Mitochondrial Genome Variation in Polish Elite Athletes
Abstract
:1. Introduction
2. Results
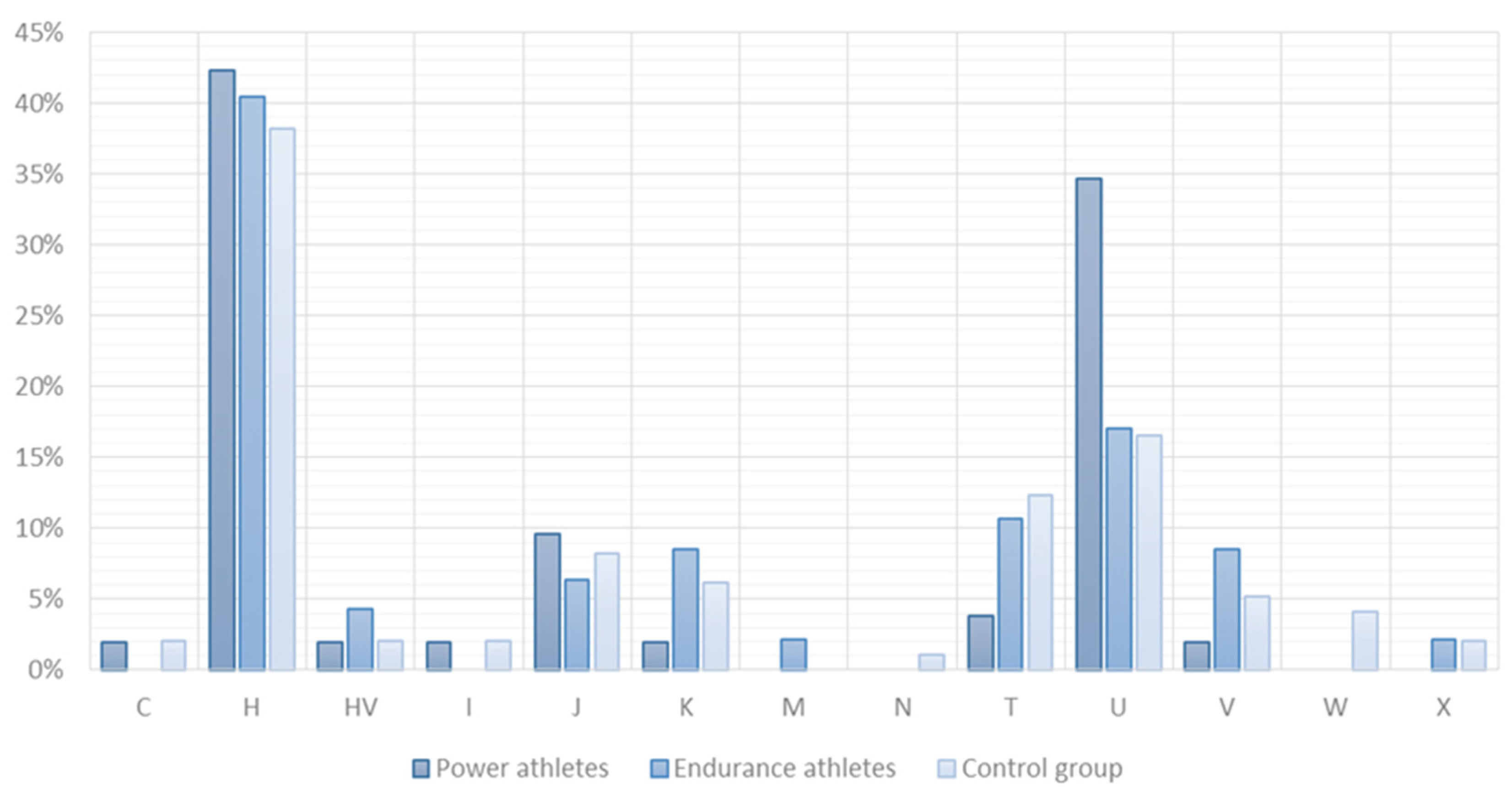
2.1. mtDNA Haplogroup Distribution
2.2. SNV Association Analysis
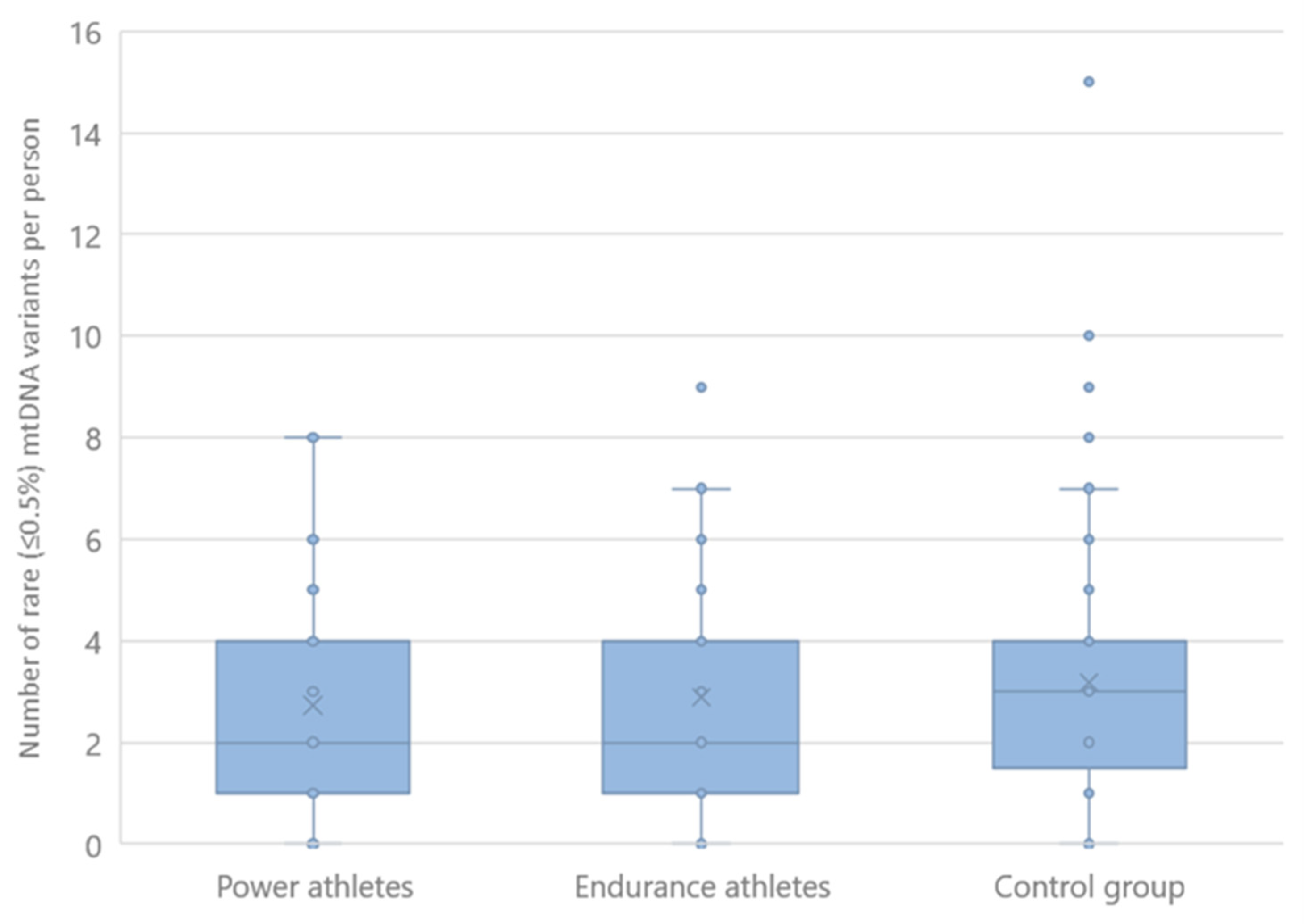
2.3. Rare mtDNA Variants
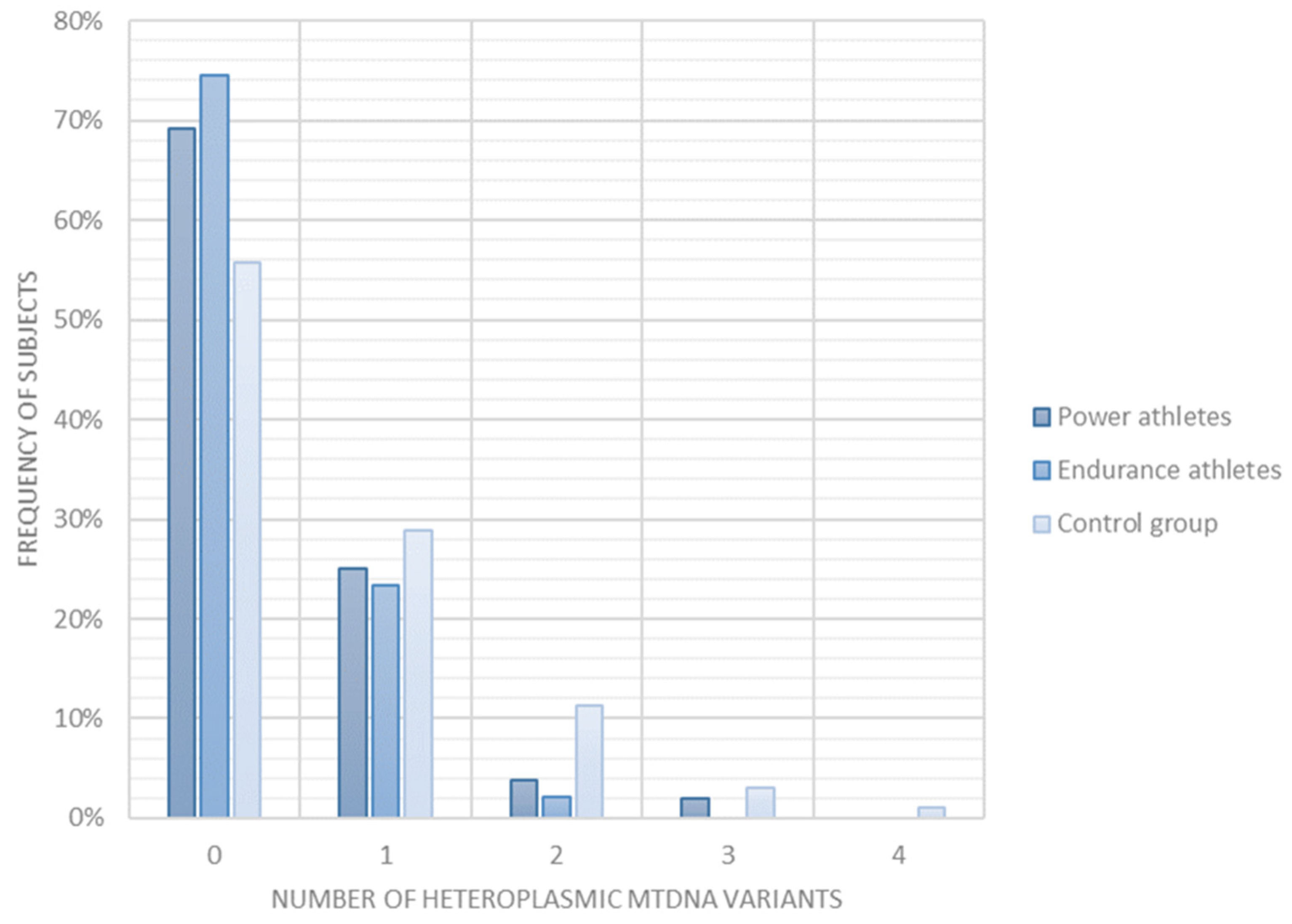
2.4. Heteroplasmic mtDNA Variants
2.5. Non-Synonymous and Synonymous mtDNA Variants
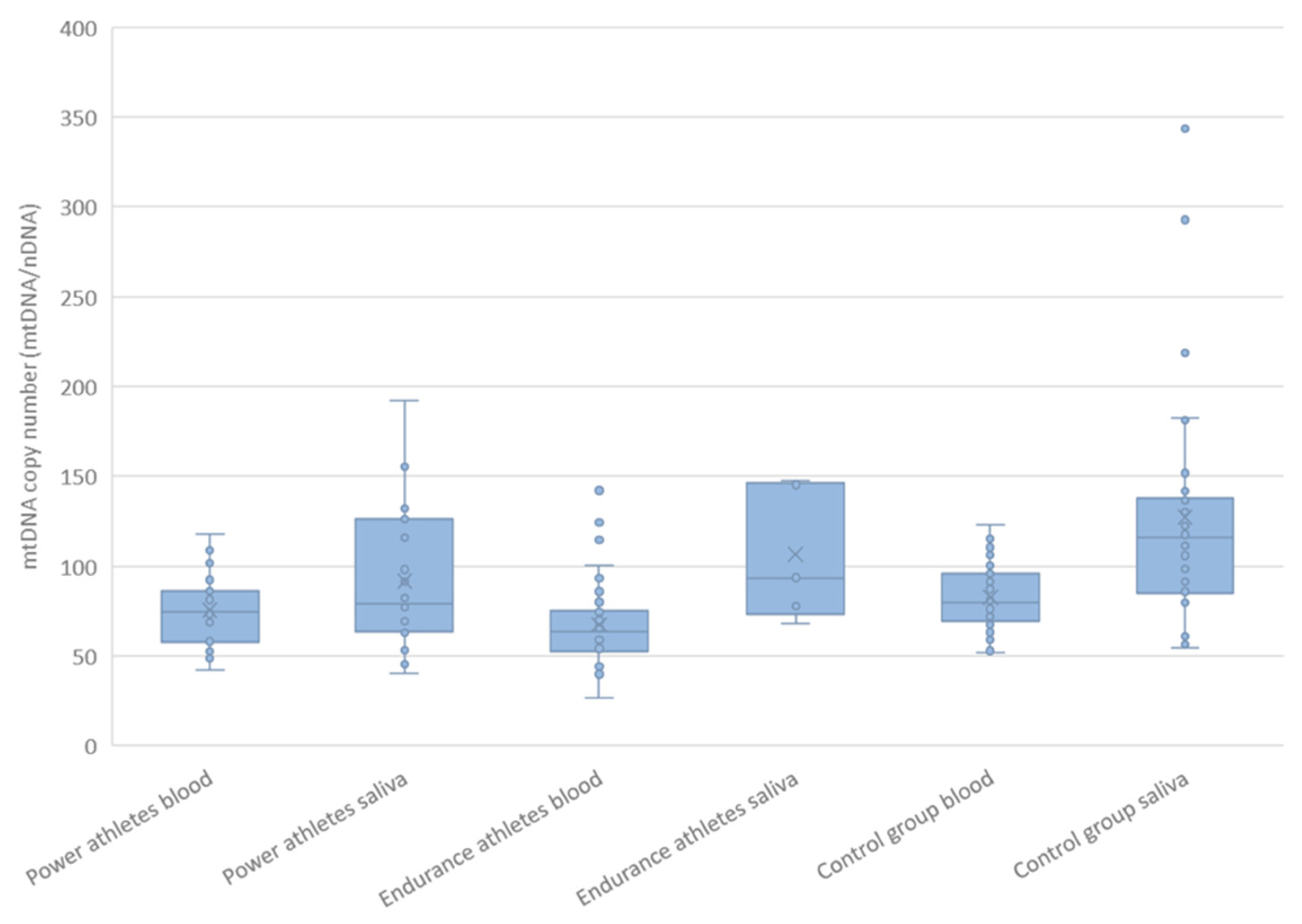
2.6. mtDNA Copy Number
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. DNA Isolation
4.3. Structure of the Study
4.4. mtDNA Sequencing
4.5. Determination of mtDNA Copy Number
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- De Moor, M.H.M.; Spector, T.D.; Cherkas, L.F.; Falchi, M.; Hottenga, J.J.; Boomsma, D.I.; De Geus, E.J.C. Genome-Wide Linkage Scan for Athlete Status in 700 British Female DZ Twin Pairs. Twin Res. Hum. Genet. 2007, 10, 812–820. [Google Scholar] [CrossRef]
- Miyamoto-Mikami, E.; Zempo, H.; Fuku, N.; Kikuchi, N.; Miyachi, M.; Murakami, H. Heritability Estimates of Endurance-Related Phenotypes: A Systematic Review and Meta-Analysis. Scand. J. Med. Sci. Sports 2018, 28, 834–845. [Google Scholar] [CrossRef] [PubMed]
- Ahmetov, I.I.; Hall, E.C.R.; Semenova, E.A.; Pranckevičienė, E.; Ginevičienė, V. Advances in Sports Genomics. Adv. Clin. Chem. 2022, 107, 215–263. [Google Scholar] [CrossRef]
- Lesage, R.; Simoneau, J.A.; Jobin, J.; Leblanc, J.; Bouchard, C. Familial Resemblance in Maximal Heart Rate, Blood Lactate and Aerobic Power. Hum. Hered. 1985, 35, 182–189. [Google Scholar] [CrossRef]
- Pérusse, L.; Gagnon, J.; Province, M.A.; Rao, D.C.; Wilmore, J.H.; Leon, A.S.; Bouchard, C.; Skinner, J.S. Familial Aggregation of Submaximal Aerobic Performance in the HERITAGE Family Study. Med. Sci. Sports Exerc. 2001, 33, 597–604. [Google Scholar] [CrossRef]
