S-Adenosylhomocysteine Is a Useful Metabolic Factor in the Early Prediction of Septic Disease Progression and Death in Critically Ill Patients: A Prospective Cohort Study
Abstract
1. Introduction
2. Results
2.1. Enrollment and Grouping of Patients by Time Course of Severity of Illness
2.2. Baseline and Clinical Characteristics of Study Participants
2.3. Biomarkers of Energetic Failure at Baseline
2.4. Prediction of Progression of Disease and Mortality in Univariate and Multiple Logistic Regression Analyses
3. Discussion
Study Limitations
4. Materials and Methods
4.1. Ethics
4.2. Study Population
4.3. Collection of Data and Blood Sampling
4.4. Determination of Plasma Concentrations of SAH and SAM
4.5. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pool, R.; Gomez, H.; Kellum, J.A. Mechanisms of Organ Dysfunction in Sepsis. Crit. Care Clin. 2018, 34, 63–80. [Google Scholar] [CrossRef] [PubMed]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Arina, P.; Singer, M. Pathophysiology of sepsis. Curr. Opin. Anaesthesiol. 2021, 34, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Caraballo, C.; Jaimes, F. Organ Dysfunction in Sepsis: An Ominous Trajectory from Infection to Death. Yale J. Biol. Med. 2019, 92, 629–640. [Google Scholar] [PubMed]
- Singer, M. The role of mitochondrial dysfunction in sepsis-induced multi-organ failure. Virulence 2014, 5, 66–72. [Google Scholar] [CrossRef]
- Fink, M.P. Bench-to-bedside review: Cytopathic hypoxia. Crit. Care 2002, 6, 491–499. [Google Scholar] [CrossRef]
- Singer, M. Critical illness and flat batteries. Crit. Care 2017, 21 (Suppl. S3), 309. [Google Scholar] [CrossRef]
- Ryoo, S.M.; Lee, J.; Lee, Y.S.; Lee, J.H.; Lim, K.S.; Huh, J.W.; Hong, S.B.; Lim, C.M.; Koh, Y.; Kim, W.Y. Lactate Level versus Lactate Clearance for Predicting Mortality in Patients with Septic Shock Defined by Sepsis-3. Crit. Care Med. 2018, 46, e489–e495. [Google Scholar] [CrossRef]
- Lee, S.G.; Song, J.; Park, D.W.; Moon, S.; Cho, H.J.; Kim, J.Y.; Park, J.; Cha, J.H. Prognostic value of lactate levels and lactate clearance in sepsis and septic shock with initial hyperlactatemia: A retrospective cohort study according to the Sepsis-3 definitions. Medicine 2021, 100, e24835. [Google Scholar] [CrossRef]
- Rhodes, A.; Evans, L.E.; Alhazzani, W.; Levy, M.M.; Antonelli, M.; Ferrer, R.; Kumar, A.; Sevransky, J.E.; Sprung, C.L.; Nunnally, M.E.; et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Intensive Care Med. 2017, 43, 304–377. [Google Scholar] [CrossRef]
- Hernández, G.; Ospina-Tascón, G.A.; Damiani, L.P.; Estenssoro, E.; Dubin, A.; Hurtado, J.; Friedman, G.; Castro, R.; Alegría, L.; Teboul, J.L.; et al. Effect of a Resuscitation Strategy Targeting Peripheral Perfusion Status vs. Serum Lactate Levels on 28-Day Mortality among Patients with Septic Shock: The ANDROMEDA-SHOCK Randomized Clinical Trial. JAMA 2019, 321, 654–664. [Google Scholar] [CrossRef]
