Catalyst Design through Grafting of Diazonium Salts—A Critical Review on Catalyst Stability
Abstract
1. Introduction
2. Diazonium Salts
3. Different Approaches to Catalyst Production via Diazonium Salt Chemistry
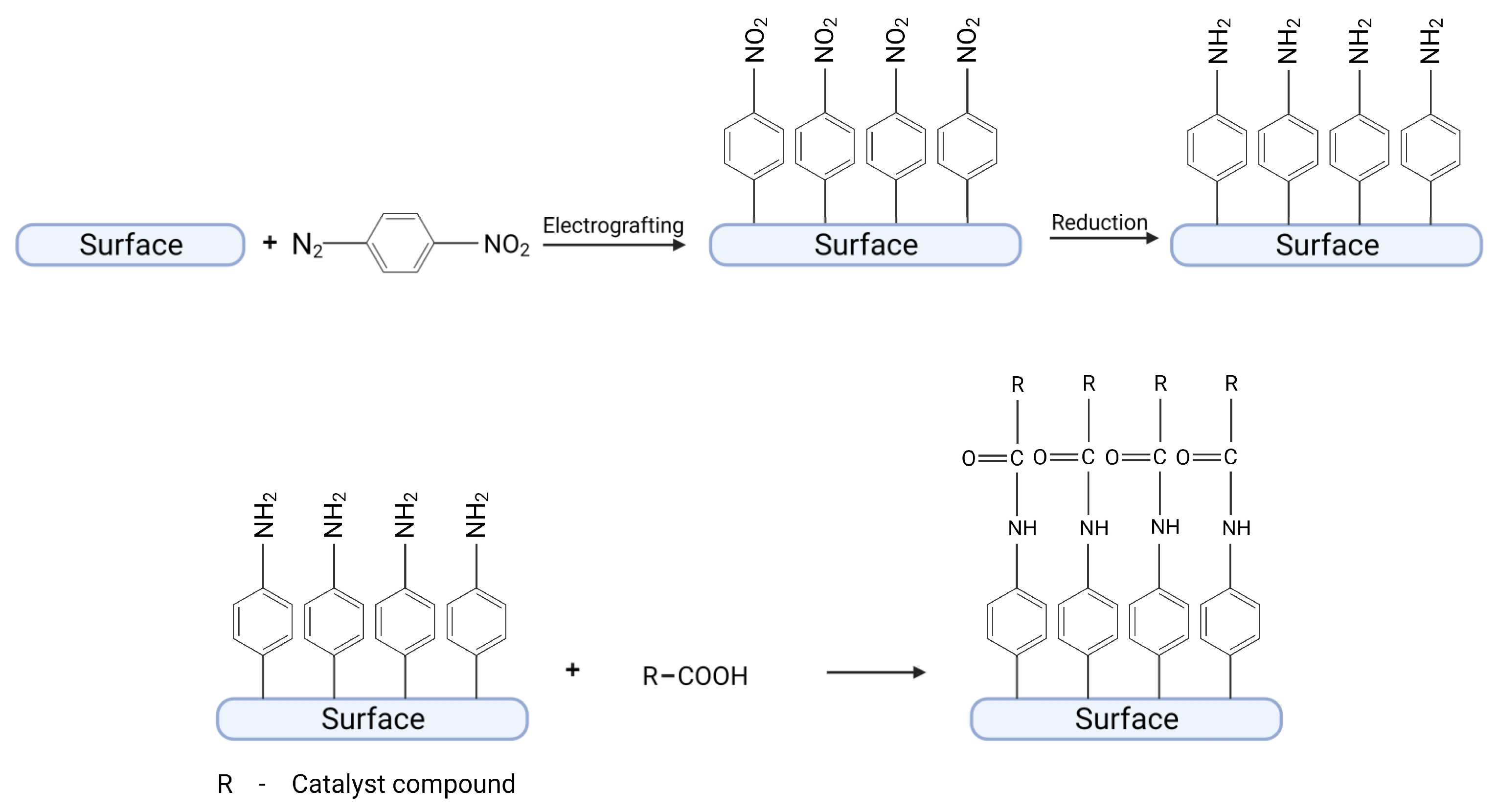
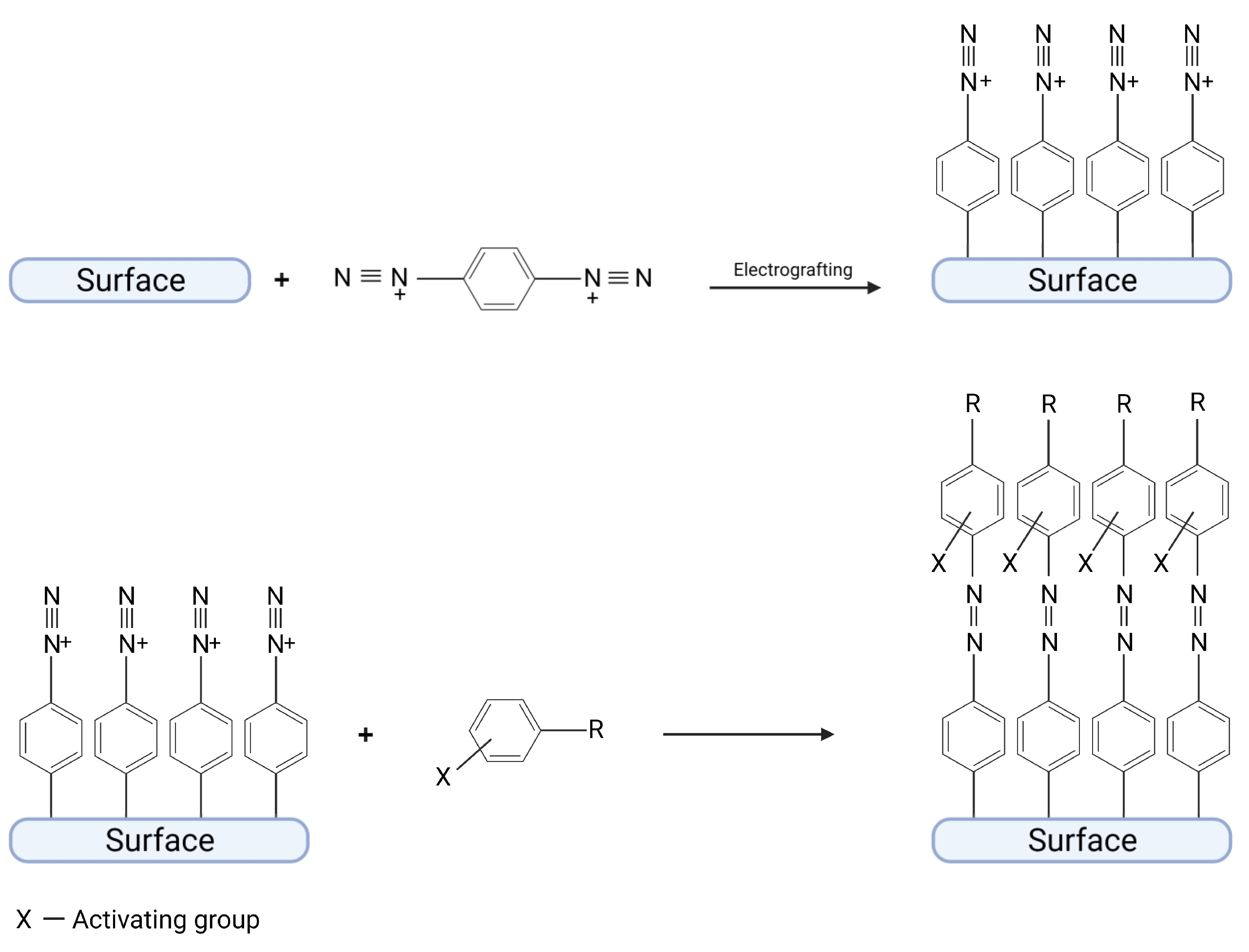
3.1. Immobilization of Catalysts by a Chemical Reaction with a Diazonium Moiety
3.2. Diazonium Salts and Nanoparticles as Catalysts
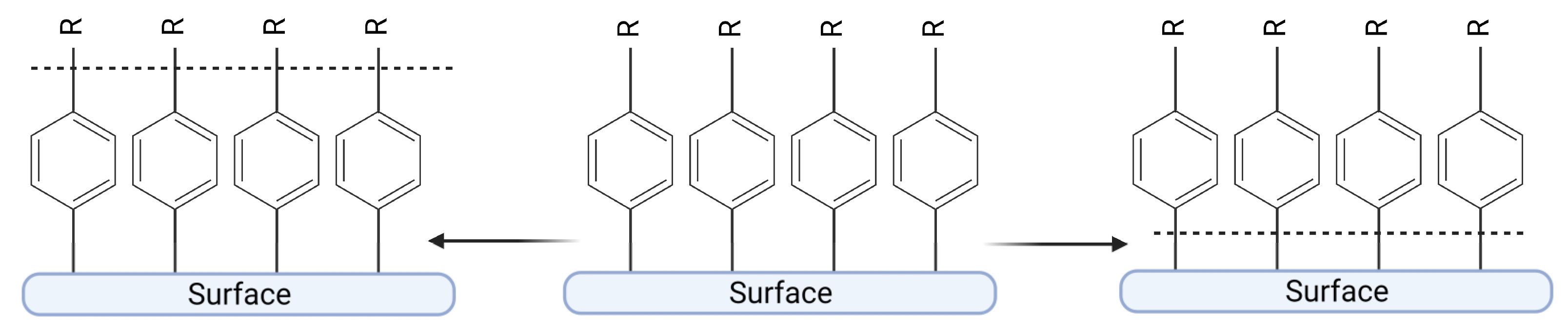
3.3. Modulating Wettability of a Carrier
3.4. Transforming a Catalyst into a Corresponding Diazonium Salt
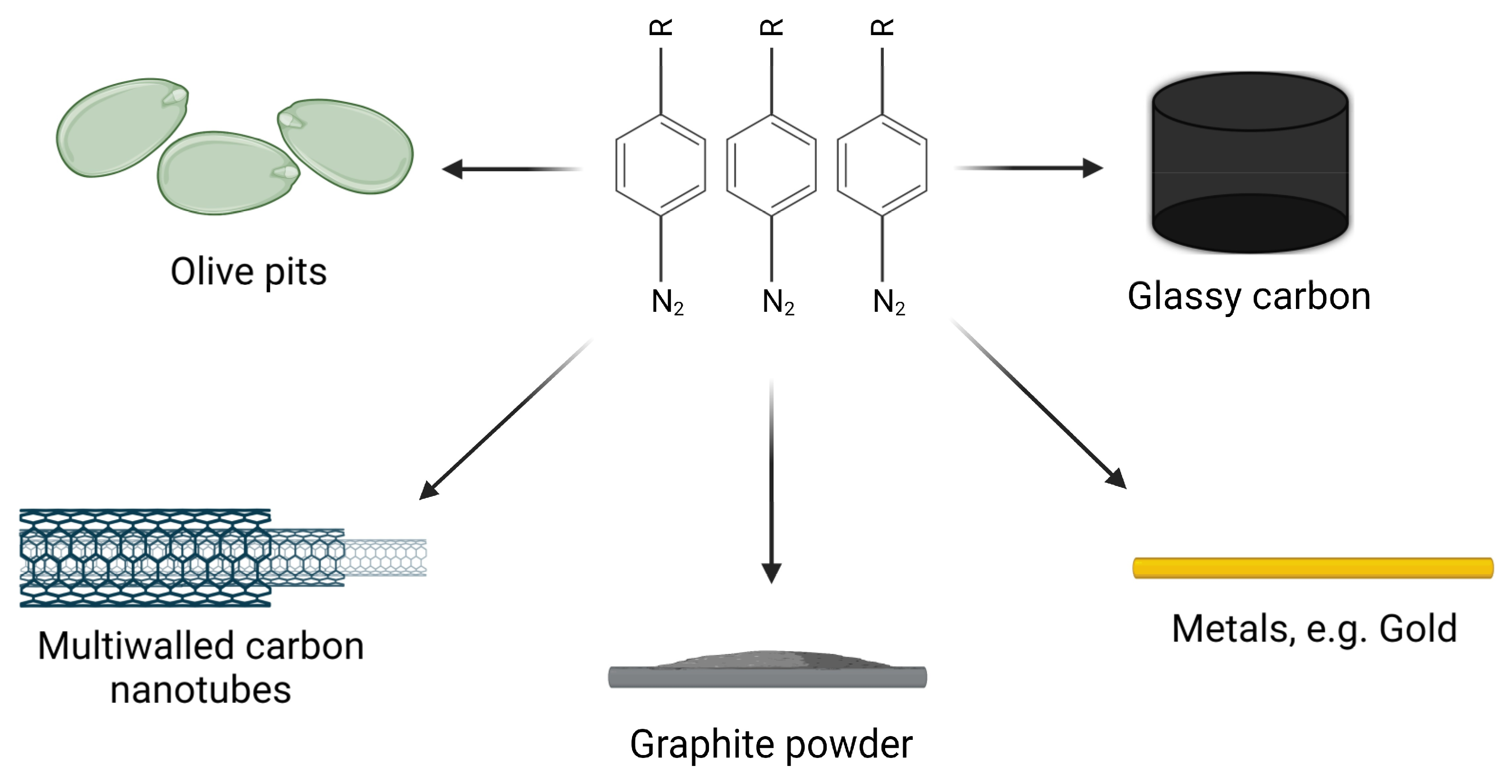
4. Choice of a Carrier
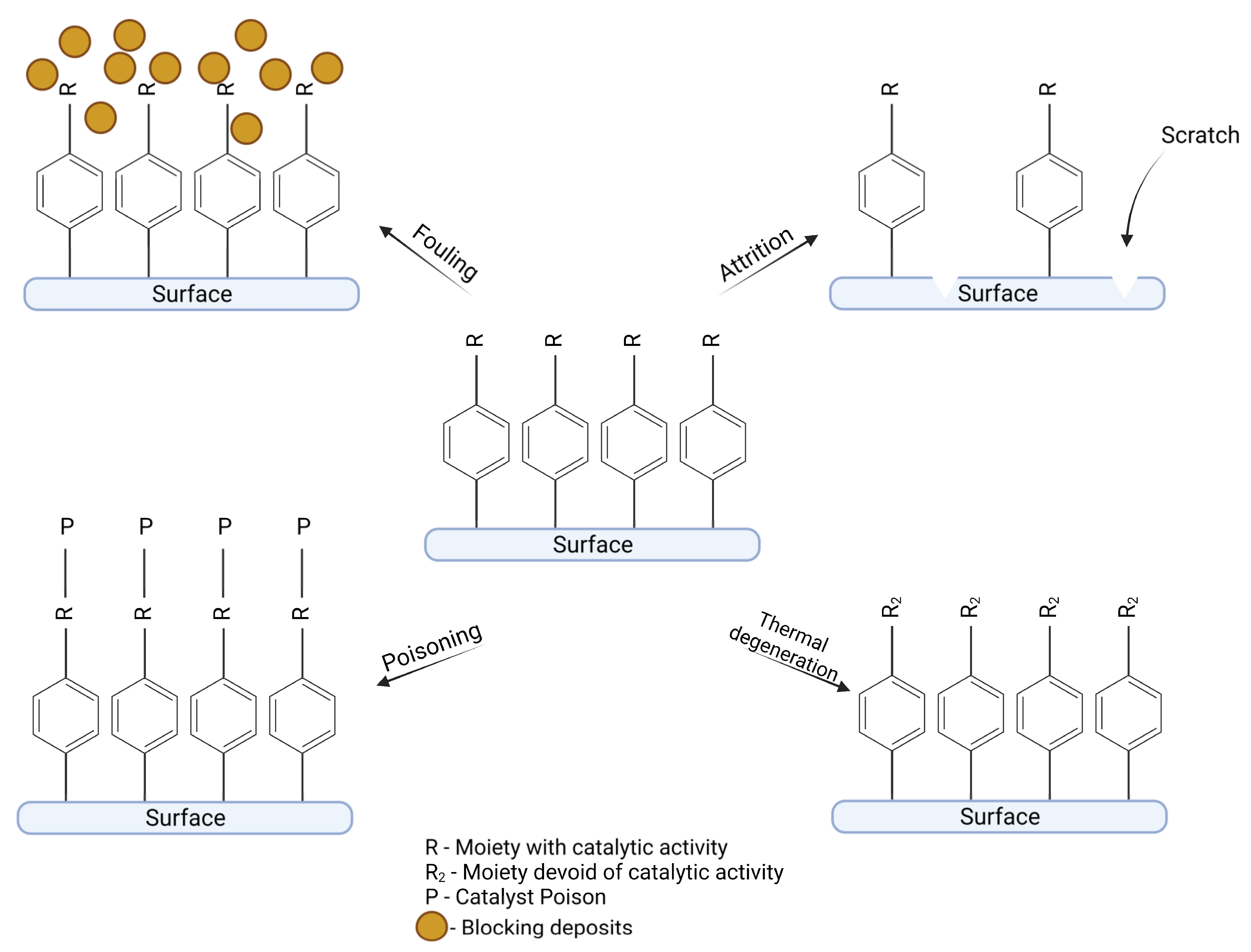
5. Deactivation Routes of Diazonium-Based Catalysts
5.1. Mechanical Stability
5.2. Thermal Stability
5.3. Chemical Stability
5.4. Deactivation through Poisoning
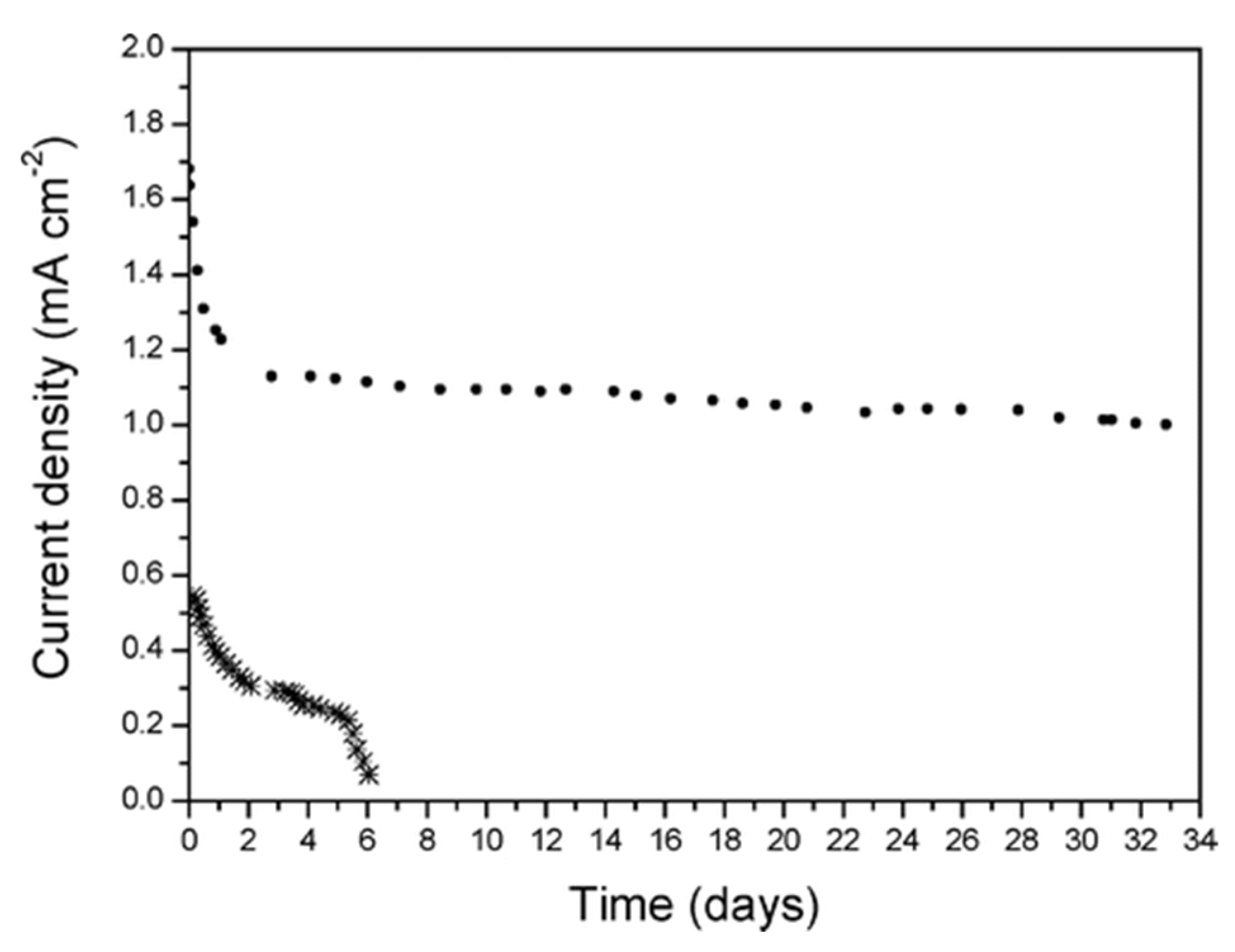
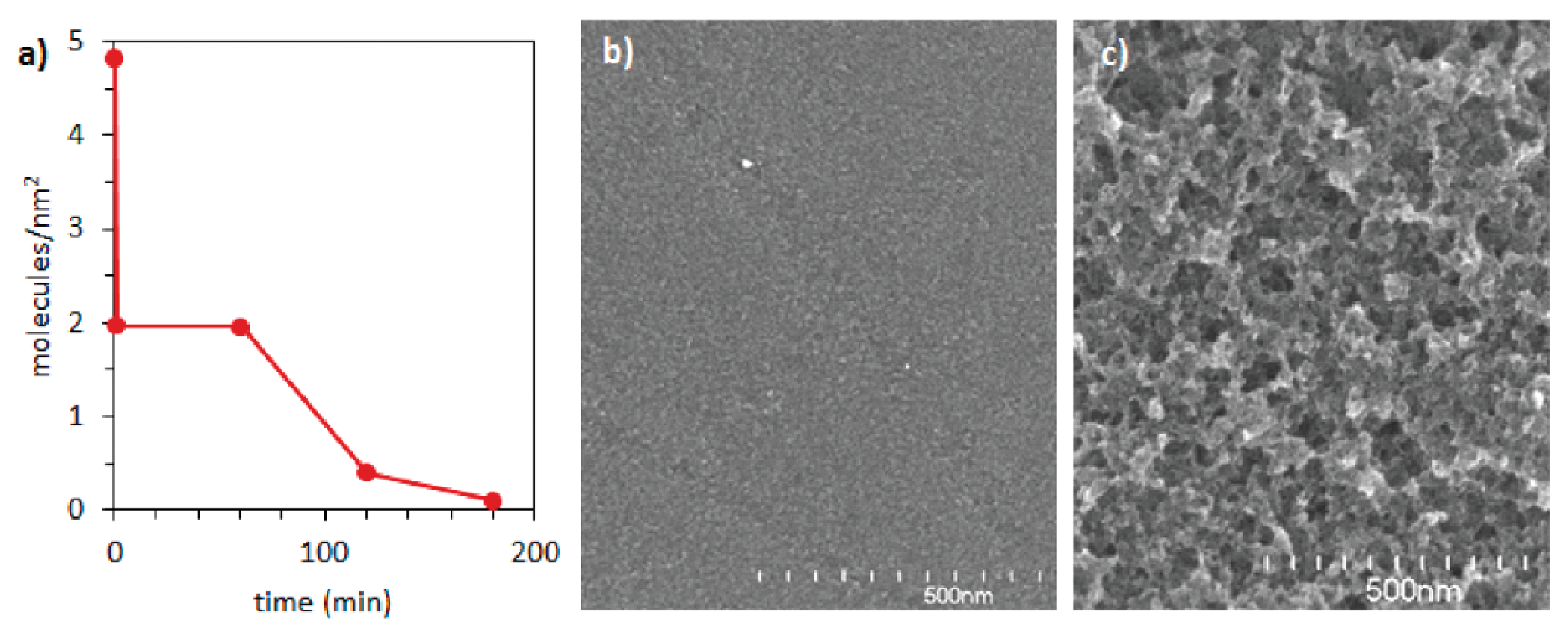
6. Stability of Diazonium-Based Catalysts: Experimental Studies
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sunol, A.K.; Sunol, S.G.; Cogswell, K.; Rooney, D.; Jacquemin, J.; García-álvarez, J.; Villanueva-Bermejo, D.; Fornari, T. Substitution of solvents by safer products. In Handbook of Solvents, Volume 2: Use, Health, and Environment; Elsevier: Amsterdam, The Netherlands, 2019; pp. 1455–1634. [Google Scholar] [CrossRef]
- Fadhel, A.Z.; Pollet, P.; Liotta, C.L.; Eckert, C.A. Combining the Benefits of Homogeneous and Heterogeneous Catalysis with Tunable Solvents and Nearcritical Water. Molecules 2010, 15, 8400. [Google Scholar] [CrossRef]
