CBD Inhibits In Vivo Development of Human Breast Cancer Tumors
Abstract
:1. Introduction
2. Results
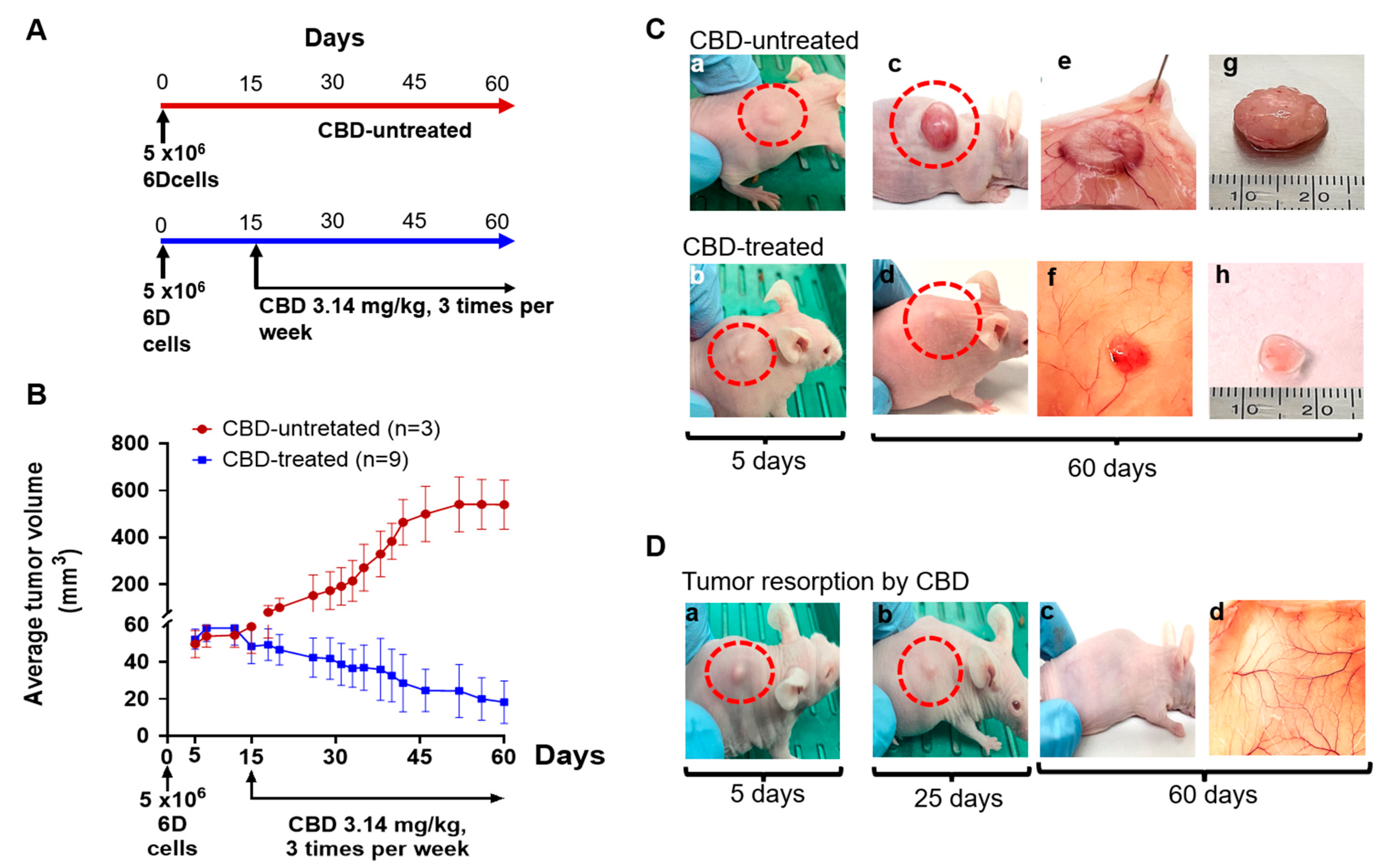
2.1. CBD Antitumor Effect under Therapeutic Conditions
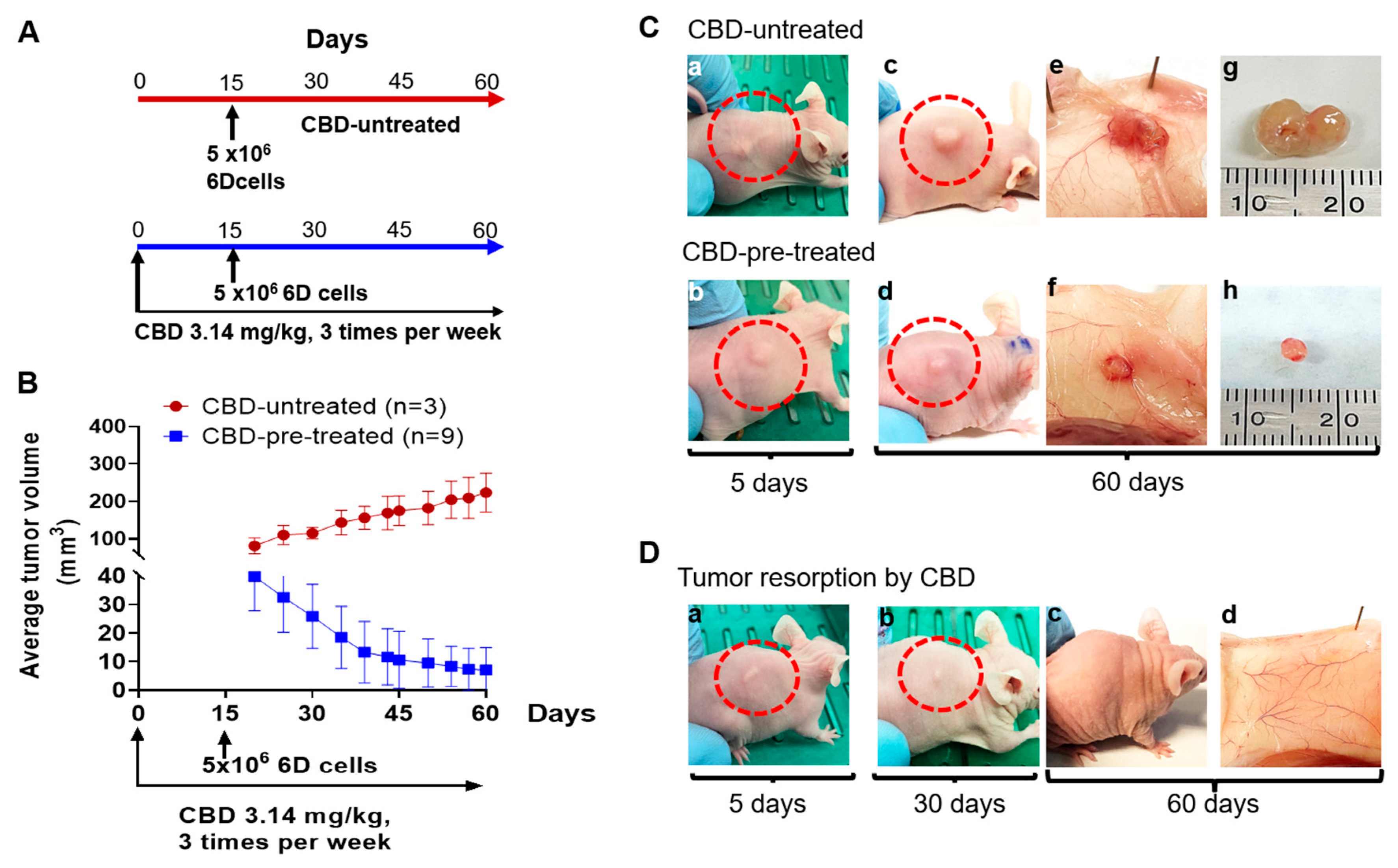
2.2. CBD Treatment of Mice under Prophylactic Conditions
2.3. CBD Effects on Tumor Histology
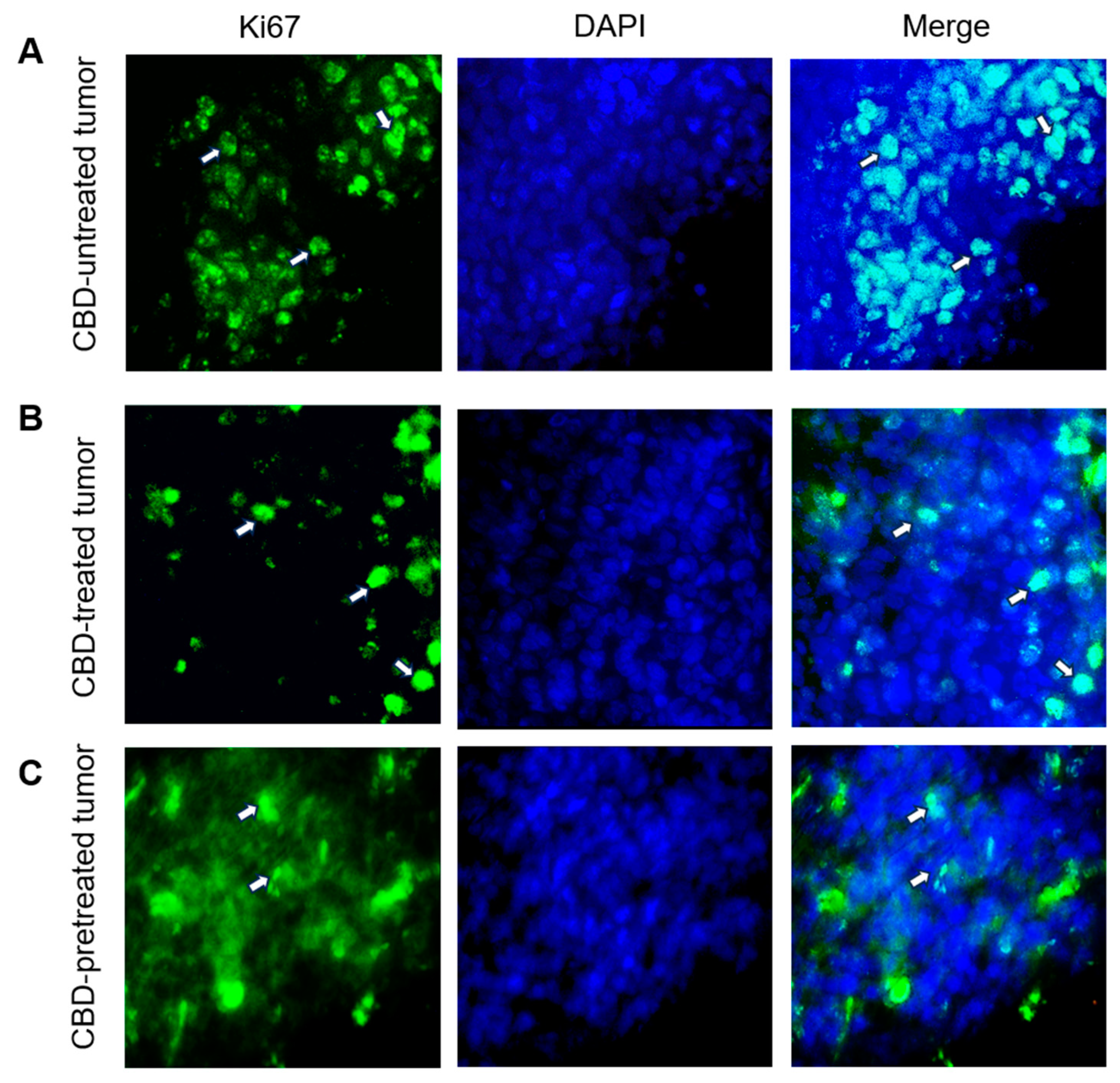
2.4. CBD Modifies the Expression of Tumor Development Markers Ki67, Bcl2 and P53
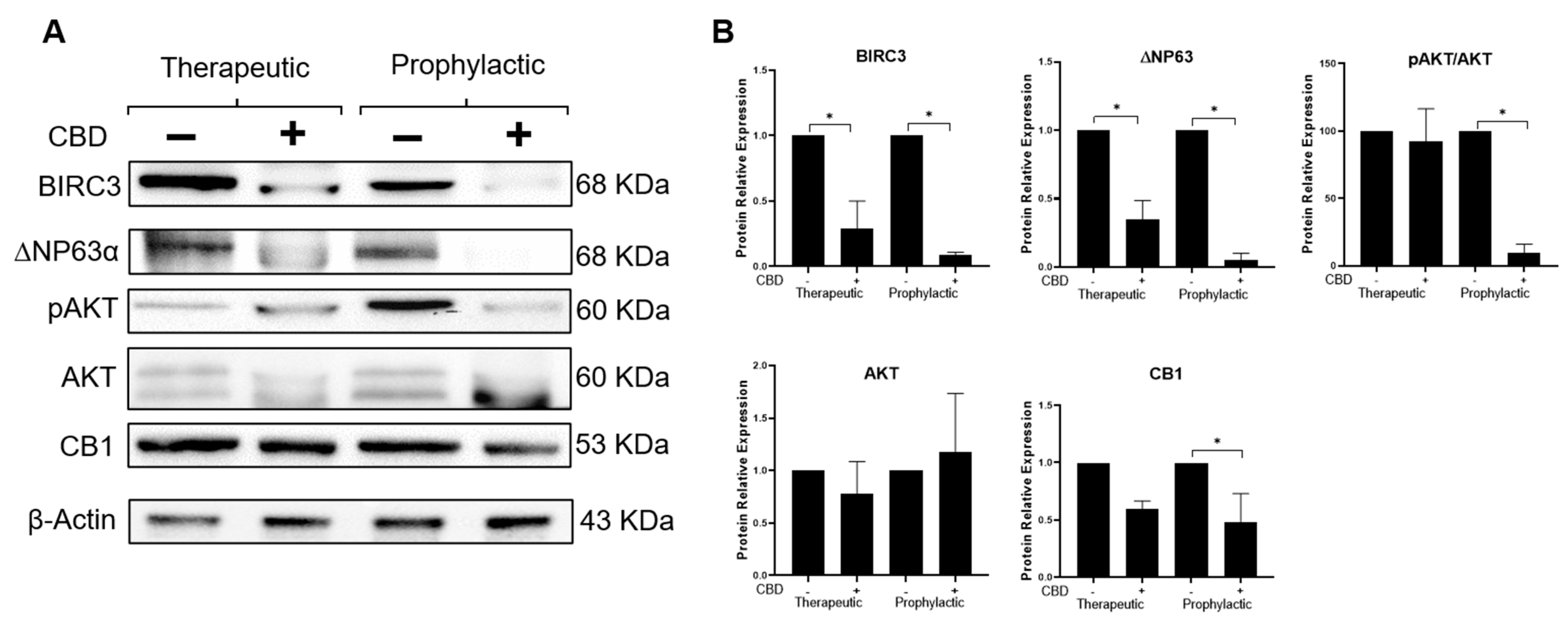
2.5. CBD Modifies Expression of Effectors Participating in the IL-1β/IL-1R/β-Catenin Pathway
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Primary Antibodies
4.3. Cell Culture
4.4. Animal Model
4.5. SDS-PAGE and Western Blotting
4.6. Histologic Analysis and Immunofluorescence Staining
4.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yang, L.; Lin, P.C. Mechanisms That Drive Inflammatory Tumor Microenvironment, Tumor Heterogeneity, and Metastatic Progression. Semin. Cancer Biol. 2017, 47, 185–195. [Google Scholar] [CrossRef]
- Jang, J.-H.; Kim, D.-H.; Surh, Y.-J. Dynamic Roles of Inflammasomes in Inflammatory Tumor Microenvironment. NPJ Precis. Oncol. 2021, 5, 18. [Google Scholar] [CrossRef]
- Pérez-Yépez, E.A.; Ayala-Sumuano, J.-T.; Reveles-Espinoza, A.M.; Meza, I. Selection of a MCF-7 Breast Cancer Cell Subpopulation with High Sensitivity to IL-1 Β: Characterization of and Correlation between Morphological and Molecular Changes Leading to Increased Invasiveness. Int. J. Breast Cancer 2012, 2012, 609148. [Google Scholar] [CrossRef]
