Endothelial Cell Response to Combined Photon or Proton Irradiation with Doxorubicin
Abstract
:1. Introduction
2. Results
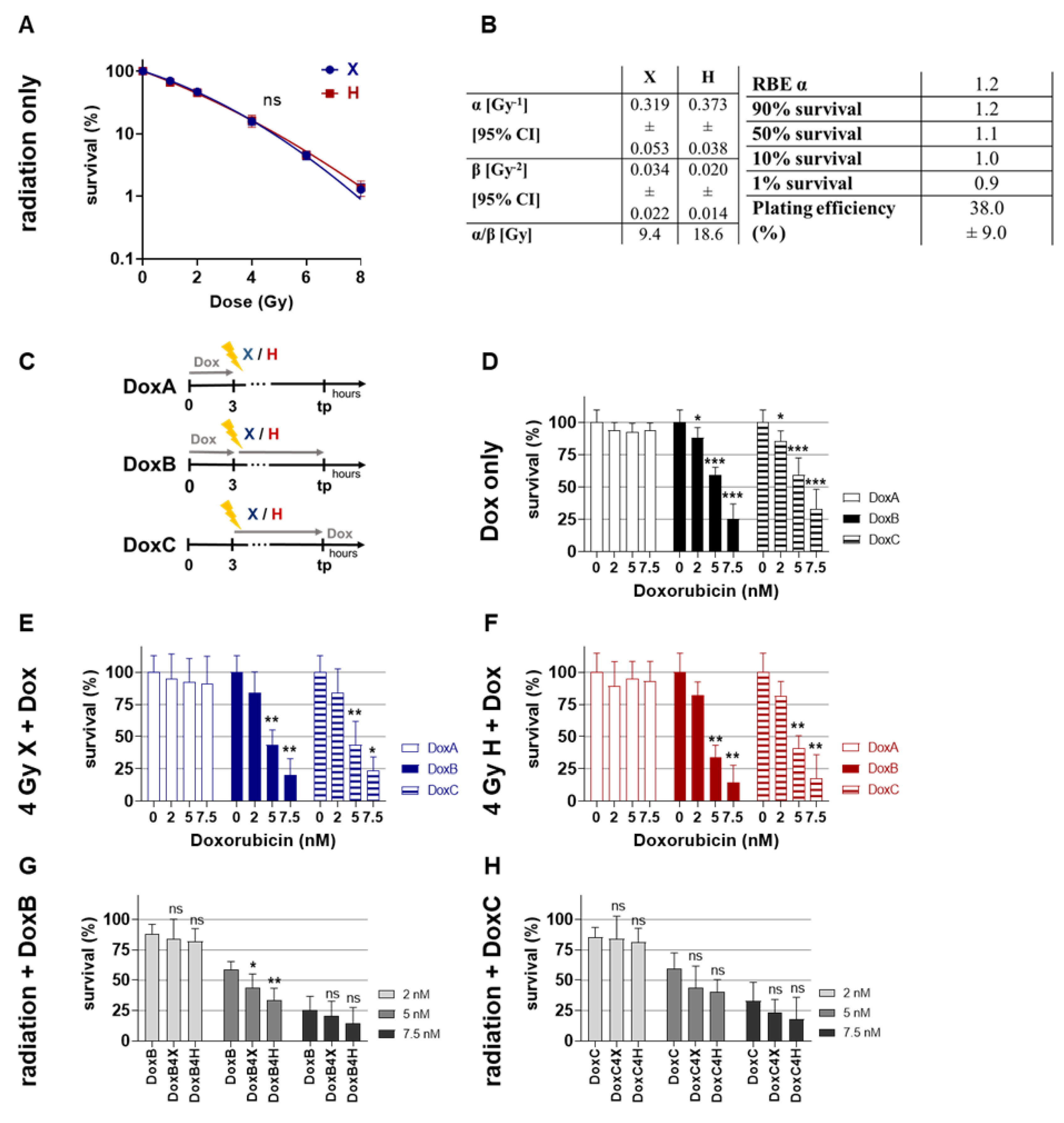
2.1. Clonogenic Survival: Combined Dox Treatment Reduced Clonogenic Survival of HMEC-1 Cells More Efficently upon Prolonged Treatment
2.2. Apoptosis: HMEC-1 Cells Are Chemoresistant for Dox Treatment Alone Independent of Treatment Schedule, but Sensitive for Combined Treatment with Radiation
2.3. Cell Cycle Distribution: Accumulation of G2/M Population in HMEC-1 Cells Following Mono- or Combined Treatment with Radiation and Dox
2.4. Proliferation: Prolonged Dox Treatment Combined with Radiation Have Additive Effects on Reduction of Proliferation Activity in HMEC-1 Cells
2.5. Cell Viability: X Radiation Alone and in Combination with Dox Treatment Increase Cell Viability at 72 h Post-Treatment
2.6. Cell Motility: X Radiation Reduced Motility of HMEC-1 Cells Stronger Than H Radiation
2.7. Cell Morphology Analysis Post-Treatment: Combined Treatment with Radiation and Prolonged Dox Treatment Show Additive Effects on Morphology
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Photon Irradiation
4.3. Proton Irradiation
4.4. Doxorubicin Treatment
4.5. Colony Formation Assay (CFA)
4.6. Flow Cytometry Analysis
4.7. Cell Viability and Proliferation Assay
4.8. Migration Assay
4.9. Data Analysis and Statistics
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yamazaki, T.; Young, K.H. Effects of radiation on tumor vasculature. Mol. Carcinog. 2022, 61, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Klein, D. The Tumor Vascular Endothelium as Decision Maker in Cancer Therapy. Front. Oncol. 2018, 8, 367. [Google Scholar] [CrossRef] [PubMed]
- Wijerathne, H.; Langston, J.C.; Yang, Q.; Sun, S.; Miyamoto, C.; Kilpatrick, L.E.; Kiani, M.F. Mechanisms of radiation-induced endothelium damage: Emerging models and technologies. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 2021, 158, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Jarosz-Biej, M.; Smolarczyk, R.; Cichoń, T.; Kułach, N. Tumor Microenvironment as a “Game Changer” in Cancer Radiotherapy. Int. J. Mol. Sci. 2019, 20, 3212. [Google Scholar] [CrossRef]
- Gabryś, D.; Greco, O.; Patel, G.; Prise, K.M.; Tozer, G.M.; Kanthou, C. Radiation effects on the cytoskeleton of endothelial cells and endothelial monolayer permeability. Int. J. Radiat. Oncol. Biol. Phys. 2007, 69, 1553–1562. [Google Scholar] [CrossRef] [PubMed]
