Insulin-like Growth Factor 1, Growth Hormone, and Anti-Müllerian Hormone Receptors Are Differentially Expressed during GnRH Neuron Development
Abstract
1. Introduction
2. Results
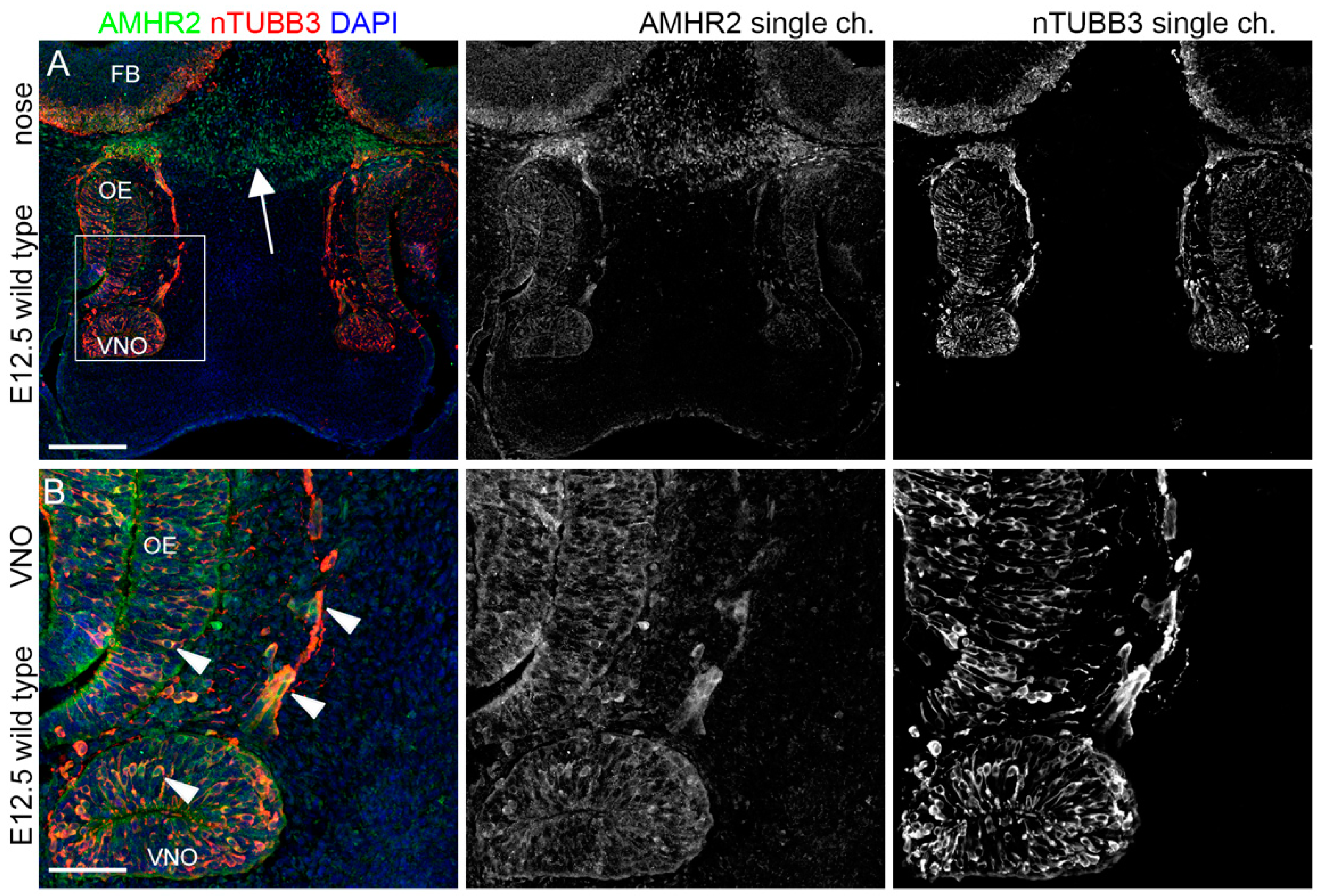
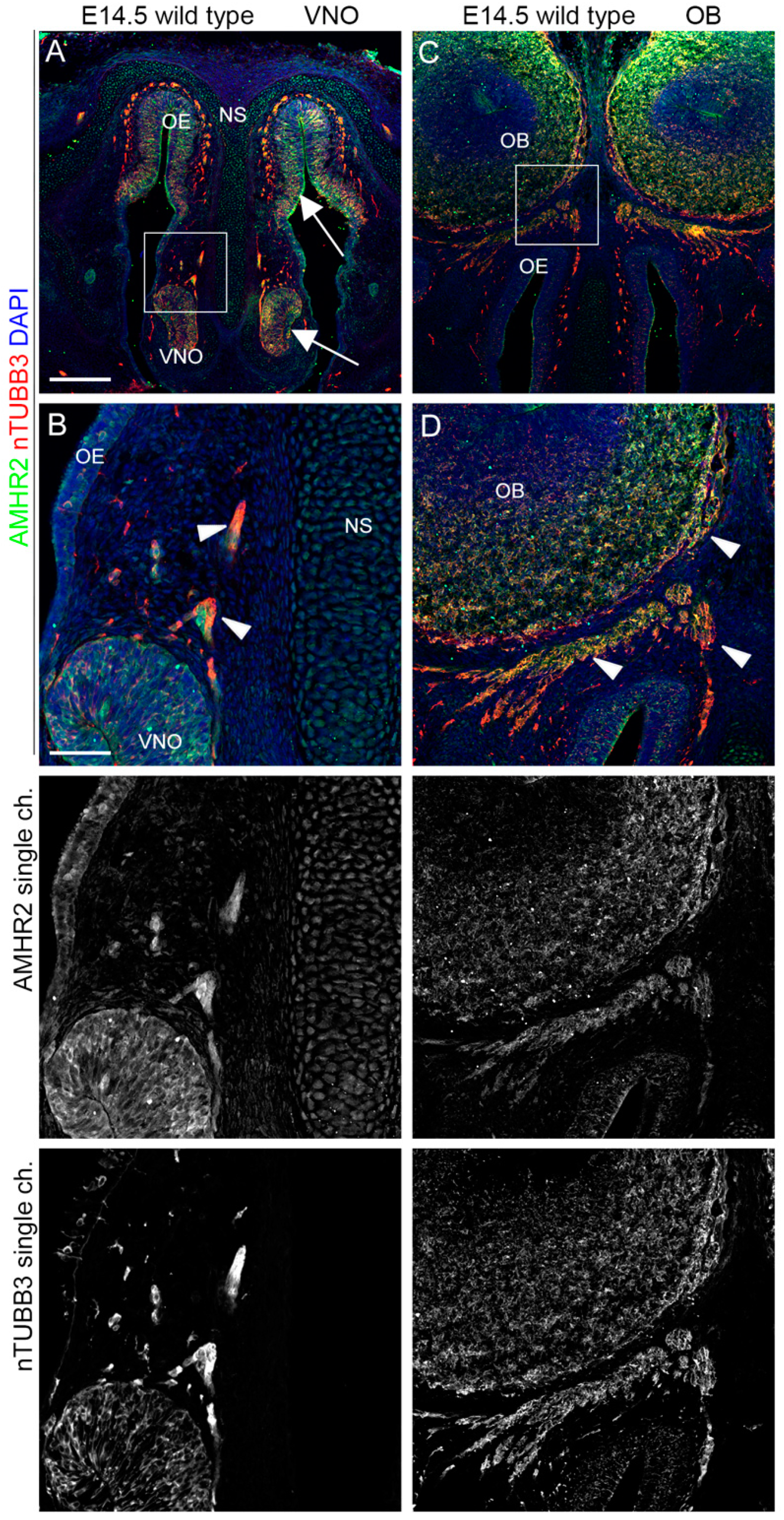
2.1. IGF1R, GHR, and AMHR2 Are Expressed in Neurogenic Niches of the Olfactory and Vomeronasal Systems during Early Embryo Development
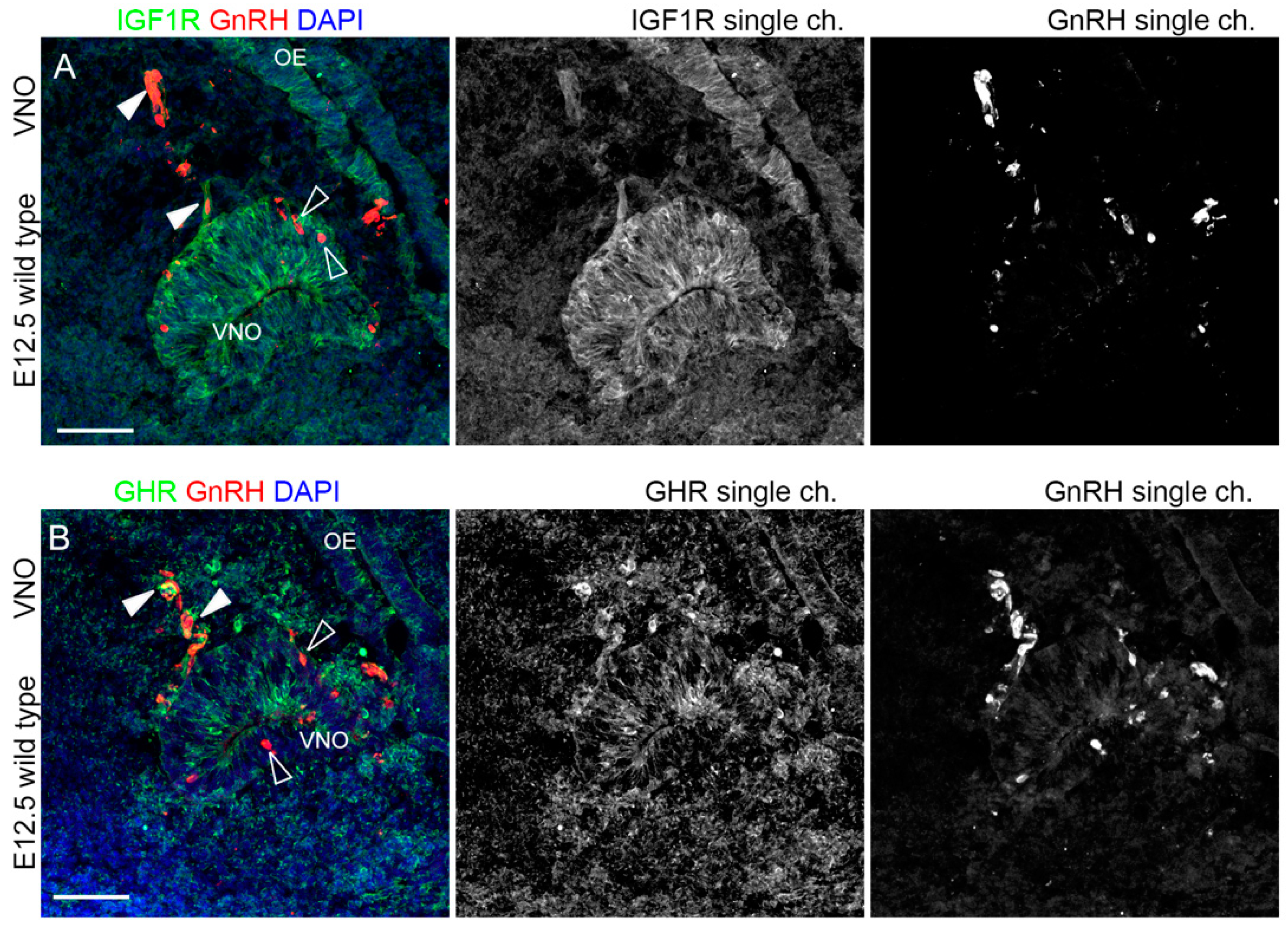
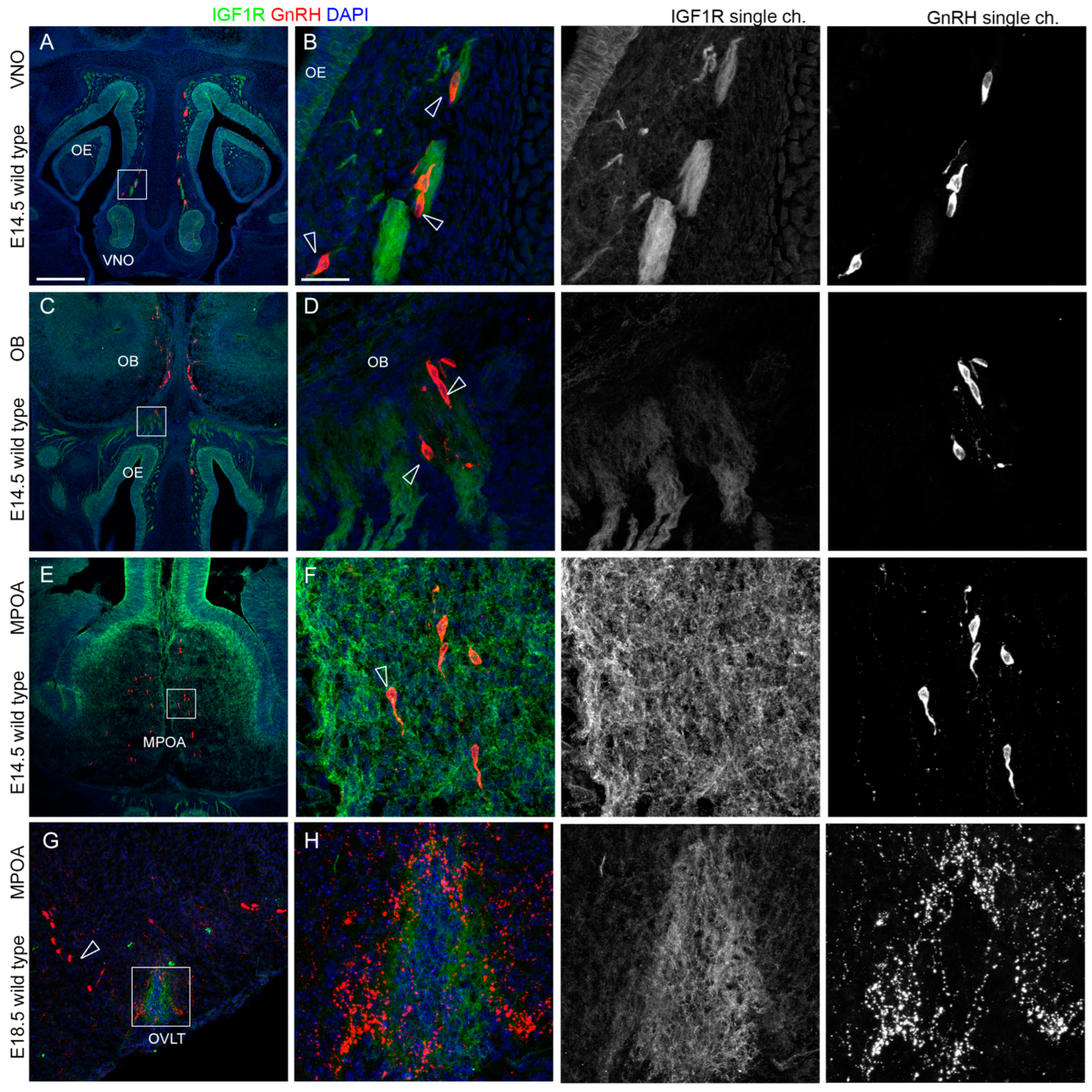
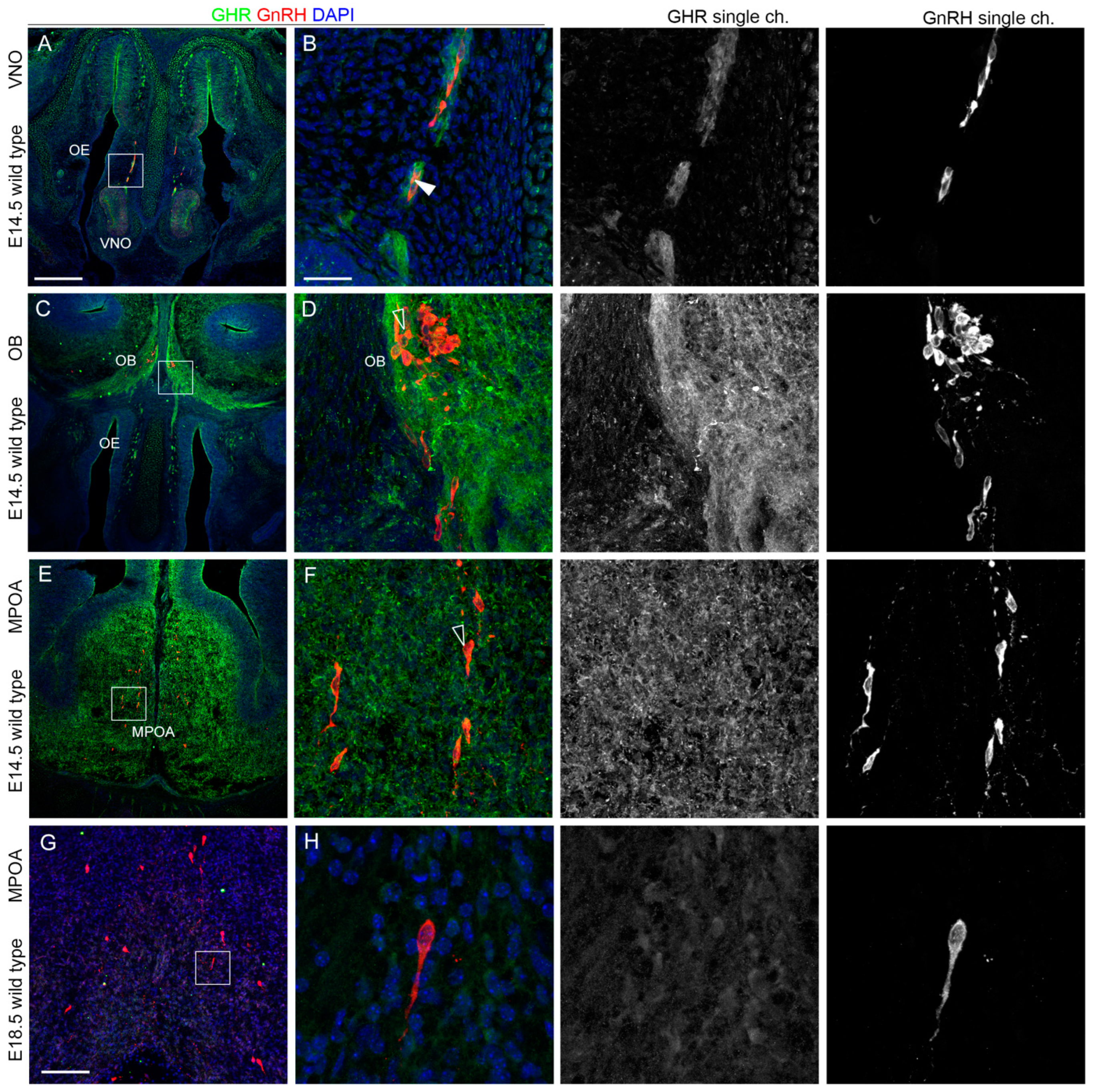
2.2. IGF1R and GHR Are Expressed by GnRH Neurons Migrating in the Nasal Parenchyma
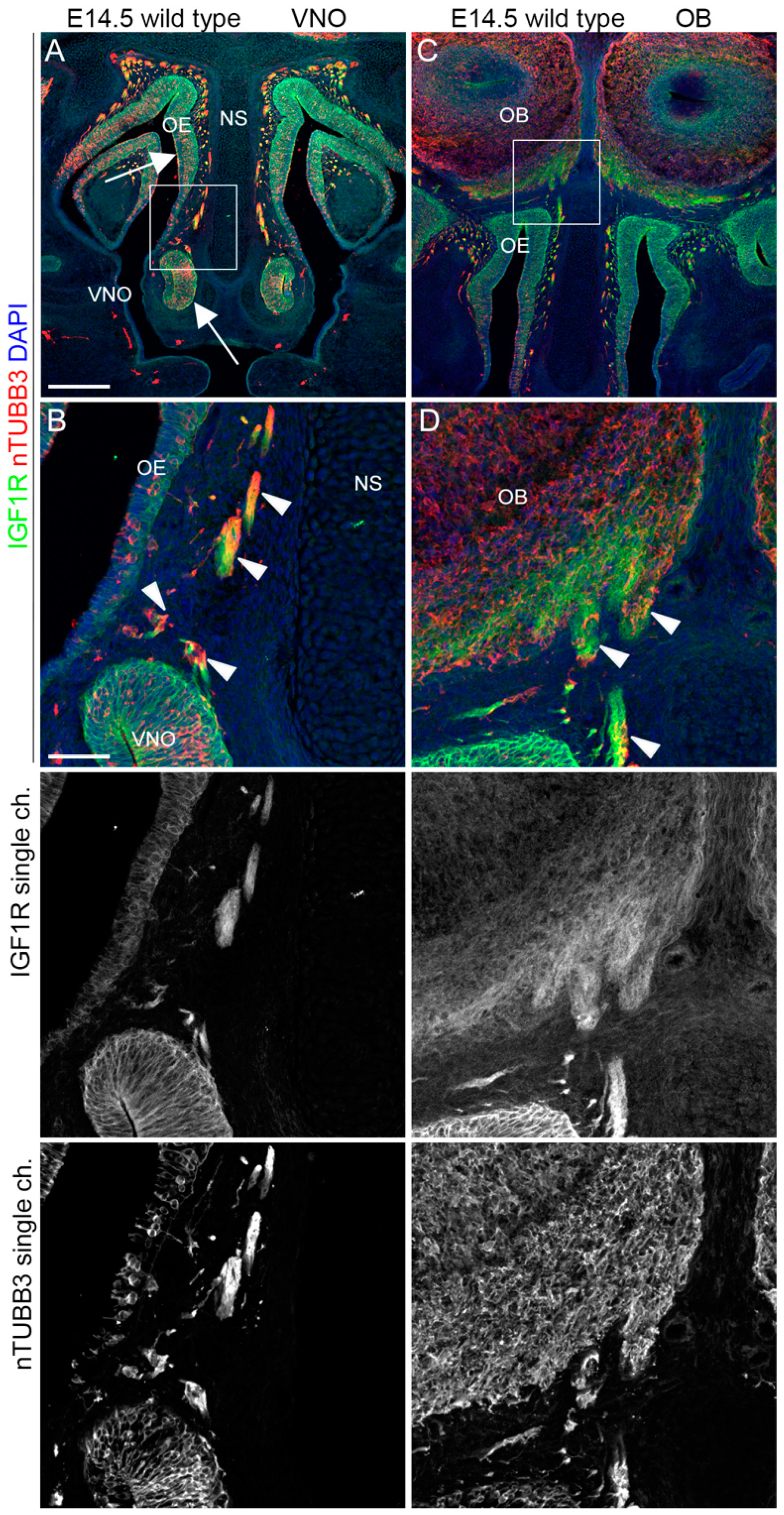
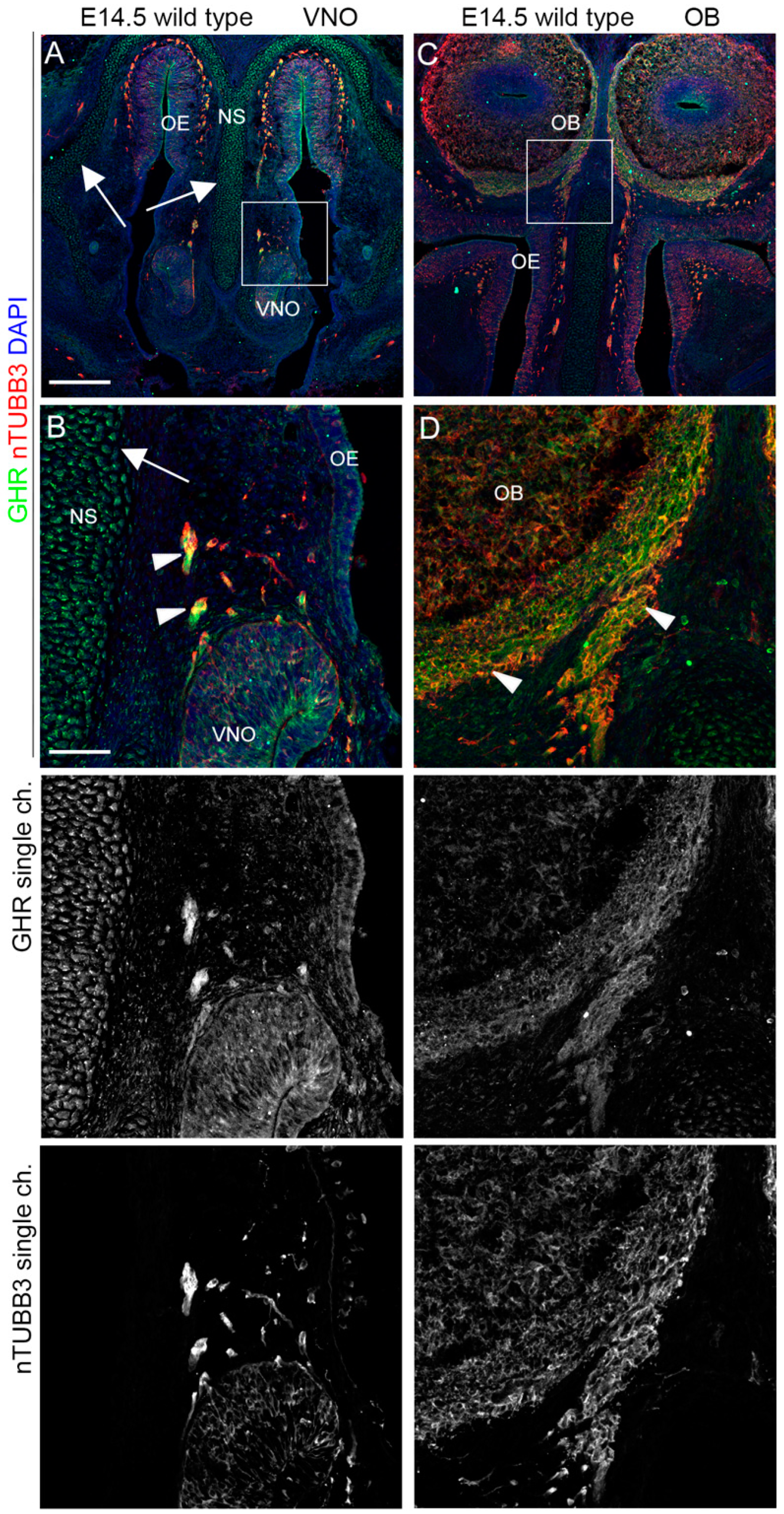
2.3. IGF1R, GHR, and AMHR2 Expression Is Maintained in Nasal Axons and Migrating Cells at the Intermediate Embryo Developmental Stage
2.4. AMHR2 Expression Is Maintained by GnRH Neurons through Their Developmental Trajectory, Whereas GHR and IGF1R Expression Follows a Decreasing Pattern
3. Discussion
4. Materials and Methods
4.1. Mouse Embryos Preparation
4.2. Double Immunofluorescence Studies
4.3. Image Acquisition
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Festa, A.; Umano, G.R.; Miraglia Del Giudice, E.; Grandone, A. Genetic Evaluation of Patients With Delayed Puberty and Congenital Hypogonadotropic Hypogonadism: Is it Worthy of Consideration? Front. Endocrinol. 2020, 11, 253. [Google Scholar] [CrossRef]
- Schwanzel-Fukuda, M.; Pfaff, D.W. Origin of luteinizing hormone-releasing hormone neurons. Nature 1989, 338, 161–164. [Google Scholar] [CrossRef]
