Refining Evaluation of Bone Mass and Adipose Distribution in Dunnigan Syndrome
Abstract
1. Introduction
2. Results
2.1. Clinical and Laboratory Findings
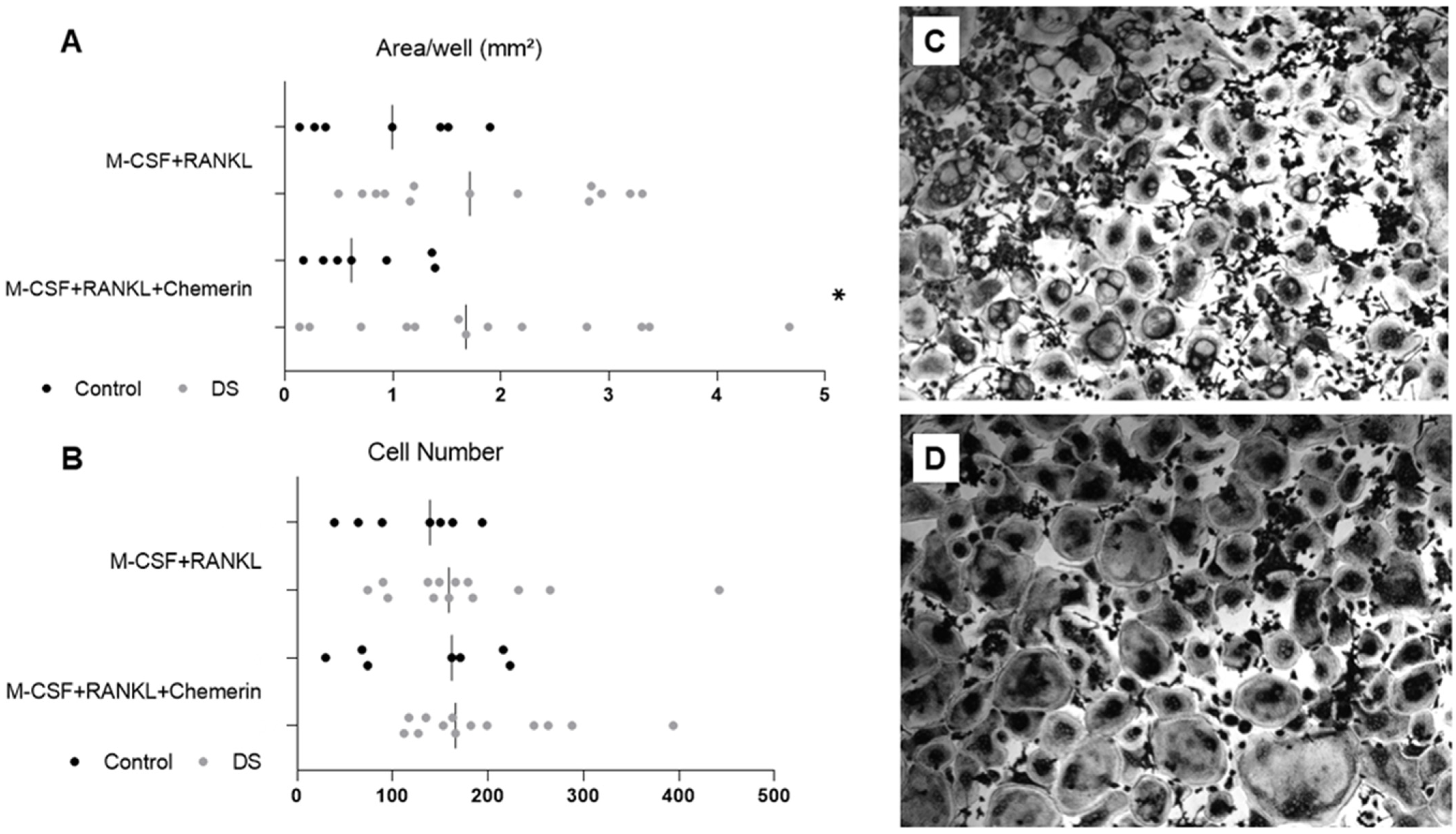
2.2. Bone Phenotype, Adipose Tissue Distribution and Osteoclastogenesis
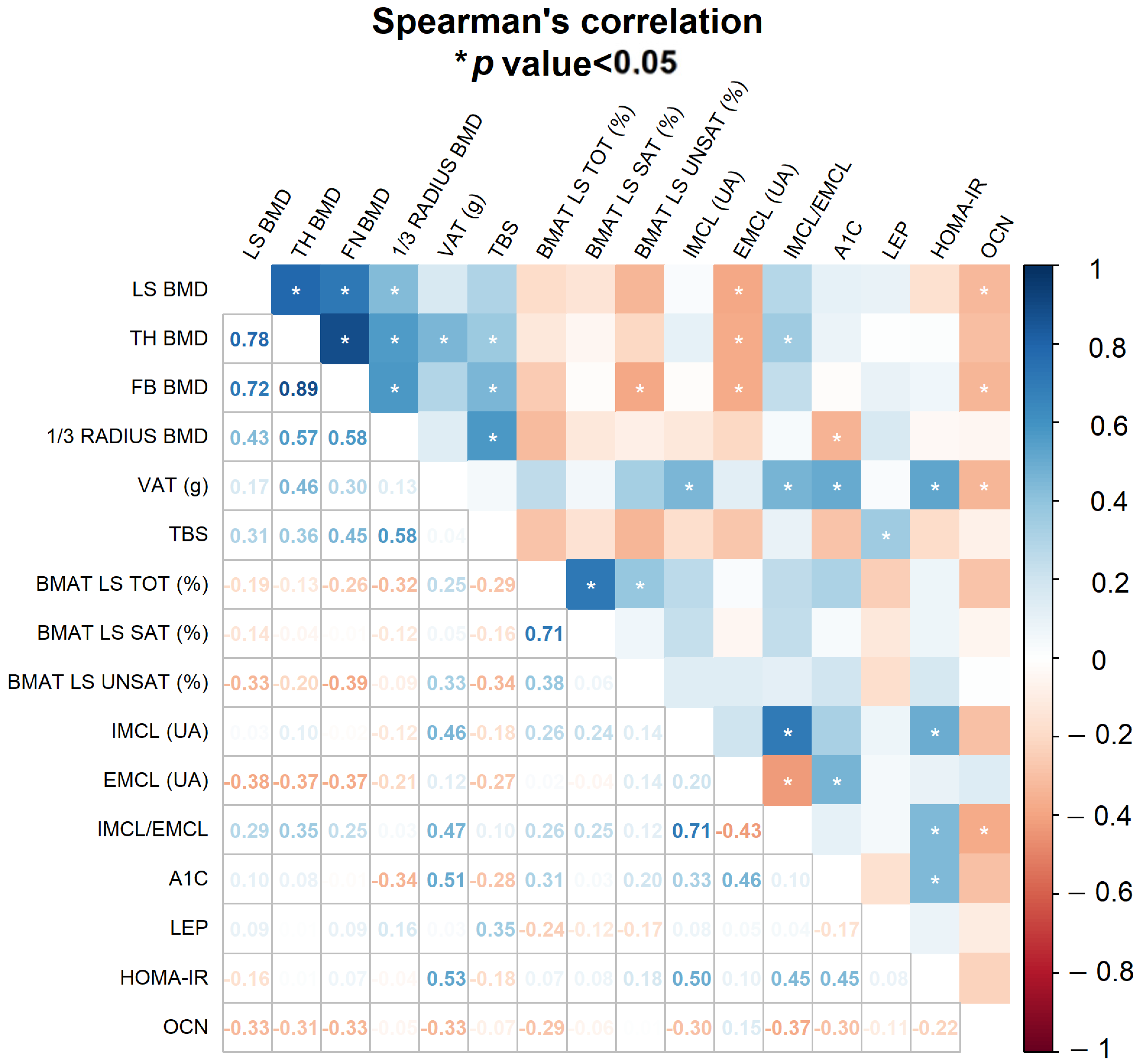
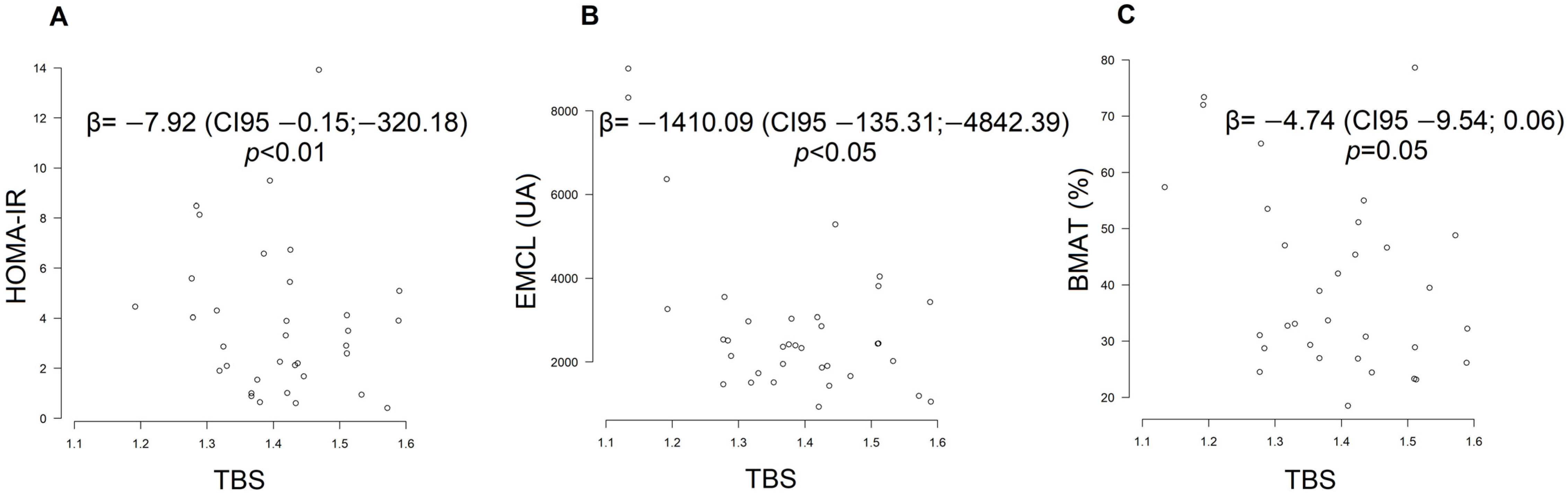
2.3. Influence of IR Markers on Bone Quantity, Quality and Lipid Distribution
3. Discussion
4. Materials and Methods
4.1. Study Design and Population
4.2. Biochemistry
4.3. LMNA Mutation Analysis
4.4. Dual-Energy X-ray Absorptiometry
4.5. ¹H-MR Spectroscopy
4.5.1. Lumbar Spine
4.5.2. Soleus Muscle
4.6. Osteoclastogenesis
4.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Agrawal, S.; Klarqvist, M.D.R.; Diamant, N.; Stanley, T.L.; Ellinor, P.T.; Mehta, N.N.; Philippakis, A.; Ng, K.; Claussnitzer, M.; Grinspoon, S.K.; et al. BMI-Adjusted Adipose Tissue Volumes Exhibit Depot-Specific and Divergent Associations with Cardiometabolic Diseases. Nat. Commun. 2023, 14, 266. [Google Scholar] [CrossRef]
- Fernández-Pombo, A.; Ossandon-Otero, J.A.; Guillín-Amarelle, C.; Sánchez-Iglesias, S.; Castro, A.I.; González-Méndez, B.; Rodríguez-García, S.; Rodriguez-Cañete, L.; Casanueva, F.F.; Araújo-Vilar, D. Bone Mineral Density in Familial Partial Lipodystrophy. Clin. Endocrinol. 2018, 88, 44–50. [Google Scholar] [CrossRef]
- de Araújo, I.M.; Moreira, M.L.M.; de Paula, F.J.A. Diabetes and Bone. Arch. Endocrinol. Metab. 2022, 66, 633–641. [Google Scholar] [CrossRef]
- De Paula, F.J.A.; Rosen, C.J. Structure and Function of Bone Marrow Adipocytes. Compr. Physiol. 2018, 8, 315–349. [Google Scholar] [CrossRef]
- de Araújo, I.M.; Parreiras-e-Silva, L.T.; Carvalho, A.L.; Elias, J.; Salmon, C.E.G.; de Paula, F.J.A. Insulin Resistance Negatively Affects Bone Quality Not Quantity: The Relationship between Bone and Adipose Tissue. Osteoporos. Int. 2020, 31, 1125–1133. [Google Scholar] [CrossRef]
- Palomo, T.; Muszkat, P.; Weiler, F.G.; Dreyer, P.; Brandão, C.M.A.; Silva, B.C. Update on Trabecular Bone Score. Arch. Endocrinol. Metab. 2022, 66, 694–706. [Google Scholar] [CrossRef]
- Fernandez-Pombo, A.; Diaz-Lopez, E.J.; Castro, A.I.; Sanchez-Iglesias, S.; Cobelo-Gomez, S.; Prado-Moraña, T.; Araujo-Vilar, D. Clinical Spectrum of LMNA-Associated Type 2 Familial Partial Lipodystrophy: A Systematic Review. Cells 2023, 12, 725. [Google Scholar] [CrossRef]
