Genetic Variation and Sensory Perception of a Pediatric Formulation of Ibuprofen: Can a Medicine Taste Too Good for Some?
Abstract
:1. Introduction
2. Results
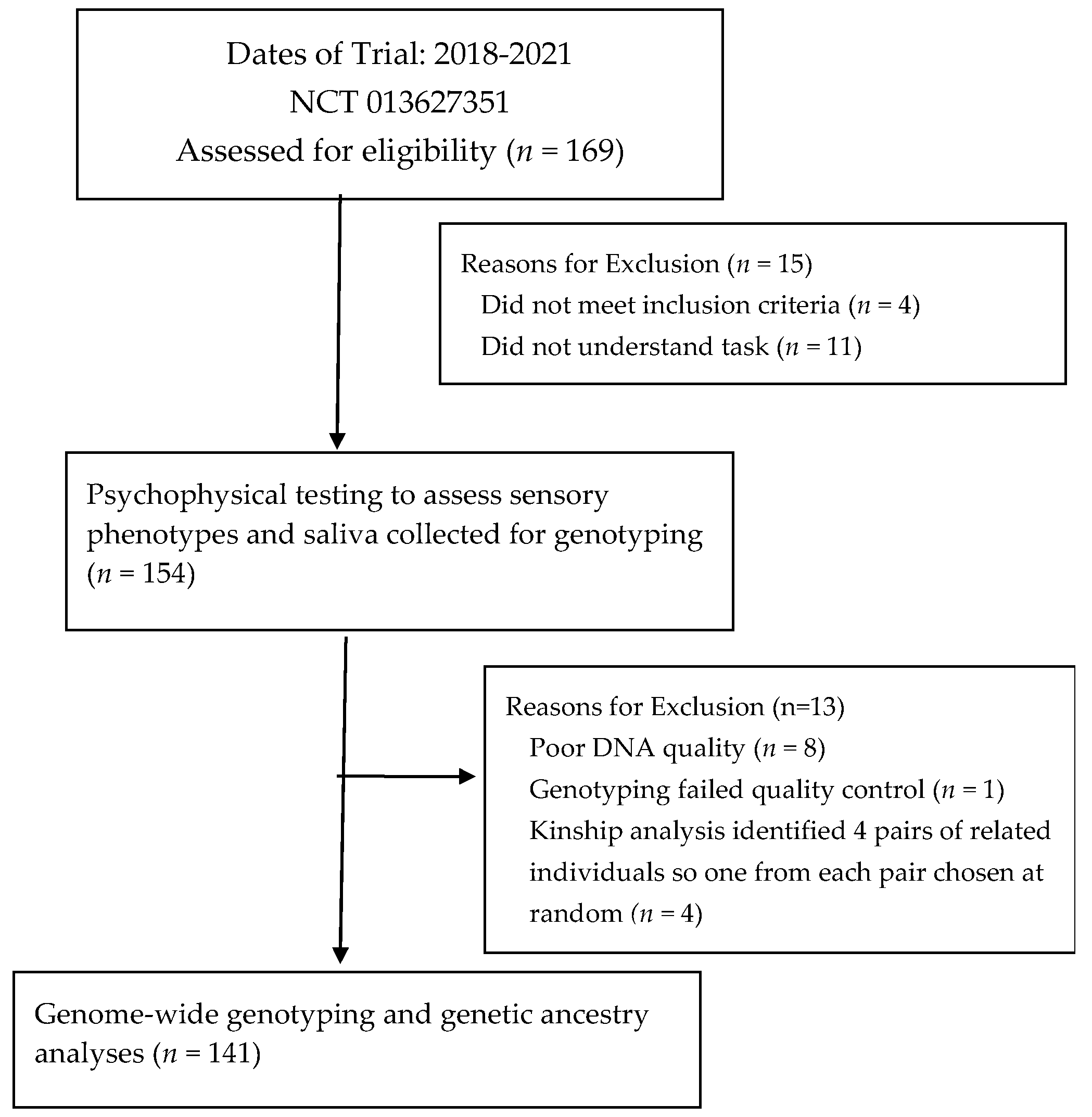
2.1. Panel Characteristics
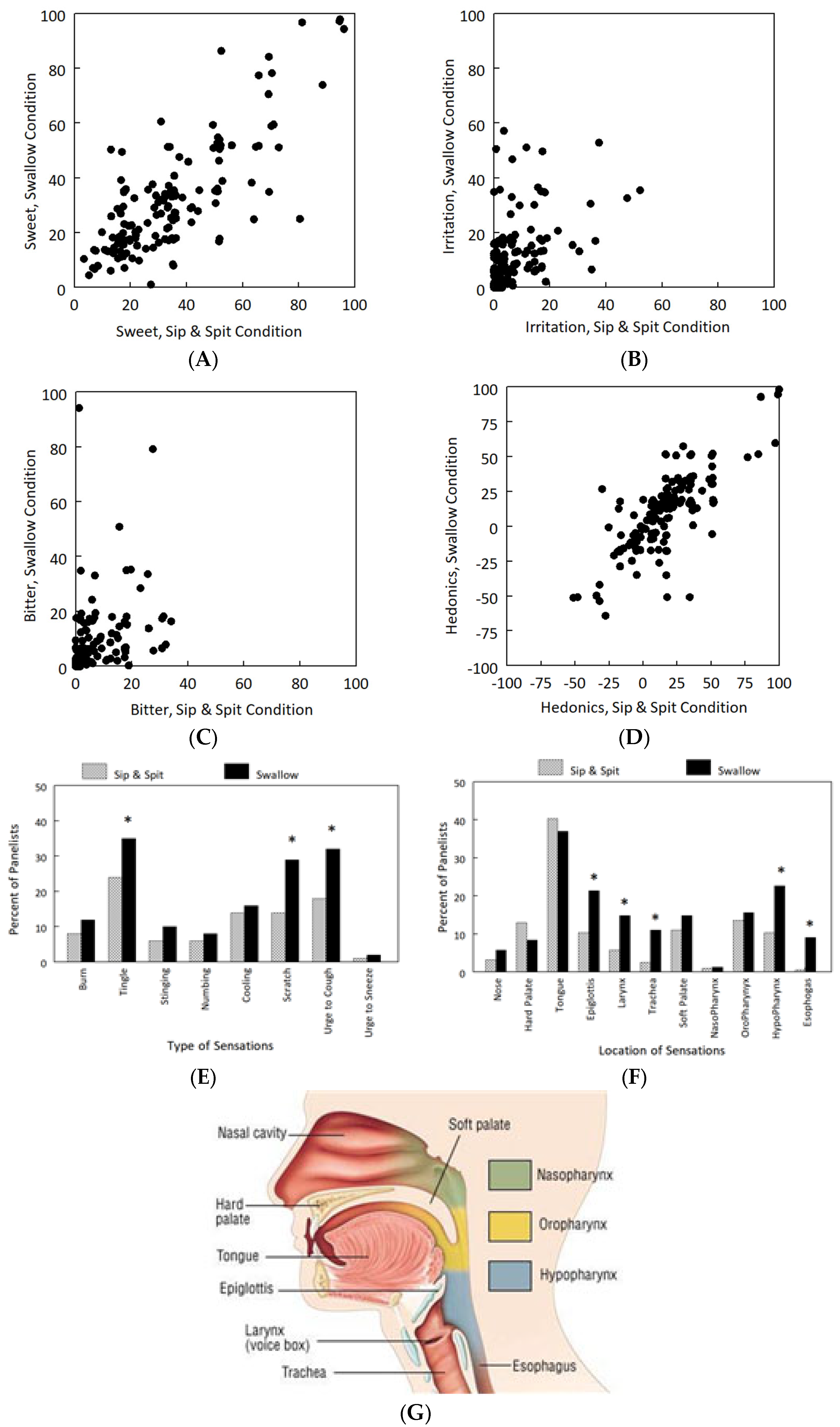
2.2. Chemosensory Phenotypes
2.3. Genetic Associations with Chemosensory Phenotypes
3. Discussion
4. Materials and Methods
4.1. Participants and Stimuli
4.2. Phenotyping
4.3. Genotyping, Genetic Ancestry, and Candidate Single-Nucleotide Polymorphisms (SNPs)
4.4. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Kroeze, J.H.; Bartoshuk, L.M. Bitterness suppression as revealed by split-tongue taste stimulation in humans. Physiol. Behav. 1985, 35, 779–783. [Google Scholar] [CrossRef]
- Mennella, J.A.; Reed, D.R.; Roberts, K.M.; Mathew, P.S.; Mansfield, C.J. Age-related differences in bitter taste and efficacy of bitter blockers. PLoS ONE 2014, 9, e103107. [Google Scholar] [CrossRef]
- Schwieterman, M.L.; Colquhoun, T.A.; Jaworski, E.A.; Bartoshuk, L.M.; Gilbert, J.L.; Tieman, D.M.; Odabasi, A.Z.; Moskowitz, H.R.; Folta, K.M.; Klee, H.J.; et al. Strawberry flavor: Diverse chemical compositions, a seasonal influence, and effects on sensory perception. PLoS ONE 2014, 9, e88446. [Google Scholar] [CrossRef] [PubMed]
- Mennella, J.A.; Spector, A.C.; Reed, D.R.; Coldwell, S.E. The bad taste of medicines: Overview of basic research on bitter taste. Clin. Ther. 2013, 35, 1225–1246. [Google Scholar] [CrossRef] [PubMed]
- Electronic Medicines Compendium (EMC) Lemsip Cold and Flu Lemon. Available online: https://www.medicines.org.uk/emc/product/617/smpc#gref (accessed on 5 June 2023).