- Schwanzel-Fukuda, M.; Crossin, K.; Pfaff, D.; Bouloux, P.; Hardelin, J.; Petit, C. Migration of luteinizing hormone-releasing hormone (LHRH) neurons in early human embryos. J. Comp. Neurol. 1996, 366, 547–557. [Google Scholar] [CrossRef]
- Teixeira, L.; Guimiot, F.; Dodé, C.; Fallet-Bianco, C.; Millar, R.; Delezoide, A.; Hardelin, J. Defective migration of neuroendocrine GnRH cells in human arrhinencephalic conditions. J. Clin. Investig. 2010, 120, 3668–3672. [Google Scholar] [CrossRef]
- Laitinen, E.; Vaaralahti, K.; Tommiska, J.; Eklund, E.; Tervaniemi, M.; Valanne, L.; Raivio, T. Incidence, phenotypic features and molecular genetics of Kallmann syndrome in Finland. Orphanet J. Rare Dis. 2011, 6, 41. [Google Scholar] [CrossRef]
- Franco, B.; Guioli, S.; Pragliola, A.; Incerti, B.; Bardoni, B.; Tonlorenzi, R.; Carrozzo, R.; Maestrini, E.; Pieretti, M.; Taillon-Miller, P.; et al. A gene deleted in Kallmann’s syndrome shares homology with neural cell adhesion and axonal path-finding molecules. Nature 1991, 353, 529–536. [Google Scholar] [CrossRef]
- Dodé, C.; Hardelin, J. Kallmann syndrome. Eur. J. Hum. Genet. EJHG 2009, 17, 139–146. [Google Scholar] [CrossRef]
- Oleari, R.; Massa, V.; Cariboni, A.; Lettieri, A. The Differential Roles for Neurodevelopmental and Neuroendocrine Genes in Shaping GnRH Neuron Physiology and Deficiency. Int. J. Mol. Sci. 2021, 22, 9425. [Google Scholar] [CrossRef]
- Cangiano, B.; Swee, D.S.; Quinton, R.; Bonomi, M. Genetics of congenital hypogonadotropic hypogonadism: Peculiarities and phenotype of an oligogenic disease. Hum. Genet. 2021, 140, 77–111. [Google Scholar] [CrossRef]
