The Role of Sensory Innervation in Homeostatic and Injury-Induced Corneal Epithelial Renewal
Abstract
1. Introduction
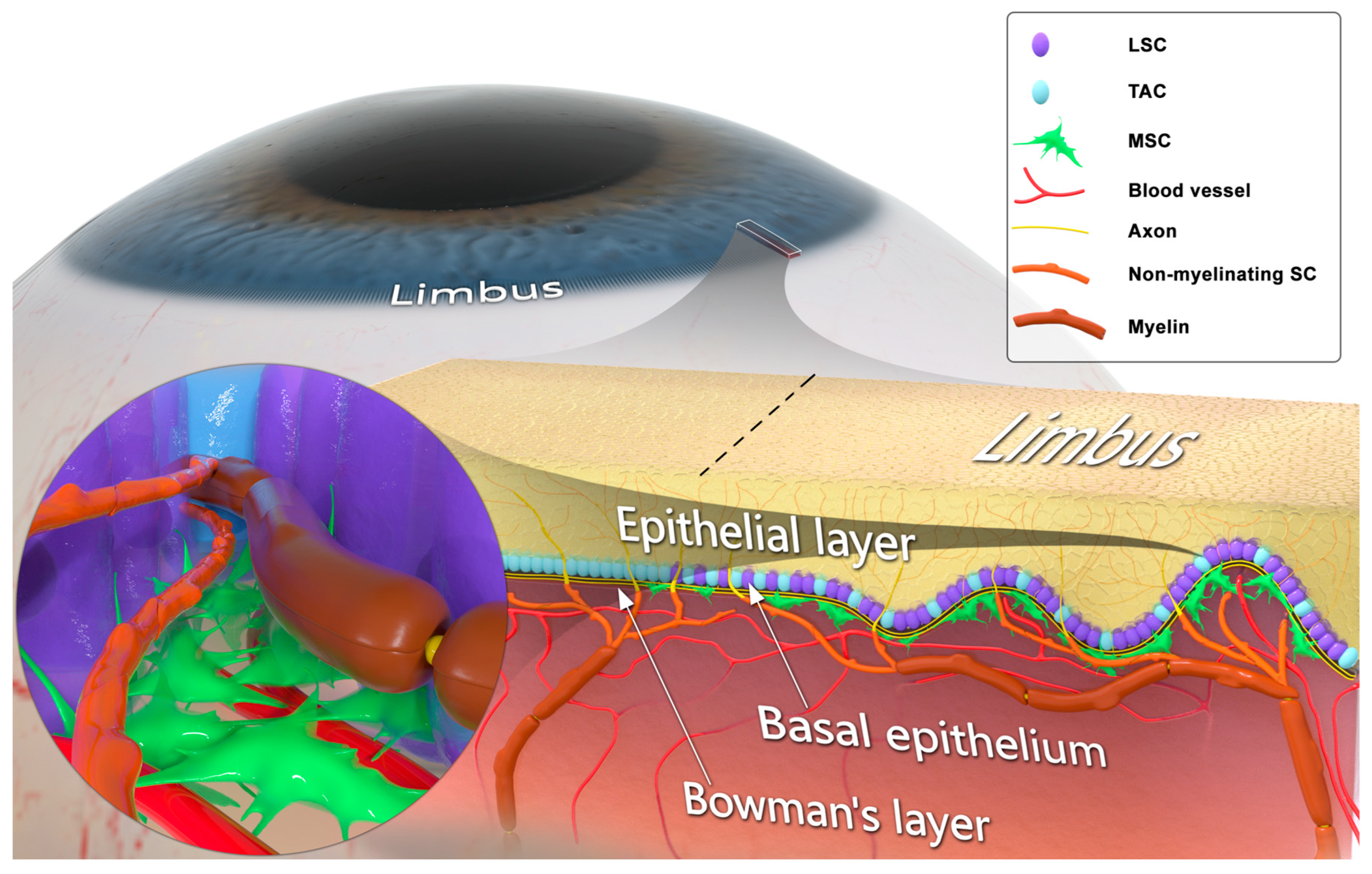
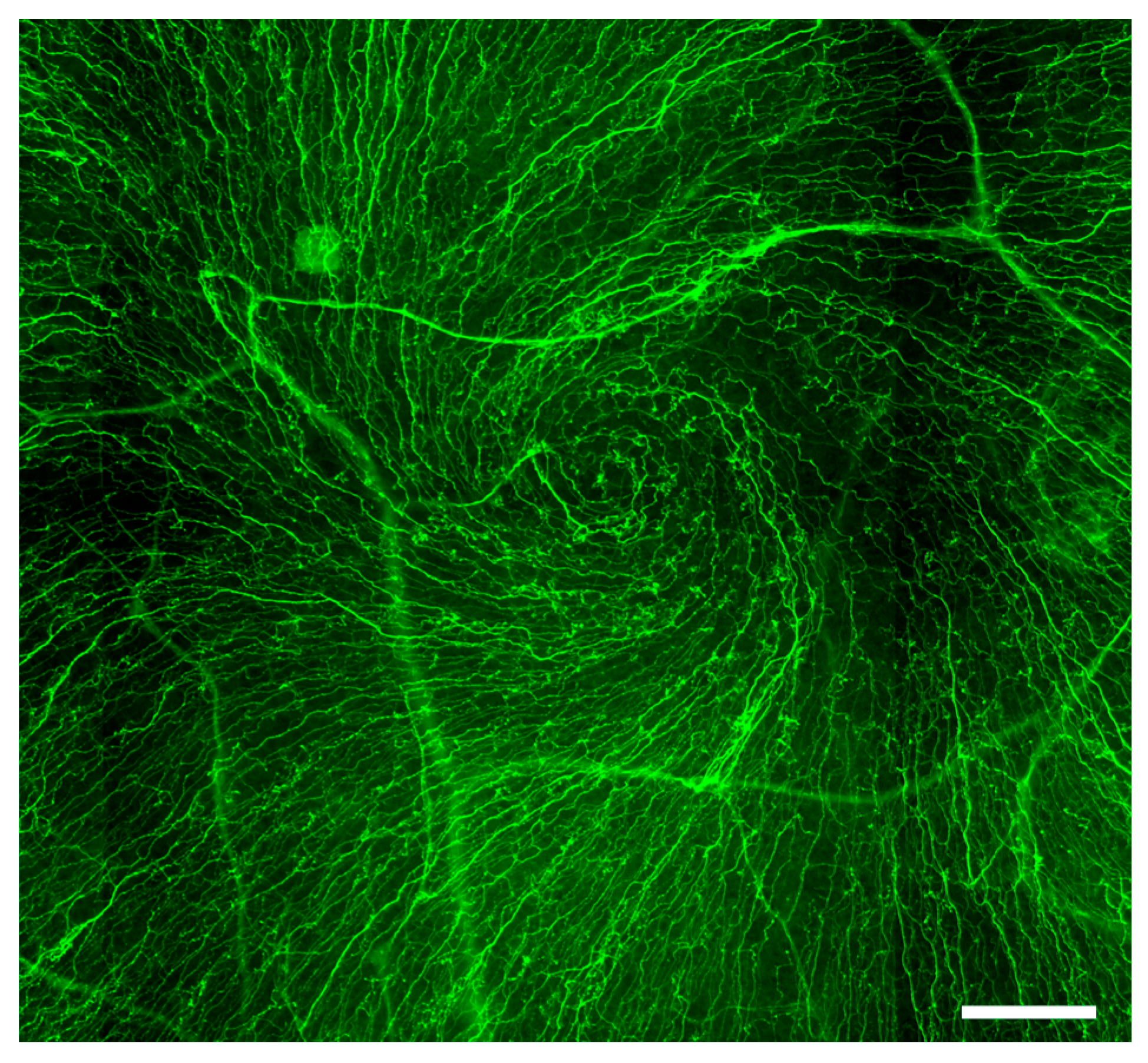
2. Corneal Sensory Innervation
3. Schwann Cells and Innervation-Dependent Corneal Epithelial Renewal
4. Trophic Regulation of Sensory Innervation-Mediated Corneal Epithelial Renewal
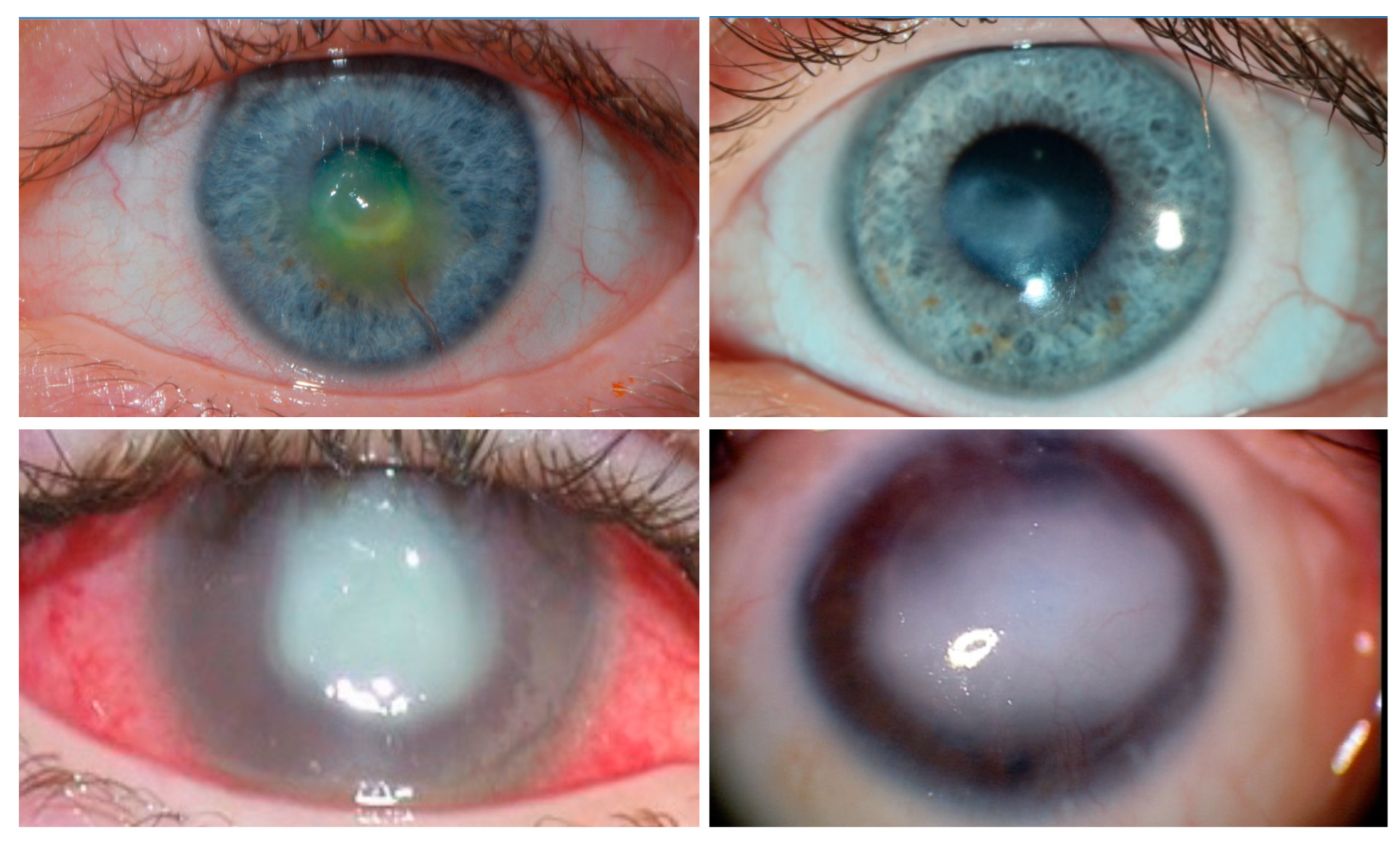
5. Currently Available Pharmacological Approaches to Treat NK
Nerve Growth Factor (NGF)
6. Strategies to Promote Corneal Reinnervation
6.1. Potential Treatment with Tacrolimus
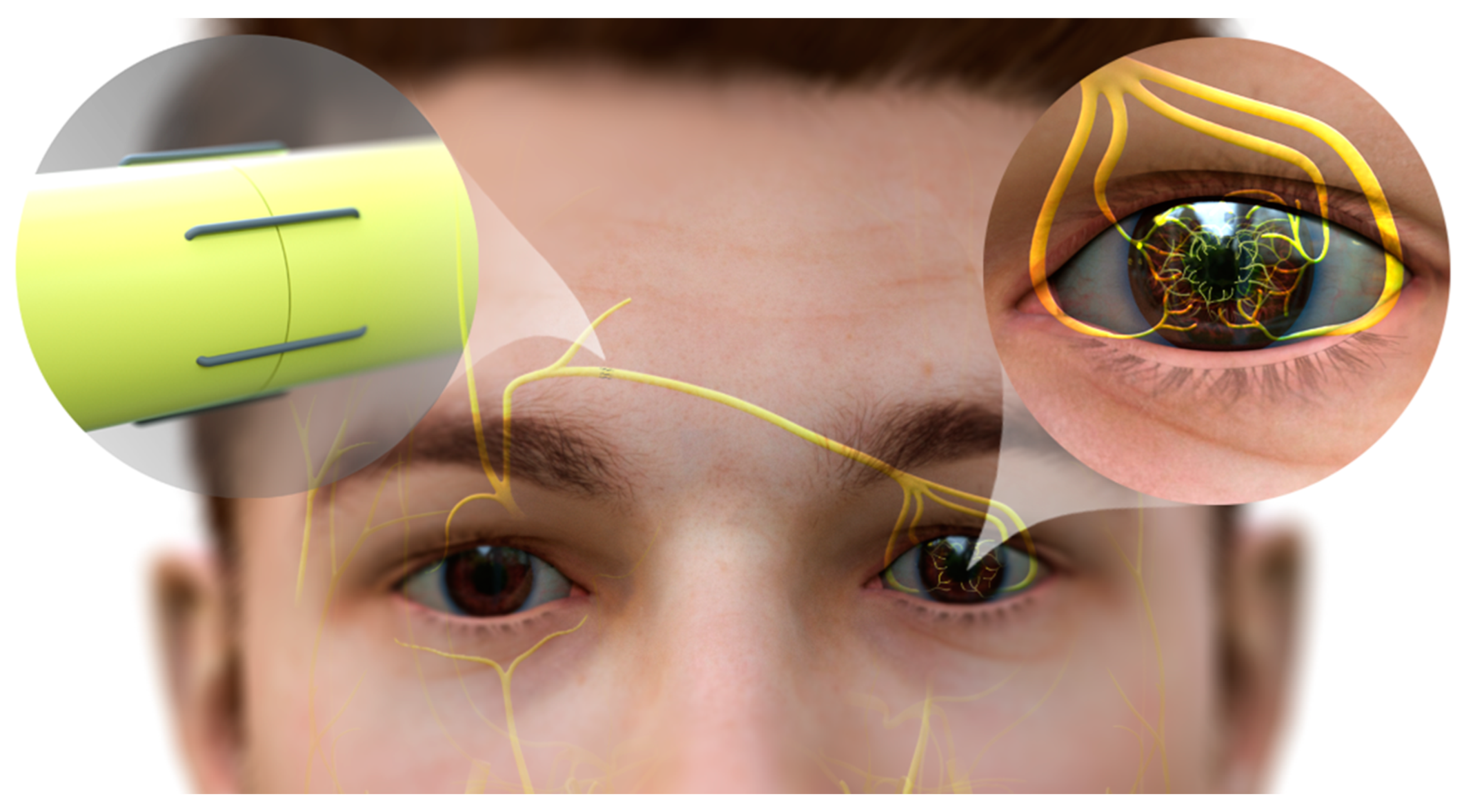
6.2. Corneal Neurotization
7. Summary and Discussion
8. Methods of Literature Review
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hertsenberg, A.J.; Funderburgh, J.L. Stem Cells in the Cornea. Prog. Mol. Biol. Transl. Sci. 2015, 134, 25–41. [Google Scholar]
- Hanna, C.; Bicknell, D.S.; O’Brien, J.E. Cell turnover in the adult human eye. Arch. Ophthalmol. 1961, 65, 695–698. [Google Scholar] [CrossRef] [PubMed]
- Cenedella, R.J.; Fleschner, C.R. Kinetics of corneal epithelium turnover in vivo. Studies of lovastatin. Investig. Ophthalmol. Vis. Sci. 1990, 31, 1957–1962. [Google Scholar]
- Bentley, A.J.; Nakamura, T.; Hammiche, A.; Pollock, H.M.; Martin, F.L.; Kinoshita, S.; Fullwood, N.J. Characterization of human corneal stem cells by synchrotron infrared micro-spectroscopy. Mol. Vis. 2007, 13, 237–242. [Google Scholar] [PubMed]
- Chen, Z.; de Paiva, C.S.; Luo, L.; Kretzer, F.L.; Pflugfelder, S.C.; Li, D.Q. Characterization of putative stem cell phenotype in human limbal epithelia. Stem. Cells 2004, 22, 355–366. [Google Scholar] [CrossRef]
- Schlotzer-Schrehardt, U.; Kruse, F.E. Identification and characterization of limbal stem cells. Exp. Eye Res. 2005, 81, 247–264. [Google Scholar] [CrossRef]
- Romano, A.C.; Espana, E.M.; Yoo, S.H.; Budak, M.T.; Wolosin, J.M.; Tseng, S.C. Different cell sizes in human limbal and central corneal basal epithelia measured by confocal microscopy and flow cytometry. Investig. Ophthalmol. Vis. Sci. 2003, 44, 5125–5129. [Google Scholar] [CrossRef]
- Pellegrini, G.; Golisano, O.; Paterna, P.; Lambiase, A.; Bonini, S.; Rama, P.; De Luca, M. Location and clonal analysis of stem cells and their differentiated progeny in the human ocular surface. J. Cell Biol. 1999, 145, 769–782. [Google Scholar] [CrossRef] [PubMed]
- Amin, S.; Jalilian, E.; Katz, E.; Frank, C.; Yazdanpanah, G.; Guaiquil, V.H.; Rosenblatt, M.I.; Djalilian, A.R. The Limbal Niche and Regenerative Strategies. Vision 2021, 5, 43. [Google Scholar] [CrossRef]
- Yazdanpanah, G.; Haq, Z.; Kang, K.; Jabbehdari, S.; Rosenblatt, M.L.; Djalilian, A.R. Strategies for reconstructing the limbal stem cell niche. Ocul. Surf. 2019, 17, 230–240. [Google Scholar] [CrossRef]
- Altshuler, A.; Amitai-Lange, A.; Tarazi, N.; Dey, S.; Strinkovsky, L.; Hadad-Porat, S.; Bhattacharya, S.; Nasser, W.; Imeri, J.; Ben-David, G.; et al. Discrete limbal epithelial stem cell populations mediate corneal homeostasis and wound healing. Cell Stem Cell 2021, 28, 1248–1261.e8. [Google Scholar] [CrossRef]
- Li, W.; Hayashida, Y.; Chen, Y.T.; Tseng, S.C. Niche regulation of corneal epithelial stem cells at the limbus. Cell Res. 2007, 17, 26–36. [Google Scholar] [CrossRef] [PubMed]
- Cotsarelis, G.; Cheng, S.Z.; Dong, G.; Sun, T.T.; Lavker, R.M. Existence of slow-cycling limbal epithelial basal cells that can be preferentially stimulated to proliferate: Implications on epithelial stem cells. Cell 1989, 57, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Mo, V.; Nagasaki, T. Distribution of label-retaining cells in the limbal epithelium of a mouse eye. J. Histochem. Cytochem. 2009, 57, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Pajoohesh-Ganji, A.; Pal-Ghosh, S.; Simmens, S.J.; Stepp, M.A. Integrins in slow-cycling corneal epithelial cells at the limbus in the mouse. Stem Cells 2006, 24, 1075–1086. [Google Scholar] [CrossRef]
- Figueira, E.C.; Di Girolamo, N.; Coroneo, M.T.; Wakefield, D. The phenotype of limbal epithelial stem cells. Investig. Ophthalmol. Vis. Sci. 2007, 48, 144–156. [Google Scholar] [CrossRef]
- Raji, B.; Dansault, A.; Leemput, J.; de la Houssaye, G.; Vieira, V.; Kobetz, A.; Arbogast, L.; Masson, C.; Menasche, M.; Abitbol, M. The RNA-binding protein Musashi-1 is produced in the developing and adult mouse eye. Mol. Vis. 2007, 13, 1412–1427. [Google Scholar]
- Thomas, P.B.; Liu, Y.H.; Zhuang, F.F.; Selvam, S.; Song, S.W.; Smith, R.E.; Trousdale, M.D.; Yiu, S.C. Identification of Notch-1 expression in the limbal basal epithelium. Mol. Vis. 2007, 13, 337–344. [Google Scholar]
