Metabolic Brain Changes Can Predict the Underlying Pathology in Neurodegenerative Brain Disorders: A Case Report of Sporadic Creutzfeldt–Jakob Disease with Concomitant Parkinson’s Disease
Abstract
:1. Background
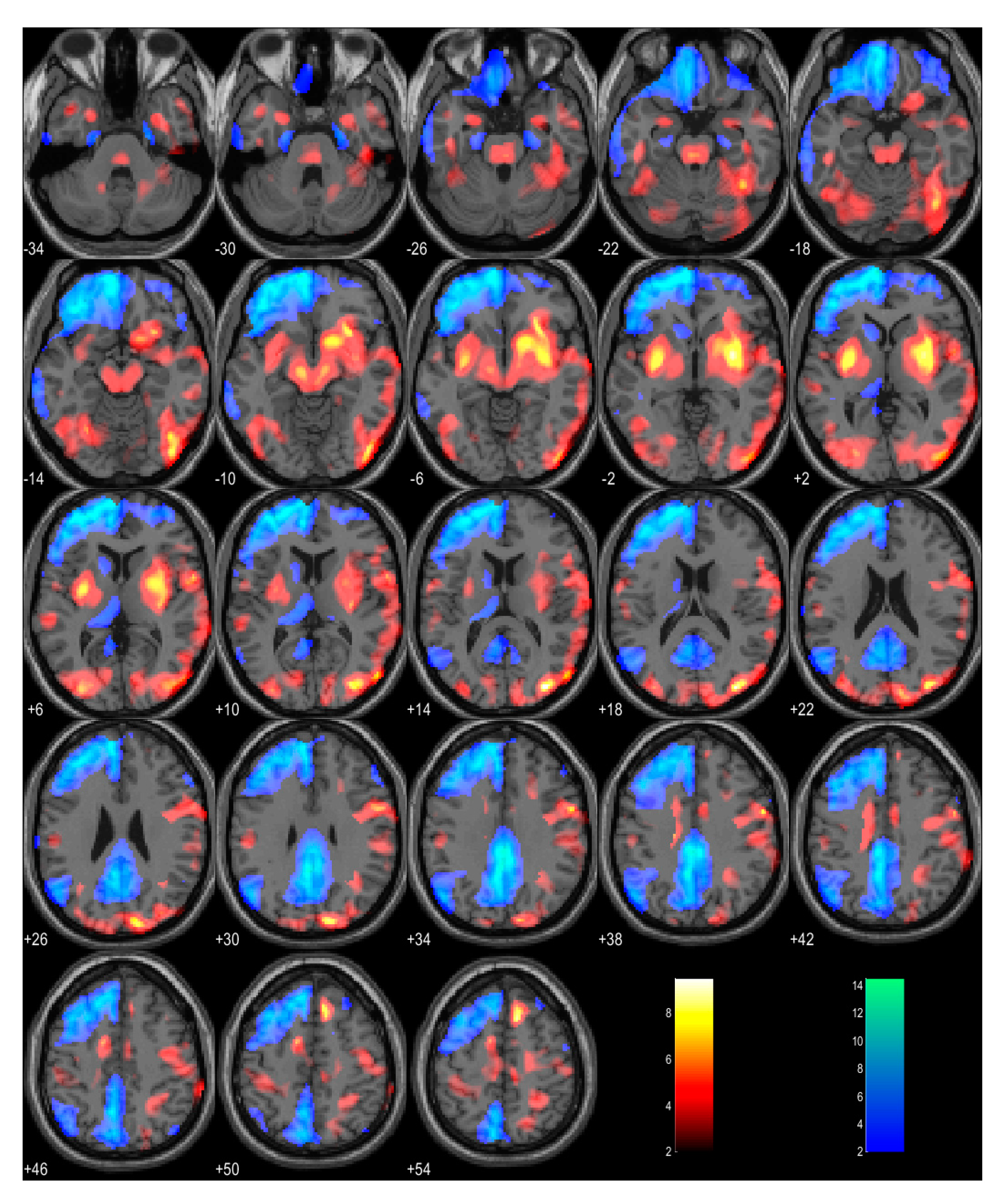
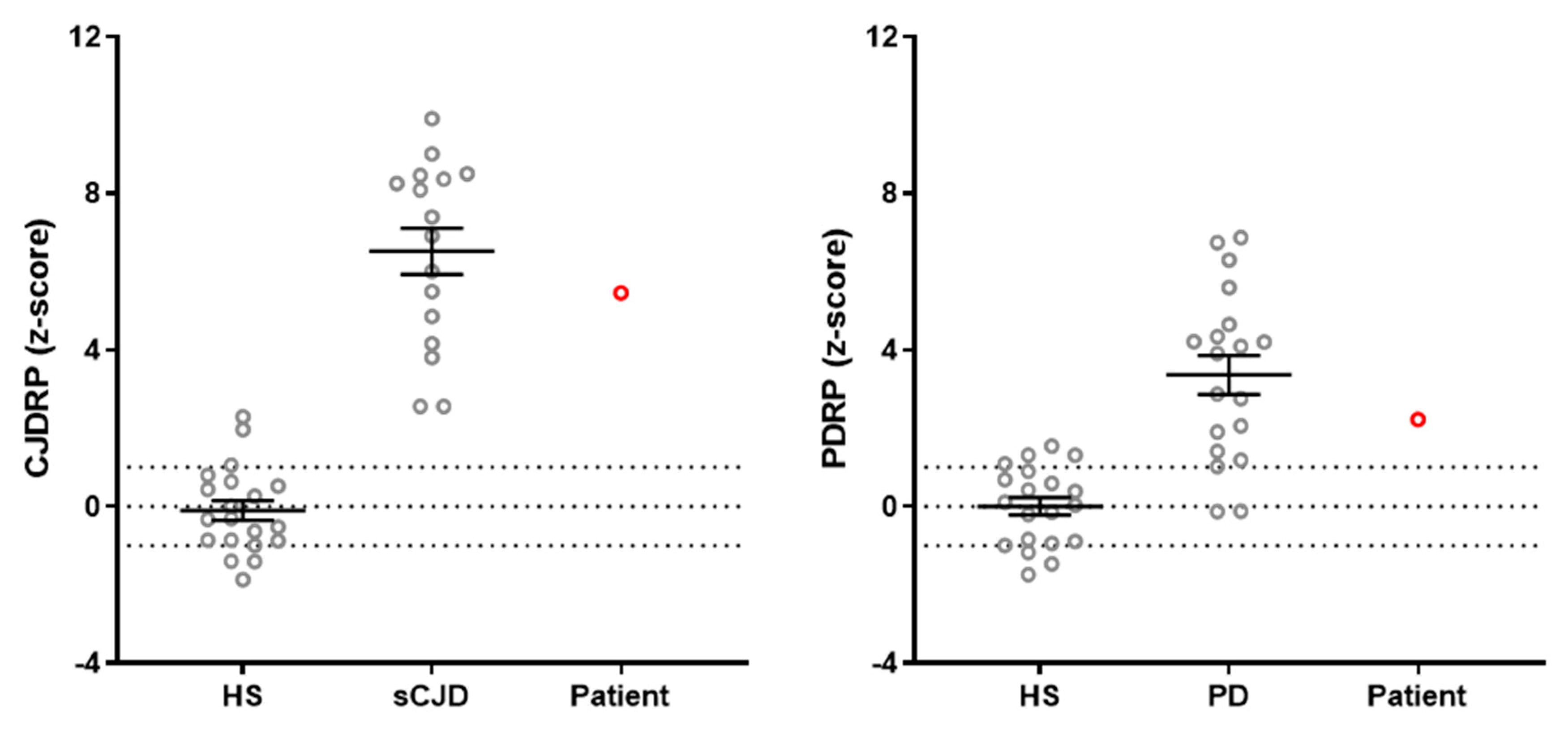
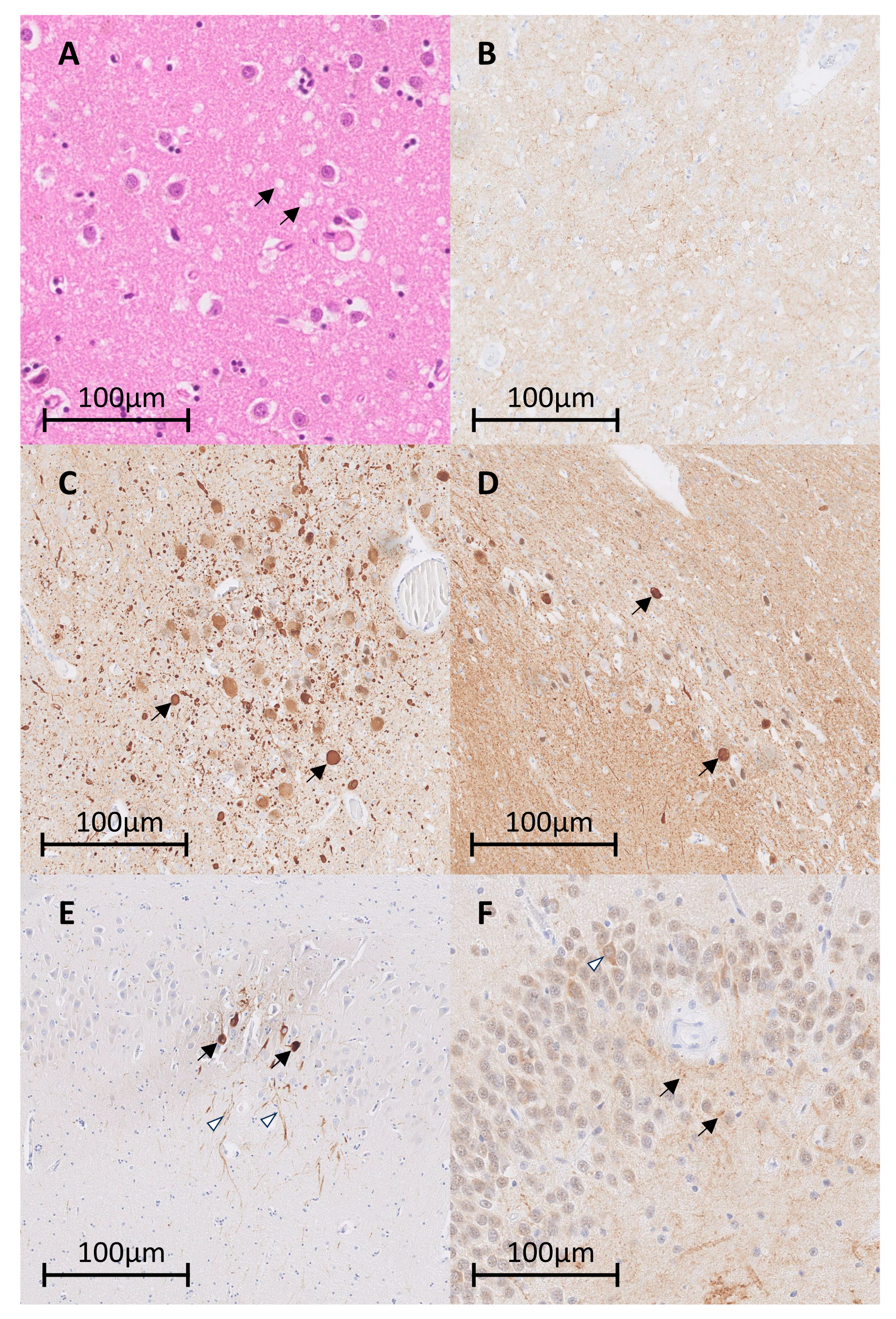
2. Case Report
3. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kovacs, G.G.; Alafuzoff, I.; Al-Sarraj, S.; Arzberger, T.; Bogdanovic, N.; Capellari, S.; Ferrer, I.; Gelpi, E.; Kövari, V.; Kretzschmar, H.; et al. Mixed brain pathologies in dementia: The BrainNet Europe consortium experience. Dement. Geriatr. Cogn. Disord. 2008, 26, 343–350. [Google Scholar] [CrossRef]
- Rahimi, J.; Kovacs, G.G. Prevalence of mixed pathologies in the aging brain. Alzheimers Res. Ther. 2014, 6, 82. [Google Scholar] [CrossRef] [PubMed]
- Villemagne, V.L.; Fodero-Tavoletti, M.T.; Masters, C.L.; Rowe, C.C. Tau imaging: Early progress and future directions. Lancet Neurol. 2015, 14, 114–124. [Google Scholar] [CrossRef] [PubMed]
- Parobkova, E.; van der Zee, J.; Dillen, L.; Van Broeckhoven, C.; Rusina, R.; Matej, R. Sporadic Creutzfeldt-Jakob Disease and Other Proteinopathies in Comorbidity. Front. Neurol. 2020, 11, 596108. [Google Scholar] [CrossRef] [PubMed]
- Rossi, M.; Kai, H.; Baiardi, S.; Bartoletti-Stella, A.; Carlà, B.; Zenesini, C.; Capellari, S.; Kitamoto, T. The characterization of AD/PART co-pathology in CJD suggests independent pathogenic mechanisms and no cross-seeding between misfolded Aβ and prion proteins. Acta Neuropathol. Commun. 2019, 7, 53. [Google Scholar] [CrossRef]