- Sykiotis, G.; Plummer, L.; Hughes, V.; Au, M.; Durrani, S.; Nayak-Young, S.; Dwyer, A.; Quinton, R.; Hall, J.; Gusella, J.; et al. Oligogenic basis of isolated gonadotropin-releasing hormone deficiency. Proc. Natl. Acad. Sci. USA 2010, 107, 15140–15144. [Google Scholar] [CrossRef]
- Boehm, U.; Bouloux, P.; Dattani, M.; de Roux, N.; Dodé, C.; Dunkel, L.; Dwyer, A.; Giacobini, P.; Hardelin, J.; Juul, A.; et al. Expert consensus document: European Consensus Statement on congenital hypogonadotropic hypogonadism--pathogenesis, diagnosis and treatment. Nature reviews. Endocrinology 2015, 11, 547–564. [Google Scholar] [CrossRef]
- Cimino, I.; Casoni, F.; Liu, X.; Messina, A.; Parkash, J.; Jamin, S.P.; Catteau-Jonard, S.; Collier, F.; Baroncini, M.; Dewailly, D.; et al. Novel role for anti-Müllerian hormone in the regulation of GnRH neuron excitability and hormone secretion. Nat. Commun. 2016, 7, 10055. [Google Scholar] [CrossRef]
- Malone, S.A.; Papadakis, G.E.; Messina, A.; Mimouni, N.E.H.; Trova, S.; Imbernon, M.; Allet, C.; Cimino, I.; Acierno, J.; Cassatella, D.; et al. Defective AMH signaling disrupts GnRH neuron development and function and contributes to hypogonadotropic hypogonadism. Elife 2019, 8, 47198. [Google Scholar] [CrossRef]
- Laron, Z.; Sarel, R. Penis and testicular size in patients with growth hormone insufficiency. Acta Endocrinol. 1970, 63, 625–633. [Google Scholar] [CrossRef]
- Laron, Z.; Lilos, P.; Klinger, B. Growth curves for Laron syndrome. Arch. Dis. Child. 1993, 68, 768–770. [Google Scholar] [CrossRef]
- Guevara-Aguirre, J.; Rosenbloom, A.L.; Fielder, P.J.; Diamond, F.B.; Rosenfeld, R.G. Growth hormone receptor deficiency in Ecuador: Clinical and biochemical phenotype in two populations. J. Clin. Endocrinol. Metab. 1993, 76, 417–423. [Google Scholar] [CrossRef]