- Walsh, J.S.; Evans, A.L.; Bowles, S.; Naylor, K.E.; Jones, K.S.; Schoenmakers, I.; Jacques, R.M.; Eastell, R. Free 25-Hydroxyvitamin D Is Low in Obesity, but There Are No Adverse Associations with Bone Health. Am. J. Clin. Nutr. 2016, 103, 1465–1471. [Google Scholar] [CrossRef]
- Garg, A.; Peshock, R.M.; Fleckenstein, J.L. Adipose Tissue Distribution Pattern in Patients with Familial Partial Lipodystrophy (Dunnigan Variety). J. Clin. Endocrinol. Metab. 1999, 84, 170–174. [Google Scholar] [CrossRef][Green Version]
- Savage, D.B.; Watson, L.; Carr, K.; Adams, C.; Brage, S.; Chatterjee, K.K.; Hodson, L.; Boesch, C.; Kemp, G.J.; Sleigh, A. Accumulation of Saturated Intramyocellular Lipid Is Associated with Insulin Resistance. J. Lipid Res. 2019, 60, 1323–1332. [Google Scholar] [CrossRef]
- Machann, J.; Häring, H.; Shick, F.; Stumvoll, M. Intramyocellular Lipids and Insulin Resistance. Diabetes Obes. Metab. 2004, 6, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Ghani, M.A.; Defronzo, R.A. Pathogenesis of Insulin Resistance in Skeletal Muscle. J. Biomed. Biotechnol. 2010, 2010, 476279. [Google Scholar] [CrossRef] [PubMed]
- Bachmann, O.P.; Dahl, D.B.; Brechtel, K.; Rgen Machann, J.; Haap, M.; Maier, T.; Loviscach, M.; Stumvoll, M.; Claussen, C.D.; Schick, F.; et al. Effects of Intravenous and Dietary Lipid Challenge on Intramyocellular Lipid Content and the Relation with Insulin Sensitivity in Humans. Diabetes 2001, 50, 2579–2584. [Google Scholar] [CrossRef] [PubMed]
- Patsch, J.M.; Li, X.; Baum, T.; Yap, S.P.; Karampinos, D.C.; Schwartz, A.V.; Link, T.M. Bone Marrow Fat Composition as a Novel Imaging Biomarker in Postmenopausal Women with Prevalent Fragility Fractures. J. Bone Miner. Res. 2013, 28, 1721–1728. [Google Scholar] [CrossRef]
- Devlin, M.J. Bone Marrow Composition, Diabetes, and Fracture Risk: More Bad News for Saturated Fat. J. Bone Miner. Res. 2013, 28, 1718–1720. [Google Scholar] [CrossRef] [PubMed]
- De Araújo, I.M.; Salmon, C.E.G.; Nahas, A.K.; Nogueira-Barbosa, M.H.; Elias, J.; De Paula, F.J.A. Marrow Adipose Tissue Spectrum in Obesity and Type 2 Diabetes Mellitus. Eur. J. Endocrinol. 2017, 176, 21–30. [Google Scholar] [CrossRef]
- Dos Reis, R.C.P.; Duncan, B.B.; Malta, D.C.; Iser, B.P.M.; Schmidt, M.I. Evolution of Diabetes in Brazil: Prevalence Data from the 2013 and 2019 Brazilian National Health Survey. Cad. Saúde Pública 2022, 38, e00149321. [Google Scholar] [CrossRef]
