Low and Ultra-Low HER2 in Human Breast Cancer: An Effort to Define New Neoplastic Subtypes
Abstract
1. Introduction
2. How to Currently Establish the HER2 Status
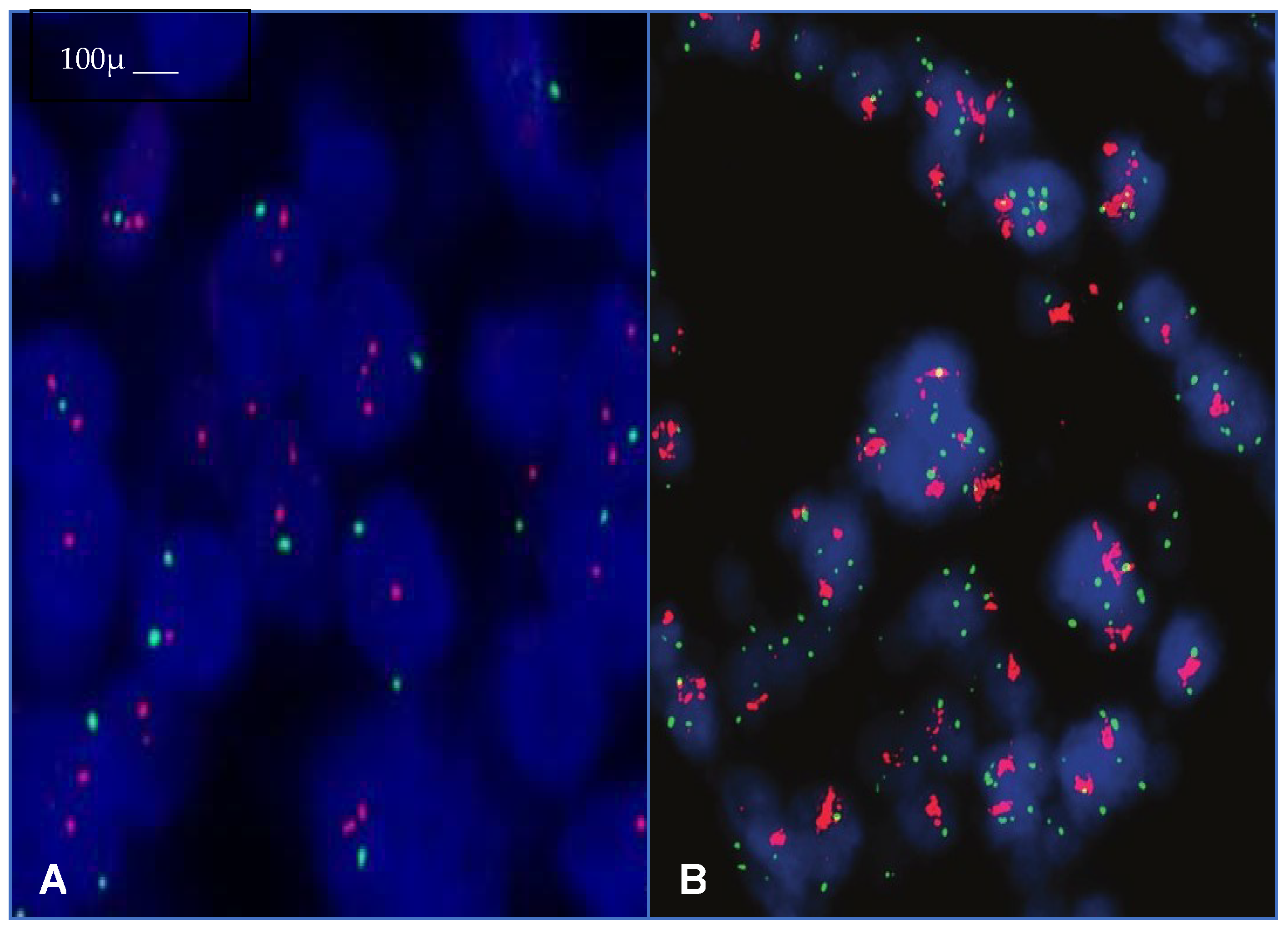
3. Identification and Definition of HER2-Low BC
4. Identification and Definition of HER2-Ultra-Low BC
5. New Treatment Options for HER2-Low and Ultra-Low Patients
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Miglietta, F.; Dieci, M.V.; Griguolo, G.; Guarneri, V. Neoadjuvant approach as a platform for treatment personalization: Focus on HER2-positive and triple-negative breast cancer. Cancer Treat. Rev. 2021, 98, 102222. [Google Scholar] [CrossRef] [PubMed]
- Lamb, C.A.; Vanzulli, S.I.; Lanari, C. Hormone receptors in breast cancer: More than estrogen receptors. Medicina 2019, 79, 540–545. [Google Scholar]
- Johnson, K.S.; Conant, E.F.; Soo, M.S. Molecular Subtypes of Breast Cancer: A Review for Breast Radiologists. J. Breast Imaging 2021, 3, 12–24. [Google Scholar] [CrossRef]
- Yersal, O. Biological subtypes of breast cancer: Prognostic and therapeutic implications. World J. Clin. Oncol. 2014, 5, 412. [Google Scholar] [CrossRef]
- Inic, Z.; Zegarac, M.; Inic, M.; Markovic, I.; Kozomara, Z.; Djurisic, I.; Inic, I.; Pupic, G.; Jancic, S. Difference between Luminal A and Luminal B Subtypes According to Ki-67, Tumor Size, and Progesterone Receptor Negativity Providing Prognostic Information. Clin. Med. Insights Oncol. 2014, 8, 107–111. [Google Scholar] [CrossRef]
- Sakach, E.; O’Regan, R.; Meisel, J.; Li, X. Molecular Classification of Triple Negative Breast Cancer and the Emergence of Targeted Therapies. Clin. Breast Cancer 2021, 21, 509–520. [Google Scholar] [CrossRef]
- Wolff, A.C.; Hammond, M.E.H.; Allison, K.H.; Harvey, B.E.; Mangu, P.B.; Bartlett, J.M.; Bilous, M.; Ellis, I.O.; Fitzgibbons, P.; Hanna, W.; et al. Human Epidermal Growth Factor Receptor 2 Testing in Breast Cancer: ASCO-College of American Pathologists Guideline Update. Arch. Pathol. Lab. Med. 2023, 142, 1364–1382. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Yu, J.; Liu, D.; Ren, F.; Wu, J.; Shen, K. Distribution, dynamic evolution, and clinical outcomes of patients with advanced breast cancer according to HER2 expression. BMC Cancer 2023, 23, 173. [Google Scholar] [CrossRef] [PubMed]
- Oh, D.-Y.; Bang, Y.-J. HER2-targeted therapies—A role beyond breast cancer. Nat. Rev. Clin. Oncol. 2020, 17, 33–48. [Google Scholar] [CrossRef] [PubMed]
- Slamon, D.; Eiermann, W.; Robert, N.; Pienkowski, T.; Martin, M.; Press, M.; Mackey, J.; Glaspy, J.; Chan, A.; Pawlicki, M.; et al. Adjuvant Trastuzumab in HER2-Positive Breast Cancer. N. Engl. J. Med. 2011, 365, 1273–1283. [Google Scholar] [CrossRef]
- Fehrenbacher, L.; Cecchini, R.S.; Geyer, C.E., Jr.; Rastogi, P.; Costantino, J.P.; Atkins, J.N.; Crown, J.P.; Polikoff, J.; Boileau, J.-F.; Provencher, L.; et al. NSABP B-47/NRG Oncology Phase III Randomized Trial Comparing Adjuvant Chemotherapy with or without Trastuzumab in High-Risk Invasive Breast Cancer Negative for HER2 by FISH and with IHC 1+ or 2+. J. Clin. Oncol. 2020, 38, 444–453. [Google Scholar] [CrossRef] [PubMed]
- Ishii, K.; Morii, N.; Yamashiro, H. Pertuzumab in the treatment of HER2-positive breast cancer: An evidence-based review of its safety, efficacy, and place in therapy. Core Evid. 2019, 14, 51–70. [Google Scholar] [CrossRef]
- Takada, M.; Toi, M. Neoadjuvant treatment for HER2-positive breast cancer. Chin. Clin. Oncol. 2020, 9, 32. [Google Scholar] [CrossRef]
- Geyer, C.E.; Forster, J.; Lindquist, D.; Chan, S.; Romieu, C.G.; Pienkowski, T.; Jagiello-Gruszfeld, A.; Crown, J.; Chan, A.; Kaufman, B.; et al. Lapatinib plus Capecitabine for HER2-Positive Advanced Breast Cancer. N. Engl. J. Med. 2006, 355, 2733–2743. [Google Scholar] [CrossRef]