- Di Girolamo, N.; Sarris, M.; Chui, J.; Cheema, H.; Coroneo, M.T.; Wakefield, D. Localization of the low-affinity nerve growth factor receptor p75 in human limbal epithelial cells. J. Cell Mol. Med. 2008, 12, 2799–2811. [Google Scholar] [CrossRef]
- Barbaro, V.; Testa, A.; Di Iorio, E.; Mavilio, F.; Pellegrini, G.; De Luca, M. C/EBPdelta regulates cell cycle and self-renewal of human limbal stem cells. J. Cell Biol. 2007, 177, 1037–1049. [Google Scholar] [CrossRef]
- Di Iorio, E.; Barbaro, V.; Ruzza, A.; Ponzin, D.; Pellegrini, G.; De Luca, M. Isoforms of DeltaNp63 and the migration of ocular limbal cells in human corneal regeneration. Proc. Natl. Acad. Sci. USA 2005, 102, 9523–9528. [Google Scholar] [CrossRef]
- Amitai-Lange, A.; Altshuler, A.; Bubley, J.; Dbayat, N.; Tiosano, B.; Shalom-Feuerstein, R. Lineage tracing of stem and progenitor cells of the murine corneal epithelium. Stem Cells 2015, 33, 230–239. [Google Scholar] [CrossRef] [PubMed]
- Nasser, W.; Amitai-Lange, A.; Soteriou, D.; Hanna, R.; Tiosano, B.; Fuchs, Y.; Shalom-Feuerstein, R. Corneal-Committed Cells Restore the Stem Cell Pool and Tissue Boundary following Injury. Cell Rep. 2018, 22, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Farrelly, O.; Suzuki-Horiuchi, Y.; Brewster, M.; Kuri, P.; Huang, S.; Rice, G.; Bae, H.; Xu, J.; Dentchev, T.; Lee, V.; et al. Two-photon live imaging of single corneal stem cells reveals compartmentalized organization of the limbal niche. Cell Stem Cell 2021, 28, 1233–1247.e4. [Google Scholar] [CrossRef]
- Mann, I. A Study of Epithelial Regeneration in the Living Eye. Br. J. Ophthalmol. 1944, 28, 26–40. [Google Scholar] [CrossRef] [PubMed]
- Davanger, M.; Evensen, A. Role of the pericorneal papillary structure in renewal of corneal epithelium. Nature 1971, 229, 560–561. [Google Scholar] [CrossRef]
- Pfister, R.R.; Burstein, N. The alkali burned cornea I. Epithelial and stromal repair. Exp. Eye. Res. 1976, 23, 519–535. [Google Scholar] [CrossRef]
- Buck, R.C. Cell migration in repair of mouse corneal epithelium. Investig. Ophthalmol. Vis. Sci. 1979, 18, 767–784. [Google Scholar]
- Buck, R.C. Measurement of centripetal migration of normal corneal epithelial cells in the mouse. Investig. Ophthalmol. Vis. Sci. 1985, 26, 1296–1299. [Google Scholar]
- Collinson, J.M.; Chanas, S.A.; Hill, R.E.; West, J.D. Corneal development, limbal stem cell function, and corneal epithelial cell migration in the Pax6+/− mouse. Investig. Ophthalmol. Vis. Sci. 2004, 45, 1101–1108. [Google Scholar] [CrossRef]
- Nagasaki, T.; Zhao, J. Centripetal movement of corneal epithelial cells in the normal adult mouse. Investig. Ophthalmol. Vis. Sci. 2003, 44, 558–566. [Google Scholar] [CrossRef] [PubMed]
- Di Girolamo, N.; Bobba, S.; Raviraj, V.; Delic, N.C.; Slapetova, I.; Nicovich, P.R.; Halliday, G.M.; Wakefield, D.; Whan, R.; Lyons, J.G. Tracing the fate of limbal epithelial progenitor cells in the murine cornea. Stem Cells 2015, 33, 157–169. [Google Scholar] [CrossRef] [PubMed]
- Peterson, D.C.; Hamel, R.N. Corneal Reflex. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Muller, L.J.; Marfurt, C.F.; Kruse, F.; Tervo, T.M. Corneal nerves: Structure, contents and function. Exp. Eye Res. 2003, 76, 521–542. [Google Scholar] [CrossRef] [PubMed]
- Ueno, H.; Ferrari, G.; Hattori, T.; Saban, D.R.; Katikireddy, K.R.; Chauhan, S.K.; Dana, R. Dependence of corneal stem/progenitor cells on ocular surface innervation. Investig. Ophthalmol. Vis. Sci. 2012, 53, 867–872. [Google Scholar] [CrossRef] [PubMed]
- Cavanagh, H.D.; Colley, A.M. The molecular basis of neurotrophic keratitis. Acta Ophthalmol. Suppl. 1989, 192, 115–134. [Google Scholar] [CrossRef] [PubMed]
- Bonini, S.; Rama, P.; Olzi, D.; Lambiase, A. Neurotrophic keratitis. Eye 2003, 17, 989–995. [Google Scholar] [CrossRef]
- Sigelman, S.; Friedenwald, J.S. Mitotic and wound-healing activities of the corneal epithelium; effect of sensory denervation. AMA Arch. Ophthalmol. 1954, 52, 46–57. [Google Scholar] [CrossRef]
- Nakamura, M.; Nishida, T.; Ofuji, K.; Reid, T.W.; Mannis, M.J.; Murphy, C.J. Synergistic effect of substance P with epidermal growth factor on epithelial migration in rabbit cornea. Exp. Eye Res. 1997, 65, 321–329. [Google Scholar] [CrossRef]
- Yamada, N.; Yanai, R.; Inui, M.; Nishida, T. Sensitizing effect of substance P on corneal epithelial migration induced by IGF-1, fibronectin, or interleukin-6. Investig. Ophthalmol. Vis. Sci. 2005, 46, 833–839. [Google Scholar] [CrossRef]
- Murphy, C.J.; Marfurt, C.F.; McDermott, A.; Bentley, E.; Abrams, G.A.; Reid, T.W.; Campbell, S. Spontaneous chronic corneal epithelial defects (SCCED) in dogs: Clinical features, innervation, and effect of topical SP, with or without IGF-1. Investig. Ophthalmol. Vis. Sci. 2001, 42, 2252–2261. [Google Scholar]