- McCloskey, E.V.; Odén, A.; Harvey, N.C.; Leslie, W.D.; Hans, D.; Johansson, H.; Barkmann, R.; Boutroy, S.; Brown, J.; Chapurlat, R.; et al. A Meta-Analysis of Trabecular Bone Score in Fracture Risk Prediction and Its Relationship to FRAX. J. Bone Miner. Res. 2016, 31, 940–948. [Google Scholar] [CrossRef]
- Hegele, R.A.; Cao, H.; Huff, M.W.; Anderson, C.M. LMNA R482Q Mutation in Partial Lipodystrophy Associated with Reduced Plasma Leptin Concentration. J. Clin. Endocrinol. Metab. 2000, 85, 3089–3093. [Google Scholar] [CrossRef][Green Version]
- Brown, R.J.; Gorden, P. Leptin Therapy in Patients with Lipodystrophy and Syndromic Insulin Resistance. In Leptin; Springer International Publishing: Cham, Switzerland, 2015; pp. 225–236. [Google Scholar]
- Reid, I.R.; Ames, R.; Evans, M.C.; Sharpe, S.; Gamble, G.; France, J.T.; Lim, T.M.; Cundy, T.F. Determinants of Total Body and Regional Bone Mineral Density in Normal Postmenopausal Women—A Key Role for Fat Mass. J. Clin. Endocrinol. Metab. 1992, 75, 45–51. [Google Scholar] [CrossRef]
- Walowski, C.O.; Herpich, C.; Enderle, J.; Braun, W.; Both, M.; Hasler, M.; Müller, M.J.; Norman, K.; Bosy-Westphal, A. Determinants of Bone Mass in Older Adults with Normal- and Overweight Derived from the Crosstalk with Muscle and Adipose Tissue. Sci. Rep. 2023, 13, 5030. [Google Scholar] [CrossRef] [PubMed]
- Lima, J.G.; Nobrega, L.H.C.; Lima, N.N.; dos Santos, M.C.F.; Baracho, M.d.F.P.; Winzenrieth, R.; Bandeira, F.; Mendes-Aguiar, C.d.O.; Neto, F.P.F.; Ferreira, L.C.; et al. Normal Bone Density and Trabecular Bone Score, but High Serum Sclerostin in Congenital Generalized Lipodystrophy. Bone 2017, 101, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Zou, W.; Rohatgi, N.; Brestoff, J.R.; Zhang, Y.; Scheller, E.L.; Craft, C.S.; Brodt, M.D.; Migotsky, N.; Silva, M.J.; Harris, C.A.; et al. Congenital Lipodystrophy Induces Severe Osteosclerosis. PLoS Genet. 2019, 15, e1008244. [Google Scholar] [CrossRef] [PubMed]
- Lima, J.G.; Nobrega, L.H.C.; Lima, N.N.; dos Santos, M.C.F.; Baracho, M.d.F.P.; Bandeira, F.; Capistrano, L.; Freire Neto, F.P.; Jeronimo, S.M.B. Bone Density in Patients with Berardinelli-Seip Congenital Lipodystrophy Is Higher in Trabecular Sites and in Type 2 Patients. J. Clin. Densitom. 2018, 21, 61–67. [Google Scholar] [CrossRef]