- Yuan, Y.; Liu, X.; Cai, Y.; Li, W. Lapatinib and lapatinib plus trastuzumab therapy versus trastuzumab therapy for HER2 positive breast cancer patients: An updated systematic review and meta-analysis. Syst. Rev. 2022, 11, 264. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, L.; Yu, Q.; Liu, Z.; Li, C.; Wang, F.; Yu, Z. The Effectiveness of Lapatinib in HER2-Positive Metastatic Breast Cancer Patients Pretreated with Multiline Anti-HER2 Treatment: A Retrospective Study in China. Technol. Cancer Res. Treat. 2021, 20, 153303382110378. [Google Scholar] [CrossRef] [PubMed]
- Dieci, M.V.; Miglietta, F. HER2: A never ending story. Lancet Oncol. 2021, 22, 1051–1052. [Google Scholar] [CrossRef]
- Surveillance, Epidemiology and End-Results program Web site. Available online: https://https://seer.cancer.gov/statfacts/html/breast-subtypes.html (accessed on 6 March 2023).
- Hamilton, E.; Shastry, M.; Shiller, S.M.; Ren, R. Targeting HER2 heterogeneity in breast cancer. Cancer Treat. Rev. 2021, 100, 102286. [Google Scholar] [CrossRef]
- Yang, J.; Ju, J.; Guo, L.; Ji, B.; Shi, S.; Yang, Z.; Gao, S.; Yuan, X.; Tian, G.; Liang, Y.; et al. Prediction of HER2-positive breast cancer recurrence and metastasis risk from histopathological images and clinical information via multimodal deep learning. Comput. Struct. Biotechnol. J. 2022, 20, 333–342. [Google Scholar] [CrossRef]
- Shui, R.; Liang, X.; Li, X.; Liu, Y.; Li, H.; Xu, E.; Zhang, Z.; Lian, Y.; Guo, S.; Yao, M.; et al. Hormone Receptor and Human Epidermal Growth Factor Receptor 2 Detection in Invasive Breast Carcinoma: A Retrospective Study of 12,467 Patients from 19 Chinese Representative Clinical Centers. Clin. Breast Cancer 2020, 20, e65–e74. [Google Scholar] [CrossRef] [PubMed]
- Swain, S.M.; Macharia, H.; Cortes, J.; Dang, C.; Gianni, L.; Hurvitz, S.A.; Jackisch, C.; Schneeweiss, A.; Slamon, D.; Valagussa, P.; et al. Event-Free Survival in Patients with Early HER2-Positive Breast Cancer with a Pathological Complete Response after HER2-Targeted Therapy: A Pooled Analysis. Cancers 2022, 14, 5051. [Google Scholar] [CrossRef] [PubMed]
- Lambertini, M.; Agbor-Tarh, D.; Metzger-Filho, O.; Ponde, N.F.; Poggio, F.; Hilbers, F.S.; Korde, L.A.; Chumsri, S.; Werner, O.; Del Mastro, L.; et al. Prognostic role of distant disease-free interval from completion of adjuvant trastuzumab in HER2-positive early breast cancer: Analysis from the ALTTO (BIG 2-06) trial. ESMO Open 2020, 5, e000979. [Google Scholar] [CrossRef] [PubMed]
- Ellegård, S.; Engvall, K.; Asowed, M.; Hallbeck, A.-L.; Elander, N.; Stål, O. Long-term follow-up of early stage HER2-positive breast cancer patients treated with trastuzumab: A population-based real world multicenter cohort study. Front. Oncol. 2022, 12, 861324. [Google Scholar] [CrossRef] [PubMed]
- Tarantino, P.; Hamilton, E.; Tolaney, S.M.; Cortes, J.; Morganti, S.; Ferraro, E.; Marra, A.; Viale, G.; Trapani, D.; Cardoso, F.; et al. HER2-Low Breast Cancer: Pathological and Clinical Landscape. J. Clin. Oncol. 2020, 38, 1951–1962. [Google Scholar] [CrossRef]
- Ferraro, E.; Drago, J.Z.; Modi, S. Implementing antibody-drug conjugates (ADCs) in HER2-positive breast cancer: State of the art and future directions. Breast Cancer Res. 2021, 23, 84. [Google Scholar] [CrossRef]
- Najjar, M.K.; Manore, S.G.; Regua, A.T.; Lo, H.-W. Antibody-Drug Conjugates for the Treatment of HER2-Positive Breast Cancer. Genes 2022, 13, 2065. [Google Scholar] [CrossRef]
- Denkert, C.; Seither, F.; Schneeweiss, A.; Link, T.; Blohmer, J.-U.; Just, M.; Wimberger, P.; Forberger, A.; Tesch, H.; Jackisch, C.; et al. Clinical and molecular characteristics of HER2-low-positive breast cancer: Pooled analysis of individual patient data from four prospective, neoadjuvant clinical trials. Lancet Oncol. 2021, 22, 1151–1161. [Google Scholar] [CrossRef]
- Sun, Q.; Li, Q.; Gao, F.; Wu, H.; Fu, Y.; Yang, J.; Fan, X.; Cui, X.; Pu, X. HER2 overexpression/amplification status in colorectal cancer: A comparison between immunohistochemistry and fluorescence in situ hybridization using five different immunohistochemical scoring criteria. J. Cancer Res. Clin. Oncol. 2023, 149, 579–592. [Google Scholar] [CrossRef]