- Lühr, A.; von Neubeck, C.; Pawelke, J.; Seidlitz, A.; Peitzsch, C.; Bentzen, S.M.; Bortfeld, T.; Debus, J.; Deutsch, E.; Langendijk, J.A.; et al. “Radiobiology of Proton Therapy”: Results of an international expert workshop. Radiother. Oncol. 2018, 128, 56–67. [Google Scholar] [CrossRef] [PubMed]
- Jang, G.H.; Ha, J.-H.; Huh, T.-L.; Lee, Y.M. Effect of proton beam on blood vessel formation in early developing zebrafish (Danio rerio) embryos. Arch. Pharm. Res. 2008, 31, 779–785. [Google Scholar] [CrossRef] [PubMed]
- Girdhani, S.; Lamont, C.; Hahnfeldt, P.; Abdollahi, A.; Hlatky, L. Proton Irradiation Suppresses Angiogenic Genes and Impairs Cell Invasion and Tumor Growth. Radiat. Res. 2012, 178, 33–45. [Google Scholar] [CrossRef]
- Yang, E.H.; Marmagkiolis, K.; Balanescu, D.V.; Hakeem, A.; Donisan, T.; Finch, W.; Virmani, R.; Herrman, J.; Cilingiroglu, M.; Grines, C.L.; et al. Radiation-Induced Vascular Disease—A State-of-the-Art Review. Front. Cardiovasc. Med. 2021, 8, 652761. [Google Scholar] [CrossRef]
- Thomas, H.; Timmermann, B. Paediatric proton therapy. Br. J. Radiol. 2020, 93, 20190601. [Google Scholar] [CrossRef]
- Pasquali, S.; Pizzamiglio, S.; Touati, N.; Litiere, S.; Marreaud, S.; Kasper, B.; Gelderblom, H.; Stacchiotti, S.; Judson, I.; Tos, A.P.D.; et al. The impact of chemotherapy on survival of patients with extremity and trunk wall soft tissue sarcoma: Revisiting the results of the EORTC-STBSG 62931 randomised trial. Eur. J. Cancer 2019, 109, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Graziani, S.; Scorrano, L.; Pontarin, G. Transient Exposure of Endothelial Cells to Doxorubicin Leads to Long-Lasting Vascular Endothelial Growth Factor Receptor 2 Downregulation. Cells 2022, 11, 210. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Zhang, H.; Lin, H.; Gao, L.; Zhang, H.; Zhang, J.; Wang, C.; Gu, J. Irisin ameliorates doxorubicin-induced cardiac perivascular fibrosis through inhibiting endothelial-to-mesenchymal transition by regulating ROS accumulation and autophagy disorder in endothelial cells. Redox Biol. 2021, 46, 102120. [Google Scholar] [CrossRef] [PubMed]
- Podyacheva, E.; Danilchuk, M.; Toropova, Y. Molecular mechanisms of endothelial remodeling under doxorubicin treatment. Biomed. Pharmacother. Biomed. Pharmacother. 2023, 162, 114576. [Google Scholar] [CrossRef]
- Bernardo, T.; Behrends, C.; Klein, D.; Kuntze, A.; Timmermann, B.; von Neubeck, C. Similar additive effects of doxorubicin in combination with photon or proton irradiation in soft tissue sarcoma models. Front. Oncol. 2023, 13, 1211984. [Google Scholar] [CrossRef] [PubMed]
- Kabakov, A.E.; Gabai, V.L. Cell Death and Survival Assays. In Chaperones: Methods and Protocols; Calderwood, S.K., Prince, T.L., Eds.; Methods in Molecular Biology; Springer: New York, NY, USA, 2018; pp. 107–127. ISBN 978-1-4939-7477-1. [Google Scholar]