- Leslie, W.D.; Johansson, H.; McCloskey, E.V.; Harvey, N.C.; Kanis, J.A.; Hans, D. Comparison of Methods for Improving Fracture Risk Assessment in Diabetes: The Manitoba BMD Registry. J. Bone Miner. Res. 2018, 33, 1923–1930. [Google Scholar] [CrossRef]
- Spuler, S.; Kalbhenn, T.; Zabojszcza, J.; van Landeghem, F.K.H.; Ludtke, A.; Wenzel, K.; Koehnlein, M.; Schuelke, M.; Ludemann, L.; Schmidt, H.H. Muscle and Nerve Pathology in Dunnigan Familial Partial Lipodystrophy. Neurology 2007, 68, 677–683. [Google Scholar] [CrossRef]
- Szczepaniak, L.S.; Babcock, E.E.; Schick, F.; Dobbins, R.L.; Garg, A.; Burns, D.K.; Mcgarry, J.D.; Stein, D.T. Measurement of Intracellular Triglyceride Stores by 1 H Spectroscopy: Validation In Vivo. Am. J. Physiol.-Endocrinol. Metab. 1999, 276, E977–E989. [Google Scholar] [CrossRef]
- Krebs, M.; Roden, M. Molecular Mechanisms of Lipid-Induced Insulin Resistance in Muscle, Liver and Vasculature. Diabetes Obes. Metab. 2005, 7, 621–632. [Google Scholar] [CrossRef]
- De Paula, F.J.A.; De Araújo, I.M.; Carvalho, A.L.; Elias, J.; Salmon, C.E.G.; Nogueira-Barbosa, M.H. The Relationship of Fat Distribution and Insulin Resistance with Lumbar Spine Bone Mass in Women. PLoS ONE 2015, 10, e0129764. [Google Scholar] [CrossRef]
- Batista, S.L.; De Araújo, I.M.; Carvalho, A.L.; Alencar, M.A.V.S.D.; Nahas, A.K.; Elias, J.; Nogueira-Barbosa, M.H.; Salmon, C.E.G.; Elias, P.C.L.; Moreira, A.C.; et al. Beyond the Metabolic Syndrome: Visceral and Marrow Adipose Tissues Impair Bone Quantity and Quality in Cushing’s Disease. PLoS ONE 2019, 14, e0223432. [Google Scholar] [CrossRef]
- De Paula, F.J.A.; Rosen, C.J. Vitamin D Safety and Requirements. Arch. Biochem. Biophys. 2012, 523, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Bozaoglu, K.; Bolton, K.; McMillan, J.; Zimmet, P.; Jowett, J.; Collier, G.; Walder, K.; Segal, D. Chemerin Is a Novel Adipokine Associated with Obesity and Metabolic Syndrome. Endocrinology 2007, 148, 4687–4694. [Google Scholar] [CrossRef] [PubMed]
- Goralski, K.B.; McCarthy, T.C.; Hanniman, E.A.; Zabel, B.A.; Butcher, E.C.; Parlee, S.D.; Muruganandan, S.; Sinal, C.J. Chemerin, a Novel Adipokine That Regulates Adipogenesis and Adipocyte Metabolism. J. Biol. Chem. 2007, 282, 28175–28188. [Google Scholar] [CrossRef]
- Muruganandan, S.; Parlee, S.D.; Rourke, J.L.; Ernst, M.C.; Goralski, K.B.; Sinal, C.J. Chemerin, a Novel Peroxisome Proliferator-Activated Receptor γ (PPARγ) Target Gene That Promotes Mesenchymal Stem Cell Adipogenesis. J. Biol. Chem. 2011, 286, 23982–23995. [Google Scholar] [CrossRef]