- Maruszak, A.; Adamczyk, J.G.; Siewierski, M.; Sozański, H.; Gajewski, A.; Żekanowski, C. Mitochondrial DNA Variation Is Associated with Elite Athletic Status in the Polish Population. Scand. J. Med. Sci. Sports 2014, 24, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto-Mikami, E.; Fuku, N. Chapter Six-Variation of Mitochondrial DNA and Elite Athletic Performance. In Sports, Exercise, and Nutritional Genomics; Barh, D., Ahmetov, I.I., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 129–145. ISBN 978-0-12-816193-7. [Google Scholar]
- Filograna, R.; Mennuni, M.; Alsina, D.; Larsson, N. Mitochondrial DNA Copy Number in Human Disease: The More the Better? FEBS Lett. 2021, 595, 976–1002. [Google Scholar] [CrossRef]
- Wisløff, U.; Najjar, S.M.; Ellingsen, O.; Haram, P.M.; Swoap, S.; Al-Share, Q.; Fernström, M.; Rezaei, K.; Lee, S.J.; Koch, L.G.; et al. Cardiovascular Risk Factors Emerge after Artificial Selection for Low Aerobic Capacity. Science 2005, 307, 418–420. [Google Scholar] [CrossRef] [PubMed]
- Yaghoob Nezhad, F.; Verbrugge, S.A.J.; Schönfelder, M.; Becker, L.; Hrabě de Angelis, M.; Wackerhage, H. Genes Whose Gain or Loss-of-Function Increases Endurance Performance in Mice: A Systematic Literature Review. Front. Physiol. 2019, 10, 262. [Google Scholar] [CrossRef]
- Lee, H.-C.; Wei, Y.-H. Mitochondrial Biogenesis and Mitochondrial DNA Maintenance of Mammalian Cells under Oxidative Stress. Int. J. Biochem. Cell Biol. 2005, 37, 822–834. [Google Scholar] [CrossRef]
- Baykara, O.; Sahin, S.K.; Akbas, F.; Guven, M.; Onaran, I. The Effects of Mitochondrial DNA Deletion and Copy Number Variations on Different Exercise Intensities in Highly Trained Swimmers. Cell Mol. Biol. 2016, 62, 109–115. [Google Scholar]