- Tsai, Y.-F.; Tseng, L.-M.; Lien, P.-J.; Hsu, C.-Y.; Lin, Y.-S.; King, K.-L.; Wang, Y.-L.; Chao, T.-C.; Liu, C.-Y.; Chiu, J.-H.; et al. HER2 immunohistochemical scores provide prognostic information for patients with HER2-type invasive breast cancer. Histopathology 2019, 74, 578–586. [Google Scholar] [CrossRef]
- Wolff, A.C.; Hammond, M.E.H.; Schwartz, J.N.; Hagerty, K.L.; Allred, D.C.; Cote, R.J.; Dowsett, M.; Fitzgibbons, P.L.; Hanna, W.M.; Langer, A.; et al. American Society of Clinical Oncology/College of American Pathologists Guideline Recommendations for Human Epidermal Growth Factor Receptor 2 Testing in Breast Cancer. J. Clin. Oncol. 2007, 25, 118–145. [Google Scholar] [CrossRef] [PubMed]
- Tuccari, G.; Ieni, A.; Barresi, V.; Caltabiano, R.; Zeppa, P.; Del Sordo, R.; Cabibi, D.; Lanzafame, S.; Sidoni, A.; Franco, V.; et al. Discordance rate of HER2 status in primary breast carcinomas versus synchronous axillary lymph node metastases: A multicenter retrospective investigation. Onco Targets Ther. 2014, 7, 1267. [Google Scholar] [CrossRef] [PubMed]
- Wolff, A.C.; Hammond, M.E.H.; Hicks, D.G.; Dowsett, M.; McShane, L.M.; Allison, K.H.; Allred, D.C.; Bartlett, J.M.S.; Bilous, M.; Fitzgibbons, P.; et al. Recommendations for Human Epidermal Growth Factor Receptor 2 Testing in Breast Cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Update. J. Clin. Oncol. 2013, 31, 3997–4013. [Google Scholar] [CrossRef]
- Rugo, H.S.; Im, S.A.; Cardoso, F.; Cortés, J.; Curigliano, G.; Musolino, A.; Pegram, M.D.; Wright, G.S.; Saura, C.; Escrivá-de-Romaní, S.; et al. Efficacy of Margetuximab vs. Trastuzumab in Patients with Pretreated ERBB2-Positive Advanced Breast Cancer: A Phase 3 Randomized Clinical Trial. JAMA Oncol. 2021, 7, 573–584. [Google Scholar] [CrossRef]
- Cardoso, F.; Kyriakides, S.; Ohno, S.; Penault-Llorca, F.; Poortmans, P.; Rubio, I.T.; Zackrisson, S.; Senkus, E. Early breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2019, 30, 1194–1220. [Google Scholar] [CrossRef]
- Swain, S.M.; Baselga, J.; Kim, S.-B.; Ro, J.; Semiglazov, V.; Campone, M.; Ciruelos, E.; Ferrero, J.-M.; Schneeweiss, A.; Heeson, S.; et al. Pertuzumab, Trastuzumab, and Docetaxel in HER2-Positive Metastatic Breast Cancer. N. Engl. J. Med. 2015, 372, 724–734. [Google Scholar] [CrossRef] [PubMed]
- Başaran, G.A.; Twelves, C.; Diéras, V.; Cortés, J.; Awada, A. Ongoing unmet needs in treating estrogen receptor-positive/HER2-negative metastatic breast cancer. Cancer Treat. Rev. 2018, 63, 144–155. [Google Scholar] [CrossRef]
- Lev, S. Targeted therapy and drug resistance in triple-negative breast cancer: The EGFR axis. Biochem. Soc. Trans. 2020, 48, 657–665. [Google Scholar] [CrossRef]
- Fisusi, F.A.; Akala, E.O. Drug Combinations in Breast Cancer Therapy. Pharm. Nanotechnol. 2019, 7, 3–23. [Google Scholar] [CrossRef]
- Ieni, A.; Angelico, G.; Giuffrè, G.; Tuccari, G. Discordance Rate of HER2 Status in Primary Gastric Cancer and Synchronous Lymph Node Metastases: Its Impact on Therapeutic Decision and Clinical Management. Pathol. Oncol. Res. 2018, 24, 695–696. [Google Scholar] [CrossRef]
- Ieni, A.; Cardia, R.; Lentini, M.; Tuccari, G. Intratumoral HER2 heterogeneity in early gastric carcinomas: Potential bias in therapeutic management. Virchows Arch. 2019, 474, 401–402. [Google Scholar] [CrossRef]
- Marchiò, C.; Annaratone, L.; Marques, A.; Casorzo, L.; Berrino, E.; Sapino, A. Evolving concepts in HER2 evaluation in breast cancer: Heterogeneity, HER2-low carcinomas and beyond. Semin. Cancer Biol. 2021, 72, 123–135. [Google Scholar] [CrossRef] [PubMed]
- Seol, H.; Lee, H.J.; Choi, Y.; Lee, H.E.; Kim, Y.J.; Kim, J.H.; Kang, E.; Kim, S.-W.; Park, S.Y. Intratumoral heterogeneity of HER2 gene amplification in breast cancer: Its clinicopathological significance. Mod. Pathol. 2012, 25, 938–948. [Google Scholar] [CrossRef]
