Interleukin 6 (IL-6) Regulates GABAA Receptors in the Dorsomedial Hypothalamus Nucleus (DMH) through Activation of the JAK/STAT Pathway to Affect Heart Rate Variability in Stressed Rats
Abstract
:1. Introduction
2. Results
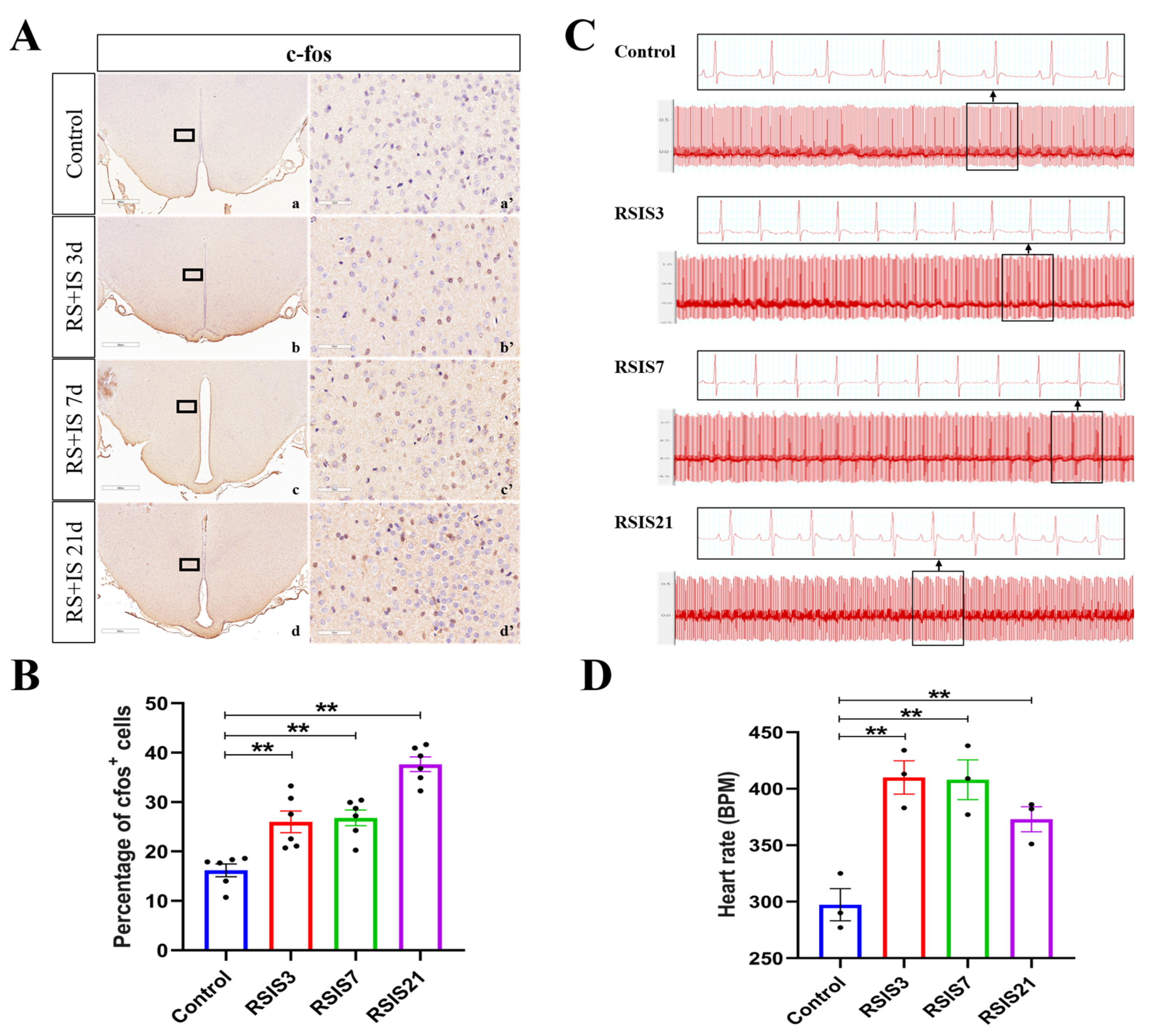
2.1. Stress-Induced Enhancement of DMH Neuronal Activity Increased Heart Rate
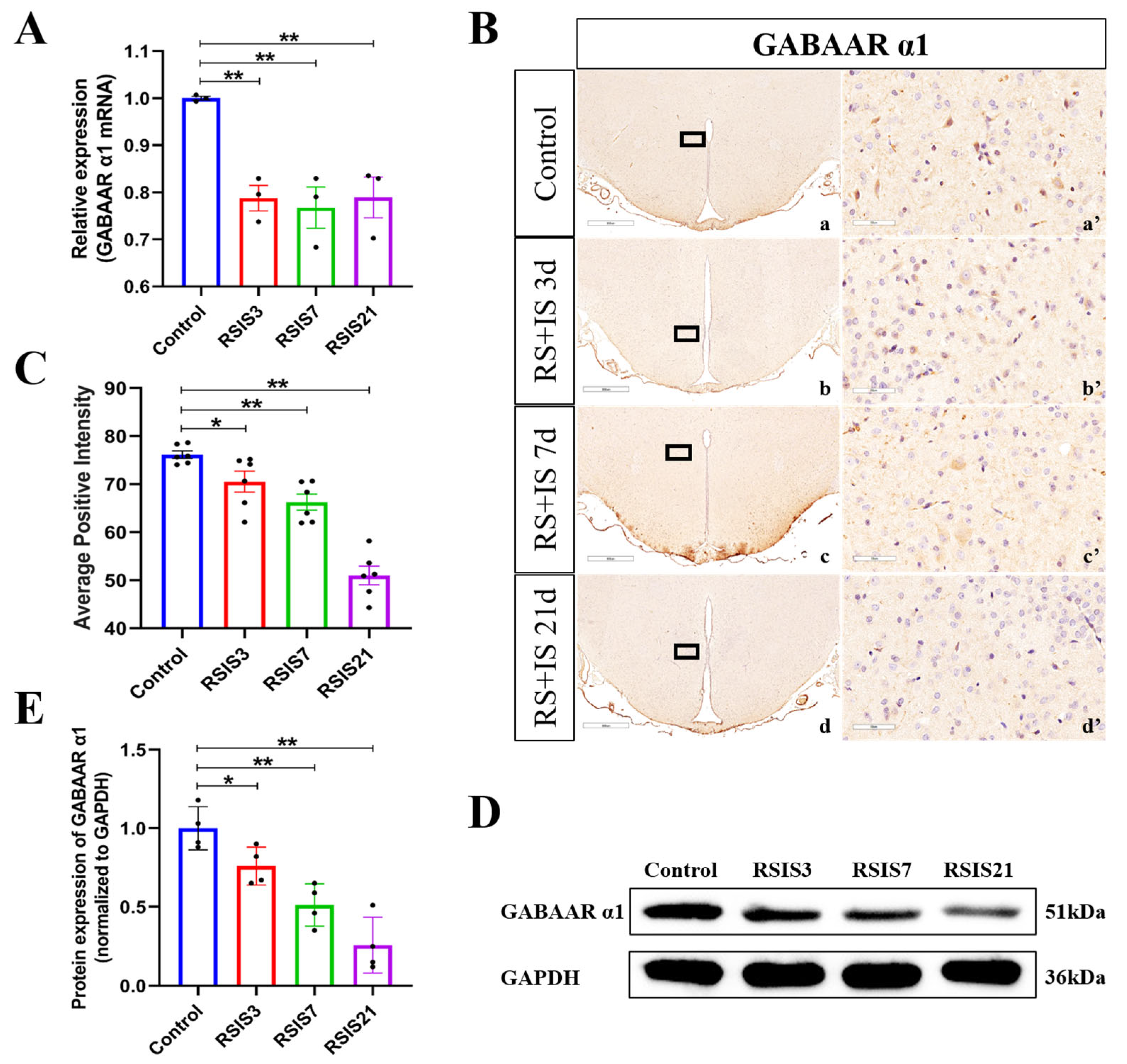
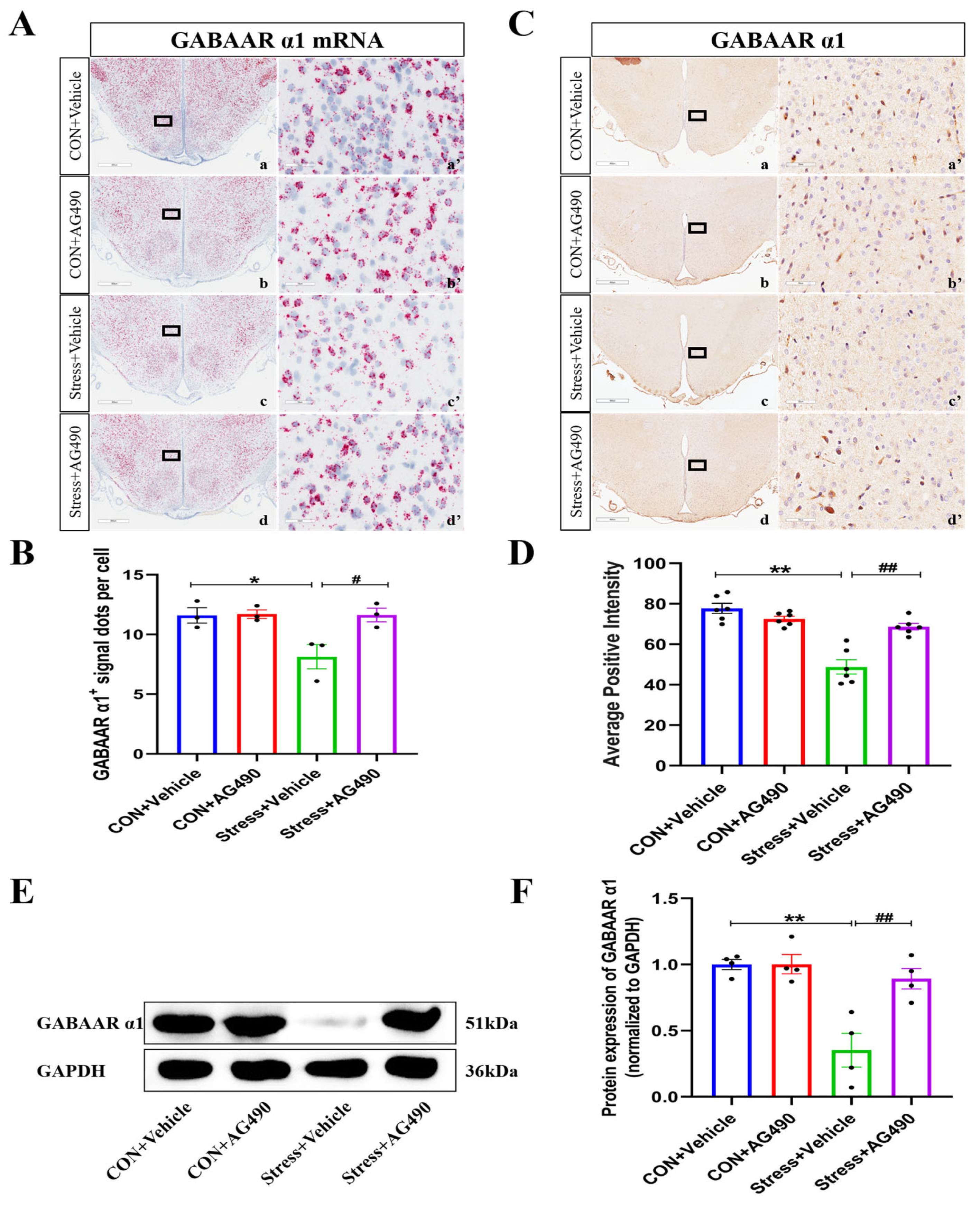
2.2. Stress Decreased the Expression of the GABAAR α1 Subunit
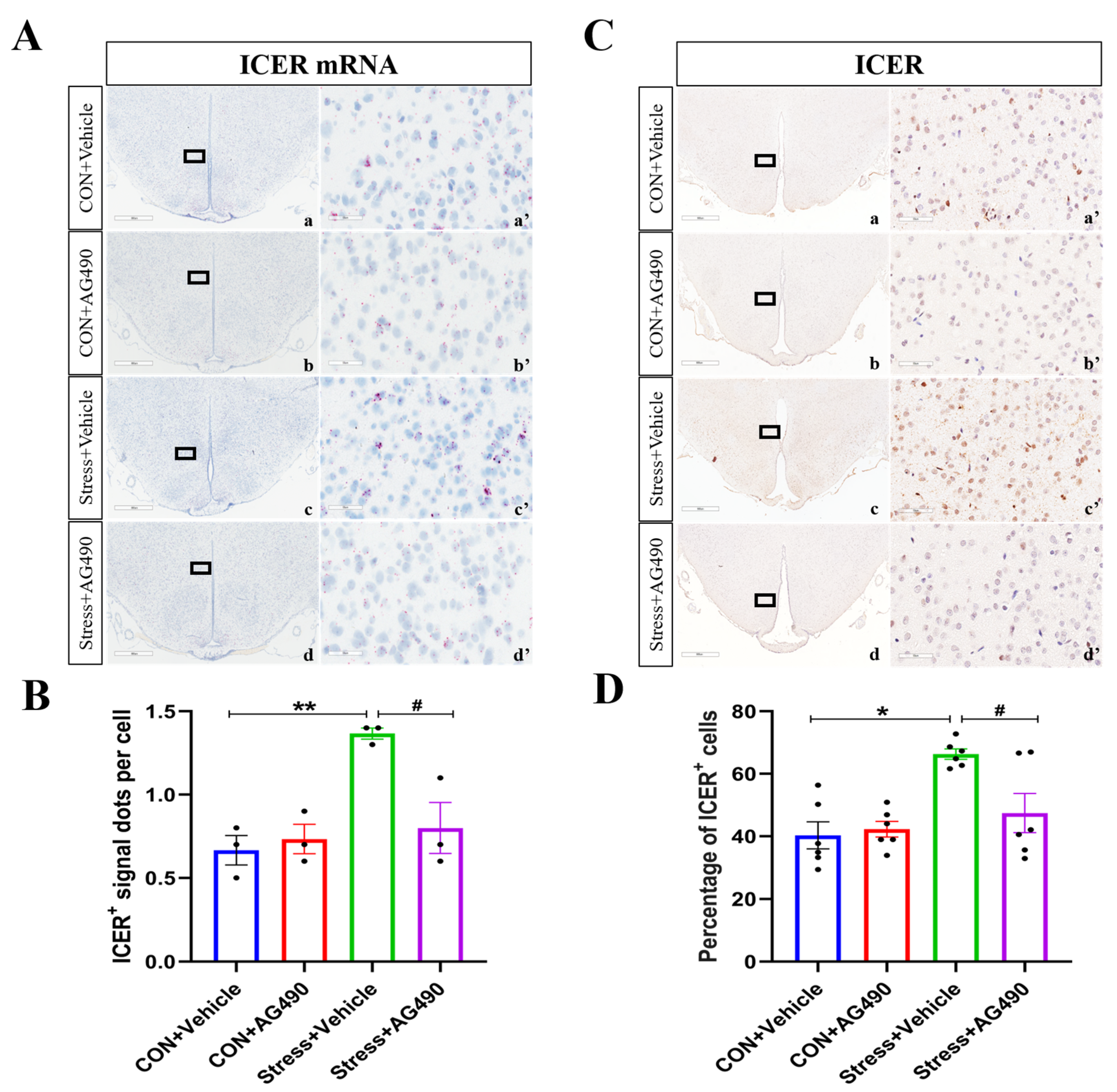
2.3. Stress-Induced Increase in IL-6 Activated the JAK/STAT Pathway, Promoting the Expression of the Downstream Molecule ICER
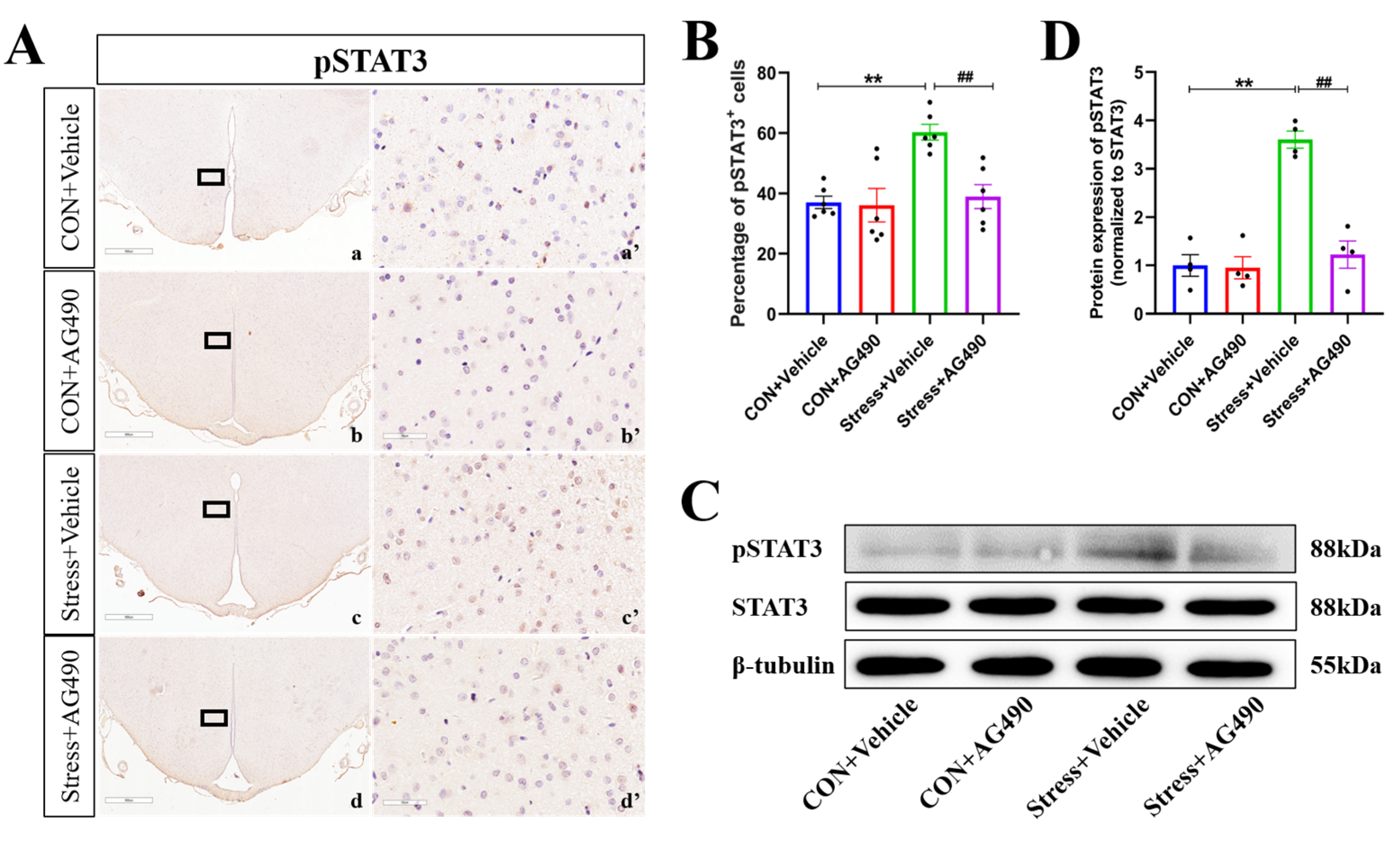
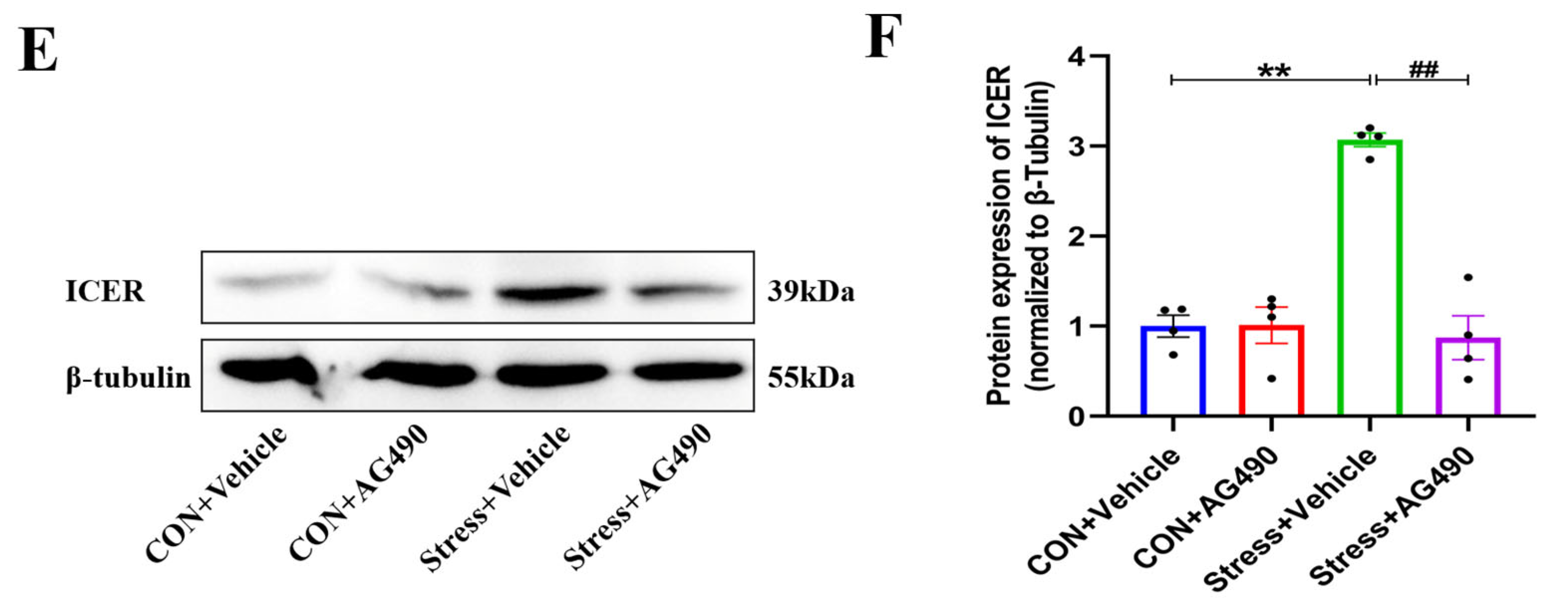
2.4. AG490 Treatment Inhibited the Levels of pSTAT3 and Its Downstream Molecule ICER in Stressed Rats
2.5. AG490 Treatment Alleviated the Stress-Induced Decrease in the GABAAR α1 Subunit
2.6. AG490 Treatment Reduced DMH Neuronal Activity and Improved Tachycardia in Stressed Rats
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. RSIS Procedure
4.3. Intraventricular Injection
4.4. Electrocardiogram Measurement
4.5. Enzyme-Linked Immunosorbent Assay (ELISA)
4.6. Real-Time Quantitative Polymerase Chain Reaction (RT-qPCR)
4.7. Immunohistochemistry (IHC)
4.8. Analysis of IHC Staining
4.9. RNAscope Assay
4.10. Western Blot Analysis
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hammadah, M.; Kim, J.H.; Al Mheid, I.; Samman Tahhan, A.; Wilmot, K.; Ramadan, R.; Alkhoder, A.; Khayata, M.; Mekonnen, G.; Levantsevych, O.; et al. Coronary and Peripheral Vasomotor Responses to Mental Stress. J. Am. Heart Assoc. 2018, 7, e008532. [Google Scholar] [CrossRef] [PubMed]
- Steptoe, A.; Kivimäki, M. Stress and cardiovascular disease. Nat. Rev. Cardiol. 2012, 9, 360–370. [Google Scholar] [CrossRef] [PubMed]