- Lott, M.T.; Leipzig, J.N.; Derbeneva, O.; Xie, H.M.; Chalkia, D.; Sarmady, M.; Procaccio, V.; Wallace, D.C. MtDNA Variation and Analysis Using Mitomap and Mitomaster. Curr. Protoc. Bioinform. 2013, 44, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Iwanicka-Pronicka, K.; Pollak, A.; Skórka, A.; Lechowicz, U.; Pajdowska, M.; Furmanek, M.; Rzeski, M.; Korniszewski, L.; Skarżyński, H.; Płoski, R. Postlingual Hearing Loss as a Mitochondrial 3243A>G Mutation Phenotype. PLoS ONE 2012, 7, e44054. [Google Scholar] [CrossRef] [PubMed]
- Gorman, G.S.; Schaefer, A.M.; Ng, Y.; Gomez, N.; Blakely, E.L.; Alston, C.L.; Feeney, C.; Horvath, R.; Yu-Wai-Man, P.; Chinnery, P.F.; et al. Prevalence of Nuclear and Mitochondrial DNA Mutations Related to Adult Mitochondrial Disease. Ann. Neurol. 2015, 77, 753–759. [Google Scholar] [CrossRef] [PubMed]
- Laricchia, K.M.; Lake, N.J.; Watts, N.A.; Shand, M.; Haessly, A.; Gauthier, L.; Benjamin, D.; Banks, E.; Soto, J.; Garimella, K.; et al. Mitochondrial DNA Variation across 56,434 Individuals in GnomAD. Genome Res. 2022, 32, 569–582. [Google Scholar] [CrossRef] [PubMed]
- Wallace, D.C. Bioenergetics, the Origins of Complexity, and the Ascent of Man. Proc. Natl. Acad. Sci. USA 2010, 107, 8947–8953. [Google Scholar] [CrossRef] [PubMed]
- Wallace, D.C. Mitochondrial DNA Variation in Human Radiation and Disease. Cell 2015, 163, 33–38. [Google Scholar] [CrossRef]
- Niemi, A.-K.; Majamaa, K. Mitochondrial DNA and ACTN3 Genotypes in Finnish Elite Endurance and Sprint Athletes. Eur. J. Hum. Genet. 2005, 13, 965–969. [Google Scholar] [CrossRef]