- Marfurt, C.F.; Echtenkamp, S.F. The effect of diabetes on neuropeptide content in the rat cornea and iris. Investig. Ophthalmol. Vis. Sci. 1995, 36, 1100–1106. [Google Scholar]
- Elbadri, A.A.; Shaw, C.; Johnston, C.F.; Archer, D.B.; Buchanan, K.D. The distribution of neuropeptides in the ocular tissues of several mammals: A comparative study. Comp. Biochem. Physiol. C 1991, 100, 625–627. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Di, G.; Qi, X.; Qu, M.; Wang, Y.; Duan, H.; Danielson, P.; Xie, L.; Zhou, Q. Substance P promotes diabetic corneal epithelial wound healing through molecular mechanisms mediated via the neurokinin-1 receptor. Diabetes 2014, 63, 4262–4274. [Google Scholar] [CrossRef]
- Marfurt, C.F.; Kingsley, R.E.; Echtenkamp, S.E. Sensory and sympathetic innervation of the mammalian cornea. A retrograde tracing study. Investig. Ophthalmol. Vis. Sci. 1989, 30, 461–472. [Google Scholar]
- Jones, M.A.; Marfurt, C.F. Calcitonin gene-related peptide and corneal innervation: A developmental study in the rat. J. Comp. Neurol. 1991, 313, 132–150. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Hirschfeld, J.; Lopez-Briones, L.G.; Belmonte, C. Neurotrophic influences on corneal epithelial cells. Exp. Eye Res. 1994, 59, 597–605. [Google Scholar] [CrossRef] [PubMed]
- Lambiase, A.; Rama, P.; Bonini, S.; Caprioglio, G.; Aloe, L. Topical treatment with nerve growth factor for corneal neurotrophic ulcers. N. Engl. J. Med. 1998, 338, 1174–1180. [Google Scholar] [CrossRef]
- Lambiase, A.; Manni, L.; Bonini, S.; Rama, P.; Micera, A.; Aloe, L. Nerve growth factor promotes corneal healing: Structural, biochemical, and molecular analyses of rat and human corneas. Investig. Ophthalmol. Vis. Sci. 2000, 41, 1063–1069. [Google Scholar]
- Tan, M.H.; Bryars, J.; Moore, J. Use of nerve growth factor to treat congenital neurotrophic corneal ulceration. Cornea 2006, 25, 352–355. [Google Scholar] [CrossRef]
- Ivanusic, J.J.; Wood, R.J.; Brock, J.A. Sensory and sympathetic innervation of the mouse and guinea pig corneal epithelium. J. Comp. Neurol. 2013, 521, 877–893. [Google Scholar] [CrossRef]
- Alper, M.G. The anesthetic eye: An investigation of changes in the anterior ocular segment of the monkey caused by interrupting the trigeminal nerve at various levels along its course. Trans. Am. Ophthalmol. Soc. 1975, 73, 323–365. [Google Scholar]
- Beuerman, R.W.; Schimmelpfennig, B. Sensory denervation of the rabbit cornea affects epithelial properties. Exp. Neurol. 1980, 69, 196–201. [Google Scholar] [CrossRef] [PubMed]
- Sacchetti, M.; Lambiase, A. Diagnosis and management of neurotrophic keratitis. Clin. Ophthalmol. 2014, 8, 571–579. [Google Scholar] [PubMed]
- Dana, R.; Farid, M.; Gupta, P.K.; Hamrah, P.; Karpecki, P.; McCabe, C.M.; Nijm, L.; Pepose, J.S.; Pflugfelder, S.; Rapuano, C.J.; et al. Expert consensus on the identification, diagnosis, and treatment of neurotrophic keratopathy. BMC Ophthalmol. 2021, 21, 327. [Google Scholar] [CrossRef] [PubMed]
- Schimmelpfennig, B.; Beuerman, R. A technique for controlled sensory denervation of the rabbit cornea. Graefes. Arch. Clin. Exp. Ophthalmol. 1982, 218, 287–293. [Google Scholar] [CrossRef]
- Araki, K.; Ohashi, Y.; Kinoshita, S.; Hayashi, K.; Kuwayama, Y.; Tano, Y. Epithelial wound healing in the denervated cornea. Curr. Eye Res. 1994, 13, 203–211. [Google Scholar] [CrossRef]
- Gallar, J.; Pozo, M.A.; Rebollo, I.; Belmonte, C. Effects of capsaicin on corneal wound healing. Investig. Ophthalmol. Vis. Sci. 1990, 31, 1968–1974. [Google Scholar]
- Ferrari, G.; Chauhan, S.K.; Ueno, H.; Nallasamy, N.; Gandolfi, S.; Borges, L.; Dana, R. A novel mouse model for neurotrophic keratopathy: Trigeminal nerve stereotactic electrolysis through the brain. Investig. Ophthalmol. Vis. Sci. 2011, 52, 2532–2539. [Google Scholar] [CrossRef]
- Ramaesh, K.; Stokes, J.; Henry, E.; Dutton, G.N.; Dhillon, B. Congenital corneal anesthesia. Surv. Ophthalmol. 2007, 52, 50–60. [Google Scholar] [CrossRef]
- Rosenberg, M.L. Congenital trigeminal anaesthesia. A review and classification. Brain 1984, 107 Pt 4, 1073–1082. [Google Scholar] [CrossRef]
- Lambley, R.G.; Pereyra-Munoz, N.; Parulekar, M.; Mireskandari, K.; Ali, A. Structural and functional outcomes of anaesthetic cornea in children. Br. J. Ophthalmol. 2015, 99, 418–424. [Google Scholar] [CrossRef]
- Agranat, J.S.; Kitos, N.R.; Jacobs, D.S. Prosthetic replacement of the ocular surface ecosystem: Impact at 5 years. Br. J. Ophthalmol. 2016, 100, 1171–1175. [Google Scholar] [CrossRef]