- Kivimaki, M.; Virtanen, M.; Elovainio, M.; Kouvonen, A.; Vaananen, A.; Vahtera, J. Work stress in the etiology of coronary heart disease—A meta-analysis. Scand. J. Work Environ. Health 2006, 32, 431–442. [Google Scholar] [CrossRef] [PubMed]
- Kyrou, I.; Tsigos, C. Stress hormones: Physiological stress and regulation of metabolism. Curr. Opin. Pharmacol. 2009, 9, 787–793. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.J.; Blumenthal, J.A. Psychiatric and Behavioral Aspects of Cardiovascular Disease: Epidemiology, Mechanisms, and Treatment. Rev. Esp. Cardiol. 2011, 64, 924–933. [Google Scholar] [CrossRef] [PubMed]
- Vaccarino, V.; Shah, A.J.; Mehta, P.K.; Pearce, B.; Raggi, P.; Bremner, J.D.; Quyyumi, A.A. Brain-heart connections in stress and cardiovascular disease: Implications for the cardiac patient. Atherosclerosis 2021, 328, 74–82. [Google Scholar] [CrossRef]
- Buijs, R.M.; Van Eden, C.G. The integration of stress by the hypothalamus, amygdala and prefrontal cortex: Balance between the autonomic nervous system and the neuroendocrine system. Prog. Brain Res. 2000, 126, 117–132. [Google Scholar]
- Kono, Y.; Yokota, S.; Fukushi, I.; Arima, Y.; Onimaru, H.; Okazaki, S.; Takeda, K.; Yazawa, I.; Yoshizawa, M.; Hasebe, Y.; et al. Structural and functional connectivity from the dorsomedial hypothalamus to the ventral medulla as a chronological amplifier of sympathetic outflow. Sci. Rep. 2020, 10, 13325. [Google Scholar] [CrossRef]
- Fontes, M.A.P.; Limborco, M.; Machado, N.L.S.; de Paula, C.A.; Cordeiro, L.M.S.; Xavier, C.H.; Marins, F.R.; Henderson, L.; Macefield, V.G. Asymmetric sympathetic output: The dorsomedial hypothalamus as a potential link between emotional stress and cardiac arrhythmias. Auton. Neurosci.-Basic 2017, 207, 22–27. [Google Scholar] [CrossRef]