- Fernández-Vega, I.; Ruiz-Ojeda, J.; Juste, R.A.; Geijo, M.; Zarranz, J.J.; Menoyo, J.L.S.; Vicente-Etxenausia, I.; Mediavilla-García, J.; Guerra-Merino, I. Coexistence of mixed phenotype Creutzfeldt-Jakob disease, Lewy body disease and argyrophilic grain disease plus histological features of possible Alzheimer’s disease: A multi-protein disorder in an autopsy case. Neuropathology 2015, 35, 56–63. [Google Scholar] [CrossRef]
- Kubo S ichiro Matsubara, T.; Taguchi, T.; Sengoku, R.; Takeuchi, A.; Saito, Y. Parkinson’s disease with a typical clinical course of 17 years overlapped by Creutzfeldt–Jakob disease: An autopsy case report. BMC Neurol. 2021, 21, 480. [Google Scholar] [CrossRef]
- Klotz, S.; König, T.; Erdler, M.; Ulram, A.; Nguyen, A.; Ströbel, T.; Zimprich, A.; Stögmann, E.; Regelsberger, G.; Höftberger, R.; et al. Co-incidental C9orf72 expansion mutation-related frontotemporal lobar degeneration pathology and sporadic Creutzfeldt-Jakob disease. Eur. J. Neurol. 2021, 28, 1009–1015. [Google Scholar] [CrossRef]
- Guedj, E.; Varrone, A.; Boellaard, R.; Albert, N.L.; Barthel, H.; van Berckel, B.; Brendel, M.; Cecchin, D.; Ekmekcioglu, O.; Garibotto, V.; et al. EANM procedure guidelines for brain PET imaging using [18F]FDG, version 3. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 632. [Google Scholar] [CrossRef]