- Catapano, J.; Fung, S.S.M.; Halliday, W.; Jobst, C.; Cheyne, D.; Ho, E.S.; Zuker, R.M.; Borschel, G.H.; Ali, A. Treatment of neurotrophic keratopathy with minimally invasive corneal neurotisation: Long-term clinical outcomes and evidence of corneal reinnervation. Br. J. Ophthalmol. 2019, 103, 1724–1731. [Google Scholar] [CrossRef]
- Daeschler, S.C.; Mirmoeini, K.; Gordon, T.; Chan, K.; Zhang, J.; Ali, A.; Feinberg, K.; Borschel, G.H. Sustained Release of Tacrolimus From a Topical Drug Delivery System Promotes Corneal Reinnervation. Transl. Vis. Sci. Technol. 2022, 11, 20. [Google Scholar] [CrossRef]
- Pflugfelder, S.C.; Massaro-Giordano, M.; Perez, V.L.; Hamrah, P.; Deng, S.X.; Espandar, L.; Foster, C.S.; Affeldt, J.; Seedor, J.A.; Afshari, N.A.; et al. Topical Recombinant Human Nerve Growth Factor (Cenegermin) for Neurotrophic Keratopathy: A Multicenter Randomized Vehicle-Controlled Pivotal Trial. Ophthalmology 2020, 127, 14–26. [Google Scholar] [CrossRef]
- Bargagna-Mohan, P.; Ishii, A.; Lei, L.; Sheehy, D.; Pandit, S.; Chan, G.; Bansal, R.; Mohan, R. Sustained activation of ERK1/2 MAPK in Schwann cells causes corneal neurofibroma. J. Neurosci. Res. 2017, 95, 1712–1729. [Google Scholar] [CrossRef] [PubMed]
- Bargagna-Mohan, P.; Schultz, G.; Rheaume, B.; Trakhtenberg, E.F.; Robson, P.; Pal-Ghosh, S.; Stepp, M.A.; Given, K.S.; Macklin, W.B.; Mohan, R. Corneal nonmyelinating Schwann cells illuminated by single-cell transcriptomics and visualized by protein biomarkers. J. Neurosci. Res. 2021, 99, 731–749. [Google Scholar] [CrossRef] [PubMed]
- Johnston, A.P.; Yuzwa, S.A.; Carr, M.J.; Mahmud, N.; Storer, M.A.; Krause, M.P.; Jones, K.; Paul, S.; Kaplan, D.R.; Miller, F.D. Dedifferentiated Schwann Cell Precursors Secreting Paracrine Factors Are Required for Regeneration of the Mammalian Digit Tip. Cell Stem Cell 2016, 19, 433–448. [Google Scholar] [CrossRef]
- Johnston, A.P.; Naska, S.; Jones, K.; Jinno, H.; Kaplan, D.R.; Miller, F.D. Sox2-mediated regulation of adult neural crest precursors and skin repair. Stem Cell Rep. 2013, 1, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Mirmoeini, K.; Tajdaran, K.; Zhang, J.; Gordon, T.; Ali, A.; Kaplan, D.R.; Feinberg, K.; Borschel, G.H. Schwann Cells are Key Regulators of Corneal Epithelial Renewal. IOVS 2023, 64, 7. [Google Scholar] [CrossRef]
- Rozsa, A.J.; Beuerman, R.W. Density and organization of free nerve endings in the corneal epithelium of the rabbit. Pain 1982, 14, 105–120. [Google Scholar] [CrossRef] [PubMed]
- Utsunomiya, T.; Nagaoka, T.; Hanada, K.; Omae, T.; Yokota, H.; Abiko, A.; Haneda, M.; Yoshida, A. Imaging of the Corneal Subbasal Whorl-like Nerve Plexus: More Accurate Depiction of the Extent of Corneal Nerve Damage in Patients With Diabetes. Investig. Ophthalmol. Vis. Sci. 2015, 56, 5417–5423. [Google Scholar] [CrossRef] [PubMed]
- Catapano, J.; Antonyshyn, K.; Zhang, J.J.; Gordon, T.; Borschel, G.H. Corneal Neurotization Improves Ocular Surface Health in a Novel Rat Model of Neurotrophic Keratopathy and Corneal Neurotization. Investig. Ophthalmol. Vis. Sci. 2018, 59, 4345–4354. [Google Scholar]
- Semeraro, F.; Forbice, E.; Romano, V.; Angi, M.; Romano, M.R.; Filippelli, M.E.; Di Iorio, R.; Costagliola, C. Neurotrophic keratitis. Ophthalmologica 2014, 231, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Labetoulle, M.; Baudouin, C.; Calonge, M.; Merayo-Lloves, J.; Boboridis, K.G.; Akova, Y.A.; Aragona, P.; Geerling, G.; Messmer, E.M.; Benitez-Del-Castillo, J. Role of corneal nerves in ocular surface homeostasis and disease. Acta Ophthalmol. 2019, 97, 137–145. [Google Scholar] [CrossRef]
- Adachi, W.; Ulanovsky, H.; Li, Y.; Norman, B.; Davis, J.; Piatigorsky, J. Serial analysis of gene expression (SAGE) in the rat limbal and central corneal epithelium. Investig. Ophthalmol. Vis. Sci. 2006, 47, 3801–3810. [Google Scholar] [CrossRef]
- Yamazaki, S.; Ema, H.; Karlsson, G.; Yamaguchi, T.; Miyoshi, H.; Shioda, S.; Taketo, M.M.; Karlsson, S.; Iwama, A.; Nakauchi, H. Nonmyelinating Schwann cells maintain hematopoietic stem cell hibernation in the bone marrow niche. Cell 2011, 147, 1146–1158. [Google Scholar] [CrossRef]
- JeJessen, K.R.; Mirsky, R. Embryonic Schwann cell development: The biology of Schwann cell precursors and early Schwann cells. J. Anat. 1997, 191 Pt 4, 501–505. [Google Scholar] [CrossRef]
- Mirsky, R.; Jessen, K.R. The neurobiology of Schwann cells. Brain Pathol. 1999, 9, 293–311. [Google Scholar] [CrossRef]
- Taylor, J.S.; Bampton, E.T. Factors secreted by Schwann cells stimulate the regeneration of neonatal retinal ganglion cells. J. Anat. 2004, 204, 25–31. [Google Scholar] [CrossRef]
- Shimizu, T.; Izumi, K.; Fujita, S.; Koja, T.; Sorimachi, M.; Ohba, N.; Fukuda, T. Capsaicin-induced corneal lesions in mice and the effects of chemical sympathectomy. J. Pharmacol. Exp. Ther. 1987, 243, 690–695. [Google Scholar] [PubMed]