- Levy, B.; Desebbe, O.; Montemont, C.; Gibot, S. Increased aerobic glycolysis through beta2 stimulation is a common mechanism involved in lactate formation during shock states. Shock 2008, 30, 417–421. [Google Scholar] [CrossRef]
- Alamdari, N.; Constantin-Teodosiu, D.; Murton, A.J.; Gardiner, S.M.; Bennett, T.; Layfield, R.; Greenhaff, P.L. Temporal changes in the involvement of pyruvate dehydrogenase complex in muscle lactate accumulation during lipopolysaccharide infusion in rats. J. Physiol. 2008, 586, 1767–1775. [Google Scholar] [CrossRef] [PubMed]
- Sterling, S.A.; Puskarich, M.A.; Jones, A.E. The effect of liver disease on lactate normalization in severe sepsis and septic shock: A cohort study. Clin. Exp. Emerg. Med. 2015, 2, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Wittayachamnankul, B.; Chentanakij, B.; Sruamsiri, K.; Chattipakorn, N. The role of central venous oxygen saturation, blood lactate, and central venous-to-arterial carbon dioxide partial pressure difference as a goal and prognosis of sepsis treatment. J. Crit. Care 2016, 36, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Hölle, T.; Rehn, P.; Leventogiannis, K.; Kotsaki, A.; Kanni, T.; Antonakos, N.; Psarrakis, C.; Damoraki, G.; Schenz, J.; Schmitt, F.C.F.; et al. Evaluation of the Novel Sepsis Biomarker Host-Derived Delta-like Canonical Notch Ligand 1—A Secondary Analysis of 405 Patients Suffering from Inflammatory or Infectious Diseases. Int. J. Mol. Sci. 2023, 24, 9164. [Google Scholar] [CrossRef]
- Semmler, A.; Prost, J.C.; Smulders, Y.; Smith, D.; Blom, H.; Bigler, L.; Linnebank, M. Methylation metabolism in sepsis and systemic inflammatory response syndrome. Scand. J. Clin. Lab. Investig. 2013, 73, 368–372. [Google Scholar] [CrossRef]
- Wexler, O.; Gough, M.S.; Morgan, M.A.M.; Mack, C.M.; Apostolakos, M.J.; Doolin, K.P.; Mooney, R.A.; Arning, E.; Bottiglieri, T.; Pietropaoli, A.P. Methionine Metabolites in Patients with Sepsis. J. Intensive Care Med. 2018, 33, 37–47. [Google Scholar] [CrossRef]
- Deussen, A.; Borst, M.; Kroll, K.; Schrader, J. Formation of S-adenosylhomocysteine in the heart. II: A sensitive index for regional myocardial underperfusion. Circ. Res. 1988, 63, 250–261. [Google Scholar] [CrossRef]
- Schrader, J.; Deussen, A.; Smolenski, R.T. Adenosine is a sensitive oxygen sensor in the heart. Experientia 1990, 46, 1172–1175. [Google Scholar] [CrossRef]
- Deussen, A.; Borst, M.; Schrader, J. Formation of S-adenosylhomocysteine in the heart. I: An index of free intracellular adenosine. Circ. Res. 1988, 63, 240–249. [Google Scholar] [CrossRef] [PubMed]