- Beauchamp, G.K. The flavor of serendipity. Am. Sci. 2019, 107, 170–177. [Google Scholar]
- Schatzker, M. The Dorito Effect: The Surprising New Truth about Food and Flavor; Simon and Schuster: New York, NY, USA, 2015. [Google Scholar]
- Breslin, P.A.; Gingrich, T.N.; Green, B.G. Ibuprofen as a chemesthetic stimulus: Evidence of a novel mechanism of throat irritation. Chem. Senses 2001, 26, 55–65. [Google Scholar] [CrossRef]
- Mennella, J.A.; Reed, D.R.; Mathew, P.S.; Roberts, K.M.; Mansfield, C.J. “A spoonful of sugar helps the medicine go down”: Bitter masking by sucrose among children and adults. Chem. Senses 2015, 40, 17–25. [Google Scholar] [CrossRef]
- Hines, E.Q. Pediatric poisonings: The risk of over-the-counter pharmaceuticals. Pediatr. Ann. 2017, 46, e454–e458. [Google Scholar] [CrossRef]
- Barnett, S.; Bhatt, A. A chewable pediatric preparation of ibuprofen is palatable and acceptable to children. Paediatr. Neonatal Pain 2020, 2, 2–6. [Google Scholar] [CrossRef]
- Ross, J.A.; Woodfin, M.H.; Rege, S.V.; Holstege, C.P. Pediatric suicides reported to U.S. poison centers. Clin. Toxicol. 2022, 60, 869–871. [Google Scholar] [CrossRef]
- Rakowsky, S.; Spiller, H.A.; Casavant, M.J.; Chounthirath, T.; Hodges, N.L.; Kim, E.H.; Smith, G.A. Antipyretic medication exposures among young children reported to US Poison Centers, 2000–2015. Clin. Pediatr. 2018, 57, 266–276. [Google Scholar] [CrossRef]
- White, N.D.; Kibalama, W. Prevention of pediatric pharmaceutical poisonings. Am. J. Lifestyle Med. 2018, 12, 117–119. [Google Scholar] [CrossRef]
- Silver, K. Why Medicine Flavor Matters in Drug Design, Especially for Kids. Available online: https://www.pfizer.com/news/articles/why_medicine_flavor_matters_in_drug_design_especially_for_kids (accessed on 9 August 2023).
- Smethers, A.D.; Mennella, J.A. Sweet taste and added sugar consumption in infancy and childhood. In Sensory Science and Chronic Disease: Clinical Implications and Disease Management; Joseph, P., Duffy, V., Eds.; Springer International Publishing: Cham, Switzerland, 2021. [Google Scholar]
- Mennella, J.A.; Mathew, P.S.; Lowenthal, E.D. Use of adult sensory panel to study individual differences in the palatability of a pediatric HIV treatment drug. Clin. Ther. 2017, 39, 2038–2048. [Google Scholar] [CrossRef]
- Marques, C.; Correia, E.; Dinis, L.T.; Vilela, A. An overview of sensory characterization techniques: From classical descriptive analysis to the emergence of novel profiling methods. Foods 2022, 11, 255. [Google Scholar] [CrossRef] [PubMed]
- Khan, D.; Kirby, D.; Bryson, S.; Shah, M.; Rahman Mohammed, A. Paediatric specific dosage forms: Patient and formulation considerations. Int. J. Pharm. 2022, 616, 121501. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency Committee for Medicinal Products for Human Use and Paedatric Committee Guideline on Pharmaceutical Development of Medicines for Paediatric Use (Adopted) 2013. Available online: eu/docs/en_GB/document_library/Scientific_guideline/2013/07/WC500147002.pdf (accessed on 7 July 2023).