- Muruganandan, S.; Govindarajan, R.; McMullen, N.M.; Sinal, C.J. Chemokine-Like Receptor 1 Is a Novel Wnt Target Gene That Regulates Mesenchymal Stem Cell Differentiation. Stem Cells 2017, 35, 711–724. [Google Scholar] [CrossRef] [PubMed]
- Muruganandan, S.; Dranse, H.J.; Rourke, J.L.; Mcmullen, N.M.; Sinal, C.J. Chemerin Neutralization Blocks Hematopoietic Stem Cell Osteoclastogenesis. Stem Cells 2013, 31, 2172–2182. [Google Scholar] [CrossRef]
- Lin, Y.; Xiao, L.; Cai, Q.; Zhu, C.; Li, S.; Li, B.; Liu, T.; Zhang, Q.; Wang, Y.; Li, Y.; et al. The Chemerin-CMKLR1 Axis Limits Thermogenesis by Controlling a Beige Adipocyte/IL-33/Type 2 Innate Immunity Circuit. Sci. Immunol. 2021, 6, eabg9698. [Google Scholar] [CrossRef]
- Hussain, I.; Garg, A. Lipodystrophy Syndromes. Endocrinol. Metab. Clin. N. Am. 2016, 45, 783–797. [Google Scholar] [CrossRef]
- Cao, H.; Hegele, R.A. Nuclear Lamin A/C R482Q Mutation in Canadian Kindreds with Dunnigan-Type Familial Partial Lipodystrophy. Hum. Mol. Genet. 2000, 9, 109–112. [Google Scholar] [CrossRef]
- Speckman, R.A.; Garg, A.; Du, F.; Bennett, L.; Veile, R.; Arioglu, E.; Taylor, S.I.; Lovett, M.; Bowcock, A.M. Mutational and Haplotype Analyses of Families with Familial Partial Lipodystrophy (Dunnigan Variety) Reveal Recurrent Missense Mutations in the Globular C-Terminal Domain of Lamin A/C. J. Hum. Genet. 2000, 66, 1192–1198. [Google Scholar] [CrossRef]
- Shackleton, S.; Lloyd, D.J.; Jackson, S.N.J.; Evans, R.; Niermeijer, M.F.; Singh, B.M.; Schmidt, H.; Brabant, G.; Kumar, S.; Durrington, P.N.; et al. LMNA, Encoding Lamin A/C, Is Mutated in Partial Lipodystrophy. Nat. Genet. 2000, 24, 153–156. [Google Scholar] [CrossRef] [PubMed]
- Chiquette, E.; Oral, E.A.; Garg, A.; Araújo-Vilar, D.; Dhankhar, P. Estimating the Prevalence of Generalized and Partial Lipodystrophy: Findings and Challenges. Diabetes Metab. Syndr. Obes. 2017, 10, 375–383. [Google Scholar] [CrossRef]
- Brown, R.J.; Araujo-Vilar, D.; Cheung, P.T.; Dunger, D.; Garg, A.; Jack, M.; Mungai, L.; Oral, E.A.; Patni, N.; Rother, K.I.; et al. The Diagnosis and Management of Lipodystrophy Syndromes: A Multi-Society Practice Guideline. J. Clin. Endocrinol. Metab. 2016, 101, 4500–4511. [Google Scholar] [CrossRef] [PubMed]
