Multiple Factors Involved in Bone Damage Caused by Chikungunya Virus Infection
Abstract
:1. Introduction
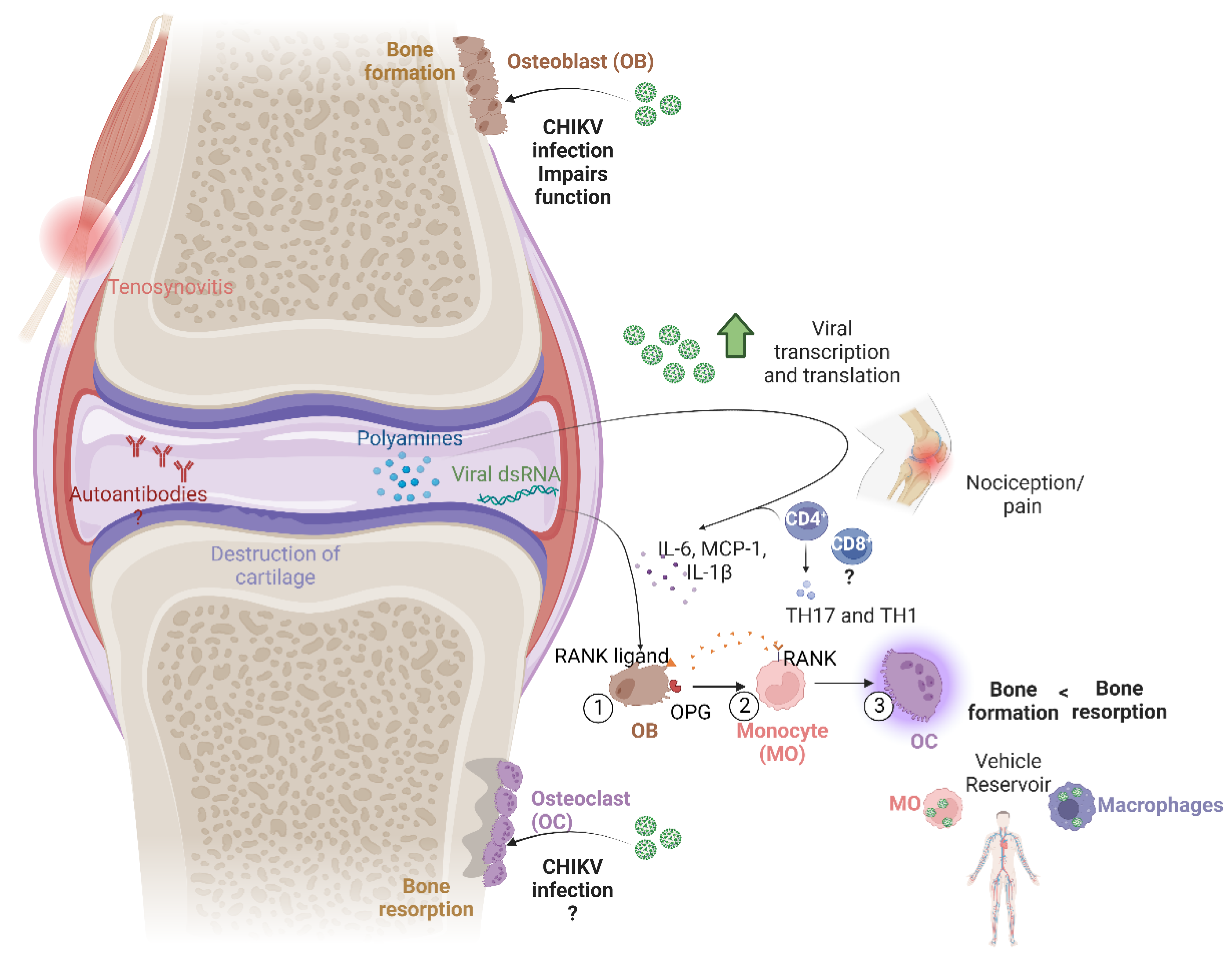
2. Bone Damage and Its Mechanisms
3. Cell Signaling in Bone Resorption
4. Persistence of Viral RNA and Proteins in Chronic Cases
5. The Role of the Immune Response
6. Comorbidities
7. Genetic Factors
8. Viral Arthritis Markers
9. Autoimmunity and Bone Erosion
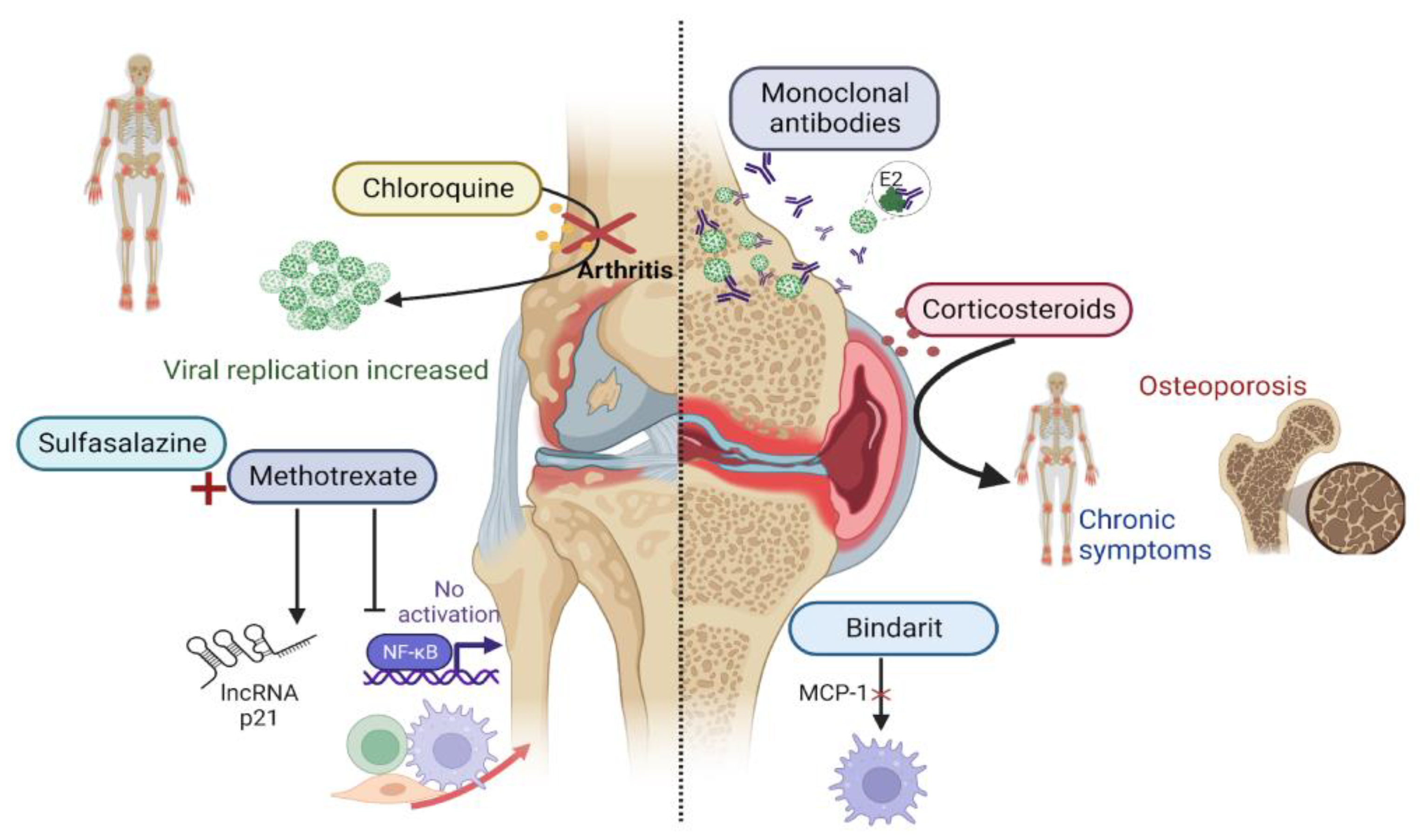
10. Treatment Perspectives
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Ross, R.W. The Newala Epidemic. Epidemiol. Infect. 1956, 54, 177–191. [Google Scholar]
- Amdekar, S.; Parashar, D.; Alagarasu, K. Chikungunya Virus-Induced Arthritis: Role of Host and Viral Factors in the Pathogenesis. Viral Immunol. 2017, 30, 691–702. [Google Scholar] [CrossRef] [PubMed]
- Schnierle, B.S. Cellular Attachment and Entry Factors for Chikungunya Virus. Viruses 2019, 11, 1078. [Google Scholar] [CrossRef]
- Garmashova, N.; Gorchakov, R.; Volkova, E.; Paessler, S.; Frolova, E.; Frolov, I. The Old World and New World Alphaviruses Use Different Virus-Specific Proteins for Induction of Transcriptional Shutoff. J. Virol. 2007, 81, 2472–2484. [Google Scholar] [CrossRef]
- Sutaria, R.B.; Amaral, J.K.; Schoen, R.T. Emergence and treatment of chikungunya arthritis. Curr. Opin. Rheumatol. 2018, 30, 256–263. [Google Scholar] [CrossRef]
- Amaral, J.K.; Bilsborrow, J.B.; Schoen, R.T. Chronic Chikungunya Arthritis and Rheumatoid Arthritis: What They Have in Common. Am. J. Med. 2020, 133, e91–e97. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.C.; Wu, Q.; Teh, S.W.; Peli, A.; Bu, G.; Qiu, Y.S.; Benelli, G.; Kumar, S.S. Bone breaking infections–A focus on bacterial and mosquito-borne viral infections. Microb. Pathog. 2018, 122, 130–136. [Google Scholar] [CrossRef]
- Malvy, D.; Ezzedine, K.; Mamani-Matsuda, M.; Autran, B.; Tolou, H.; Receveur, M.-C.; Pistone, T.; Rambert, J.; Moynet, D.; Mossalayi, D. Destructive arthritis in a patient with chikungunya virus infection with persistent specific IgM antibodies. BMC Infect. Dis. 2009, 9, 200. [Google Scholar] [CrossRef]
- Krutikov, M.; Manson, J. Chikungunya Virus Infection: An Update on Joint Manifestations and Management. Rambam Maimonides Med. J. 2016, 7, e0033. [Google Scholar] [CrossRef]
- Krishnamoorthy, K.; Harichandrakumar, K.T.; Kumari, A.K.; Das, L.K. Burden of chikungunya in India: Estimates of disability adjusted life years (DALY) lost in 2006 epidemic. J. Vector Borne Dis. 2009, 46, 26. [Google Scholar]
- Dapurkar, D.; Telang, M. A patent perspective on chikungunya. Acta Trop. 2019, 199, 105131. [Google Scholar] [CrossRef] [PubMed]
- Sharp, J.T.; Lidsky, M.D.; Collins, L.C.; Moreland, J. Methods of scoring the progression of radiologic changes in rheumatoid arthritis. Correlation of radiologic, clinical and laboratory abnormalities. Arthritis Rheum. 1971, 14, 706–720. [Google Scholar] [CrossRef] [PubMed]
- Baum, R.; Gravallese, E.M. Impact of Inflammation on the Osteoblast in Rheumatic Diseases. Curr. Osteoporos. Rep. 2014, 12, 9. [Google Scholar] [CrossRef] [PubMed]