- de Vos, D.E.; Sels, B.F.; Jacobs, P.A. Immobilization of Homogeneous Oxidation Catalysts. Adv. Catal. 2001, 46, 1–87. [Google Scholar] [CrossRef]
- Vankelecom, I. Catalyst Immobilization in Membranes. In Encyclopedia of Membranes; Springer: Berlin/Heidelberg, Germany, 2016; pp. 315–316. [Google Scholar] [CrossRef]
- Stasiak, M.; Röben, C.; Rosenberger, N.; Schleth, F.; Studer, A.; Greiner, A.; Wendorff, J.H. Design of Polymer Nanofiber Systems for the Immobilization of Homogeneous Catalysts—Preparation and Leaching Studies. Polymer 2007, 48, 5208–5218. [Google Scholar] [CrossRef]
- Bélanger, D.; Pinson, J. Electrografting: A Powerful Method for Surface Modification. Chem. Soc. Rev. 2011, 40, 3995–4048. [Google Scholar] [CrossRef]
- Radi, A.E.; Muñoz-Berbel, X.; Cortina-Puig, M.; Marty, J.L. Novel Protocol for Covalent Immobilization of Horseradish Peroxidase on Gold Electrode Surface. Electroanalysis 2009, 21, 696–700. [Google Scholar] [CrossRef]
- Guo, D.J.; Li, H.L. Highly Dispersed Ag Nanoparticles on Functional MWNT Surfaces for Methanol Oxidation in Alkaline Solution. Carbon 2005, 43, 1259–1264. [Google Scholar] [CrossRef]
- Revenga-Parra, M.; Villa-Manso, A.M.; Briones, M.; Mateo-Martí, E.; Martínez-Periñán, E.; Lorenzo, E.; Pariente, F. Bioelectrocatalytic Platforms Based on Chemically Modified Nanodiamonds by Diazonium Salt Chemistry. Electrochim. Acta 2020, 357, 136876. [Google Scholar] [CrossRef]
