The sGC Activator Runcaciguat Has Kidney Protective Effects and Prevents a Decline of Kidney Function in ZSF1 Rats
Abstract
:1. Introduction
2. Results
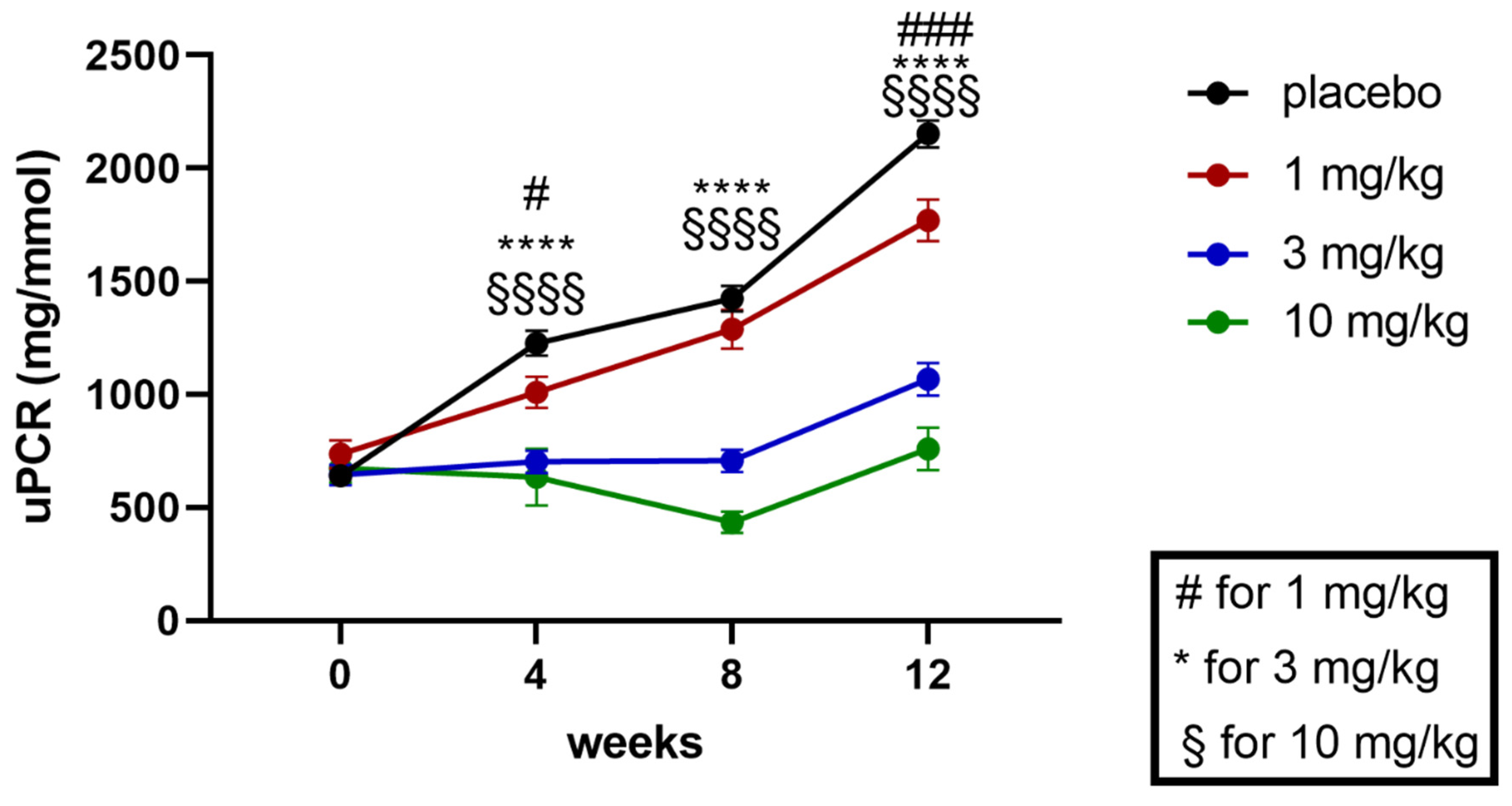
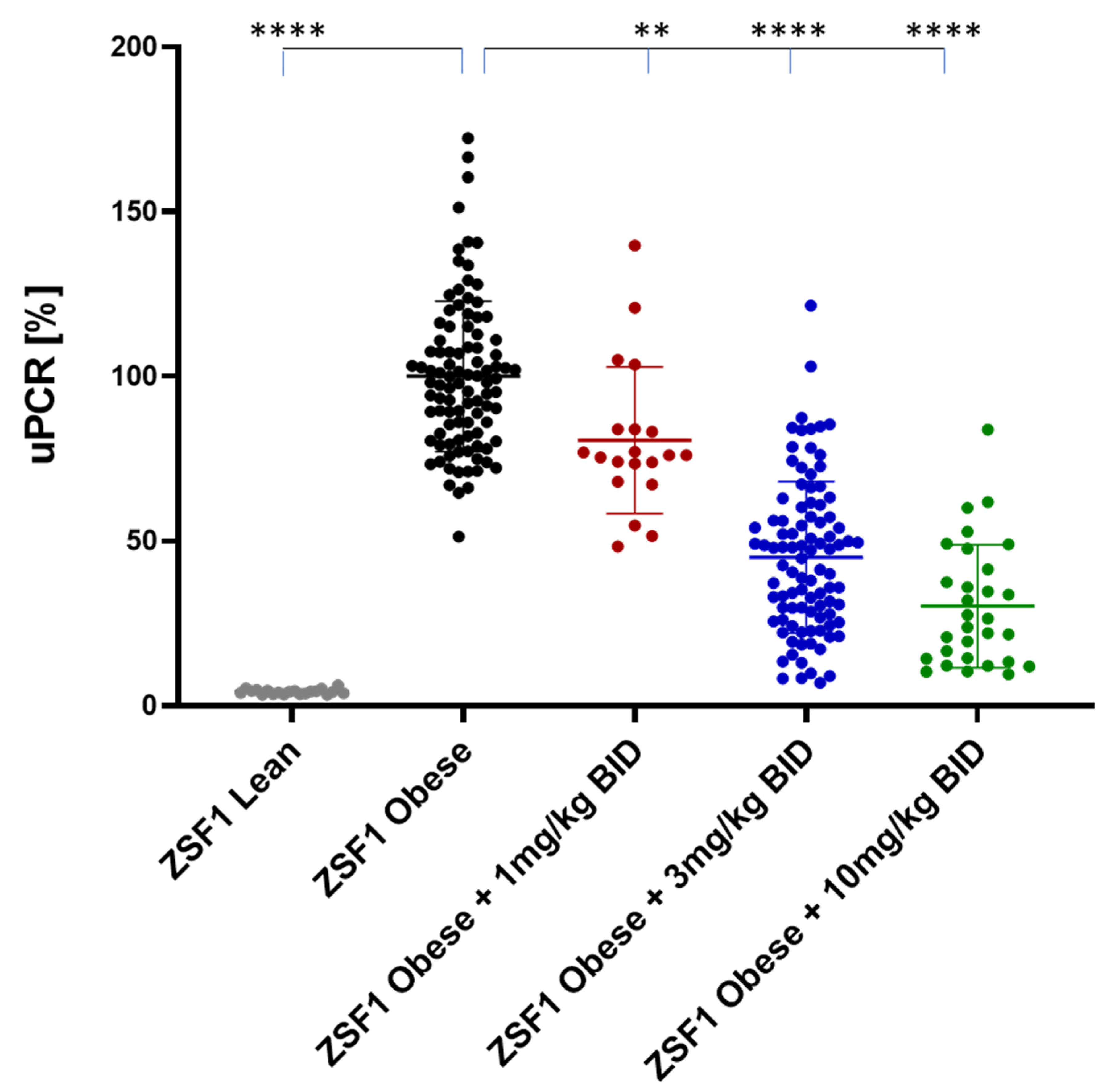
2.1. Effects of Runcaciguat on Proteinuria in ZSF1 Rats
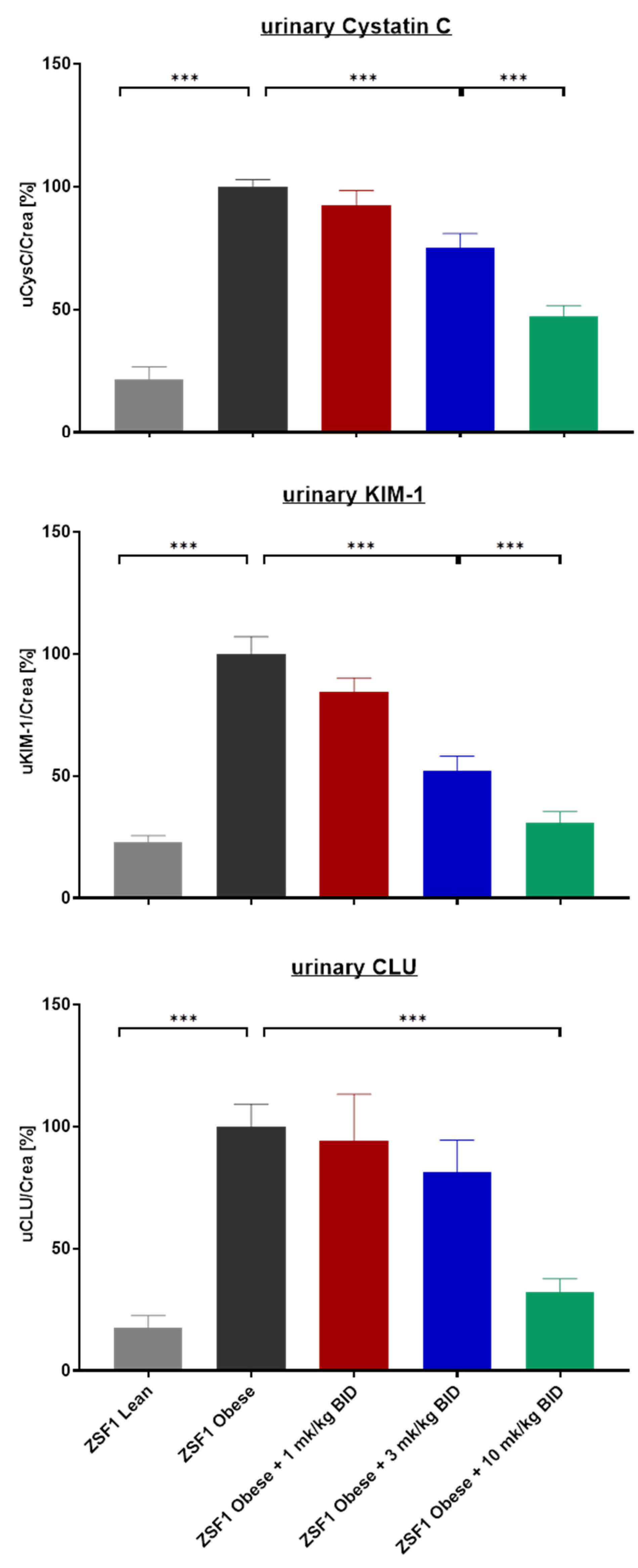
2.2. Effects of Runcaciguat on Urinary Biomarkers of Kidney Injury
2.3. Effects of Runcaciguat on Renal Weights and Histopathology
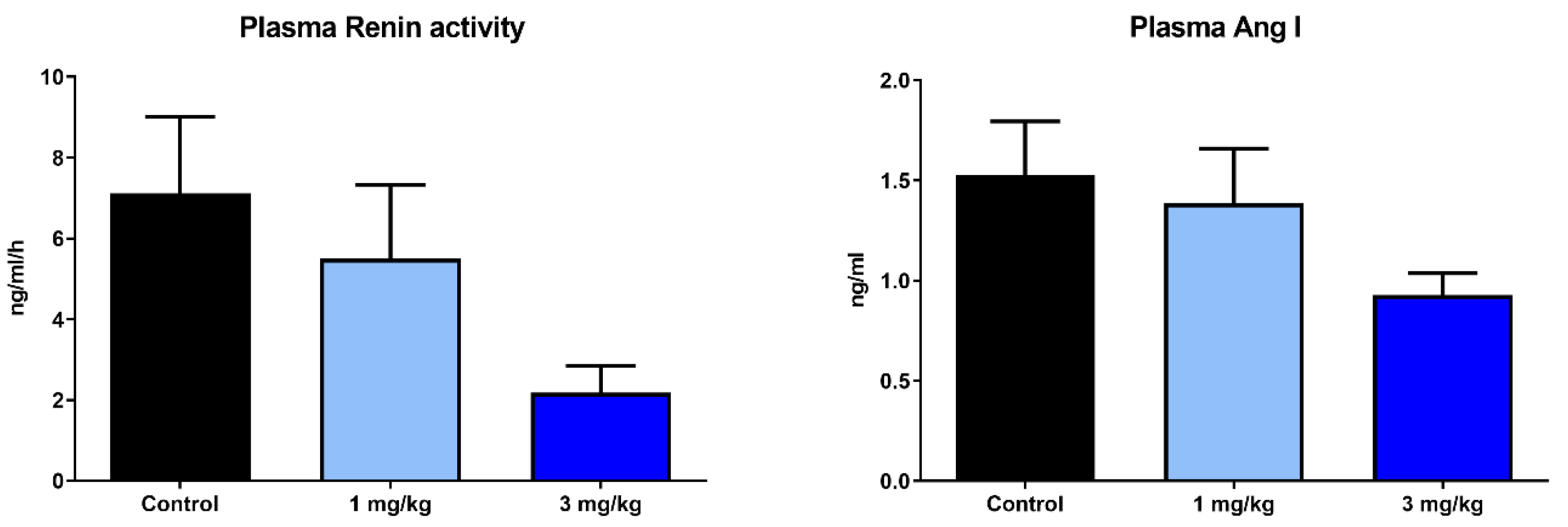
2.4. Effects of Runcaciguat on Blood Pressure and RAAS
2.5. Effects of Runcaciguat on Diabetic and Metabolic Phenotypes
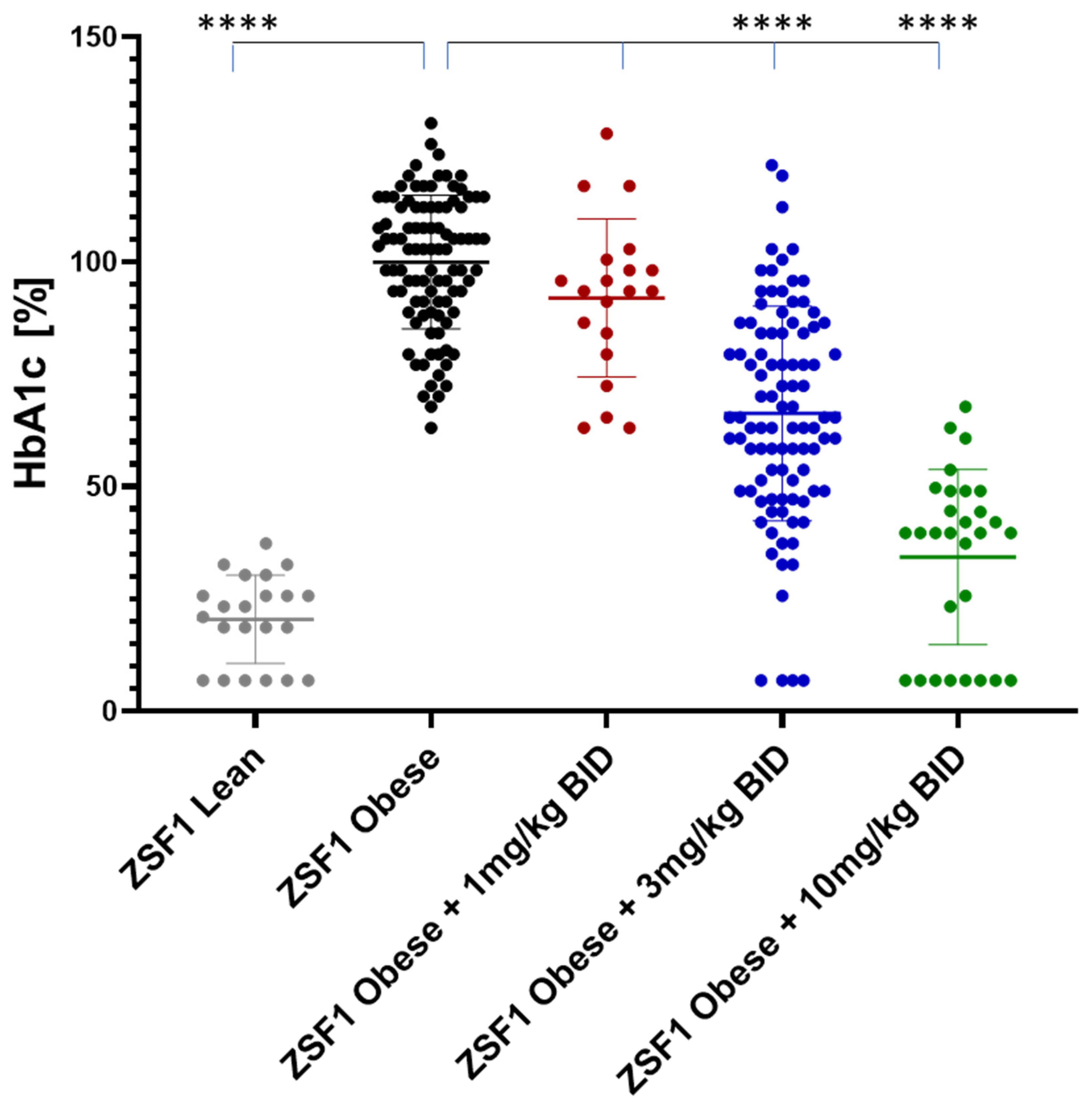
2.5.1. Effects on Blood Glucose
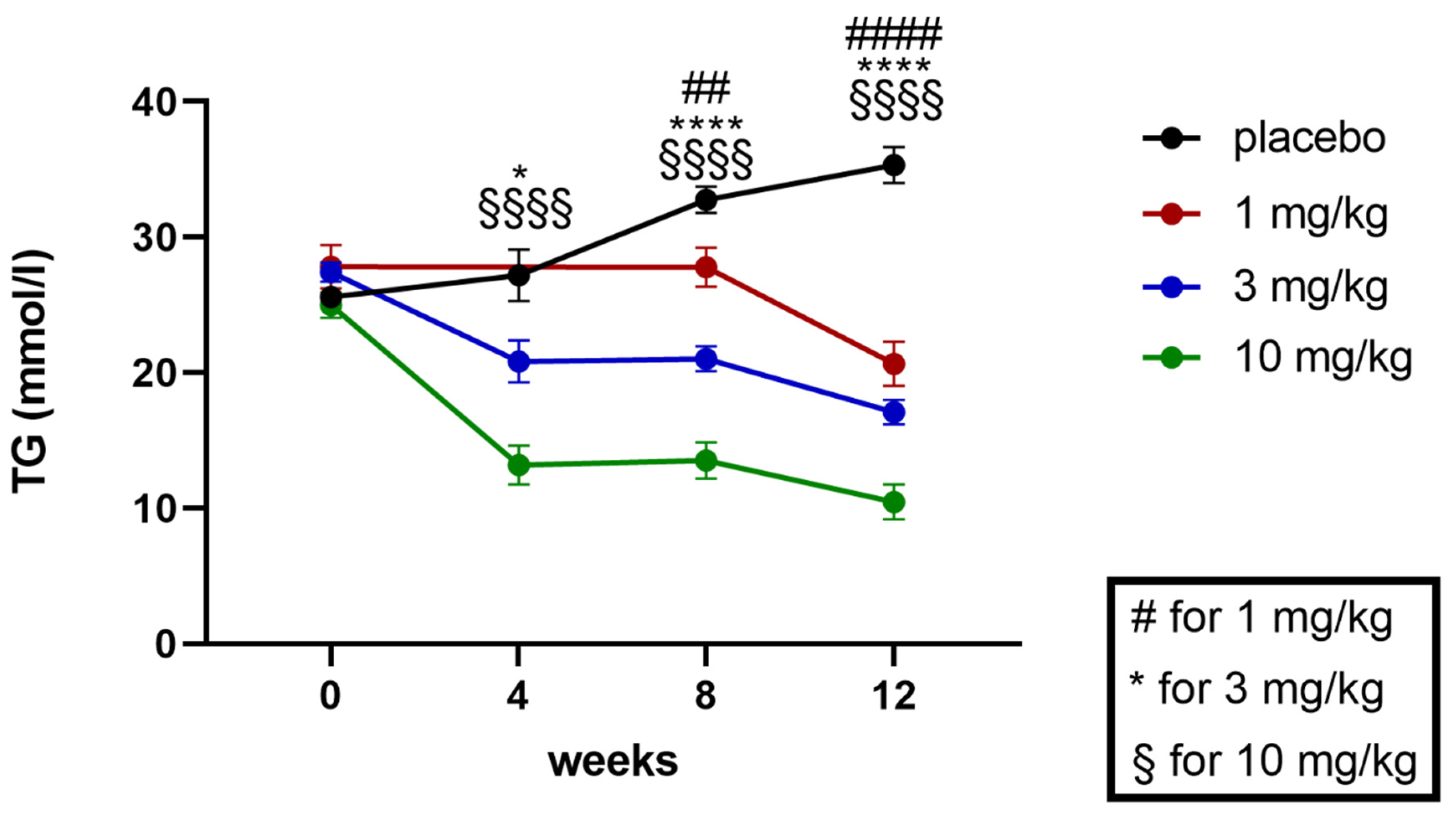
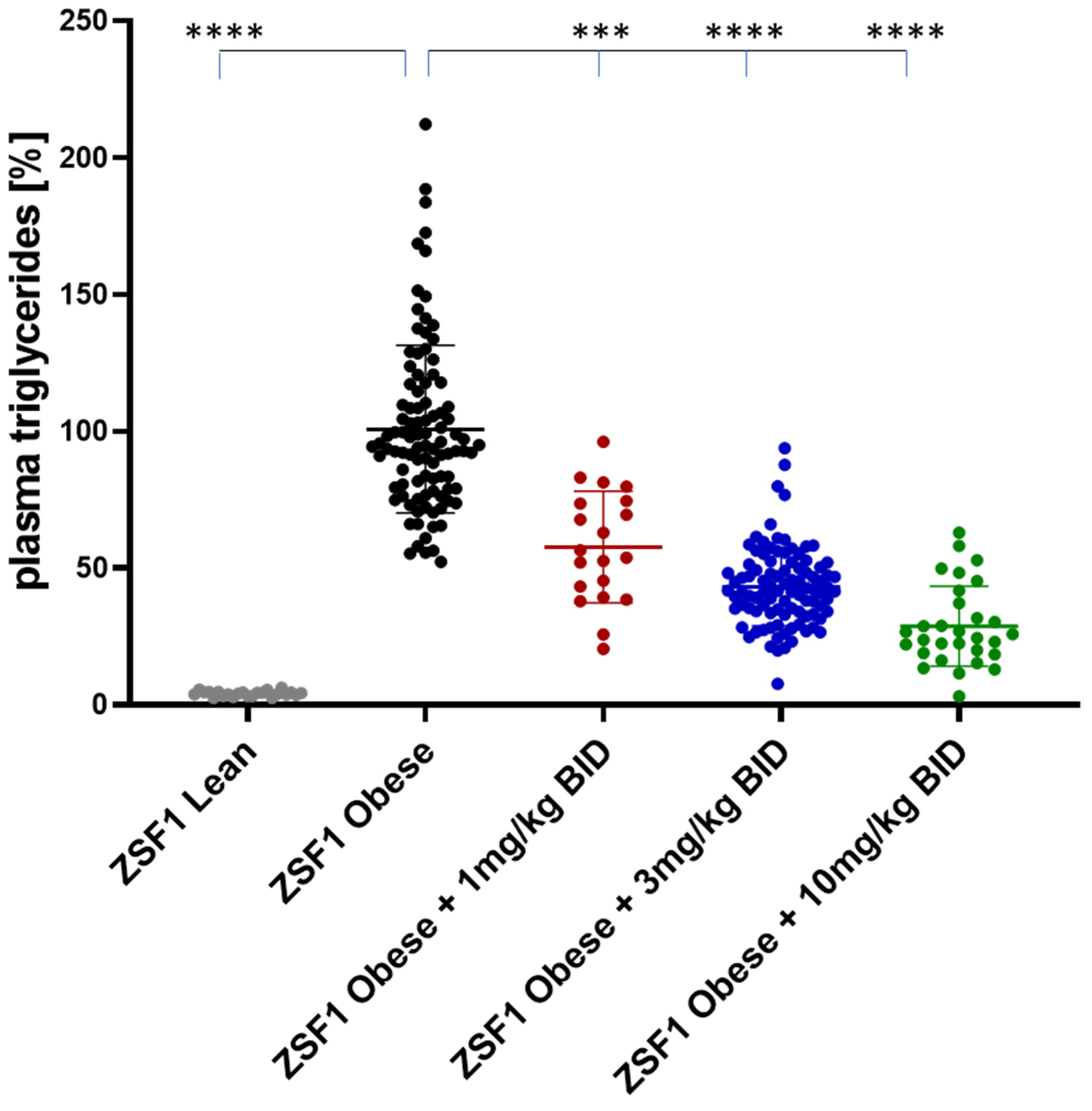
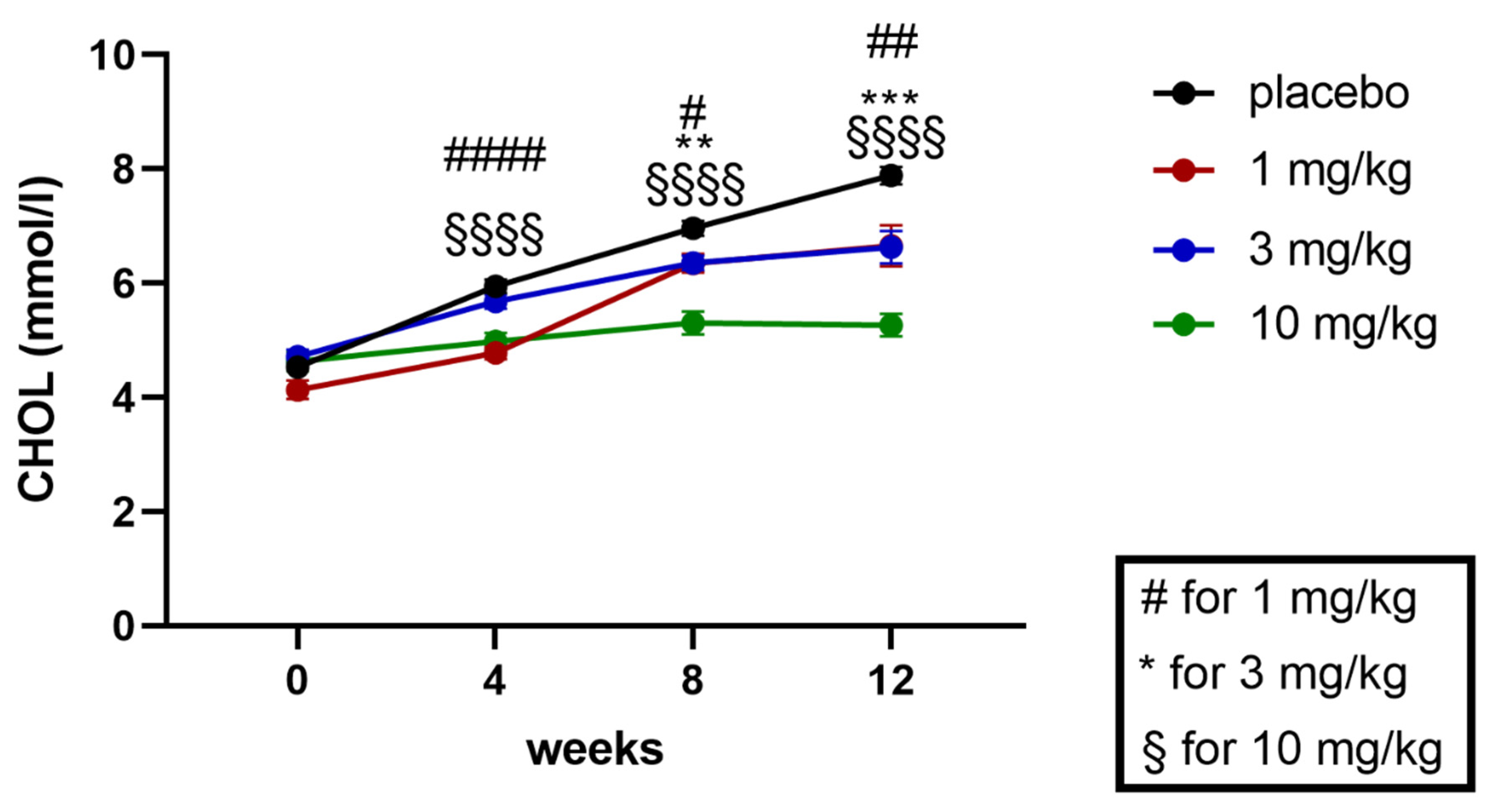
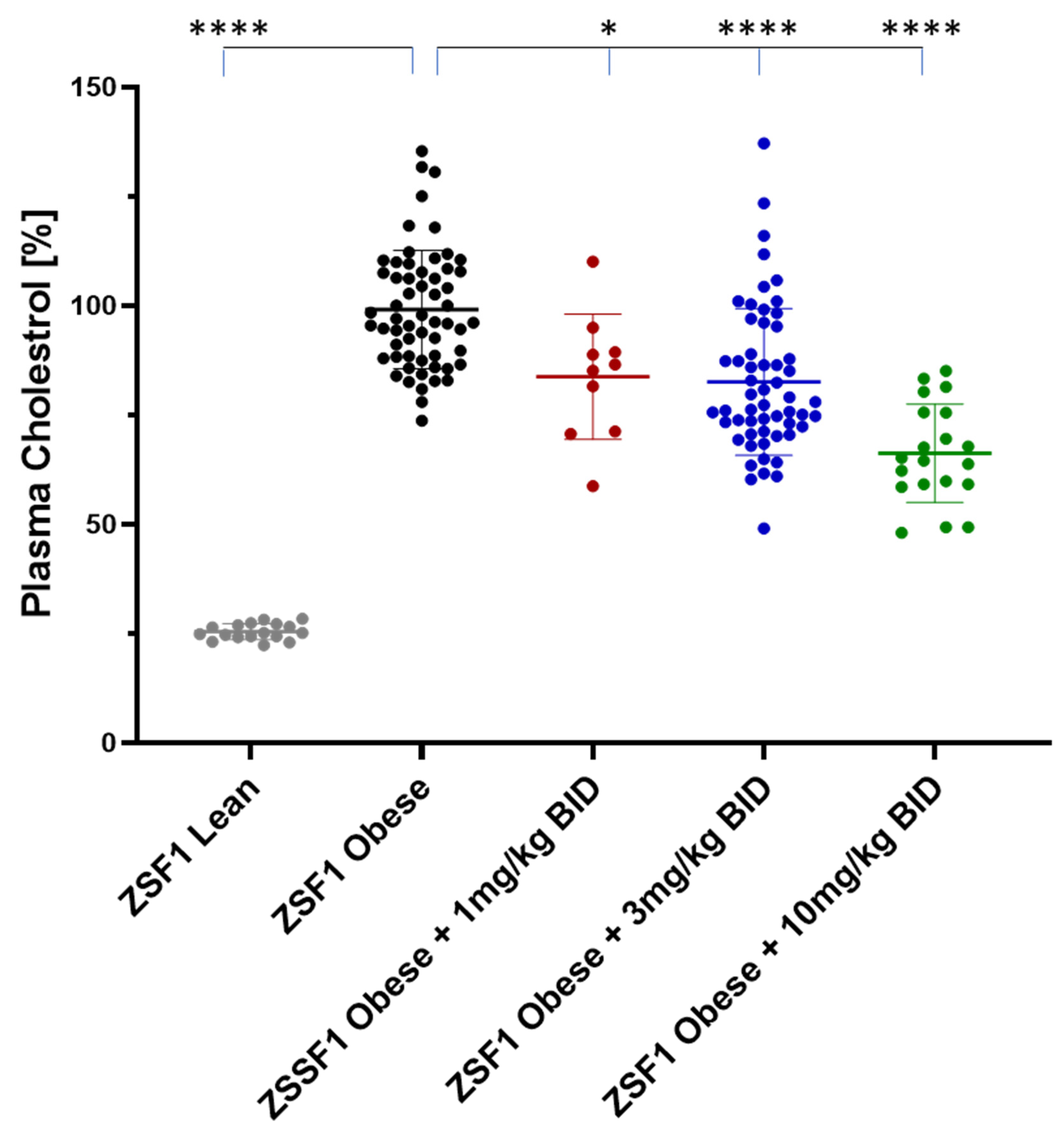
2.5.2. Effects on Plasma Triglycerides and Cholesterol
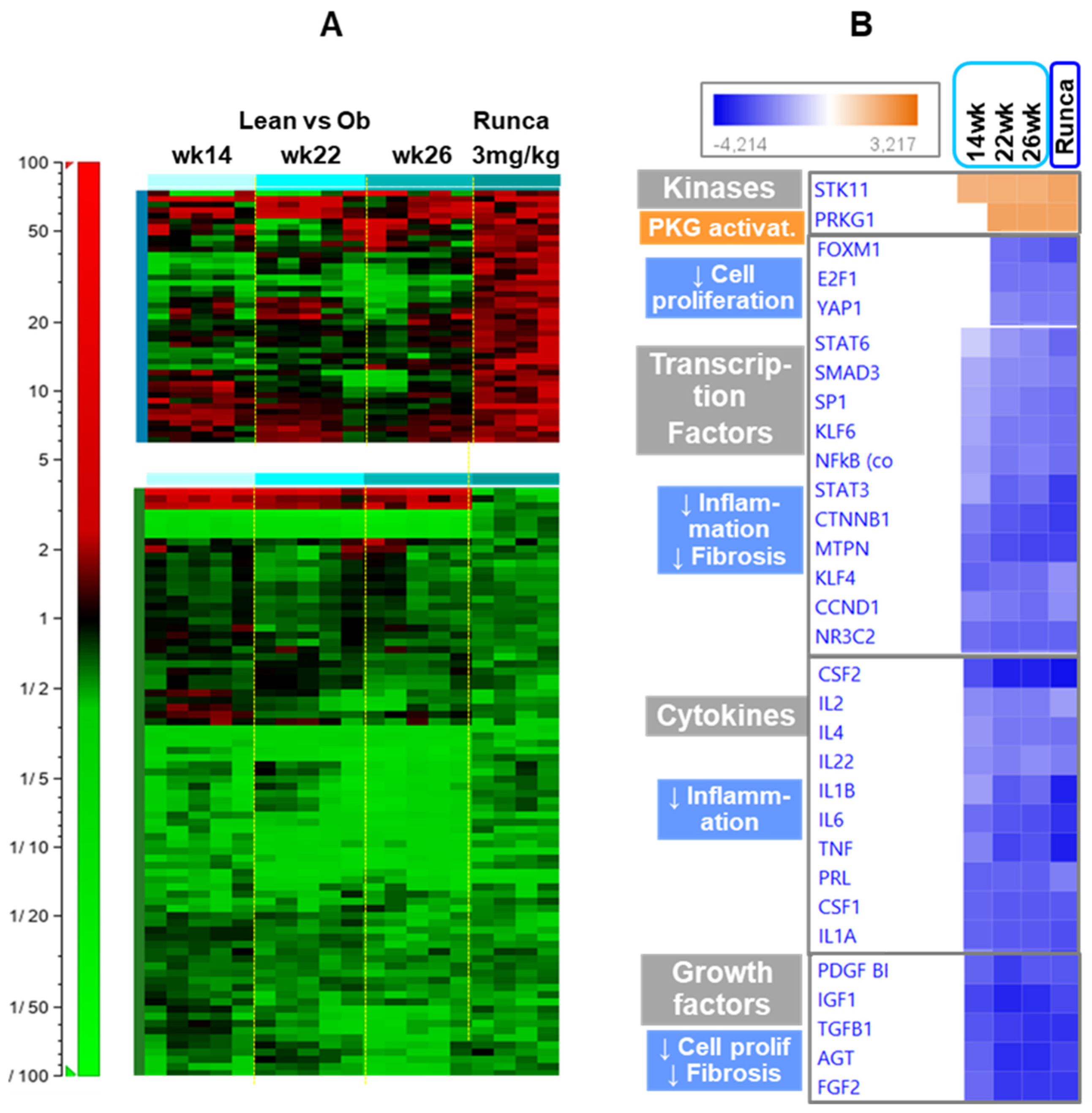
2.6. Effects of Runcaciguat on the Regulation of Different Pathways
3. Discussion
4. Material and Methods
4.1. Animal Experiments
4.2. Study Protocol
4.3. Measurement of Urinary Biomarkers
4.4. Measurements of Plasma Parameters
4.5. Microarray Expression Profiling in Kidney
4.6. Necropsy and Histopathology
4.7. Blood Pressure Measurements
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Evgenov, O.V.; Pacher, P.; Schmidt, P.M.; Hasko, G.; Schmidt, H.H.; Stasch, J.P. NO-independent stimulators and activators of soluble guanylate cyclase: Discovery and therapeutic potential. Nat. Rev. Drug Discov. 2006, 5, 755–768. [Google Scholar] [CrossRef] [PubMed]