- Teitelbaum, S.L.; Ross, F.P. Genetic regulation of osteoclast development and function. Nat. Rev. Genet. 2003, 4, 638–649. [Google Scholar] [CrossRef]
- Lacey, D.L.; Timms, E.; Tan, H.L.; Kelley, M.J.; Dunstan, C.R.; Burgess, T.; Elliott, R.; Colombero, A.; Elliott, G.; Scully, S.; et al. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell 1998, 93, 165–176. [Google Scholar] [CrossRef]
- Simonet, W.S.; Lacey, D.L.; Dunstan, C.R.; Kelley, M.; Chang, M.S.; Luthy, R.; Nguyen, H.Q.; Wooden, S.; Bennett, L.; Boone, T.; et al. Osteoprotegerin: A Novel Secreted Protein Involved in the Regulation of Bone Density. Cell 1997, 89, 309–319. [Google Scholar] [CrossRef]
- Li, M.; Xu, D. Antiresorptive activity of osteoprotegerin requires an intact heparan sulfate-binding site. Proc. Natl. Acad. Sci. USA 2020, 117, 17187–17194. [Google Scholar] [CrossRef]
- Li, M.; Yang, S.; Xu, D. Heparan Sulfate Regulates the Structure and Function of Osteoprotegerin in Osteoclastogenesis. J. Biol. Chem. 2016, 291, 24160–24171. [Google Scholar] [CrossRef]
- Xiong, J.; Cawley, K.; Piemontese, M.; Fujiwara, Y.; Zhao, H.; Goellner, J.J.; O’brien, C.A. Soluble RANKL contributes to osteoclast formation in adult mice but not ovariectomy-induced bone loss. Nat. Commun. 2018, 9, 2909. [Google Scholar] [CrossRef]
- Woo, K.M.; Choi, Y.; Ko, S.-H.; Ko, J.S.; Oh, K.-O.; Kim, K.-K.; Hong, S.H.; Lee, G.D.; Chung, J.Y.; Cho, K.S.; et al. Osteoprotegerin is present on the membrane of osteoclasts isolated from mouse long bones. Exp. Mol. Med. 2002, 34, 347–352. [Google Scholar] [CrossRef]
- Feng, W.; Liu, H.; Luo, T.; Liu, D.; Du, J.; Sun, J.; Wang, W.; Han, X.; Yang, K.; Guo, J.; et al. Author Correction: Combination of IL-6 and sIL-6R differentially regulate varying levels of RANKL-induced osteoclastogenesis through NF-κB, ERK and JNK signaling pathways. Sci. Rep. 2022, 12, 41411. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Zhou, X.; Huang, D.; Ji, Y.; Kang, F. IL-6 Enhances Osteocyte-Mediated Osteoclastogenesis by Promoting JAK2 and RANKL Activity In Vitro. Cell. Physiol. Biochem. 2017, 41, 1360–1369. [Google Scholar] [CrossRef]
- Feng, W.; Yang, P.; Liu, H.; Zhang, F.; Li, M. IL-6 promotes low concentration of RANKL-induced osteoclastic differentiation by mouse BMMs through trans-signaling pathway. J. Mol. Histol. 2022, 53, 599–610. [Google Scholar] [CrossRef] [PubMed]
- Yokota, K.; Sato, K.; Miyazaki, T.; Aizaki, Y.; Tanaka, S.; Sekikawa, M.; Kozu, N.; Kadono, Y.; Oda, H.; Mimura, T. Characterization and Function of Tumor Necrosis Factor and Interleukin-6–Induced Osteoclasts in Rheumatoid Arthritis. Arthritis Rheumatol. 2021, 73, 1145–1154. [Google Scholar] [CrossRef] [PubMed]
- Chang, A.Y.; Martins, K.A.O.; Encinales, L.; Reid, S.P.; Acuña, M.; Encinales, C.; Matranga, C.B.; Pacheco, N.; Cure, C.; Shukla, B.; et al. Chikungunya Arthritis Mechanisms in the Americas: A Cross-Sectional Analysis of Chikungunya Arthritis Patients Twenty-Two Months After Infection Demonstrating No Detectable Viral Persistence in Synovial Fluid. Arthritis Rheumatol. 2018, 70, 585–593. [Google Scholar] [CrossRef] [PubMed]