- Friston, K.; Ashburner, J.; Kiebel, S.; Nichols, T.; Penny, W. (Eds.) Statistical Parametric Mapping: The Analysis of Functional Brain Images; Elsevier: Amsterdam, The Netherlands, 2007. [Google Scholar]
- Perovnik, M.; Rus, T.; Schindlbeck, K.A.; Eidelberg, D. Functional brain networks in the evaluation of patients with neurodegenerative disorders. Nat. Rev. Neurol. 2023, 19, 73–90. [Google Scholar] [CrossRef]
- Rus, T.; Mlakar, J.; Ležaić, L.; Vo, A.; Nguyen, N.; Tang, C.; Fiorini, M.; Prieto, E.; Marti-Andres, G.; Arbizu, J.; et al. Sporadic Creutzfeldt-Jakob disease is associated with reorganization of metabolic connectivity in a pathological brain network. Eur. J. Neurol. 2023, 30, 1035–1047. [Google Scholar] [CrossRef] [PubMed]
- Rus, T.; Perovnik, M.; Vo, A.; Nguyen, N.; Tang, C.; Jamšek, J.; Popović, K.Š.; Grimmer, T.; Yakushev, I.; Diehl-Schmid, J.; et al. Disease specific and nonspecific metabolic brain networks in behavioral variant of frontotemporal dementia. Hum. Brain Mapp. 2023, 44, 1079–1093. [Google Scholar] [CrossRef] [PubMed]
- Teune, L.K.; Strijkert, F.; Renken, R.J.; Gerbrand, J.I.; Jeroen, J.d.V.; Marcel, S.; Jos, B.T.M.R.; Rudi, A.J.O.D.; Klaus, J.L. The Alzheimer’s disease-related glucose metabolic brain pattern. Curr. Alzheimer Res. 2014, 11, 725–732. [Google Scholar] [CrossRef] [PubMed]
- Rus, T.; Tomše, P.; Jensterle, L.; Grmek, M.; Pirtošek, Z.; Eidelberg, D.; Tang, C.; Trošt, M. Differential diagnosis of parkinsonian syndromes: A comparison of clinical and automated-metabolic brain patterns’ based approach. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 2901–2910. [Google Scholar] [CrossRef] [PubMed]