- Sandner, P.; Zimmer, D.P.; Milne, G.T.; Follmann, M.; Hobbs, A.; Stasch, J.P. Soluble Guanylate Cyclase Stimulators and Activators. Handb. Exp. Pharmacol. 2021, 264, 355–394. [Google Scholar] [PubMed]
- Sasser, J.M. Nitric Oxide in the Kidney; Morgan & Claypool Life Sciences: San Rafael, CA, USA, 2015; ISBN 13: 9781615046669. [Google Scholar]
- Stehle, D.; Xu, M.Z.; Schomber, T.; Hahn, M.G.; Schweda, F.; Feil, S.; Kraehling, J.R.; Eitner, F.; Patzak, A.; Sandner, P.; et al. Novel sGC activators increase glomerular cGMP, induce vasodilation, and improve blood flow in the murine kidney. Br. J. Pharmacol. 2021, 179, 2476–2489. [Google Scholar] [CrossRef] [PubMed]
- Kurtz, A.; Wagner, C. Role of nitric oxide in the control of renin secretion. Am. J. Physiol. 1998, 275 Pt 2, F849–F862. [Google Scholar] [CrossRef]
- Schramm, A.; Schweda, F.; Sequeira-Lopez, M.L.S.; Hofmann, F.; Sandner, P.; Schlossmann, J. Protein Kinase G Is Involved in Acute but Not in Long-Term Regulation of Renin Secretion. Front. Pharmacol. 2019, 10, 800. [Google Scholar] [CrossRef]
- Carlstrom, M. Nitric oxide signalling in kidney regulation and cardiometabolic health. Nat. Rev. Nephrol. 2021, 17, 575–590. [Google Scholar] [CrossRef]
- Schrammel, A.; Behrends, S.; Schmidt, K.; Koesling, D.; Mayer, B. Characterization of 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one as a heme-site inhibitor of nitric oxide-sensitive guanylyl cyclase. Mol. Pharmacol. 1996, 50, 1–5. [Google Scholar]