- Fontes, M.A.P.; Xavier, C.H.; Marins, F.R.; Limborco, M.; Vaz, G.C.; Muller-Ribeiro, F.C.; Nalivaiko, E. Emotional stress and sympathetic activity: Contribution of dorsomedial hypothalamus to cardiac arrhythmias. Brain Res. 2014, 1554, 49–58. [Google Scholar] [CrossRef]
- Zaretskaia, M.V.; Zaretsky, D.V.; Sarkar, S.; Shekhar, A.; Dimicco, J.A. Induction of Fos-immunoreactivity in the rat brain following disinhibition of the dorsomedial hypothalamus. Brain Res. 2008, 1200, 39–50. [Google Scholar] [CrossRef]
- Sallard, E.; Letourneur, D.; Legendre, P. Electrophysiology of ionotropic GABA receptors. Cell. Mol. Life Sci. 2021, 78, 5341–5370. [Google Scholar] [CrossRef]
- Le Magueresse, C.; Monyer, H. GABAergic Interneurons Shape the Functional Maturation of the Cortex. Neuron 2013, 77, 388–405. [Google Scholar] [CrossRef]
- Skilbeck, K.J.; Johnston, G.A.R.; Hinton, T. Stress and GABA(A) receptors. J. Neurochem. 2010, 112, 1115–1130. [Google Scholar] [CrossRef] [PubMed]
- Bavelier, D.; Levi, D.M.; Li, R.W.; Dan, Y.; Hensch, T.K. Removing Brakes on Adult Brain Plasticity: From Molecular to Behavioral Interventions. J. Neurosci. 2010, 30, 14964–14971. [Google Scholar] [CrossRef] [PubMed]
- Lewis, D.A. Cortical circuit dysfunction and cognitive deficits in schizophrenia—Implications for preemptive interventions. Eur. J. Neurosci. 2012, 35, 1871–1878. [Google Scholar] [CrossRef]
- Fritschy, J.M.; Panzanelli, P. GABAA receptors and plasticity of inhibitory neurotransmission in the central nervous system. Eur. J. Neurosci. 2014, 39, 1845–1865. [Google Scholar] [CrossRef] [PubMed]
- Rudolph, U.; Knoflach, F. Beyond classical benzodiazepines: Novel therapeutic potential of GABAA receptor subtypes. Nat. Rev. Drug Discov. 2011, 10, 685–697. [Google Scholar] [CrossRef] [PubMed]