- Hosonaga, M.; Arima, Y.; Sampetrean, O.; Komura, D.; Koya, I.; Sasaki, T.; Sato, E.; Okano, H.; Kudoh, J.; Ishikawa, S.; et al. HER2 Heterogeneity Is Associated with Poor Survival in HER2-Positive Breast Cancer. Int. J. Mol. Sci. 2018, 19, 2158. [Google Scholar] [CrossRef] [PubMed]
- Allott, E.H.; Geradts, J.; Sun, X.; Cohen, S.M.; Zirpoli, G.R.; Khoury, T.; Bshara, W.; Chen, M.; Sherman, M.E.; Palmer, J.R.; et al. Intratumoral heterogeneity as a source of discordance in breast cancer biomarker classification. Breast Cancer Res. 2016, 18, 68. [Google Scholar] [CrossRef] [PubMed]
- Ieni, A.; Barresi, V.; Caltabiano, R.; Caleo, A.; Bonetti, L.R.; Lanzafame, S.; Zeppa, P.; Caruso, R.A.; Tuccari, G. Discordance Rate of HER2 Status in Primary Gastric Carcinomas and Synchronous Lymph Node Metastases: A Multicenter Retrospective Analysis. Int. J. Mol. Sci. 2014, 15, 22331–22341. [Google Scholar] [CrossRef]
- Lee, H.J.; Na Seo, A.; Kim, E.J.; Jang, M.H.; Suh, K.J.; Ryu, H.S.; Kim, Y.J.; Kim, J.H.; Im, S.-A.; Gong, G.; et al. HER2 Heterogeneity Affects Trastuzumab Responses and Survival in Patients with HER2-Positive Metastatic Breast Cancer. Am. J. Clin. Pathol. 2014, 142, 755–766. [Google Scholar] [CrossRef]
- Ahn, S.; Woo, J.W.; Lee, K.; Park, S.Y. HER2 status in breast cancer: Changes in guidelines and complicating factors for interpretation. J. Pathol. Transl. Med. 2020, 54, 34–44. [Google Scholar] [CrossRef]
- Hou, Y.; Nitta, H.; Wei, L.; Banks, P.M.; Portier, B.; Parwani, A.V.; Li, Z. HER2 intratumoral heterogeneity is independently associated with incomplete response to anti-HER2 neoadjuvant chemotherapy in HER2-positive breast carcinoma. Breast Cancer Res. Treat. 2017, 166, 447–457. [Google Scholar] [CrossRef]
- Memon, R.; Granada, C.N.P.; Harada, S.; Winokur, T.; Reddy, V.; Kahn, A.G.; Siegal, G.P.; Wei, S. Discordance Between Immunohistochemistry and In Situ Hybridization to Detect HER2 Overexpression/Gene Amplification in Breast Cancer in the Modern Age: A Single Institution Experience and Pooled Literature Review Study. Clin. Breast Cancer 2022, 22, e123–e133. [Google Scholar] [CrossRef]
- Gibbons-Fideler, I.-S.; Nitta, H.; Murillo, A.; Tozbikian, G.; Banks, P.; Parwani, A.V.; Li, Z. Identification of HER2 Immunohistochemistry-Negative, FISH-Amplified Breast Cancers and Their Response to Anti-HER2 Neoadjuvant Chemotherapy. Am. J. Clin. Pathol. 2019, 151, 176–184. [Google Scholar] [CrossRef]
- Yamashita, H.; Ishida, N.; Hatanaka, Y.; Hagio, K.; Oshino, T.; Takeshita, T.; Kanno-Okada, H.; Shimizu, A.; Hatanaka, K.C.; Matsuno, Y. HER2 Gene Amplification in ER-positive HER2 Immunohistochemistry 0 or 1+ Breast Cancer with Early Recurrence. Anticancer. Res. 2020, 40, 645–652. [Google Scholar] [CrossRef] [PubMed]
- Bahreini, F.; Soltanian, A.R.; Mehdipour, P. A meta-analysis on concordance between immunohistochemistry (IHC) and fluorescence in situ hybridization (FISH) to detect HER2 gene overexpression in breast cancer. Breast Cancer 2015, 22, 615–625. [Google Scholar] [CrossRef] [PubMed]
- Eswarachary, V.; Mohammed, I.G.; Jayanna, P.K.; Patilokaly, G.V.; Nargund, A.R.; Dhondalay, G.K.; Prabhudesai, S.; Sahoo, R. HER2/neu Testing In 432 Consecutive Breast Cancer Cases using FISH and IHC—A Comparative Study. J. Clin. Diagn. Res. 2017, 11, EC01–EC05. [Google Scholar] [CrossRef]
- Furrer, D.; Jacob, S.; Caron, C.; Sanschagrin, F.; Provencher, L.; Diorio, C. Concordance of HER2 Immunohistochemistry and Fluorescence In Situ Hybridization Using Tissue Microarray in Breast Cancer. Anticancer. Res. 2017, 37, EC01. [Google Scholar]
- Fusco, N.; Ragazzi, M.; Sajjadi, E.; Venetis, K.; Piciotti, R.; Morganti, S.; Santandrea, G.; Fanelli, G.N.; Despini, L.; Invernizzi, M.; et al. Assessment of estrogen receptor low positive status in breast cancer: Implications for pathologists and oncologists. Histol. Histopathol. 2021, 36, 1235–1245. [Google Scholar]