- Dionne, F.T.; Turcotte, L.; Thibault, M.C.; Boulay, M.R.; Skinner, J.S.; Bouchard, C. Mitochondrial DNA Sequence Polymorphism, VO2max, and Response to Endurance Training. Med. Sci. Sports Exerc. 1991, 23, 177–185. [Google Scholar] [CrossRef]
- Harvey, N.R.; Voisin, S.; Lea, R.A.; Yan, X.; Benton, M.C.; Papadimitriou, I.D.; Jacques, M.; Haupt, L.M.; Ashton, K.J.; Eynon, N.; et al. Investigating the Influence of MtDNA and Nuclear Encoded Mitochondrial Variants on High Intensity Interval Training Outcomes. Sci. Rep. 2020, 10, 11089. [Google Scholar] [CrossRef]
- Vellers, H.L.; Massett, M.P.; Avila, J.J.; Kim, S.K.; Marzec, J.M.; Santos, J.H.; Lightfoot, J.T.; Kleeberger, S.R. Mitochondrial DNA Lesions and Copy Number Are Strain Dependent in Endurance-Trained Mice. Physiol. Rep. 2020, 8, e14605. [Google Scholar] [CrossRef] [PubMed]
- Kumagai, H.; Miller, B.; Kim, S.-J.; Leelaprachakul, N.; Kikuchi, N.; Yen, K.; Cohen, P. Novel Insights into Mitochondrial DNA: Mitochondrial Microproteins and MtDNA Variants Modulate Athletic Performance and Age-Related Diseases. Genes 2023, 14, 286. [Google Scholar] [CrossRef] [PubMed]
- Mikami, E.; Fuku, N.; Kong, Q.-P.; Takahashi, H.; Ohiwa, N.; Murakami, H.; Miyachi, M.; Higuchi, M.; Tanaka, M.; Pitsiladis, Y.P.; et al. Comprehensive Analysis of Common and Rare Mitochondrial DNA Variants in Elite Japanese Athletes: A Case-Control Study. J. Hum. Genet. 2013, 58, 780–787. [Google Scholar] [CrossRef] [PubMed]
- Kiiskilä, J.; Moilanen, J.S.; Kytövuori, L.; Niemi, A.-K.; Majamaa, K. Analysis of Functional Variants in Mitochondrial DNA of Finnish Athletes. BMC Genom. 2019, 20, 784. [Google Scholar] [CrossRef] [PubMed]
- Venter, M.; van der Westhuizen, F.H.; Elson, J.L. The Aetiology of Cardiovascular Disease: A Role for Mitochondrial DNA? Cardiovasc. J. Afr. 2018, 29, 122–132. [Google Scholar] [CrossRef] [PubMed]