- Savage, D.B.; Semple, R.K.; Chatterjee, V.K.K.; Wales, J.K.H.; Ross, R.J.M.; O’Rahilly, S. A clinical approach to severe insulin resistance. Endocr. Dev. 2007, 11, 122–132. [Google Scholar] [CrossRef]
- Daftary, S.S.; Gore, A.C. The hypothalamic insulin-like growth factor-1 receptor and its relationship to gonadotropin-releasing hormones neurones during postnatal development. J. Neuroendocrinol. 2004, 16, 160–169. [Google Scholar] [CrossRef]
- Magni, P.; Vettor, R.; Pagano, C.; Calcagno, A.; Beretta, E.; Messi, E.; Zanisi, M.; Martini, L.; Motta, M. Expression of a leptin receptor in immortalized gonadotropin-releasing hormone-secreting neurons. Endocrinology 1999, 140, 1581–1585. [Google Scholar] [CrossRef][Green Version]
- Longo, K.M.; Sun, Y.; Gore, A.C. Insulin-like growth factor-I effects on gonadotropin-releasing hormone biosynthesis in GT1-7 cells. Endocrinology 1998, 139, 1125–1132. [Google Scholar] [CrossRef][Green Version]
- Cannarella, R.; Paganoni, A.J.J.; Cicolari, S.; Oleari, R.; Condorelli, R.A.; La Vignera, S.; Cariboni, A.; Calogero, A.E.; Magni, P. Anti-Müllerian Hormone, Growth Hormone, and Insulin-Like Growth Factor 1 Modulate the Migratory and Secretory Patterns of GnRH Neurons. Int. J. Mol. Sci. 2021, 22, 2445. [Google Scholar] [CrossRef]
- Choi, I.; Rickert, E.; Fernandez, M.; Webster, N.J.G. SIRT1 in Astrocytes Regulates Glucose Metabolism and Reproductive Function. Endocrinology 2019, 160, 1547–1560. [Google Scholar] [CrossRef]