- Yamada, M.; Ogata, M.; Kawai, M.; Mashima, Y. Decreased substance P concentrations in tears from patients with corneal hypesthesia. Am. J. Ophthalmol. 2000, 129, 671–672. [Google Scholar] [CrossRef] [PubMed]
- Nishida, T.; Tanaka, T. Extracellular matrix and growth factors in corneal wound healing. Curr. Opin. Ophthalmol. 1996, 7, 2–11. [Google Scholar] [CrossRef]
- Reid, T.W.; Murphy, C.J.; Iwahashi, C.K.; Foster, B.A.; Mannis, M.J. Stimulation of epithelial cell growth by the neuropeptide substance P. J. Cell Biochem. 1993, 52, 476–485. [Google Scholar] [CrossRef] [PubMed]
- Kruse, F.E.; Tseng, S.C. Growth factors modulate clonal growth and differentiation of cultured rabbit limbal and corneal epithelium. Investig. Ophthalmol. Vis. Sci. 1993, 34, 1963–1976. [Google Scholar]
- Nishida, T. Neurotrophic mediators and corneal wound healing. Ocul. Surf. 2005, 3, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Nishida, T. Translational research in corneal epithelial wound healing. Eye Contact Lens 2010, 36, 300–304. [Google Scholar] [CrossRef]
- Saika, S.; Okada, Y.; Miyamoto, T.; Yamanaka, O.; Ohnishi, Y.; Ooshima, A.; Liu, C.Y.; Weng, D.; Kao, W.W. Role of p38 MAP kinase in regulation of cell migration and proliferation in healing corneal epithelium. Investig. Ophthalmol. Vis. Sci. 2004, 45, 100–109. [Google Scholar] [CrossRef]
- Terai, K.; Call, M.K.; Liu, H.; Saika, S.; Liu, C.Y.; Hayashi, Y.; Chikama, T.; Zhang, J.; Terai, N.; Kao, C.W.; et al. Crosstalk between TGF-β and MAPK signaling during corneal wound healing. Investig. Ophthalmol. Vis. Sci. 2011, 52, 8208–8215. [Google Scholar] [CrossRef]
- Wilson, S.E. Corneal wound healing. Exp. Eye Res. 2020, 197, 108089. [Google Scholar] [CrossRef]
- Zhou, Q.; Chen, P.; Di, G.; Zhang, Y.; Wang, Y.; Qi, X.; Duan, H.; Xie, L. Ciliary neurotrophic factor promotes the activation of corneal epithelial stem/progenitor cells and accelerates corneal epithelial wound healing. Stem Cells 2015, 33, 1566–1576. [Google Scholar] [CrossRef]
- Ghalibafan, S.; Osei, K.; Amescua, G.; Sabater, A. Efficacy of Plasma Rich in Growth Factors (PRGF) in Stage 1 Neurotrophic Keratitis. Res. Sq. 2023, 64, 5151. [Google Scholar]
- Castro Mora, M.P.; Palacio Varona, J.; Perez Riano, B.; Laverde Cubides, C.; Rey-Rodriguez, D.V. Effectiveness of topical insulin for the treatment of surface corneal pathologies. Arch. Soc. Esp. Oftalmol. (Engl. Ed.) 2023, 98, 220–232. [Google Scholar]
- Wang, A.L.; Weinlander, E.; Metcalf, B.M.; Barney, N.P.; Gamm, D.M.; Nehls, S.M.; Struck, M.C. Use of Topical Insulin to Treat Refractory Neurotrophic Corneal Ulcers. Cornea 2017, 36, 1426–1428. [Google Scholar] [CrossRef] [PubMed]
- Takanashi, N.; Haruki, T.; Miyazaki, D.; Inoue, Y. Effects of the pharmaceutical formulation of topical medications on corneal epithelial healing after phototherapeutic keratectomy. Jpn. J. Ophthalmol. 2023, 67, 424–430. [Google Scholar] [CrossRef] [PubMed]
- Bonini, S.; Lambiase, A.; Rama, P.; Sinigaglia, F.; Allegretti, M.; Chao, W.; Mantelli, F.; Group, R.S. Phase II Randomized, Double-Masked, Vehicle-Controlled Trial of Recombinant Human Nerve Growth Factor for Neurotrophic Keratitis. Ophthalmology 2018, 125, 1332–1343. [Google Scholar] [CrossRef]
- Kolli, S.; Bojic, S.; Ghareeb, A.E.; Kurzawa-Akanbi, M.; Figueiredo, F.C.; Lako, M. The Role of Nerve Growth Factor in Maintaining Proliferative Capacity, Colony-Forming Efficiency, and the Limbal Stem Cell Phenotype. Stem Cells 2019, 37, 139–149. [Google Scholar] [CrossRef]
- Deeks, E.D.; Lamb, Y.N. Cenegermin: A Review in Neurotrophic Keratitis. Drugs 2020, 80, 489–494. [Google Scholar] [CrossRef]
- Sheha, H.; Tighe, S.; Hashem, O.; Hayashida, Y. Update On Cenegermin Eye Drops In The Treatment Of Neurotrophic Keratitis. Clin. Ophthalmol. 2019, 13, 1973–1980. [Google Scholar] [CrossRef]
- Dai, X.; Jensen, A.; Dun, C.; Karakus, S.; Rajaii, F.; Woreta, F. Cost and Prescriber and Patient Characteristics of Cenegermin Use in the Medicare Population. Am. J. Ophthalmol. 2023, 250, 12–19. [Google Scholar] [CrossRef]
- Blanco-Mezquita, T.; Martinez-Garcia, C.; Proenca, R.; Zieske, J.D.; Bonini, S.; Lambiase, A.; Merayo-Lloves, J. Nerve growth factor promotes corneal epithelial migration by enhancing expression of matrix metalloprotease-9. Investig. Ophthalmol. Vis. Sci. 2013, 54, 3880–3890. [Google Scholar] [CrossRef] [PubMed]
- Bonini, S.; Lambiase, A.; Rama, P.; Caprioglio, G.; Aloe, L. Topical treatment with nerve growth factor for neurotrophic keratitis. Ophthalmology 2000, 107, 1347–1351, Discussion 1351—1352. [Google Scholar] [CrossRef]