- Thompson, C.; Lombardi, D.; Sjostedt, P.; Squires, L. Best practice recommendations regarding the assessment of palatability and swallowability in the development of oral dosage forms for pediatric patients. Ther. Innov. Regul. Sci. 2015, 49, 647–658. [Google Scholar] [CrossRef] [PubMed]
- Pew Charitable Trusts Philadelphia 2023: The State of the City. Available online: https://www.pewtrusts.org/en/research-and-analysis/reports/2023/04/philadelphia-2023-state-of-the-city (accessed on 5 July 2023).
- Bennett, S.M.; Hayes, J.E. Differences in the chemesthetic subqualities of capsaicin, ibuprofen, and olive oil. Chem. Senses 2012, 37, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Rose, T.M.; Reilly, C.A.; Deering-Rice, C.E.; Brewster, C.; Brewster, C. Inhibition of FAAH, TRPV1, and COX2 by NSAID-serotonin conjugates. Bioorg. Med. Chem. Lett. 2014, 24, 5695–5698. [Google Scholar] [CrossRef] [PubMed]
- Caceres, A.I.; Liu, B.; Jabba, S.V.; Achanta, S.; Morris, J.B.; Jordt, S.E. Transient Receptor Potential Cation Channel Subfamily M Member 8 channels mediate the anti-inflammatory effects of eucalyptol. Br. J. Pharmacol. 2017, 174, 867–879. [Google Scholar] [CrossRef] [PubMed]
- Peterson, R.E.; Kuchenbaecker, K.; Walters, R.K.; Chen, C.Y.; Popejoy, A.B.; Periyasamy, S.; Lam, M.; Iyegbe, C.; Strawbridge, R.J.; Brick, L.; et al. Genome-wide association studies in ancestrally diverse populations: Opportunities, methods, pitfalls, and recommendations. Cell 2019, 179, 589–603. [Google Scholar] [CrossRef] [PubMed]
- Duello, T.M.; Rivedal, S.; Wickland, C.; Weller, A. Race and genetics versus ‘race’ in genetics: A systematic review of the use of African ancestry in genetic studies. Evol. Med. Public Health 2021, 9, 232–245. [Google Scholar] [CrossRef]
- Khan, A.T.; Gogarten, S.M.; McHugh, C.P.; Stilp, A.M.; Sofer, T.; Bowers, M.L.; Wong, Q.; Cupples, L.A.; Hidalgo, B.; Johnson, A.D.; et al. Recommendations on the use and reporting of race, ethnicity, and ancestry in genetic research: Experiences from the NHLBI TOPMed program. Cell Genom. 2022, 2, 100155. [Google Scholar] [CrossRef] [PubMed]
- Peyrot des Gachons, C.; Lescoat, E.; Slade, L.; Beauchamp, G.K. Comparative perceptual properties of the TRPA1 ligand oleocanthal and the TRPV1 ligand capsaicin in the pharyngeal area. In Proceedings of the 41st Annual Meeting for the Association for Chemoreception Sciences, Bonita Springs, FL, USA, 14–17 April 2019. [Google Scholar]
- Allen, A.L.; McGeary, J.E.; Knopik, V.S.; Hayes, J.E. Bitterness of the non-nutritive sweetener acesulfame potassium varies with polymorphisms in TAS2R9 and TAS2R31. Chem. Senses 2013, 38, 379–389. [Google Scholar] [CrossRef] [PubMed]
- Green, B.G.; Lim, J.; Osterhoff, F.; Blacher, K.; Nachtigal, D. Taste mixture interactions: Suppression, additivity, and the predominance of sweetness. Physiol. Behav. 2010, 101, 731–737. [Google Scholar] [CrossRef] [PubMed]
- Prescott, J.; Stevenson, R.J. Effects of oral chemical irritation on tastes and flavors in frequent and infrequent users of chili. Physiol. Behav. 1995, 58, 1117–1127. [Google Scholar] [CrossRef]