- Wierman, M.E.; Kiseljak-Vassiliades, K.; Tobet, S. Gonadotropin-releasing hormone (GnRH) neuron migration: Initiation, maintenance and cessation as critical steps to ensure normal reproductive function. Front. Neuroendocrinol. 2011, 32, 43–52. [Google Scholar] [CrossRef]
- Miller, A.M.; Treloar, H.B.; Greer, C.A. Composition of the migratory mass during development of the olfactory nerve. J. Comp. Neurol. 2010, 518, 4825–4841. [Google Scholar] [CrossRef]
- Oleari, R.; Caramello, A.; Campinoti, S.; Lettieri, A.; Ioannou, E.; Paganoni, A.; Fantin, A.; Cariboni, A.; Ruhrberg, C. PLXNA1 and PLXNA3 cooperate to pattern the nasal axons that guide gonadotropin-releasing hormone neurons. Development 2019, 146, dev176461. [Google Scholar] [CrossRef]
- Macchi, C.; Steffani, L.; Oleari, R.; Lettieri, A.; Valenti, L.; Dongiovanni, P.; Romero-Ruiz, A.; Tena-Sempere, M.; Cariboni, A.; Magni, P.; et al. Iron overload induces hypogonadism in male mice via extrahypothalamic mechanisms. Mol. Cell Endocrinol. 2017, 454, 135–145. [Google Scholar] [CrossRef]
- Oleari, R.; Lettieri, A.; Manzini, S.; Paganoni, A.; André, V.; Grazioli, P.; Busnelli, M.; Duminuco, P.; Vitobello, A.; Philippe, C.; et al. Autism-linked NLGN3 is a key regulator of gonadotropin-releasing hormone deficiency. Dis. Model. Mech. 2023, 16, dmm049996. [Google Scholar] [CrossRef]
- Cariboni, A.; André, V.; Chauvet, S.; Cassatella, D.; Davidson, K.; Caramello, A.; Fantin, A.; Bouloux, P.; Mann, F.; Ruhrberg, C. Dysfunctional SEMA3E signaling underlies gonadotropin-releasing hormone neuron deficiency in Kallmann syndrome. J. Clin. Investig. 2015, 125, 2413–2428. [Google Scholar] [CrossRef]