- Schmidt, H.H.; Schmidt, P.M.; Stasch, J.P. NO- and haem-independent soluble guanylate cyclase activators. Handb. Exp. Pharmacol. 2009, 191, 309–339. [Google Scholar]
- Hoffmann, L.S.; Kretschmer, A.; Lawrenz, B.; Hocher, B.; Stasch, J.P. Chronic Activation of Heme Free Guanylate Cyclase Leads to Renal Protection in Dahl Salt-Sensitive Rats. PLoS ONE 2015, 10, e0145048. [Google Scholar] [CrossRef]
- Stasch, J.-P.; Schlossmann, J.; Hocher, B. Renal effects of soluble guanylate cyclase stimulators and activators: A review of the preclinical evidence. Curr. Opin. Pharmacol. 2015, 21, 95–104. [Google Scholar] [CrossRef]
- Hahn, M.G.; Lampe, T.; El Sheikh, S.; Griebenow, N.; Woltering, E.; Schlemmer, K.H.; Dietz, L.; Gerisch, M.; Wunder, F.; Becker-Pelster, E.M.; et al. Discovery of the Soluble Guanylate Cyclase Activator Runcaciguat (BAY 1101042). J. Med. Chem. 2021, 64, 5323–5344. [Google Scholar] [CrossRef] [PubMed]
- Benardeau, A.; Kahnert, A.; Schomber, T.; Meyer, J.; Pavkovic, M.; Kretschmer, A.; Lawrenz, B.; Hartmann, E.; Mathar, I.; Hueser, J.; et al. Runcaciguat, a novel soluble guanylate cyclase activator, shows renoprotection in hypertensive, diabetic, and metabolic preclinical models of chronic kidney disease. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2021, 394, 2363–2379. [Google Scholar] [CrossRef] [PubMed]
- Tofovic, S.P.; Kusaka, H.; Kost, C.K., Jr.; Bastacky, S. Renal function and structure in diabetic, hypertensive, obese ZDFxSHHF-hybrid rats. Ren. Fail. 2000, 22, 387–406. [Google Scholar] [CrossRef] [PubMed]
- Carney, E.F. The impact of chronic kidney disease on global health. Nat. Rev. Nephrol. 2020, 16, 251. [Google Scholar] [CrossRef]