- Hénault, M.; Marsit, S.; Charron, G.; Landry, C.R. Hybridization Drives Mitochondrial DNA Degeneration and Metabolic Shift in a Species with Biparental Mitochondrial Inheritance. Genome Res. 2022, 32, 2043–2056. [Google Scholar] [CrossRef]
- Hagman, M.; Fristrup, B.; Michelin, R.; Krustrup, P.; Asghar, M. Football and Team Handball Training Postpone Cellular Aging in Women. Sci. Rep. 2021, 11, 11733. [Google Scholar] [CrossRef]
- Mengel-From, J.; Thinggaard, M.; Dalgård, C.; Kyvik, K.O.; Christensen, K.; Christiansen, L. Mitochondrial DNA Copy Number in Peripheral Blood Cells Declines with Age and Is Associated with General Health among Elderly. Hum. Genet. 2014, 133, 1149–1159. [Google Scholar] [CrossRef]
- Knez, J.; Winckelmans, E.; Plusquin, M.; Thijs, L.; Cauwenberghs, N.; Gu, Y.; Staessen, J.A.; Nawrot, T.S.; Kuznetsova, T. Correlates of Peripheral Blood Mitochondrial DNA Content in a General Population. Am. J. Epidemiol. 2016, 183, 138–146. [Google Scholar] [CrossRef]
- Rice, A.C.; Keeney, P.M.; Algarzae, N.K.; Ladd, A.C.; Thomas, R.R.; Bennett, J.P. Mitochondrial DNA Copy Numbers in Pyramidal Neurons Are Decreased and Mitochondrial Biogenesis Transcriptome Signaling Is Disrupted in Alzheimer’s Disease Hippocampi. J. Alzheimers Dis. 2014, 40, 319–330. [Google Scholar] [CrossRef]
- Dölle, C.; Flønes, I.; Nido, G.S.; Miletic, H.; Osuagwu, N.; Kristoffersen, S.; Lilleng, P.K.; Larsen, J.P.; Tysnes, O.-B.; Haugarvoll, K.; et al. Defective Mitochondrial DNA Homeostasis in the Substantia Nigra in Parkinson Disease. Nat. Commun. 2016, 7, 13548. [Google Scholar] [CrossRef]
- Nilsson, M.I.; Tarnopolsky, M.A. Mitochondria and Aging-The Role of Exercise as a Countermeasure. Biology 2019, 8, 40. [Google Scholar] [CrossRef] [PubMed]