- Bonnaud, S.; Niaudet, C.; Pottier, G.; Gaugler, M.-H.; Millour, J.; Barbet, J.; Sabatier, L.; Paris, F. Sphingosine-1-phosphate protects proliferating endothelial cells from ceramide-induced apoptosis but not from DNA damage-induced mitotic death. Cancer Res. 2007, 67, 1803–1811. [Google Scholar] [CrossRef]
- Thorn, C.F.; Oshiro, C.; Marsh, S.; Hernandez-Boussard, T.; McLeod, H.; Klein, T.E.; Altman, R.B. Doxorubicin pathways: Pharmacodynamics and adverse effects. Pharmacogenet. Genom. 2011, 21, 440–446. [Google Scholar] [CrossRef]
- Huang, R.-X.; Zhou, P.-K. DNA damage response signaling pathways and targets for radiotherapy sensitization in cancer. Signal Transduct. Target. Ther. 2020, 5, 60. [Google Scholar] [CrossRef]
- Nowak-Sliwinska, P.; Alitalo, K.; Allen, E.; Anisimov, A.; Aplin, A.C.; Auerbach, R.; Augustin, H.G.; Bates, D.O.; van Beijnum, J.R.; Bender, R.H.F.; et al. Consensus guidelines for the use and interpretation of angiogenesis assays. Angiogenesis 2018, 21, 425–532. [Google Scholar] [CrossRef]
- Pippa, L.F.; de Oliveira, M.L.; Rocha, A.; de Andrade, J.M.; Lanchote, V.L. Total, renal and hepatic clearances of doxorubicin and formation clearance of doxorubicinol in patients with breast cancer: Estimation of doxorubicin hepatic extraction ratio. J. Pharm. Biomed. Anal. 2020, 185, 113231. [Google Scholar] [CrossRef]
- Moding, E.J.; Castle, K.D.; Perez, B.A.; Oh, P.; Min, H.D.; Norris, H.; Ma, Y.; Cardona, D.M.; Lee, C.-L.; Kirsch, D.G. Tumor cells, but not endothelial cells, mediate eradication of primary sarcomas by stereotactic body radiation therapy. Sci. Transl. Med. 2015, 7, 278ra34. [Google Scholar] [CrossRef] [PubMed]
- Rohrer Bley, C.; Furmanova, P.; Orlowski, K.; Grosse, N.; Broggini-Tenzer, A.; McSheehy, P.M.J.; Pruschy, M. Microtubule stabilising agents and ionising radiation: Multiple exploitable mechanisms for combined treatment. Eur. J. Cancer 2013, 49, 245–253. [Google Scholar] [CrossRef]
- Yagi, M.; Takahashi, Y.; Minami, K.; Matsuura, T.; Nam, J.-M.; Onodera, Y.; Akagi, T.; Maeda, T.; Okimoto, T.; Shirato, H.; et al. A Consistent Protocol Reveals a Large Heterogeneity in the Biological Effectiveness of Proton and Carbon-Ion Beams for Various Sarcoma and Normal-Tissue-Derived Cell Lines. Cancers 2022, 14, 2009. [Google Scholar] [CrossRef] [PubMed]
- Doxorubicin Hydrochloride—NCI. 8 Oktober 2007. Available online: https://www.cancer.gov/about-cancer/treatment/drugs/doxorubicinhydrochloride (accessed on 30 June 2023).