- Watson, N.; Hermann, P.; Ladogana, A.; Denouel, A.; Baiardi, S.; Colaizzo, E.; Giaccone, G.; Glatzel, M.; Green, A.J.E.; Haik, S. Validation of Revised International Creutzfeldt-Jakob Disease Surveillance Network Diagnostic Criteria for Sporadic Creutzfeldt-Jakob Disease. JAMA Netw. Open 2022, 5, e2146319. [Google Scholar] [CrossRef]
- Spetsieris, P.G.; Eidelberg, D. Scaled subprofile modeling of resting state imaging data in Parkinson’s disease: Methodological issues. Neuroimage 2011, 54, 2899–2914. [Google Scholar] [CrossRef] [PubMed]
- Tomše, P.; Jensterle, L.; Grmek, M.; Zaletel, K.; Pirtošek, Z.; Dhawan, V.; Peng, S.; Eidelberg, D.; Ma, Y. Abnormal metabolic brain network associated with Parkinson’s disease: Replication on a new European sample. Neuroradiology 2017, 59, 507–515. [Google Scholar] [CrossRef]
- Renard, D.; Castelnovo, G.; Collombier, L.; Thouvenot, E.; Boudousq, V. FDG-PET in Creutzfeldt-Jakob disease: Analysis of clinical-PET correlation. Prion 2017, 11, 440–453. [Google Scholar] [CrossRef]
- Prieto, E.; Domínguez-Prado, I.; Riverol, M.; Ortega-Cubero, S.; Ribelles, M.J.; Luquin, M.R.; de Castro, P. Metabolic patterns in prion diseases: An FDG PET voxel-based analysis. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 1522–1529. [Google Scholar] [CrossRef]
- Berti, V.; Mosconi, L.; Pupi, A. Brain: Normal Variations and Benign Findings in FDG PET/CT imaging. PET Clin. 2014, 9, 129. [Google Scholar] [CrossRef]
