, F.; Frigiola, A.; Cappello, F.; Riccioni, G.; Murabito, P.; D’Orazio, N.; Torella, M.; Gazzolo, D.; Li Volti, G. Potential therapeutic effects of natural heme oxygenase-1 inducers in cardiovascular diseases. Antioxid. Redox Signal. 2013, 18, 507–521. [Google Scholar] [CrossRef]
- Waza, A.A.; Hamid, Z.; Ali, S.; Bhat, S.A.; Bhat, M.A. A review on heme oxygenase-1 induction: Is it a necessary evil. Inflamm. Res. 2018, 67, 579–588. [Google Scholar] [CrossRef]
- Allwood, M.A.; Kinobe, R.T.; Ballantyne, L.; Romanova, N.; Melo, L.G.; Ward, C.A.; Brunt, K.R.; Simpson, J.A. Heme oxygenase-1 overexpression exacerbates heart failure with aging and pressure overload but is protective against isoproterenol-induced cardiomyopathy in mice. Cardiovasc. Pathol. 2014, 23, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Nemes, A.; Homoki, J.R.; Kiss, R.; Hegedus, C.; Kovacs, D.; Peitl, B.; Gal, F.; Stundl, L.; Szilvassy, Z.; Remenyik, J. Effect of Anthocyanin-Rich Tart Cherry Extract on Inflammatory Mediators and Adipokines Involved in Type 2 Diabetes in a High Fat Diet Induced Obesity Mouse Model. Nutrients 2019, 11, 1966. [Google Scholar] [CrossRef] [PubMed]
- Homoki, J.R.; Nemes, A.; Fazekas, E.; Gyemant, G.; Balogh, P.; Gal, F.; Al-Asri, J.; Mortier, J.; Wolber, G.; Babinszky, L.; et al. Anthocyanin composition, antioxidant efficiency, and alpha-amylase inhibitor activity of different Hungarian sour cherry varieties (Prunus cerasus L.). Food Chem. 2016, 194, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Priksz, D.; Bombicz, M.; Varga, B.; Kurucz, A.; Gesztelyi, R.; Balla, J.; Toth, A.; Papp, Z.; Szilvassy, Z.; Juhasz, B. Upregulation of Myocardial and Vascular Phosphodiesterase 9A in A Model of Atherosclerotic Cardiovascular Disease. Int. J. Mol. Sci. 2018, 19, 2882. [Google Scholar] [CrossRef] [PubMed]
- Abela, G.S.; Picon, P.D.; Friedl, S.E.; Gebara, O.C.; Miyamoto, A.; Federman, M.; Tofler, G.H.; Muller, J.E. Triggering of plaque disruption and arterial thrombosis in an atherosclerotic rabbit model. Circulation 1995, 91, 776–784. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Xu, Z.; Zhao, H.; Wang, X.; Pang, J.; Li, Q.; Yang, Y.; Ling, W. Anthocyanin supplementation improves anti-oxidative and anti-inflammatory capacity in a dose-response manner in subjects with dyslipidemia. Redox Biol. 2020, 32, 101474. [Google Scholar] [CrossRef]
- Xu, Z.; Xie, J.; Zhang, H.; Pang, J.; Li, Q.; Wang, X.; Xu, H.; Sun, X.; Zhao, H.; Yang, Y.; et al. Anthocyanin supplementation at different doses improves cholesterol efflux capacity in subjects with dyslipidemia-a randomized controlled trial. Eur. J. Clin. Nutr. 2021, 75, 345–354. [Google Scholar] [CrossRef]
- Sozanski, T.; Kucharska, A.Z.; Szumny, A.; Magdalan, J.; Bielska, K.; Merwid-Lad, A.; Wozniak, A.; Dzimira, S.; Piorecki, N.; Trocha, M. The protective effect of the Cornus mas fruits (cornelian cherry) on hypertriglyceridemia and atherosclerosis through PPARalpha activation in hypercholesterolemic rabbits. Phytomedicine 2014, 21, 1774–1784. [Google Scholar] [CrossRef]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 233–270. [Google Scholar] [CrossRef]


| Serum Parameter | Control | C + PCE | HC | HC + PCE |
|---|---|---|---|---|
| Total cholesterol (mmol/L) | 0.72 ± 0.083 | 0.478 ± 0.066 | 34.79 ± 1.319 **** | 25.02 ± 2.546 ##**** |