- Hayat, A.; Sassolas, A.; Marty, J.L.; Radi, A.E. Highly Sensitive Ochratoxin A Impedimetric Aptasensor Based on the Immobilization of Azido-Aptamer onto Electrografted Binary Film via Click Chemistry. Talanta 2013, 103, 14–19. [Google Scholar] [CrossRef]
- Alonso-Lomillo, M.A.; Rüdiger, O.; Maroto-Valiente, A.; Velez, M.; Rodríguez-Ramos, I.; Muñoz, F.J.; Fernández, V.M.; De Lacey, A.L. Hydrogenase-Coated Carbon Nanotubes for Efficient H2 Oxidation. Nano Lett. 2007, 7, 1603–1608. [Google Scholar] [CrossRef]
- Marwan, J.; Addou, T.; Bélanger, D. Functionalization of Glassy Carbon Electrodes with Metal-Based Species. Chem. Mater. 2005, 17, 2395–2403. [Google Scholar] [CrossRef]
- Belbekhouche, S.; Kebe, S.I.; Mahouche-Chergui, S.; Guerrouache, M.; Carbonnier, B.; Jaziri, M.; Chehimi, M.M. Aryl Diazonium-Modified Olive Waste: A Low Cost Support for the Immobilization of Nanocatalysts. Colloids Surf. A Physicochem. Eng. Asp. 2017, 529, 541–549. [Google Scholar] [CrossRef]
- Mirkhalaf, F.; Graves, J.E. Nanostructured Electrocatalysts Immobilised on Electrode Surfaces and Organic Film Templates. Chem. Pap. 2012, 66, 472–483. [Google Scholar] [CrossRef]
- Mousli, F.; Snoussi, Y.; Chehimi, M.M.; Wojcieszak, R. On the Use of Diazonium Salts in the Design of Catalytic Hybrid Materials and Coatings. In Aryl Diazonium Salts and Related Compounds: Surface Chemistry and Applications; Springer International Publishing: Cham, Switzerland, 2022; pp. 287–308. [Google Scholar] [CrossRef]