- Raible, D.J.; Frey, L.C.; Cruz Del Angel, Y.; Russek, S.J.; Brooks-Kayal, A.R. GABA(A) receptor regulation after experimental traumatic brain injury. J. Neurotrauma. 2012, 29, 2548–2554. [Google Scholar] [CrossRef]
- Schwartz-Bloom, R.D.; Sah, R. gamma-Aminobutyric acid(A) neurotransmission and cerebral ischemia. J. Neurochem. 2001, 77, 353–371. [Google Scholar] [CrossRef]
- Wu, C.; Sun, D. GABA receptors in brain development, function, and injury. Metab. Brain Dis. 2015, 30, 367–379. [Google Scholar] [CrossRef] [PubMed]
- Bobbo, V.C.D.; Jara, C.P.; Mendes, N.F.; Morari, J.; Velloso, L.A.; Araujo, E.P. Interleukin-6 Expression by Hypothalamic Microglia in Multiple Inflammatory Contexts: A Systematic Review. Biomed. Res. Int. 2019, 2019, 1365210. [Google Scholar] [CrossRef] [PubMed]
- Jiang, N.; Wang, H.; Huang, H.; Lv, J.; Zeng, G.; Wang, Q.; Bao, Y.; Chen, Y.; Liu, X. The Antidepressant-Like Effects of Shen Yuan in a Chronic Unpredictable Mild Stress Rat Model. Front. Psychiatry 2021, 12, 622204. [Google Scholar] [CrossRef] [PubMed]
- Hunter, C.A.; Jones, S.A. IL-6 as a keystone cytokine in health and disease. Nat. Immunol. 2015, 16, 448–457. [Google Scholar] [CrossRef]
- Zhu, H.; Jian, Z.H.; Zhong, Y.; Ye, Y.Z.; Zhang, Y.G.; Hu, X.Y.; Pu, B.; Gu, L.J.; Xiong, X.X. Janus Kinase Inhibition Ameliorates Ischemic Stroke Injury and Neuroinflammation Through Reducing NLRP3 Inflammasome Activation via JAK2/STAT3 Pathway Inhibition. Front. Immunol. 2021, 12, 714943. [Google Scholar] [CrossRef]
- Guan, X.F.; Wang, Q.; Liu, M.X.; Sun, A.J.; Li, X.B. Possible Involvement of the IL-6/JAK2/STAT3 Pathway in the Hypothalamus in Depressive-Like Behavior of Rats Exposed to Chronic Mild Stress. Neuropsychobiology 2021, 80, 279–287. [Google Scholar] [CrossRef]