- Moreno-Ajona, D.; Prieto, E.; Grisanti, F.; Esparragosa, I.; Sánchez Orduz, L.; Gállego Pérez-Larraya, J.; Arbizu, J.; Riverol, M. 18F-FDG-PET Imaging Patterns in Autoimmune Encephalitis: Impact of Image Analysis on the Results. Diagnostics 2020, 10, 356. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Shan, W.; Zhao, X.; Ren, J.; Ren, G.; Chen, C.; Shi, W.; Lv, R.; Li, Z.; Liu, Y.; et al. The Clinical Value of 18F-FDG-PET in Autoimmune Encephalitis Associated With LGI1 Antibody. Front. Neurol. 2020, 11, 418. [Google Scholar] [CrossRef] [PubMed]
- Capuron, L.; Pagnoni, G.; Demetrashvili, M.; Lawson, D.; Fornwalt, F.; Woolwine, B.; Berns, G.; Nemeroff, C. Basal Ganglia Hypermetabolism and Symptoms of Fatigue during Interferon-α Therapy. Neuropsychopharmacology 2007, 32, 2384–2392. [Google Scholar] [CrossRef] [PubMed]
- Rus, T.; Schindlbeck, K.A.; Tang, C.C.; Vo, A.; Dhawan, V.; Trošt, M.; Eidelberg, D. Stereotyped relationship between motor and cognitive metabolic networks in Parkinson’s disease. Mov. Disord. 2022, 37, 2247–2256. [Google Scholar] [CrossRef]
- Braak, H.; Tredici, K.; Del Rüb, U.; de Vos, R.A.I.; Jansen Steur, E.N.H.; Braak, E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol. Aging 2003, 24, 197–211. [Google Scholar] [CrossRef]
- Burke, R.E.; Dauer, W.T.; Vonsattel, J.P.G. A Critical Evaluation of The Braak Staging Scheme for Parkinson’s Disease. Ann. Neurol. 2008, 64, 485. [Google Scholar] [CrossRef]