- Mo, F.; Dong, G.; Zhang, Y.; Wang, J. Recent Applications of Arene Diazonium Salts in Organic Synthesis. Org. Biomol. Chem. 2013, 11, 1582–1593. [Google Scholar] [CrossRef]
- Yates, E.; Yates, A. Johann Peter Griess FRS (1829–88): Victorian Brewer and Synthetic Dye Chemist. Notes Rec. R. Soc. Lond. 2016, 70, 65. [Google Scholar] [CrossRef]
- Li, X.; Ye, X.; Wei, C.; Shan, C.; Wojtas, L.; Wang, Q.; Shi, X. Diazo Activation with Diazonium Salts: Synthesis of Indazole and 1,2,4-Triazole. Org. Lett. 2020, 22, 4151–4155. [Google Scholar] [CrossRef]
- Mahouche-Chergui, S.; Gam-Derouich, S.; Mangeney, C.; Chehimi, M.M. Aryl Diazonium Salts: A New Class of Coupling Agents for Bonding Polymers, Biomacromolecules and Nanoparticles to Surfaces. Chem. Soc. Rev. 2011, 40, 4143–4166. [Google Scholar] [CrossRef]
- Hetemi, D.; Noël, V.; Pinson, J. Grafting of Diazonium Salts on Surfaces: Application to Biosensors. Biosensors 2020, 10, 4. [Google Scholar] [CrossRef]
- Noël, J.M.; Sjöberg, B.; Marsac, R.; Zigah, D.; Bergamini, J.F.; Wang, A.; Rigaut, S.; Hapiot, P.; Lagrost, C. Flexible Strategy for Immobilizing Redox-Active Compounds Using in Situ Generation of Diazonium Salts. Investigations of the Blocking and Catalytic Properties of the Layers. Langmuir 2009, 25, 12742–12749. [Google Scholar] [CrossRef] [PubMed]
- Vaz-Dominguez, C.; Campuzano, S.; Rüdiger, O.; Pita, M.; Gorbacheva, M.; Shleev, S.; Fernandez, V.M.; de Lacey, A.L. Laccase Electrode for Direct Electrocatalytic Reduction of O2 to H2O with High-Operational Stability and Resistance to Chloride Inhibition. Biosens. Bioelectron. 2008, 24, 531–537. [Google Scholar] [CrossRef]