- Grabenstatter, H.L.; Russek, S.J.; Brooks-Kayal, A.R. Molecular pathways controlling inhibitory receptor expression. Epilepsia 2012, 53, 71–78. [Google Scholar] [CrossRef]
- Raible, D.J.; Frey, L.C.; Del Angel, Y.C.; Carlsen, J.; Hund, D.; Russek, S.J.; Smith, B.; Brooks-Kayal, A.R. JAK/STAT pathway regulation of GABAA receptor expression after differing severities of experimental TBI. Exp. Neurol. 2015, 271, 445–456. [Google Scholar] [CrossRef]
- Garcia-Oscos, F.; Salgado, H.; Hall, S.; Thomas, F.; Farmer, G.E.; Bermeo, J.; Galindo, L.C.; Ramirez, R.D.; D’Mello, S.; Rose-John, S.; et al. The Stress-Induced Cytokine Interleukin-6 Decreases the Inhibition/Excitation Ratio in the Rat Temporal Cortex via Trans-Signaling. Biol. Psychiat. 2012, 71, 574–582. [Google Scholar] [CrossRef]
- Naylor, D.E.; Liu, H.T.; Wasterlain, C.G. Trafficking of GABA(A) receptors, loss of inhibition, and a mechanism for pharmacoresistance in status epilepticus. J. Neurosci. 2005, 25, 7724–7733. [Google Scholar] [CrossRef]
- Lamotte, G.; Shouman, K.; Benarroch, E.E. Stress and central autonomic network. Auton. Neurosci. 2021, 235, 102870. [Google Scholar] [CrossRef] [PubMed]
- Li, T.L.; Chen, J.Y.S.; Huang, S.C.; Dai, Y.E.; Hwang, L.L. Cardiovascular pressor effects of orexins in the dorsomedial hypothalamus. Eur. J. Pharmacol. 2018, 818, 343–350. [Google Scholar] [CrossRef] [PubMed]
- DiMicco, J.A.; Samuels, B.C.; Zaretskaia, M.V.; Zaretsky, D.V. The dorsomedial hypothalamus and the response to stress: Part renaissance, part revolution. Pharmacol. Biochem. Behav. 2002, 71, 469–480. [Google Scholar] [CrossRef] [PubMed]
- Rudolph, U.; Mohler, H. GABA-based therapeutic approaches: GABAA receptor subtype functions. Curr. Opin. Pharmacol. 2006, 6, 18–23. [Google Scholar] [CrossRef]