- Donnerer, J.; Amann, R.; Schuligoi, R.; Skofitsch, G. Complete recovery by nerve growth factor of neuropeptide content and function in capsaicin-impaired sensory neurons. Brain Res. 1996, 741, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Streppel, M.; Azzolin, N.; Dohm, S.; Guntinas-Lichius, O.; Haas, C.; Grothe, C.; Wevers, A.; Neiss, W.F.; Angelov, D.N. Focal application of neutralizing antibodies to soluble neurotrophic factors reduces collateral axonal branching after peripheral nerve lesion. Eur. J. Neurosci. 2002, 15, 1327–1342. [Google Scholar] [CrossRef] [PubMed]
- Staniszewska, I.; Sariyer, I.K.; Lecht, S.; Brown, M.C.; Walsh, E.M.; Tuszynski, G.P.; Safak, M.; Lazarovici, P.; Marcinkiewicz, C. Integrin alpha9 beta1 is a receptor for nerve growth factor and other neurotrophins. J. Cell Sci. 2008, 121 Pt 4, 504–513. [Google Scholar] [CrossRef]
- Gavazzi, I.; Kumar, R.D.; McMahon, S.B.; Cohen, J. Growth responses of different subpopulations of adult sensory neurons to neurotrophic factors in vitro. Eur. J. Neurosci. 1999, 11, 3405–3414. [Google Scholar] [CrossRef]
- Diamond, J.; Foerster, A.; Holmes, M.; Coughlin, M. Sensory nerves in adult rats regenerate and restore sensory function to the skin independently of endogenous NGF. J. Neurosci. 1992, 12, 1467–1476. [Google Scholar] [CrossRef][Green Version]
- Campenot, R.B. Local control of neurite sprouting in cultured sympathetic neurons by nerve growth factor. Brain Res. 1987, 465, 293–301. [Google Scholar] [CrossRef]
- Kaplan, D.; Zirrgiebel, U.; Atwal, J. Center stage for NGF in peripheral (but not central) sensory neuron outgrowth. Neuron 2000, 25, 253–254. [Google Scholar] [CrossRef][Green Version]
- Moore, K.; MacSween, M.; Shoichet, M. Immobilized concentration gradients of neurotrophic factors guide neurite outgrowth of primary neurons in macroporous scaffolds. Tissue Eng. 2006, 12, 267–278. [Google Scholar] [CrossRef]
- Gundersen, R.W.; Barrett, J.N. Neuronal chemotaxis: Chick dorsal-root axons turn toward high concentrations of nerve growth factor. Science 1979, 206, 1079–1080. [Google Scholar] [CrossRef] [PubMed]
- Parrinello, S.; Napoli, I.; Ribeiro, S.; Wingfield Digby, P.; Fedorova, M.; Parkinson, D.B.; Doddrell, R.D.; Nakayama, M.; Adams, R.H.; Lloyd, A.C. EphB signaling directs peripheral nerve regeneration through Sox2-dependent Schwann cell sorting. Cell 2010, 143, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.Y.; McDonald, D.; Cheng, C.; Magnowski, B.; Durand, J.; Zochodne, D.W. Axon and Schwann cell partnership during nerve regrowth. J. Neuropathol. Exp. Neurol. 2005, 64, 613–622. [Google Scholar] [CrossRef] [PubMed]
- Stepp, M.A.; Tadvalkar, G.; Hakh, R.; Pal-Ghosh, S. Corneal epithelial cells function as surrogate Schwann cells for their sensory nerves. Glia 2017, 65, 851–863. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, D.R.; Hempstead, B.L.; Martin-Zanca, D.; Chao, M.V.; Parada, L.F. The trk proto-oncogene product: A signal transducing receptor for nerve growth factor. Science 1991, 252, 554–558. [Google Scholar] [CrossRef]
- Kaplan, D.R.; Miller, F.D. Neurotrophin signal transduction in the nervous system. Curr. Opin. Neurobiol. 2000, 10, 381–391. [Google Scholar] [CrossRef]
- Miller, F.D.; Kaplan, D.R. On Trk for retrograde signaling. Neuron 2001, 32, 767–770. [Google Scholar] [CrossRef]
- Vanathi, M.; Raj, N.; Kusumesh, R.; Aron, N.; Gupta, N.; Tandon, R. Update on pediatric corneal diseases and keratoplasty. Surv. Ophthalmol. 2022, 67, 1647–1684. [Google Scholar] [CrossRef]
- Yavuz Saricay, L.; Bayraktutar, B.N.; Lilley, J.; Mah, F.S.; Massaro-Giordano, M.; Hamrah, P. Efficacy of Recombinant Human Nerve Growth Factor in Stage 1 Neurotrophic Keratopathy. Ophthalmology 2022, 129, 1448–1450. [Google Scholar] [CrossRef]
- Shoughy, S.S. Topical tacrolimus in anterior segment inflammatory disorders. Eye Vis. 2017, 4, 7. [Google Scholar] [CrossRef]
- Erdinest, N.; Ben-Eli, H.; Solomon, A. Topical tacrolimus for allergic eye diseases. Curr. Opin. Allergy Clin. Immunol. 2019, 19, 535–543. [Google Scholar] [CrossRef] [PubMed]
- Magalhaes, O.A.; Marinho, D.R.; Kwitko, S. Topical 0.03% tacrolimus preventing rejection in high-risk corneal transplantation: A cohort study. Br. J. Ophthalmol. 2013, 97, 1395–1398. [Google Scholar] [CrossRef] [PubMed]
- Lyons, W.E.; George, E.B.; Dawson, T.M.; Steiner, J.P.; Snyder, S.H. Immunosuppressant FK506 promotes neurite outgrowth in cultures of PC12 cells and sensory ganglia. Proc. Natl. Acad. Sci. USA 1994, 91, 3191. [Google Scholar] [CrossRef] [PubMed]
- Gold, B.G.; Densmore, V.; Shou, W.; Matzuk, M.M.; Gordon, H.S. Immunophilin FK506-binding protein 52 (not FK506-binding protein 12) mediates the neurotrophic action of FK506. J. Pharmacol. Exp. Ther. 1999, 289, 1202–1210. [Google Scholar] [PubMed]