- Zappia, V.; Zydek-Cwick, R.; Schlenk, F. The specificity of S-adenosylmethionine derivatives in methyl transfer reactions. J. Biol. Chem. 1969, 244, 4499–4509. [Google Scholar] [CrossRef] [PubMed]
- Melnyk, S.; Pogribna, M.; Pogribny, I.; Yi, P.; James, S. Measurement of plasma and intracellular S-adenosylmethionine and S-adenosylhomocysteine utilizing coulometric electrochemical detection: Alterations with plasma homocysteine and pyridoxal 5′-phosphate concentrations. Clin. Chem. 2000, 46, 265–272. [Google Scholar] [CrossRef]
- Rudd, K.E.; Johnson, S.C.; Agesa, K.M.; Shackelford, K.A.; Tsoi, D.; Kievlan, D.R.; Colombara, D.V.; Ikuta, K.S.; Kissoon, N.; Finfer, S.; et al. Global, regional, and national sepsis incidence and mortality, 1990–2017: Analysis for the Global Burden of Disease Study. Lancet 2020, 395, 200–211. [Google Scholar] [CrossRef]
- Fleischmann-Struzek, C.; Mellhammar, L.; Rose, N.; Cassini, A.; Rudd, K.E.; Schlattmann, P.; Allegranzi, B.; Reinhart, K. Incidence and mortality of hospital- and ICU-treated sepsis: Results from an updated and expanded systematic review and meta-analysis. Intensive Care Med. 2020, 46, 1552–1562. [Google Scholar] [CrossRef] [PubMed]
- Marx, G. Incidence of severe sepsis and septic shock in German intensive care units: The prospective, multicentre INSEP study. Intensive Care Med. 2016, 42, 1980–1989. [Google Scholar] [CrossRef]
- Bauer, M.; Gerlach, H.; Vogelmann, T.; Preissing, F.; Stiefel, J.; Adam, D. Mortality in sepsis and septic shock in Europe, North America and Australia between 2009 and 2019—Results from a systematic review and meta-analysis. Crit. Care 2020, 24, 239. [Google Scholar] [CrossRef]
- Russo, A.; Pallone, R.; Trecarichi, E.M.; Torti, C. Lights and Shadows of Sepsis Management: Challenges and Future Perspectives. Int. J. Mol. Sci. 2023, 24, 9426. [Google Scholar] [CrossRef]
- Evans, L.; Rhodes, A.; Alhazzani, W.; Antonelli, M.; Coopersmith, C.M.; French, C.; Machado, F.R.; McIntyre, L.; Ostermann, M.; Prescott, H.C.; et al. Surviving sepsis campaign: International guidelines for management of sepsis and septic shock 2021. Intensive Care Med. 2021, 47, 1181–1247. [Google Scholar] [CrossRef]
- Gattinoni, L.; Vasques, F.; Camporota, L.; Meessen, J.; Romitti, F.; Pasticci, I.; Duscio, E.; Vassalli, F.; Forni, L.G.; Payen, D.; et al. Understanding Lactatemia in Human Sepsis. Potential Impact for Early Management. Am. J. Respir. Crit. Care Med. 2019, 200, 582–589. [Google Scholar] [CrossRef]
- Kailing, L.L.; Bertinetti, D.; Herberg, F.W.; Pavlidis, I.V. A coupled photometric assay for characterization of S-adenosyl-l-homocysteine hydrolases in the physiological hydrolytic direction. New Biotechnol. 2017, 39 Pt A, 11–17. [Google Scholar] [CrossRef]