- Evans, M.; Lewis, R.D.; Morgan, A.R.; Whyte, M.B.; Hanif, W.; Bain, S.C.; Davies, S.; Dashora, U.; Yousef, Z.; Patel, D.C.; et al. A Narrative Review of Chronic Kidney Disease in Clinical Practice: Current Challenges and Future Perspectives. Adv. Ther. 2022, 39, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Collaboration, G.B.D.C.K.D. Global, regional, and national burden of chronic kidney disease, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2020, 395, 709–733. [Google Scholar]
- Emdin, C.A.; Khera, A.V.; Klarin, D.; Natarajan, P.; Zekavat, S.M.; Nomura, A.; Haas, M.; Aragam, K.; Ardissino, D.; Wilson, J.G.; et al. Phenotypic Consequences of a Genetic Predisposition to Enhanced Nitric Oxide Signaling. Circulation 2018, 137, 222–232. [Google Scholar] [CrossRef]
- Sandner, P.; Follmann, M.; Becker-Pelster, E.; Hahn, M.G.; Meier, C.; Freitas, C.; Roessig, L.; Stasch, J.P. Soluble GC stimulators and activators: Past, present and future. Br. J. Pharmacol. 2021. [Google Scholar] [CrossRef]
- Boustany-Kari, C.M.; Harrison, P.C.; Chen, H.; Lincoln, K.A.; Qian, H.S.; Clifford, H.; Wang, H.; Zhang, X.; Gueneva-Boucheva, K.; Bosanac, T.; et al. A Soluble Guanylate Cyclase Activator Inhibits the Progression of Diabetic Nephropathy in the ZSF1 Rat. J. Pharmacol. Exp. Ther. 2016, 356, 712–719. [Google Scholar] [CrossRef]