- Amar, D.; Gay, N.R.; Jimenez-Morales, D.; Jean Beltran, P.M.; Ramaker, M.E.; Raja, A.N.; Zhao, B.; Sun, Y.; Marwaha, S.; Gaul, D.; et al. The Mitochondrial Multi-Omic Response to Exercise Training across Tissues. bioRxiv 2023. [Google Scholar] [CrossRef]
- Ylikallio, E.; Tyynismaa, H.; Tsutsui, H.; Ide, T.; Suomalainen, A. High Mitochondrial DNA Copy Number Has Detrimental Effects in Mice. Hum. Mol. Genet. 2010, 19, 2695–2705. [Google Scholar] [CrossRef] [PubMed]
- Piotrowska-Nowak, A.; Elson, J.L.; Sobczyk-Kopciol, A.; Piwonska, A.; Puch-Walczak, A.; Drygas, W.; Ploski, R.; Bartnik, E.; Tonska, K. New MtDNA Association Model, MutPred Variant Load, Suggests Individuals With Multiple Mildly Deleterious MtDNA Variants Are More Likely to Suffer From Atherosclerosis. Front. Genet. 2018, 9, 702. [Google Scholar] [CrossRef]
- Piotrowska-Nowak, A.; Kosior-Jarecka, E.; Schab, A.; Wrobel-Dudzinska, D.; Bartnik, E.; Zarnowski, T.; Tonska, K. Investigation of Whole Mitochondrial Genome Variation in Normal Tension Glaucoma. Exp. Eye Res. 2019, 178, 186–197. [Google Scholar] [CrossRef] [PubMed]
- Weissensteiner, H.; Pacher, D.; Kloss-Brandstätter, A.; Forer, L.; Specht, G.; Bandelt, H.-J.; Kronenberg, F.; Salas, A.; Schönherr, S. HaploGrep 2: Mitochondrial Haplogroup Classification in the Era of High-Throughput Sequencing. Nucleic Acids Res. 2016, 44, W58–W63. [Google Scholar] [CrossRef] [PubMed]


| mtDNA Variant | Power Athletes (n = 52) | Endurance Athletes (n = 47) | Control Group (n = 97) |
|---|---|---|---|
| m.194C>T | 0% | 0% | 5% $,^ |
| m.709G>A | 4% $,# | 19% | 18% |
| m.930G>A | 0% # | 6% | 9% |
| m.8994G>A | 0% | 0% | 5% $,^ |
| m.11812A>G | 2% # | 6% | 11% |