- Finkelstein, J.D. Metabolic regulatory properties of S-adenosylmethionine and S-adenosylhomocysteine. Clin. Chem. Lab. Med. 2007, 45, 1694–1699. [Google Scholar] [CrossRef] [PubMed]
- Kloor, D.; Delabar, U.; Mühlbauer, B.; Luippold, G.; Osswald, H. Tissue levels of S-adenosylhomocysteine in the rat kidney: Effects of ischemia and homocysteine. Biochem. Pharmacol. 2002, 63, 809–815. [Google Scholar] [CrossRef] [PubMed]
- Rangel-Frausto, M.S.; Pittet, D.; Costigan, M.; Hwang, T.; Davis, C.S.; Wenzel, R.P. The natural history of the systemic inflammatory response syndrome (SIRS): A prospective study. JAMA 1995, 273, 117–123. [Google Scholar] [CrossRef]
- Barichello, T.; Generoso, J.S.; Singer, M.; Dal-Pizzol, F. Biomarkers for sepsis: More than just fever and leukocytosis—A narrative review. Crit. Care 2022, 26, 14. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Ko, E.R.; Gilchrist, J.J.; Pittman, K.J.; Rautanen, A.; Pirinen, M.; Thompson, J.W.; Dubois, L.G.; Langley, R.J.; Jaslow, S.L.; et al. Human genetic and metabolite variation reveals that methylthioadenosine is a prognostic biomarker and an inflammatory regulator in sepsis. Sci. Adv. 2017, 3, e1602096. [Google Scholar] [CrossRef]
- Lindner, H.A.; Schamoni, S.; Kirschning, T.; Worm, C.; Hahn, B.; Centner, F.S.; Schoettler, J.J.; Hagmann, M.; Krebs, J.; Mangold, D.; et al. Ground truth labels challenge the validity of sepsis consensus definitions in critical illness. J. Transl. Med. 2022, 20, 27. [Google Scholar] [CrossRef]
- Bone, R.C.; Balk, R.A.; Cerra, F.B.; Dellinger, R.P.; Fein, A.M.; Knaus, W.A.; Schein, R.M.; Sibbald, W.J. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest 1992, 101, 1644–1655. [Google Scholar] [CrossRef]
- Dellinger, R.P.; Levy, M.M.; Rhodes, A.; Annane, D.; Gerlach, H.; Opal, S.M.; Sevransky, J.E.; Sprung, C.L.; Douglas, I.S.; Jaeschke, R. Surviving Sepsis Campaign: International guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med. 2013, 39, 165–228. [Google Scholar] [CrossRef]
- Levy, M.M.; Fink, M.P.; Marshall, J.C.; Abraham, E.; Angus, D.; Cook, D.; Cohen, J.; Opal, S.M.; Vincent, J.-L.; Ramsay, G. 2001 sccm/esicm/accp/ats/sis international sepsis definitions conference. Intensive Care Med. 2003, 29, 530–538. [Google Scholar] [CrossRef]
- Centner, F.S.; Oster, M.E.; Dally, F.J.; Sauter-Servaes, J.; Pelzer, T.; Schoettler, J.J.; Hahn, B.; Fairley, A.M.; Abdulazim, A.; Hackenberg, K.A.M.; et al. Comparative Analyses of the Impact of Different Criteria for Sepsis Diagnosis on Outcome in Patients with Spontaneous Subarachnoid Hemorrhage. J. Clin. Med. 2022, 11, 3873. [Google Scholar] [CrossRef]