- Genick, U.K.; Kutalik, Z.; Ledda, M.; Destito, M.C.; Souza, M.M.; Cirillo, C.A.; Godinot, N.; Martin, N.; Morya, E.; Sameshima, K.; et al. Sensitivity of genome-wide-association signals to phenotyping strategy: The PROP-TAS2R38 taste association as a benchmark. PLoS ONE 2011, 6, e27745. [Google Scholar] [CrossRef]
- Bobowski, N.; Reed, D.R.; Mennella, J.A. Variation in the TAS2R31 bitter taste receptor gene relates to liking for the nonnutritive sweetener Acesulfame-K among children and adults. Sci. Rep. 2016, 6, 39135. [Google Scholar] [CrossRef]
- Mennella, J.A.; Lukasewycz, L.D.; Griffith, J.W.; Beauchamp, G.K. Evaluation of the Monell forced-choice, paired-comparison tracking procedure for determining sweet taste preferences across the lifespan. Chem. Senses 2011, 36, 345–355. [Google Scholar] [CrossRef]
- Bowser, S.; Farnsworth, N.; Russell, K.; Schlechter, H.; Bernstein, S.; Courville, A.B.; Zambell, K.; Skarulis, M.; Muniyappa, R. Sweet taste perception is greater in non-Hispanic black than in non-Hispanic white adults. Nutrition 2019, 59, 103–107. [Google Scholar] [CrossRef]
- Kuzawa, C.W.; Sweet, E. Epigenetics and the embodiment of race: Developmental origins of US racial disparities in cardiovascular health. Am. J. Hum. Biol. 2009, 21, 2–15. [Google Scholar] [CrossRef]
- Aneli, S.; Mezzavilla, M.; Bortolini, E.; Pirastu, N.; Girotto, G.; Spedicati, B.; Berchialla, P.; Gasparini, P.; Pagani, L. Impact of cultural and genetic structure on food choices along the Silk Road. Proc. Natl. Acad. Sci. USA 2022, 119, e2209311119. [Google Scholar] [CrossRef] [PubMed]
- Deng, M.; Hida, N.; Yamazaki, T.; Morishima, R.; Kato, Y.; Fujita, Y.; Nakamura, A.; Harada, T. Comparison of bitterness intensity between Prednisolone and quinine in a human sensory test indicated individual differences in bitter-taste perception. Pharmaceutics 2022, 14, 2454. [Google Scholar] [CrossRef] [PubMed]
- Ivanovska, V.; Rademaker, C.M.; van Dijk, L.; Mantel-Teeuwisse, A.K. Pediatric drug formulations: A review of challenges and progress. Pediatrics 2014, 134, 361–372. [Google Scholar] [CrossRef]
- Mennella, J.A.; Pepino, M.Y.; Duke, F.F.; Reed, D.R. Age modifies the genotype-phenotype relationship for the bitter receptor TAS2R38. BMC Genet. 2010, 11, 60. [Google Scholar] [CrossRef] [PubMed]
- Borrell, L.N.; Elhawary, J.R.; Fuentes-Afflick, E.; Witonsky, J.; Bhakta, N.; Wu, A.H.B.; Bibbins-Domingo, K.; Rodriguez-Santana, J.R.; Lenoir, M.A.; Gavin, J.R., 3rd; et al. Race and genetic ancestry in medicine–A time for reckoning with racism. N. Engl. J. Med. 2021, 384, 474–480. [Google Scholar] [CrossRef]
- Venables, R.; Marriott, J.; Stirling, H. FIND OUT: Key problems with children’s medicines formulations... It’s a taste issue! Int. J. Pharm. Pract. 2012, 20, 23. [Google Scholar]
- White, N.C.; Litovitz, T.; Benson, B.E.; Horowitz, B.Z.; Marr-Lyon, L.; White, M.K. The impact of bittering agents on pediatric ingestions of antifreeze. Clin. Pediatr. 2009, 48, 913–921. [Google Scholar] [CrossRef]