- Hoarau, J.-J.; Bandjee, M.-C.J.; Trotot, P.K.; Das, T.; Li-Pat-Yuen, G.; Dassa, B.; Denizot, M.; Guichard, E.; Ribera, A.; Henni, T.; et al. Persistent Chronic Inflammation and Infection by Chikungunya Arthritogenic Alphavirus in Spite of a Robust Host Immune Response. J. Immunol. 2010, 184, 5914–5927. [Google Scholar] [CrossRef] [PubMed]
- Frolov, I.; Kim, D.Y.; Akhrymuk, M.; Mobley, J.A.; Frolova, E.I. Hypervariable Domain of Eastern Equine Encephalitis Virus nsP3 Redundantly Utilizes Multiple Cellular Proteins for Replication Complex Assembly. J. Virol. 2017, 91, e00371-17. [Google Scholar] [CrossRef]
- Jin, J.; Galaz-Montoya, J.G.; Sherman, M.B.; Sun, S.Y.; Goldsmith, C.S.; O’Toole, E.T.; Ackerman, L.; Carlson, L.-A.; Weaver, S.C.; Chiu, W.; et al. Neutralizing Antibodies Inhibit Chikungunya Virus Budding at the Plasma Membrane. Cell Host Microbe 2018, 24, 417–428.e5. [Google Scholar] [CrossRef]
- Narendra, S.C.; Chalise, J.P.; Höök, N.; Magnusson, M. Dendritic cells activated by double-stranded RNA induce arthritis via autocrine type I IFN signaling. J. Leukoc. Biol. 2014, 95, 661–666. [Google Scholar] [CrossRef]
- Magnusson, M.; Zare, F.; Tarkowski, A. Requirement of type I interferon signaling for arthritis triggered by double-stranded RNA. Arthritis Rheum. 2006, 54, 148–157. [Google Scholar] [CrossRef]
- Zare, F.; Bokarewa, M.; Nenonen, N.; Bergström, T.; Alexopoulou, L.; Flavell, R.A.; Tarkowski, A. Arthritogenic Properties of Double-Stranded (Viral) RNA. J. Immunol. 2004, 172, 5656–5663. [Google Scholar] [CrossRef] [PubMed]
- Labadie, K.; Larcher, T.; Joubert, C.; Mannioui, A.; Delache, B.; Brochard, P.; Guigand, L.; Dubreil, L.; Lebon, P.; Verrier, B.; et al. Chikungunya disease in nonhuman primates involves long-term viral persistence in macrophages. J. Clin. Investig. 2010, 120, 894–906. [Google Scholar] [CrossRef] [PubMed]
- Young, A.R.; Locke, M.C.; Cook, L.E.; Hiller, B.E.; Zhang, R.; Hedberg, M.L.; Monte, K.J.; Veis, D.J.; Diamond, M.S.; Lenschow, D.J. Dermal and muscle fibroblasts and skeletal myofibers survive chikungunya virus infection and harbor persistent RNA. PLoS Pathog. 2019, 15, e1007993. [Google Scholar] [CrossRef] [PubMed]
- Monsion, B.; Incarbone, M.; Hleibieh, K.; Poignavent, V.; Ghannam, A.; Dunoyer, P.; Daeffler, L.; Tilsner, J.; Ritzenthaler, C. Efficient detection of long dsRNA in vitro and in vivo using the dsRNA binding domain from FHV B2 protein. Front. Plant Sci. 2018, 9, 70. [Google Scholar] [CrossRef]
- Mateer, E.; Paessler, S.; Huang, C. Confocal Imaging of Double-Stranded RNA and Pattern Recognition Receptors in Negative-Sense RNA Virus Infection. J. Vis. Exp. 2019, 143, e59095. [Google Scholar] [CrossRef]
- Poo, Y.S.; Rudd, P.A.; Gardner, J.; Wilson, J.A.C.; Larcher, T.; Colle, M.-A.; Le, T.T.; Nakaya, H.I.; Warrilow, D.; Allcock, R.; et al. Multiple Immune Factors Are Involved in Controlling Acute and Chronic Chikungunya Virus Infection. PLoS Negl. Trop. Dis. 2014, 8, e3354. [Google Scholar] [CrossRef]