- Centner, F.S.; Schoettler, J.J.; Fairley, A.M.; Lindner, H.A.; Schneider-Lindner, V.; Weiss, C.; Thiel, M.; Hagmann, M. Impact of different consensus definition criteria on sepsis diagnosis in a cohort of critically ill patients-Insights from a new mathematical probabilistic approach to mortality-based validation of sepsis criteria. PLoS ONE 2020, 15, e0238548. [Google Scholar] [CrossRef]
- Gellekink, H.; van Oppenraaij-Emmerzaal, D.; van Rooij, A.; Struys, E.A.; den Heijer, M.; Blom, H.J. Stable-isotope dilution liquid chromatography-electrospray injection tandem mass spectrometry method for fast, selective measurement of S-adenosylmethionine and S-adenosylhomocysteine in plasma. Clin. Chem. 2005, 51, 1487–1492. [Google Scholar] [CrossRef]
- Moreno, R.; Vincent, J.-L.; Matos, R.; Mendonca, A.; Cantraine, F.; Thijs, L.; Takala, J.; Sprung, C.; Antonelli, M.; Bruining, H. The use of maximum SOFA score to quantify organ dysfunction/failure in intensive care. Results of a prospective, multicentre study. Intensive Care Med. 1999, 25, 686–696. [Google Scholar] [CrossRef]
- Le Gall, J.R.; Lemeshow, S.; Saulnier, F. A new Simplified Acute Physiology Score (SAPS II) based on a European/North American multicenter study. JAMA 1993, 270, 2957–2963. [Google Scholar] [CrossRef]
- Hosmer, D.W.; Lemeshow, S. Applied Logistic Regression, 2nd ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2000; p. 177. [Google Scholar]

| All (N = 99) | Groups by Sepsis-1/2 Definition * | Groups by Sepsis-3 Definition ** | Groups by In-Hospital Survival | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| G121 (N = 67) | G122 (N = 26) | G31 (N = 39) | G32 (N = 27) | Survivor, S (N = 75) | Non-Survivor, NS (N = 24) | ||||||||||||
| n | n | n | G121 vs. G122 | n | n | G31 vs. G32 | n | n | S vs. NS | ||||||||
| Demographics | |||||||||||||||||
| Age (years) | 99 | 63 (53–76) | 67 | 62 (53–76) | 26 | 63 (53–78) | ns | 39 | 61 (49–73) | 27 | 56 (49–73) | ns | 75 | 61 (49–74) | 24 | 73 (60–79) | 0.002 |
| Male | 65 (66) | 43 (64) | 18 (69) | ns | 23 (59) | 24 (89) | 0.012 | 46 (61) | 19 (79) | ns | |||||||
| Clinical Course | |||||||||||||||||
| Pressor therapy | 68 (69) | 41 (61) | 22 (85) | 0.037 | 23 (59) | 24 (89) | 0.013 | 48 (64) | 20 (83) | ns | |||||||
| ICU-LOS (days) | 99 | 25 (16–47) | 67 | 27 (16–46) | 26 | 23 (18–69) | ns | 39 | 25 (16–39) | 27 | 37 (18–60) | ns | 75 | 27 (18–53) | 24 | 19 (13–34) | 0.028 |
| In-hospital mortality | 24 (24) | 8 (12) | 11 (42) | 0.001 | 4 (10) | 9 (33) | 0.029 | ||||||||||
| Clinical chemistry at baseline | |||||||||||||||||
| Creatinine (mg/dL) | 96 | 0.98 (0.73–1.50) | 64 | 0.88 (0.68–1.29) | 26 | 1.19 (0.79–1.68) | ns | 38 | 0.80 (0.66–1.22) | 27 | 1.10 (0.75–1.38) | ns | 72 | 0.88 (0.68–1.26) | 24 | 1.72 (1.05–2.72) | 0.003 |
| Urea (mg/dL) | 96 | 45.1 (33.4–63.8) | 64 | 41.9 (29.3–58.1) | 26 | 51.4 (41.8–76.8) | 0.015 | 38 | 37.5 (27.1–56.1) | 27 | 45.2 (31.7–55.4) | ns | 72 | 42.2 (30.8–55.8) | 24 | 60.1 (43.7–81.7) | 0.007 |