- Le Goff, A.; Artero, V.; Jousselme, B.; Tran, P.D.; Guillet, N.; Métayé, R.; Fihri, A.; Palacin, S.; Fontecave, M. From Hydrogenases to Noble Metal-Free Catalytic Nanomaterials for H2 Production and Uptake. Science 2009, 326, 1384–1387. [Google Scholar] [CrossRef]
- Harper, J.C.; Polsky, R.; Dirk, S.M.; Wheeler, D.R.; Brozik, S.M. Electroaddressable Selective Functionalization of Electrode Arrays: Catalytic NADH Detection Using Aryl Diazonium Modified Gold Electrodes. Electroanalysis 2007, 19, 1268–1274. [Google Scholar] [CrossRef]
- Bourdillon, C.; Delamar, M.; Demaille, C.; Hitmi, R.; Moiroux, J.; Pinson, J. Immobilization of Glucose Oxidase on a Carbon Surface Derivatized by Electrochemical Reduction of Diazonium Salts. J. Electroanal. Chem. 1992, 336, 113–123. [Google Scholar] [CrossRef]
- Radi, A.E.; Lates, V.; Marty, J.L. Mediatorless Hydrogen Peroxide Biosensor Based on Horseradish Peroxidase Immobilized on 4-Carboxyphenyl Film Electrografted on Gold Electrode. Electroanalysis 2008, 20, 2557–2562. [Google Scholar] [CrossRef]
- Marshall, N.; Locklin, J. Reductive Electrografting of Benzene (p-Bisdiazonium Hexafluorophosphate): A Simple and Effective Protocol for Creating Diazonium-Functionalized Thin Films. Langmuir 2011, 27, 13367–13373. [Google Scholar] [CrossRef] [PubMed]
- Press Release: The Nobel Prize in Chemistry 2022. Available online: https://www.nobelprize.org/prizes/chemistry/2022/press-release/ (accessed on 26 October 2022).