- Hawman, D.W.; Stoermer, K.A.; Montgomery, S.A.; Pal, P.; Oko, L.; Diamond, M.S.; Morrison, T.E. Chronic Joint Disease Caused by Persistent Chikungunya Virus Infection Is Controlled by the Adaptive Immune Response. J. Virol. 2013, 87, 13878–13888. [Google Scholar] [CrossRef]
- Shim, J.-H.; Stavre, Z.; Gravallese, E.M. Bone Loss in Rheumatoid Arthritis: Basic Mechanisms and Clinical Implications. Calcif. Tissue Int. 2018, 102, 533–546. [Google Scholar] [CrossRef]
- Mounce, B.C.; Poirier, E.Z.; Passoni, G.; Simon-Loriere, E.; Cesaro, T.; Prot, M.; Stapleford, K.A.; Moratorio, G.; Sakuntabhai, A.; Levraud, J.-P.; et al. Interferon-Induced Spermidine-Spermine Acetyltransferase and Polyamine Depletion Restrict Zika and Chikungunya Viruses. Cell Host Microbe 2016, 20, 167–177. [Google Scholar] [CrossRef]
- Silva, M.A.; Klafke, J.Z.; Rossato, M.F.; Gewehr, C.; Guerra, G.P.; Rubin, M.A.; Ferreira, J. Role of peripheral polyamines in the development of inflammatory pain. Biochem. Pharmacol. 2011, 82, 269–277. [Google Scholar] [CrossRef]
- Wilder, R.L. Hypothesis for retroviral causation of rheumatoid arthritis. Curr. Opin. Rheumatol. 1994, 6, 295–299. [Google Scholar] [CrossRef]
- Ospelt, C.; Neidhart, M.; Gay, E.R.; Gay, S. Synovial activation in rheumatoid arthritis. Front. Biosci. 2004, 9, 2323–2334. [Google Scholar] [CrossRef] [PubMed]
- Balada, E.; Vilardell-Tarrés, M.; Ordi-Ros, J. Implication of Human Endogenous Retroviruses in the Development of Autoimmune Diseases. Int. Rev. Immunol. 2010, 29, 351–370. [Google Scholar] [CrossRef] [PubMed]
- Neidhart, M.; Karouzakis, E.; Jüngel, A.; Gay, R.E.; Gay, S. Inhibition of Spermidine/Spermine N1-Acetyltransferase Activity: A New Therapeutic Concept in Rheumatoid Arthritis. Arthritis Rheumatol. 2014, 66, 1723–1733. [Google Scholar] [CrossRef] [PubMed]
- Nexø, B.A.; Jensen, S.B.; Nissen, K.K.; Hansen, B.; Laska, M.J. Two endogenous retroviral loci appear to contribute to Multiple Sclerosis. BMC Neurol. 2016, 16, 57. [Google Scholar] [CrossRef] [PubMed]
- Schilte, C.; Couderc, T.; Chretien, F.; Sourisseau, M.; Gangneux, N.; Guivel-Benhassine, F.; Kraxner, A.; Tschopp, J.; Higgs, S.; Michault, A.; et al. Type I IFN controls chikungunya virus via its action on nonhematopoietic cells. J. Exp. Med. 2010, 207, 429–442. [Google Scholar] [CrossRef] [PubMed]
- Gardner, C.L.; Burke, C.W.; Higgs, S.T.; Klimstra, W.B.; Ryman, K.D. Interferon-alpha/beta deficiency greatly exacerbates arthritogenic disease in mice infected with wild-type chikungunya virus but not with the cell culture-adapted live-attenuated 181/25 vaccine candidate. Virology 2012, 425, 103–112. [Google Scholar] [CrossRef]
- Borgherini, G.; Poubeau, P.; Staikowsky, F.; Lory, M.; Moullec, N.L.; Becquart, J.P.; Wengling, C.; Michault, A.; Paganin, F. Outbreak of Chikungunya on Reunion Island: Early Clinical and Laboratory Features in 157 Adult Patients. Clin. Infect. Dis. 2007, 44, 1401–1407. [Google Scholar] [CrossRef]