| CRP (mg/dL) | 96 | 150 (90–218) | 64 | 132 (80–176) | 26 | 221 (141–253) | 0.001 | 38 | 124 (59–171) | 27 | 150 (92–244) | ns | 72 | 152 (91–220) | 24 | 146 (86–216) | ns |
| K+ (mmol/L) | 98 | 4.1 (3.8–4.3) | 66 | 4.0 (3.8–4.2) | 26 | 4.3 (4.1–4.5) | 0.012 | 38 | 4.0 (3.8–4.2) | 27 | 4.2 (4.0–4.4) | ns | 74 | 4.0 (3.8–4.2) | 24 | 4.3 (4.1–4.5) | 0.041 |
| Vital signs at baseline | |||||||||||||||||
| Temperature (°C) | 96 | 37.1 (36.8–37.7) | 65 | 37.0 (36.6–37.6) | 25 | 37.4 (37.0–37.9) | 0.016 | 37 | 37.1 (36.8–37.5) | 27 | 37.4 (37.1–37.9) | 0.006 | 72 | 37.1 (36.8–37.7) | 24 | 37.1 (36.8–37.6) | ns |
| Resp. rate (1/min) | 98 | 19 (16–22) | 66 | 18 (15–22) | 26 | 20 (18–22) | ns | 38 | 17 (15–20) | 27 | 21 (18–24) | 0.006 | 74 | 19 (16–22) | 24 | 20 (16–25) | ns |
| Shock index | 98 | 0.70 (0.61–0.88) | 66 | 0.68 (0.58–0.85) | 26 | 0.79 (0.62–0.90) | 0.039 | 38 | 0.68 (0.59–0.86) | 27 | 0.82 (0.65–0.93) | 0.024 | 74 | 0.71 (0.60–0.87) | 24 | 0.69 (0.62–0.95) | ns |
| SpO2 (%) | 98 | 98 (95–100) | 66 | 99 (96–100) | 26 | 97 (96–99) | ns | 38 | 99 (97–100) | 27 | 97 (96–99) | 0.035 | 74 | 99 (96–100) | 24 | 97 (95–98) | ns |
| Horovitz (mmHg) | 98 | 280 (220–343) | 66 | 302 (234–362) | 26 | 235 (199–320) | 0.010 | 38 | 303 (244–362) | 27 | 266 (220–326) | ns | 74 | 301 (229–347) | 24 | 218 (185–298) | ns |
| Clinical scores at baseline | |||||||||||||||||
| SAPS II | 98 | 35 (28–43) | 66 | 33 (25–40) | 26 | 37 (32–45) | 0.039 | 38 | 33 (25–39) | 27 | 33 (28–38) | ns | 74 | 33 (25–39) | 24 | 42 (36–51) | <0.001 |
| SOFA | 99 | 8 (5–11) | 67 | 7 (5–10) | 26 | 9 (7–13) | 0.024 | 39 | 8 (5–11) | 27 | 11 (8–13) | 0.004 | 75 | 8 (5–10) | 24 | 11 (7–13) | 0.013 |
| Groups by Sepsis-1/2 Definition | Groups by Sepsis-3 Definition | Groups by In-Hospital Survival | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| G121 (N = 67) | G122 (N = 26) | G31 (N = 39) | G32 (N = 27) | Survivor (S) (N = 75) | Non-Survivor (NS) (N = 24) | ||||||||||
| n | n | G121 vs. G122 | n | n | G31 vs. G32 | n | n | S vs. NS | |||||||
| Lactate (mmol/L) | 66 | 0.9 (0.7–1.5) | 26 | 1.1 (0.8–1.6) | ns | 38 | 1.0 (0.8–1.6) | 27 | 0.9 (0.6–1.5) | ns | 74 | 0.9 (0.7–1.5) | 24 | 1.1 (0.9–1.7) | 0.045 |
| SAM (nmol/L) | 66 | 161 (123–238) | 23 | 195.3 (130–281) | ns | 38 | 155 (121–238) | 26 | 170 (130–230) | ns | 72 | 158 (123–234) | 23 | 207 (148–310) | 0.019 |
| SAH (nmol/L) | 67 | 18.5 (12.4–33.7) | 26 | 29.2 (16.5–58.2) | 0.041 | 39 | 18.5 (11.8–33.7) | 27 | 18.0 (13.4–43.2) | ns | 75 | 18.6 (13.4–32.0) | 24 | 47.6 (23.4–91.3) | <0.001 |
| SAM/SAH | 66 | 9.2 (5.0–14.5) | 23 | 8.0 (3.6–12.6) | ns | 38 | 8.4 (4.0–14.5) | 26 | 8.0 (5.2–13.7) | ns | 72 | 8.9 (5.1–14.3) | 23 | 4.9 (2.8–9.5) | 0.026 |