- Reinhart, G.A.; Harrison, P.C.; Lincoln, K.; Chen, H.; Sun, P.; Hill, J.; Qian, H.S.; McHugh, M.C.; Clifford, H.; Ng, K.J.; et al. The Novel, Clinical-Stage Soluble Guanylate Cyclase Activator BI 685509 Protects from Disease Progression in Models of Renal Injury and Disease. J. Pharmacol. Exp. Ther. 2023, 384, 382–392. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, X.; Cao, X. Dendritic cells in systemic lupus erythematosus: From pathogenesis to therapeutic applications. J. Autoimmun. 2022, 132, 102856. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Chen, Y.; Zhou, X.; Hoek, M.; Cox, J.; Lin, K.; Liu, Y.; Blumenschein, W.; Grein, J.; Swaminath, G. Effects of soluble guanylate cyclase stimulator on renal function in ZSF-1 model of diabetic nephropathy. PLoS ONE 2022, 17, e0261000. [Google Scholar] [CrossRef] [PubMed]
- Hanrahan, J.P.; de Boer, I.H.; Bakris, G.L.; Wilson, P.J.; Wakefield, J.D.; Seferovic, J.P.; Chickering, J.G.; Chien, Y.T.; Carlson, K.; Cressman, M.D.; et al. Effects of the Soluble Guanylate Cyclase Stimulator Praliciguat in Diabetic Kidney Disease: A Randomized Placebo-Controlled Clinical Trial. Clin. J. Am. Soc. Nephrol. CJASN 2020, 16, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Shea, C.M.; Jones, J.E.; Price, G.M.; Warren, W.; Lonie, E.; Yan, S.; Currie, M.G.; Profy, A.T.; Masferrer, J.L.; et al. Praliciguat inhibits progression of diabetic nephropathy in ZSF1 rats and suppresses inflammation and apoptosis in human renal proximal tubular cells. Am. J. Physiol. Ren. Physiol. 2020, 319, F697–F711. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.K.; Chen, H.; Ng, K.J.; Villalona, J.; McHugh, M.; Zeveleva, S.; Wilks, J.; Brilisauer, K.; Bretschneider, T.; Qian, H.S.; et al. Soluble Guanylyl Cyclase Activator BI 685509 Reduces Portal Hypertension and Portosystemic Shunting in a Rat Thioacetamide-Induced Cirrhosis Model. J. Pharmacol. Exp. Ther. 2023, 386, 70–79. [Google Scholar] [CrossRef]