- Bussolati, G.; Annaratone, L.; Maletta, F. The pre-analytical phase in surgical pathology. Recent. Results Cancer Res. 2015, 199, 1–13. [Google Scholar] [PubMed]
- Alves, F.R.; Gil, L.; de Matos, L.V.; Baleiras, A.; Vasques, C.; Neves, M.T.; Ferreira, A.; Fontes-Sousa, M.; Miranda, H.; Martins, A.; et al. Impact of Human Epidermal Growth Factor Receptor 2 (HER2) Low Status in Response to Neoadjuvant Chemotherapy in Early Breast Cancer. Cureus 2022. [CrossRef] [PubMed]
- Agostinetto, E.; Rediti, M.; Fimereli, D.; Debien, V.; Piccart, M.; Aftimos, P.; Sotiriou, C.; de Azambuja, E. HER2-Low Breast Cancer: Molecular Characteristics and Prognosis. Cancers 2021, 13, 2824. [Google Scholar] [CrossRef]
- Bardia, A.; Viale, G. HER2-Low Breast Cancer—Diagnostic Challenges and Opportunities for Insights from Ongoing Studies: A Podcast. Target. Oncol. 2023, 18, 313–319. [Google Scholar] [CrossRef]
- Popović, M.; Silovski, T.; Križić, M.; Dedić Plavetić, N. HER2 Low Breast Cancer: A New Subtype or a Trojan for Cytotoxic Drug Delivery? Int. J. Mol. Sci. 2023, 24, 8206. [Google Scholar] [CrossRef]
- Iwata, H.; Tamura, K.; Doi, T.; Tsurutani, J.; Modi, S.; Park, H.; Krop, I.E.; Sagara, Y.; Redfern, C.H.; Murthy, R.K.; et al. Trastuzumab deruxtecan (DS-8201a) in subjects with HER2-expressing solid tumors: Long-term results of a large phase 1 study with multiple expansion cohorts. J. Clin. Oncol. 2018, 36 (Suppl. S15), 2501. [Google Scholar] [CrossRef]
- Banerji, U.; van Herpen, C.M.L.; Saura, C.; Thistlethwaite, F.; Lord, S.; Moreno, V.; Macpherson, I.R.; Boni, V.; Rolfo, C.; de Vries, E.G.E.; et al. Trastuzumab duocarmazine in locally advanced and metastatic solid tumours and HER2-expressing breast cancer: A phase 1 dose-escalation and dose-expansion study. Lancet Oncol. 2019, 20, 1124–1135. [Google Scholar] [CrossRef]
- Cortés, J.; Kim, S.-B.; Chung, W.-P.; Im, S.-A.; Park, Y.H.; Hegg, R.; Kim, M.H.; Tseng, L.-M.; Petry, V.; Chung, C.-F.; et al. Trastuzumab Deruxtecan versus Trastuzumab Emtansine for Breast Cancer. N. Engl. J. Med. 2022, 386, 1143–1154. [Google Scholar] [CrossRef] [PubMed]
- Nicolò, E.; Boscolo Bielo, L.; Curigliano, G.; Tarantino, P. The HER2-low revolution in breast oncology: Steps forward and emerging challenges. Ther. Adv. Med. Oncol. 2023, 15, 17588359231152842. [Google Scholar] [CrossRef]
- Hurvitz, S.A.; Hegg, R.; Chung, W.-P.; Im, S.-A.; Jacot, W.; Ganju, V.; Chiu, J.W.Y.; Xu, B.; Hamilton, E.; Madhusudan, S.; et al. Trastuzumab deruxtecan versus trastuzumab emtansine in patients with HER2-positive metastatic breast cancer: Updated results from DESTINY-Breast03, a randomised, open-label, phase 3 trial. Lancet 2023, 401, 105–117. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Peng, Y. Current Biological, Pathological and Clinical Landscape of HER2-Low Breast Cancer. Cancers 2022, 15, 126. [Google Scholar] [CrossRef]
- Modi, S.; Jacot, W.; Yamashita, T.; Sohn, J.; Vidal, M.; Tokunaga, E.; Tsurutani, J.; Ueno, N.T.; Prat, A.; Chae, Y.S.; et al. Trastuzumab Deruxtecan in Previously Treated HER2-Low Advanced Breast Cancer. N. Engl. J. Med. 2022, 387, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Berrino, E.; Annaratone, L.; Bellomo, S.E.; Ferrero, G.; Gagliardi, A.; Bragoni, A.; Grassini, D.; Guarrera, S.; Parlato, C.; Casorzo, L.; et al. Integrative genomic and transcriptomic analyses illuminate the ontology of HER2-low breast carcinomas. Genome Med. 2022, 29, 98. [Google Scholar] [CrossRef]
- Roche Receives FDA Approval for First Companion Diagnostic to Identify Patients with HER2 Low Metastatic Breast Cancer Eligible for Enhertu. 2022. Available online: https://diagnostics.roche.com/global/en/news-listing/2022/roche-receives-fda-ap-proval-for-first-companion-diagnostic-to-id.html (accessed on 23 July 2023).