- Kharlamov, E.A.; Lepsveridze, E.; Meparishvili, M.; Solomonia, R.O.; Lu, B.; Miller, E.R.; Kelly, K.M.; Mtchedlishvili, Z. Alterations of GABA(A) and glutamate receptor subunits and heat shock protein in rat hippocampus following traumatic brain injury and in posttraumatic epilepsy. Epilepsy Res. 2011, 95, 20–34. [Google Scholar] [CrossRef]
- Lund, I.V.; Hu, Y.; Raol, Y.H.; Benham, R.S.; Faris, R.; Russek, S.J.; Brooks-Kayal, A.R. BDNF selectively regulates GABAA receptor transcription by activation of the JAK/STAT pathway. Sci. Signal. 2008, 1, ra9. [Google Scholar] [CrossRef]
- Galic, M.A.; Riazi, K.; Pittman, Q.J. Cytokines and brain excitability. Front Neuroendocrinol 2012, 33, 116–125. [Google Scholar] [CrossRef]
- Vezzani, A.; Viviani, B. Neuromodulatory properties of inflammatory cytokines and their impact on neuronal excitability. Neuropharmacology 2015, 96, 70–82. [Google Scholar] [CrossRef]
- Zhong, Y.; Yin, B.; Ye, Y.; Dekhel, O.; Xiong, X.; Jian, Z.; Gu, L. The bidirectional role of the JAK2/STAT3 signaling pathway and related mechanisms in cerebral ischemia-reperfusion injury. Exp. Neurol. 2021, 341, 113690. [Google Scholar] [CrossRef]
- Campbell, I.L.; Erta, M.; Lim, S.L.; Frausto, R.; May, U.; Rose-John, S.; Scheller, J.; Hidalgo, J. Trans-Signaling Is a Dominant Mechanism for the Pathogenic Actions of Interleukin-6 in the Brain. J. Neurosci. 2014, 34, 2503–2513. [Google Scholar] [CrossRef]
- Narasimhamurthy, R.K.; Andrade, D.; Mumbrekar, K.D. Modulation of CREB and its associated upstream signaling pathways in pesticide-induced neurotoxicity. Mol. Cell. Biochem. 2022, 477, 2581–2593. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Liu, Z.; Wang, L.; Wu, G.; Liu, T. Influence of hippocampal low-frequency stimulation on GABA(A) R alpha1, ICER and BNDF expression level in brain tissues of amygdala-kindled drug-resistant temporal lobe epileptic rats. Brain Res. 2018, 1698, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Borlikova, G.; Endo, S. Inducible cAMP early repressor (ICER) and brain functions. Mol. Neurobiol. 