- Sandner, P.; Stasch, J.P. Anti-fibrotic effects of soluble guanylate cyclase stimulators and activators: A review of the preclinical evidence. Respir. Med. 2017, 122 (Suppl. S1), S1–S9. [Google Scholar] [CrossRef]
- Schinner, E.; Wetzl, V.; Schramm, A.; Kees, F.; Sandner, P.; Stasch, J.P.; Hofmann, F.; Schlossmann, J. Inhibition of the TGFbeta signalling pathway by cGMP and cGMP-dependent kinase I in renal fibrosis. FEBS Open Bio. 2017, 7, 550–561. [Google Scholar] [CrossRef]
- Balzer, M.S.; Pavkovic, M.; Frederick, J.; Abedini, A.; Freyberger, A.; Vienenkotter, J.; Mathar, I.; Siudak, K.; Eitner, F.; Sandner, P.; et al. Treatment effects of soluble guanylate cyclase modulation on diabetic kidney disease at single-cell resolution. Cell Rep. Med. 2023, 4, 100992. [Google Scholar] [CrossRef]
- Obeidat, M.; Li, L.; Ballermann, B.J. TIMAP promotes angiogenesis by suppressing PTEN-mediated Akt inhibition in human glomerular endothelial cells. Am. J. Physiol. Ren. Physiol. 2014, 307, F623–F633. [Google Scholar] [CrossRef]
- Dlugosova, K.; Weismann, P.; Bernatova, I.; Sotnikova, R.; Slezak, J.; Okruhlicova, L. Omega-3 fatty acids and atorvastatin affect connexin 43 expression in the aorta of hereditary hypertriglyceridemic rats. Can. J. Physiol. Pharmacol. 2009, 87, 1074–1082. [Google Scholar] [CrossRef]