- Geloneze, B.; Carolina Junqueira Vasques, A.; França Camargo Stabe, C.; Carlos Pareja, J.; Enriqueta Frandsen Paez de Lima Rosado, L.; Cristina de Queiroz, E.; Antonio Tambascia, M.; Investigators, B.; Letícia Murro, A.; Claudia Felici, A.; et al. HOMA1-IR and HOMA2-IR Indexes in Identifying Insulin Resistance and Metabolic Syndrome-Brazilian Metabolic Syndrome Study (BRAMS) Índices HOMA1-IR e HOMA2-IR Para Identificação de Resistência à Insulina e Síndrome Metabólica-Estudo Brasileiro de Síndrome Metabólica (BRAMS). Arq. Bras. Endocrinol. Metabol. 2009, 53, 281–287. [Google Scholar] [PubMed]
- Parreiras-e-Silva, L.T.; de Araújo, I.M.; Elias, J.; Nogueira-Barbosa, M.H.; Suen, V.M.M.; Marchini, J.S.; Salmon, C.E.G.; de Paula, F.J.A. Osteoporosis and Hepatic Steatosis: 2 Closely Related Complications in Short-Bowel Syndrome. J. Parenter. Enter. Nutr. 2020, 44, 1271–1279. [Google Scholar] [CrossRef]
- Ramos-Junior, E.S.; Leite, G.A.; Carmo-Silva, C.C.; Taira, T.M.; Neves, K.B.; Colón, D.F.; da Silva, L.A.B.; Salvador, S.L.; Tostes, R.C.; Cunha, F.Q.; et al. Adipokine Chemerin Bridges Metabolic Dyslipidemia and Alveolar Bone Loss in Mice. J. Bone Miner. Res. 2017, 32, 974–984. [Google Scholar] [CrossRef]
- Taira, T.M.; Ramos-Junior, E.S.; Melo, P.H.; Costa-Silva, C.C.; Alteen, M.G.; Vocadlo, D.J.; Dias, W.B.; Cunha, F.Q.; Alves-Filho, J.C.; Søe, K.; et al. HBP/O-GlcNAcylation Metabolic Axis Regulates Bone Resorption Outcome. J. Dent. Res. 2023, 102, 440–449. [Google Scholar] [CrossRef]
- Montgomery, D.C. Design and Analysis of Experiments; Springer: New York, NY, USA, 1999; ISBN 9781118146927. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing 2022; R Core Team: Vienna, Austria, 2022. [Google Scholar]


| Reference Values | Control (n= 23) | DS (n = 18) | p-Value | |
|---|---|---|---|---|
| Female/male | 18/5 | 15/3 | ||
| Age, years | 38.6 ± 9.6 | 39.2 ± 11.0 | 0.76 | |
| Height, m | 1.67 ± 0.08 | 1.62 ± 0.09 | 0.67 | |
| Weight, kg | 73.9 ± 15.2 | 70.6 ± 21.2 | 0.76 | |
| BMI, kg/m² | (18–25) | 26.3 ± 4.5 | 26.5 ± 5.2 | 0.75 |
| Glucose, mg/dL | (70.0–100.0) | 91.2 ± 9.7 | 175.4 ± 107.7 | <0.05 |
| HbA1c, % | (4.7–5.6) | 5.0 (4.6; 5.6) | 7.4 (5.5; 10; 6) | <0.05 |
| Fasting insulin, µIU/mL | (2.0–25.0) | 10.7 ± 6.8 | 26.4 ± 17.0 # | <0.05 |
| HOMA-IR | <2.7 | 2.1 (1.0; 3.5) | 5.6 (4.1; 8.5) # | <0.05 |
| Total cholesterol, mg/dL | <190.0 | 169.8 ± 30.5 | 186.4 ± 48.2 | 0.19 |