- Mullins, M.E.; Zane Horowitz, B. Was it necessary to add Bitrex (denatonium benzoate) to automotive products? Vet. Hum. Toxicol. 2004, 46, 150–152. [Google Scholar]
- Dalton, P.; Doty, R.L.; Murphy, C.; Frank, R.; Hoffman, H.J.; Maute, C.; Kallen, M.A.; Slotkin, J. Olfactory assessment using the NIH Toolbox. Neurology 2013, 80 (Suppl. S3), S32–S36. [Google Scholar] [CrossRef]
- Hoffman, H.J.; Rawal, S.; Li, C.M.; Duffy, V.B. New chemosensory component in the U.S. National Health and Nutrition Examination Survey (NHANES): First-year results for measured olfactory dysfunction. Rev. Endocr. Metab. Disord. 2016, 17, 221–240. [Google Scholar] [CrossRef]
- Bartoshuk, L.M.; Duffy, V.B.; Green, B.G.; Hoffman, H.J.; Ko, C.W.; Lucchina, L.A.; Marks, L.E.; Snyder, D.J.; Weiffenbach, J.M. Valid across-group comparisons with labeled scales: The gLMS versus magnitude matching. Physiol. Behav. 2004, 82, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Meiselman, H.L.; Dzendolet, E. Variability in gustatory quality identification. Percept. Psychophys. 1967, 2, 496–498. [Google Scholar] [CrossRef]
- Alfaro, R.; Crowder, S.; Sarma, K.P.; Arthur, A.E.; Pepino, M.Y. Taste and smell function in head and neck cancer survivors. Chem. Senses 2021, 46, bjab026. [Google Scholar] [CrossRef] [PubMed]
- Purcell, S.; Neale, B.; Todd-Brown, K.; Thomas, L.; Ferreira, M.A.; Bender, D.; Maller, J.; Sklar, P.; de Bakker, P.I.; Daly, M.J.; et al. PLINK: A tool set for whole-genome association and population-based linkage analyses. Am. J. Hum. Genet. 2007, 81, 559–575. [Google Scholar] [CrossRef]
- Manichaikul, A.; Mychaleckyj, J.C.; Rich, S.S.; Daly, K.; Sale, M.; Chen, W.M. Robust relationship inference in genome-wide association studies. Bioinformatics 2010, 26, 2867–2873. [Google Scholar] [CrossRef]
- Price, A.L.; Patterson, N.J.; Plenge, R.M.; Weinblatt, M.E.; Shadick, N.A.; Reich, D. Principal components analysis corrects for stratification in genome-wide association studies. Nat. Genet. 2006, 38, 904–909. [Google Scholar] [CrossRef]
- Genomes Project Consortium; Auton, A.; Brooks, L.D.; Durbin, R.M.; Garrison, E.P.; Kang, H.M.; Korbel, J.O.; Marchini, J.L.; McCarthy, S.; McVean, G.A.; et al. A global reference for human genetic variation. Nature 2015, 526, 68–74. [Google Scholar]
- Nelson, G.; Hoon, M.A.; Chandrashekar, J.; Zhang, Y.; Ryba, N.J.; Zuker, C.S. Mammalian sweet taste receptors. Cell 2001, 106, 381–390. [Google Scholar] [CrossRef]
- Liman, E.R. TRPM5 and taste transduction. Handb. Exp. Pharmacol. 2007, 179, 287–298. [Google Scholar]
- Allen, A.L.; McGeary, J.E.; Hayes, J.E. Polymorphisms in TRPV1 and TAS2Rs associate with sensations from sampled ethanol. Alcohol. Clin. Exp. Res. 2014, 38, 2550–2560. [Google Scholar] [CrossRef]
- Meyerhof, W.; Batram, C.; Kuhn, C.; Brockhoff, A.; Chudoba, E.; Bufe, B.; Appendino, G.; Behrens, M. The molecular receptive ranges of human TAS2R bitter taste receptors. Chem. Senses 2010, 35, 157–170. [Google Scholar] [CrossRef] [PubMed]

| Experimental Condition | p Value 2 | ||||
|---|---|---|---|---|---|
| Sip & Spit | Swallow | Delay | Sip v Swallow | Sip v Delay | |
| Intensity, gLMS rating | |||||