- Kavvadas, P.; Abed, A.; Poulain, C.; Authier, F.; Labejof, L.P.; Calmont, A.; Afieri, C.; Prakoura, N.; Dussaule, J.C.; Chatziantoniou, C.; et al. Decreased Expression of Connexin 43 Blunts the Progression of Experimental GN. J. Am. Soc. Nephrol. JASN 2017, 28, 2915–2930. [Google Scholar] [CrossRef] [PubMed]
- Mann, P.C.; Vahle, J.; Keenan, C.M.; Baker, J.F.; Bradley, A.E.; Goodman, D.G.; Harada, T.; Herbert, R.; Kaufmann, W.; Kellner, R.; et al. International harmonization of toxicologic pathology nomenclature: An overview and review of basic principles. Toxicol. Pathol. 2012, 40 (Suppl. S4), 7s–13s. [Google Scholar] [CrossRef] [PubMed]
- Follmann, M.; Ackerstaff, J.; Redlich, G.; Wunder, F.; Lang, D.; Kern, A.; Fey, P.; Griebenow, N.; Kroh, W.; Becker-Pelster, E.M.; et al. Discovery of the Soluble Guanylate Cyclase Stimulator Vericiguat (BAY 1021189) for the Treatment of Chronic Heart Failure. J. Med. Chem. 2017, 60, 5146–5161. [Google Scholar] [CrossRef] [PubMed]
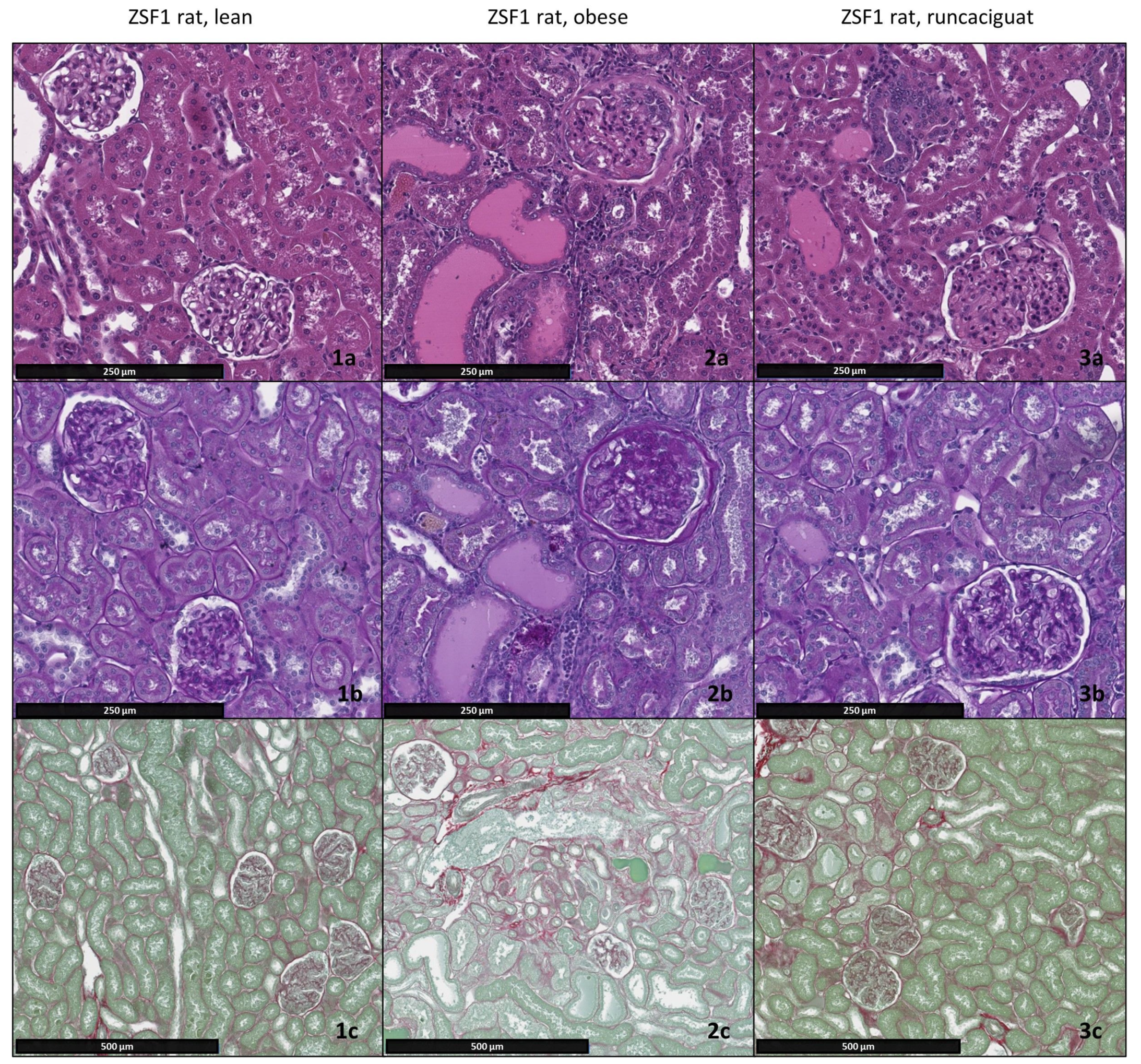


| ZSF1 Obese Rats Treatment Groups | Kidney Weight/Body Weight [g/kg] | Glomerulopathy [Grade] | Tubular Degeneration [Grade] | Protein Casts [Grade] | Interstital Fibrosis [Grade] |
|---|---|---|---|---|---|
| ZSF1 obese + placebo | 7.17 ± 0.34 | 3.28 ± 0.57 | 2.94 ± 0.31 | 2.63 ± 0.34 | 2.57 ± 0.31 |
| ZSF1 obese + 1 mg/kg BID | 6.95 ± 0.50 | 3.1 ± 0.41 | 2.85 ±0.01 | 2.25 ± 0.73 | 2.4 ± 0.6 |
| ZSF1 obese + 3 mg/kg BID | 5.95 ± 0.33 * | 2.09 ± 0.68 | 2.10 ± 0.63 | 1.69 ± 0.68 | 1.37 ± 0.67 |