- Kam, Y.-W.; Simarmata, D.; Chow, A.; Her, Z.; Teng, T.-S.; Ong, E.K.S.; Rénia, L.; Leo, Y.-S.; Ng, L.F.P. Early Appearance of Neutralizing Immunoglobulin G3 Antibodies Is Associated with Chikungunya Virus Clearance and Long-term Clinical Protection. J. Infect. Dis. 2012, 205, 1147–1154. [Google Scholar] [CrossRef]
- Teo, T.-H.; Lum, F.-M.; Claser, C.; Lulla, V.; Lulla, A.; Merits, A.; Rénia, L.; Ng, L.F.P. A Pathogenic Role for CD4+ T Cells during Chikungunya Virus Infection in Mice. J. Immunol. 2013, 190, 259–269. [Google Scholar] [CrossRef]
- Bin Joo, Y.; Park, Y.; Kim, K.; Bang, S.-Y.; Bae, S.-C.; Lee, H.-S. Association of CD8+ T-cells with bone erosion in patients with rheumatoid arthritis. Int. J. Rheum. Dis. 2017, 21, 440–446. [Google Scholar] [CrossRef]
- Messaoudi, I.; Vomaske, J.; Totonchy, T.; Kreklywich, C.N.; Haberthur, K.; Springgay, L.; Brien, J.D.; Diamond, M.S.; DeFilippis, V.R.; Streblow, D.N. Chikungunya Virus Infection Results in Higher and Persistent Viral Replication in Aged Rhesus Macaques Due to Defects in Anti-Viral Immunity. PLoS Negl. Trop. Dis. 2013, 7, e2343. [Google Scholar] [CrossRef] [PubMed]
- Gérardin, P.; Fianu, A.; Michault, A.; Mussard, C.; Boussaïd, K.; Rollot, O.; Grivard, P.; Kassab, S.; Bouquillard, E.; Borgherini, G.; et al. Predictors of Chikungunya rheumatism: A prognostic survey ancillary to the TELECHIK cohort study. Arthritis Res. Ther. 2013, 15, R9. [Google Scholar] [CrossRef] [PubMed]
- Suhrbier, A.; Mahalingam, S. The immunobiology of viral arthritides. Pharmacol. Ther. 2009, 124, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Gardner, J.; Anraku, I.; Le, T.T.; Larcher, T.; Major, L.; Roques, P.; Schroder, W.A.; Higgs, S.; Suhrbier, A. Chikungunya Virus Arthritis in Adult Wild-Type Mice. J. Virol. 2010, 84, 8021–8032. [Google Scholar] [CrossRef]
- Rulli, N.E.; Melton, J.; Wilmes, A.; Ewart, G.; Mahalingam, S. The Molecular and Cellular Aspects of Arthritis Due to Alphavirus Infections: Lesson learned from Ross River virus. Ann. N. Y. Acad. Sci. 2007, 1102, 96–108. [Google Scholar] [CrossRef]
- Phuklia, W.; Kasisith, J.; Modhiran, N.; Rodpai, E.; Thannagith, M.; Thongsakulprasert, T.; Smith, D.R.; Ubol, S. Osteoclastogenesis induced by CHIKV-infected fibroblast-like synoviocytes: A possible interplay between synoviocytes and monocytes/macrophages in CHIKV-induced arthralgia/arthritis. Virus Res. 2013, 177, 179–188. [Google Scholar] [CrossRef]
- Noret, M.; Herrero, L.; Rulli, N.; Rolph, M.; Smith, P.N.; Li, R.W.; Roques, P.; Gras, G.; Mahalingam, S. Interleukin 6, RANKL, and Osteoprotegerin Expression by Chikungunya Virus-Infected Human Osteoblasts. J. Infect. Dis. 2012, 206, 455–457. [Google Scholar] [CrossRef]
- Sissoko, D.; Malvy, D.; Ezzedine, K.; Renault, P.; Moscetti, F.; Ledrans, M.; Pierre, V. Post-Epidemic Chikungunya Disease on Reunion Island: Course of Rheumatic Manifestations and Associated Factors over a 15-Month Period. PLoS Negl. Trop. Dis. 2009, 3, e389. [Google Scholar] [CrossRef]