| m.11947A>G | 0% | 0% | 5% $,^ |
| m.12414T>C | 0% | 0% | 5% $,^ |
| m.14233A>G | 2% # | 6% | 11% |
| m.16294C>T | 4% # | 11% | 14% |
| m.16296C>T | 0% # | 6% | 8% |
| Category of Hypotheses | Number of Hypotheses Tested | Associations Considered True Positive * | p-Value | q-Value |
|---|---|---|---|---|
| Haplogroup distribution | 1 | - | 0.41 | 0.41 |
| mtDNA SNV associations | 475 | - | >0.015 | >0.73 |
| Rare mtDNA variants | 4 | - | >0.17 | >0.35 |
| Heteroplasmic mtDNA variants | 4 | number of heteroplasmic mtDNA variants is lower in athletes than in controls | 0.0079 | 0.027 |
| number of heteroplasmic mtDNA variants is lower in endurance athletes than in controls | 0.014 | 0.027 | ||
| Synonymous and non-synonymous mtDNA variants | 12 | - | >0.14 | >0.86 |
| mtDNA copy number | 12 | mtDNA copy number in blood is lower in endurance athletes than in controls | 0.00005 | 0.0003 |
| mtDNA copy number in blood is lower in athletes than in controls | 0.00028 | 0.0011 | ||
| mtDNA copy number is higher in saliva than in blood in controls | 0.000009 | 0.00011 | ||
| mtDNA copy number is higher in saliva than in blood in athletes | 0.0033 | 0.0099 | ||
| mtDNA copy number is higher in saliva than in blood in endurance athletes | 0.012 | 0.028 |
| Sport | n | International Elite Class | National Elite Class | |
|---|---|---|---|---|
| Power sports | Athletics, sprints (100–400 m) and throws | 27 | 25 | 2 |
| Weightlifting | 23 | 0 | 23 | |
| Powerlifting | 1 | 0 | 1 | |
| Swimming (sprint) | 1 | 1 | 0 | |
| Total | 52 | 26 | 26 | |
| Endurance sports | Athletics, long-distance runs (3000 m—marathon) | 10 | 9 | 1 |
| Cross-country skiing | 9 | 4 | 5 | |
| Swimming (400–1500 m) | 22 | 8 | 14 | |
| Triathlon | 3 | 2 | 1 | |