- Perez-Yepez, E.A.; Ayala-Sumuano, J.-T.; Lezama, R.; Meza, I. A Novel β-Catenin Signaling Pathway Activated by IL-1β Leads to the Onset of Epithelial-Mesenchymal Transition in Breast Cancer Cells. Cancer Lett. 2014, 354, 164–171. [Google Scholar] [CrossRef]
- Jiménez-Garduño, A.M.; Mendoza-Rodríguez, M.G.; Urrutia-Cabrera, D.; Domínguez-Robles, M.C.; Pérez-Yépez, E.A.; Ayala-Sumuano, J.T.; Meza, I. IL-1β Induced Methylation of the Estrogen Receptor ERα Gene Correlates with EMT and Chemoresistance in Breast Cancer Cells. Biochem. Biophys. Res. Commun. 2017, 490, 780–785. [Google Scholar] [CrossRef]
- Mendoza-Rodríguez, M.; Arévalo Romero, H.; Fuentes-Pananá, E.M.; Ayala-Sumuano, J.-T.; Meza, I. IL-1β Induces up-Regulation of BIRC3, a Gene Involved in Chemoresistance to Doxorubicin in Breast Cancer Cells. Cancer Lett. 2017, 390, 39–44. [Google Scholar] [CrossRef]
- Mendoza-Rodríguez, M.; Ayala-Sumuano, J.; García-Morales, L.; Zamudio-Meza, H.; Pérez-Yepez, E.; Meza, I. IL-1β Inflammatory Cytokine-Induced TP63 Isoform ∆NP63α Signaling Cascade Contributes to Cisplatin Resistance in Human Breast Cancer Cells. Int. J. Mol. Sci. 2019, 20, 270. [Google Scholar] [CrossRef]
- Fu, Z.; Zhao, P.-Y.; Yang, X.-P.; Li, H.; Hu, S.-D.; Xu, Y.-X.; Du, X.-H. Cannabidiol Regulates Apoptosis and Autophagy in Inflammation and Cancer: A Review. Front. Pharmacol. 2023, 14, 1094020. [Google Scholar] [CrossRef]