- Schilte, C.; Staikovsky, F.; Couderc, T.; Madec, Y.; Carpentier, F.; Kassab, S.; Albert, M.L.; Lecuit, M.; Michault, A. Chikungunya Virus-associated Long-term Arthralgia: A 36-month Prospective Longitudinal Study. PLoS Negl. Trop. Dis. 2013, 7, e2137. [Google Scholar] [CrossRef]
- Javelle, E.; Ribera, A.; Degasne, I.; Gaüzère, B.-A.; Marimoutou, C.; Simon, F. Specific Management of Post-Chikungunya Rheumatic Disorders: A Retrospective Study of 159 Cases in Reunion Island from 2006-2012. PLoS Negl. Trop. Dis. 2015, 9, e0003603. [Google Scholar] [CrossRef] [PubMed]
- Carrillo, F.B.; Gordon, A.; Harris, E. Reply to Gérardin et al. Clin. Infect. Dis. 2019, 68, 172–174. [Google Scholar] [CrossRef] [PubMed]
- Sudarsanareddy; Sarojamma, V.; Ramakrishna, V. Genetic predisposition to chikungunya a blood group study in chikungunya affected families. Virol. J. 2009, 6, 77. [Google Scholar] [CrossRef]
- Thanapati, S.; Ganu, M.; Giri, P.; Kulkarni, S.; Sharma, M.; Babar, P.; Ganu, A.; Tripathy, A.S. Impaired NK cell functionality and increased TNF-α production as biomarkers of chronic chikungunya arthritis and rheumatoid arthritis. Hum. Immunol. 2017, 78, 370–374. [Google Scholar] [CrossRef] [PubMed]
- Bouquillard, E.; Combe, B. Rheumatoid arthritis after Chikungunya fever: A prospective follow-up study of 21 cases. Ann. Rheum. Dis. 2009, 68, 1505–1506. [Google Scholar] [CrossRef]
- Ruyssen-Witrand, A.; Degboé, Y.; Cantagrel, A.; Nigon, D.; Lukas, C.; Scaramuzzino, S.; Allanore, Y.; Vittecoq, O.; Schaeverbeke, T.; Morel, J.; et al. Association between RANK, RANKL and OPG polymorphisms with ACPA and erosions in rheumatoid arthritis: Results from a meta-analysis involving three French cohorts. RMD Open 2016, 2, e000226. [Google Scholar] [CrossRef]
- Ercolini, A.M.; Miller, S.D. The role of infections in autoimmune disease. Clin. Exp. Immunol. 2009, 155, 1–15. [Google Scholar] [CrossRef]
- Rueda, J.C.; Arcos-Burgos, M.; Santos, A.M.; Martin-Arsanios, D.; Villota-Erazo, C.; Reyes, V.; Bernal-Macías, S.; Peláez-Ballestas, I.; Cardiel, M.H.; Londono, J. Human Genetic Host Factors and Its Role in the Pathogenesis of Chikungunya Virus Infection. Front. Med. 2022, 9, 654395. [Google Scholar] [CrossRef]
- Sharma, S.K.; Jain, S. Chikungunya: A rheumatologist’s perspective. Int. J. Rheum. Dis. 2018, 21, 584–601. [Google Scholar] [CrossRef]
- Tiwari, V.; Bergman, M.J. Viral Arthritis. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Naciute, M.; Mieliauskaite, D.; Rugiene, R.; Maciunaite, G.; Mauricas, M.; Murovska, M.; Girkontaite, I. Parvovirus B19 infection modulates the levels of cytokines in the plasma of rheumatoid arthritis patients. Cytokine 2017, 96, 41–48. [Google Scholar] [CrossRef]
- Maslennikov, R.; Ivashkin, V.; Efremova, I.; Shirokova, E. Immune disorders and rheumatologic manifestations of viral hepatitis. World J. Gastroenterol. 2021, 27, 2073–2089. [Google Scholar] [CrossRef] [PubMed]
- Carroll, M.B.; Fields, J.; Clerc, P. Rheumatoid arthritis in patients with HIV: Management challenges. Open Access Rheumatol. Res. Rev. 2016, 8, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Trier, N.H.; Holm, B.E.; Heiden, J.; Slot, O.; Locht, H.; Lindegaard, H.; Svendsen, A.; Nielsen, C.T.; Jacobsen, S.; Theander, E.; et al. Antibodies to a strain-specific citrullinated Epstein-Barr virus peptide diagnoses rheumatoid arthritis. Sci. Rep. 2018, 8, 3684. [Google Scholar] [CrossRef]