| LDL-C (mmol/L) | 0.096 ± 0.007 | 0.071 ± 0.012 | 32.99 ±0.694 **** | 21.80 ± 2.054 ####**** |
| HDL-C (mmol/L) | 0.452 ± 0.049 | 0.282 ± 0.060 | 1.714 ± 0.408 ** | 1.458 ± 0.197 ** |
| Atherogenic index of plasma (AIP) (TC/HDL) | 1.613 ± 0.075 | 2.102 ± 0.313 | 28.17 ± 4.225 **** | 18.09 ± 0.756 ##**** |
| Triglyceride (mmol/L) | 0.968 ± 0.227 | 0.829 ± 0.157 | 1.239 ± 0.195 | 1.635 ± 0.368 |
| CRP (mg/L) | 0.2 ± 0.104 | 0.241 ± 0.091 | 0.815 ± 0.649 | 0.15 ± 0.023 |
| CK-MB (U/L) | 217.7 ± 58.62 | 446.0 ± 125.1 | 477.4 ± 75.19 | 480.9 ± 82.50 |
| AST (GOT) (U/L). | 20.60 ± 2.768 | 20.78 ± 2.159 | 30.75 ± 6.421 | 30.22 ± 4.300 |
| ALT (GPT) (U/L) | 56.76 ± 7.115 | 44.62 ± 3.042 | 37.98 ± 7.127 | 43.01 ± 7.825 |
| Glucose (mmol/L) | 5.473 ± 0.217 | 6.774 ± 0.348 | 6.940 ± 0.552 | 7.040 ± 0.255 |
| Parameter | Control | C + PCE | HC | HC + PCE |
|---|---|---|---|---|
| Tei-index | 0.7939 ± 0.044 | 0.7792 ± 0.021 | 0.7670 ± 0.050 | 0.7637 ± 0.027 |
| HR (bpm) | 174.4 ± 5.066 | 195.5 ± 5.726 | 153.4 ± 8.394 | 158.3 ± 4.040 |
| EF (%) | 65.09 ± 3.497 | 71.31 ± 2.690 | 63.38 ± 4.946 | 72.52 ± 1.896 |
| FS (%) | 33.55 ± 2.602 | 38.23 ± 2.107 | 32.88 ± 3.351 | 39.81 ± 1.677 |
| s’ (mm/s) | 71.30 ± 4.858 | 77.62 ± 2.973 | 61.88 ± 4.951 | 70.86 ± 3.621 |
| MAPSE (mm) | 4.628 ± 0.175 | 4.507 ± 0.229 | 3.990 ± 0.289 | 4.955 ± 0.268 |
| LVOT Vmax (m/s) | 1.027 ± 0.047 | 1.133 ± 0.046 | 1.119 ± 0.067 | 1.125 ± 0.039 |
| LVOT Vmean (m/s) | 0.656 ± 0.027 | 0.725 ± 0.035 | 0.71 ± 0.036 | 0.69 ± 0.026 |
| LVOT maxPG (mmHg) | 4.284 ± 0.385 | 5.219 ± 0.423 | 5.109 ± 0.615 | 5.185 ± 0.378 |
| LVOT meanPG (mmHg) | 2.110 ± 0.180 | 2.417 ± 0.278 | 2.566 ± 0.264 | 2.453 ± 0.186 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szekeres, R.; Priksz, D.; Kiss, R.; Romanescu, D.D.; Bombicz, M.; Varga, B.; Gesztelyi, R.; Szilagyi, A.; Takacs, B.; Tarjanyi, V.; et al. Therapeutic Aspects of Prunus cerasus Extract in a Rabbit Model of Atherosclerosis-Associated Diastolic Dysfunction. Int. J. Mol. Sci. 2023, 24, 13253. https://doi.org/10.3390/ijms241713253
Szekeres R, Priksz D, Kiss R, Romanescu DD, Bombicz M, Varga B, Gesztelyi R, Szilagyi A, Takacs B, Tarjanyi V, et al. Therapeutic Aspects of Prunus cerasus Extract in a Rabbit Model of Atherosclerosis-Associated Diastolic Dysfunction. International Journal of Molecular Sciences. 2023; 24(17):13253. https://doi.org/10.3390/ijms241713253
Chicago/Turabian StyleSzekeres, Reka, Daniel Priksz, Rita Kiss, Dana Diana Romanescu, Mariann Bombicz, Balazs Varga, Rudolf Gesztelyi, Anna Szilagyi, Barbara Takacs, Vera Tarjanyi, and et al. 2023. "Therapeutic Aspects of Prunus cerasus Extract in a Rabbit Model of Atherosclerosis-Associated Diastolic Dysfunction" International Journal of Molecular Sciences 24, no. 17: 13253. https://doi.org/10.3390/ijms241713253
APA StyleSzekeres, R., Priksz, D., Kiss, R., Romanescu, D. D., Bombicz, M., Varga, B., Gesztelyi, R., Szilagyi, A., Takacs, B., Tarjanyi, V., Pelles-Tasko, B., Forgacs, I., Remenyik, J., Szilvassy, Z., & Juhasz, B. (2023). Therapeutic Aspects of Prunus cerasus Extract in a Rabbit Model of Atherosclerosis-Associated Diastolic Dysfunction. International Journal of Molecular Sciences, 24(17), 13253. https://doi.org/10.3390/ijms241713253