- Hosami, F.; Ghadimkhah, M.H.; Salimi, V.; Ghorbanhosseini, S.S.; Tavakoli-Yaraki, M. The Strengths and Limits of Cannabinoids and Their Receptors in Cancer: Insights into the Role of Tumorigenesis-Underlying Mechanisms and Therapeutic Aspects. Biomed. Pharmacother. 2021, 144, 112279. [Google Scholar] [CrossRef]
- Hinz, B.; Ramer, R. Cannabinoids as Anticancer Drugs: Current Status of Preclinical Research. Br. J. Cancer 2022, 127, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Wang, Y.L.; Wang, G.W.; Wong, Y.C.; Wang, X.F.; Wang, Y.; Xu, K.X. A Novel Role of Id-1 in Regulation of Epithelial-to-Mesenchymal Transition in Bladder Cancer. Urol. Oncol. Semin. Orig. Investig. 2013, 31, 1242–1253. [Google Scholar] [CrossRef] [PubMed]
- Pellati, F.; Borgonetti, V.; Brighenti, V.; Biagi, M.; Benvenuti, S.; Corsi, L. Cannabis Sativa L. and Nonpsychoactive Cannabinoids: Their Chemistry and Role against Oxidative Stress, Inflammation, and Cancer. BioMed Res. Int. 2018, 2018, 1691428. [Google Scholar] [CrossRef] [PubMed]
- García-Morales, L.; Castillo, A.M.; Tapia Ramírez, J.; Zamudio-Meza, H.; Domínguez-Robles, M.D.C.; Meza, I. CBD Reverts the Mesenchymal Invasive Phenotype of Breast Cancer Cells Induced by the Inflammatory Cytokine IL-1β. Int. J. Mol. Sci. 2020, 21, 2429. [Google Scholar] [CrossRef]