| SAM/Crea | 63 | 165 (109–236) | 23 | 151 (116–295) | ns | 37 | 169 (118–240) | 26 | 161 (109–210) | ns | 69 | 165 (114–240) | 23 | 138 (78–235) | ns |
| SAH/Crea | 64 | 20.4 (15.4–31.3) | 26 | 29.0 (17.1–36.1) | ns | 38 | 20.0 (15.4–31.6) | 27 | 20.8 (13.7–31.8) | ns | 72 | 20.7 (15.6–31.6) | 24 | 31.7 (17.7–40.8) | ns |
| Groups by Sepsis-1/2 Definition | Groups by Sepsis-3 Definition | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| G121 + G122 (N = 93) | G123 (N = 6) | G31 + G32 (N = 66) | G33 (N = 29) | G33 + G34 (N = 33) | |||||||||
| n | n | G121 + G122 vs. G123 | n | n | n | G31 + G32 vs. G33 | G31 + G32 vs. G33 + G34 | ||||||
| Lactate (mmol/L) | 92 | 0.9 (0.7–1.6) | 6 | 1.3 (1.1–2.2) | ns | 65 | 0.9 (0.7–1.5) | 29 | 0.9 (0.7–1.2) | 33 | 1.0 (0.7–1.6) | ns | ns |
| SAM (nmol/L) | 89 | 174 (127–257) | 6 | 197 (168–263) | ns | 64 | 158 (127–238) | 28 | 208 (144–334) | 31 | 197 (140–331) | 0.043 | ns |
| SAH (nmol/L) | 93 | 20.2 (13.5–43.2) | 6 | 72.9 (52.1–135.0) | 0.001 | 66 | 18.3 (12.4–42.5) | 29 | 29.0 (20.0–77.2) | 33 | 29.7 (20.5–77.2) | 0.005 | 0.003 |
| SAM/SAH | 89 | 8.8 (4.9–13.8) | 6 | 2.8 (2.3–3.9) | 0.003 | 64 | 8.0 (4.5–14.3) | 28 | 8.8 (3.7–11.0) | 31 | 8.8 (2.8–10.1) | ns | ns |
| SAM/Crea | 86 | 165 (114–240) | 6 | 93 (69–163) | ns | 63 | 165 (114–229) | 26 | 164 (69–341) | 29 | 153 (75–292) | ns | ns |
| SAH/Crea | 90 | 21.4 (15.4–35.3) | 6 | 44.9 (26.9–57.9) | 0.008 | 65 | 20.6 (15.4–31.7) | 27 | 30.1 (17.4–39.4) | 31 | 30.1 (17.4–39.5) | 0.022 | 0.011 |
| Sepsis-1/2 Definition | Sepsis-3 Definition | ||
|---|---|---|---|
| Variable | p-Value | Variable | p-Value |
| CRP (mg/L) | 0.002 | SOFA | 0.006 |
| Urea (mg/dL) | 0.011 | Temperature (°C) | 0.010 |
| Potassium (mmol/L) | 0.017 | Gender | 0.013 |
| SOFA | 0.018 | Vasopressor therapy | 0.018 |
| SAH (nmol/L) | 0.019 | Shock index | 0.028 |
| Temperature (°C) | 0.024 | Respiratory rate (1/min) | 0.029 |
| Horovitz index (mmHg) | 0.025 | CRP (mg/L) | 0.041 |
| Shock index | 0.028 | ||
| SAPS II | 0.041 | ||
| Vasopressor therapy | 0.044 | ||
| Variable | p-Value |
|---|---|
| SAPS II | <0.001 |
| SAH (nmol/L) | <0.001 |
| Urea (mg/dL) | 0.002 |
| Creatinine (mg/dL) | 0.005 |
| SOFA | 0.008 |
| Acidosis (pH < 7.35) | 0.010 |
| Age (years) | 0.013 |
| Potassium (mmol/L) | 0.026 |
| Standard base excess (mmol/L) | 0.029 |
| Standard bicarbonate (mmol/L) | 0.030 |
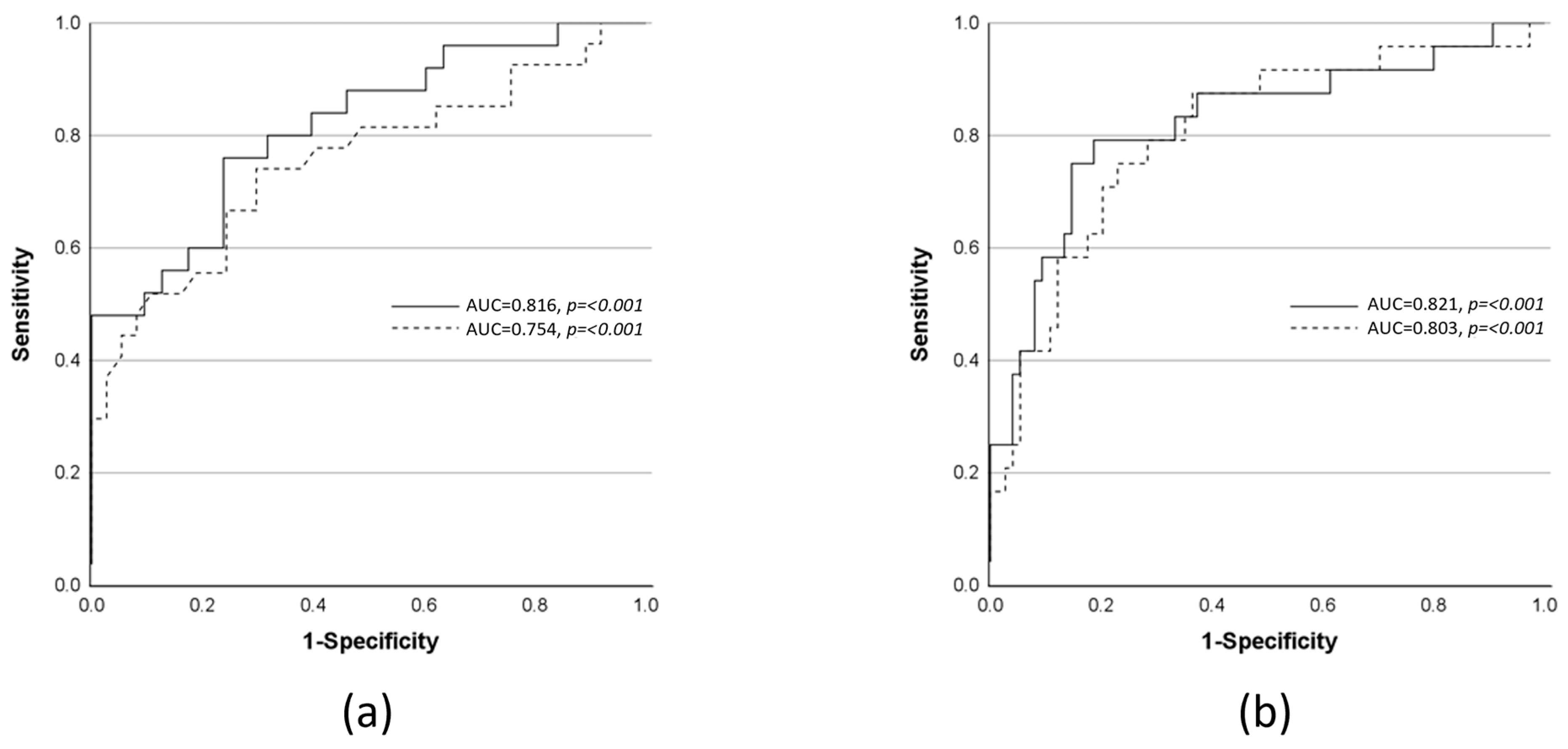
| Predicted Outcome | N | AUROC (p-Value) | TP | FP | FN | TN | Sensitivity (%) (95% CI) | Specificity (%) (95% CI) | Diagnostic OR (95% CI) | Accuracy (%) | Model Parameters | OR (95% CI) | p-Value of OR |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Progression from SIRS or sepsis to severe sepsis or septic shock (sepsis-1/2 definition) | 88 | 0.816 (<0.001) | 19 | 15 | 6 | 48 | 76 (57–89) | 76 (64–85) | 10.13 (3.42–30.01) | 76.1 | CRP | 1.01 (1.00–1.02) | 0.005 |