2009, 40, 73–86. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Zhou, L. AG490 protects cerebral ischemia/reperfusion injury via inhibiting the JAK2/3 signaling pathway. Brain Behav. 2021, 11, e01911. [Google Scholar] [CrossRef]
- Xuan, Y.T.; Guo, Y.; Han, H.; Zhu, Y.; Bolli, R. An essential role of the JAK-STAT pathway in ischemic preconditioning. Proc. Natl. Acad. Sci. USA 2001, 98, 9050–9055. [Google Scholar] [CrossRef]
- Fang, S.; Xu, H.; Lu, J.; Zhu, Y.; Jiang, H. Neuroprotection by the kappa-opioid receptor agonist, BRL52537, is mediated via up-regulating phosphorylated signal transducer and activator of transcription-3 in cerebral ischemia/reperfusion injury in rats. Neurochem. Res. 2013, 38, 2305–2312. [Google Scholar] [CrossRef]
- Damm, J.; Harden, L.M.; Gerstberger, R.; Roth, J.; Rummel, C. The putative JAK-STAT inhibitor AG490 exacerbates LPS-fever, reduces sickness behavior, and alters the expression of pro- and anti-inflammatory genes in the rat brain. Neuropharmacology 2013, 71, 98–111. [Google Scholar] [CrossRef]
- Yi, S.Y.; Chen, K.; Zhang, L.H.; Shi, W.B.; Zhang, Y.X.; Niu, S.B.; Jia, M.M.; Cong, B.; Li, Y.M. Endoplasmic Reticulum Stress Is Involved in Stress-Induced Hypothalamic Neuronal Injury in Rats via the PERK-ATF4-CHOP and IRE1-ASK1-JNK Pathways. Front. Cell. Neurosci. 2019, 13, 190. [Google Scholar] [CrossRef]
- Mazieres, J.; Brugger, W.; Cappuzzo, F.; Middel, P.; Frosch, A.; Bara, I.; Klingelschmitt, G.; Klughammer, B. Evaluation of EGFR protein expression by immunohistochemistry using H-score and the magnification rule: Re-analysis of the SATURN study. Lung Cancer 2013, 82, 231–237. [Google Scholar] [CrossRef]