- Hass, R.; von der Ohe, J.; Ungefroren, H. Impact of the Tumor Microenvironment on Tumor Heterogeneity and Consequences for Cancer Cell Plasticity and Stemness. Cancers 2020, 12, 3716. [Google Scholar] [CrossRef] [PubMed]
- Deepak, K.G.K.; Vempati, R.; Nagaraju, G.P.; Dasari, V.R.; Nagini, S.; Rao, D.N.; Malla, R.R. Tumor Microenvironment: Challenges and Opportunities in Targeting Metastasis of Triple Negative Breast Cancer. Pharmacol. Res. 2020, 153, 104683. [Google Scholar] [CrossRef]
- Seltzer, E.S.; Watters, A.K.; MacKenzie, D., Jr.; Granat, L.M. Cannabidiol (CBD) as a Promising Anti-Cancer Drug. Cancers 2020, 12, 3203. [Google Scholar] [CrossRef]
- Go, Y.Y.; Kim, S.R.; Kim, D.Y.; Chae, S.-W.; Song, J.-J. Cannabidiol Enhances Cytotoxicity of Anti-Cancer Drugs in Human Head and Neck Squamous Cell Carcinoma. Sci. Rep. 2020, 10, 20622. [Google Scholar] [CrossRef] [PubMed]
- Mouhamed, Y.; Vishnyakov, A.; Qorri, B.; Sambi, M.; Frank, S.S.; Nowierski, C.; Lamba, A.; Bhatti, U.; Szewczuk, M.R. Therapeutic Potential of Medicinal Marijuana: An Educational Primer for Health Care Professionals. Drug. Healthc. Patient Saf. 2018, 10, 45–66. [Google Scholar] [CrossRef]
- Makki, J. Diversity of Breast Carcinoma: Histological Subtypes and Clinical Relevance. Clin. Med. Insights Pathol. 2015, 8, CPath.S31563. [Google Scholar] [CrossRef]
- Zhang, S.; Yang, X.; Wang, L.; Zhang, C. Interplay between Inflammatory Tumor Microenvironment and Cancer Stem Cells (Review). Oncol. Lett. 2018, 16, 679–686. [Google Scholar] [CrossRef]