- Potiron, V.; Clément-Colmou, K.; Jouglar, E.; Pietri, M.; Chiavassa, S.; Delpon, G.; Paris, F.; Supiot, S. Tumor vasculature remodeling by radiation therapy increases doxorubicin distribution and efficacy. Cancer Lett. 2019, 457, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Roohani, S.; Ehret, F.; Kobus, M.; Flörcken, A.; Märdian, S.; Striefler, J.K.; Rau, D.; Öllinger, R.; Jarosch, A.; Budach, V.; et al. Preoperative hypofractionated radiotherapy for soft tissue sarcomas: A systematic review. Radiat. Oncol. Lond. Engl. 2022, 17, 159. [Google Scholar] [CrossRef] [PubMed]
- Greco, W.R.; Faessel, H.; Levasseur, L. The search for cytotoxic synergy between anticancer agents: A case of Dorothy and the ruby slippers? J. Natl. Cancer Inst. 1996, 88, 699–700. [Google Scholar] [CrossRef] [PubMed]
- Sabbatinelli, J.; Prattichizzo, F.; Olivieri, F.; Procopio, A.D.; Rippo, M.R.; Giuliani, A. Where Metabolism Meets Senescence: Focus on Endothelial Cells. Front. Physiol. 2019, 10, 1523. [Google Scholar] [CrossRef]
- Geng, J.; Zhang, Y.; Gao, Q.; Neumann, K.; Dong, H.; Porter, H.; Potter, M.; Ren, H.; Argyle, D. Switching on prodrugs using radiotherapy. Nat. Chem. 2021, 13, 805–810. [Google Scholar] [CrossRef]
- Ades, E.W.; Candal, F.J.; Swerlick, R.A.; George, V.G.; Summers, S.; Bosse, D.C.; Lawley, T.J. HMEC-1: Establishment of an immortalized human microvascular endothelial cell line. J. Investig. Dermatol. 1992, 99, 683–690. [Google Scholar] [CrossRef]
- Riccardi, C.; Nicoletti, I. Analysis of apoptosis by propidium iodide staining and flow cytometry. Nat. Protoc. 2006, 1, 1458–1461. [Google Scholar] [CrossRef]
- Klein, D.; Schmitz, T.; Verhelst, V.; Panic, A.; Schenck, M.; Reis, H.; Drab, M.; Sak, A.; Herskind, C.; Maier, P.; et al. Endothelial Caveolin-1 regulates the radiation response of epithelial prostate tumors. Oncogenesis 2015, 4, e148. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bernardo, T.; Kuntze, A.; Klein, D.; Heinzelmann, F.; Timmermann, B.; von Neubeck, C. Endothelial Cell Response to Combined Photon or Proton Irradiation with Doxorubicin. Int. J. Mol. Sci. 2023, 24, 12833. https://doi.org/10.3390/ijms241612833
Bernardo T, Kuntze A, Klein D, Heinzelmann F, Timmermann B, von Neubeck C. Endothelial Cell Response to Combined Photon or Proton Irradiation with Doxorubicin. International Journal of Molecular Sciences. 2023; 24(16):12833. https://doi.org/10.3390/ijms241612833
Chicago/Turabian StyleBernardo, Teresa, Anna Kuntze, Diana Klein, Feline Heinzelmann, Beate Timmermann, and Cläre von Neubeck. 2023. "Endothelial Cell Response to Combined Photon or Proton Irradiation with Doxorubicin" International Journal of Molecular Sciences 24, no. 16: 12833. https://doi.org/10.3390/ijms241612833
APA StyleBernardo, T., Kuntze, A., Klein, D., Heinzelmann, F., Timmermann, B., & von Neubeck, C. (2023). Endothelial Cell Response to Combined Photon or Proton Irradiation with Doxorubicin. International Journal of Molecular Sciences, 24(16), 12833. https://doi.org/10.3390/ijms241612833