| Temperature | 4.01 (1.48–10.86) | 0.006 | |||||||||||
| K+ | 5.75 (1.31–25.21) | 0.020 | |||||||||||
| SAH | 1.02 (1.00–1.04) | 0.033 | |||||||||||
| Progression from non-sepsis to sepsis or septic shock (sepsis-3 definition) | 64 | 0.754 (<0.001) | 20 | 11 | 7 | 26 | 74 (55–89) | 70 (54–83) | 6.75 (2.22–20.55) | 71.9 | SOFA | 1.24 (1.04–1.48) | 0.018 |
| Temperature | 3.37 (1.23–9.27) | 0.019 | |||||||||||
| Progression to death (SAPS II-based model) | 98 | 0.803 (<0.001) | 18 | 17 | 6 | 57 | 75 (55–88) | 77 (66–85) | 10.06 (3.45–29.35) | 76.5 | SAPS II | 1.08 (1.01–1.15) | 0.021 |
| SAH | 1.02 (1.00–1.04) | 0.030 | |||||||||||
| Progression to death (SOFA-based model) | 99 | 0.821 (<0.001) | 19 | 14 | 5 | 61 | 79 (60–91) | 81 (71–89) | 16.56 (5.28–51.96) | 80.8 | SOFA | 1.25 (1.05–1.50) | 0.015 |
| SAH | 1.02 (1.00–1.04) | 0.014 | |||||||||||
| Age | 1.06 (1.01–1.11) | 0.011 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Centner, F.-S.; Schoettler, J.J.; Brohm, K.; Mindt, S.; Jäger, E.; Hahn, B.; Fuderer, T.; Lindner, H.A.; Schneider-Lindner, V.; Krebs, J.; et al. S-Adenosylhomocysteine Is a Useful Metabolic Factor in the Early Prediction of Septic Disease Progression and Death in Critically Ill Patients: A Prospective Cohort Study. Int. J. Mol. Sci. 2023, 24, 12600. https://doi.org/10.3390/ijms241612600
Centner F-S, Schoettler JJ, Brohm K, Mindt S, Jäger E, Hahn B, Fuderer T, Lindner HA, Schneider-Lindner V, Krebs J, et al. S-Adenosylhomocysteine Is a Useful Metabolic Factor in the Early Prediction of Septic Disease Progression and Death in Critically Ill Patients: A Prospective Cohort Study. International Journal of Molecular Sciences. 2023; 24(16):12600. https://doi.org/10.3390/ijms241612600
Chicago/Turabian StyleCentner, Franz-Simon, Jochen J. Schoettler, Kathrin Brohm, Sonani Mindt, Evelyn Jäger, Bianka Hahn, Tanja Fuderer, Holger A. Lindner, Verena Schneider-Lindner, Joerg Krebs, and et al. 2023. "S-Adenosylhomocysteine Is a Useful Metabolic Factor in the Early Prediction of Septic Disease Progression and Death in Critically Ill Patients: A Prospective Cohort Study" International Journal of Molecular Sciences 24, no. 16: 12600. https://doi.org/10.3390/ijms241612600
APA StyleCentner, F.-S., Schoettler, J. J., Brohm, K., Mindt, S., Jäger, E., Hahn, B., Fuderer, T., Lindner, H. A., Schneider-Lindner, V., Krebs, J., Neumaier, M., & Thiel, M. (2023). S-Adenosylhomocysteine Is a Useful Metabolic Factor in the Early Prediction of Septic Disease Progression and Death in Critically Ill Patients: A Prospective Cohort Study. International Journal of Molecular Sciences, 24(16), 12600. https://doi.org/10.3390/ijms241612600