- Sengupta, S.; Bhattacharya, N.; Tripathi, A. Increased CRP, anti-CCP antibody, IL-2R, COMP levels in prognosis of post-chikungunya chronic arthritis and protective role of their specific genotypes against arthritic manifestation. Virus Res. 2023, 323, 198998. [Google Scholar] [CrossRef]
- Wimalasiri-Yapa, B.; Yapa, H.E.; Huang, X.; Hafner, L.M.; Kenna, T.J.; Frentiu, F.D. Zika Virus and Arthritis/Arthralgia: A Systematic Review and Meta-Analysis. Viruses 2020, 12, 1137. [Google Scholar] [CrossRef]
- Reddy, V.; Desai, A.; Krishna, S.S.; Vasanthapuram, R. Molecular Mimicry between Chikungunya Virus and Host Components: A Possible Mechanism for the Arthritic Manifestations. PLoS Negl. Trop. Dis. 2017, 11, e0005238. [Google Scholar] [CrossRef] [PubMed]
- Tanay, A. Chikungunya virus and autoimmunity. Curr. Opin. Rheumatol. 2017, 29, 389–393. [Google Scholar] [CrossRef]
- Raad, J.J.; Rosero, A.S.; Martínez, J.V.; Parody, A.; Raad, R.J.; Tovar, D.C.; López, P.C.; Ramírez, M.G.; Magdaniel, J.B.; Celedón, L.A. Respuesta inmunitaria de una población del Caribe colombiano infectada con el virus chikungunya. Rev. Colomb. Reumatol. 2016, 23, 85–91. [Google Scholar] [CrossRef]
- Rodríguez-Morales, A.J.; Cardona-Ospina, J.A.; Urbano-Garzón, S.F.; Hurtado-Zapata, J.S. Prevalence of Post-Chikungunya Infection Chronic Inflammatory Arthritis: A Systematic Review and Meta-Analysis. Arthritis Care Res. 2016, 68, 1849–1858. [Google Scholar] [CrossRef]
- Suhrbier, A. Rheumatic manifestations of chikungunya: Emerging concepts and interventions. Nat. Rev. Rheumatol. 2019, 15, 597–611. [Google Scholar] [CrossRef]
- Maek-A-Nantawat, W.; Silachamroon, U. Presence of Autoimmune Antibody in Chikungunya Infection. Case Rep. Med. 2009, 2009, 840183. [Google Scholar] [CrossRef] [PubMed]
- Simon, F.; Javelle, E.; Cabie, A.; Bouquillard, E.; Troisgros, O.; Gentile, G.; Leparc-Goffart, I.; Hoen, B.; Gandjbakhch, F.; Rene-Corail, P.; et al. French guidelines for the management of chikungunya (acute and persistent presentations). November 2014. Med. Mal. Infect. 2015, 45, 243–263. [Google Scholar] [CrossRef] [PubMed]
- Bilsborrow, J.B.; Amaral, J.K.; Schoen, R.T. Clinical Features and Management of Chronic Chikungunya Arthritis. In Current Topics in Neglected Tropical Diseases; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef]
- Boisson, V.; Reynier, J.-C.; Enault, S.; Flahault, A.; Roques, P.; Le Grand, R.; Schwameis, M.; Buchtele, N.; Wadowski, P.P.; Schoergenhofer, C.; et al. On Chikungunya Acute Infection and Chloroquine Treatment. Vector Borne Zoonotic Dis. 2008, 8, 837–840. [Google Scholar] [CrossRef]
- Maheshwari, R.K.; Srikantan, V.; Bhartiya, D. Chloroquine enhances replication of Semliki Forest virus and encephalomyocarditis virus in mice. J. Virol. 1991, 65, 992–995. [Google Scholar] [CrossRef] [PubMed]