- Federici, S.; Cangiano, B.; Goggi, G.; Messetti, D.; Munari, E.V.; Amer, M.; Giovanelli, L.; Hrvat, F.; Vezzoli, V.; Persani, L.; et al. Genetic and phenotypic differences between sexes in congenital hypogonadotropic hypogonadism (CHH): Large cohort analysis from a single tertiary centre. Front. Endocrinol. 2022, 13, 965074. [Google Scholar] [CrossRef]
- Juul, A.; Skakkebæk, N.E. Why Do Normal Children Have Acromegalic Levels of IGF-I During Puberty? J. Clin. Endocrinol. Metab. 2019, 104, 2770–2776. [Google Scholar] [CrossRef]
- Celik, N.B.; Losekoot, M.; Isık, E.; Gonc, E.N.; Alikasifoglu, A.; Kandemir, N.; Ozon, Z.A. Long Term Growth Hormone Therapy in a Patient with. J. Clin. Res. Pediatr. Endocrinol. 2023. [Google Scholar] [CrossRef]
- Cannarella, R.; Crafa, A.; La Vignera, S.; Condorelli, R.A.; Calogero, A.E. Role of the GH-IGF1 axis on the hypothalamus-pituitary-testicular axis function: Lessons from Laron syndrome. Endocr. Connect. 2021, 10, 1006–1017. [Google Scholar] [CrossRef]
- Zhou, C.; Niu, Y.; Xu, H.; Li, Z.; Wang, T.; Yang, W.; Wang, S.; Wang, D.W.; Liu, J. Mutation profiles and clinical characteristics of Chinese males with isolated hypogonadotropic hypogonadism. Fertil. Steril. 2018, 110, 486–495.e485. [Google Scholar] [CrossRef]
- Vastagh, C.; Csillag, V.; Solymosi, N.; Farkas, I.; Liposits, Z. Gonadal Cycle-Dependent Expression of Genes Encoding Peptide, Growth Factor-, and Orphan G-Protein-Coupled Receptors in Gonadotropin- Releasing Hormone Neurons of Mice. Front. Mol. Neurosci. 2020, 13, 594119. [Google Scholar] [CrossRef]
- García-Aragón, J.; Lobie, P.E.; Muscat, G.E.; Gobius, K.S.; Norstedt, G.; Waters, M.J. Prenatal expression of the growth hormone (GH) receptor/binding protein in the rat: A role for GH in embryonic and fetal development? Development 1992, 114, 869–876. [Google Scholar] [CrossRef]