- Gold, B.G.; Yew, J.Y.; Zeleny-Pooley, M. The immunosuppressant FK506 increases GAP-43 mRNA levels in axotomized sensory neurons. Neurosci. Lett. 1998, 241, 25–28. [Google Scholar] [CrossRef]
- Steiner, J.P.; Connolly, M.A.; Valentine, H.L.; Hamilton, G.S.; Dawson, T.M.; Hester, L.; Snyder, S.H. Neurotrophic actions of nonimmunosuppressive analogues of immunosuppressive drugs FK506, rapamycin and cyclosporin A. Nat. Med. 1997, 3, 421–428. [Google Scholar] [CrossRef]
- Yang, R.K.; Lowe, J.B., 3rd; Sobol, J.B.; Sen, S.K.; Hunter, D.A.; Mackinnon, S.E. Dose-dependent effects of FK506 on neuroregeneration in a rat model. Plast. Reconstr. Surg. 2003, 112, 1832–1840. [Google Scholar] [CrossRef]
- Iwasaki, K.; Shiraga, T.; Matsuda, H.; Teramura, Y.; Kawamura, A.; Hata, T.; Ninomiya, S.; Esumi, Y. Absorption, Distribution, Metabolism and Excretion of Tacrolimus (FK506) in the Rat. Drug Metab. Pharmacokinet. 1998, 13, 259–265. [Google Scholar] [CrossRef]
- Gold, B.G.; Storm-Dickerson, T.; Austin, D.R. The immunosuppressant FK506 increases functional recovery and nerve regeneration following peripheral nerve injury. Restor. Neurol. Neurosci. 1994, 6, 287–296. [Google Scholar] [CrossRef]
- Sulaiman, O.A.; Voda, J.; Gold, B.G.; Gordon, T. FK506 increases peripheral nerve regeneration after chronic axotomy but not after chronic schwann cell denervation. Exp. Neurol. 2002, 175, 127–137. [Google Scholar] [CrossRef]
- Jo, S.; Pan, D.; Halevi, A.E.; Roh, J.; Schellhardt, L.; Hunter Ra, D.A.; Snyder-Warwick, A.K.; Moore, A.M.; Mackinnon, S.E.; Wood, M.D. Comparing electrical stimulation and tacrolimus (FK506) to enhance treating nerve injuries. Muscle Nerve 2019, 60, 629–636. [Google Scholar] [CrossRef] [PubMed]
- Daneri-Becerra, C.; Patiño-Gaillez, M.G.; Galigniana, M.D. Proof that the high molecular weight immunophilin FKBP52 mediates the in vivo neuroregenerative effect of the macrolide FK506. Biochem. Pharmacol. 2020, 182, 114204. [Google Scholar] [CrossRef] [PubMed]
- Quintá, H.R.; Galigniana, M.D. The neuroregenerative mechanism mediated by the Hsp90-binding immunophilin FKBP52 resembles the early steps of neuronal differentiation. Br. J. Pharmacol. 2012, 166, 637–649. [Google Scholar] [CrossRef] [PubMed]
- Shim, S.; Yuan, J.P.; Kim, J.Y.; Zeng, W.; Huang, G.; Milshteyn, A.; Kern, D.; Muallem, S.; Ming, G.-l.; Worley, P.F. Peptidyl-Prolyl Isomerase FKBP52 Controls Chemotropic Guidance of Neuronal Growth Cones via Regulation of TRPC1 Channel Opening. Neuron 2009, 64, 471–483. [Google Scholar] [CrossRef]
- Udina, E.; Ceballos, D.; Verdú, E.; Gold, B.G.; Navarro, X. Bimodal dose-dependence of FK506 on the rate of axonal regeneration in mouse peripheral nerve. Muscle Nerve 2002, 26, 348–355. [Google Scholar] [CrossRef]
- Gold, B.G.; Katoh, K.; Storm-Dickerson, T. The immunosuppressant FK506 increases the rate of axonal regeneration in rat sciatic nerve. J. Neurosci. Off. J. Soc. Neurosci. 1995, 15, 7509–7516. [Google Scholar] [CrossRef]
- Zuo, K.J.; Shafa, G.; Chan, K.; Zhang, J.; Hawkins, C.; Tajdaran, K.; Gordon, T.; Borschel, G.H. Local FK506 drug delivery enhances nerve regeneration through fresh, unprocessed peripheral nerve allografts. Exp. Neurol. 2021, 341, 113680. [Google Scholar] [CrossRef]
- Tajdaran, K.; Shoichet, M.S.; Gordon, T.; Borschel, G.H. A novel polymeric drug delivery system for localized and sustained release of tacrolimus (FK506). Biotechnol. Bioeng. 2015, 112, 1948–1953. [Google Scholar] [CrossRef]
- Tajdaran, K.; Chan, K.; Shoichet, M.S.; Gordon, T.; Borschel, G.H. Local delivery of FK506 to injured peripheral nerve enhances axon regeneration after surgical nerve repair in rats. Acta Biomater. 2019, 96, 211–221. [Google Scholar] [CrossRef]
- Islam, F.; Westcott, M.; Rees, A.; Robson, A.G.; Kapoor, B.; Holder, G.; Pavesio, C. Safety profile and efficacy of tacrolimus in the treatment of birdshot retinochoroiditis: A retrospective case series review. Br. J. Ophthalmol. 2018, 102, 983–990. [Google Scholar] [CrossRef]
- Ghaffari, R.; Ghassemi, H.; Zarei-Ghanavati, M.; Latifi, G.; Dehghani, S.; Haq, Z.; Djalilian, A.R. Tacrolimus Eye Drops as Adjunct Therapy in Severe Corneal Endothelial Rejection Refractory to Corticosteroids. Cornea 2017, 36, 1195–1199. [Google Scholar] [CrossRef] [PubMed]
- Shoughy, S.S.; Jaroudi, M.O.; Tabbara, K.F. Efficacy and safety of low-dose topical tacrolimus in vernal keratoconjunctivitis. Clin. Ophthalmol. 2016, 10, 643–647. [Google Scholar] [CrossRef] [PubMed]