| HDLc, mg/dL | >40.0 | 53.6 ± 13.9 | 33.1 ± 10.9 | <0.05 |
| LDLc, mg/dL | <130.0 | 97.7 ± 26.5 | 91.3 ± 32.6 | 0.50 |
| Triglyceride, mg/dL | <150.0 | 75.0 (51.0; 116.0) | 378.0 (197.0; 612.0) | <0.05 |
| Serum calcium, mg/dL | 8.5–10.5 | 9.0 ± 0.3 | 10.0 ± 2.4 | <0.05 |
| Albumin, g/dL | 3.5–5.0 | 4.4 ± 0.3 | 4.6 ± 1.4 | 0.63 |
| Serum phosphorus, mg/dL | 2.5–5.5 | 3.9 ± 0.6 | 4.5 ± 1.3 | 0.06 |
| Alkaline phosphatase, U/L | 40.0–150.0 | 58.5 (50.0; 71.8) | 101; 5 (75.0; 123.0) | <0.05 |
| Urea, mg/dL | 10.0–45.0 | 29.0 (26.4; 35.8) | 28.5 (23.0; 33.0) | 0.29 |
| Creatinine, mg/dL | 0.6–1.2 | 0.9 ± 0.2 | 0.8 ± 0.1 | <0.05 |
| AST, U/L | 10.0–40.0 | 21.0 (17.0; 27.0) | 21.0 (17.0; 36.0) | 0.14 |
| ALT, U/L | 5.0–40.0 | 19.7 (13.6; 28.0) | 26.5 (21.0; 56.0) | <0.05 |
| 25(OH) vitamin-D, ng/mL | >20.0 | 25.5 ± 8.3 | 18.7 ± 8.0 | <0.05 |
| PTH, pg/mL | 15.0–65.0 | 69.3 ± 28.6 | 37.9 ± 15.2 | <0.05 |
| Osteocalcin, ng/mL | 11.0–46.0 | 22.6 ± 5.5 | 17.8 ± 6.1 | <0.05 |
| Leptin, ng/mL | 6.5–19.1 | 7.0 (4.1; 32.8) | 4.7 (3.8; 12.2) | 0.07 |
| Chemerin, ng/mL | 0.3–8.0 | 4.7 ± 0.9 | 4.8 ± 1.1 | 0.90 |
| DXA | Control (n = 23) | DS (n = 18) | p-Value | |
|---|---|---|---|---|
| Lumbar spine (L1–L4) | BMD, g/cm² | 1.199 ± 0.161 | 1.180 ± 0.210 | 0.79 |
| Z-score. SD | 0.2 ± 1.5 | 0.2 ± 1.7 | 0.97 | |
| Total hip | BMD, g/cm² | 1.000 ± 0.146 | 1.040 ± 0.190 | 0.50 |
| Z-score, SD | 0.1 ± 1.1 | 0.4 ± 1.5 | 0.41 | |
| Femoral neck | BMD, g/cm² | 1.002 ± 0.132 | 1.020 ± 0.210 | 0.56 |
| Z-score, SD | 0.0 ± 1.1 | 0.4 ± 1.6 | 0.47 | |
| 1/3 radius | BMD, g/cm² | 0.890 ± 0.090 | 0.810 ± 0.120 | <0.05 |
| Z-score, SD | −0.1 ± 0.8 | −0.9 ± 1.1 | <0.05 | |
| TBS (L1–L4) | 1.440 ± 0.080 | 1.310 ± 0.110 | <0.05 |
| ρ | p-Value | |
|---|---|---|
| Osteoclast area (RANKL) × lumbar spine BMD | 0.57 | <0.05 |
| Osteoclast area (RANKL + chemerin) × lumbar spine BMD | 0.40 | 0.08 |
| Osteoclast area (RANKL) × total hip BMD | 0.55 | <0.05 |
| Osteoclast area (RANKL + chemerin) × total hip BMD | 0.46 | <0.05 |
| Osteoclast area (RANKL) × femoral neck BMD | 0.39 | 0.09 |
| Osteoclast area (RANKL + chemerin) × femoral neck BMD | 0.40 | 0.08 |
| Osteoclast area (RANKL) × BMAT | 0.14 | 0.58 |
| Osteoclast area (RANKL + chemerin) × BMAT | 0.11 | 0.69 |
| Osteoclast area (RANKL) × TBS | 0 | 0.98 |
| Osteoclast area (RANKL + chemerin) × TBS | 0.03 | 0.91 |
| Osteoclast number (RANKL) × BMAT | 0.25 | 0.34 |