| Sweetness | 33.9 ± 1.6 | 31.3 ± 1.6 | 16.4 ± 1.3 | 0.02 | 0.001 |
| Irritation | 6.7 ± 0.7 | 10.9 ± 1.0 | 8.9 ± 1.0 | <0.001 | 0.02 |
| Bitterness | 7.3 ± 0.8 | 8.6 ± 0.8 | 6.6 ± 0.8 | 0.03 | 0.34 |
| Palatability | |||||
| Hedonic gLMS rating | 16.4 ± 2.1 | 10.4 ± 2.2 | 8.2 ± 2.1 | <0.001 | 0.06 |
| Gene | SNP Identifier/ Function | Major/Minor Allele 1 | Minor Allele Frequency | Phenotype, Stimulus 2 | SNP-Phenotype p Value 3 | SNP-Phenotype, Linear or Logistic Regression 4 | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| All Ancestries | African Ancestry | European Ancestry | Nonadjusted | PC Adjusted | |||||||
| Effect Size | p Value | Effect Size | p Value | ||||||||
| TRPM8 | rs7593557/S419N | G/A | 0.326 | 0.563 | 0.049 | Sweet rating, Motrin | 0.02 | 5.92 ± 2.15 | 0.007 | 3.49 ± 2.88 | 0.23 |
| Cough/scratch, Motrin | 0.009 | 0.49 (0.30–0.77) | 0.003 | 0.60 (0.32–1.10) | 0.10 | ||||||
| TRPV1 | rs224534/T469I | G/A | 0.28 | 0.143 | 0.324 | Tingle, Motrin | 0.04 | — | 0.23 | — | 0.96 |
| TRPA1 | rs11988795/Intronic | C/T | 0.337 | 0.310 | 0.353 | Tingle, Motrin | 0.06 | 1.88 (1.12–3.23) | 0.02 | 1.80 (1.05–3.15) | 0.03 |
| TAS1R3 | rs35744813/Intergenic | C/T | 0.369 | 0.619 | 0.127 | Sweet rating, Ace-K | 0.01 | — | 0.46 | — | 0.74 |
| Sweet rating, sucrose | 0.07 | — | 0.18 | — | 0.93 | ||||||
| TAS2R9 | rs3741845/V187A | A/G | 0.426 | 0.222 | 0.569 | Bitter rating, Ace-K | <0.001 | −0.99 ± 0.21 | <0.001 | −1.00 ± 0.25 | <0.001 |
| TAS2R31 | rs10845293 A227V/ | G/A | 0.39 | 0.325 | 0.510 | Bitter rating, Ace-K | 0.002 | 0.69 ± 0.23 | 0.003 | 0.78 ± 0.23 | 0.001 |
| TAS2R38 | rs713598/A49P | C/G | 0.465 | 0.508 | 0.412 | Bitter rating, PTU | <0.001 | 1.72 ± 0.24 | <0.001 | 1.73 ± 0.25 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mennella, J.A.; Kan, M.; Lowenthal, E.D.; Saraiva, L.R.; Mainland, J.D.; Himes, B.E.; Pepino, M.Y. Genetic Variation and Sensory Perception of a Pediatric Formulation of Ibuprofen: Can a Medicine Taste Too Good for Some? Int. J. Mol. Sci. 2023, 24, 13050. https://doi.org/10.3390/ijms241713050
Mennella JA, Kan M, Lowenthal ED, Saraiva LR, Mainland JD, Himes BE, Pepino MY. Genetic Variation and Sensory Perception of a Pediatric Formulation of Ibuprofen: Can a Medicine Taste Too Good for Some? International Journal of Molecular Sciences. 2023; 24(17):13050. https://doi.org/10.3390/ijms241713050
Chicago/Turabian StyleMennella, Julie A., Mengyuan Kan, Elizabeth D. Lowenthal, Luis R. Saraiva, Joel D. Mainland, Blanca E. Himes, and M. Yanina Pepino. 2023. "Genetic Variation and Sensory Perception of a Pediatric Formulation of Ibuprofen: Can a Medicine Taste Too Good for Some?" International Journal of Molecular Sciences 24, no. 17: 13050. https://doi.org/10.3390/ijms241713050
APA StyleMennella, J. A., Kan, M., Lowenthal, E. D., Saraiva, L. R., Mainland, J. D., Himes, B. E., & Pepino, M. Y. (2023). Genetic Variation and Sensory Perception of a Pediatric Formulation of Ibuprofen: Can a Medicine Taste Too Good for Some? International Journal of Molecular Sciences, 24(17), 13050. https://doi.org/10.3390/ijms241713050