- Rüschoff, J.; Friedrich, M.; Nagelmeier, I.; Kirchner, M.; Andresen, L.M.; Salomon, K.; Portier, B.; Sredni, S.T.; Schildhaus, H.U.; Jasani, B.; et al. Comparison of HercepTestTM mAb pharmDx (Dako Omnis, GE001) with Ventana PATHWAY anti-HER-2/neu (4B5) in breast cancer: Correlation with HER2 amplification and HER2 low status. Virchows Arch. 2022, 481, 685–694. [Google Scholar] [CrossRef]
- Huang, Y.; Burns, D.J.; Rich, B.E.; MacNeil, I.A.; Dandapat, A.; Soltani, S.M.; Myhre, S.; Sullivan, B.F.; Lange, C.A.; Furcht, L.T.; et al. Development of a test that measures real-time HER2 signaling function in live breast cancer cell lines and primary cells. BMC Cancer 2017, 17, 199. [Google Scholar] [CrossRef]
- Farahmand, S.; Fernandez, A.I.; Ahmed, F.S.; Rimm, D.L.; Chuang, J.H.; Reisenbichler, E.; Zarringhalam, K. Deep learning trained on hematoxylin and eosin tumor region of Interest predicts HER2 status and trastuzumab treatment response in HER2+ breast cancer. Mod. Pathol. 2022, 35, 44–51. [Google Scholar] [CrossRef]
- Yousif, M.; Huang, Y.; Sciallis, A.; Kleer, C.G.; Pang, J.; Smola, B.; Naik, K.; McClintock, D.S.; Zhao, L.; Kunju, L.P.; et al. Quantitative Image Analysis as an Adjunct to Manual Scoring of ER, PgR, and HER2 in Invasive Breast Carcinoma. Am. J. Clin. Pathol. 2022, 157, 899–907. [Google Scholar] [CrossRef] [PubMed]
- La Barbera, D.; Polónia, A.; Roitero, K.; Conde-Sousa, E.; Della Mea, V. Detection of HER2 from Haematoxylin-Eosin Slides Through a Cascade of Deep Learning Classifiers via Multi-Instance Learning. J. Imaging 2020, 6, 82. [Google Scholar] [CrossRef]
- Ercoli, G.; Lopez, G.; Ciapponi, C.; Corti, C.; Despini, L.; Gambini, D.; Runza, L.; Blundo, C.; Sciarra, A.; Fusco, N. Building Up a High-throughput Screening Platform to Assess the Heterogeneity of HER2 Gene Amplification in Breast Cancers. J. Vis. Exp. 2017, 130, e56686. [Google Scholar]
- Lai, H.-Z.; Han, J.-R.; Fu, X.; Ren, Y.-F.; Li, Z.-H.; You, F.-M. Targeted Approaches to HER2-Low Breast Cancer: Current Practice and Future Directions. Cancers 2022, 14, 3774. [Google Scholar] [CrossRef]
- Indini, A.; Rijavec, E.; Grossi, F. Trastuzumab Deruxtecan: Changing the Destiny of HER2 Expressing Solid Tumors. Int. J. Mol. Sci. 2021, 22, 4774. [Google Scholar] [CrossRef]
- Denkert, C.; Lebeau, A.; Schildhaus, H.U.; Jackisch, C.; Rüschoff, J. New treatment options for metastatic HER2-low breast cancer. Die Pathol. 2022. [Google Scholar] [CrossRef] [PubMed]
- Miglietta, F.; Griguolo, G.; Bottosso, M.; Giarratano, T.; Lo Mele, M.; Fassan, M.; Cacciatore, M.; Genovesi, E.; De Bartolo, D.; Vernaci, G.; et al. Evolution of HER2-low expression from primary to recurrent breast cancer. npj Breast Cancer 2021, 7, 137. [Google Scholar] [CrossRef]
- Venetis, K.; Sajjadi, E.; Haricharan, S.; Fusco, N. Mismatch repair testing in breast cancer: The path to tumor-specific immuno-oncology biomarkers. Transl. Cancer Res. 2020, 9, 4060–4064. [Google Scholar] [CrossRef] [PubMed]
- Exman, P.; Garrido-Castro, A.C.; Hughes, M.E.; Freedman, R.A.; Li, T.; Trippa, L.; Bychkovsky, B.L.; Barroso-Sousa, R.; Di Lascio, S.; Mackichan, C.; et al. Identifying ERBB2 Activating Mutations in HER2-Negative Breast Cancer: Clinical Impact of Institute-Wide Genomic Testing and Enrollment in Matched Therapy Trials. JCO Precis. Oncol. 2019, 3, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Bose, R.; Kavuri, S.M.; Searleman, A.C.; Shen, W.; Shen, D.; Koboldt, D.C.; Monsey, J.; Goel, N.; Aronson, A.B.; Li, S.; et al. Activating HER2 Mutations in HER2 Gene Amplification Negative Breast Cancer. Cancer Discov. 2013, 3, 224–237. [Google Scholar] [CrossRef] [PubMed]
- Sajjadi, E.; Venetis, K.; Piciotti, R.; Invernizzi, M.; Guerini-Rocco, E.; Haricharan, S.; Fusco, N. Mismatch repair-deficient hormone receptor-positive breast cancers: Biology and pathological characterization. Cancer Cell Int. 2021, 21, 266. [Google Scholar] [CrossRef]
- Punturi, N.B.; Seker, S.; Devarakonda, V.; Mazumder, A.; Kalra, R.; Chen, C.H.; Li, S.; Primeau, T.; Ellis, M.J.; Kavuri, S.M.; et al. Mismatch repair deficiency predicts response to HER2 blockade in HER2-negative breast cancer. Nat. Commun. 2021, 12, 2940. [Google Scholar] [CrossRef]