- Ran, Q.; Peng, R.; Liang, C.; Ye, S.; Xian, Y.; Zhang, W.; Jin, L. Covalent Immobilization of Horseradish Peroxidase via Click Chemistry and Its Direct Electrochemistry. Talanta 2011, 83, 1381–1385. [Google Scholar] [CrossRef]
- Zhang, L.; Vilà, N.; Klein, T.; Kohring, G.W.; Mazurenko, I.; Walcarius, A.; Etienne, M. Immobilization of Cysteine-Tagged Proteins on Electrode Surfaces by Thiol-Ene Click Chemistry. ACS Appl. Mater. Interfaces 2016, 8, 17591–17598. [Google Scholar] [CrossRef]
- Zhang, L.; Vilà, N.; Walcarius, A.; Etienne, M. Molecular and Biological Catalysts Coimmobilization on Electrode by Combining Diazonium Electrografting and Sequential Click Chemistry. ChemElectroChem 2018, 5, 2208–2217. [Google Scholar] [CrossRef]
- Bensghaïer, A.; Bhullar, V.; Kaur, N.; Lo, M.; Bdiri, M.; Mahajan, A.; Chehimi, M.M. “Painted CNT”@Au Nanoparticles: A Nanohybrid Electrocatalyst of Direct Methanol Oxidation. Emergent Mater. 2021, 4, 515–524. [Google Scholar] [CrossRef]
- Aghajani, A.; Santoni, M.P.; Mirzaei, P.; Mohamed, A.A.; Chehimi, M.M.; Jouini, M. Tuning Arylation of Gold Nanoparticles for the Electrocatalyzed Oxidation of Ethanol. Appl. Organomet. Chem. 2022, 36, e6835. [Google Scholar] [CrossRef]
- Ait-Touchente, Z.; Falah, S.; Scavetta, E.; Chehimi, M.M.; Touzani, R.; Tonelli, D.; Taleb, A. Different Electrochemical Sensor Designs Based on Diazonium Salts and Gold Nanoparticles for Pico Molar Detection of Metals. Molecules 2020, 25, 3903. [Google Scholar] [CrossRef]
- Yang, H.; Li, S.; Shen, S.; Jin, Z.; Jin, J.; Ma, J. Unraveling the Cooperative Synergy of Palladium/Tin Oxide/Aniline-Functionalized Carbon Nanotubes Enabled by Layer-by-Layer Synthetic Strategy for Ethanol Electrooxidation. ACS Sustain. Chem. Eng. 2019, 7, 10008–10015. [Google Scholar] [CrossRef]
- Mirzaei, P.; Bastide, S.; Aghajani, A.; Bourgon, J.; Leroy, É.; Zhang, J.; Snoussi, Y.; Bensghaier, A.; Hamouma, O.; Chehimi, M.M.; et al. Bimetallic Cu-Rh Nanoparticles on Diazonium-Modified Carbon Powders for the Electrocatalytic Reduction of Nitrates. Langmuir 2019, 35, 14428–14436. [Google Scholar] [CrossRef]
- Bollella, P.; Hibino, Y.; Kano, K.; Gorton, L.; Antiochia, R. Enhanced Direct Electron Transfer of Fructose Dehydrogenase Rationally Immobilized on a 2-Aminoanthracene Diazonium Cation Grafted Single-Walled Carbon Nanotube Based Electrode. ACS Catal. 2018, 8, 10279–10289. [Google Scholar] [CrossRef]
- Park, Y.B.; You, E.; Pak, C.; Min, M. Preparation and Characterization of Durable Catalyst via Diazonium Reaction in PEMFC. Electrochim. Acta 2018, 284, 242–252. [Google Scholar] [CrossRef]
- Gross, A.J.; Tanaka, S.; Colomies, C.; Giroud, F.; Nishina, Y.; Cosnier, S.; Tsujimura, S.; Holzinger, M. Diazonium Electrografting vs. Physical Adsorption of Azure A at Carbon Nanotubes for Mediated Glucose Oxidation with FAD-GDH. ChemElectroChem 2020, 7, 4543–4549. [Google Scholar] [CrossRef]
- Revenga-Parra, M.; Gómez-Anquela, C.; García-Mendiola, T.; Gonzalez, E.; Pariente, F.; Lorenzo, E. Grafted Azure A Modified Electrodes as Disposable β-Nicotinamide Adenine Dinucleotide Sensors. Anal. Chim. Acta 2012, 747, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Karthik, M.; Suresh, P. Brønsted Acidic Reduced Graphene Oxide as a Sustainable Carbocatalyst: A Selective Method for the Synthesis of C-2-Substituted Benzimidazole. New J. Chem. 2018, 42, 17931–17938. [Google Scholar] [CrossRef]
- Firth, J.D.; Fairlamb, I.J.S. A Need for Caution in the Preparation and Application of Synthetically Versatile Aryl Diazonium Tetrafluoroborate Salts. Org. Lett. 2020, 22, 7057–7059. [Google Scholar] [CrossRef]
- Nasri, Z.; Shams, E.; Ahmadi, M. Direct Modification of a Glassy Carbon Electrode with Toluidine Blue Diazonium Salt: Application to NADH Determination and Biosensing of Ethanol. Electroanalysis 2013, 25, 1917–1925. [Google Scholar] [CrossRef]
- Yang, X.; Hall, S.B.; Tan, S.N. Electrochemical Reduction of a Conjugated Cinnamic Acid Diazonium Salt as an Immobilization Matrix for Glucose Biosensor. Electroanalysis 2003, 15, 885–891. [Google Scholar] [CrossRef]
- DeKrafft, K.E.; Wang, C.; Xie, Z.; Su, X.; Hinds, B.J.; Lin, W. Electrochemical Water Oxidation with Carbon-Grafted Iridium Complexes. ACS Appl. Mater. Interfaces 2012, 4, 608–613. [Google Scholar] [CrossRef] [PubMed]