| ZSF1 obese + 10 mg/kg BID | 5.45 ± 0.16 * | 1.55 ± 0.92 | 1.15 ± 0.49 | 1.20 ± 0.14 | 0.9 ± 0.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kraehling, J.R.; Benardeau, A.; Schomber, T.; Popp, L.; Vienenkoetter, J.; Ellinger-Ziegelbauer, H.; Pavkovic, M.; Hartmann, E.; Siudak, K.; Freyberger, A.; et al. The sGC Activator Runcaciguat Has Kidney Protective Effects and Prevents a Decline of Kidney Function in ZSF1 Rats. Int. J. Mol. Sci. 2023, 24, 13226. https://doi.org/10.3390/ijms241713226
Kraehling JR, Benardeau A, Schomber T, Popp L, Vienenkoetter J, Ellinger-Ziegelbauer H, Pavkovic M, Hartmann E, Siudak K, Freyberger A, et al. The sGC Activator Runcaciguat Has Kidney Protective Effects and Prevents a Decline of Kidney Function in ZSF1 Rats. International Journal of Molecular Sciences. 2023; 24(17):13226. https://doi.org/10.3390/ijms241713226
Chicago/Turabian StyleKraehling, Jan R., Agnes Benardeau, Tibor Schomber, Laura Popp, Julia Vienenkoetter, Heidrun Ellinger-Ziegelbauer, Mira Pavkovic, Elke Hartmann, Krystyna Siudak, Alexius Freyberger, and et al. 2023. "The sGC Activator Runcaciguat Has Kidney Protective Effects and Prevents a Decline of Kidney Function in ZSF1 Rats" International Journal of Molecular Sciences 24, no. 17: 13226. https://doi.org/10.3390/ijms241713226
APA StyleKraehling, J. R., Benardeau, A., Schomber, T., Popp, L., Vienenkoetter, J., Ellinger-Ziegelbauer, H., Pavkovic, M., Hartmann, E., Siudak, K., Freyberger, A., Hagelschuer, I., Mathar, I., Hueser, J., Hahn, M. G., Geiss, V., Eitner, F., & Sandner, P. (2023). The sGC Activator Runcaciguat Has Kidney Protective Effects and Prevents a Decline of Kidney Function in ZSF1 Rats. International Journal of Molecular Sciences, 24(17), 13226. https://doi.org/10.3390/ijms241713226