- Amaral, J.K.; Taylor, P.C.; Teixeira, M.M.; Morrison, T.E.; Schoen, R.T. The Clinical Features, Pathogenesis and Methotrexate Therapy of Chronic Chikungunya Arthritis. Viruses 2019, 11, 289. [Google Scholar] [CrossRef] [PubMed]
- Amaral, J.K.; Bingham, C.O.; Schoen, R.T. Schoen. Successful Methotrexate Treatment of Chronic Chikungunya Arthritis. J. Clin. Rheumatol. 2020, 26, 119–124. [Google Scholar] [CrossRef]
- Ganu, M.A.; Ganu, A.S. Post-chikungunya chronic arthritis--our experience with DMARDs over two year follow up-PubMed. J. Assoc. Physicians India 2011, 59, 83–86. Available online: https://pubmed.ncbi.nlm.nih.gov/21751641/ (accessed on 24 February 2022).
- Long, F.; Fong, R.H.; Austin, S.K.; Chen, Z.; Klose, T.; Fokine, A.; Liu, Y.; Porta, J.; Sapparapu, G.; Akahata, W.; et al. Cryo-EM structures elucidate neutralizing mechanisms of anti-chikungunya human monoclonal antibodies with therapeutic activity. Proc. Natl. Acad. Sci. USA 2015, 112, 13898–13903. [Google Scholar] [CrossRef]
- Hawman, D.W.; Fox, J.M.; Ashbrook, A.W.; May, N.A.; Schroeder, K.M.; Torres, R.M.; Crowe, J.E.; Dermody, T.S.; Diamond, M.S.; Morrison, T.E. Pathogenic Chikungunya Virus Evades B Cell Responses to Establish Persistence. Cell Rep. 2016, 16, 1326–1338. [Google Scholar] [CrossRef]
- Chen, W.; Foo, S.-S.; Taylor, A.; Lulla, A.; Merits, A.; Hueston, L.; Forwood, M.R.; Walsh, N.C.; Sims, N.A.; Herrero, L.J.; et al. Bindarit, an Inhibitor of Monocyte Chemotactic Protein Synthesis, Protects against Bone Loss Induced by Chikungunya Virus Infection. J. Virol. 2015, 89, 581–593. [Google Scholar] [CrossRef]
- Herrero, L.J.; Foo, S.-S.; Sheng, K.-C.; Chen, W.; Forwood, M.R.; Bucala, R.; Mahalingam, S. Pentosan Polysulfate: A Novel Glycosaminoglycan-Like Molecule for Effective Treatment of Alphavirus-Induced Cartilage Destruction and Inflammatory Disease. J. Virol. 2015, 89, 8063–8076. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Avila-Trejo, A.M.; Rodríguez-Páez, L.I.; Alcántara-Farfán, V.; Aguilar-Faisal, J.L. Multiple Factors Involved in Bone Damage Caused by Chikungunya Virus Infection. Int. J. Mol. Sci. 2023, 24, 13087. https://doi.org/10.3390/ijms241713087
Avila-Trejo AM, Rodríguez-Páez LI, Alcántara-Farfán V, Aguilar-Faisal JL. Multiple Factors Involved in Bone Damage Caused by Chikungunya Virus Infection. International Journal of Molecular Sciences. 2023; 24(17):13087. https://doi.org/10.3390/ijms241713087
Chicago/Turabian StyleAvila-Trejo, Amanda M., Lorena I. Rodríguez-Páez, Verónica Alcántara-Farfán, and J. Leopoldo Aguilar-Faisal. 2023. "Multiple Factors Involved in Bone Damage Caused by Chikungunya Virus Infection" International Journal of Molecular Sciences 24, no. 17: 13087. https://doi.org/10.3390/ijms241713087
APA StyleAvila-Trejo, A. M., Rodríguez-Páez, L. I., Alcántara-Farfán, V., & Aguilar-Faisal, J. L. (2023). Multiple Factors Involved in Bone Damage Caused by Chikungunya Virus Infection. International Journal of Molecular Sciences, 24(17), 13087. https://doi.org/10.3390/ijms241713087