- Cesbron, M.; Dabos-Seignon, S.; Gautier, C.; Breton, T. Enhanced Electrocatalytic Activity on TEMPO Mixed Film Grafted by Diazonium Reduction. Electrochim. Acta 2020, 345, 136190. [Google Scholar] [CrossRef]
- Kudas, Z.; Atmaca, U.; Saruhan, T.; Celik, M.; Ekinci, D. Electrocatalytic Reduction of Oxygen at Glassy Carbon Electrodes Coated with Diazonium-Derived Porphyrin/Metalloporphyrin Films. Electroanalysis 2020, 32, 1379–1390. [Google Scholar] [CrossRef]
- Bartholomew, C.H. Mechanisms of Catalyst Deactivation. Appl. Catal. A Gen. 2001, 212, 17–60. [Google Scholar] [CrossRef]
- Anariba, F.; DuVall, S.H.; McCreery, R.L. Mono- and Multilayer Formation by Diazonium Reduction on Carbon Surfaces Monitored with Atomic Force Microscopy “Scratching”. Anal. Chem. 2003, 75, 3837–3844. [Google Scholar] [CrossRef]
- Hinge, M.; Gonçalves, E.S.; Pedersen, S.U.; Daasbjerg, K. On the Electrografting of Stainless Steel from Para-Substituted Aryldiazonium Salts and the Thermal Stability of the Grafted Layer. Surf. Coat. Technol. 2010, 205, 820–827. [Google Scholar] [CrossRef]
- Toupin, M.; Bélanger, D. Thermal Stability Study of Aryl Modified Carbon Black by in Situ Generated Diazonium Salt. J. Phys. Chem. C 2007, 111, 5394–5401. [Google Scholar] [CrossRef]
- Civit, L.; Fragoso, A.; O’Sullivan, C.K. Thermal Stability of Diazonium Derived and Thiol-Derived Layers on Gold for Application in Genosensors. Electrochem. Commun. 2010, 12, 1045–1048. [Google Scholar] [CrossRef]
- Mahesh, S.; Tang, K.C.; Raj, M. Amide Bond Activation of Biological Molecules. Molecules 2018, 23, 2615. [Google Scholar] [CrossRef]
- Li, G.; Ma, S.; Szostak, M. Amide Bond Activation: The Power of Resonance. Trends Chem. 2020, 2, 914–928. [Google Scholar] [CrossRef]
- Huang, L.; Shi, Y.I.; Chen, L.; Jin, X.; Liu, R.; Winnik, M.A.; Mitchell, D. Thermal Decomposition of Amide and Imide Derivatives of Maleated Polyethylene. J. Polym. Sci. Part A Polym. Chem. 2000, 38, 730–740. [Google Scholar] [CrossRef]
- Çanakçı, D. Thermal Stability, Degradation Kinetic and Structural Characterization of Novel Aromatic Amide Compounds. J. Mol. Struct. 2020, 1205, 127645. [Google Scholar] [CrossRef]
- Nguyen, T.L.; Saleh, M.A. Thermal Degradation of Azobenzene Dyes. Results Chem. 2020, 2, 100085. [Google Scholar] [CrossRef]
- Arabahmadi, R. Synthesis, Spectral Characterization, Thermal Analysis and Electrochemistry Properties of Ni(II) Complexes Derived from Azo Dyes. J. Therm. Anal. Calorim. 2016, 123, 595–605. [Google Scholar] [CrossRef]
- Guo, S.; Wan, W.; Chen, C.; Chen, W.H. Thermal Decomposition Kinetic Evaluation and Its Thermal Hazards Prediction of AIBN. J. Therm. Anal. Calorim. 2013, 113, 1169–1176. [Google Scholar] [CrossRef]
- Yamashina, M.; Sei, Y.; Akita, M.; Yoshizawa, M. Safe Storage of Radical Initiators within a Polyaromatic Nanocapsule. Nat. Commun. 2014, 5, 4662. [Google Scholar] [CrossRef]
- Kazem-Rostami, M. Factors Influencing the Thermal Stability of Azo and Bisazo Compounds. J. Therm. Anal. Calorim. 2020, 140, 613–623. [Google Scholar] [CrossRef]
- Zhou, A.; Wan, L.; Hu, Y.; Huang, F.; Du, L. Synthesis and Characterization of Novel Polytriazoleimides by CuAAC Step-Growth Polymerization. Polym. J. 2010, 42, 216–222. [Google Scholar] [CrossRef]
- Ma, Q.; Lu, H.; Qu, Y.; Liao, L.; Li, J.; Fan, G.; Chen, Y. A Facile Synthesis of 3,3’-Dinitro-5,5’-Diamino-Bi-1,2,4-Triazole and a Study of Its Thermal Decomposition. J. Energ. Mater. 2017, 2017, 281–295. [Google Scholar] [CrossRef]
- Sykam, K.; Donempudi, S.; Basak, P. 1,2,3-Triazole Rich Polymers for Flame Retardant Application: A Review. J. Appl. Polym. Sci. 2022, 139, e52771. [Google Scholar] [CrossRef]
- Griffete, N.; Herbst, F.; Pinson, J.; Ammar, S.; Mangeney, C. Preparation of Water-Soluble Magnetic Nanocrystals Using Aryl Diazonium Salt Chemistry. J. Am. Chem. Soc. 2011, 133, 1646–1649. [Google Scholar] [CrossRef] [PubMed]
- Fantoni, N.Z.; El-Sagheer, A.H.; Brown, T. A Hitchhiker’s Guide to Click-Chemistry with Nucleic Acids. Chem. Rev. 2021, 121, 7122–7154. [Google Scholar] [CrossRef] [PubMed]
- Hein, J.E.; Fokin, V.V. Copper-Catalyzed Azide–Alkyne Cycloaddition (CuAAC) and beyond: New Reactivity of Copper(i) Acetylides. Chem. Soc. Rev. 2010, 39, 1302. [Google Scholar] [CrossRef] [PubMed]
- Ji Ram, V.; Sethi, A.; Nath, M.; Pratap, R. Five-Membered Heterocycles. In The Chemistry of Heterocycles; Elsevier: Amsterdam, The Netherlands, 2019; pp. 149–478. [Google Scholar] [CrossRef]