- Greten, F.R.; Grivennikov, S.I. Inflammation and Cancer: Triggers, Mechanisms, and Consequences. Immunity 2019, 51, 27–41. [Google Scholar] [CrossRef] [PubMed]
- Niu, N.; Wang, L. In Vitro Human Cell Line Models to Predict Clinical Response to Anticancer Drugs. Pharmacogenomics 2015, 16, 273–285. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Pacheco, S.; O’Driscoll, L. Pre-Clinical In Vitro Models Used in Cancer Research: Results of a Worldwide Survey. Cancers 2021, 13, 6033. [Google Scholar] [CrossRef]
- Fleming, J.M.; Miller, T.C.; Meyer, M.J.; Ginsburg, E.; Vonderhaar, B.K. Local Regulation of Human Breast Xenograft Models. J. Cell. Physiol. 2010, 224, 795–806. [Google Scholar] [CrossRef]
- Behzadi, R.; Ahmadpour, S.; Amiri, F.T.; Kavosian, S.; Asori, M.; Hosseinimehr, S.J. Choosing the Right Protocol to Establish MCF-7 Tumor Xenograft in Nude Mice. Anticancer Agents Med. Chem. 2023, 23, 222–226. [Google Scholar] [CrossRef]
- Ghallab, A. In Vitro Test Systems and Their Limitations. EXCLI J. 2013, 12, 1024–1026. [Google Scholar]
- Huestis, M.A.; Solimini, R.; Pichini, S.; Pacifici, R.; Carlier, J.; Busardò, F.P. Cannabidiol Adverse Effects and Toxicity. Curr. Neuropharmacol. 2019, 17, 974–989. [Google Scholar] [CrossRef] [PubMed]
- Massi, P.; Vaccani, A.; Bianchessi, S.; Costa, B.; Macchi, P.; Parolaro, D. The Non-Psychoactive Cannabidiol Triggers Caspase Activation and Oxidative Stress in Human Glioma Cells. Cell. Mol. Life Sci. 2006, 63, 2057–2066. [Google Scholar] [CrossRef]