- Sajjadi, E.; Venetis, K.; Ivanova, M.; Fusco, N. Improving HER2 testing reproducibility in HER2-low breast cancer. Cancer Drug Resist. 2022, 5, 882–888. [Google Scholar] [CrossRef] [PubMed]
- Haricharan, S.; Punturi, N.; Singh, P.; Holloway, K.R.; Anurag, M.; Schmelz, J.; Schmidt, C.; Lei, J.T.; Suman, V.; Hunt, K.; et al. Loss of MutL Disrupts CHK2-Dependent Cell-Cycle Control through CDK4/6 to Promote Intrinsic Endocrine Therapy Resistance in Primary Breast Cancer. Cancer Discov. 2017, 7, 1168–1183. [Google Scholar] [CrossRef]
- Sajjadi, E.; Venetis, K.; Piciotti, R.; Gambini, D.; Blundo, C.; Runza, L.; Ferrero, S.; Guerini-Rocco, E.; Fusco, N. Combined analysis of PTEN, HER2, and hormone receptors status: Remodeling breast cancer risk profiling. BMC Cancer 2021, 21, 1152. [Google Scholar] [CrossRef] [PubMed]
- Piciotti, R.; Venetis, K.; Sajjadi, E.; Fusco, N. Mismatch Repair Status Characterization in Oncologic Pathology: Taking Stock of the Real-World Possibilities. J. Mol. Pathol. 2021, 2, 93–100. [Google Scholar] [CrossRef]
- Lambein, K.; Van Bockstal, M.; Vandemaele, L.; Geenen, S.; Rottiers, I.; Nuyts, A.; Matthys, B.; Praet, M.; Denys, H.; Libbrecht, L. Distinguishing score 0 from score 1+ in HER2 immunohistochemistry-negative breast cancer: Clinical and pathobiological relevance. Am. J. Clin. Pathol. 2013, 140, 561–566. [Google Scholar] [CrossRef]
- Fernandez, A.I.; Liu, M.; Bellizzi, A.; Brock, J.; Fadare, O.; Hanley, K.; Harigopal, M.; Jorns, J.M.; Kuba, M.G.; Ly, A.; et al. Examination of Low ERBB2 Protein Expression in Breast Cancer Tissue. JAMA Oncol. 2022, 8, 607–610. [Google Scholar] [CrossRef]
- Ogitani, Y.; Aida, T.; Hagihara, K.; Yamaguchi, J.; Ishii, C.; Harada, N.; Soma, M.; Okamoto, H.; Oitate, M.; Arakawa, S.; et al. DS-8201a, A Novel HER2-Targeting ADC with a Novel DNA Topoisomerase I Inhibitor, Demonstrates a Promising Antitumor Efficacy with Differentiation from T-DM1. Clin. Cancer Res. 2016, 22, 5097–5108. [Google Scholar] [CrossRef] [PubMed]
- Ogitani, Y.; Hagihara, K.; Oitate, M.; Naito, H.; Agatsuma, T. Bystander killing effect of DS-8201a, a novel anti-human epidermal growth factor receptor 2 antibody–drug conjugate, in tumors with human epidermal growth factor receptor 2 heterogeneity. Cancer Sci. 2016, 107, 1039–1046. [Google Scholar] [CrossRef] [PubMed]
- Modi, S.; Park, H.; Murthy, R.K.; Iwata, H.; Tamura, K.; Tsurutani, J.; Moreno-Aspitia, A.; Doi, T.; Sagara, Y.; Redfern, C.; et al. Antitumor Activity and Safety of Trastuzumab Deruxtecan in Patients with HER2-Low–Expressing Advanced Breast Cancer: Results from a Phase Ib Study. J. Clin. Oncol. 2020, 38, 1887–1896. [Google Scholar] [CrossRef] [PubMed]
- Iwata, T.N.; Ishii, C.; Ishida, S.; Ogitani, Y.; Wada, T.; Agatsuma, T. A HER2-Targeting Antibody–Drug Conjugate, Trastuzumab Deruxtecan (DS-8201a), Enhances Antitumor Immunity in a Mouse Model. Mol. Cancer Ther. 2018, 17, 1494–1503. [Google Scholar] [CrossRef] [PubMed]
- Iwata, T.N.; Sugihara, K.; Wada, T.; Agatsuma, T. [Fam-] trastuzumab deruxtecan (DS-8201a)-induced antitumor immunity is facilitated by the anti–CTLA-4 antibody in a mouse model. PLoS ONE 2019, 14, e0222280. [Google Scholar] [CrossRef] [PubMed]
- Schmid, P.; Im, S.-A.; Armstrong, A.; Park, Y.H.; Chung, W.-P.; Nowecki, Z.; Lord, S.; Wysocki, P.J.; Lu, Y.-S.; Dry, H.; et al. BEGONIA: Phase 1b/2 study of durvalumab (D) combinations in locally advanced/metastatic triple-negative breast cancer (TNBC)—Initial results from arm 1, d+paclitaxel (P), and arm 6, d+trastuzumab deruxtecan (T-DXd). J. Clin. Oncol. 2021, 39 (Suppl. S15), 1023. [Google Scholar] [CrossRef]
- Hamilton, E.P.; Shapiro, C.L.; Boni, V.; Jimenez, M.M.; Del Conte, G.; Cortés, J.; Agrawal, L.; Arkenau, H.T.; Tan, A.R.; Debruyne, P.R.; et al. 162O Primary analysis from DS8201-A-U105: A 2-part, open label, phase Ib trial assessing trastuzumab deruxtecan (T-DXd) with nivolumab (nivo) in patients (pts) with HER2-expressing advanced breast cancer. Ann. Oncol. 2022, 33, S196. [Google Scholar] [CrossRef]
- Wang, J.; Liu, Y.; Zhang, Q.; Feng, J.; Fang, J.; Chen, X.; Han, Y.; Li, Q.; Zhang, P.; Yuan, P.; et al. RC48-ADC, a HER2-targeting antibody-drug conjugate, in patients with HER2-positive and HER2-low expressing advanced or metastatic breast cancer: A pooled analysis of two studies. J. Clin. Oncol. 2021, 39 (Suppl. S15), 1022. [Google Scholar] [CrossRef]