- O’Connor, C. Acidic and Basic Amide Hydrolysis. Q. Rev. Chem. Soc. 1970, 24, 553–564. [Google Scholar] [CrossRef]
- Broqvist, P.; Molina, L.M.; Grönbeck, H.; Hammer, B. Promoting and Poisoning Effects of Na and Cl Coadsorption on CO Oxidation over MgO-Supported Au Nanoparticles. J. Catal. 2004, 227, 217–226. [Google Scholar] [CrossRef]
- Shekhar, M.; Wang, J.; Lee, W.S.; Cem Akatay, M.; Stach, E.A.; Nicholas Delgass, W.; Ribeiro, F.H. Counting Au Catalytic Sites for the Water-Gas Shift Reaction. J. Catal. 2012, 293, 94–102. [Google Scholar] [CrossRef]
- Ma, W.; Lv, X.; Han, D.; Li, F.; Dong, X.; Niu, L. Decoration of Electro-Reduced Graphene Oxide with Uniform Gold Nanoparticles Based on in Situ Diazonium Chemistry and Their Application in Methanol Oxidation. J. Electroanal. Chem. 2013, 690, 111–116. [Google Scholar] [CrossRef]
- Jlassi, K.; Zavahir, S.; Kasak, P.; Krupa, I.; Mohamed, A.A.; Chehimi, M.M. Emerging Clay-Aryl-Gold Nanohybrids for Efficient Electrocatalytic Proton Reduction. Energy Convers. Manag. 2018, 168, 170–177. [Google Scholar] [CrossRef]
- Bangle, R.; Sampaio, R.N.; Troian-Gautier, L.; Meyer, G.J. Surface Grafting of Ru(II) Diazonium-Based Sensitizers on Metal Oxides Enhances Alkaline Stability for Solar Energy Conversion. ACS Appl. Mater. Interfaces 2018, 10, 3121–3132. [Google Scholar] [CrossRef]
- Ahmad, A.A.L.; Panicker, S.; Chehimi, M.M.; Monge, M.; Lopez-De-Luzuriaga, J.M.; Mohamed, A.A.; Bruce, A.E.; Bruce, M.R.M. Synthesis of Water-Soluble Gold–Aryl Nanoparticles with Distinct Catalytic Performance in the Reduction of the Environmental Pollutant 4-Nitrophenol. Catal. Sci. Technol. 2019, 9, 6059–6071. [Google Scholar] [CrossRef]



| Catalysts | Type of Bonds | Application | Advantages | Ref. |
|---|---|---|---|---|
| Au/MWCNTs/4-aminophenyl/hydrogenase | amide bonds | hydrogen oxidation | impressive stability compared to adsorbed hydrogenase | [11] |
| MWCNT/4-aminophenyl/AgNPs | C-C bonds | methanol oxidation in alkaline solution | prevention of NPs nucleation | [8] |
| GC/N,N-diethylaniline/Cu | C-C bonds | electrochemical reduction of nitrate | much lower current response compared to catalysts without Cu | [12] |
| GC/4-sulfonatephenyl/Ru | C-C bonds | electrochemical oxidation of H2O2 | unmodified electrode showed no current response, when modified showed strong peak typical for H2O2 oxidation | [12] |
| Au/4-aminophenyl/PQQ | amide bonds | electrooxidation of NADH | protection against non-specific adsorption and mild chemical reactions | [24] |
| Au/p-diazoniumphenyl/HPP | azo-coupling | electrochemical reduction of H2O2 | electrocatalytic activity towards the reduction of H2O2 without any mediator; fast amperometric response to H2O2; acceptable sensitivity, good reproducibility and long-term stability | [7] |
| Carbon electrode/4-((trimethylsilyl)ethynyl)benzene/p-nitrobenzene/aptamer | Click chemistry | detection of ochratoxin A | wide detection range (from 1.25 ng/L to 500 ng/L), detection limit of 0.25 ng/L | [10] |
| GC/SWCNT/2-aminoantraceneFDH | π-π interactions | detection of fructose | efficient direct electron transfer reaction between FDH and GC electrode | [37] |
| Screen printed carbon electrodes/Azure A | C-C bonds | NADH oxidation | high and stable electrocatalytic response | [40] |
| Olive pits/-NH2/AuNP Olive pits/-SH/AuNP Olive pits/-COOH/AgNP | C-C bonds | reduction of nitrophenol | remarkable catalytic activity | [13] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smołka, S.; Krukiewicz, K. Catalyst Design through Grafting of Diazonium Salts—A Critical Review on Catalyst Stability. Int. J. Mol. Sci. 2023, 24, 12575. https://doi.org/10.3390/ijms241612575
Smołka S, Krukiewicz K. Catalyst Design through Grafting of Diazonium Salts—A Critical Review on Catalyst Stability. International Journal of Molecular Sciences. 2023; 24(16):12575. https://doi.org/10.3390/ijms241612575
Chicago/Turabian StyleSmołka, Szymon, and Katarzyna Krukiewicz. 2023. "Catalyst Design through Grafting of Diazonium Salts—A Critical Review on Catalyst Stability" International Journal of Molecular Sciences 24, no. 16: 12575. https://doi.org/10.3390/ijms241612575
APA StyleSmołka, S., & Krukiewicz, K. (2023). Catalyst Design through Grafting of Diazonium Salts—A Critical Review on Catalyst Stability. International Journal of Molecular Sciences, 24(16), 12575. https://doi.org/10.3390/ijms241612575