- Chard, T. Hormonal control of growth in the human fetus. J. Endocrinol. 1989, 123, 3–9. [Google Scholar] [CrossRef]
- Liao, S.; Vickers, M.H.; Stanley, J.L.; Baker, P.N.; Perry, J.K. Human Placental Growth Hormone Variant in Pathological Pregnancies. Endocrinology 2018, 159, 2186–2198. [Google Scholar] [CrossRef]
- Onuma, T.A.; Ding, Y.; Abraham, E.; Zohar, Y.; Ando, H.; Duan, C. Regulation of temporal and spatial organization of newborn GnRH neurons by IGF signaling in zebrafish. J. Neurosci. 2011, 31, 11814–11824. [Google Scholar] [CrossRef]
- Divall, S.A.; Williams, T.R.; Carver, S.E.; Koch, L.; Brüning, J.C.; Kahn, C.R.; Wondisford, F.; Radovick, S.; Wolfe, A. Divergent roles of growth factors in the GnRH regulation of puberty in mice. J. Clin. Investig. 2010, 120, 2900–2909. [Google Scholar] [CrossRef]
- Scolnick, J.A.; Cui, K.; Duggan, C.D.; Xuan, S.; Yuan, X.B.; Efstratiadis, A.; Ngai, J. Role of IGF signaling in olfactory sensory map formation and axon guidance. Neuron 2008, 57, 847–857. [Google Scholar] [CrossRef]
- de Lima, J.B.M.; Ubah, C.; Debarba, L.K.; Ayyar, I.; Didyuk, O.; Sadagurski, M. Hypothalamic GHR-SIRT1 Axis in Fasting. Cells 2021, 10, 891. [Google Scholar] [CrossRef] [PubMed]
- Oleari, R.; André, V.; Lettieri, A.; Tahir, S.; Roth, L.; Paganoni, A.; Eberini, I.; Parravicini, C.; Scagliotti, V.; Cotellessa, L.; et al. A Novel SEMA3G Mutation in Two Siblings Affected by Syndromic GnRH Deficiency. Neuroendocrinol. Off. Organ Int. Soc. Neuroendocrinol. 2021, 111, 421–441. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paganoni, A.J.J.; Cannarella, R.; Oleari, R.; Amoruso, F.; Antal, R.; Ruzza, M.; Olivieri, C.; Condorelli, R.A.; La Vignera, S.; Tolaj, F.; et al. Insulin-like Growth Factor 1, Growth Hormone, and Anti-Müllerian Hormone Receptors Are Differentially Expressed during GnRH Neuron Development. Int. J. Mol. Sci. 2023, 24, 13073. https://doi.org/10.3390/ijms241713073
Paganoni AJJ, Cannarella R, Oleari R, Amoruso F, Antal R, Ruzza M, Olivieri C, Condorelli RA, La Vignera S, Tolaj F, et al. Insulin-like Growth Factor 1, Growth Hormone, and Anti-Müllerian Hormone Receptors Are Differentially Expressed during GnRH Neuron Development. International Journal of Molecular Sciences. 2023; 24(17):13073. https://doi.org/10.3390/ijms241713073
Chicago/Turabian StylePaganoni, Alyssa J. J., Rossella Cannarella, Roberto Oleari, Federica Amoruso, Renata Antal, Marco Ruzza, Chiara Olivieri, Rosita A. Condorelli, Sandro La Vignera, Fationa Tolaj, and et al. 2023. "Insulin-like Growth Factor 1, Growth Hormone, and Anti-Müllerian Hormone Receptors Are Differentially Expressed during GnRH Neuron Development" International Journal of Molecular Sciences 24, no. 17: 13073. https://doi.org/10.3390/ijms241713073
APA StylePaganoni, A. J. J., Cannarella, R., Oleari, R., Amoruso, F., Antal, R., Ruzza, M., Olivieri, C., Condorelli, R. A., La Vignera, S., Tolaj, F., Cariboni, A., Calogero, A. E., & Magni, P. (2023). Insulin-like Growth Factor 1, Growth Hormone, and Anti-Müllerian Hormone Receptors Are Differentially Expressed during GnRH Neuron Development. International Journal of Molecular Sciences, 24(17), 13073. https://doi.org/10.3390/ijms241713073