| Rowing | 2 | 2 | 0 | |
| Speed skating | 1 | 1 | 0 | |
| Total | 47 | 26 | 21 |
| Olympic Games | World Championships | European Championships | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Participant, Only | Finalist, Only | Medalists | Participant, Only | Finalist, Only | Medalists | Participant, Only | Finalist, Only | Medalist | |
| Power sports | 16 | 9 | 4 | 21 | 18 | 11 | 26 | 19 | 14 |
| Endurance sports | 9 | 4 | 2 | 10 | 8 | 5 | 24 | 10 | 6 |
| Total | 25 | 13 | 6 | 31 | 26 | 16 | 50 | 29 | 20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piotrowska-Nowak, A.; Safranow, K.; Adamczyk, J.G.; Sołtyszewski, I.; Cięszczyk, P.; Tońska, K.; Żekanowski, C.; Borzemska, B. Mitochondrial Genome Variation in Polish Elite Athletes. Int. J. Mol. Sci. 2023, 24, 12992. https://doi.org/10.3390/ijms241612992
Piotrowska-Nowak A, Safranow K, Adamczyk JG, Sołtyszewski I, Cięszczyk P, Tońska K, Żekanowski C, Borzemska B. Mitochondrial Genome Variation in Polish Elite Athletes. International Journal of Molecular Sciences. 2023; 24(16):12992. https://doi.org/10.3390/ijms241612992
Chicago/Turabian StylePiotrowska-Nowak, Agnieszka, Krzysztof Safranow, Jakub G. Adamczyk, Ireneusz Sołtyszewski, Paweł Cięszczyk, Katarzyna Tońska, Cezary Żekanowski, and Beata Borzemska. 2023. "Mitochondrial Genome Variation in Polish Elite Athletes" International Journal of Molecular Sciences 24, no. 16: 12992. https://doi.org/10.3390/ijms241612992
APA StylePiotrowska-Nowak, A., Safranow, K., Adamczyk, J. G., Sołtyszewski, I., Cięszczyk, P., Tońska, K., Żekanowski, C., & Borzemska, B. (2023). Mitochondrial Genome Variation in Polish Elite Athletes. International Journal of Molecular Sciences, 24(16), 12992. https://doi.org/10.3390/ijms241612992