- Gaudio, F.; Tamma, R.; Ingravallo, G.; Perrone, T.; Laddaga, F.E.; De Candia, M.; Maiorano, E.; Ribatti, D.; Specchia, G. Computer-driven quantitative image analysis in the assessment of tumor cell and T cell features in diffuse large B cell lymphomas. Ann. Hematol. 2018, 97, 663–668. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Shi, W.; Liu, J.; Chen, K.; Zhang, G.; Zhang, S.; Cong, B.; Li, Y. Interleukin 6 (IL-6) Regulates GABAA Receptors in the Dorsomedial Hypothalamus Nucleus (DMH) through Activation of the JAK/STAT Pathway to Affect Heart Rate Variability in Stressed Rats. Int. J. Mol. Sci. 2023, 24, 12985. https://doi.org/10.3390/ijms241612985
Zhang L, Shi W, Liu J, Chen K, Zhang G, Zhang S, Cong B, Li Y. Interleukin 6 (IL-6) Regulates GABAA Receptors in the Dorsomedial Hypothalamus Nucleus (DMH) through Activation of the JAK/STAT Pathway to Affect Heart Rate Variability in Stressed Rats. International Journal of Molecular Sciences. 2023; 24(16):12985. https://doi.org/10.3390/ijms241612985
Chicago/Turabian StyleZhang, Lihua, Weibo Shi, Jingmin Liu, Ke Chen, Guowei Zhang, Shengnan Zhang, Bin Cong, and Yingmin Li. 2023. "Interleukin 6 (IL-6) Regulates GABAA Receptors in the Dorsomedial Hypothalamus Nucleus (DMH) through Activation of the JAK/STAT Pathway to Affect Heart Rate Variability in Stressed Rats" International Journal of Molecular Sciences 24, no. 16: 12985. https://doi.org/10.3390/ijms241612985
APA StyleZhang, L., Shi, W., Liu, J., Chen, K., Zhang, G., Zhang, S., Cong, B., & Li, Y. (2023). Interleukin 6 (IL-6) Regulates GABAA Receptors in the Dorsomedial Hypothalamus Nucleus (DMH) through Activation of the JAK/STAT Pathway to Affect Heart Rate Variability in Stressed Rats. International Journal of Molecular Sciences, 24(16), 12985. https://doi.org/10.3390/ijms241612985