| HER2 Score 3+ | HER2 Score 2+ | HER2 Score 1+ | HER2 Score 0 | |
|---|---|---|---|---|
| IHC | Complete, intense, and circumferential membrane staining in >10% of tumor cells | Incomplete and/or weak to moderate circumferential membrane staining in >10% of the tumor cells or the presence of intense, complete, and circumferential membrane staining in ≤10% of the tumor cells | Incomplete or faint/barely perceptible membrane staining in >10% of tumor cells | Incomplete or faint/barely perceptible membrane staining in ≤10% of tumor cells |
| No staining | ||||
| ISH | AMPLIFIED HER2/CEP17 ratio of ≥2.0 or HER2/CEP17 ratio < 2.0 with average HER2 copy number ≥ 6.0 | NOT AMPLIFIED HER2/CEP17 ratio < 2.0 with an average HER2 copy number of <4.0. Notes: If the IHC result is 2+, recount ISH by having an additional observer, blinded to previous ISH results, count at least 20 cells that include the area of invasive cancer with IHC 2+ staining If reviewing the count by the additional observer changes the result into another ISH category, the result should be adjudicated as per internal procedures to define the final category. If the count remains an average of <4.0 HER2 signals/cell and an HER2/CEP17 ratio > 2.0, the diagnosis is HER2-negative with a comment. | ||
| HER2-POSITIVE | HER2-LOW | HER2-ULTRA-LOW | ||
| HER2-NEGATIVE | ||||
| Treatment | Trastuzumab-emtansine (T-DM1) | Trastuzumab-deruxtecan (T-DXd) | Other recommended regimens NCCN Guidelines® Insights: Breast Cancer, Version 4.2023 | |
| ISH Interpretation | ISH Rejection Criteria |
|---|---|
| Reject and repeat if:
|
| Drug | Population | Clinical Trial | Result |
|---|---|---|---|
| Trastuzumab-Deruxtecan (T-DXd) | Pretreated HER2-low MBC | 0 [95] | ORR = 37% PFS = 11.1 months |
| Trastuzumab-Deruxtecan (T-DXd) | HER2-low MBC pretreated with chemotherapy | NCT03734029 (DESTINY Breast 04—phase III) [69] | In HR+ patients: PFS = 9.9 months OS = 23.4 months In HR− patients: PFS = 8.5 months OS = 18.2 months |
| Trastuzumab-Deruxtecan (T-DXd) | HR+ HER2-low MBC | NCT04494425 (DESTINY Breast 06—phase III) | ongoing |
| T-DXd + anti-PD-L1 | Preclinical study [96] | Enhanced antitumor effect by an increase in T-cell activity and upregulation of PD-L1 expression in xenograft mouse models | |
| T-DXd + CTLA-4 | Preclinical study [97] | Enhanced antitumor effect by increases in tumor-infiltrating CD4 and CD8 | |
| T-Dxd + Durvalumab | HER2-low locally advanced/metastatic TNBC | NCT03742102 (BEGONIA—phase Ib/II) [98] | ORR = 66.7% ongoing |
| T-Dxd + Nivolumab | Pretreated HER2-low MBC | NCT03523572 (phase Ib) [99] | ORR = 50% PFS = 7 months |
| Trastuzumab + duocarmazine | Pretreated HER2-low MBC | NCT02277717 (phase I) [64] | In HR+ patients: ORR = 28% PFS = 4.9 months In HR− patients: ORR = 40% PFS = 4.1 months |
| Hertuzumab + Disitamab Vedotin | Pretreated HER2-low MBC | NCT02881138 NCT03052634 (phase I/Ib) [100] | ORR = 40% PFS = 5.7 months |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Franchina, M.; Pizzimenti, C.; Fiorentino, V.; Martini, M.; Ricciardi, G.R.R.; Silvestris, N.; Ieni, A.; Tuccari, G. Low and Ultra-Low HER2 in Human Breast Cancer: An Effort to Define New Neoplastic Subtypes. Int. J. Mol. Sci. 2023, 24, 12795. https://doi.org/10.3390/ijms241612795
Franchina M, Pizzimenti C, Fiorentino V, Martini M, Ricciardi GRR, Silvestris N, Ieni A, Tuccari G. Low and Ultra-Low HER2 in Human Breast Cancer: An Effort to Define New Neoplastic Subtypes. International Journal of Molecular Sciences. 2023; 24(16):12795. https://doi.org/10.3390/ijms241612795
Chicago/Turabian StyleFranchina, Mariausilia, Cristina Pizzimenti, Vincenzo Fiorentino, Maurizio Martini, Giuseppina Rosaria Rita Ricciardi, Nicola Silvestris, Antonio Ieni, and Giovanni Tuccari. 2023. "Low and Ultra-Low HER2 in Human Breast Cancer: An Effort to Define New Neoplastic Subtypes" International Journal of Molecular Sciences 24, no. 16: 12795. https://doi.org/10.3390/ijms241612795
APA StyleFranchina, M., Pizzimenti, C., Fiorentino, V., Martini, M., Ricciardi, G. R. R., Silvestris, N., Ieni, A., & Tuccari, G. (2023). Low and Ultra-Low HER2 in Human Breast Cancer: An Effort to Define New Neoplastic Subtypes. International Journal of Molecular Sciences, 24(16), 12795. https://doi.org/10.3390/ijms241612795