- Callejas, B.E.; Mendoza-Rodríguez, M.G.; Villamar-Cruz, O.; Reyes-Martínez, S.; Sánchez-Barrera, C.A.; Rodríguez-Sosa, M.; Delgado-Buenrostro, N.L.; Martínez-Saucedo, D.; Chirino, Y.I.; León-Cabrera, S.A.; et al. Helminth-Derived Molecules Inhibit Colitis-Associated Colon Cancer Development through NF-ΚB and STAT3 Regulation. Int. J. Cancer 2019, 145, 3126–3139. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Morales, L.; Mendoza-Rodríguez, M.G.; Tapia Ramírez, J.; Meza, I. CBD Inhibits In Vivo Development of Human Breast Cancer Tumors. Int. J. Mol. Sci. 2023, 24, 13235. https://doi.org/10.3390/ijms241713235
García-Morales L, Mendoza-Rodríguez MG, Tapia Ramírez J, Meza I. CBD Inhibits In Vivo Development of Human Breast Cancer Tumors. International Journal of Molecular Sciences. 2023; 24(17):13235. https://doi.org/10.3390/ijms241713235
Chicago/Turabian StyleGarcía-Morales, Lázaro, Mónica G. Mendoza-Rodríguez, José Tapia Ramírez, and Isaura Meza. 2023. "CBD Inhibits In Vivo Development of Human Breast Cancer Tumors" International Journal of Molecular Sciences 24, no. 17: 13235. https://doi.org/10.3390/ijms241713235
APA StyleGarcía-Morales, L., Mendoza-Rodríguez, M. G., Tapia Ramírez, J., & Meza, I. (2023). CBD Inhibits In Vivo Development of Human Breast Cancer Tumors. International Journal of Molecular Sciences, 24(17), 13235. https://doi.org/10.3390/ijms241713235