| Osteoclast number (RANKL + chemerin) × BMAT | 0.14 | 0.60 |
| Osteoclast number (RANKL) × TBS | 0.12 | 0.63 |
| Osteoclast number (RANKL + chemerin) × TBS | 0.10 | 0.68 |
| Unadjusted | Adjusted | |||||
|---|---|---|---|---|---|---|
| β Effect | CI | p-Value | β Effect | CI | p-Value | |
| Glucose × femoral neck BMD | 3.96 | −12.08:19.99 | 0.62 | 12.18 | 0.71:23.65 | <0.05 |
| HOMA-IR × 1/3 radius BMD | −0.43 | −0.04:89.5 | 0.52 | −0.90 | −0.03:0.57 | 0.05 |
| Osteocalcin × lumbar spine BMD | −1.01 | −2.06:0.04 | 0.06 | −1.04 | −1.99:−0.09 | <0.05 |
| HOMA-IR × TBS | −2.95 | −0.04:818.47 | 0.25 | −7.92 | −0.15:−320.18 | <0.01 |
| EMCL × TBS | −3577.45 | −598.14:−13,308.65 | <0.01 | −1410.09 | −135.31:−4842.39 | <0.05 |
| EMCL × lumbar spine BMD | −681.17 | −299.67:−706.46 | <0.05 | −250.57 | −68.33:−392.41 | <0.05 |
| EMCL × total hip BMD | −774.27 | −328.41:−925.64 | <0.01 | −300.16 | −76.91:−576.98 | <0.05 |
| EMCL × femoral neck BMD | −681.09 | −297.10:−667.18 | <0.05 | −236.45 | −59.36:26.01 | 0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moreira, M.L.M.; de Araújo, I.M.; Fukada, S.Y.; Venturini, L.G.R.; Guidorizzi, N.R.; Garrido, C.E.; Rosen, C.J.; de Paula, F.J.A. Refining Evaluation of Bone Mass and Adipose Distribution in Dunnigan Syndrome. Int. J. Mol. Sci. 2023, 24, 13118. https://doi.org/10.3390/ijms241713118
Moreira MLM, de Araújo IM, Fukada SY, Venturini LGR, Guidorizzi NR, Garrido CE, Rosen CJ, de Paula FJA. Refining Evaluation of Bone Mass and Adipose Distribution in Dunnigan Syndrome. International Journal of Molecular Sciences. 2023; 24(17):13118. https://doi.org/10.3390/ijms241713118
Chicago/Turabian StyleMoreira, Mariana Lima Mascarenhas, Iana Mizumukai de Araújo, Sandra Yasuyo Fukada, Lucas Gabriel R. Venturini, Natalia Rossin Guidorizzi, Carlos Ernesto Garrido, Clifford J. Rosen, and Francisco José Albuquerque de Paula. 2023. "Refining Evaluation of Bone Mass and Adipose Distribution in Dunnigan Syndrome" International Journal of Molecular Sciences 24, no. 17: 13118. https://doi.org/10.3390/ijms241713118
APA StyleMoreira, M. L. M., de Araújo, I. M., Fukada, S. Y., Venturini, L. G. R., Guidorizzi, N. R., Garrido, C. E., Rosen, C. J., & de Paula, F. J. A. (2023). Refining Evaluation of Bone Mass and Adipose Distribution in Dunnigan Syndrome. International Journal of Molecular Sciences, 24(17), 13118. https://doi.org/10.3390/ijms241713118