- Kovacs, G.G. Molecular pathology of neurodegenerative diseases: Principles and practice. J. Clin. Pathol. 2019, 72, 725–735. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rus, T.; Mlakar, J.; Jamšek, J.; Trošt, M. Metabolic Brain Changes Can Predict the Underlying Pathology in Neurodegenerative Brain Disorders: A Case Report of Sporadic Creutzfeldt–Jakob Disease with Concomitant Parkinson’s Disease. Int. J. Mol. Sci. 2023, 24, 13081. https://doi.org/10.3390/ijms241713081
Rus T, Mlakar J, Jamšek J, Trošt M. Metabolic Brain Changes Can Predict the Underlying Pathology in Neurodegenerative Brain Disorders: A Case Report of Sporadic Creutzfeldt–Jakob Disease with Concomitant Parkinson’s Disease. International Journal of Molecular Sciences. 2023; 24(17):13081. https://doi.org/10.3390/ijms241713081
Chicago/Turabian StyleRus, Tomaž, Jernej Mlakar, Jan Jamšek, and Maja Trošt. 2023. "Metabolic Brain Changes Can Predict the Underlying Pathology in Neurodegenerative Brain Disorders: A Case Report of Sporadic Creutzfeldt–Jakob Disease with Concomitant Parkinson’s Disease" International Journal of Molecular Sciences 24, no. 17: 13081. https://doi.org/10.3390/ijms241713081
APA StyleRus, T., Mlakar, J., Jamšek, J., & Trošt, M. (2023). Metabolic Brain Changes Can Predict the Underlying Pathology in Neurodegenerative Brain Disorders: A Case Report of Sporadic Creutzfeldt–Jakob Disease with Concomitant Parkinson’s Disease. International Journal of Molecular Sciences, 24(17), 13081. https://doi.org/10.3390/ijms241713081