- Paller, A.S.; Fölster-Holst, R.; Chen, S.C.; Diepgen, T.L.; Elmets, C.; Margolis, D.J.; Pollock, B.H. No evidence of increased cancer incidence in children using topical tacrolimus for atopic dermatitis. J. Am. Acad. Dermatol. 2020, 83, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Bentata, Y. Tacrolimus: 20 years of use in adult kidney transplantation. What we should know about its nephrotoxicity. Artif. Organs 2020, 44, 140–152. [Google Scholar] [CrossRef]
- Cury Martins, J.; Martins, C.; Aoki, V.; Gois, A.F.; Ishii, H.A.; da Silva, E.M. Topical tacrolimus for atopic dermatitis. Cochrane Database Syst. Rev. 2015, 2015, Cd009864. [Google Scholar] [CrossRef]
- Provenzani, A.; Santeusanio, A.; Mathis, E.; Notarbartolo, M.; Labbozzetta, M.; Poma, P.; Provenzani, A.; Polidori, C.; Vizzini, G.; Polidori, P.; et al. Pharmacogenetic considerations for optimizing tacrolimus dosing in liver and kidney transplant patients. World J. Gastroenterol. 2013, 19, 9156–9173. [Google Scholar] [CrossRef]
- Böttiger, Y.; Brattström, C.; Tydén, G.; Säwe, J.; Groth, C.G. Tacrolimus whole blood concentrations correlate closely to side-effects in renal transplant recipients. Br. J. Clin. Pharmacol. 1999, 48, 445–448. [Google Scholar] [CrossRef]
- Luaces-Rodríguez, A.; Touriño-Peralba, R.; Alonso-Rodríguez, I.; García-Otero, X.; González-Barcia, M.; Rodríguez-Ares, M.T.; Martínez-Pérez, L.; Aguiar, P.; Gómez-Lado, N.; Silva-Rodríguez, J.; et al. Preclinical characterization and clinical evaluation of tacrolimus eye drops. Eur. J. Pharm. Sci. 2018, 120, 152–161. [Google Scholar] [CrossRef] [PubMed]
- Terzis, J.K.; Dryer, M.M.; Bodner, B.I. Corneal neurotization: A novel solution to neurotrophic keratopathy. Plast. Reconstr. Surg. 2009, 123, 112–120. [Google Scholar] [CrossRef]
- Elbaz, U.; Bains, R.; Zuker, R.M.; Borschel, G.H.; Ali, A. Restoration of corneal sensation with regional nerve transfers and nerve grafts: A new approach to a difficult problem. JAMA Ophthalmol. 2014, 132, 1289–1295. [Google Scholar] [CrossRef]
- Woo, J.H.; Daeschler, S.C.; Mireskandari, K.; Borschel, G.H.; Ali, A. Minimally Invasive Corneal Neurotization Provides Sensory Function, Protects Against Recurrent Ulceration, and Improves Visual Acuity. Am. J. Ophthalmol. 2022, 241, 179–189. [Google Scholar] [CrossRef]
- Swanson, M.A.; Swanson, R.D.; Kotha, V.S.; Cai, Y.; Clark, R.; Jin, A.; Kumar, A.R.; Davidson, E.H. Corneal Neurotization: A Meta-analysis of Outcomes and Patient Selection Factors. Ann. Plast. Surg. 2022, 88, 687–694. [Google Scholar] [CrossRef] [PubMed]
- Jowett, N.; Pineda Ii, R. Acellular nerve allografts in corneal neurotisation: An inappropriate choice. Br. J. Ophthalmol. 2020, 104, 149–150. [Google Scholar] [CrossRef]
- Ebner, R.; Fridrich, G.; Socolovsky, M.; Luna, A.; Croxatto, J.O. In Vivo Corneal Confocal Microscopy: Pre- and Post-operative Evaluation in a Case of Corneal Neurotization. Neuroophthalmology 2020, 44, 193–196. [Google Scholar] [CrossRef] [PubMed]
- Meier, C.; Parmantier, E.; Brennan, A.; Mirsky, R.; Jessen, K.R. Developing Schwann cells acquire the ability to survive without axons by establishing an autocrine circuit involving insulin-like growth factor, neurotrophin-3, and platelet-derived growth factor-BB. J. Neurosci. 1999, 19, 3847–3859. [Google Scholar] [CrossRef] [PubMed][Green Version]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feinberg, K.; Tajdaran, K.; Mirmoeini, K.; Daeschler, S.C.; Henriquez, M.A.; Stevens, K.E.; Mulenga, C.M.; Hussain, A.; Hamrah, P.; Ali, A.; et al. The Role of Sensory Innervation in Homeostatic and Injury-Induced Corneal Epithelial Renewal. Int. J. Mol. Sci. 2023, 24, 12615. https://doi.org/10.3390/ijms241612615
Feinberg K, Tajdaran K, Mirmoeini K, Daeschler SC, Henriquez MA, Stevens KE, Mulenga CM, Hussain A, Hamrah P, Ali A, et al. The Role of Sensory Innervation in Homeostatic and Injury-Induced Corneal Epithelial Renewal. International Journal of Molecular Sciences. 2023; 24(16):12615. https://doi.org/10.3390/ijms241612615
Chicago/Turabian StyleFeinberg, Konstantin, Kiana Tajdaran, Kaveh Mirmoeini, Simeon C. Daeschler, Mario A. Henriquez, Katelyn E. Stevens, Chilando M. Mulenga, Arif Hussain, Pedram Hamrah, Asim Ali, and et al. 2023. "The Role of Sensory Innervation in Homeostatic and Injury-Induced Corneal Epithelial Renewal" International Journal of Molecular Sciences 24, no. 16: 12615. https://doi.org/10.3390/ijms241612615
APA StyleFeinberg, K., Tajdaran, K., Mirmoeini, K., Daeschler, S. C., Henriquez, M. A., Stevens, K. E., Mulenga, C. M., Hussain, A., Hamrah, P., Ali, A., Gordon, T., & Borschel, G. H. (2023). The Role of Sensory Innervation in Homeostatic and Injury-Induced Corneal Epithelial Renewal. International Journal of Molecular Sciences, 24(16), 12615. https://doi.org/10.3390/ijms241612615





